Congenital Chest Wall Deformities
Pectus excavatum, also known as an ‘excavated, sunken, or funnel chest,’ is the most common chest wall anomaly, constituting about 80% of deformities seen at our hospital (Table 20-1). Pectus carinatum, a chest wall protuberance, comprises approximately 12% of chest wall deformities, whereas combined excavatum/carinatum deformities are found in about 5%. Jeune syndrome, or asphyxiating chondrodystrophy, is an extreme form of mixed pectus excavatum/carinatum and is very rare.
TABLE 20-1
Incidence and Etiology of Congenital Chest Wall Anomalies Seen at Children’s Hospital of the King’s Daughters

Many of these deformities are present at birth. Some cases, such as ectopia cordis, are incompatible with life and have rarely been successfully repaired. Chest wall deformities are frequently associated with a systemic weakness of the connective tissues and with poor muscular development of the abdominal region, thorax, and spine. An association with Marfan syndrome, Ehlers–Danlos syndrome, and scoliosis, as well as with omphalocele in the case of bifid sternum, have been identified, all of which complicate the management of these patients (see Table 20-1).
Pectus Excavatum
Pectus excavatum is a depression of the anterior chest wall of variable severity and can usually be characterized as mild, moderate, or severe. The deformity may be localized and deep (‘cup-shaped’; Fig. 20-1A), or diffuse and shallow (‘saucer-shaped’; see Fig. 20-1B), or asymmetric (see Fig. 20-1C). The depth and extent of the depression determine the degree of cardiac and pulmonary compression, which, in turn, determines the degree of physiologic effect. Only one-third of patients referred from our own region had a deformity severe enough to require surgical correction. Even with referral of patients with a severe deformity from other centers, our ratio of operative correction has been only about 60% (Box 20-1).
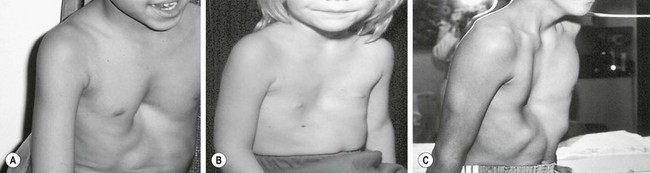
FIGURE 20-1 (A) Localized or ‘cup-shaped’ pectus excavatum. (B) Diffuse or ‘saucer-shaped deformity. (C) Eccentric deformity.
History
Pectus excavatum was recognized as early as the 16th century. Johan Schenck collected literature on the subject.1 In 1594, Bauhinus described the clinical features of pectus excavatum in a patient who had pulmonary compression with dyspnea and paroxysmal cough, both attributed to the severe pectus excavatum.2 The familial predisposition was first noted by Coulson in 1820, who cited a family of three brothers with pectus excavatum.3 In 1872, Williams described a 17-year-old patient who was born with a pectus excavatum, and whose father and brother also had the condition.4
Numerous other case reports appeared in the 19th century, including a five-case report by Ebstein in 1882 that covered the clinical spectrum of this condition.5 Treatment at that time was limited to ‘fresh air, breathing exercises, aerobic activities, and lateral pressure’.6,7
Thoracic surgery remained ‘forbidden territory’ until the early years of the 20th century. The first attempt at correction was a tentative approach in 1911 by Meyer who removed the second and third costal cartilages on the right side without improvement.8 Sauerbruch, one of the pioneers of thoracic surgery, used a more aggressive approach in 1913 by excising a section of the anterior chest wall, which included the left fifth to ninth costal cartilages as well as a segment of the adjacent sternum.7 Before his operation, the patient was incapacitated by severe dyspnea and palpitations, even at rest, and was unable to work in his father’s watch factory. After recovery, the heart could be seen to pulsate under the muscle flap, but the patient was able to work without dyspnea and was married three years later.
In the 1920s, Sauerbruch performed the first pectus repair that used the bilateral costal cartilage resection and sternal osteotomy technique later popularized by Ravitch.9 He also advocated external traction to hold the sternum in its corrected position for six postoperative weeks. His technique was soon adapted by others in Europe and rapidly gained popularity in the USA. In 1939, Ochsner and DeBakey published their experience with this approach, and reviewed the entire surgical literature on the subject.10 Also in 1939, Lincoln Brown published his experience in two patients and reviewed the literature with particular reference to the etiology of pectus excavatum.11 He was impressed with the theory that short diaphragmatic ligaments and the pull of the diaphragm on the posterior sternum were causative factors.
Ravitch initially subscribed to the short ligament theory as well. As a result, he advocated even more radical mobilization of the sternum, with transection of all sternal attachments, including the intercostal bundles, rectus muscles, diaphragmatic attachments, and excision of the xiphisternum. In 1949, he published his experience with eight patients in which he used this radically extended modification of Sauerbruch’s technique of bilateral cartilage resection and sternal osteotomy, but without external traction.12
The lack of external traction may have led to an increased recurrence of the condition. As a result, Wallgren and Sulamaa introduced the concept of internal support in 1956 by using a slightly curved stainless steel bar that was pushed through the caudal end of the sternum from side to side and bridged the newly created gap between the sternum and ribs.13,14 In 1961, Adkins and Blades modified internal bracing further by passing a straight stainless steel bar behind, rather than through, the sternum.15 This technique was rapidly adapted for patients of all ages.
As early as 1958, Welch and Gross advocated a less radical approach than that of Ravitch.16,17 Welch showed excellent results in 75 patients without dividing all the intercostal bundles or the pectus muscle attachments. However, he still advocated performing the procedure in young patients. Conversely, Pena was very disturbed by the idea of resecting the rib cartilages from very young patients and demonstrated that asphyxiating chondrodystrophy developed in baby rabbits after cartilage resection during their growth phase.18 Later, Haller also reported the risk of acquired asphyxiating chondrodystrophy as well.19 As a result, many surgeons stopped performing open pectus repair in young children and waited until they had reached puberty. They also reduced the amount of cartilage resected and spoke about a ‘modified Ravitch procedure,’ which was really the original Sauerbruch procedure.
In 1997, we published our 10-year experience with a minimally invasive technique that did not utilize cartilage resection or sternal osteotomy, but instead relied on internal bracing made possible by the flexibility and malleability of the costal cartilages.20 The rationale for this technique was based on the three following observations:
1. Malleability of the chest. Children have a soft and malleable chest. In young children, the chest is so soft that even minor respiratory obstruction can cause severe sternal retraction. Trauma rarely causes rib fractures and flail chest because the chest is so soft and malleable.21–23 Thus, the American Heart Association recommends ‘using only two fingers’ when performing cardiac resuscitation in young children and ‘only one hand in older children’ for fear of crushing the heart.
2. Chest reconfiguration. In middle-aged and older adults, a barrel-shaped chest configuration develops in response to chronic obstructive respiratory diseases such as emphysema. If older adults are able to reconfigure the chest wall, children and teenagers should be able to remodel as well, especially with the increased malleability of their anterior chest wall.
3. Bracing. The role of braces and serial casting in successfully correcting skeletal anomalies such as scoliosis, clubfoot, and maxillomandibular malocclusion by orthopedic and orthodontic surgeons is well established. The anterior chest wall, being even more malleable than the previously mentioned skeletal structures, is ideally suited for this type of correction.
Incidence and Etiology
Pectus excavatum occurs in approximately 1 in 1000 children and constitutes 80% of all chest wall deformities in our center (see Table 20-1). However, this is not the case in all countries. In Argentina, pectus carinatum is more common than pectus excavatum.24 Pectus excavatum also is rare in African-Americans and in Africans. A genetic predisposition, already noted in the 19th century, has been found in almost 40% of our patients. We have seen families with three siblings, as well as cousins and other family members, who had a pectus deformity severe enough to require correction. We also have seen patients whose fathers and grandfathers have the deformity. The male-to-female ratio of 4 : 1 in our series of pectus excavatum patients is similar to that of other large series.25 Female patients have an increased risk of associated scoliosis. Inheritance is autosomal dominant, autosomal recessive, X-linked, and multifactorial in different families. 26, 27
Clinical Features
Pectus excavatum is noted in infancy in approximately one-third of patients,25 and usually progresses slowly as the child grows. Because young children have significant cardiac and pulmonary reserve and their chest wall is still very pliable, the majority of young children are asymptomatic. However, as they become older, the deformity becomes more severe and the chest wall becomes more rigid. Eventually, they find that they have difficulty keeping up with their peers when playing aerobic sports. A vicious cycle may develop as patients stop participating in aerobic activities because of their inability to keep up. Subsequently, their exercise capacity diminishes further. The downward spiral is further promoted by the fact that these patients, already embarrassed by their deformity, will avoid situations in which they have to remove their shirts in front of other children, inhibiting participation in school and team activities.
By withdrawing from participation in activities with their peers, they also become depressed, which may affect their schoolwork. Most pectus patients have a typical geriatric or ‘pectus posture’ that includes thoracic kyphosis, forward-sloping shoulders, and a protuberant abdomen (Fig. 20-2). A sedentary ‘couch potato’ lifestyle may aggravate this posture, and the poor posture depresses the sternum even farther. For this reason, we always recommend an aggressive pectus posture exercise and breathing program, both preoperatively and postoperatively.

FIGURE 20-2 Classic pectus posture with thoracic kyphosis, forward-sloping shoulders, and lumbar lordosis.
The earliest complaints are shortness of breath and lack of endurance with exercise. As the deformity progresses, chest pain and palpitations with exercise may develop, giving rise to exercise intolerance. Other symptoms include frequent and prolonged respiratory tract infections, which may lead to symptoms of asthma (Table 20-2).
TABLE 20-2
Presenting of Symptoms Patients Who Have Undergone Operative Correction of Pectus Excavatum at Children’s’ Hospital of the King’s Daughters
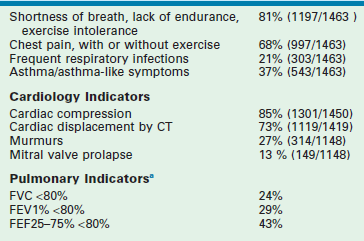
Number of patients with echocardiograms = 1148.
Number of patients with mention of cardiac compression (or not) on CT scan = 1450.
Number of patients with mention of cardiac displacement (or not) on CT scan = 1419.
a<80% defined as 2 SD below average of normal population; in a ‘normal’ dataset, only 2.5% of all persons.
A recent study showed that these patients have a poor body image, which has a major impact on their self-worth.28 Therefore, it is important to correct the deformity before it affects their ability to function normally. Just as one would not consider leaving a child with a cleft lip untreated, one should not leave a child with a severe pectus untreated. Both have a physiologic and psychological impact on the patient.
Cardiac and Pulmonary Effects of Pectus Excavatum
A great deal has been written about cardiopulmonary function in patients with pectus excavatum.29 In the last ten years, there has been increasing acknowledgement in the medical community that a severe pectus excavatum has a significant detrimental effect on cardiopulmonary function. Though studies conflict, many older studies treated the condition as either present or absent, with no quantification of the anatomic severity.30,31 Later work demonstrated the effects more clearly, and showed that in a minority of patients there are no deleterious effects on cardiopulmonary function.32 Several factors play a role when testing cardiopulmonary function. These include the severity of the deformity, the inherent physical fitness of the individual patient, the patient’s age, associated conditions, whether the tests are done supine or erect, and whether they are done at rest or during exercise. Recently, a study has shown statistically significant and clinically meaningful improvements in stroke volume, cardiac output, and cardiac index after minimally invasive repair of pectus excavatum.33 Also, an improvement in exercise cardiopulmonary function has been noted as well.
Cardiac effects include decreased cardiac output, mitral valve prolapse, and arrhythmias (see Table 20-2). Compression of the heart results in incomplete filling and decreased stroke volume, which in turn, results in decreased cardiac output.30,31,34,35 Cardiac compression may interfere with normal valve function. Mitral valve prolapse has been found in 13% of our patients and in up to 65% in other series.36,37 Prolapse occurs in only 1% in the normal pediatric population.38 In one study, the mitral prolapse resolved in about half of patients who underwent correction.36 Thus, both mechanical and connective tissue derangements may be involved. Dysrhythmias, including first-degree heart block, right bundle branch block, and Wolff–Parkinson–White syndrome have been found in 16% of patients.39
Pulmonary effects result from poor motion of the depressed part of the chest wall. Normal chest wall motion includes ‘pump handle’ movement of the sternum. The lower sternum moves up and out, like the handle of a mechanical water pump. Physical examination, and now motion capture analysis, show that this motion is almost absent in the depressed area of the pectus excavatum chest. Instead, patients compensate by increased abdominal diaphragmatic breathing. Following correction, the motion is indistinguishable from normal chest wall.40
Pulmonary function testing shows statistically and clinically meaningful diminution in static pulmonary function tests (forced vital capacity, forced expiratory volume in one second, and others) even though most patients do not have any problem with their airways or pulmonary parenchyma (see Table 20-2).35 These values improve after operation. Exercise pulmonary function testing also shows improvement. Stress testing has shown an increase in oxygen consumption for a given exercise when compared with that of normal patients.41 This shows that the work of breathing is increased and explains why they lack endurance.
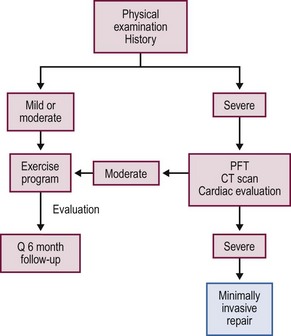
Evaluation and Indications for Operation
A complete history and physical examination is performed on all patients and includes documenting photographs. Younger patients with a mild to moderate deformity are treated with a posture and exercise program in an attempt to halt the progression and are followed at yearly or longer intervals (Fig. 20-3).
Patients with a severe deformity or those with documented progression also are treated with the exercise and posture program. Additionally, they undergo objective studies to evaluate whether their condition is severe enough to warrant repair. These studies include pulmonary function tests (PFTs), a thoracic computed tomography (CT), or magnetic resonance imaging (MRI) scan, and a cardiac evaluation that includes an electrocardiogram (ECG) and an echocardiogram.
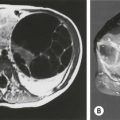
CT scans are very helpful because they clearly show the degree of cardiac compression and displacement, the degree of pulmonary compression and atelectasis, asymmetry of the chest, sternal torsion, compensatory development of a barrel-chest deformity in long-standing deformities, and ossification of the cartilages in patients with previous repairs (Fig. 20-4A, B). They also are used to calculate the CT index, which gives an objective measurement for comparing the severity among patients. The CT index is calculated by dividing the transverse diameter by the anteroposterior diameter (see Fig. 20-4C).42
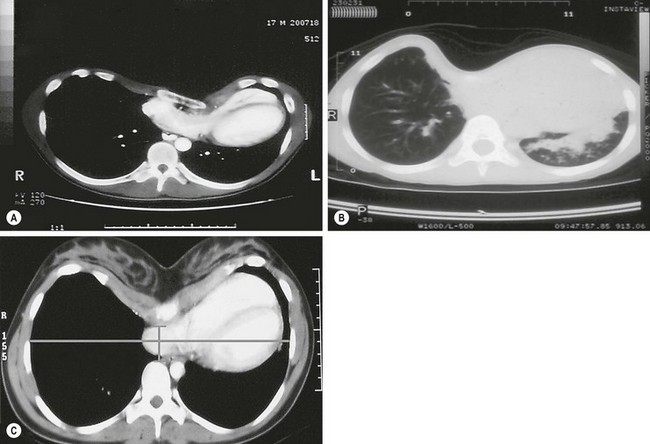
FIGURE 20-4 (A) CT scan showing cardiac compression and displacement, pulmonary compression, asymmetry of the chest, and sternal torsion. (B) CT scan showing severe pulmonary compression and atelectasis. (C) CT index is calculated by dividing the transverse diameter by the anteroposterior diameter.
Determination of a severe pectus excavatum and the need for repair include two or more of the following criteria: (1) a CT index greater than 3.2; (2) pulmonary function studies that indicate restrictive airway disease; (3) a cardiology evaluation in which compression is causing murmurs, mitral valve prolapse, cardiac displacement, or conduction abnormalities on the echocardiogram or ECG tracings; (4) documentation of progression of the deformity with associated physical symptoms other than isolated concerns of body image; (5) a failed Ravitch procedure; or (6) a failed minimally invasive procedure. With these criteria, only about 60% of patients referred to us are found to have a deformity severe enough to warrant correction.20,43
The age parameters for surgical correction depend on the type of repair selected. Unlike the more invasive procedures (e.g., Ravitch procedure, sternal turnover), there is no interference with growth plates with the minimally invasive approach. Therefore, it can be done at any age, as evidenced by the fact that we have successfully operated on patients from ages 13 months through to 31 years (Fig. 20-5). However, the concern with patients younger than 11 years is that if the procedure is performed at too young an age, many years of subsequent growth remain during which the excavatum can recur.
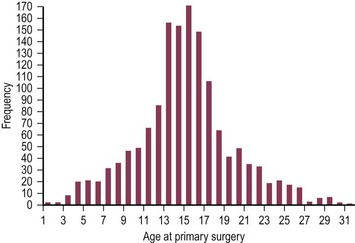
FIGURE 20-5 Age distribution of the authors’ primary pectus excavatum repairs (n = 1463; median age at surgery 15 years, range 1 to 31 years; data collected from 5/18/1987 through to 1/1/2012).
Our experience suggests that the optimal age for repair is 11 to 14 years. At this age, the patient is prepubertal, the chest is still soft and malleable, there is a quick recovery with a rapid return to normal activities, and results are excellent. After puberty, the flexibility of the chest wall is decreased, sometimes requiring the insertion of two bars, which makes the operation more difficult. It also takes patients longer to recover. However, patients older than 20 years have been uniformly pleased with their results. Several other centers have reported success with patients up to age 44 years.34,44,45
Operative Approaches
Minimally Invasive Pectus Repair
The minimally invasive pectus repair (Fig. 20-6) involves making incisions on each side of the chest and creating a subcutaneous tunnel from the lateral thoracic incision to the top of the pectus ridge on each side. At the top of the ridge, bilateral thoracostomy incisions are bluntly created, and a large introducer is inserted into the chest cavity under thoracoscopic visualization. Very carefully, the pleura and pericardium are dissected off the undersurface of the sternum and the introducer is slowly advanced across the mediastinum and exteriorized through the thoracostomy incision on the contralateral side. When the introducer is in place, the sternum is lifted out of its depressed position by the introducer. Once the sternal depression has been corrected, an umbilical tape is attached to the introducer, and the introducer is slowly withdrawn. The pectus support bar is then attached to the umbilical tape and is slowly guided through the substernal tunnel with its convexity facing posteriorly until it emerges on the contralateral side. All the maneuvers are performed using thoracoscopy to see inside the chest.
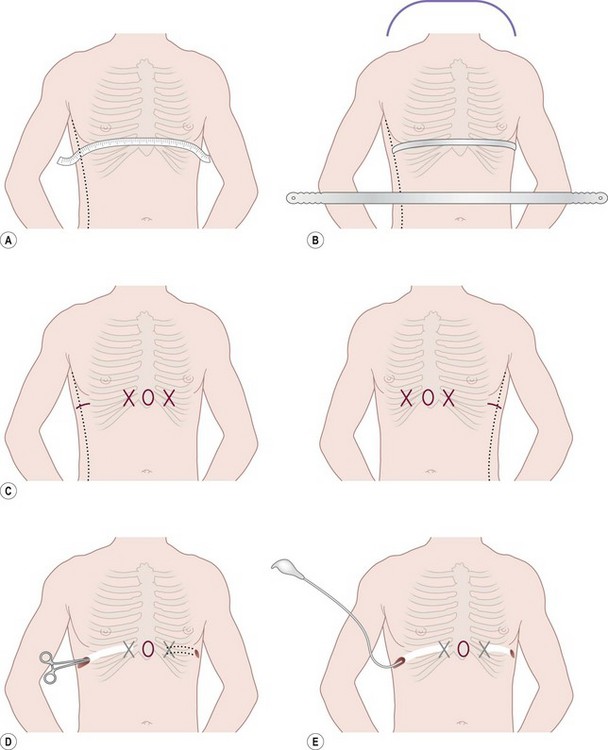
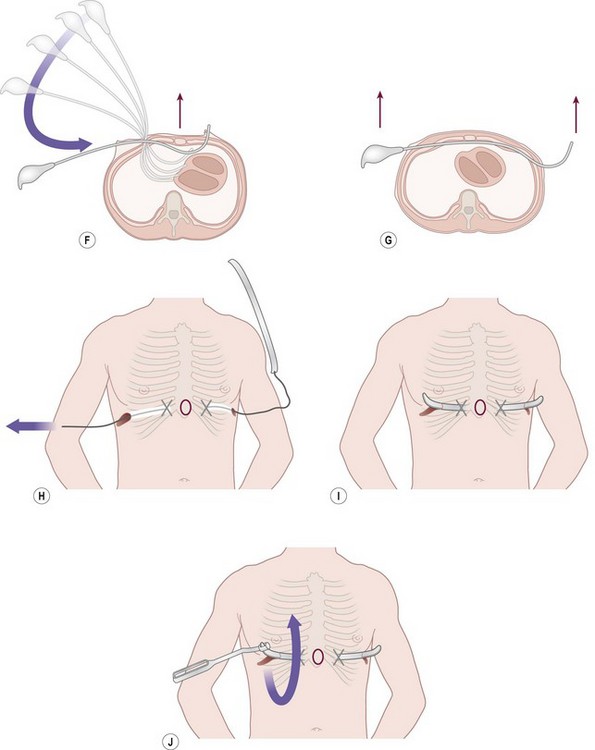
FIGURE 20-6 (A) To calculate the length of the pectus bar, measure the distance from right to left midaxillary line and subtract 1–2 cm (1 inch). (B) Bend the Lorenz pectus support bar to conform to the desired chest wall curvature. (C) Mark the deepest point of the pectus excavatum with a circle by using a marking pen. If this point is inferior to the sternum, then move the circle superiorly to the lower end of the sternum just above the xiphoid. This point sets the horizontal plane bar for insertion. (D) After confirming by thoracoscopy that the internal and external anatomy match up well, make lateral thoracic skin incisions and raise skin flaps anteriorly toward the ‘X’ marked on the external skin at the top of the pectus ridge. (E) Retract skin incision anteriorly to allow visualization of the intercostal space previously marked with an ‘X’. Under thoracoscopic control, insert the appropriate size Lorenz introducer through the right intercostal space at the top of the pectus ridge and at the previously marked ‘X.’ (F) When the substernal tunnel has been completed, gently push the tip of the introducer through the contralateral intercostal space at the previously marked ‘X,’ medial to the top of the pectus ridge on the left side. (G) Use the introducer to elevate the sternum. The surgeon lifts on the right side, and the assistant lifts the left side of the introducer. (H) Attach the previously prepared pectus bar to the umbilical tape and slowly guide the bar through the tunnel by using the umbilical tape for traction. (I) The bar is inserted with the convexity facing posteriorly. (J) When the bar is in position, use the specially designed Lorenz bar rotational instrument (bar flipper) to turn the bar over.
A recent prospective randomized trial evaluated the use of a thoracic epidural catheter vs patient-controlled analgesia (PCA) with intravenous narcotics for postoperative pain management after the minimally invasive pectus repair. One hundred and ten patients were randomized with fixed protocols for each arm. The primary outcome variable was length of postoperative hospitalization with a power of 0.8 and an alpha of 0.05. No difference was found in length of hospitalization between the two treatment arms. There was a longer operative time, more calls to anesthesia, and greater hospital charges in the epidural group. Pain scores favored the epidural pathway in the first few days and the PCA pathway thereafter. Interestingly, the epidural catheter could not be placed or was removed within 24 hours in 12 patients (22%).46
Open Technique
The preoperative preparation and evaluation are the same for the open approach for pectus repair as for minimally invasive technique. However, because of the risk of interference with growth plates in young children and the development of asphyxiating chondrodystrophy, the procedure should be reserved for patients who have completed their growth.18,19 The open procedure is best suited for the older patients, especially those who have asymmetric or eccentric deformities, and patients with mixed pectus carinatum/excavatum deformities.
The open technique involves making an anterior thoracic incision and elevating skin and muscle flaps until all the costal cartilages from T3 to T6 are exposed. The perichondrium is then incised longitudinally, and the deformed cartilages are either partially or completely removed. Most surgeons now advocate removing only a small section (1–2 cm) of the deformed cartilages, as was originally advocated by Sauerbruch and Gross.9,17 An anterior table, wedge-shaped, sternal osteotomy is performed at the angle of Louis. The sternum is elevated, and the osteotomy is closed with nonabsorbable sutures. Some surgeons insert a metal strut under the sternum to bridge the gap between the ribs and the sternum to prevent the sternum from sinking back into the chest. The perichondrial ‘sleeves’ are approximated with absorbable sutures, drains are left, the muscle flaps are sutured back into position, and the incisions are closed. Postoperative management is similar to that for the minimally invasive technique except that patients are required to refrain from contact sports for at least three months.
Results
The minimally invasive approach received rapid acceptance by the surgical community because the technique requires neither rib incision nor resection nor sternal osteotomy. The blood loss is minimal, the operating time is short, and the patient rapidly returns to regular activity.47–53
Although our initial report in 1998 presented a ten-year experience, the numbers were limited (42 patients) and the long-term results were affected by the early learning curve of using a support bar that was too soft.20 Moreover, in some of these patients, the bar was removed too soon. From 1988 through December 2011, 1463 patients have had their initial operation at our institution (see Box 20-1). Since the original presentation, numerous important modifications have been made, both to the operative technique (e.g., routine use of thoracoscopy) and instruments, to minimize the risks of the procedure and facilitate insertion and stabilization of the support bar. These modifications have markedly reduced the risks and complications and have been previously reported.43,45
In our series of 1463 patients with pectus excavatum, only seven (0.48%) had a mixed pectus excavatum and carinatum. One (0.07%) patient had Poland syndrome, and one (0.1%) had an associated complex cardiac anomaly (atrioventricular canal). The male-to-female ratio in patients undergoing repair was more than 4 : 1. The median age was 15 years, with a range from 13 months to 32 years. Preoperative evaluation included CT scan with a median CT index of 4.6 (range, 2.4 to 21). Cardiac compression was noted on echocardiography or CT scan, or both, in 1,301 of 1,450 patients (89.72%). Results of PFT’s are shown in Table 20-2.
Complications
Early Complications
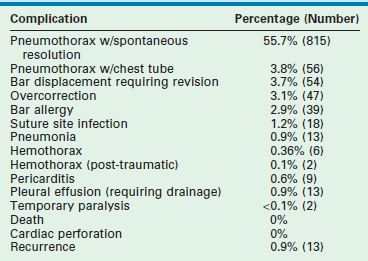
No deaths or cardiac perforations occurred during the 1463 repairs at our institution. Pneumothorax requiring chest tube drainage developed in 56 (3.8%) repairs. Blood loss in most patients was minimal (±10 mL), with the exception of 6 (0.4%) patients in whom a hemothorax formed. Thirteen (0.9%) pleural effusions required treatment with either a chest tube or aspiration (Table 20-3).
Late Complications
Thirty-nine (2.7%) of 1463 patients have had unsuspected allergies to the metal in the bar. These allergies initially presented as rashes in the area of the bar or stabilizer, and required revision with custom-made bars using other alloys. Mild overcorrection occurred in 47 (3.1%) patients. In four, a true carinatum deformity developed. Of the patients in whom a true carinatum deformity developed, three had Marfan syndrome and the other had Ehlers–Danlos syndrome. No patient has developed thoracic chondrodystrophy.
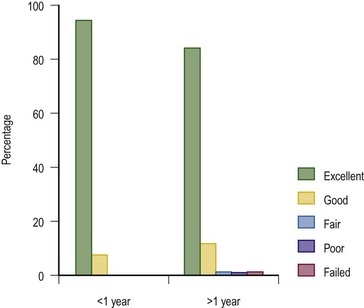
Overall Results and Long-Term Follow-up
Bar Removal
We advise the pectus bar be left in place for two to three years. We evaluate patients on an annual basis and monitor their growth, activity level, and PFT results, and encourage them to perform their pectus exercises and participate in aerobic sports. Patients between the ages of 6 and 10 years often do not grow rapidly. Therefore, they tolerate the bar well for three or even four years. Conversely, we have had teenagers who have undergone a massive growth spurt, completely outgrowing the bar, and requiring bar removal after 2 years. We consider the exercise programs to be as important as the operation. Many children and adults lead sedentary lifestyles and never perform aerobic activities. Therefore, their lungs never expand beyond the resting tidal volume (approximately 10% of total lung capacity). Deep breathing with breath holding for ten to 15 seconds and aerobic activities, such as running (e.g., soccer, basketball) and swimming, are strongly encouraged. We have seen a mild recurrence over the long term in patients who do not follow our exercise protocol (Fig. 20-7).
Pectus Carinatum
Pectus carinatum, or protrusion deformity of the chest, occurs less frequently than does pectus excavatum in most countries. It comprises about 5% of patients with chest wall deformities.54 The prominence may be in the upper manubrium of the sternum, which is called a chondromanubrial deformity. The most common protrusion is found in the lower segment or body of the sternum (the gladiolus) and is called chondrogladiolar (Fig. 20-8). The protrusion may be unilateral, bilateral, or mixed.55 About 80% of patients who develop pectus carinatum are boys. Although the etiology is unknown, a genetic component of causation is suggested by the approximately 25% of patients with a family history of a chest wall defect.53,56,57 Pectus carinatum has also been reported to occur after treatment for pectus excavatum.58
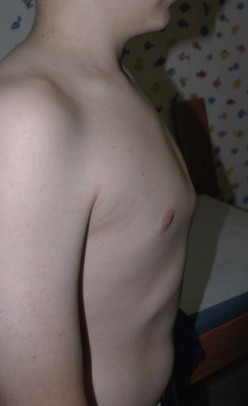
FIGURE 20-8 This teenager has a chondrogladiolar pectus carinatum. This is the most common form of pectus carinatum and involves protrusion of the lower portion of the sternum (the gladiolus). In this patient, the protrusion is fairly symmetrical.
Pectus carinatum is usually noted in adolescence and is seen most commonly around the time of a growth spurt, rather than at birth as is often seen with pectus excavatum. Symptoms of dyspnea, reduced endurance, or tachypnea with exertion were noted in all 260 patients in one study.59 Associated mitral valve disease has been reported as well.60,61 Other associations include Marfan syndrome and scoliosis (in 15%53).
Orthotic bracing has been applied successfully in some patients with pectus carinatum. Reports have described correction or improvement in this condition by means of a brace analogous to that used for treatment of scoliosis but that exerts pressure in the anteroposterior direction.24,62
Following adoption of a new brace in 2009, we have seen marked improvement in success with brace treatment (Fig. 20-9). About 85% of our brace patients have been successfully treated without operation.62 In the remainder, minimally invasive or open operation is performed.25,55,62–66 Postoperative complications are uncommon, and recurrence rates are low in centers with a large experience.
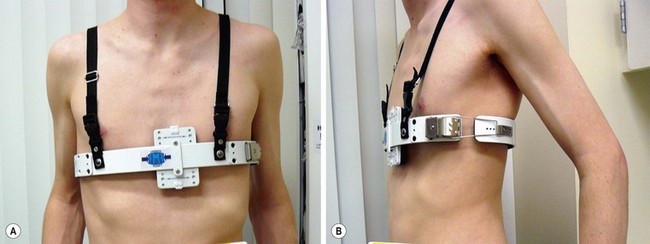
FIGURE 20-9 A patient being treated with dynamic compression bracing for a prominent pectus carinatum. The advantage of this brace is that it allows a preset pressure to be used to compress the carinatum.
An unusual form of pectus carinatum occurs with a short nonsegmented sternum and marked posterior angulation at the site of the normal chondromanubrial junction (Currarino–Silverman syndrome).60 Also, congenital heart disease is often present. Although its etiology is unclear, early fusion of the sternal plates is postulated as the cause of this deformity.67 In a large review from our hospital, we found about 1% of pectus patients had this anomaly.68 Repair is best performed using an open technique with subparachondral resection of the second to seventh costal cartilages and a broad wedge-shaped osteotomy through the anterior cortex of the sternum at the point of maximal angularization. The lower sternum is then displaced anteriorly with sutures while the costal cartilages regenerate.69 The confusing part about this anomaly is that it may appear to be a pectus excavatum deformity when, in fact, it is an uncommon variant of pectus carinatum (Fig. 20-10).
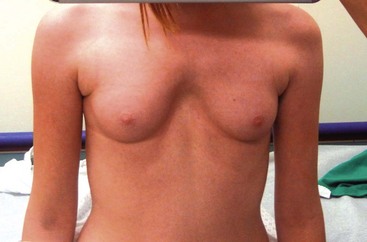
FIGURE 20-10 This girl has the typical features of Currarino–Silverman syndrome. It looks like she has a pectus excavatum, but really has a variant of pectus carinatum. The sternum is short and there is marked posterior angulation at the site of the normal chondromanubrial junction. Congenital heart disease is often present.
Poland Syndrome
Poland syndrome affects 1 in 30,000 live births and is sporadic in occurrence.70 It is a constellation of anomalies that present in a variety of ways. Clinical manifestations can include any or all of the following: absence of the pectoralis major, pectoralis minor, serratus anterior, rectus abdominis, and latissimus dorsi muscles (Fig. 20-11). Athelia or amastia, nipple deformities, limb deformities (syndactyly, brachydactyly), absent axillary hair, and limited subcutaneous fat can also be found.
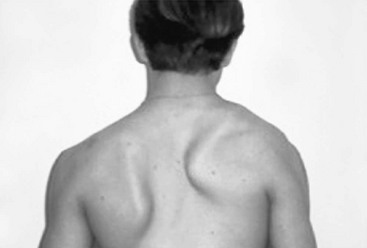
FIGURE 20-11 A 12-year-old boy with Poland syndrome and absence of the serratus anterior muscles, leading to a winged scapula on the right.
In 1841, Alfred Poland, an English medical student, published a partial description of the deformity.71 However, the syndrome had been initially described in the French and German literature in 1826 and 1839.72,73
Poland syndrome does not appear to be genetic, although occasional occurrences within families have occurred. The right side is more commonly affected and is present in boys 70% of the time.74 Approximately 15% of patients with breast hypoplasia/aplasia have Poland syndrome. The etiology is unclear, but theories include abnormal migration of the embryonic tissues forming the pectoralis muscles, hypoplasia of the subclavian artery, or in utero trauma.
Repair is rarely required, except in those patients with aplasia of the ribs or a major depression deformity.74,75 When necessary, chest wall reconstruction with correction of contralateral carinatum-type protrusions can usually be performed at the same time (Fig. 20-12). Autologous rib grafts, or a variety of bioprosthetic agents, can be used with or without a latissimus dorsi flap. The use of custom-made chest wall prostheses has been associated with significant problems such as migration, erosion of local tissues, and less than optimal cosmesis. Chest wall reconstruction should be performed before breast reconstruction in a girl with hypoplasia or aplasia of the breast.
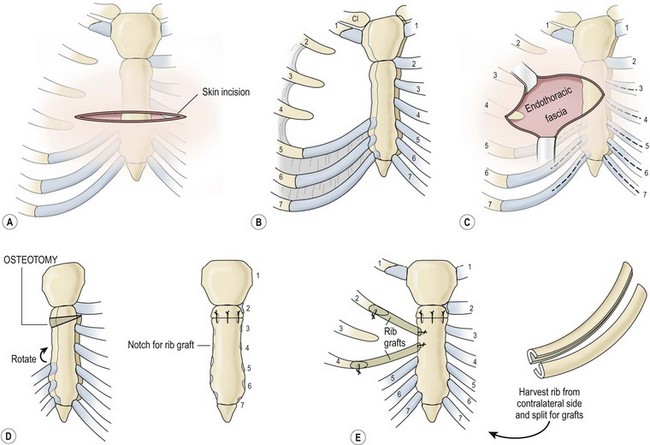
FIGURE 20-12 (A) The transverse incision is placed below and within the nipples. In girls, it is placed in the future inframammary crease. (B) A schematic depiction of the deformity with rotation of the sternum, depression of the cartilages of the involved side, and carinate protrusion of the contralateral side. (C) In cases with aplasia of the ribs, the endothoracic fascia is encountered directly below the attenuated subcutaneous tissue and pectoral fascia. The pectoral muscle flap is elevated on the contralateral side, with the pectoral fascia, if present, on the involved side. Subperichondrial resection of the costal cartilages is carried out as shown (dashed line), preserving the costochondral junction. Rarely this resection must be carried to the level of the second costal cartilage. (D) A transverse, offset, wedge-shaped sternal osteotomy is created below the second costal cartilage. Closure of this defect with heavy silk sutures or elevation of the sternum with a strut corrects both the posterior displacement and the rotation of the sternum. (E) In cases with rib aplasia, rib grafts are harvested from the contralateral fifth or sixth ribs, split, and secured medially with wire sutures into notches created in the sternum and with wire to the native ribs laterally. Ribs are split as shown, along their short axes, to maintain maximal mechanical strength. (Adapted from Shamberger RC, Welch KJ, Upton J III. Surgical treatment of thoracic deformity in Poland’s syndrome. J Pediatr Surg 1989;24:760–6.)
Sternal Defects
Cleft sternum (bifid sternum, partial ectopia cordis) is a rare malformation (0.15% of all chest wall malformations in some series), and is due to partial or total failure of sternal fusion at an early stage of embryonic development. Sternal clefts can be classified as either complete (the rarest form), superior, or inferior.76
Superior clefts are either U-shaped (proximal to the fourth cartilage) or V-shaped (reaching the xiphoid process). They are most often isolated, with only minor associated lesions. The heart is in a normal position, and cardiac anomalies are rare. Operative repair, which is usually very successful, is warranted once the diagnosis is made and can be scheduled electively. Optimally, it is performed in the neonatal period when the sternal edges can be approximated easily because of flexibility and minimal compression of mediastinal structures (Fig. 20-13). After age 1 year, primary repair is difficult and more extensive techniques may be needed, such as the use of autologous structures (costal cartilage, ribs) or prosthetic materials.77,78
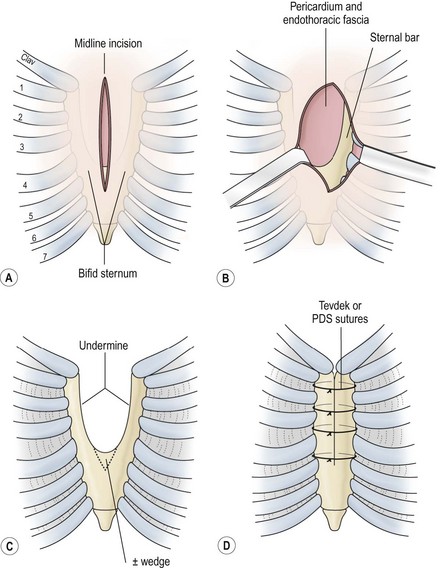
FIGURE 20-13 (A) Repair of a bifid sternum is best performed through a longitudinal incision extending the length of the defect. These defects are characteristically cleft superiorly, as shown. (B) Directly beneath the subcutaneous tissues, the sternal bars are encountered, with the origin of the pectoral muscles on the lateral aspect of the bars. The endothoracic fascia and pericardium are just below these structures. (C) The endothoracic fascia is mobilized off the sternal bars posteriorly with blunt dissection to allow safe placement of the sutures. Approximation of the sternal bars may be facilitated by excising a wedge of cartilage inferiorly. Repair is best accomplished in the neonatal period because of the flexibility of the chest wall. (D) Closure of the defect is achieved with 2-0 Tevdek or polydioxanone sutures. (Adapted from Shamberger RC, Welch KJ. Sternal defects. Pediatr Surg Int 1990;5:156–64.)
Thoracic ectopia cordis (true ectopia cordis) is a lesion in which the heart has no overlying somatic structures. It is very rare (incidence 5.5 to 7.9 per million births) and usually occurs with some form of an abdominal wall defect, with the heart sitting on the chest and the apex pointed toward the chin (Fig. 20-14).79 Intrinsic cardiac anomalies are frequent, especially tetralogy of Fallot, pulmonary artery stenosis, transposition of the great arteries, and ventricular septal defects (VSD). Survival in patients with thoracic ectopia cordis is rare. Most patients die because of torsion of the great vessels and compression of the heart while attempting to reduce it back in the chest. The goals of therapy are to cover the heart, prevent kinking of the great vessels, repair the associated abdominal wall defect, and stabilize the thoracic cavity so that spontaneous ventilation can be effective.79–81
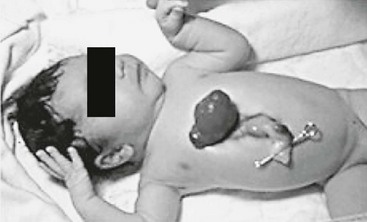
FIGURE 20-14 An infant with thoracic ectopia cordis with no significant abdominal wall defect present. Note the characteristic high insertion of the umbilicus and anterior projection of the apex of the heart.
Thoracoabdominal ectopia cordis (Cantrell pentalogy) involves lesions in which the heart is covered by an omphalocele-like membrane (Fig. 20-15).82 Intrinsic cardiac anomalies also are common in these patients, with tetralogy of Fallot and VSDs being the most common. Cantrell’s pentalogy consists of an inferior sternal cleft, ectopia cordis, midline abdominal wall defects or omphalocele, pericardial defects, and one or more cardiac defects. Repair in these patients is much more successful than in thoracic ectopia cordis. Initial management addresses the lack of skin overlying the heart and abdominal cavity. After stabilization, the goal of the first operation is to provide coverage of the midline defects, separate the abdominal and pericardial compartments, and repair the diaphragm. Various techniques for closure include flap mobilization, skin closure only, and a variety of bioprosthetic agents. The congenital heart defect is repaired at a later date.
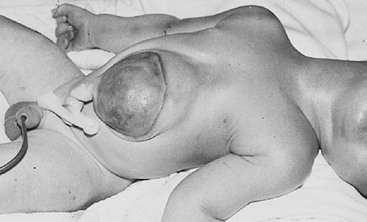
FIGURE 20-15 A newborn with the external features of Cantrell pentalogy is seen. Flaring of the lower thoracic cavity is present, with a large epigastric omphalocele. The transverse septum of the diaphragm and the inferior portion of the pericardium are absent. The patient also has tetralogy of Fallot.
Thoracic Insufficiency Syndrome Associated with Diffuse Skeletal Disorders
Thoracic insufficiency syndrome may be defined as any disorder that produces the inability of the thorax to support normal respiration or lung growth.83 It includes a spectrum of disorders including asphyxiating thoracic dystrophy (Jeune syndrome), acquired asphyxiating thoracic dystrophy (after open pectus excavatum repair), spondylothoracic dysplasia (Jarcho–Levin syndrome), congenital scoliosis with multiple vertebral anomalies and fused or absent ribs (jumbled spine), and severe kyphoscoliosis. These disorders have been viewed and treated as separate entities, with little coordinated effort between specialties. However, they are best addressed with a unified approach integrating pediatric general and orthopedic surgeons as well as pediatric pulmonologists.
Jeune syndrome is an autosomal recessive inherited osteochondrodystrophy with variable expression.84 In mild forms, the chest may support adequate respiration. In more severe cases, the thorax is narrowed both transversely and vertically, with short, wide horizontal ribs and irregular costochondral junctions (Fig. 20-16). This chest wall configuration produces a rigid chest with very little intercostal excursion for normal respiration, leading to ventilator dependence and eventual death from respiratory failure.85,86 The presence or absence of intrinsic pulmonary abnormalities varies among patients. However, most have normal bronchial development with variable alveolar density.87,88 This suggests that the extrinsic chest wall plays a significant role in the underlying hypoplasia. Other associated skeletal abnormalities in Jeune syndrome include short stubby extremities, fixed elevated clavicles, hypoplastic iliac wings, and a high incidence of C1 spinal stenosis.89–91 These patients also have varying degrees of renal dysplasia.92
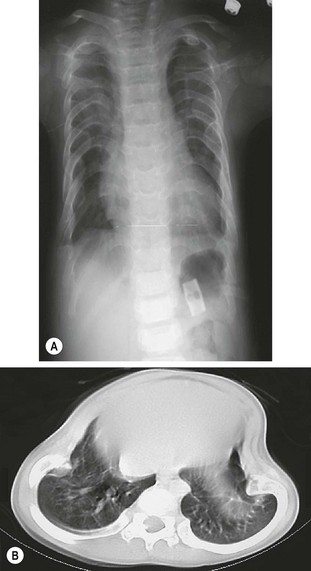
FIGURE 20-16 (A) Chest radiograph of a patient with Jeune syndrome (asphyxiating thoracic dystrophy). The thorax is narrow, and the ribs are short and wide. (B) CT scan demonstrating Jeune asphyxiating thoracic dystrophy.
Spondylothoracic dysplasia (Jarcho–Levin) syndrome occurs in two forms with different inheritance patterns. Type I is an autosomal recessive deformity characterized by multiple vertebral hemivertebrae and posterior rib fusions.93 This produces a marked shortening of the thoracic spine and a crab-like appearance of the chest on a chest radiograph (Fig. 20-17).94 Associated malformations are noted in 30% of patients and include cardiac and renal anomalies. This form is often fatal by age 15 months, and a high incidence is reported in Puerto Rican families.95 Type II spondylothoracic dysplasia has an autosomal dominant inheritance pattern and is associated with near-normal longevity. It is seen most commonly in white children.91
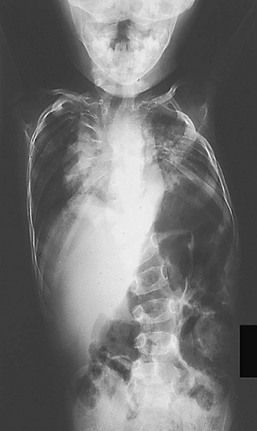
FIGURE 20-17 Chest radiograph of a patient with Jarcho–Levin syndrome with a markedly shortened thoracic spine producing a crab-like appearance.
Thoracic insufficiency also may arise secondary to over-extensive Ravitch-type pectus excavation repairs or repairs performed too early in life.19 Complex spine anomalies producing the so-called jumbled spine, unilateral thoracic hypoplasia seen with the VACTERL (vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities) syndrome, and kyphoscoliosis may be a cause of thoracic insufficiency as well.96–100
Operative techniques to correct the spectrum of these complex disorders have attempted to address the issue of thoracic volume by various approaches. In both congenital (Jeune) and acquired (post-pectus) thoracic dystrophy, one approach has been an anterior longitudinal sternal split with widening of the sternum. This has been accomplished with methylmethacrylate, bone grafts or rib, and metal plates.101–103 A staged approach with a methylmethacrylate plate followed by secondary removal of the plate and latissimus dorsi flaps to cover the created sternal cleft also has been described.104 In cases of acquired thoracic dystrophy, elevation of the sternum has been performed using both the open and the minimally invasive techniques used for standard pectus repair.19,41 A lateral staged approach with staggered rib osteotomies, staggered division of the chest wall, intercostal muscles, and pleura, with transposition of alternating ribs by using metal plate fixation, also has been described.105 These approaches have had variable results because they are not easily revised to allow continued growth of the chest wall to allow lung expansion. The lateral thoracic expansion may also interfere with intercostal muscle function after division of multiple intercostal muscles and nerves.
A promising technique to address this problem rejoins the disciplines of pediatric general, thoracic, and orthopedic surgery. Developed by Campbell and Smith, expansion thoracoplasty and the use of a vertically expandable prosthetic titanium rib (VEPTR) addresses many of the problems found in the spectrum of these disorders. This technique allows serial expansion of the chest wall to provide continued growth of the thorax and spine until skeletal maturity is achieved. More than 300 patients with various disorders have been treated with this approach.106 In Jeune asphyxiating thoracic dystrophy, 14 patients have undergone staged bilateral expansions.107–109 With this technique, anterior rib osteotomies adjacent to the costochondral junction and posterior osteotomies in the 3rd to 9th ribs next to the transverse process of the spine are performed. This creates a segment of chest wall that is distracted posterolaterally and anchored to a curved VEPTR that is attached to the 2nd and 10th ribs (Fig. 20-18). The distracted segment is anchored to the VEPTR with 2 mm titanium rings, stabilizing the segment, and allowing reossification of the multiple osteotomies (Fig. 20-19). The second stage is performed three months later, and then the devices are expanded every six months.
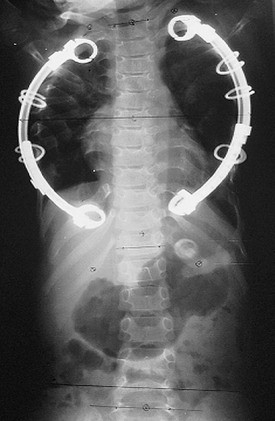
FIGURE 20-18 Bilateral vertical expandable prosthetic titanium rib (VEPTR) fixed with titanium rings to ribs of the patient with Jeune’s asphyxiating thoracic dystrophy seen in Figure 20-16A.
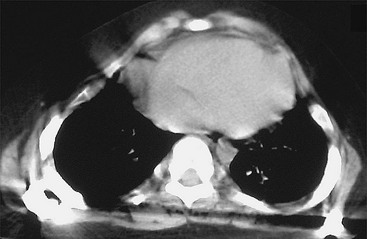
FIGURE 20-19 Postoperative CT scan of the patient from Figure 20-16B after VEPTR placement, demonstrating expansion of the thorax.
In patients with fused or absent ribs and scoliosis, a wedge thoracostomy through the fused segment of ribs not only allows expansion of the chest, but also correction of the scoliosis and the rotational spinal deformity (producing a windswept thorax).107 It also stimulates increased spinal height in both congenital scoliosis and Jarcho–Levin syndrome, in which bilateral devices are placed (Fig. 20-20).
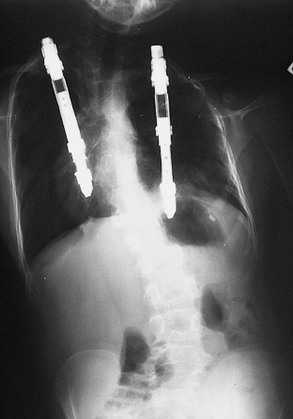
FIGURE 20-20 Bilateral VEPTRs were placed in the patient in Figure 20-17 with Jarcho–Levin syndrome.
References
1. Ebstein, E. Die Trichterbrust in ihren Beziehungen zur Konstitution. Z Konstitutionslehre. 1921; 8:103.
2. Bauhinus J. Observationum Medicariam. Liber II, Observ. 264, Francfurti 1600.507.
3. Coulson, W. Deformities of the chest. London Med Gaz. 1820; 4:69–73.
4. Williams, CT. Congenital malformation of the thorax: Great depression of the sternum. Trans Pathol Soc Lond. 1872; 24:50.
5. Ebstein, W. Ueber die Trichterbrust. Dtsch Arch. 1882; 30:411.
6. Meade, RH. A History of Thoracic Surgery. Springfield, IL: Charles C Thomas; 1961.
7. Sauerbruch, F. Die Chirurgie der Brustorgane. Berlin: Springer; 1920.
8. Meyer, L. Zurchirurqishen Behandlung der augeborenen Trichterbrust. Verh Bel Med Gest. 1911; 42:364.
9. Sauerbruch, F. Operative Beseitigung der Angeborenen Trichterbrust. Dtsch Z Chir. 1931; 234:760.
10. Ochsner, A, DeBakey, M, Chone-Chondrosternon. J Thorac Surg. 1939; 8:469–511.
11. Brown, AL. Pectus excavatum. J Thorac Surg. 1939; 9:164–184.
12. Ravitch, MM. The operative treatment of pectus excavatum. Ann Surg. 1949; 129:429–444.
13. Wallgren GR and Sulamaa M. Surgical treatment of funnel chest. Exhib. VIII, presented at the International Congress of Paediatrics 1956.32.
14. Paltia, V, Parkkulainen, KV, Sulamaa, M, et al. Operative technique in funnel chest. Acta Chir Scand. 1958/1959; 116:90–98.
15. Adkins, PC, Blades, BA. Stainless steel strut for correction of pectus excavatum. Surg Gynecol Obstet. 1961; 113:111–113.
16. Welch, KJ. Satisfactory surgical correction of pectus excavatum deformity in childhood. J Thorac Surg. 1958; 36:697–713.
17. Gross, RE. The Surgery of Infancy and Childhood. Philadelphia: WB Saunders; 1953.
18. Martinez, D, Juame, J, Stein, T, et al. The effect of costal cartilage resection on chest wall development. Pediatr Surg Int. 1990; 5:170–173.
19. Haller, JA, Colombani, PM, Humphries, CT, et al. Chest wall constriction after too extensive and too early operations for pectus excavatum. Ann Thorac Surg. 1996; 61:1618–1625.
20. Nuss, D, Kelly, RE, Jr., Croitoru, DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998; 33:545–552.
21. Kelley, SW. Surgical Diseases of Children: Dislocations, Congenital and Acquired, 3rd ed. St. Louis: CV Mosby; 1929.
22. Haller, JA, Jr., Thoracic injuries. Pediatric Surgery. 4th ed. Welch, KJ, Randolph, JG, Ravitch, MM, et al, eds. Pediatric Surgery; vol. 1. Year Book Medical Publishers, Chicago, 1986:147.
23. Wesson, DE, et al, Thoracic injuries. Pediatric Surgery. 5th ed. O’Neill, JA, Jr., Rowe, MI, Grosfeld, JL, eds. Pediatric Surgery; vol. 1. Mosby Grosfeld, St. Louis, 1998:245.
24. Martinez-Ferro, M, Fraire, C, Bernard, S. Dynamic compression system for the correction of pectus carinatum. Semin Pediatr Surg. 2008; 17:194–200.
25. Shamberger, RC. Congenital chest wall deformities. In: Grosfeld JL, O’Neill JA, Jr., Fonkalsrud EW, Coran AG, eds. Pediatric Surgery. 5th ed. Philadelphia: Elsevier; 1998:787–817.
26. Creswick, HA, Stacey, MW, Kelly, RE, et al. Family study of the inheritance of pectus excavatum. J Pediatr Surg. 2006; 41:1699–1703.
27. Horth, L, Stacey, M, Kelly, RE, Jr., et al. Advancing our understanding of the inheritance and transmission of pectus excavatum – Inheritance of pectus exavatum. J Pediatr Genetics. 2012; 161–173.
28. Lawson, ML, Cash, TF, Akers, RA, et al. A pilot study of the impact of surgical repair on disease-specific quality of life among patients with pectus excavatum. J Pediatr Surg. 2003; 38:916–918.
29. Shamberger, RC. Cardiopulmonary effects of anterior chest wall deformities. Chest Surg Clin North Am. 2000; 10:245–251.
30. Haller, JA, Jr., Peters, GN, Mazur, D, et al. Pectus excavatum: A 20-year surgical experience. J Thorac Cardiovasc Surg. 1970; 60:375–383.
31. Zhao, L, Feinberg, MS, Gaides, M, et al. Why is exercise capacity reduced in subjects with pectus excavatum? J Pediatr. 2000; 136:163–167.
32. Mocchegiani, R, Badano, L, Lestuzzi, C, et al. Relation of right ventricular morphology and function in pectus excavatum to the severity of the chest wall deformity. Am J Cardiol. 1995; 76:941–946.
33. Sigalet, DL, Montgomery, M, Harder, J, et al. Long-term cardiopulmonary effects of closed repair of pectus excavatum. Pediatr Surg Int. 2007; 23:493–497.
34. Coln, D, Gunning, T, Ramsay, M, et al. Early experience with the Nuss minimally invasive correction of pectus excavatum in adults. World J Surg. 2002; 26:1217–1221.
35. Malek, MH, Berger, DE, Housh, TJ. Cardiovascular function following surgical repair of pectus excavatum: A meta-analysis. Chest. 2006; 130:506–516.
36. Shamberger, RC, Welch, KJ, Sanders, SP. Mitral valve prolapse associated with pectus excavatum. J Pediatr. 1987; 111:404–407.
37. Saint-Mezard, G, Duret, JC, Chanudet, X, et al. Mitral valve prolapse and pectus excavatum. Presse Med. 1986; 15:439.
38. Warth, DC, King, ME, Cohen, JM, et al. Prevalence of mitral valve prolapse in normal children. J Am Coll Cardiol. 1985; 5:1173–1177.
39. Park, JM, Farmer, AR. Wolff-Parkinson-White syndrome in children with pectus excavatum. J Pediatr Surg. 1988; 112:926–928.
40. Redlinger, RE, Jr., Wootton, A, Kelly, RE, et al. Optoelectronic plethysmography demonstrates abrogation of regional chest wall motion dysfunction in patients with pectus excavatum after Nuss repair. J Pediatr Surg. 2012; 47:160–164.
41. Haller, JA, Jr., Loughlin, GM. Cardiorespiratory function is significantly improved following corrective surgery for severe pectus excavatum. J Cardiovasc Surg. 2000; 41:125–130.
42. Haller, JA, Jr., Kramer, SS, Lietman, SA. Use of CT scans in selection of patients for pectus excavatum surgery: A preliminary report. J Pediatr Surg. 1987; 22:904–908.
43. Croitoru, DP, Kelly, RE, Jr., Nuss, D, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg. 2002; 37:437–445.
44. Columbani P. Personal communication.
45. Park HJ, Lee SY, Lee CS, et al. The Nuss procedure for pectus excavatum: An evolution of techniques and results on 322 patients. Presented at the 39th annual meeting of the Society of Thoracic Surgeons, San Diego, CA, January 31 to February 2, 2003.
46. St Peter, SD, Weesner, KA, Weissend, EE, et al. Epidural vs. patient-controlled analgesia for postoperative pain after pectus excavatum repair: A prospective, randomized trial. J Pediatr Surg. 2012; 47:148–153.
47. Azizkhan, RG. What’s new in pediatric surgery? J Am Coll Surg. 1998; 186:203–211.
48. Adzick, NS, Nance, ML. Pediatric surgery. N Engl J Med. 2000; 342:1651–1657.
49. Hebra, A, Swoveland, B, Egbert, M, et al. Outcome analysis of minimally invasive repair of pectus excavatum: Review of 251 cases. J Pediatr Surg. 2000; 35:252–258.
50. Miller, KA, Woods, RK, Sharp, RJ, et al. Minimally invasive repair of pectus excavatum: A single institution’s experience. Surgery. 2001; 130:652–659.
51. Molik, KA, Engum, SA, Rescoda, FJ, et al. Pectus excavatum repair: Experience with standard and minimally invasive techniques. J Pediatr Surg. 2001; 36:324–328.
52. Wu, PC, Knauer, EM, McGowan, GE, et al. Repair of pectus excavatum deformities in children: A new perspective of treatment using minimal access surgical technique. Arch Surg. 2001; 136:419–424.
53. Hosie, S, Sitkiewicz, T, Peterson, C, et al. Minimally invasive repair of pectus excavatum: The Nuss procedure: A European Multicenter Experience. Eur J Pediatr Surg. 2002; 12:235–238.
54. Shamberger, RC, Welch, KJ. Surgical correction of pectus carinatum. J Pediatr Surg. 1987; 22:48–53.
55. Chin, EF. Surgery of funnel chest and congenital sternal prominence. Br J Surg. 1957; 44:360–376.
56. Robisek, F, Cook, JW, Daugherty, HK, et al. Pectus carinatum. J Thorac Cardiovasc Surg. 1979; 78:52–61.
57. Pena, A, Perez, L, Nurka, S, et al. Pectus carinatum and pectus excavatum: Are they the same disease? Am Surg. 1981; 47:215–218.
58. Hebra, A, Thomas, PB, Tagge, EP, et al. Pectus carinatum as a sequela of minimally invasive pectus excavatum repair. Pediatr Endosurg Innovat Techn. 2002; 6:41–44.
59. Fonkalsrud, EW. Surgical correction of pectus carinatum: Lessons learned from 260 patients. J Pediatr Surg. 2008; 43:1235–1243.
60. Currarino, G, Silverman, FN. Premature obliteration of the sternal sutures and pigeon breast deformity. Radiology. 1958; 70:532–540.
61. Chidambaram, B, Mehta, AV. Currarino-Silverman syndrome (pectus carinatum type 2 deformity) and mitral valve disease. Chest. 1992; 102:780–782.
62. Cohee, A, Lin, JR, Frantz, FW, et al. Staged management of pectus carinatum. Eur J Pediatr Surg. 2013; 48(2):315–320.
63. Haje, SA, Bowen, JR. Preliminary results of orthotic treatment of pectus deformities in children and adolescents. J Pediatr Orthop. 1992; 12:795–800.
64. Ravitch, MM. The operative correction of pectus carinatum (pigeon breast). Ann Surg. 1960; 151:705–714.
65. Welch, KJ, Vos, A. Surgical correction of pectus carinatum (pigeon breast). J Pediatr Surg. 1973; 8:659–667.
66. Abramson, H, D’Agostino, JD, Wuscovi, S. A 5-year experience with a minimally invasive technique for pectus carinatum repair. J Pediatr Surg. 2009; 44:118–124.
67. Allwyn, JS, Shetty, L, Pare, VS, et al. Chondro-manubrial deformity and bifid rib, rare variations seen in pectus carinatum: A radiological finding. Surg Radiol Anat. 2012.
68. Kelly, RE, Jr., Quinn, A, Varela, P, et al. Dysmorphology of chest wall deformities: Frequency distribution of subtypes of typical pectus excavatum and rare subtypes. Arch Bronconeumol. 2012.
69. Shamberger, RC, Welch, KJ. Surgical correction of chondromanubrial deformity (Currarino Silverman syndrome). J Pediatr Surg. 1988; 23:319–322.
70. Freire-Maia, N, Chautard, EA, Opitz, JM. The Poland syndrome: Clinical and genealogical data, dermatoglyphic analysis, and incidence. Hum Hered. 1973; 23:97–104.
71. Poland, A. Deficiency of the pectoralis muscles. Guys Hosp Rep. 1841; 6:191–193.
72. Froriep, R. Beobachtung eines Falles Von Mangel der Brustdrüse. Notizen aus dem Gebiete der Naturund Heilkinde. 1839; 10:9–14.
73. Lallemand, LM. Ephermerides. Medicales de Montpellier. 1826; 1:144–147.
74. Seyfer, AE, Icochea, R, Graber, GM. Poland’s anomaly: Natural history and long-term results of chest wall reconstruction in 33 patients. Ann Surg. 1988; 208:776–782.
75. Shamberger, RC, Welch, KJ, Upton, J, III. Surgical treatment of thoracic deformity in Poland’s syndrome. J Pediatr Surg. 1989; 24:760–765.
76. Samarrai, AR, Charmockley, HA, Attr, AA. Complete cleft sternum: Classification and surgical repair. Int Surg. 1985; 70:71–73.
77. Shamberger, RC, Welch, KJ. Sternal defects. Pediatr Surg Int. 1990; 5:156–164.
78. Knox, L, Tuggle, D, Knott-Craig, CJ. Repair of congenital sternal clefts in adolescence and infancy. J Pediatr Surg. 1994; 29:1513–1516.
79. Amato, J, Douglas, W, Desai, U, et al. Ectopia cordis. Chest Surg Clin N Am. 2000; 10:297–316.
80. Groner, JI. Ectopia cordis and sternal defects. In: Zeigler MM, Azizkhan RG, Weber TR, eds. Operative Pediatric Surgery. New York: McGraw-Hill; 2003:279–293.
81. Amato, U, Zelen, J, Talwalker, NG. Single-stage repair of thoracic ectopia cordis. Ann Thorac Surg. 1995; 59:518–520.
82. Engum, SA. Embryology, sternal clefts, ectopia cordis, and Cantrell’s pentalogy. Semin Pediatr Surg. 2008; 17:154–160.
83. Campbell, RM, Smith, MD, Mayes, TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003; 85:399–408.
84. Jeune, M, Carron, R, Beraud, C, et al. Polychondrodystrophie avec blocage thoracique d’evolution fatale. Pediatrie. 1954; 9:390–392.
85. Borland, LM. Anesthesia for children with Jeune’s syndrome (asphyxiating thoracic dystrophy). Anesthesiology. 1987; 66:86–88.
86. Tahernia, AC, Stamps, P. Jeune’s syndrome (asphyxiating thoracic dystrophy). Chin Pediatr. 1977; 16:903–907.
87. Williams, AJ, Vawter, G, Reid, LM. Lung structure in asphyxiating thoracic dystrophy. Arch Pathol Lab Med. 1984; 108:658–661.
88. Finegold, J, Katzew, H, Genieser, NB, et al. Lung structure in thoracic dystrophy. Am J Dis Child. 1971; 122:153–159.
89. Langer, LO. Thoracic pelvic phalangeal dystrophy: Asphyxiating thoracic dystrophy of the newborn, infantile thoracic dystrophy. Radiology. 1968; 91:447–456.
90. Oberklaid, F, Danks, DM, Mayne, V, et al. Asphyxiating thoracic dysplasia. Arch Dis Child. 1977; 52:758–765.
91. Campbell RM. The incidence of proximal cervical spine stenosis in Jeune’s asphyxiating dystrophy. Paper presented at the Scoliosis Research Society 2001.
92. Herdman, RC, Langer, LO. The thoracic asphyxiant dystrophy and renal disease. Am J Dis Child. 1977; 52:192–201.
93. Jarcho, S, Levin, PM. Hereditary malformations of the vertebral bodies. Bull Johns Hopkins Hosp. 1938; 62:216–226.
94. Roberts, AP, Conner, AN, Tolmie, JL, et al. Spondylothoracic and spondylocostal dysostosis: Hereditary forms of spinal deformity. J Bone Joint Surg Br. 1988; 70:123–126.
95. Heilbronner, DM, Renshaw, TS. Spondylothoracic dysplasia. J Bone Joint Surg Am. 1984; 66:302–303.
96. McMaster, MJ. Congenital scoliosis. In: Weinstein SL, ed. The Pediatric Spine: Principles and Practice. New York: Raven Press, 1994.
97. McMaster, MJ. Congenital scoliosis caused by unilateral failure of vertebral segmentation with contralateral hemivertebrae. Spine. 1998; 23:998–1005.
98. McMaster, MJ, David, C. Hemivertebrae as a cause of scoliosis: A study of 104 patients. J Bone Joint Surg Br. 1986; 68:588–595.
99. Campbell, RM. Congenital scoliosis due to multiple vertebrae anomalies associated with thoracic insufficiency syndrome. Spine. 2000; 14:209–218.
100. Campbell, RM, Smith, MD, Mayes, T, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg. 2003; 85:399–408.
101. Todd, DW, Tinguely, ST, Norberg, WJ. A thoracic expansion technique for Jeune’s asphyxiating thoracic dystrophy. J Pediatr Surg. 1986; 21:161–163.
102. Barnes, ND, Hall, D, Milner, AD, et al. Chest reconstruction in asphyxiating thoracic dystrophy. Arch Dis Child. 1971; 46:833–837.
103. Weber, TR, Kurkchubasche, AG. Operative management of asphyxiating thoracic dystrophy after pectus repair. J Pediatr Surg. 1998; 33:262–265.
104. Sharoni, E, Erez, E, Chorer, G, et al. Chest reconstruction in asphyxiating thoracic dystrophy. J Pediatr Surg. 1998; 33:1578–1581.
105. Davis, JT, Heistein, JB, Castile, RG, et al. Lateral thoracic expansion for Jeune’s syndrome: Mid-term results. Ann Thorac Surg. 2001; 72:872–878.
106. Campbell, RM, Hell-Vocke, AK. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am. 2003; 85:409–419.
107. Phillips, JD, van Aalst, JA. Jeune’s syndrome (asphyxiating thoracic dystrophy): Congenital and acquired. Semin Pediatr Surg. 2008; 17:167–172.
108. Ramirez, N, Flynn, JM, Emans, JB. Vertical expandable prosthetic titanium rib as treatment of thoracic insufficiency syndrome in spondylocostal dysplasia. J Pediatr Orthop. 2010; 30:521–526.
109. Gadepalli, SK, Hirschl, RB, Tsai, WC, et al. Vertical expandable prosthetic titanium rib device insertion: Does it improve pulmonary function? J Pediatr Surg. 2011; 46:77–80.