CHAPTER 21 Congenital Cardiac Anesthesia: Non-Bypass Procedures
The population of children and adults who has undergone successful repair or palliation of congenital heart disease (CHD) continues to increase (Warnes and Deanfield, 2001; Warnes et al., 2001). Approximately 85% of infants born with CHD can expect to reach adulthood (Moller et al., 1994), and because children of parents with CHD have an increased incidence of CHD (Hoffman et al., 2004), the total incidence and prevalence of CHD are likely to increase generation by generation. Although CHD is synonymous with childhood, the number of adults with CHD currently equals, and is predicted to exceed, the number of children with CHD (Webb and Williams, 2001); the total number in the United States alone may be approaching 500,000 individuals with significant versions of palliated CHD. The heterogeneous nature of the CHD population is made more so by the use of different treatment strategies for the same or similar lesions, in conjunction with advances in pediatric cardiac surgery, interventional catheterization, and electrophysiologic techniques. For example, in transposition of the great arteries (D-TGA), the majority of adults have had the atrial switch operation (the Mustard or Senning procedure), whereas most children have had the arterial switch operation (the Jatene). These two operations for the same underlying condition carry vastly different intermediate and long-term outcomes (Williams and Webb, 2000; Warnes, 2006).
Although it is common to differentiate between corrective and palliative surgery, total correction or cure is not the rule for the majority of children with CHD (Stark, 1989). Cure or definitive repair, in the strictest sense, means that normal cardiovascular structure and function are achieved and maintained; life expectancy is normal; further medical, surgical, and catheter-based treatments for the CHD are unnecessary; and noncardiovascular (e.g., renal, neurologic) consequences are nonexistent. With cure, there are no cardiac or vascular residua (abnormalities that were part of the original defect and are still present after repair), sequelae (disorders intentionally incurred at the time of reparative surgery and deemed unavoidable), or complications (unintentional aftermath) after surgery (Perloff, 1997). Palliative repair implies that future procedures are anticipated or necessary to maintain or restore the patient to a state of normal (or at least compensated) physiology and to improve life span. Lesions that lend themselves to cure are uncomplicated closure at an early age of an uncomplicated, nonpulmonary hypertensive patent ductus arteriosus (PDA), atrial septal defect (ASD), ventricular septal defect (VSD) (Talner et al., 1980), and in some instances catheter ablation of tachyarrhythmias (Walsh, 2007). Virtually all other forms of CHD require long-term surveillance. Many carry substantial risk for residual and potentially progressive structural, contractile, hemodynamic, electrophysiologic, and end-organ abnormalities.
Cardiac factors influencing outcome and anesthetic risk
The factors that determine the natural history and pathophysiologic consequences of congenital cardiovascular malformations also affect perioperative risk. Although the majority of anesthesiologists are not familiar with the natural history of each and every lesion, it is possible to develop a rational approach to the anesthetic management of this group of patients by focusing on the factors listed in Box 21-1. Identification of patients at increased risk, and development of an appropriate strategy to prevent adverse events is the cornerstone of anesthetic management.
Shunting
A shunt is an abnormal communication between the systemic and pulmonary circulations, allowing blood to flow directly from one circulatory system to the other. A left-to-right shunt allows oxygenated pulmonary venous blood to return directly to the lungs rather than being pumped to the body, whereas a right-to-left shunt allows deoxygenated systemic venous blood to bypass the lungs and return to the body (Sommer et al., 2008). An increased workload is placed on the ventricles, with the degree (volume) of shunting determining the severity of symptoms. Factors influencing the direction and degree of shunting include the size of the shunt orifice, the pressure gradient between the chambers or arteries involved in the shunt, the relative compliance of the right and left ventricles, the ratio of pulmonary vascular resistance (PVR) to systemic vascular resistance (SVR), and the blood viscosity (hematocrit). Total pulmonary blood flow (QP) is the sum of effective pulmonary blood flow and recirculated pulmonary blood flow, whereas total systemic blood flow (QS) is the sum of effective systemic blood flow and recirculated systemic blood flow. Total QP and total QS do not have to be equal.
The lesions most likely to be encountered before repair that are associated with the potential for left-to-right shunting include large PDA, VSD, and atrioventricular canal defects; patients palliated with large, unobstructed modified Blalock-Taussig shunts (e.g., stage I repair of hypoplastic left heart syndrome) can behave similarly. Left-to-right shunting results in increased pulmonary blood flow, increased pulmonary vascular resistance, increased pulmonary artery pressures (PAP), increased left atrial volume or pressure, pulmonary edema, and volume overload of both the right and left ventricles leading to biventricular failure. Low aortic diastolic pressure accompanying large systemic-to-pulmonary artery shunts can lead to myocardial ischemia as well as organ hypoperfusion (e.g., bowel ischemia). Pulmonary overcirculation is associated with decreased lung compliance, increased airway resistance (Stayer et al., 2004), and airway compression (Berlinger et al., 1983). Alterations in pulmonary function lead to increased work of breathing (with increased energy expenditure), atelectasis, air trapping, and respiratory infections. The time course for developing pulmonary vascular occlusive disease (PVOD) with shunt reversal (right-to-left) and Eisenmenger’s syndrome depends on the size and site of the shunt and age at repair; patients with underlying anatomic or genetic predisposition to the development of pulmonary hypertension—for example, trisomy 21 children—can develop earlier, more severe PVOD for a given shunt (Chi and Krovetz, 1975; Gorenflo et al., 2002).
Right-to-left shunting results in decreased oxygen content of the systemic arterial blood, with the decrease in proportion to the volume of deoxygenated systemic venous blood mixing with the oxygenated pulmonary venous blood. Even with normal cardiac output, the decrease in tissue oxygen delivery limits exercise tolerance (Sommer et al., 2008).
Ventricular Dysfunction
Progressive ventricular dysfunction leading to congestive heart failure (CHF) is the most common cause of disability and death in patients with CHD (Warnes and Deanfield, 2001). The etiology is multifactorial and may result from the primary disease, many years of abnormal volume and pressure loading (which cause pathologic remodeling of numerous cardiomyocyte and nonmyocyte functions and processes), chronic hypoxemia, damage during surgical repair (inadequate myocardial preservation, scarring, poor repair, damage to coronary arteries), acquired disease (Graham, 1982), and arrhythmias (Shinbane et al., 1997; Deal, 2001). Ventricular volume overload occurs with intracardiac or extracardiac left-to-right shunts, valvular regurgitation, and single-ventricle lesions. The time course over which irreversible ventricular dysfunction develops is variable, but if surgical intervention to correct the volume overload is undertaken within the first year of life, residual dysfunction is uncommon (Cordell et al., 1976; Graham et al., 1976). Ventricular pressure overload results from residual or recurrent ventricular outflow obstruction or elevated PAP or PVR. The time to develop significant ventricular dysfunction is longer compared with a chronic volume load, so symptoms are uncommon unless the obstruction is severe and prolonged, or it is combined with a volume load (Graham, 1991). Chronic hypoxemia and cyanosis decrease ventricular oxygen supply and increase oxygen demand through increased work related to increases in pulmonary and systemic vascular resistance with associated polycythemia. Myocardial ischemia resulting from coronary artery anomalies or kinking or torsion after reimplantation may also cause ventricular dysfunction.
Ventricular Outflow Obstruction
Ventricular outflow obstruction may be subvalvar, valvar, supravalvar, or a combination thereof, isolated or part of more complex malformations, residual or recurrent, and fixed or dynamic. Outflow obstruction results in pressure overload on the ventricle, ventricular hypertrophy, a “stiff” ventricle (diastolic dysfunction), and ultimately systolic ventricular dysfunction (Carabello, 2006). Left ventricular outflow obstruction may occur with aortic stenosis, coarctation of the aorta, interrupted aortic arch, and variants of HLHS and Shone’s anomaly (Shone et al., 1963). Right ventricular outflow obstruction is seen with pulmonary stenosis, TOF, hypoplastic pulmonary arteries, right ventricle–to–pulmonary artery (RV-PA) conduits (performed in repair of pulmonary atresia, truncus arteriosus, TGA with pulmonary stenosis [Rastelli procedure], some double-outlet right ventricle defects), and pulmonary hypertension. Conduits calcify and narrow, and together with the increasing stroke volume that occurs with growth, significant obstruction can develop. The septal shift associated with severe right ventricular pressure overload can compromise left ventricular function via a reduction in left ventricular filling and systemic outflow obstruction.
Hypoxemia and Cyanosis
Cyanosis is associated with decreased pulmonary blood flow or intracardiac mixing lesions, or both. Prior to repair, cyanotic lesions include tricuspid atresia, pulmonary atresia, tetralogy of Fallot, transposition of the great arteries, and truncus arteriosus; tricuspid atresia, pulmonary atresia, and TOF are all lesions that typically have reduced PBF. Increased PBF occurs in truncus arteriosus. In lesions with intracardiac mixing (e.g., TGA), cyanosis can occur with decreased or increased PBF depending on whether there is obstruction to the PBF. Cyanosis may also be found in the setting of very low cardiac output, increased arteriovenous oxygen difference, and respiratory disease. With the advent of early infant repair, chronic hypoxemia is now most frequently encountered in the young child undergoing staged repair, and in the adult with unrepaired or palliated CHD. Indeed, one stimulus for early, definitive repair is to eliminate hypoxemia and the compensatory polycythemia, with its rheologic, neurologic, hemostatic, renal, and metabolic consequences. Although the analyses are limited and imperfect for obvious reasons, the data that does exist suggests that chronic hypoxemia during infancy and early childhood is a significant risk factor for reduced cognitive performance. As blood viscosity increases, systemic (including coronary) and pulmonary vascular resistances increase markedly. Hemostatic abnormalities that can result from cyanosis and polycythemia include thrombocytopenia, platelet dysfunction, shortened platelet survival, disseminated intravascular coagulation, decreased production of coagulation factors, and primary fibrinolysis (Tempe and Virmani, 2002; Odegard et al., 2003, 2009). Sludging of red blood cells increases the risk for thromboembolism and stroke, particularly when the hemoglobin approaches or exceeds, 20 g/dL, and in conjunction with dehydration. Phlebotomy regimens are being used less frequently in the absence of symptoms, as they may be associated with an increased risk for cerebrovascular events (Ammash and Warnes, 1996). The duration and degree of hypoxemia and polycythemia are important historical factors in the evaluation of possible long-term residual cardiac muscle blood flow abnormalities.
Rhythm and Conduction Abnormalities
Arrhythmias and conduction defects have a major impact on the prognosis and management of patients who have undergone palliation or repair of CHD (Warnes and Deanfield, 2001). Rhythm disturbances that may be well tolerated in a structurally normal heart may be life threatening in a structurally or functionally abnormal heart. The etiology is multifactorial and includes damage to the arterial supply or direct injury to the sinoatrial node, atrioventricular (AV) node, and conduction system, atrial or ventricular scarring, chamber dilation or hypertrophy with resultant pathologic remodeling, and myocardial ischemia.
Arrhythmias may occur in the postoperative period or many years after surgery, and they tend to vary with the type of underlying heart disease and surgical procedures that have been performed (Vetter, 1991). Supraventricular tachycardias (atrial flutter/intraatrial reentrant tachycardia, atrial fibrillation) and sinoatrial node dysfunction (bradycardia, tachy-brady syndrome, exit block, sinus arrest) are more common in lesions that required extensive intraatrial surgery or have residual elevations in right atrial pressure, such as the atrial switch (Mustard or Senning) procedure for TGA, the Fontan procedure, and TOF repair. Isolated right bundle branch block is frequent after ventriculotomy. The QRS duration may be an independent predictor of arrhythmia, right ventricular dysfunction, and sudden death risk in patients after TOF repair (Gatzoulis et al., 2000a). Whether aberrant conduction and ventricular dyssynchrony are independent causes of ventricular dysfunction and, consequently, whether using biventricular pacing to restore normal activation-contraction patterns can prevent or reverse ventricular function in patients with dyssynchrony is currently under investigation (Cecchin et al., 2009). Ventricular arrhythmias are more common in lesions with residual ventricular pressure or volume load, as in aortic stenosis, hypertrophic cardiomyopathy, and TOF repair. Tachyarrhythmias and ventricular dysfunction are a dangerous combination and a cause of late sudden death (Vetter, 1991).
Existing pacemakers should be checked preoperatively and the appropriate precautions taken in patients with complete AV block. There is the need for preoperative evaluation and temporary resetting or shutting down of pacemakers and implantable cardioverter defibrillators (Practice advisory, 2005), as well as backup pacing (transcutaneous pacing) in the event of pacemaker malfunction. For high-risk patients, an external pacing or defibrillator unit should be in the operating room and pads applied around the time of induction of anesthesia. The need for and use of electrocautery (monopolar versus bipolar) in pacemaker-dependent patients should be discussed with both the surgeon and electrophysiologist. Implanted pacemakers and cardioverter defibrillators typically need to be interrogated and reprogrammed after the surgical procedure. The electrophysiologist may also be helpful for patient optimization. For example, consideration should be given to temporary pacing in select unpaced patients with bradycardia, as well as to radiofrequency catheter ablation in patients with amenable tachyarrhythmias, especially before major procedures.
Pulmonary Hypertension
In the unrepaired child, unrestricted left-to-right shunting with increased pulmonary blood flow produces a volume load on the heart and structural changes in the pulmonary vascular bed (medial hypertrophy progressing to necrotizing arteritis [Heath and Edwards, 1958]), leading to decreased myocardial function and pulmonary hypertension (mean PAP greater than 25 mm Hg). The time course for developing PVOD depends on the size and site of the shunt and age at surgery. Progression is more rapid when both the volume and the pressure load on the pulmonary circulation are increased, such as with a large VSD. For the majority of infants with an unrestrictive shunt, repair of the defect in the first year of life is usually associated with regression of the pulmonary vascular changes. Pulmonary hypertension develops more slowly with increased pulmonary blood flow in the absence of elevated pulmonary artery pressures, as with an ASD, where the absence of pulmonary hypertension into the third decade or beyond is not uncommon. Eisenmenger’s syndrome is characterized by irreversible PVOD and cyanosis related to reversal of the left-to-right shunt (Wood, 1958).
Anesthetic considerations: Severe pulmonary hypertension appears to impart major anesthetic risk, even for minor procedures (Ammash and Warnes, 1996; Raines et al., 1996; Daliento et al., 1998; Martin et al., 2002; Carmosino et al., 2007). This topic has been the subject of recent reviews (Subramaniam and Yared, 2007; Friesen and Williams, 2008). Suprasystemic pulmonary artery pressure is a significant predictor of major complications in children with pulmonary hypertension undergoing noncardiac surgery (Carmosino et al., 2007). It is not possible to recommend a specific anesthetic technique as all anesthetic techniques have been used successfully. Of note, many perioperative deaths occur in the postoperative period (Lyons et al., 1995; Martin et al., 2002). In older patients with Eisenmenger’s syndrome, most deaths appear to occur either as a result of the surgical procedure and not of anesthesia, or in the postoperative period because of complications such as atelectasis, pneumonia, worsening hypoxemia, or other end-organ dysfunction (Martin et al., 2002). Preoperative knowledge of the degree of pulmonary hypertension, pulmonary vascular reactivity, right ventricular dysfunction, and the presence of an intracardiac communication is imperative. For example, patients with severe pulmonary arterial hypertension, significant RV dysfunction, and no “pop-off” communication (i.e., right-to-left shunt) are probably the most at risk.
Acute right ventricular dysfunction and resultant low systemic cardiac output are the major pathophysiologic consequences of acute exacerbations in pulmonary artery pressure. The overall goals of anesthesia, therefore, are to provide adequate analgesia and anesthesia while minimizing increases in PVR and depression of myocardial function (Friesen and Williams, 2008). Likely methods include providing adequate preoperative sedation, high inspired oxygen, hyperventilation (respiratory alkalosis) if possible, adequate depth of anesthesia, maintenance of a normal to increased preload, early use of an inotrope to support RV function and systemic blood pressure (i.e., RV perfusion), and use of induction and maintenance agents that do not significantly reduce contractility or systemic blood pressure. Endotracheal intubation as a potential mechanical trigger of pulmonary vasoreactivity should be recognized; this also applies to extubation on emergence from anesthesia. In addition to adequate anesthetic depth, lidocaine spray to the vocal cords and trachea may offer some degree of protection. Noninvasive ventilation may be an attractive alternative to support adequate gas exchange during anesthesia and surgery under some conditions. Although allowing better control of oxygenation and ventilation, positive pressure ventilation increases RV afterload and decreases RV filling so that excessive inspiratory pressures and volumes, and positive end-expiratory pressure (PEEP), should be avoided. It is critical that pulmonary vasodilator therapy not be interrupted perioperatively, particularly prostacyclin (Flolan) infusions, whose discontinuation can result in severe rebound pulmonary hypertension in as little as 10 to 15 minutes. With severe pulmonary hypertension and known responsiveness of the pulmonary vasculature to NO, it is advisable to have NO available for immediate or even prophylactic administration. The use of invasive monitoring (e.g., arterial blood pressure) is usually determined by the nature of the surgical procedure. Because of the significant morbidity and mortality of anesthesia and surgery for the patient with severe pulmonary hypertension, a risk-benefit analysis involving the cardiologist, surgeon, and anesthesiologist is essential before performing elective procedures.
Infective Endocarditis
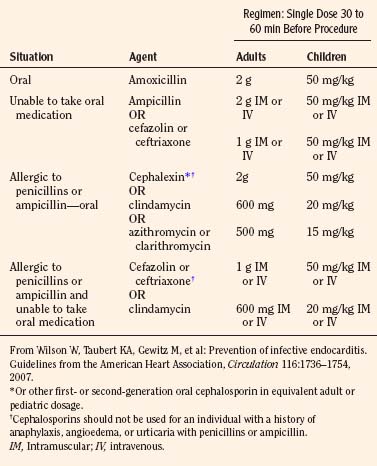
The most recent guidelines of the American Heart Association concluded that only an extremely small number of cases of infective endocarditis could be prevented by antibiotic prophylaxis for dental procedures (Wilson et al., 2007). As a result, infective endocarditis prophylaxis for dental procedures is recommended only for patients with underlying cardiac conditions (Box 21-2) associated with the highest occurrence and risk for adverse outcome from infective endocarditis. Prophylaxis in this group is recommended for all dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa. The antibiotic is administered in a single dose before the procedure (Table 21-1). Administration of antibiotics solely to prevent endocarditis is not recommended for patients who undergo a genitourinary or gastrointestinal tract procedure.
* Except for the conditions listed above, antibiotic prophylaxis is no longer recommended for any form of CHD.
† Prophylaxis is recommended because endothelialization of prosthetic material occurs within 6 months after the procedure.
From Wilson W, Taubert KA, Gewitz M, et al: Prevention of infective endocarditis. Guidelines from the American Heart Association, Circulation 116:1736–1754, 2007.
End-Organ Dysfunction and Injury
Unrestricted left-to-right shunting, in addition to increasing PAP and PVR, produces alterations in lung mechanics and airway compression. The primary effects on lung mechanics are a decrease in lung compliance and an increase in airway resistance (Bancalari et al., 1977; Stayer et al., 2004). Decreased compliance will necessitate higher than expected airway pressures, with care being taken not to insufflate the stomach during mask ventilation. Airway compression can result from dilated pulmonary arteries, left (or right) atrial dilation, massive cardiomegaly, or intraluminal bronchial obstruction (Berlinger et al., 1983). The pulmonary lymphatics are also compressed in these circumstances, perhaps explaining an increased incidence of pulmonary infectious symptoms in patients with large left-to-right shunts.
Neurologic injury and adverse neurodevelopmental outcome in CHD patients are multifactorial, with various contributions from genetic, lesion, and procedural elements (Newburger and Bellinger, 2006). Relatively pervasive issues include hyperactivity, diminished executive function, and various other neurocognitive abnormalities, especially in areas pertaining to speech and language and executive functions. Less commonly seen in the current era are seizures, stroke, and choreoathetosis. Structural brain abnormalities have been found with magnetic resonance imaging (MRI) in infants with CHD before any intervention, as well as after surgery or balloon atrial septostomy (Mahle et al., 2002; McQuillen et al., 2006; McQuillen et al., 2007; Miller et al., 2007; Andropoulos et al., 2010). Complex CHD may have anywhere from a mild to a profound impact on a child’s psychosocial development (Horner et al., 2000). These issues necessitate additional sensitivity with the family, altering the amount of detail discussed in front of the child when obtaining informed consent, and precluding certain sedation or regional anesthetic techniques.
Chronic cyanosis, low systemic cardiac output, or high venous pressures (Damman et al., 2009) may over time contribute to the development of renal and hepatic insufficiency. This may not be evident on routine laboratory tests (e.g., serum creatinine or liver enzymes) but may predispose to perioperative dysfunction in response to relatively minor changes in organ perfusion and oxygen delivery, or to otherwise relatively mild toxic stresses (e.g., ketorolac). Cardiac catheterization (contrast nephropathy) increases the risk for perioperative renal dysfunction (Briguori et al., 2007).
Extracardiac Anomalies
Extracardiac anomalies occur in 25% of infants seen during the first year of life in patients with cardiac disease (Greenwood et al., 1975). The anomalies are often multiple and may result from chromosomal, genetic, teratogenic, or unknown causes.
Heart Transplant Recipients
The worldwide annual transplant rate in children is around 400, with the major indications being cardiomyopathy and congenital heart disease (Kirk et al., 2008). Factors to consider in this population are cardiac physiology and functional status, cardiac allograft vasculopathy, rejection, the side effects of immunosuppressive agents, and the development of renal dysfunction, hypertension, and malignancy. Efferent denervation results in a resting tachycardia (withdrawal of vagal tone) and impaired chronotropic response to stress. Afferent denervation results in lack of angina during myocardial ischemia and alterations in cardiac baroreceptors and mechanoreceptors. Cardiac physiology is restrictive, with mildly elevated filling pressures and a low-normal ejection fraction (Cotts and Oren, 1997). There may be sinus node dysfunction and there is a shift from β1 to β2 receptors.
Anesthetic considerations (see Chapter 20, Anesthesia for Congenital Heart Surgery, for a more complete discussion): The response to hemodynamic instability is slower (dependence on circulating catecholamines) and less robust. The denervated heart is preload dependent, with a reduced chronotropic response to hypotension or sympathetic stimulation. Restrictive physiology, particularly with rejection, increases the risk for pulmonary edema when fluid administration is not judicious. Sensitivity is increased to direct-acting catecholamines, β-blockers, adenosine, and verapamil, and it is decreased to digoxin and indirect-acting sympathomimetic agents. Myocardial ischemia is an ever-present threat from coronary artery vasculopathy. A new onset of dysrhythmias or heart block is ominous, suggesting rejection or myocardial ischemia. Immunosuppression requires strict aseptic technique, and the hypertension and nephrotoxicity associated with some agents and possible need for stress-dose corticosteroids need to be addressed.
Preoperative assessment
CHD adds significant risk for morbidity and mortality in patients requiring noncardiac surgery (Warner et al., 1998; Coran et al., 1999; Baum et al., 2000; Martin et al., 2002; Carmosino et al., 2007). The preoperative evaluation should be complete enough to provide a clear understanding of the pathophysiology of the cardiac defect, the implications of any corrective or palliative procedures, and the likely interactions with the planned surgical procedure. As a general rule, patients with CHD who are doing well clinically (i.e., have good functional status, few or no medications, and only routine medical examinations) tend to do well with anesthesia and surgery. Not surprisingly, the unrepaired or palliated patient presents a greater risk, as does the more complex and stressful surgical procedure. Broadly speaking, there are three categories of patients with CHD: those who have undergone a reparative (corrective) procedure, those who have undergone a palliative procedure, and those who have not undergone any procedure. The principles of anesthetic management are the same whether the patient is a child or an adult, and whether they have had a procedure or not.
History and Physical Examination
As the history may be incomplete or misleading with complex CHD (Colman, 2003), close collaboration with the patient’s cardiologist is valuable. Additionally, the cardiologist can help identify patients at high risk, clarify pathophysiologic issues, establish if the current clinical status is the best possible, and provide the findings of recent cardiologic studies. The focus of the history should be on the type of lesion and factors listed in Box 21-1, prior surgical and catheterization procedures and complications thereof, anesthetic experience, medications, allergies, and current functional status. Specific symptoms that should be sought are feeding difficulties and sweating in infants, poor growth, cyanotic spells, decreased activity level such as inability to keep up with healthy peers, fatigue, dyspnea, palpitations, chest pain, and syncope. New or worsening symptoms require cardiology consultation. Recent respiratory tract infections can cause changes in pulmonary vascular resistance and airway reactivity, increasing anesthetic risk in the setting of decreased pulmonary compliance, pulmonary hypertension, systemic-to-pulmonary artery shunts, and cavopulmonary anastomosis.
The physical examination should include general appearance, level of activity, presence of distress, and vital signs. Arterial oxygen saturation (SpO2) varies with clinical status, but is expected to be above 94% after definitive procedures and in the range of 75% to 85% after palliative interventions that create shunted or intracardiac mixing circulations. Evidence of tachycardia, cyanosis, tachypnea, labored breathing, congestive heart failure, and poor peripheral perfusion should be sought. Airway assessment is important, because extracardiac anomalies may be present in up to one quarter of patients with CHD (Friedman, 1997). Peripheral pulses and four extremity blood pressures should be assessed in the setting of known or suspected aortic arch obstruction, previous or present Blalock-Taussig shunts, or after multiple cardiac catheterizations.
Special Investigations
The extent of laboratory testing depends on the child’s clinical status and the complexity of the planned procedure. General recommendations for blood testing include the following: (1) hematocrit or hemoglobin if the child is pale, cyanotic, or undergoing a procedure with the potential for significant blood loss; (2) serum electrolytes for patients with renal dysfunction or those receiving diuretics, angiotensin-converting enzyme inhibitors, or digoxin (although preoperative electrolyte disturbances in children and young adults presenting for cardiac surgery are uncommon [Hastings et al., 2008]); (3) platelet count and coagulation studies for cyanotic children or those on anticoagulants or antiplatelet agents; and (4) blood typing and cross-matching if significant blood loss is anticipated. A chest radiograph should be obtained with new cardiorespiratory symptoms or abnormal findings on clinical examination, or if dictated by the surgical procedure. Cardiologic studies should be coordinated with the child’s cardiologist, because some tests will have been recently completed, and investigations such as cardiac catheterization, Holter monitoring, exercise stress tests, and cardiac MRI mandate the cardiologist’s input. The electrocardiogram is reviewed for rhythm abnormalities, impaired conduction, chamber enlargement, and ischemia. Changes from prior studies need to be explained before proceeding. Preoperative consultation with the cardiologist is essential for patients with pacemakers, with evaluation of the pacemaker and a clear plan made for appropriate adjustment on the day of surgery (see earlier) (Practice advisory, 2005). In all but the simplest lesions, recent echocardiography is probably useful to document the current status of anatomy and ventricular function.
Procedure Scheduling
Institutional practices vary in the provision of anesthetic care for the highest-risk children with CHD. The aging of the CHD population has also resulted in adults with CHD undergoing noncardiac procedures at pediatric institutions (Warnes et al., 2001). In some institutions, anesthesia is provided by those who routinely practice pediatric cardiac anesthesia, and in others (including our own) pediatric anesthesiologists who do not practice cardiac anesthesia provide the care (Walker et al., 2009). The important point is that the case be assigned to an anesthesiologist who will understand the cardiac pathophysiology and know how to prevent and promptly deal with cardiac-related complications.
Intraoperative management
Premedication
Midazolam, 0.5 to 0.75 mg/kg orally, is usually sufficient, but for patients who have had multiple surgeries and catheterizations and thus are likely to be more anxious and perhaps more tolerant to sedative medications, it is often necessary to increase the dose to 1 mg/kg (Masue et al., 2003); sometimes oral ketamine, 3 to 10 mg/kg, is added as well (Auden et al., 2000; Funk, et al., 2000). Troublesome ketamine-stimulated secretions can usually be controlled with glycopyrrolate once intravenous access is established. If the patient has intravenous access in situ or after placement, a small bolus of midazolam, 0.05 mg/kg, repeated as necessary, will provide anxiolysis and facilitate separation from the parents. Intramuscular sedation with ketamine, 3 to 5 mg/kg, with or without midazolam, 0.05 to 0.1 mg/kg, may be necessary for the uncooperative or combative child who will not accept oral premedication and for whom an intravenous induction is most desirable. A combination of oral meperidine and pentobarbital for heavy premedication of CHD patients has been used with a substantial track record of safety and success (Nicolson et al., 1989), but insufficient effect and rage-like reactions (most likely attributable to pentobarbital) are known side effects. After any heavy premedication, the anesthesiologist should remain with the patient, and, particularly for patients with cyanotic CHD, oxygen saturation should be monitored and oxygen administered as needed (Stow et al., 1988; DeBock et al., 1990).
Anesthetic Technique
For general anesthesia, an intravenous induction is recommended for patients with limited cardiac reserve, particularly those at risk for a marked decrease in cardiac output or circulatory instability with induction. Such high-risk cases include the more severe presentations of ventricular dysfunction, outflow tract obstruction, and pulmonary hypertension. Patients with cyanotic lesions resulting from right-to-left shunts have a slower inhalation induction because of the effects of reduced QP (Tanner et al., 1985). Dependency on a systemic-to-pulmonary artery shunt (and hence maintenance of adequate cardiac output and peripheral resistance) may also be better served by a hemodynamically stable intravenous induction, particularly if there is evidence (e.g., echocardiographic, history of decreasing arterial oxygen saturation or rising hemoglobin) of shunt narrowing or outgrowth over time.
Etomidate lacks significant hemodynamic effects in clinical dosages in children and adults (Gooding et al., 1979; Sarkar et al., 2005), and ketamine provides hemodynamic stability through sympathetically mediated increases in heart rate and systemic vascular resistance, albeit with direct myocardial depressant effects (Morray et al., 1984). There is no technique that guarantees preservation of myocardial function and hemodynamic stability during anesthetic induction at the extreme of ventricular dysfunction (e.g., severe end-stage dilated cardiomyopathy), valvar obstruction, or pulmonary hypertension; reasons are multiple and include increased aortic impedance (both ketamine and etomidate) in the face of absent myocardial reserve to respond, deleterious effects of institution of positive pressure ventilation, and altered catecholamine tone. Clearly, only absolutely necessary or emergent procedures should be undertaken for such patients. Preoperative treatment with inotropes or inodilators (e.g., milrinone), or both, for patients with severe ventricular dysfunction or balloon dilation of severe valvar stenosis are examples of preoperative optimization techniques that should be considered. In addition to including inotropic support, the ability to rapidly convert to support with extracorporeal membrane oxygenation should be available (Duncan et al., 1998; Thiagarajan et al., 2007).
Although reports of the effect of ketamine on pulmonary vascular resistance are conflicting (Morray et al., 1984; Hickey et al., 1985; Berman et al., 1990; Maruyama et al., 1995), it can be used without risk of major increases in PVR in patients with CHD provided elevations in arterial CO2 are prevented (Hickey et al., 1985). Opioid-benzodiazepine techniques are also suitable, particularly if postoperative mechanical ventilation is planned (Hickey and Hansen, 1984). Remifentanil provides profound analgesia with short recovery times, but it can be associated with bradycardia and hypotension in high dosages (Ross et al., 2001) and in association with sevoflurane (0.6 minimum alveolar concentration) (Foubert et al., 2002); bradycardia can be prevented with pretreatment with glycopyrrolate, 0.006 mg/kg (Reyntjens et al., 2005). Propofol use should probably be limited to children with adequate cardiovascular reserve who can tolerate mild to moderate decreases in systemic vascular resistance, contractility, and heart rate (Williams et al., 1999). Sevoflurane is associated with less myocardial depression and better hemodynamic stability than halothane, particularly in infants (Holzman et al., 1996; Wodey et al., 1997; Rivenes et al., 2001; Russell et al., 2001; Laird et al., 2002). Exposing neonates to large amounts of opioids and benzodiazepines can lead to tolerance that may persist (Mao and Mayer, 2001; Suresh and Anand, 2001), whereas inadequate analgesia for infants may lead to specific centrally mediated pain sensitization and thus increased sensitivity to pain and greater fear of painful procedures (Porter et al., 1999; Lowery et al., 2007). This has implications for premedication, opioid-benzodiazepine anesthesia, and postoperative pain control.
Regional anesthesia, alone or in combination with general anesthesia, has been used successfully in patients with CHD (Selsby and Sugden, 1989; Holzman et al., 1992; Martin et al., 2002; Sacrista et al., 2003; Katznelson et al., 2005; Walker et al., 2009), including those with shunt physiology and left-sided obstructive lesions. In infants and young children (<3 to 4 years of age), this success is likely to be at least in part due to minimal effects of regional blockade on α-adrenergic tone and SVR. As complex CHD can have a profound effect on emotional life and psychosocial development (Horner et al., 2000; Reid et al., 2006), substantial persuasion may be necessary in the older child or young adult when primarily regional or local anesthetic techniques are deemed to be indicated to reduce anesthetic risk.
Monitoring and Vascular Access
Venous access may be limited as the result of multiple surgical procedures, cardiac catheterizations, and intensive care admissions. Multiple cardiac catheterizations can also lead to femoral vein occlusion. It is imperative to avoid the introduction of air into the venous system, leading to systemic air embolization in patients with intracardiac or intravascular communications and the potential for right-to-left shunting. For the more complex surgical procedures, central venous catheters allow monitoring of trends in central venous pressure and oxygen saturation (Dueck et al., 2005), as well as fluid and inotrope administration. Internal jugular or subclavian vein catheters can be quite useful but also carry a risk for thrombosis in the superior vena cava, as well as postoperative catheter-associated infection. The thrombotic complication can be disastrous in patients with a cavopulmonary anastomosis; consideration should be given to placing a femoral venous catheter if central access is necessary in such patients. Central venous catheters should be used for the shortest duration possible, access frequency limited, and special access ports and disinfection techniques employed to reduce the risk for catheter-associated infection. A low-dosage postoperative heparin infusion (10 IU/kg per hr) through the catheter can reduce the risk for thrombosis. Urinary catheters are placed as a guide to the adequacy of cardiac output and renal perfusion if major fluid shifts or blood losses are anticipated or if the procedure will be prolonged.
Transesophageal echocardiography (TEE) has been shown in adults to be useful for intraoperative assessment of ventricular preload and function (Schulmeyer et al., 2006), but the often complex anatomy in patients with CHD mandates additional expertise (Rivenes et al., 2001; Ikemba et al., 2004). TEE is likely to be used with increasing frequency in patients with and without CHD undergoing noncardiac procedures for monitoring of ventricular function and preload, particularly as the technology becomes more available and anesthesiologists become more practiced in its use (Mahmood et al., 2008). Intermittent transthoracic echocardiography is an alternative approach, provided that it is practical, there are adequate acoustic windows, and personnel with expertise are available.
Use of pulmonary artery catheterization (PAC) is controversial (Bernard et al., 2000), as added benefits have not convincingly been demonstrated with acquired heart disease. The risks are increased in the setting of pulmonary hypertension, and correct placement may be more difficult in some patients with CHD (absence of pulsatile flow, need to traverse atrial baffles). PAC can be placed under fluoroscopic guidance in the cardiac catheterization laboratory before elective, major noncardiac surgery (e.g., spinal fusion) in patients with a Fontan circulation or severe ventricular dysfunction. In these circumstances, PAC can be useful as an indicator of systemic ventricular filling (pulmonary artery capillary wedge pressure) and oxygen delivery (pulmonary artery saturation). In the future, this role may be supplanted by TEE.
Postoperative management
The preoperative clinical status, nature of the surgical procedure, and intraoperative course determine whether postoperative admission will be to a general surgical ward, a cardiology ward, or an intensive care unit. Hemorrhage, hypoxia, hypoventilation, fever, uncontrolled pain, or myocardial ischemia may convert a well-tolerated surgical procedure into a crisis. Day surgery is possible for those patients whose cardiac status is well controlled, who have undergone a minor surgical procedure, and who have attained their baseline status prior to discharge. Higher-risk patients, particularly those undergoing major surgical procedures, are usually best managed in an intensive care setting. It is our practice to admit the child to the cardiology ward if changes in cardiac physiology pose a greater risk than complications from the surgical procedure. The healthier cardiac patient with a more complex surgical procedure is usually admitted to the appropriate surgical ward with cardiology consultation and follow-up. Standard fluid replacement guidelines are employed, and postoperative laboratory testing is guided by intraoperative blood loss, fluid shifts, and the child’s preoperative status. Effective pain management is important and can be especially difficult if the patient is tolerant to sedatives and narcotics, or has recently been discharged after prolonged hospitalization and still on a withdrawal regimen. Involvement of colleagues with pediatric pain management expertise is recommended (see Chapter 15, Pain Management). Despite the best-possible anesthetic and procedure, it is in the postoperative period that complications are still likely to develop. In our experience, these are usually in patients with subtle or occult preexisting end-organ dysfunction, occurring as a consequence of chronic cyanosis, low cardiac output, or high venous pressures (or a combination of these). Certainly, the ability of various anesthetic agents, other drugs, and the surgical procedure to compromise organ blood flow or alter organ function plays a role as well.
Outcomes of common congenital heart defects
Tetralogy of Fallot
Tetralogy of Fallot is the most common cyanotic lesion and accounts for approximately 10% of all CHD (Hoffman and Kaplan, 2002; Therrien and Webb, 2003). See related video online at www.expertconsult.com. The defect is characterized by anterocephalad deviation of the outlet septum, resulting in right ventricular outflow tract obstruction, a nonrestrictive VSD, aortic override, and right ventricular hypertrophy. The subpulmonary obstruction may be fixed or dynamic, it is invariably associated with some degree of pulmonary valve stenosis, and there may be hypoplasia of the main and branch pulmonary arteries as well. Associated cardiac anomalies include a right-sided aortic arch (25%), a second VSD (3%), and coronary artery anomalies (anterior descending coronary artery arising from the right coronary artery and crossing the RV outflow tract [3%]). TOF is frequently associated with a 22q11 deletion, and DiGeorge and velocardiofacial syndromes.
The defect is characterized by anterocephalad deviation of the outlet septum, resulting in right ventricular outflow tract obstruction, a nonrestrictive VSD, aortic override, and right ventricular hypertrophy. The subpulmonary obstruction may be fixed or dynamic, it is invariably associated with some degree of pulmonary valve stenosis, and there may be hypoplasia of the main and branch pulmonary arteries as well. Associated cardiac anomalies include a right-sided aortic arch (25%), a second VSD (3%), and coronary artery anomalies (anterior descending coronary artery arising from the right coronary artery and crossing the RV outflow tract [3%]). TOF is frequently associated with a 22q11 deletion, and DiGeorge and velocardiofacial syndromes.
If the spell persists, the child can be anesthetized, the trachea intubated, and mechanical ventilation instituted with 100% oxygen, low inspiratory pressures, and long expiratory times to promote venous return and antegrade flow across the RV outflow. An inhalation agent may be beneficial to reduce hyperdynamic right ventricular outflow obstruction. Manual compression of the abdominal aorta can be an effective means to temporarily increase SVR and decrease cyanosis in the anesthetized patient. In the operating room, there may be the need to proceed very rapidly to cardiopulmonary bypass (CPB) if the spell is severe and not resolving. Resuscitation with extracorporeal membrane oxygenation (ECMO) should be considered for refractory episodes when immediate operative intervention is not possible (Duncan et al., 1998; Thiagarajan et al., 2007).
Primary complete repair for the neonate or infant has largely replaced the traditional two-stage repair sequence of arteriopulmonary shunting in early infancy followed by later repair (Pigula et al., 1999). Thus, it is becoming fairly unusual to encounter an unrepaired or shunted infant with TOF. Right ventricular outflow obstruction is relieved by resection of hypertrophied muscle bundles and enlargement of the outflow tract with a pericardial patch. Although pulmonary valve-sparing techniques are increasingly being employed, a very small pulmonic annulus or a very stenotic pulmonary valve dictates placement of the patch across the annulus (transannular patch), resulting in pulmonary insufficiency. The VSD is closed with a Dacron patch. If a major coronary artery crosses the RV outflow tract (RVOT), or with long-segment pulmonary atresia, a right ventricle–to–pulmonary artery conduit rather than a pericardial patch is placed.
The majority of problems after TOF repair relate to chronic abnormal RV loading—that is, pressure loading from residual or recurrent RVOT obstruction and volume loading primarily from pulmonary regurgitation (issues shared by other lesions that require RVOT reconstruction or RV-PA conduit such as truncus arteriosus, pulmonary atresia, and the Rastelli procedure for TGA). In the long term, there is progressive RV dysfunction and the development of arrhythmias with increased risk for sudden death. Factors that have been associated with reduced long-term survival include older age (>4 years) at repair, initial palliative shunting procedures, significant RV hypertension, and volume loading of the RV (Gatzoulis et al., 2000a; Karamlou et al., 2006). Timely pulmonary valve replacement in children and young adults, before irreversible severe RV dilation and systolic ventricular dysfunction accrue, has become a major consideration (Therrien et al., 2000). Many patients after repair are asymptomatic with normal activity, although right ventricular dysfunction may progress and be evident only on exercise testing (decreased maximal aerobic capacity and endurance), stress echocardiography, or radionuclide techniques. Cardiac MRI is particularly useful to quantify RV systolic function, RV volume, the degree of pulmonary regurgitation, and sites of RVOT obstruction (Geva and Powell, 2008). The presence of tricuspid regurgitation is a likely surrogate for substantial RV dysfunction. Right bundle branch block is commonly seen after repair, whereas atrial tachyarrhythmias (atrial flutter or intraatrial reentrant tachycardia) arise in about one third of adults. Although the presence of premature ventricular contractions (PVCs) is common in asymptomatic patients (approximately 10% on routine electrocardiogram, 30% on exercise stress testing, and up to 50% during Holter monitoring), it is often of low grade and has not been a predictor of patients at risk for sudden death. Although a QRS duration of 180 msec or greater is a highly sensitive marker for sustained ventricular tachycardia and sudden death, its positive predictive value is low (Gatzoulis et al., 1995). LV dysfunction and dyssynchrony have been observed in patients with TOF and was associated with QRS duration, such that abnormal LV mechanics in combination with RV dysfunction may explain the relation between QRS duration and adverse cardiac outcomes (Tzemos et al., 2009).
However, the presence of monomorphic ventricular tachycardia in a symptomatic patient (syncope and palpitations) is significant and necessitates treatment. Electrophysiologic methods are to assess and ablate atrial or ventricular arrhythmias and for deciding on the need for an implantable cardioverter-defibrillator (Khairy et al., 2008b).
Anesthetic considerations: The degree of RV dysfunction needs to be defined and consideration given to preoperative interventional cardiac catheterization to reduce the impact of significant residual lesions (RVOT or PA obstruction, residual VSD, collaterals causing volume loading, arrhythmias). In the presence of pulmonary regurgitation or RV dysfunction, factors that increase PVR should be avoided, as should factors detrimental to RV myocardial supply-demand relationship (tachycardia, hypotension, anemia, and acidosis are detrimental). The role of pulmonary valve replacement in patients with severe pulmonary regurgitation, RV volume overload, and RV dysfunction (all best assessed by cardiac MRI) remains to be defined. Right ventricular filling should be maintained, understanding that excessive volume (or dysfunction) can lead to RV dilation and resultant LV dysfunction (ventricular interdependence). Prophylactic administration of an inotrope to improve RV contractile performance should be considered in patients with significant RV dysfunction. (See Pulmonary Hypertension, earlier.) A means for external defibrillation and pacing should be readily available. Late development of LV dysfunction is another consideration in patients with TOF as they age.
Transposition of the Great Arteries
Transposition of the great arteries (D-TGA) accounts for 3% to 10% of all CHD (Hoffman and Kaplan, 2002). See related video online at www.expertconsult.com. Ventriculoarterial discordance results in the aorta arising from the anatomic right ventricle and the pulmonary artery from the left ventricle. Associated defects include a VSD (40% to 45%), VSD with LV outflow tract obstruction (10%), or variability in coronary artery pattern (Mayer et al., 1990). A much less common form of TGA is congenitally (physiologically) “corrected” TGA (L-TGA), in which there is both ventriculoarterial and atrioventricular discordance (i.e., a series circulation in which the right atrium connects via the mitral valve to the LV and then the PA, and the left atrium connects via the tricuspid valve to the RV and then the aorta). Congenitally corrected transposition (without associated cardiac lesions) may go undetected for decades until the RV, which is the systemic ventricle, begins to fail. This group is at increased risk for the spontaneous development of complete heart block.
Ventriculoarterial discordance results in the aorta arising from the anatomic right ventricle and the pulmonary artery from the left ventricle. Associated defects include a VSD (40% to 45%), VSD with LV outflow tract obstruction (10%), or variability in coronary artery pattern (Mayer et al., 1990). A much less common form of TGA is congenitally (physiologically) “corrected” TGA (L-TGA), in which there is both ventriculoarterial and atrioventricular discordance (i.e., a series circulation in which the right atrium connects via the mitral valve to the LV and then the PA, and the left atrium connects via the tricuspid valve to the RV and then the aorta). Congenitally corrected transposition (without associated cardiac lesions) may go undetected for decades until the RV, which is the systemic ventricle, begins to fail. This group is at increased risk for the spontaneous development of complete heart block.
D-TGA is currently repaired anatomically by the arterial switch (Jatene) operation (ASO) (Jatene et al., 1976). The pulmonary artery and aorta are transected distal to their respective valves. The coronary arteries are excised with a button of surrounding tissue and reimplanted into the proximal pulmonary artery (neoaorta). The great arteries are then switched, with the pulmonary artery brought anterior (Lecompte maneuver) and anastomosed to the proximal aorta (right ventricular outflow) and the aorta anastomosed to the proximal pulmonary artery (left ventricular outflow). Most patients with TGA have coronary anatomy that is suitable for coronary reimplantation. The variant of D-TGA with VSD and severe LVOT obstruction precludes the ASO. In this setting, the Rastelli procedure is performed, in which the VSD is repaired by a patch that directs blood from the left ventricle through the defect into the aorta (and also closes the VSD), and a valved conduit is placed from the right ventricle to the main pulmonary artery. With the Rastelli procedure, the LV functions as the systemic ventricle, but conduit degeneration and stenosis are inevitable (Warnes, 2006).
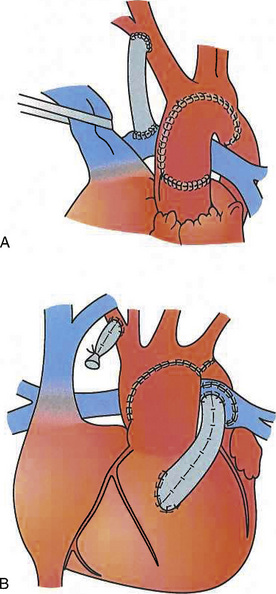
The atrial switch procedure (Mustard, Senning), which revolutionized the management of infants with TGA, is now rarely used. It is a physiologic repair in which a baffle in the atrium directs systemic venous blood to the mitral valve (and consequently to the LV and pulmonary artery) and pulmonary venous blood to the tricuspid valve (and consequently to the RV and aorta) (Fig. 21-1).
The atrial switch procedures provide excellent midterm results (15-year survival, 77% to 94%; 20-year survival, 80%) (Merlo et al., 1991; Helbing et al., 1994; Wilson et al., 1998; Sarkar et al., 1999; Williams et al., 2003), with many patients able to lead fairly normal lives into their third and fourth decades. In the long term, there is progressive deterioration in RV function (Piran et al., 2002) and development of tricuspid regurgitation (which is the systemic atrioventricular valve in this circulation) with heart failure, arrhythmias, and risk for sudden death (Wilson et al., 1998). Even in asymptomatic patients, exercise testing demonstrates moderate to severe limitations in RV function and maximal aerobic capacity. Sinus rhythm is maintained in only 40% to 50% of patients (Deanfield et al., 1988), with the frequent occurrence of sick sinus syndrome. Atrial flutter parallels the development of ventricular dysfunction (Gatzoulis et al., 2000b) and is experienced in one third or more of patients, 20 years after surgery (Puley et al., 1999). Intraatrial baffle leaks can result in shunting and hypoxemia. Baffle obstruction of the systemic venous return can cause superior vena cava (SVC) syndrome, hepatic congestion, ascites, and peripheral edema, whereas pulmonary venous baffle obstruction can cause pulmonary edema and pulmonary hypertension. Subendocardial perfusion defects of the systemic (right) ventricle have been noted in a significant number of patients.
The ASO is associated with an early hospital mortality of less than 5% in experienced centers (Hutter et al., 2002; Warnes, 2006; Raisky et al., 2007). At 15 years, good LV function and sinus rhythm are maintained in 96.4% and 98.1% of patients, respectively (Losay et al., 2001). The ongoing risk for death is less than after the atrial switch operation, and it is related to coronary events and arrhythmias. In long-term follow-up, patients remain in good condition, and the majority are New York Heart Association functional class I (Hutter et al., 2002). The most frequent need for reintervention has been the development of supravalvar (anastomotic) pulmonary artery stenosis, less commonly supravalvar aortic stenosis. These are frequently amenable to balloon dilation, although reoperation may occasionally be necessary. Neoaortic insufficiency (i.e., the anatomic pulmonary valve now located in the aortic position) appears to be occurring with increasing frequency as more patients accrue and age. At present, it is usually of mild or lesser severity and thus far has infrequently required reintervention; however, the long-term implications of this problem are concerning. Another potential complication that is being found more frequently and requires ongoing attention is the development of coronary artery stenoses. Coronary obstruction is an infrequent clinical complication outside of the immediate perioperative period, where technical difficulties associated with coronary reimplantation can result in myocardial ischemia and problems related thereto. Overall, the incidence of coronary events has a bimodal pattern, with a high early incidence and low late incidence (Legendre et al., 2003). The arteries display varying degrees of proximal eccentric intimal proliferation (Pedra et al., 2004) and concentric intimal smooth muscle hyperplasia with preserved tunica media (Bartoloni et al., 2006), suggesting the potential for the development of early atherosclerosis in the reimplanted coronary arteries. Noninvasive tests are not sufficiently sensitive to detect delayed coronary artery stenosis, and coronary artery angiography or intracoronary ultrasound is required (or both); it is presently unclear whether such testing should be a mandatory component of surveillance in all patients who have undergone an ASO (Pedra et al., 2004). If needed, coronary revascularization can be achieved using coronary angioplasty in most cases (Raisky et al., 2007).
Anesthetic considerations: After an uncomplicated ASO, most patients can be managed in the same way as those with a structurally normal heart, but with an index of suspicion for coronary artery disease. Severe pulmonary artery stenosis can be managed with interventional cardiac catheterization prior to elective procedures. Anesthesia for patients after the atrial switch operation should be based on the knowledge that this can become an increasingly fragile circulation with limited physiologic reserve as patients age. The potential for decreased ventricular function is substantial, particularly of the systemic right ventricle, as well as for arrhythmias and end-organ dysfunction. Although some volatile anesthetic will be tolerated by most of these patients, many will benefit from the more hemodynamically stable induction and maintenance regimens that have been discussed earlier. As elsewhere, it may be prudent to start an inotrope infusion at the time of or shortly after induction in those with more severe degrees of ventricular dysfunction. A significant number of these patients have a pacemaker, and the appropriate guidelines need to be followed (see earlier) (Practice advisory, 2005). Baffle leaks can result in cyanosis and increase the risk for systemic embolization of air or debris; consideration should be given to device closure in the catheterization laboratory prior to elective procedures associated with a high risk for embolization.
Single-Ventricle Physiology
A single ventricle is defined as the presence of two atrioventricular valves with one ventricular chamber, or a large dominant ventricle with a diminutive opposing ventricle (Keane and Fyler, 2006c). See related video online at www.expertconsult.com. Single ventricle or univentricular hearts encompass a wide variety of lesions that include tricuspid atresia or severe tricuspid stenosis, double-inlet single ventricle (usually left), single ventricle with common atrioventricular valve (unbalanced complete AV canal, heterotaxy variants), HLHS, and some forms of double-outlet right ventricle and pulmonary atresia with intact ventricular septum. Initial management is aimed at optimization of systemic oxygen delivery and perfusion pressure. Subsequent management is aimed at reducing the volume load on the ventricle (superior cavopulmonary connection or Glenn procedure) and finally achieving a series circulation with fully saturated systemic arterial blood (Fontan procedure). This is usually achieved through a staged approach. Although, collectively, single-ventricle defects are relatively rare, they account for a disproportionate share of repeated procedures and resultant morbidity and mortality.
Single ventricle or univentricular hearts encompass a wide variety of lesions that include tricuspid atresia or severe tricuspid stenosis, double-inlet single ventricle (usually left), single ventricle with common atrioventricular valve (unbalanced complete AV canal, heterotaxy variants), HLHS, and some forms of double-outlet right ventricle and pulmonary atresia with intact ventricular septum. Initial management is aimed at optimization of systemic oxygen delivery and perfusion pressure. Subsequent management is aimed at reducing the volume load on the ventricle (superior cavopulmonary connection or Glenn procedure) and finally achieving a series circulation with fully saturated systemic arterial blood (Fontan procedure). This is usually achieved through a staged approach. Although, collectively, single-ventricle defects are relatively rare, they account for a disproportionate share of repeated procedures and resultant morbidity and mortality.
Unoperated single-ventricle physiology is characterized by complete mixing of systemic and pulmonary venous blood at the atrial or ventricular level (or both). If there is no obstruction to pulmonary or systemic outflow, the amount of flow to each circulation is determined by the relative resistances of the pulmonary and systemic vascular beds. With no obstruction to pulmonary blood flow and the normal postnatal regression in PVR, PBF gradually increases and results in congestive heart failure. Obstruction to PBF will result in progressive cyanosis in the absence of a patent ductus arteriosus. Systemic outflow obstruction will result in increased PBF, CHF, and systemic hypoperfusion. In patients with single-ventricle physiology, an arterial saturation of 75% to 80% is felt to be indicative of a relatively balanced circulation with a QP:QS at or near 1:1 (assuming pulmonary venous saturation of 95% to 100% and mixed venous saturation of 50% to 55%) (Rudolph, 1974). However, even with a “balanced” QP:QS of 1:1, the systemic ventricle is essentially pumping double the normal cardiac output. This gives some appreciation for the even greater degrees of systemic ventricular volume overload that is imposed by increased amounts of PBF.
Norwood Operation
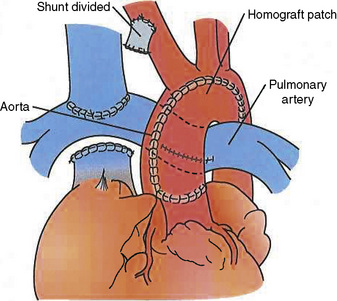
The Norwood procedure, also referred to as stage I single-ventricle palliation, is performed for patients with HLHS and its variants (Norwood et al., 1983) or when the pathway to the aorta from the systemic ventricle is obstructed. The pulmonary artery is transected just proximal to its bifurcation, and the pulmonary artery is anastomosed end to side, or side to side, to the ascending aorta, with reconstruction of the ascending and transverse aorta. In the neonatal period, PBF is supplied by a modified BT shunt or RV-to-PA conduit (Sano et al., 2003) (Fig. 21-2). When pulmonary venous blood must cross the atrial septum to reach the systemic ventricle, the atrial septum must be nonrestrictive. Creating a nonrestrictive atrial septum can be achieved in the cardiac catheterization laboratory with a Rashkind-Miller balloon atrial septostomy, or a surgical atrial septectomy can be performed at the time of initial palliation in procedures that require CPB.
Anesthetic Considerations for the Unoperated Neonate
Management principles are similar for patients whether they are dependent on the PDA or they have a surgically created shunt. A target arterial partial pressure of oxygen (Pao2) of 40 to 45 mm Hg and an oxygen saturation of hemoglobin (Sao2) of 70% to 80% are reasonable starting goals that are likely to approach achieving adequate systemic O2 delivery and PBF. With unrestricted PBF, control and manipulation of PVR and subsequently of QP:QS is accomplished most reliably through ventilatory interventions to increase PVR, usually hypercarbia in combination with 21% inspired oxygen to achieve a pH of 7.30 to 7.35; use of hypoxic (17% inspired O2 achieved by addition of nitrogen to air) or hypercarbic (Fico2, 2.7%) are alternatives (Tabbutt et al., 2001). In anesthetized patients, an appropriate degree of hypercarbia can usually be obtained with a tidal volume of 6 to 10 mL/kg, a PEEP of 3 to 5 cm H2O, and a respiratory rate of 8 to 10 breaths/min. Higher Fio2 is avoided unless the Pao2 is less than 35 to 40 mm Hg and intrapulmonary  mismatch is suspected. Denitrogenation with 100% O2 is recommended prior to laryngoscopy and tracheal intubation so as to prevent hypoxemia during this interval. Once the airway is secured, the Fio2 can be reduced.
mismatch is suspected. Denitrogenation with 100% O2 is recommended prior to laryngoscopy and tracheal intubation so as to prevent hypoxemia during this interval. Once the airway is secured, the Fio2 can be reduced.
Pulmonary Artery Band
Unrestricted PBF compromises systemic oxygen delivery, results in congestive heart failure, and ultimately leads to pulmonary artery hypertension and the development of pulmonary vascular occlusive disease. In this setting, a pulmonary artery band is placed to limit pulmonary blood flow, thereby controlling congestive heart failure and protecting the pulmonary vascular bed (Heath and Edwards, 1958).
Systemic-to-Pulmonary Artery Shunt
Infants with ductal-dependent pulmonary blood flow will require stabilization with PGE1 followed by placement of a surgical aortopulmonary shunt (clearly, an intracardiac right-to-left shunt is also required). A modified Blalock-Taussig shunt is performed most commonly and involves placement of a Gore-Tex graft between the subclavian or innominate artery and the pulmonary artery on the side opposite to the aortic arch. The aim of this procedure is to establish a stable source of PBF as the PDA closes. In patients with HLHS, a right ventricle–to–pulmonary artery conduit is an alternative to a modified BT shunt (Sano et al., 2003). A superior cavopulmonary shunt cannot be performed in the neonatal period, because the high PVR necessitates a pumping ventricle or systemic blood pressure to drive blood through the lungs.
Anesthetic Considerations after the Superior Cavopulmonary Connection
In the superior cavopulmonary connection or bidirectional Glenn operation, the SVC is transected and the cranial end is sewn in an end-to-side manner to the right pulmonary artery (which is in continuity with the left pulmonary artery); the distal (cardiac) end is oversewn (Fig. 21-3). Systemic venous return from the head and upper body thus passes directly from the SVC into the pulmonary circulation. The goal is to reduce the volume load on the ventricle and allow cardiac remodeling (compared with the prior shunted circulation) with maintenance of cardiac output at a lower ventricular filling volume. After the Glenn operation, the QP:QS will be between approximately 0.5:1 and 0.8:1, as the inferior vena cava (IVC) blood does not pass through the lungs but mixes with pulmonary venous blood in a functionally common atrium. The arterial oxygen saturation will still be reduced (75% to 85%), with a typical SVC pressure of 10 to 12 mm Hg and an atrial pressure of 5 to 6 mm Hg. The transpulmonary gradient is thus 4 to 7 mm Hg. The physiology of PBF and anesthetic considerations are similar to the Fontan circulation and discussed later.
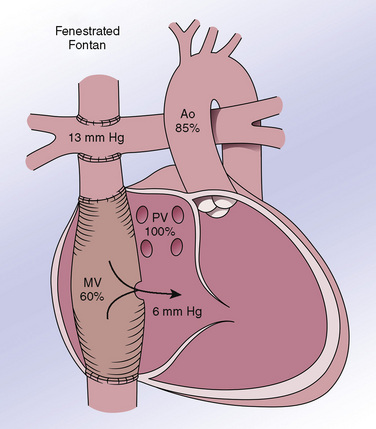
Anesthetic Considerations after the Total Cavopulmonary Connection or Fontan Operation
Patients with Fontan circulations are symbolic of how asymptomatic clinical status should not give the anesthesiologist a false sense of security about cardiovascular stability. The Fontan operation is usually performed within 1 to 2 years of a bidirectional Glenn (Jonas, 2004), and thus between 15 months and 2.5 years of age. The operative principle is diversion of the systemic venous return directly to the pulmonary arteries without the need for a subpulmonary ventricle, resulting in a series circulation with relief of cyanosis. Although many modifications of this procedure have been described, the total cavopulmonary connection (lateral tunnel Fontan) is currently the procedure of choice in most centers (Jonas, 2004) (Fig. 21-4). In the presence of a preexisting bidirectional Glenn anastomosis (the situation for most patients coming to Fontan surgery at present), an intraatrial baffle is placed to direct IVC flow from the IVC orifice along the side wall of the atrium up to the SVC orifice, with opening of the cardiac (distal) end of the SVC and anastomosis to the pulmonary artery.
The key physiologic principle in patients with Fontan physiology is maintaining filling and function of the systemic ventricle. In the absence of a pumping pulmonary ventricle, the initial consideration is to maintain an adequate pressure gradient between the systemic venous return circulation and the common atrium (i.e., the atrium that serves the systemic ventricle). The circulation can be viewed, in oversimplified fashion, as a waterfall, with the systemic venous pressure as the top, and the end-diastolic pressure in the systemic ventricle as the bottom. The ideal Fontan will have a mean systemic venous (pulmonary artery) pressure of approximately 10 to 15 mm Hg and a common atrial pressure of 7 to 10 mm Hg, resulting in a transpulmonary gradient of 3 to 8 mm Hg. Optimal physiology depends on adequate preload, unobstructed systemic venous return, low PVR, low mean intrathoracic pressure, normal lung parenchyma and alveolar ventilation, unobstructed pulmonary venous return, sinus rhythm (resulting primarily from the atrial contribution to ventricular filling [Penny et al., 1991]), atrioventricular valve competency, normal ventricular function, and low ventricular afterload. Any significant departure from these requirements can result in severe compromise. For example, ventricular dysfunction with an end-diastolic pressure of 15 mm Hg mandates a venous pressure of 20 to 25 mm Hg (i.e., the transpulmonary gradient is preserved but venous pressures must be proportionately higher) to achieve comparable pulmonary blood flow, ventricular filling, and cardiac output. Relatively mild acute lung disease (e.g., viral pneumonitis), if associated with increased PVR, can lead to an acute and severe decrease in systemic ventricular filling and cardiac output unless and until preload is restored sufficiently to restore ventricular filling (here, the transpulmonary gradient is increased)—that is, any downstream problem will necessitate a higher upstream pressure.
Complications after the Fontan operation are in part dependent on surgical technique and era, and partly on pathophysiologic sequelae of multiple factors such as multiple surgeries, long-standing systemic venous hypertension, cyanosis, and perhaps limited cardiac output as well. One can expect progressive decline in the function of the systemic ventricle, an increasing risk for development of arrhythmias, and end-organ dysfunction as these patients age. Recent data suggest that diastolic dysfunction may be an early and persistent feature of Fontan myocardial physiology (Cheung et al., 2000; Olivier et al., 2003). Other specific issues include recurrent pleuropericardial effusions, peripheral edema, ascites, cirrhosis, and protein-losing enteropathy (3.7%) (Mertens et al., 1998; Khairy et al., 2007). Thrombosis in the venous pathway (6% to 33%) may occur early or late and result in both pulmonary and systemic embolic complications, particularly stroke. Sudden total obstruction appears as sudden death. It is unclear whether and to what extent specific hemostatic derangements such as increased factor VIII, dysregulation of other procoagulant and anticoagulant factor synthesis resulting from Fontan physiology, or the superimposition of this physiology on a “normal” background of thrombophilic polymorphisms (e.g., factor V Leiden, prothrombin mutations) might be responsible for the apparent increased thrombotic risk in these patients (Odegard et al., 2003, 2009).
Arrhythmias are associated with serious morbidity (especially the development of atrial thrombi) and can lead to profound hemodynamic deterioration. The combination of atrial incisions, multiple and extensive atrial suture lines, and increased right atrial pressure and size appear to be predisposing factors. The incidence of atrial tachyarrhythmias (atrial flutter or fibrillation, or both) is lower with the lateral tunnel Fontan (Stamm et al., 2001b) than in patients operated on in the 1970s and 1980s with direct atriopulmonary anastomosis (Fishberger et al., 1997). In the current era, freedom from new supraventricular tachyarrhythmia was 98% at 5 years and 95% at 10 years (Brown et al., 2010). A recent study found less atrial arrhythmia with extracardiac conduit than lateral tunnel Fontan (Robbers-Visser et al., 2009). Bradyarrhythmia (heart block and sick sinus syndrome), which may necessitate pacemaker implantation, also appears to be more frequent in the first 10 years than atrial tachyarrhythmias with the lateral tunnel version of the procedure (Stamm et al., 2001b). Freedom from late-onset bradyarrhythmia varies from 89% (Stamm et al., 2001b) to 96% (Brown et al., 2010) at 10 years.
The probability of survival after Fontan surgery has increased in more recent years (93.9% at 8 years in one study [Stamm et al., 2001b] and 95% at 15 years in another [Brown et al., 2010]), largely as a result of improved surgical techniques, patient selection, and perioperative management (Mitchell et al., 2006; Anderson et al., 2008; Khairy et al., 2008a; Robbers-Visser et al., 2009). Progressive deterioration of ventricular function (functional status) and gradual attrition occur, predominantly from thromboembolic, heart failure–related, and sudden (presumed arrhythmic) deaths (Khairy et al., 2008a).
Objective testing of functional status has indicated that patients with stable single-ventricle physiology are able to lead a fairly normal life with moderate exercise tolerance (Robbers-Visser et al., 2010). With exercise, maximal aerobic capacity was reduced, with only 28% of patients having peak oxygen consumption within the normal range (Paridon et al., 2008). Stroke volume reserve is the most important determinant of aerobic capacity, with exercise tolerance limited by peak blood flow in the pulmonary vascular bed. With exercise or other hemodynamic stress, a fall in cardiac index and oxygen saturation may occur because of an inability to increase stroke volume (Robbers-Visser et al., 2008). This inability probably results from multiple factors, including relatively fixed myocardial contractile reserve and the fact that many Fontan patients are near-maximally venoconstricted, thereby limiting the amount of preload augmentation that can occur to support exercise-stimulated increases in cardiac output; a blunted heart rate response, for reasons discussed previously, may be an additional factor. In sum, the progressive and late decline in survival and functional status implies that these repairs are not curative and should be viewed as palliative at this time (Khairy et al., 2008a). A recent Pediatric Heart Network study found that sinus rhythm was present in 67% of patients; 13% used a pacemaker; semilunar and atrioventricular valve regurgitation was present in 49% and 74% of patients, respectively; 72% had abnormal diastolic function; and pulmonary reserve was below normal in 34% of cases (Anderson et al., 2008).
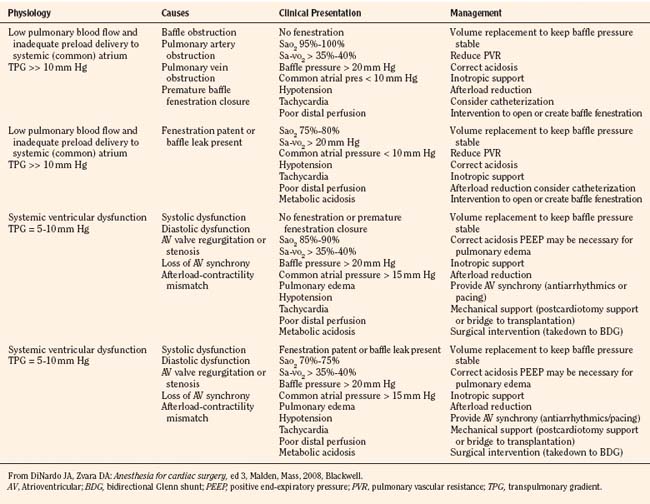
Anesthetic management: Management of Fontan physiology is summarized in Table 21-2. Hemodynamic goals are to maintain normal to increased preload, normal to slightly increased heart rate, normal to low pulmonary vascular resistance, and sinus rhythm (atrioventricular synchrony); to support ventricular function; and to avoid high ventricular afterload. In the hospitalized patient, maintenance intravenous fluids should be begun while the patient is fasting preoperatively. Particularly in outpatients, volume expansion may be necessary before induction to ensure adequate preload. A technique using sedation with local or regional anesthesia should be considered where feasible (recognizing that some patients will be on anticoagulants).
The use of mechanical ventilation deserves some consideration, as ventilation and acid-base management need to be optimized to keep PVR low. Although spontaneous ventilation enhances pulmonary blood flow (Penny and Redington, 1991), the accompanying hypoventilation likely to occur during anesthesia or procedures associated with abdominal or thoracic compression will increase PVR through atelectasis, hypoxic pulmonary vasoconstriction, and hypercarbia. In fact, increased PVR (and hence acutely reduced cardiac output) is more likely to occur during either relative hypoventilation (resulting primarily from increased Pco2 and also from alveolar hypoxia and collapse) or excessive positive pressure ventilation (resulting from increased intrathoracic and pulmonary pressures that are transmitted to the vasculature, as well as from potential microvascular distention or compression [or both] caused by overinflation). For most surgical situations (all but the shortest of procedures), we believe that the sum of effects favors judicious use of positive pressure ventilation in order to provide an effective anesthetic while maintaining lung volumes and normal gas exchange. This can usually be accomplished with a relatively large tidal volume (∼10 mL/kg), slower respiratory rates (10 to 15 breaths/min), and shorter inspiratory times (I:E of 1:3 or 1:4). Similar considerations apply to the use of PEEP: if it is targeted to maintain or improve functional residual capacity and lung compliance and thereby assist with gas exchange, it is likely to be helpful, whereas overdistention will favor deleterious effects. Additional fluid administration can be used to overcome the decrease in venous return associated with mechanical ventilation. Another benefit of positive pressure ventilation can be the decrease in afterload on the systemic ventricle (LaPlace’s law) and consequent improvement in its function.
Coarctation of the Aorta
The anatomy, pathophysiology, and anesthetic management for repair of aortic coarctation are discussed later (see Anesthesia for Closed Cardiac Procedures). The long-term consequences after coarctation repair are related to blood pressure and left ventricular mass. In long-term follow-up studies, persistent arterial hypertension in the absence of re-coarctation and type of repair is present in as many as one third of patients (de Divitiis et al., 2003; Rosenthal, 2005; Hager et al., 2007). Systolic blood pressure is positively associated with LV mass in this cohort, and elevated systolic blood pressure and LV mass are both important risk factors for late morbidity and mortality from heart failure, sudden death, and coronary and cerebrovascular disease (Toro-Salazar et al., 2002). Although it has been suggested that the risk of developing subsequent hypertension is reduced by repair in the first year of life (Seirafi et al., 1998), some series do not support this contention (de Divitiis et al., 2001). The etiology of this persistent hypertension is multifactorial and includes reduced pulsatility of the post-coarctation descending aorta (Pfammatter et al., 2004), reduced upper body vascular responses to reactive hyperemia and glyceryl trinitrate (possibly abnormal smooth muscle relaxation or structural abnormalities of the arterial wall [Murakami and Takeda, 2005]), aortic arch geometry (Ou et al., 2007, 2008), and abnormalities in baroreceptor reflexes and the renin-angiotensin-aldosterone system (Rosenthal, 2005). There is evidence to suggest that abnormalities in cardiovascular reflexes are already present in neonates with coarctation before repair (Polson et al., 2006).
These patients are at increased risk for premature coronary atherosclerosis, cerebral vascular disease, left ventricular hypertrophy with impaired diastolic function, congestive heart failure, aortic aneurysm and fistula formation, aortic dissections and rupture, and endarteritis at the repair site (Rosenthal, 2005; Hager et al., 2007). Bicuspid aortic valve and advanced age appear to be independent risk factors for aortic complications (Oliver et al., 2004).
Atrial Septal Defects
An atrial septal defect is a communication between the left and right atria, and it may be single or multiple, vary widely in size, be located anywhere in the atrial septum, and be isolated or associated with other congenital heart defects (50%). See related video online at www.expertconsult.com. There are four morphologic types: secundum ASD, primum ASD, sinus venosus ASD, and coronary sinus ASD. Patent foramen ovale is a normal interatrial communication during fetal life and is seen in almost all newborns. A probe-patent foramen ovale, present in up to 25% of adults, is not a true defect in the atrial septum but results from incomplete fusion of the septum primum and the superior limbic band of the fossa ovalis (septum secundum). Paradoxical embolism is a major anesthetic consideration in these patients.
There are four morphologic types: secundum ASD, primum ASD, sinus venosus ASD, and coronary sinus ASD. Patent foramen ovale is a normal interatrial communication during fetal life and is seen in almost all newborns. A probe-patent foramen ovale, present in up to 25% of adults, is not a true defect in the atrial septum but results from incomplete fusion of the septum primum and the superior limbic band of the fossa ovalis (septum secundum). Paradoxical embolism is a major anesthetic consideration in these patients.
ASDs allow shunts, and the amount of blood that passes through the defect is determined by the size of the defect, the pressure difference between the left and right atria, and the relative compliance of the right and left ventricles. Left-to-right shunting increases as the patient gets older (Campbell, 1970). Early in infancy, the right ventricle is less compliant, so that left-to-right shunting is minimal. With declines in pulmonary artery pressure and remodeling (increased compliance) of the right ventricle, shunting increases as the right atrial pressure becomes lower than left atrial pressure. The normally decreasing left ventricular compliance with age further increases left-to-right shunting. ASDs result in increased pulmonary blood flow and place a volume load on the right atrium, right ventricle, and left atrium.
Isolated ASDs are generally asymptomatic in infancy and childhood, but a few infants develop congestive heart failure as a result of pathophysiology that is incompletely understood. CHF is more common after 40 years of age. The incidence of atrial fibrillation (less commonly atrial flutter) also increases with advancing age (Murphy et al., 1990).
Spontaneous closure of secundum defects is related to defect diameter (<3 mm, 100%; 3 to 8 mm, 87%; 3 to 5 mm, 80%) (Radzik et al., 1993). Indications for closure are a pulmonary to systemic blood flow ratio greater than 2:1, with the second year of life currently considered the optimal time for surgical closure. Normal ventricular function after repair is the rule, but ventricular dysfunction and atrial arrhythmias can occur with late repair. Pulmonary vascular occlusive disease will develop in approximately 5% to 10% of unrepaired ASDs, most commonly in the second decade or thereafter, and can result in shunt reversal and Eisenmenger’s syndrome (Steele et al., 1987). Residua and sequelae are more commonly seen after repair of sinus venosus defects (loss of sinus node function, pulmonary venous obstruction, residual shunt) (Attenhofer Jost et al., 2005) and ostium primum defects (see Atrioventricular Septal Defects, p. 694).
Ventricular Septal Defects
A ventricular septal defect is an opening in the ventricular septum permitting communication between the left and right ventricles, and it is the most common congenital cardiac defect (2.5 per 1000 live births) after bicuspid aortic valve. VSDs are classified by their location in the septum, with multiple classifications existing. The physiologic effects are determined by the degree of shunting, which is influenced by the size of the defect and the relative pulmonary and systemic vascular resistances. Smaller (restrictive) defects limit shunting—there is a large pressure gradient across the defect. Large (nonrestrictive) defects have less pressure difference between the ventricles, so the magnitude and direction of shunting are more dependent on the relative pulmonary and systemic vascular resistances. The high PVR at birth limits left-to-right shunting, but the magnitude of the shunt increases over the first few months of life because of the normal postnatal decline in PVR; there is also a contribution from physiologic anemia of the infant (hematocrit has a disproportionately greater effect on PVR than SVR) (Lister et al., 1982). Excessive left-to-right shunting with increased pulmonary blood flow leads to pulmonary congestion, abnormal pulmonary mechanics (decreased compliance, increased airway resistance, airway compression), increased myocardial work, and pulmonary hypertension (Berlinger et al., 1983; Stayer et al., 2004). Congestive symptoms appear when the QP:QS exceeds 1.4. Pulmonary hypertension is usually reversible initially but becomes increasingly fixed as PVOD develops; although the timing can be variable, irreversible pulmonary vascular disease is uncommon in infancy.
Most VSDs are small, and many will decrease in size or close spontaneously. Indications for surgical repair in infancy are poor growth, significant CHF, pulmonary artery pressure at or near systemic level, and multiple VSDs (Keane and Fyler, 2006d). Patients with advanced PVOD and pulmonary hypertension (Eisenmenger’s complex) are generally not candidates for VSD closure. Device closure of muscular VSDs, particularly difficult-to-reach apical and anterior septal defects, is possible in the catheterization laboratory provided the device will not impinge on the atrioventricular or semilunar valves.
Anesthetic management: For unrepaired VSD, the focus should be on assessing the degree of systemic circulatory compromise and control of pulmonary vascular tone. Signs and symptoms of significant CHF in infants include (1) respiratory: tachypnea, grunting, flaring, retractions; pulmonary congestion on chest x-ray; (2) growth and nutrition: poor feeding, poor growth, failure to thrive; and (3) cardiovascular: tachycardia, diminished pulses, decreased peripheral perfusion, hepatic congestion (hepatomegaly). An inhalational induction should be reserved for patients with smaller defects, no worse than compensated CHF, well-preserved ventricular function, and no PVOD. The major concern is inducing significant decreases in PVR, resulting in decreased systemic flow and hypotension; less commonly, reactive elevations in PVR can result in right-to-left shunting with desaturation. Myocardial and pulmonary function are likely to be normal if complete repair is done within the first year or two of life (Kidd et al., 1993). Potential sequelae include residual defects, heart block, subaortic obstruction, and aortic regurgitation (Kidd et al., 1993; Chiu et al., 2007). Early and mid-term follow up of VSD closure by device found that complete atrioventricular block is a serious concern (Butera et al., 2007).
Atrioventricular Septal Defects
Atrioventricular septal defects (AVSDs), also referred to as canal defects or endocardial cushion defects, result from abnormal endocardial cushion development. See related video online at www.expertconsult.com. AVSDs may be complete (common AV valve with ostium primum ASD and moderate to large inlet VSD), partial (two distinct AV valves with ostium primum ASD and usually a cleft in the anterior leaflet of the mitral valve), or transitional (ostium primum ASD, restrictive VSD, common AV valve orifice with fused anterior and posterior bridging leaflets dividing the common AV valve into distinct mitral and tricuspid components). AVSDs are commonly associated with extracardiac defects and are present in approximately 60% of children with Down syndrome.
AVSDs may be complete (common AV valve with ostium primum ASD and moderate to large inlet VSD), partial (two distinct AV valves with ostium primum ASD and usually a cleft in the anterior leaflet of the mitral valve), or transitional (ostium primum ASD, restrictive VSD, common AV valve orifice with fused anterior and posterior bridging leaflets dividing the common AV valve into distinct mitral and tricuspid components). AVSDs are commonly associated with extracardiac defects and are present in approximately 60% of children with Down syndrome.
The physiology resembles that of atrial and ventricular septal defects, with the possible added feature of AV valve regurgitation. There is a left-to-right shunt with increased pulmonary blood flow and volume overloading of the right and left ventricles, with the magnitude of shunting largely determined by the size of the VSD component. Surgery for complete AV canal defects is usually undertaken in the first 2 to 3 months of life because of the risk for accelerated pulmonary vascular disease. Surgery is usually done later in infancy for partial and transitional AV canals because the pulmonary vasculature is protected and the normal AV valve tissue is thin and fragile (Jonas, 2004). The repair typically involves dividing the common AV valve and closing the ASD and VSD with either one or two patches, approximation and suspension of the valve apparatus, and suturing any cleft in the mitral valve.
Aortic Stenosis
Bicuspid aortic valve is the most common congenital cardiac defect. It occurs in 1% to 2% of the population and demonstrates a substantial male predominance (Hoffman and Kaplan, 2002). It may be an isolated lesion or associated with other left-sided obstructive defects (e.g., aortic coarctation), and it may be asymptomatic or become progressively obstructive or regurgitant. Critical neonatal aortic stenosis is frequently a ductal-dependant lesion, with an appearance similar to that of severe aortic coarctation. In its most severe forms, critical neonatal AS is poorly tolerated and produces left ventricular failure with poor systemic perfusion and pulmonary vascular congestion; factors that increase myocardial demand even modestly (e.g., mild fever, anemia) can markedly exacerbate the low cardiac output state and potentially precipitate cardiovascular collapse. In supravalvar aortic stenosis (SVAS), there is narrowing at the sinotubular junction, which may be associated with coronary artery stenoses, hypoplasia of the ascending aorta and arch, distal arterial tree, and pulmonary artery stenoses (Stamm et al., 2001a) (Fig. 21-5). Subaortic stenosis may comprise a discrete fibromuscular ridge or membrane, a long fibromuscular tunnel, or hypertrophy of the interventricular septum (hypertrophic cardiomyopathy). Shone’s anomaly consists of aortic coarctation, subaortic stenosis, and mitral stenosis on the basis of both a parachute mitral valve and supravalvar mitral ring (Shone et al., 1963). Although the relief of coarctation is typically straightforward, patients with Shone’s anomaly frequently have prolonged and recurrent left-sided obstruction arising from its other components, leading to reduced LV compliance and dysfunction despite one or more attempts at correction. The mitral stenosis (leading to pulmonary hypertension) and reduced ventricular function in particular can make these patients particularly difficult to manage in the perioperative period, because of associated fluid loads and shifts, anemia, fever, and stress response.
Balloon dilation of the aortic valve has replaced surgery as the initial procedure for significant aortic valve stenosis, effectively reducing the gradient and promoting growth of the valve annulus (Jonas, 2004). Aortic valvuloplasty or replacement becomes necessary when dilation of the stenosis is no longer effective or aortic regurgitation and consequent LV dilation become significant. Subaortic stenosis resulting from a discrete subaortic membrane is repaired by membrane resection. In addition to creating increased pressure within the LV, aortic valve damage and regurgitation caused by a jet arising from flow across the membrane is a common finding and an indication for surgery. Tunnel subaortic stenosis is managed by the modified Konno procedure in which LV septal tissue is excised and a patch placed to enlarge the LV outflow tract.
Supravalvar aortic stenosis, specifically that associated with Williams (Williams-Beuren) syndrome, merits particular attention. Patients with SVAS are at significantly increased risk for cardiac arrest and death during anesthesia, cardiac catheterization, and surgery (Bird et al., 1996), and the anesthetic management has recently been reviewed (Burch et al., 2008). SVAS is associated with variable-sized deletions in the elastin gene and adjacent segments and can be accompanied by a diffuse and variable arteriopathy (Pober, 2010). Common manifestations of patients with Williams syndrome include SVAS, supravalvar pulmonic stenosis, more distal PA stenoses, and at times renal artery stenosis. The increased risk for sudden death appears to occur by at least two different but potentially interacting pathophysiologic features:
Anesthetic management: Key features include avoidance of dehydration, hypotension, and other factors that compromise myocardial perfusion. Thus, minimizing the amount of time that the patient receives nothing by mouth, placing an intravenous catheter (aided by premedication as necessary), administering a fluid bolus, and administering intravenous induction with agents that maintain hemodynamic stability are important anesthetic management principles. Although uneventful inhalational inductions have been reported (Bragg et al., 2005; Medley et al., 2005; Astuto et al., 2007), we would advise against this, because significant hypotension can lead to cardiac arrest (especially when coronary artery obstruction is present) prior to establishing intravenous access; in many instances, resuscitation is unsuccessful (Bird et al., 1996). In the catheterization laboratory, both radiographic contrast injection (perhaps via its transient but significant ability to impair coronary oxygen delivery when injected into the aorta or left side of the heart) and cardiac rhythm disturbances (as might be induced by a wire or catheter) are possible events leading to decompensation. Although not based on evidence, it is our practice to have a norepinephrine infusion available for immediate administration should hypotension ensue or be predicted. The lesion itself is usually repaired by resection of the stenotic area and patch placement, although more extensive aortic (or supravalvar pulmonary) reconstruction may be necessary (Stamm et al., 2001a).
Truncus Arteriosus
Truncus arteriosus is a single arterial trunk from which the aorta and the pulmonary and coronary arteries arise, with a VSD present beneath the truncal valve. See related video online at www.expertconsult.com. Like tetralogy of Fallot, it is frequently associated with a 22q11 deletion, and with DiGeorge and velocardiofacial syndromes. It is the quintessential parallel circulation, with the QP:QS ratio determined by the ratio of pulmonary to systemic vascular resistance (Hansen and Hickey, 1986; Wong et al., 1991). Surgical repair involves patch closure of the VSD and detachment of the pulmonary arteries from the truncus with establishment of right ventricle–to–pulmonary artery continuity with a conduit (valved homograft).
Like tetralogy of Fallot, it is frequently associated with a 22q11 deletion, and with DiGeorge and velocardiofacial syndromes. It is the quintessential parallel circulation, with the QP:QS ratio determined by the ratio of pulmonary to systemic vascular resistance (Hansen and Hickey, 1986; Wong et al., 1991). Surgical repair involves patch closure of the VSD and detachment of the pulmonary arteries from the truncus with establishment of right ventricle–to–pulmonary artery continuity with a conduit (valved homograft).
Ebstein’s Anomaly
Ebstein’s anomaly consists of displacement of the septal and posterior leaflets of the tricuspid valve toward the apex of the right ventricle. See related video online at www.expertconsult.com. The right ventricle between the true annulus and the level of attachment of the septal and posterior leaflets to the ventricular septum is thin and dysplastic (“atrialized”) (Fig. 21-6). The anterior tricuspid leaflet at the annulus is larger than normal, whereas the other leaflets may be redundant, contracted, or thickened. The tricuspid valve is usually incompetent, occasionally stenotic. The right ventricular cavity beyond the atrialized portion ranges from small to large, whereas the right atrium is invariably enlarged. There is virtually always a PFO or an ASD, and an accessory AV conduction pathway (e.g., Wolff-Parkinson-White Syndrome) is present in 10% to 25% of cases (Watson, 1974; Smith et al., 1982).
The right ventricle between the true annulus and the level of attachment of the septal and posterior leaflets to the ventricular septum is thin and dysplastic (“atrialized”) (Fig. 21-6). The anterior tricuspid leaflet at the annulus is larger than normal, whereas the other leaflets may be redundant, contracted, or thickened. The tricuspid valve is usually incompetent, occasionally stenotic. The right ventricular cavity beyond the atrialized portion ranges from small to large, whereas the right atrium is invariably enlarged. There is virtually always a PFO or an ASD, and an accessory AV conduction pathway (e.g., Wolff-Parkinson-White Syndrome) is present in 10% to 25% of cases (Watson, 1974; Smith et al., 1982).
The clinical spectrum of Ebstein’s anomaly is extremely variable, ranging from the critically ill neonate with minimal to no anterograde flow out the right ventricle to the pulmonary artery, and with severe tricuspid regurgitation and heart failure, to the child with minimal or no symptoms (Brown et al., 2008). Atrial right-to-left shunting can produce cyanosis. Interventions for significant lesions include tricuspid valve repair (cone procedure) (da Silva et al., 2007) or replacement, closure of interatrial communications, and ablation of accessory pathways.
Anesthesia for closed cardiac procedures
Patent Ductus Arteriosus
The ductus arteriosus connects the origin of the left main pulmonary artery to the aorta and is a critical component of the fetal circulation. In utero, most of the blood from the RV bypasses the lungs via flow through the ductus to the descending aorta. In most newborns, the ductus closes within 72 hours of birth through contraction of a smooth muscle layer in response to an increase in postnatal systemic oxygen levels (Hermes-DeSantis and Clyman, 2006). The lumen is obliterated by 2 to 3 months of age, but if not fully obliterated, a connection remains between the systemic and pulmonary circulations. The PDA may be isolated (Fig. 21-7) or associated with almost any other congenital cardiac anomaly, in many instances of which it is lifesaving (ductal-dependent lesions). Isolated PDA is associated with increasing left-to-right shunting as the pulmonary vascular resistance falls.
A tiny or small PDA is asymptomatic and associated with normal life expectancy (Campbell, 1968); its closure may be controversial but is generally recommended because of the risk for endocarditis. With a hemodynamically significant ductus, excessive blood flow to the lungs, left atrium, left ventricle, and ascending aorta results in enlargement of these structures, and in large shunts, congestive heart failure. Elevation of left atrial pressure may cause enlargement of the foramen ovale with additional left-to-right shunting. In premature infants with respiratory distress, hypoxia promotes continued patency of the ductus, with the need for more aggressive ventilation. Runoff from the aorta into the pulmonary circulation during diastole reduces aortic diastolic pressure with decreased coronary, cerebral, and abdominal organ perfusion. Necrotizing enterocolitis and intracerebral and intraventricular hemorrhages are potential complications. Other cardiac anomalies determine the direction of shunting across a PDA. For example, with severe aortic coarctation or hypoplastic left heart syndrome, the shunting is predominantly right to left, and in fact necessary to support systemic perfusion until more definitive interventions are undertaken.
Closure of a PDA can be accomplished medically in the preterm infant with indomethacin, surgically, or in the catheterization laboratory. Surgical division is recommended in preterm infants with inadequate response to indomethacin and those with large communications (Ewert, 2005). Surgical management is typically via a small left posterolateral thoracotomy, but the video-assisted thoracoscopic (VAT) technique has become the surgical standard of care for infants and children with a small to moderate-sized ductus (Laborde et al., 1997; Jonas, 2004). Closure using interventional catheterization techniques has increased in frequency and is an attractive option because it can be done on an outpatient basis, it reduces surgical scarring, and it may be associated with less pain, faster recovery, and reduced risk for late thoracotomy complications (cosmetic defects, rib fusion, and scoliosis). It is best suited for a restrictive ductus with some length and an ampulla at the aortic end (Ewert, 2005; Keane and Fyler, 2006b).
Anesthetic Management
The majority of patients are sick, premature, ventilator-dependent neonates with respiratory distress syndrome and no better than borderline systemic blood pressure and perfusion. The major consideration is manipulations to balance the PVR and SVR. Maneuvers that decrease PVR, in particular, as well as those that reduce myocardial contractility and SVR, should be avoided. Dopamine is often already in use or may need to be added to maintain a blood pressure that is appropriate (one rule of thumb is mean arterial pressure approximately equal to the patient’s gestational age). Although direct monitoring of arterial blood pressure can be helpful in the sicker patients, it can be technically challenging and is not part of our routine practice for neonates who arrive from the neonatal ICU without it. An anesthetic technique using high-dosage fentanyl (30 to 50 mcg/kg) and pancuronium is usually well tolerated (Robinson and Gregory, 1981). In the current era, although advisable to supplement this technique with low-dosage inhaled agent or benzodiazepine, the hemodynamics often do not allow for this. Transport of these neonates to the operating room can be hazardous, with the risks of accidental extubation, inadequate attention to ventilation, line disruption, and excessive cooling. Many centers perform PDA ligation in the neonatal ICU, either as routine practice or in cases where transport seems especially risky, but the unfamiliarity of the environment, available personnel, relative lack of surgical equipment should a complication arise, adequacy of lighting, and sterility need to be considered. Surgical dissection and the lung retraction necessary to expose the duct can result in hypoxemia, hypercarbia, and hypotension. If possible, blood pressure should be monitored in an upper and lower extremity; the right arm is preferable, because the surgeon may have to place a clamp on the aorta if control of the aortic end of the ductus is lost during dissection or ligation. A second pulse oximeter on a lower extremity can provide reassuring information to the surgeon at the time the duct is clipped or ligated (the PDA can be larger than either the PA or the aorta). After ligation, there is an acute increase in blood pressure with an increase in LV afterload, so that increased inotropic support of the severely dysfunctional ventricle may be necessary. Other complications include accidental extubation, profound hemorrhage resulting from disruption or tearing of the ductus, inadvertent ligation of the left pulmonary artery or descending aorta, and recurrent laryngeal nerve (RLN) damage. One needs to be vigilant for plugging of the endotracheal tube, particularly during the surgery or after lung reexpansion, in very small infants with correspondingly small (≤ 3.0 mm) tubes.
Older infants and children with an isolated PDA undergoing surgical or catheterization procedures may be extubated at the end of the procedure, allowing a variety of anesthetic techniques. The incidence of RLN injury may be reduced by using direct intraoperative stimulation and evoked electromyogram monitoring (Odegard et al., 2000).
Coarctation of the Aorta
Coarctation of the aorta is a focal narrowing of the descending thoracic aorta consistent with a discrete posterior shelf or invagination just opposite the insertion of the ductus arteriosus (juxtaductal) and distal to the left subclavian artery (Fig. 21-8). In some instances, this shelf may be circumferential. The precise cause is unknown, but it is thought to be caused by extension of ductal tissue into the wall of the aorta or by abnormal fetal blood flow patterns associated with lesions that reduce antegrade flow into the ascending aorta. Not surprisingly, coarctation is associated with aortic valve stenosis or a bicuspid aortic valve (50%), ventricular septal defect (48%), hypoplasia of other left heart structures (mitral valve and left ventricle), and aortic arch hypoplasia (Quaegebeur et al., 1994; Aboulhosn and Child, 2006).
If the coarctation is not severe and closure of the ductus has occurred slowly, or if compensatory collaterals develop rapidly, presentation may be later in infancy, with tachypnea and failure to thrive. In such patients, the degree of coarctation commonly becomes more obstructive as the child grows. Blood pressure is elevated in the upper extremities, with an average gradient between upper and lower extremity sites of 30 to 40 mm Hg at rest (Keane and Fyler, 2006a). Collaterals may originate from the internal thoracic, intercostal, subclavian, or scapular arteries, and with extensive collateral development, patients may be asymptomatic with little difference in blood pressure between the arms and legs. Large collaterals can be a source of an auscultatable bruit over the rib cage or elsewhere, as well as rib notching on chest x-ray. Coarctation causes left ventricular hypertension and hypertrophy, as well as systemic hypertension. Symptoms (exercise intolerance, headache, chest pain, lower extremity claudication) are an absolute indication for surgical repair. For the asymptomatic patient, surgical indications include a blood pressure gradient between the upper and lower limbs of greater than 20 mm Hg, upper body blood pressure greater than two standard deviations above normal, or aortic diameter loss of 50% or greater.
The optimal treatment for neonates, infants, and children is currently felt to be complete excision of the area of coarctation and surrounding ductal tissue with end-to-end anastomosis (Dodge-Khatami et al., 2000). With a hypoplastic aortic isthmus, complete excision in conjunction with an extended end-to-end or end-to-side anastomosis is recommended (Backer, 2003). Although balloon angioplasty may be used for primary relief of coarctation (Golden and Hellenbrand, 2007), it is rather regarded as the standard of care for recurrent coarctation after previous surgical repair (Jonas, 2004). Paraplegia secondary to spinal cord ischemia is the most devastating complication associated with surgical repair. The incidence is quite low (0.14% to 0.4%), and several factors are associated with an increased risk of developing paraplegia: hyperthermia, prolonged aortic cross-clamp time, elevated cerebral spinal fluid pressures, low proximal and distal aortic blood pressures, and poorly developed collaterals to the descending aorta. In addition to recoarctation, long-term consequences of coarctation and its repair can include the development of essential hypertension (even in the absence of a residual coarctation gradient) and perhaps increased risk of premature or sudden death.
Anesthetic Management
The increase in proximal aortic pressure with application of the aortic cross-clamp in neonates and infants can usually be managed expectantly or with a volatile agent while continuing any inotropic support. In older children and adolescents, β-blockers and vasodilators may occasionally be necessary. However, a moderate increase in proximal aortic pressure during the cross-clamp period may be beneficial, as it is likely to encourage some degree of lower body perfusion via any existing collaterals. On the other hand, sodium nitroprusside results in further increases in cerebral spinal fluid pressure and further decreases in distal aortic blood pressure, reducing spinal cord blood perfusion pressure (Marini et al., 1998). In sum, we are unlikely to treat hypertension during the cross-clamp period unless it exceeds baseline arterial blood pressures by about 30% or more, or unless it approaches values that raise concern for cerebral toxicity. Spinal cord protection is accomplished with a short cross-clamp time (preferably <20 minutes) and mild induced hypothermia (34° C). Blood pressure is maintained at or a little higher than the preoperative level in the upper limbs. Removal of the cross-clamp will result in reactive hyperemia in distal tissues with vasodilation and transient hypotension, and release of acid metabolites will increase Paco2. Anticipation by decreasing or discontinuing inhaled anesthetics or vasodilators in use, volume expansion, and appropriate ventilation are usually sufficient to limit these effects. Postoperatively, rebound hypertension may occur and persist for some time. Esmolol and sodium nitroprusside infusions are used in the early postoperative period. Older children without coexisting diseases can be additionally managed with lung isolation and may be candidates for early extubation.
Systemic-to-Pulmonary Artery Shunts
Systemic-to-pulmonary artery shunts (extracardiac shunts) are palliative procedures performed in neonates and infants with severely decreased pulmonary blood flow or ductal-dependent pulmonary blood flow when a corrective procedure is not possible (del Nido et al., 1988). With increased neonatal and infant repair of complex defects, fewer systemic-to-pulmonary artery shunts are being performed. The primary indications are single-ventricle, pulmonary atresia with intact ventricular septum, and some variants of TOF with pulmonary atresia.
The classic Blalock-Taussig shunt (end-to-side anastomosis of the transected subclavian artery to the branch pulmonary artery) has been abandoned because of resultant interruption of arm blood flow, and it has been replaced by the modified Blalock-Taussig (mBT) shunt in which a synthetic tube graft of polytetrafluoroethylene (PTFE; Gore-Tex) is interposed between the subclavian (or innominate) artery and the branch pulmonary artery (Gazzaniga et al., 1976a, 1976b; de Leval et al., 1981). With a median sternotomy approach, the shunt is placed more medially from the innominate artery. The shunt is usually performed on the side opposite the aortic arch, with a 3.5-mm shunt typically used in infants weighing 2 to 3.5 kg. A central shunt, used when prior shunt procedures have failed, consists of a synthetic tube graft between the ascending aorta and the main or a branch pulmonary artery. The Potts shunt (side-to-side anastomosis of the descending aorta and left pulmonary artery) and Waterston shunt (side-to-side anastomosis of ascending aorta and right pulmonary artery) have largely been abandoned because of the high incidence of pulmonary artery distortion, the risk of too large a shunt, and the difficulty of closing the shunt at the time of subsequent repair.
Pulmonary Artery Banding
Pulmonary artery banding (PAB) is used to limit pulmonary blood flow in infants with a large left-to-right shunt not amenable to early corrective surgery, or as part of a staged palliation (Muller and Danimann, 1952). With the increase in early infant repair, PAB is performed less commonly, and it is currently most often indicated for very complex lesions (multiple VSDs, at times referred to as Swiss-cheese septum), in TGA for preparation of the left-ventricle prior to an arterial switch operation (Mee, 1986; Jonas et al., 1989), for double-inlet or double-outlet ventricles in the absence of pulmonary stenosis, or to temporize in neonates who are critically ill with significant noncardiac diseases. As congestive heart failure in the newborn period is atypical because of the high PVR, PAB is sometimes performed in the second or third month of life (del Nido et al., 1988). By reducing the extent of the left-to-right shunt, the band reduces the volume load on the ventricles, improves congestive symptoms, protects the pulmonary vascular bed against the development of pulmonary hypertension and PVOD, and improves respiratory mechanics. PAB may cause distortion of the main pulmonary artery or distortion of the branch pulmonary arteries by migration of the band distally. This distortion may complicate or even preclude subsequent attempts at definitive repair.
Vascular Rings and Slings
A vascular ring is an aortic arch anomaly in which the trachea and esophagus are surrounded completely by vascular structures (Fig. 21-9, A). See related video online at www.expertconsult.com. Rings, which need not be composed entirely of patent vascular structures, are formed by abnormal persistence or regression of components of the aortic arch complex, and they can cause compression of the trachea, bronchi, and esophagus. In a vascular sling, the trachea and esophagus are not completely encircled but can be compressed. Although accounting for less than 1% of all congenital heart defects, vascular rings and slings represent an important source of airway and esophageal obstruction. Intracardiac defects are present in approximately 29% of patients with vascular rings (Humphrey et al., 2006).
Rings, which need not be composed entirely of patent vascular structures, are formed by abnormal persistence or regression of components of the aortic arch complex, and they can cause compression of the trachea, bronchi, and esophagus. In a vascular sling, the trachea and esophagus are not completely encircled but can be compressed. Although accounting for less than 1% of all congenital heart defects, vascular rings and slings represent an important source of airway and esophageal obstruction. Intracardiac defects are present in approximately 29% of patients with vascular rings (Humphrey et al., 2006).
The vast majority of patients with a vascular ring have either a double aortic arch with right arch dominance (see Fig. 21-9, B), a right aortic arch with mirror-image branching, or a right aortic arch with an aberrant left subclavian artery and left ligamentum arteriosum (Dodge-Khatami et al., 2002; Humphrey et al., 2006). With a double aortic arch with right arch dominance, the anteriorly located left aortic arch gives rise to the left carotid and left subclavian arteries. This left arch is generally atretic or severely hypoplastic beyond the origin of either the left carotid or left subclavian artery. A right aortic arch passes rightward and posterior to the trachea, and a left aortic arch passes leftward and anterior to the trachea. With mirror-image branching, the arch gives off, in order, the common trunk of the left common carotid and left subclavian, the right common carotid, and the right subclavian arteries.
Vascular rings and slings generally appear in childhood, with the most common symptoms being inspiratory stridor, dysphagia, wheezing, dyspnea, cough, and recurrent respiratory tract infections (Humphrey et al., 2006). Feeding problems are common. MRI is the diagnostic technique of choice in many centers (van Son et al., 1994); other diagnostic tools include echocardiography (for evaluation of intracardiac defects), bronchoscopy, and barium swallow.
The goal of repair for vascular rings or slings is to divide the compressive ring, relieve the airway or esophageal compression, and maintain normal perfusion of the aortic arch. The surgical approach is typically via a left posterolateral thoracotomy, at times open, but more commonly using a video-assisted thoracoscopic technique (Burke and Chang, 1993). Simultaneous repair of vascular tracheobronchial compression syndromes and intracardiac defects carries a higher risk for morbidity and mortality (Sebening et al., 2000).
Anesthetic Management
Anesthetic management depends on the type of vascular ring, the type and severity of underlying CHD, and the planned surgical procedure. It is not clear how frequently, if ever, these lesions behave like anterior mediastinal masses (Kussman et al., 2004). The majority of these patients are best approached by careful induction, assurance of the ability to ventilate with positive pressure, and then muscle relaxation; the application of positive pressure and removal of respiratory effort usually result in improved airflow. With significant airway narrowing, the endotracheal tube tip should lie proximal to the stenotic segment to avoid edema and granulation tissue formation caused by tube impact on the narrowed area. Alternatively, cases of severe collapse may require stenting with an endotracheal tube or rigid bronchoscope to ensure adequate ventilation. For all of these reasons, previous characterization of tracheal narrowing with respect to mechanism (extrinsic compression, tracheomalacia, complete tracheal rings), location, severity, and extent (length of segment) can critically inform intubation and other aspects of management in these patients. Airway radiography (plain film, MRI, computed tomography) and bronchoscopy (routine in some centers) (Chapotte et al., 1998) can also provide this information. In patients without a prior bronchoscopy, one can be performed after induction at the time of the ring or sling surgery. In older children, the surgical approach may benefit from single-lung ventilation techniques.
The potential for a variable course and relationship of the RLN to the ligamentum arteriosum and descending aorta makes the RLN susceptible to injury during either type of surgical approach to vascular ring. In the largest reported series, there was a 5.3% incidence of unilateral vocal cord paralysis (Geva et al., 2002). Intraoperative RLN monitoring may be useful (Odegard et al., 2000), in which case muscle relaxants should not be used or allowed to wear off prior to testing. When RLN injury occurs, there is a risk for stridor or hoarseness, and the potential for aspiration.