Congenital Anomalies of the Thoracic Great Arteries
This chapter covers congenital anomalies of the aorta and the pulmonary arteries, with an emphasis on anomalies that produce clinical symptoms of airway and esophageal obstructions. Anomalies of the thoracic great arteries can be broadly classified into anomalous origins, anomalous connections, obstructions, and structural anomalies of the aortic arch. Important clinical entities under each category are listed in Box 77-1. This chapter will focus on structural anomalies of the aortic arch and pulmonary sling.
Vascular Rings
Overview: Anomalies of the aortic arch and the cervical vessels are relatively common, with a prevalence estimated at 0.5% to 3%, depending on the inclusion criteria. Most variations, such as a left aortic arch with an aberrant right subclavian artery, common origin of the left common carotid and right innominate arteries (bovine arch), and ectopic origin of the left vertebral artery from the aortic arch, are of little or no clinical consequence.
“Vascular ring” is a term that refers to encirclement of the trachea and esophagus caused by the abnormal embryologic development of the aortic arch. The principal structural components responsible for this encirclement are derived from the aortic arch or arches, subclavian artery, circumflex aortic segment, ductus arteriosus, or ligamentum arteriosum. Structural anomalies of the aortic arch can be understood as the abnormal divisions in the totipotential arch, a theoretical construct proposed by Jesse Edwards (Fig. 77-1).1 Only a small subset of aortic arch developmental anomalies leads to vascular rings, accounting for less than 1% of all congenital cardiovascular defects. Although about a dozen different types of vascular rings exist, double aortic arches and right aortic arch with left ligamentum arteriosum account for 90% of cases.2
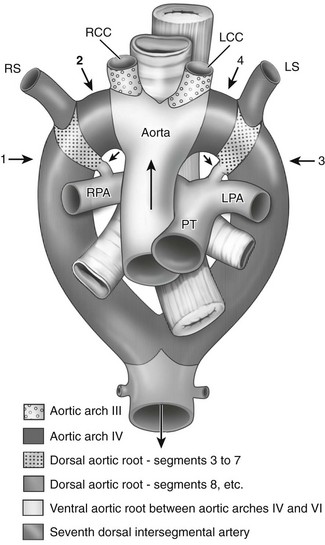
Figure 77-1 Ventral view of Dr. Jesse Edward’s “totipotential arch” or hypothetic double aortic arch and bilateral ducti arteriosi.
The numbered arrows point to the four key locations where regression occurs in various anomalies. Arrow 1 indicates the eighth segment of the right dorsal aortic root; arrow 2, the right fourth arch; and arrows 3 and 4, the corresponding two positions on the left. The shortest black arrows point to the ducti arteriosi bilaterally, and the longest black arrow indicates the direction of blood flow. LCC, Left subclavian artery; LPA, left pulmonary artery; LS, left subclavian artery; PT, pulmonary trunk; RCC, right common carotid artery; RPA, right pulmonary artery; RS, right subclavian artery. (From Stewart JR, Kincaid OW, Edwards JE: An atlas of vascular rings and related malformations of the aortic arch system, Springfield, IL, 1964, Charles C Thomas.)
Chromosome 22q11 deletion has been reported in 24% of patients with isolated arch anomalies.3,4 This deletion was first identified in persons with DiGeorge syndrome, which consists of various degrees of immunodeficiency, thymic hypoplasia or aplasia, hypoparathyroidism, outflow tract cardiac defects, and dysmorphic appearance. Chromosome 22q11 deletion is now recognized as a major factor in many congenital heart defects. For example, it is detected in up to 50% of patients born with interrupted aortic arch or truncus arteriosus. Testing for this chromosomal abnormality can be performed using fluorescence in situ hybridization.
Clinical Manifestations: The severity of symptoms and the age of onset depend on the extent of the compression about the esophagus and trachea.5 Because different ring arrangements have different constrictive effects, not all vascular rings produce the same degree of symptoms; in fact, some rings produce no symptoms. Conversely, symptomatic vascular compression of the trachea and esophagus does not require a complete ring, as can be seen in an anomalously placed or aneurysmal innominate artery or a retroesophageal subclavian artery. Most cases of vascular ring, if they are symptomatic, present during infancy or early childhood.
Imaging: Chest radiography may show tracheal compression by a vascular ring, but by itself a chest radiograph cannot confirm or exclude a vascular ring. Because a vascular ring is more likely in the presence of a right aortic arch, symptoms of tracheal compression in the presence of a right aortic arch should raise the possibility of a vascular ring.
In patients presenting with nonspecific symptoms, barium esophagography is a useful first test.6 A normal barium esophagram usually excludes a clinically significant vascular ring. Classic S-shaped indentations in the frontal projection of an esophagram are highly suggestive of double aortic arches. A posterior vascular indentation may or may not be a vascular ring but is more likely in the presence of a right aortic arch. An esophagram may reveal other causes of a patient’s symptoms, such as gastroesophageal reflux, aspiration, or tracheoesophageal fistula.
Mediastinal ultrasonography or echocardiography with gray scale and color Doppler imaging may visualize the vascular ring directly in neonates and infants because these patients have excellent sonographic windows.7 Echocardiography generally is not useful in older children or adolescents. Moreover, because ultrasound is primarily a two-dimensional imaging method, connecting tortuous vascular structures can be difficult, especially when ligamentous or interrupted vascular segments are present. The presence of a vascular ring has been diagnosed successfully in utero with fetal ultrasonography.8
Patients with severe symptoms or an abnormal results of an esophagram, chest radiograph, or mediastinal ultrasonography should undergo angiography to confirm the vascular abnormalities and to gather information for surgical planning. Conventional catheter angiography has been replaced by first-pass, contrast-enhanced computed tomographic angiography (CTA) or magnetic resonance angiography (MRA). Both modalities can visualize the aortic arch and the cervical arteries well. MRA is usually preferred because it does not subject the patient to ionizing radiation.9,10 However, in cases in which the airways and the lungs must be evaluated together with the vascular anomaly, CTA can accomplish both in a single scan and may be a better choice.
Treatment: The definitive treatment is surgical relief of the obstruction.11 A double aortic arch is repaired by dividing the nondominant arch between its last cervical artery and the point where the nondominant arch joins the descending aorta. If the ductus arteriosus or the ligamentum arteriosum forms a border of the ring, it is ligated to relieve the constriction. A thoracotomy is performed at the side of the planned ligation. Persistent stenosis or tracheomalacia may develop in the constricted trachea, requiring additional repair.
Special Considerations
Double aortic arches can be categorized as bilaterally patent or as atretic in a portion of one of the two arches, usually the left. Bilaterally patent or complete double aortic arches represent persistence of both the right and the left embryologic fourth aortic arches. Two vessels arise from the ascending aorta and course dorsally, one on each side of the trachea and esophagus, to join posteriorly in a left descending aorta in 80% of cases. In this arrangement, the left arch is usually anterior and the right arch is posterior. In 20% of cases, the descending aorta lies on the right and the posterior-anterior relationship of the double aortic arches is reversed. The larger aortic arch is the dominant arch, and in 73% of cases, the right arch is dominant. The right arch normally is situated higher than the left arch, as can be seen in a typical esophagram, where the right arch indents the esophagus higher than does the left arch (Fig. 77-2). Double aortic arches usually are found without associated cardiac anomalies.
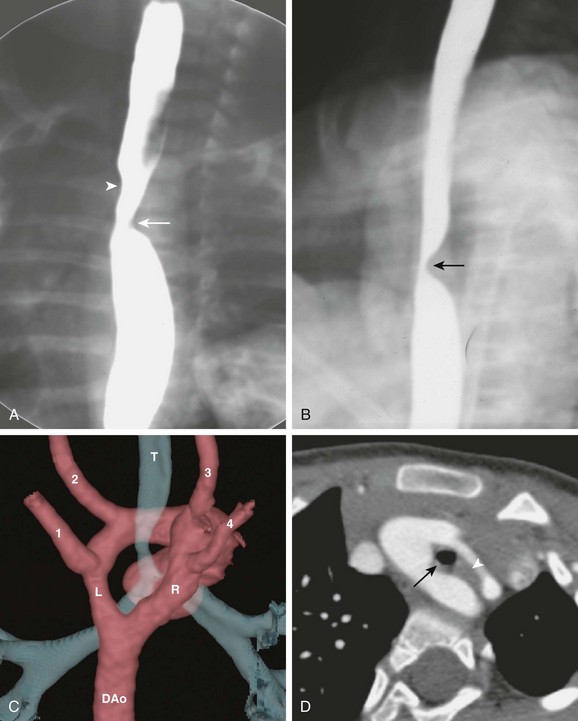
Figure 77-2 Bilaterally patent double aortic arches in a 7-year-old boy.
A, The frontal view of a barium esophagram shows bilateral indentations of the barium column. The right arch indention (arrowhead) is higher than the left (arrow). B, The lateral view of a barium esophagram shows a posterior indentation (arrow) corresponding to the posterior right arch. C, A volume-rendered image from a computed tomography (CT) angiogram shows the aorta as seen from the back. A dominant right arch (R) and a nondominant left arch (L) are present, both draining into the descending aorta (DAo). Four cervical arteries arise from the arch: left subclavian artery (1), left common carotid artery (2), right common carotid artery (3), and right subclavian artery (4). The ring encloses the trachea (T), causing tracheal narrowing just above the carina. D, An axial CT scan shows a complete double aortic arch constricting the trachea (black arrow) and the esophagus (white arrowhead).
Double aortic arches with left arch atresia develop from regression of varying segments of the left aortic arch, with fibrous continuity of the segments completing the vascular ring.12 The atretic segment may lie between the left subclavian artery and the descending aorta (Fig. 77-3) or between the left common carotid artery and the left subclavian artery. The former configuration is similar to a right aortic arch with a mirror-image branching pattern, and the latter configuration is similar to a right aortic arch with an aberrant left subclavian artery. An aortic diverticulum may be present posterior to the esophagus, which is part of the distal left aortic arch, before connecting to the aberrant left subclavian artery. Double aortic arches with right arch atresia are theoretically possible but extremely rare, with very few reported cases. The clinical presentation, imaging approach, and surgical treatment are no different from those for other types of double aortic arches.
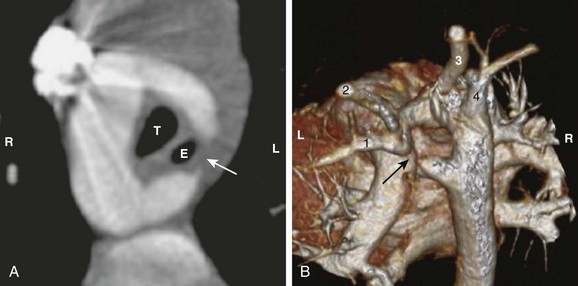
Figure 77-3 A double aortic arch with an atretic left arch in a 2-year-old girl.
A, The axial view from a computed tomography (CT) angiogram shows a patent right arch and an incomplete left arch. A ligamentous connection (arrow) can be inferred from the tapered contour of the left arch, forming the left border of the vascular ring and constricting the trachea (T) and esophagus (E). B, A volume-rendered view from a CT angiogram shows the atretic segment in the left arch (arrow). The left subclavian artery (1) and the left common carotid artery (2) are supplied by the proximal portion of the left arch. The right arch gives off the right common carotid artery (3) and the right subclavian artery (4). The patient’s right (R) and left (L) are labeled for reference.
Right Aortic Arch with Aberrant Left Subclavian Artery
A right aortic arch with an aberrant left subclavian artery is a common cause of a vascular ring. The distal portion of the rudimentary left arch may persist as a diverticulum of Kommerell, giving origin to the left subclavian artery. Unlike double aortic arches with left arch atresia, no fibrous connection is present between the left common carotid artery and the left subclavian artery. Instead, the left border of the ring is completed by the left ligamentum arteriosum, which extends from the left subclavian artery to the pulmonary artery (Fig. 77-4). In 10% of cases, the ligamentum arteriosum is on the right side, and thus there would be no vascular ring. Unlike double aortic arches, this vascular ring typically is loose, and many patients are asymptomatic or present with mild symptoms later in life.
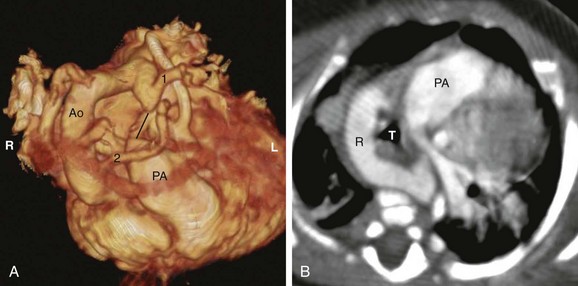
Figure 77-4 A right aortic arch with an aberrant left subclavian artery in a 1-year-old girl.
A, A volume-rendered anterior and superior view reconstructed from a computed tomography (CT) angiogram. The right aortic arch (Ao) gives off the left common carotid artery (2) as the first branch and the left subclavian artery (1) as the last branch. A kink is seen between the diverticulum of Kommerell and the left subclavian artery, suggesting the attachment of a tight ligamentum arteriosum. The probable connection of the ligamentum arteriosum is drawn as a black line, which forms the left border of the vascular ring. The patient’s right (R) and left (L) are labeled for reference. B, An axial CT angiogram of the same patient. The right aortic arch (R) forms the right and posterior border of the vascular ring; the ligamentum arteriosum forms the left border, enclosing the trachea (T). PA, Pulmonary artery.
Right Aortic Arch with Mirror-Image Branching
The right aortic arch with mirror-image branching pattern is associated with congenital heart disease in 90% of cases, typically tetralogy of Fallot and truncus arteriosus. In most instances, the aorta descends on the right. If the ductus is left sided, it usually connects between the anteriorly located innominate artery and the pulmonary artery, which does not form a vascular ring and does not result in posterior indentation on the esophagram. In rare cases, a true ring forms when a ligamentum arteriosum extends from the left pulmonary artery to an aortic diverticulum, resulting in a large posterior indentation on the esophagram.13
Right Aortic Arch with Circumflex Aorta
Unlike the typical right aortic arch, in which the descending aorta is right sided, a circumflex aorta descends on the left. To do so, the distal aortic arch travels from right to left, posterior to the esophagus, before turning downward (Fig. 77-5). A left ligamentum arteriosum connects the pulmonary artery to the descending aorta, completing the ring. The cervical branching can have a mirror-image pattern or an aberrant left subclavian artery. Neither type affects the formation of the ring. An aberrant left subclavian artery frequently arises from an aortic diverticulum with stenosis at its origin.
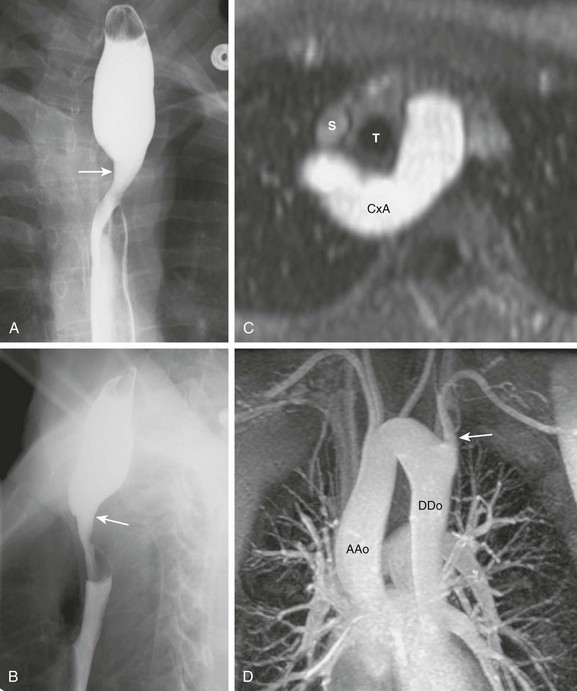
Figure 77-5 Circumflex aortic arch.
A, The frontal esophagram of an 18-year-old woman shows a large right indentation (arrow) corresponding to a right aortic arch. B, The lateral esophagram shows a posterior indentation (arrow) larger than expected for an aberrant left subclavian artery. C, Axial reconstruction of a magnetic resonance (MR) angiogram shows that the aorta (CxA) passes behind the trachea (T) and the esophagus. The superior vena cava (S) is labeled for reference. D, Maximal intensity projection of an MR angiogram shows a right ascending aorta (AAo) and a left descending aorta (DAo). There is an aberrant left subclavian artery (arrow) as the last branch off the arch.
Right Cervical Aortic Arch
A right cervical aortic arch occurs when abnormal cephalic migration of the aortic arch into the supraclavicular and neck region occurs. Embryologically, the cervical aortic arch forms from the third arch rather than the normal fourth arch. A cervical arch is more common on the right than on the left. The cervical branching pattern varies, and separate origins of the internal and external carotid arteries may arise from the cervical aortic arch. Disturbance of the carotid arteries can be anticipated, because they also are derived from the third arch. The right cervical aortic arch can give rise to a vascular ring in a manner similar to the other types of right aortic arch. Clinically, a pulsatile mass may be present in the supraclavicular region. Radiographic findings include right superior mediastinal widening, tracheal displacement to the left and anteriorly, a large oblique impression on the esophagram from cephalic right to caudal left, and a left descending aorta (Fig. 77-6).
Left Aortic Arch with Aberrant Right Subclavian Artery
Compared with a right aortic arch, vascular ring formation in a left aortic arch is rare (Fig. 77-7) because the ductus arteriosus and ligamentum arteriosum usually are left-sided. To complete a ring with a left aortic arch would require a right ductus arteriosus. In the specific case of a left aortic arch with an aberrant right subclavian artery, very few proven cases of vascular ring have been reported. Although it is not part of a vascular ring, a large diverticulum of Kommerell at the retroesophageal right subclavian artery could cause difficulty swallowing; this association has been termed “dysphagia lusoria.” This association is hard to prove, however, because although aberrant right subclavian artery is common, occurring in about 0.5% of the population, few persons experience symptoms.
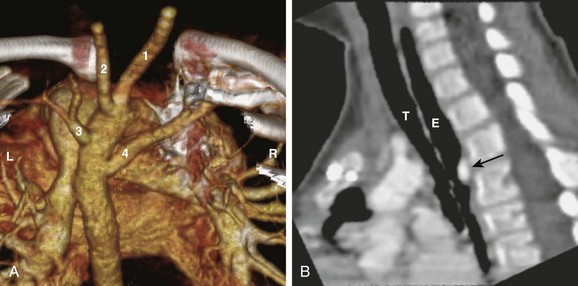
Figure 77-7 A left aortic arch with an aberrant right subclavian artery in a 4-year-old boy.
A, A volume-rendered image from a computed tomography angiogram seen from the back shows the cervical arteries: right common carotid artery (1), left common carotid artery (2), left subclavian artery (3), and aberrant right subclavian artery (4). The patient’s right (R) and left (L) are labeled for reference. B, A sagittal reformat view shows the air-filled trachea (T) and the esophagus (E). The esophagus is indented posteriorly by the aberrant subclavian artery (arrow).
Left Aortic Arch with Circumflex Aorta
A left aortic arch with a circumflex aorta is analogous to a right aortic arch with a circumflex aorta, in that the distal arch travels from left to right behind the esophagus before turning downward to become a right descending aorta. To complete a vascular ring, a right ligamentum arteriosum must connect the pulmonary artery to the descending aorta.14
Pulmonary Artery Sling
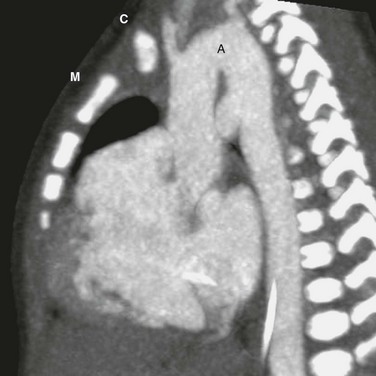
Overview: A pulmonary artery sling (PAS) is a rare congenital anomaly of the pulmonary artery that produces obstructive symptoms of the upper airway. In a person with normal anatomy, the left main pulmonary artery branches off the pulmonary trunk in a shallow turn toward the left at a level slightly above the right main pulmonary artery. It then courses above the left main bronchus. In a person with a PAS, the left main pulmonary artery originates from the posterior aspect of the right pulmonary artery. It turns left behind the trachea at or near the level of the carina toward the left pulmonary hilum. The trachea is compressed between the left and right main pulmonary arteries (Fig. 77-8). The esophagus courses posterior to both pulmonary arteries and is not obstructed, unlike the situation in a person with a vascular ring.
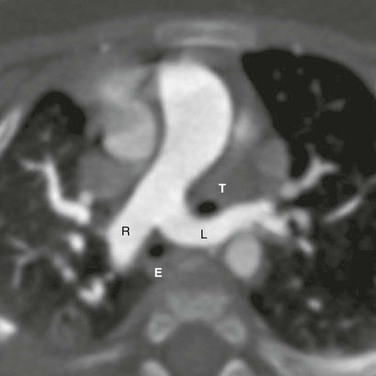
Figure 77-8 A pulmonary artery sling in a 6-month-old girl.
An axial image from a computed tomography angiogram shows an anomalous left pulmonary artery (L) arising from the right pulmonary artery (R). It courses between the trachea (T) and the esophagus (E). The trachea is entrapped between the left and right pulmonary arteries.
The incidence of PAS is not known. In a large-scale screening study of more than 180,000 school-aged children with ultrasonography, 11 cases were found,15 representing an in incidence of 1 in 17,000. A slight male predilection was noted. Patients present with clinical symptoms in childhood, and 90% of patients present with symptoms before the first year of life.16 Nonspecific respiratory symptoms include an asthmatic cough and acute and recurrent bronchopulmonary infections. Some patients are misdiagnosed with asthma for many years before PAS is discovered. Symptom severity depends on the degree of airway compression and coexisting airway abnormalities. Long-standing compression of the airways also may cause tracheobronchomalacia. Unlike with a vascular ring, the obstructive symptoms of the esophagus tend to be mild or absent in persons with a PAS.
Coexisting airway abnormalities are present in more than 50% of patients, including abnormal branching of the airways, complete tracheal rings, and tracheal stenosis.17 The vertical position of the left pulmonary artery origin, along with the branching pattern of the airways, has been used to classify PAS.18 In type 1 PAS, the left pulmonary artery originates at T4-T5 vertebral levels, just above the normal level of the carina. Subtype 1A has normal branching pattern of the airways; subtype 1B has a tracheal bronchus. In type 2 PAS, the left pulmonary artery originates below the T5 vertebral level, below the normal carina. Subtype 2A has a right main bronchus connecting to the right upper lobe only. A separate “bridging” airway arises from the left main bronchus, below the aberrant left pulmonary artery, that supplies the right middle and right lower lobes (Fig. 77-9, A). In subtype 2B, the right main bronchus is absent and the bridging airway defined in 2A supplies the entire right lung. Except for the level of the left pulmonary artery, the right main bronchus in type 2A superficially resembles the tracheal bronchus in type 1B. Regardless of the type, complete tracheal rings and stenosis frequently are present (Fig. 77-9, B).
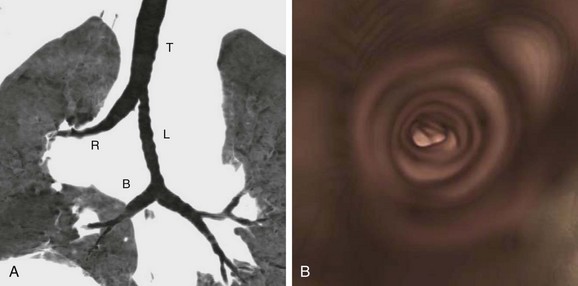
Figure 77-9 Abnormal central airways of a 40-year-old woman with a pulmonary artery sling.
A, Coronal reformation with minimum intensity projection shows type 2A configuration where the trachea (T) bifurcates into a right main bronchus (R) and a left main bronchus (L). The right main bronchus supplies the right upper lobe airways only. A bridging artery (B) supplies the rest of the right lung. Superficially, the right main bronchus resembles a tracheal bronchus, except that it arises at the expected location of the carina, instead of high up from the trachea. B, The endoscopic view in the trachea shows complete tracheal rings.
About a third of PAS cases are associated with other cardiovascular anomalies, including ventricular septal defect, atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, common ventricle, and coarctation.16 Genetic associations include trisomies 18 and 21.19
Imaging: As the left pulmonary artery wraps around the right side of the trachea, the right main bronchus or the bridging airway can be obstructed. In the newborn, this phenomenon may manifest as retained fluid in the right lung. In older children, this phenomenon may present as a hyperinflated right lung from air trapping. The most specific finding is the abnormal architecture of the central airways, often described as a low carina and an inverted T shape of the central airways (see Fig. 77-9, A). This finding is a result of the abnormal airway structure found in type 2 PAS. The apparent low carina is the result of the low origin of the bridging bronchus. The horizontal course of the bridging bronchus gives the carina a flattened look, hence the inverted T shape.
Barium esophagography is a useful screening tool for PAS. The esophagus is indented anteriorly at the level of the carina (Fig. 77-10), unlike the posterior indentation seen with a vascular ring. In addition, on the lateral projection, the trachea is separated from the esophagus by the left main pulmonary artery.
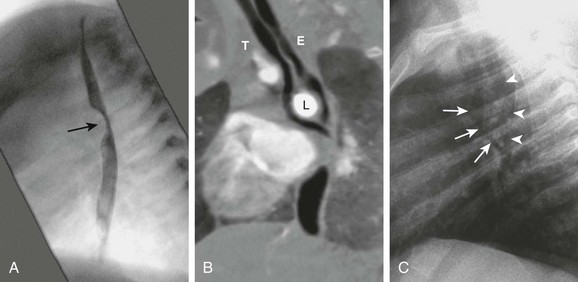
Figure 77-10 A pulmonary artery sling in a 6-month-old girl.
A, An esophagram in the lateral projection shows an anterior indentation (arrow). B, A computed tomography angiogram reformatted in the same plane shows the left pulmonary artery (L) separating the trachea (T) from the esophagus (E) at the carina level. The anterior indentation seen in the esophagram is caused by compression by the left pulmonary artery. C, A lateral chest radiograph shows the same separation of the trachea (arrows) and the air-filled esophagus (arrowheads).
Direct visualization of the anomalous left pulmonary artery can be performed with CTA or MRA.20 Because assessment of the airways and the lungs is important for PAS, CTA is the preferred imaging modality. CTA images can be postprocessed to provide virtual endoscopic views to evaluate for focal stenosis, extrinsic vascular compression, and a tracheal ring (see Fig. 77-9, B). Endoscopic bronchoscopy is recommended to evaluate dynamic airway obstruction from tracheobronchomalacia and to confirm the extent of complete tracheal rings or stenosis for surgical planning.
Treatment: The definite treatment is surgical reimplantation or relocation of the left pulmonary artery and release of the entrapped trachea. In many of these patients, stenosis of the trachea requires a sliding tracheoplasty or pericardial patch to augment the narrowed segment. A diameter of the trachea that is less than 3 mm is associated with the need for tracheoplasty or a poor outcome.21 Airway narrowing that involves the carina or the bronchi is particularly difficult to repair.22
Hellinger, JC, Daubert, M, Lee, EY, et al. Congenital thoracic vascular anomalies: evaluation with state-of-the-art MR imaging and MDCT. Radiol Clin North Am. 2011;49:969–996.
Hernanz-Schulman, M. Vascular rings: a practical approach to imaging diagnosis. Pediatr Radiol. 2005;35:961–979.
Momma, K, Matsuoka, R, Takao, A. Aortic arch anomalies associated with chromosome 22q11 deletion (CATCH 22). Pediatr Cardiol. 1999;20:97–102.
Newman, B, Cho, Y. Left pulmonary artery sling-anatomy and imaging. Semin Ultrasound CT MR. 2010;31:158–170.
Oddone, M, Granata, C, Vercellino, N, et al. Multi-modality evaluation of the abnormalities of the aortic arches in children: techniques and imaging spectrum with emphasis on MRI. Pediatr Radiol. 2005;35:947–960.
References
1. Development of the cardiovascular and lymphatic systems. In Standring S, ed.: Gray’s anatomy, 39th ed, Edinburgh: Elsevier, 2005.
2. Bonnard, A, Auber, F, Fourcade, L, et al. Vascular ring abnormalities: a retrospective study of 62 cases. J Pediatr Surg. 2003;38:539–543.
3. McElhinney, DB, Clark, BJ, III., Weinberg, PM, et al. Association of chromosome 22q11 deletion with isolated anomalies of aortic arch laterality and branching. J Am Coll Cardiol. 2001;37:2114–2119.
4. Momma, K, Matsuoka, R, Takao, A. Aortic arch anomalies associated with chromosome 22q11 deletion (CATCH 22). Pediatr Cardiol. 1999;20:97–102.
5. Woods, RK, Sharp, RJ, Holcomb, GW, et al. Vascular anomalies and tracheoesophageal compression: a single institution’s 25-year experience. Ann Thorac Surg. 2001;72:434–439.
6. Hernanz-Schulman, M. Vascular rings: a practical approach to imaging diagnosis. Pediatr Radiol. 2005;35:961–979.
7. Murdison, KA. Ultrasonic imaging of vascular rings and other anomalies causing tracheobronchial compression. Echocardiography. 1996;13:337–356.
8. Patel, CR, Lane, JR, Spector, ML, et al. Fetal echocardiographic diagnosis of vascular rings. J Ultrasound Med. 2006;25:251–257.
9. Malik, TH, Bruce, IA, Kaushik, V, et al. The role of magnetic resonance imaging in the assessment of suspected extrinsic tracheobronchial compression due to vascular anomalies. Arch Dis Child. 2006;91:52–55.
10. Oddone, M, Granata, C, Vercellino, N, et al. Multi-modality evaluation of the abnormalities of the aortic arches in children: techniques and imaging spectrum with emphasis on MRI. Pediatr Radiol. 2005;35:947–960.
11. Chun, K, Colombani, PM, Dudgeon, DL, et al. Diagnosis and management of congenital vascular rings: a 22-year experience. Ann Thorac Surg. 1992;53:597–602.
12. Holmes, KW, Bluemke, DA, Vricella, LA, et al. Magnetic resonance imaging of a distorted left subclavian artery course: an important clue to an unusual type of double aortic arch. Pediatr Cardiol. 2006;27:316–320.
13. Zachary, CH, Myers, JL, Eggli, KD. Vascular ring due to right aortic arch with mirror-image branching and left ligamentum arteriosus: complete preoperative diagnosis by magnetic resonance imaging. Pediatr Cardiol. 2001;22:71–73.
14. McLeary, MS, Frye, LL, Young, LW. Magnetic resonance imaging of a left circumflex aortic arch and aberrant right subclavian artery: the other vascular ring. Pediatr Radiol. 1998;28:263–265.
15. Yu, JM, Liao, CP, Ge, S, et al. The prevalence and clinical impact of pulmonary sling on school-aged children: a large-scale screening study. Pediatr Pulmonol. 2008;43:656–661.
16. Gikonyo, BM, Jue, KL, Edwards, JE. Pulmonary vascular sling: report of seven cases and review of the literature. Pediatr Cardiol. 1989;10:81–89.
17. Berdon, WE, Baker, DH, Wung, JT, et al. Complete cartilage-ring tracheal stenosis associated with anomalous left pulmonary artery: the ring-sling complex. Radiology. 1984;152:57–64.
18. Wells, TR, Gwinn, JL, Landing, BH, et al. Reconsideration of the anatomy of sling left pulmonary artery: the association of one form with bridging bronchus and imperforate anus. Anatomic and diagnostic aspects. J Pediatr Surg. 1988;23:892–898.
19. Newman, B, Cho, Y. Left pulmonary artery sling-anatomy and imaging. Semin Ultrasound CT MR. 2010;31:158–170.
20. Hodina, M, Wicky, S, Payot, M, et al. Non-invasive imaging of the ring-sling complex in children. Pediatr Cardiol. 2001;22:333–337.
21. Huang, SC, Wu, ET, Wang, CC, et al. Surgical management of pulmonary artery sling: trachea diameter and outcomes with or without tracheoplasty [published online ahead of print March 13, 2012]. Pediatr Pulmonol. 2012;47:903–908.
22. Oshima, Y, Yamaguchi, M, Yoshimura, N, et al. Management of pulmonary artery sling associated with tracheal stenosis. Ann Thorac Surg. 2008;56:1334–1338.