Congenital and Neonatal Abnormalities
Duodenum
Intrinsic Lesions: Duodenal Atresia and Stenosis
Overview: Duodenal atresia and stenosis represent a spectrum ranging from complete to partial obstruction, presenting from prenatal life to late childhood or even adulthood. The incidence of duodenal atresia or stenosis is cited as approximately 1 : 7000 live births and represents nearly half of intestinal atresias.1,2 Similar to atresia of the more distal bowel, duodenal atresia is classified as types I through III. Type I atresias consist of a completely or partially obstructing membrane, type II atresias are connected by a fibrous cord, and type III atresias are separated by a gap. The duodenal diverticulum or “windsock” duodenum is considered a variant of type I atresia.3
Etiology: The lumen of the duodenum is obliterated during the fourth to sixth weeks of gestation because of rapid cell division and normally recanalizes by the twelfth week. The etiology of duodenal atresia and stenosis is believed to be the result of failure of recanalization of the lumen of the duodenum.4,5
Clinical Presentation: Presentation may occur prenatally with polyhydramnios, which occurs in approximately 30% to 50% of cases,3,5 and premature birth is seen in nearly half of patients.5 Approximately one third of cases of congenital duodenal obstruction presenting in the neonatal period are due to duodenal stenosis.5 Postnatally, infants with atresia typically present with vomiting within the first 24 hours of life. Because duodenal atresia/stenosis typically occurs distal to the ampulla of Vater, these patients will present with bilious vomiting. However, obstruction occurs proximal to the ampulla of Vater in as many as 23% of patients.6,7 Such patients will present with nonbilious vomiting, simulating hypertrophic pyloric stenosis. Because duodenal obstruction is proximal, abdominal distension is not a typical finding, although fullness in the epigastric region may be present as a result of the dilated stomach and duodenum. Patients with duodenal stenosis may present later in life, depending on the degree of obstruction; presentation may be relatively nonspecific (such as failure to thrive), or it may present as pancreatitis as a result of reflux into the pancreatic duct, proximal to the site of obstruction.8,9 Presentation may be precipitated by ingestion of a foreign body, which fails to pass and may exacerbate the degree of obstruction, leading to abdominal pain and/or vomiting.10 In patients with a duodenal diaphragm, an intraluminal duodenal diverticulum or “windsock” may be found, and presentation also may be precipitated by ingestion of a foreign body.11
Associated anomalies are common in patients with duodenal atresia/stenosis and may be responsible for presenting signs and symptoms. Annular pancreas and malrotation are seen in approximately one third of cases.5 Trisomy 21 is present in approximately 25% to 40% of infants with duodenal atresia/stenosis; conversely, approximately 4% of infants with Down syndrome have duodenal obstruction.5,12 Hirschsprung disease has been reported in approximately 1% to 3% of patients with duodenal atresia and Down syndrome.13,14 Duodenal atresia may be seen in approximately 5% of patients with esophageal atresia with or without a fistula.15 Heterotaxy with polysplenia has been reported in patients with duodenal diaphragm/intraluminal diverticulum.8,16
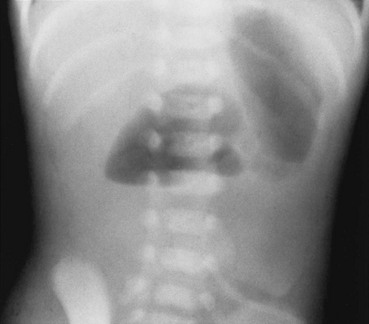
Imaging: Abdominal radiographs are the starting point in the evaluation of a child with suspected obstruction. In the neonate with duodenal atresia, the abdominal radiograph demonstrates the classic “double bubble appearance,” representing the dilated stomach and duodenum.17,18 Because obstruction has been present in utero, the obstructed proximal duodenum is typically large, approximately one half to one third the size of the stomach (Fig. 103-1); at times the pylorus is wide open, and the two bubbles are not distinctly separated (see Fig. 103-1, B). This appearance on the abdominal radiograph is diagnostic, and contrast studies to confirm the diagnosis are not needed. Rarely, air can be seen distally in a patient with complete atresia; this occurs when the atresia is flanked by the branches of an anomalous bifid common bile duct, with separate insertions into the duodenum above and below the point of atresia. Air or contrast material may be seen refluxing into the anomalous ducts, which allow the contents of the obstructed proximal duodenum to course into the distal duodenum, bypassing the point of obstruction (Fig. 103-2).7,19 In patients with duodenal stenosis, dilatation of the stomach and proximal duodenum and decrease in distal gas is seen commensurate with the degree of obstruction. If the stenosis is mild or the stomach is decompressed via an enteric tube, the plain abdominal radiographs may be nonrevealing (e-Fig. 103-3). In patients with duodenal atresia associated with esophageal atresia with a fistula, plain films are diagnostic (Fig. 103-4).
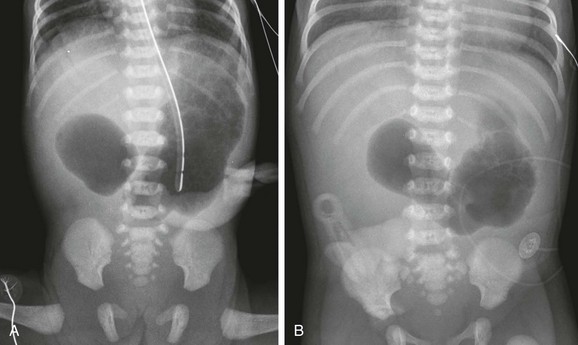
Figure 103-1 Duodenal atresia.
A, A 1-day-old premature infant born at 32 weeks’ gestation with prenatally diagnosed duodenal atresia. The radiograph shows the classic double bubble, with a distended stomach and duodenum and no distal gas. B, A full-term infant with a prenatal diagnosis of duodenal atresia. The radiograph shows a gaping pylorus through which there is wide communication between the dilated stomach and the dilated duodenum, with no distal gas. At surgery, an associated malrotation without volvulus was discovered, and a Ladd procedure was performed, in addition to a duodenoduodenostomy.
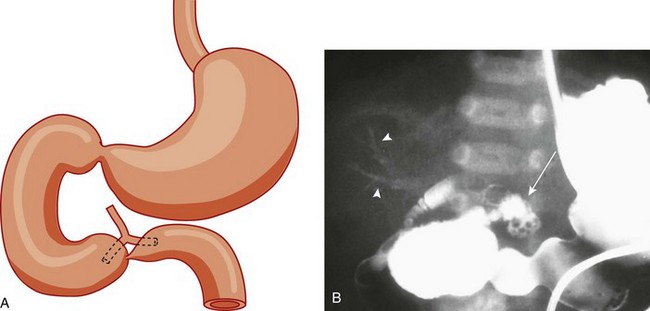
Figure 103-2 Duodenal stenosis with anomalous ducts.
A, A schematic rendering of duodenal obstruction bypass by dual anomalous ducts. B, An upper gastrointestinal series in a neonate with duodenal atresia shows contrast material within the ductal system (arrowheads) and within the bowel lumen beyond the site of obstruction.
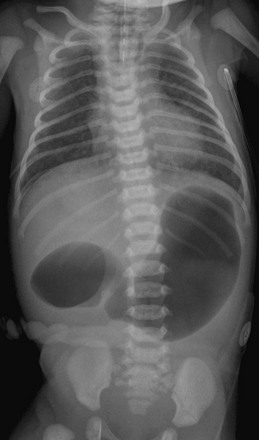
Figure 103-4 A 1-day-old infant with esophageal and duodenal atresia.
Note the orogastric tube in the proximal esophageal pouch; abdominal double bubble indicates a distal tracheoesophageal fistula and duodenal atresia.
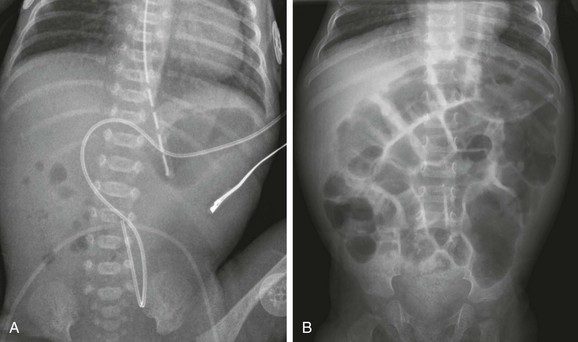
e-Figure 103-3 Duodenal stenosis.
A, A 1-day-old premature twin infant with bilious aspirates. The radiograph demonstrates paucity of distal bowel gas, which could be ascribed to decompression of the stomach by the orogastric tube. Duodenal stenosis was shown on a subsequent upper gastrointestinal examination and confirmed at surgery. B, An 8-month-old with Down syndrome who was admitted for investigation of failure to thrive. Although the radiograph does not suggest duodenal obstruction, duodenal stenosis with a membrane was demonstrated upon upper gastrointestinal examination and confirmed at subsequent surgery.
An upper gastrointestinal (GI) series may be needed in patients with duodenal stenosis or in cases in which differentiation from malrotation is a concern. Furthermore, malrotation can coexist with duodenal stenosis or atresia (e-Fig. 103-5).20 Therefore if any clinical concern for malrotation exists or if surgery is to be delayed, an upper GI series is indicated. In patients with duodenal atresia, contrast enema has been performed in the past to assess the rotation of the bowel, but this examination is not helpful if it is normal or if the cecum is high-riding.21,22
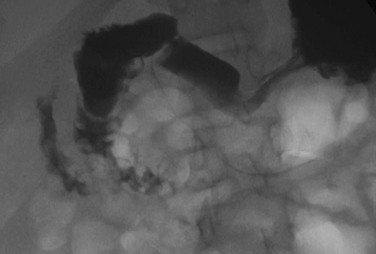
e-Figure 103-5 Duodenal web.
An upper gastrointestinal examination in an adolescent with a history of intermittent abdominal pain shows a duodenal web and malrotation.
In patients with duodenal stenosis, an upper GI series will confirm the partial obstruction (Fig. 103-6); when a web is present, the membrane may be seen as a thin linear filling defect (see Fig. 103-6, B and C). A duodenal diverticulum is more conspicuous, particularly when underscored by an ingested foreign body (e-Fig. 103-7). In patients with duodenal atresia simulating duodenal stenosis as a result of bypass of the obstruction by anomalous ducts, contrast may be seen within the ducts, as previously discussed (see Fig. 103-2).
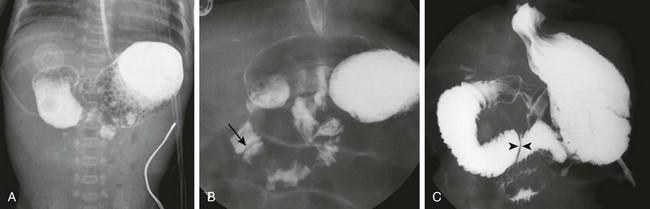
Figure 103-6 Duodenal stenosis.
A, The same infant as shown in e-Figure 103-3, A. A 30-minute radiograph after upper gastrointestinal (GI) examination shows the duodenal obstruction. B, The same infant as shown in e-Figure 103-3, B. An image from an upper GI examination shows a duodenal web (arrow). C, Image from an upper GI examination in a 9-month-old girl demonstrates a thin duodenal web (arrowheads).
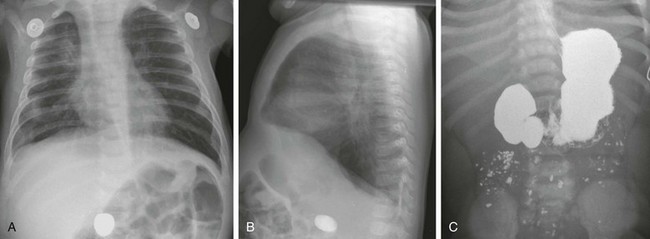
e-Figure 103-7 Duodenal web with a foreign body.
Anteroposterior (A) and lateral (B) chest radiographs in a 6-month-old demonstrate a foreign body (a rock ingested several weeks previously) in the right mid abdomen. C, A subsequent upper gastrointestinal examination demonstrates a dilated duodenum proximal to a confirmed web (the rock is obscured by the barium).
Cross-sectional imaging currently does not have a routine place in evaluation of patients with duodenal atresia or stenosis. However, occasionally a patient with duodenal stenosis proximal to the ampulla of Vater will present with nonbilious vomiting and come to ultrasound with a primary concern of pyloric stenosis. In those cases, ultrasound will reveal an abnormally distended pylorus and dilatation of the duodenal bulb (Fig. 103-8).
Treatment: Treatment of duodenal atresia and symptomatic duodenal stenosis is surgical repair. At the time when Ladd reported surgical correction of duodenal obstruction in 1932,23 the reported mortality rate was approximately 40%.24 In 1990, Kimura et al. described the technique of diamond-shaped anastomosis, which has become the standard procedure for open repair, with reported mortality of 5% to 10%, largely due to associated anomalies, particularly those involving cardiac lesions.24,25 More recently, laparoscopic duodenoduodenostomy has been introduced with increasingly good results and reported improvement in return of bowel function, leading to reduced length of hospital stay.24,26
Extrinsic Lesions: Annular Pancreas, Malrotation, Preduodenal Portal Vein, and Duplication Cysts
Overview: Annular pancreas refers to encirclement of the descending portion of the duodenum by the pancreatic head. The prevalence of annular pancreas is not known, because asymptomatic cases are not always identified. Autopsy prevalence varies between 1 to 15 : 100,000 adults, whereas endoscopic retrograde cholangiopancreatography (ERCP) studies report 1 to 4 : 1000 among symptomatic patients.27,28 In children the incidence is estimated at approximately 1 to 12 : 15,000 births.29 Annular pancreas may result in extrinsic duodenal obstruction; however, most cases of duodenal obstruction with annular pancreas are most likely the result of an associated intrinsic duodenal abnormality.3
Etiology: The pancreas arises as a small ventral and a larger dorsal bud from the duodenum. Normally the ventral bud rotates and fuses with the dorsal bud. When the ventral bud becomes tethered to the duodenum prior to rotation, or if the ventral bud fails to rotate completely before fusion, the result is an annular pancreas.3,28,30 The pancreatic annulus, the portion surrounding the duodenum, frequently has a separate duct entering the duodenum, opposite the ampulla of Vater. Duodenal contents may reflux through this duct into the annulus.
Clinical Presentation: Annular pancreas may be asymptomatic, and adult presentation has been reported (median age of 47 years) with signs and symptoms consistent with neoplasm, jaundice due to obstruction of the common bile duct, or pancreatitis, because duodenal contents may reflux through a separate pancreatic duct into the annulus.28,31 Pediatric patients typically present at a median age of 1 day at diagnosis with findings related to duodenal obstruction, which are either revealed at prenatal sonography or manifested as vomiting or feeding intolerance soon after birth.28 Older pediatric patients can present with pancreatitis and jaundice.
Associated anomalies are common in children and include malrotation, esophageal atresia, anal atresia, and cardiac defects, particularly in patients with trisomy 21 and Cornelia de Lange syndrome12,28; annular pancreas also has been reported in patients with heterotaxy.32
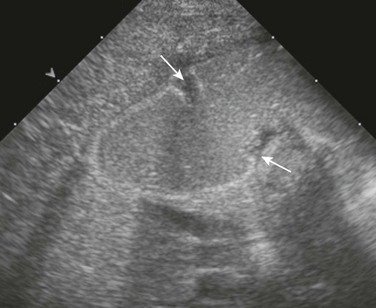
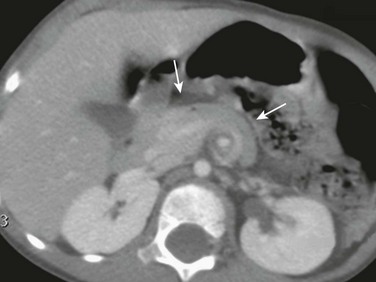
Imaging: Plain radiographs show findings of duodenal dilatation in patients who have an obstruction. An upper GI series will show narrowing of the descending portion of the duodenum (Fig. 103-9, A). Ultrasound may show a ring of pancreatic tissue about the descending portion of the duodenum, although the duodenum might be difficult to follow, and the appearance may resemble a mass at the level of the head of the pancreas (Fig. 103-9, B). A more definitive diagnosis can be made with computed tomography (CT; Fig. 103-9, C to E). Magnetic resonance imaging (MRI) also may show the ring of pancreatic tissue, but MR cholangiopancreatography will show more definitive findings, outlining the annular course of the pancreatic ducts (see Fig. 103-9 F and G), analogous to the findings seen with endoscopic retrograde cholangiopancreatography (e-Fig. 103-10).
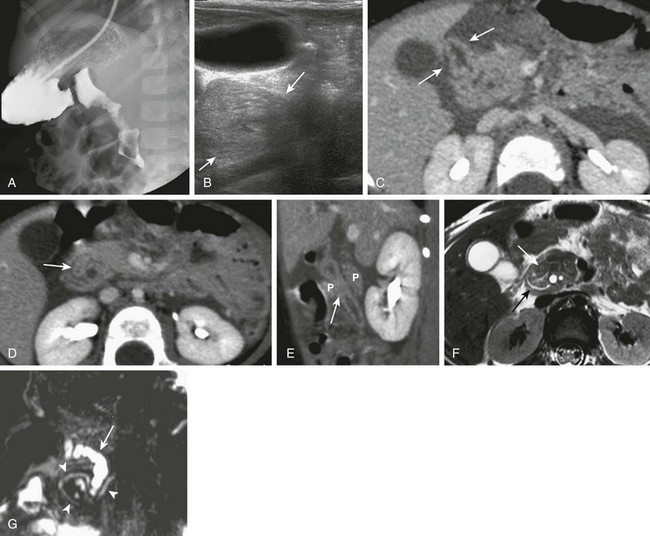
Figure 103-9 Annular pancreas.
A, An upper gastrointestinal (UGI) examination in a 2-year-old girl with annular pancreas demonstrates a circumferential impression upon the descending duodenum. The patient had presented with pancreatitis; the UGI examination was performed after her symptoms had subsided, to assess for an underlying element of duodenal obstruction. B, Ultrasound at the time of initial presentation shows a thickened head of the pancreas (arrows); the central duodenum was not identified. C-E, Abdominal computed tomography images. C, The duodenum (arrows) is seen entering the head of the pancreas. D, The duodenum (arrow) is again seen within the head of the pancreas, just lateral to the common bile duct. E, Sagittal reformat shows the duodenum (arrow) with pancreatic tissue (P) anterior and posterior to its descending portion. Free fluid is secondary to pancreatitis. F, Abdominal T2-weighted magnetic resonance (MR) shows the duodenum (white arrow) within the head of the pancreas, with circumferential pancreatic tissue, outlined by a portion of the encircling pancreatic duct dorsally (black arrow). G, MR cholangiopancreatography delineates the pancreatic duct (arrowheads) and its circumferential course at the head of the pancreas. The arrow points to the dilated common bile duct. (Courtesy Melissa A. Hilmes, Nashville, TN.)
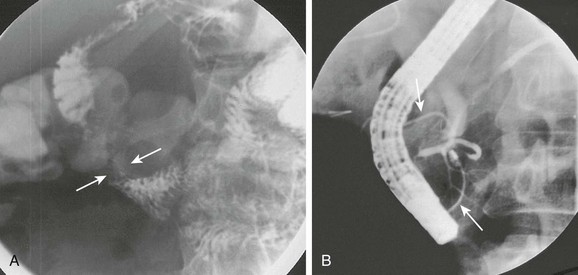
e-Figure 103-10 Annular pancreas.
A, Oblique view of the duodenal C-loop during an upper gastrointestinal series shows extrinsic narrowing (arrows). B, Similar view as in A. This endoscopic retrograde cholangiopancreatography image confirms a circumferential pancreatic duct (arrows) and annular pancreas. (Courtesy Dr. George Taylor, Boston, MA.)
Malrotation
Overview: The term “malrotation” indicates that the normal rotation of the midgut was not completed, thus presenting a spectrum of abnormalities that affect both the duodenojejunal and the cecal poles of the midgut, singly or in unison. The prevalence of malrotation is difficult to determine, inasmuch as asymptomatic cases may not be recognized; it has been quoted as high as 1 : 500 live births,33 which seems excessive, as that is similar to the incidence of pyloric stenosis (2 to 5:1000).
The bowel is thus suspended from a mesentery that is attached to the posterior abdominal wall and that extends from the left upper to the right lower quadrants (Fig. 103-11). The configuration of the duodenal C-loop mirrors the 270° of rotation that it has undergone.21,34–37 The final steps in bowel rotation and fixation involve resorption of the dorsal mesenteries of the ascending and descending colon and elongation of the ascending colon with descent of the cecum, processes that are ongoing until several months of postnatal life.33 The Meckel diverticulum is the demarcation point between the embryonic prearterial and postarterial limbs.
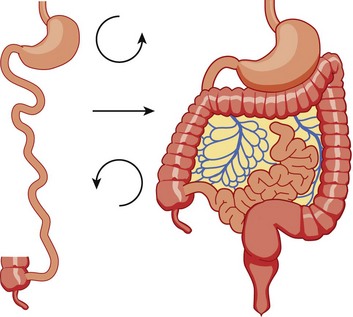
Figure 103-11 Normal rotation of midgut.
The midgut starts as a straight tube; 270 degrees of counterclockwise rotation of the duodenojejunal junction places it in the left upper quadrant, whereas 270 degrees of counterclockwise rotation of the cecum places it in the right lower quadrant. Normal fixation of the bowel after rotation is completed results in the midgut being suspended from a broad-based mesentery, with retroperitoneal attachment at both ends.
Etiology: The aforementioned background is important in the understanding of the clinical problems that arise with malrotation and malfixation of the bowel.
Malrotation arises when the process of normal rotation is arrested, which can occur at any point along the rotation of the duodenojejunal or cecal segments. If it is arrested after the initial 90° of counterclockwise rotation, the duodenojejunal junction and the small bowel will be located on the right side of the abdomen, and the cecum and the remainder of the colon will be located in the left side of the abdomen. Despite the fact that the initial 90° of counterclockwise rotation have occurred in both the duodenojejunal junction and the cecum, this arrangement is nevertheless known as nonrotation, a specific variant of malrotation, characterized by a relatively long mesenteric pedicle.38
Progression of the rotation process beyond the nonrotation stage brings the duodenojejunal junction and the cecum into proximity, and arrest along this portion of the spectrum typically results in a shorter mesenteric pedicle from which the bowel is suspended (Fig. 103-12), believed to increase the risk for subsequent volvulus. Reversed rotation is a rare form of malrotation in which the gut undergoes 90° of clockwise rotation, instead of the normal 270° of counterclockwise rotation. Starting from the initial straight tube, this rotation results in the location of the duodenojejunal junction in the left upper quadrant and of the cecum in the right lower quadrant. However, in these cases, the ceco-colic loop returns into the abdomen first, which results in the transverse colon, not the duodenum, being located between the aorta and the superior mesenteric artery.39,40
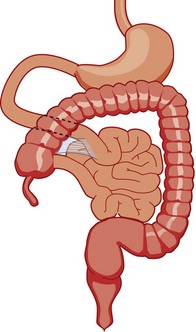
Figure 103-12 Malrotation.
As the gut rotation is arrested between the initial 90 degrees (“nonrotation”) and the normal 270 degrees, the duodenojejunal junction and the cecum approximate, resulting in suspension of the midgut from a narrow mesenteric pedicle without normal attachments. This arrangement is at high risk for midgut volvulus.
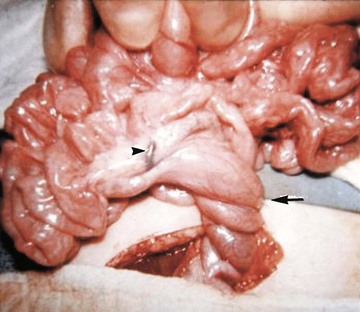
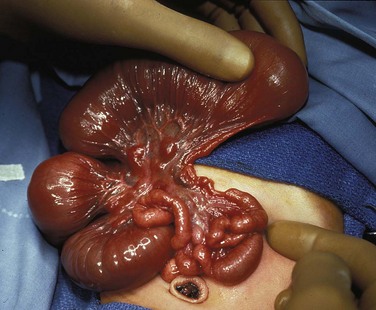
Several disorders of fixation also commonly occur in patients with malrotation. Ladd bands are the result of abnormal mesenteric attachments that are formed in patients in whom the rotational process is incomplete. These bands, named after Dr. William E. Ladd, typically extend from the edge of the liver to the malrotated cecum, crossing over and causing obstruction and kinking of the duodenum (Fig. 103-13); however, they can rarely occur more distally, in the jejunum or ileum.34
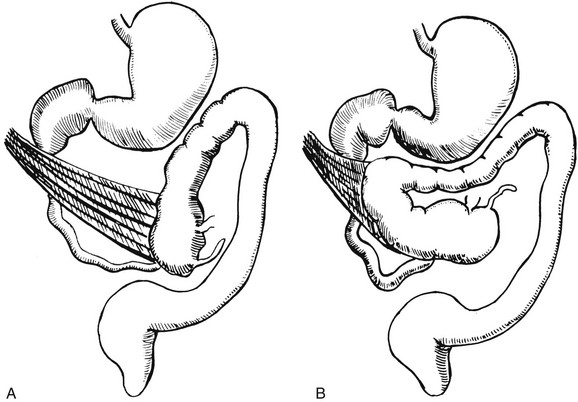
Figure 103-13 Ladd bands.
Illustration of two instances in the spectrum of malrotation, demonstrating dense peritoneal bands (Ladd bands) extending from the cecum to the right upper quadrant, crossing over and obstructing the duodenum. These bands must be divided after reduction of the volvulus to relieve the obstruction.
Internal hernias are the result of abnormal or incomplete fixation of the ascending (right paraduodenal or mesocolic) or descending portions of the colon, resulting in a defect through which the bowel can herniate. Herniation into the defect in the ascending colonic mesentery results in a right paraduodenal or mesocolic hernia, whereas herniation into the defect in the descending colonic mesentery results in a left paraduodenal or mesocolic hernia.34,37 In persons with a normally rotated bowel, abnormal or incomplete fixation of the ascending colon, cecum, or sigmoid colon can result in volvulus of these structures, which typically does not occur until late adult life.
Clinical Presentation: The presentation of patients with malrotation depends on whether obstruction is present, and if so, whether the obstruction is acute or chronic, as well as on associated malformations. Presentation may occur in utero, and the infant may be born with short bowel as a result of in utero bowel necrosis and resorption, or with the apple-peel type of atresia.41,42 In asymptomatic patients malrotation may be discovered in the course of evaluation for other clinical concerns.
Acute Volvulus: In persons with volvulus, the duodenum rotates, typically clockwise, about the axis of the superior mesenteric artery (Fig. 103-14) and is obstructed at its third portion, distal to the ampulla of Vater. Because approximately 60% to 80% of patients with volvulus present in the first month of life,33,34,38,43,44 the typical presentation is that of a previously well neonate who experiences the sudden onset of bilious vomiting. The infant may experience crampy abdominal pain, which could be confused with colic. If the obstruction is significant, the abdomen may become initially scaphoid after distal intestinal contents are evacuated. Vascular compromise may lead to intraluminal bleeding and hematochezia, seen in 10% to 15% of patients with volvulus.37 As ischemia of the midgut supervenes, the abdomen becomes distended and firm, with physical signs of peritonitis, and the patient will present in shock with cardiovascular collapse.34
Chronic Volvulus: Partial or intermittent volvulus tends to present more insidiously, with an average duration of symptoms of 28 months or greater before the correct diagnosis is made.34 The patient usually has a history of abdominal pain, which can be severe but often is vague and intermittent, and often is associated with intermittent vomiting and/or failure to thrive. Impairment of venous and lymphatic flow leads to signs and symptoms of malabsorption.45,46 The correct diagnosis is frequently delayed, with interim diagnoses including central or psychogenic vomiting, milk or other food allergies, and various malabsorption syndromes, such as celiac disease.20,34,47,48
Associated Conditions: Multiple malformations and conditions are associated with malrotation and are reported to occur in 30% to 60% of patients with malrotation.34,38 Intrinsic duodenal stenosis and annular pancreas have been discussed previously, and other intestinal atresias also occur.5,38 Other abnormalities include Hirschsprung disease and anorectal malformations, cloacal extrophy, Eagle-Barrett (prune belly) syndrome, megacystis-microcolon-intestinal hypoperistalsis or Berdon syndrome, Cornelia de Lange syndrome, Marfan syndrome, and Meckel syndrome.21,37,38,49 Malrotation also is found in some patients with trisomy 13, 18, and 21, in whom malrotation is cited as being 25 times more common than in the general population.21,50 Malrotation is part of anomalies in which the gut is unable to achieve its normal rotation—gastroschisis, omphalocele, and Bochdalek diaphragmatic hernia—and is also extremely common in patients with heterotaxy.51–53
Imaging: Plain abdominal radiographic findings in patients with malrotation without obstruction or volvulus may show an abnormal distribution of stool, with absence of stool pattern in the right lower quadrant, which has been termed the radiologic “Dance’s sign.”48 In patients with nonrotation, all colonic stool may be seen as confined to the left half of the abdomen.
In patients with volvulus, the radiographs may be completely normal at the onset of the bilious vomiting (Fig. 103-15, A). As distal bowel contents are evacuated and greater obstruction supervenes, the radiographs may show distension of the stomach, with relatively minor or subtle distension of the duodenum (Fig. 103-15, B and C). It is important to note that in malrotation with volvulus, the duodenum is not typically markedly distended, as is the case with duodenal stenosis or atresia; obstruction with Ladd bands is more likely to lead to an appearance more resembling that of the double bubble. The lack of marked bowel distension in an infant with volvulus may lead to a false sense of security or to the suspicion of a more common condition such as pyloric stenosis, if the significance of the bile-stained vomitus is not appreciated.
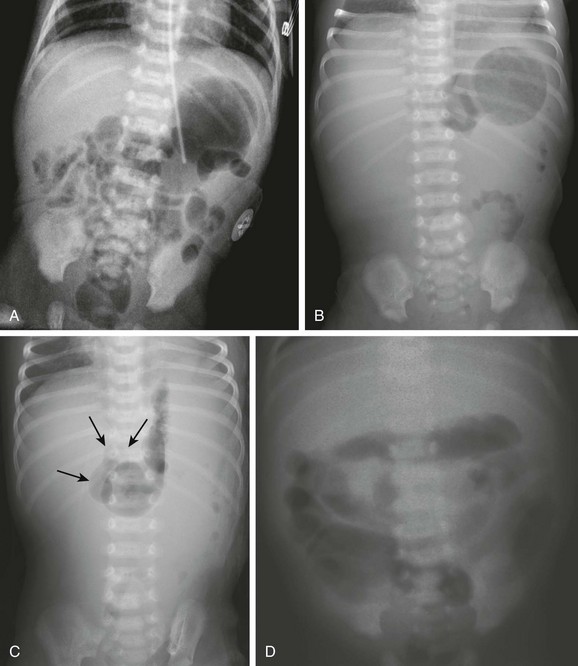
Figure 103-15 Acute midgut volvulus.
Abdominal radiographic findings. A, An abdominal radiograph in 2-day-old infant with bilious vomiting and midgut volvulus. The bowel gas pattern is easily interpreted as within normal limits. B and C, Supine (B) and left decubitus (C) radiographs in a 7-day-old infant with bilious vomiting and midgut volvulus. The bowel gas pattern is abnormal, with very little gas distal to the stomach, and a scaphoid abdomen. No distension of the duodenum is appreciated, and the stomach is normal in size, but there is discrepancy between the size of the stomach and the marked lack of gas distally. On the decubitus radiograph, distension of both the stomach and the duodenum (arrows) is appreciated, with remarkable paucity of distal gas. D, A 2-week-old infant with bilious vomiting for several days. The radiograph shows a severe ileus pattern with abdominal distension and fluid and gas distending loops of bowel throughout the abdomen. The bowel was necrotic at subsequent surgery, and the infant died.
Ominous plain radiographic findings of ischemia in patients with volvulus include abdominal distension, separation of bowel loops, tubular appearance of bowel loops, fold thickening, and thumbprinting. Diffuse fluid and gaseous distention of the bowel is a finding that suggests gangrenous bowel and a poor prognosis (Fig. 103-15, D).
Upper GI Series: An upper GI series is at present considered the standard examination to evaluate for malrotation and for its complications, particularly volvulus.
Documentation of normally rotated bowel can be challenging, and technique is very important. In pediatric patients, documentation of the normally rotated C-loop needs to be made on passage of the first bolus of contrast from the stomach; missing this opportunity can result in confusing and misleading findings. The course of the duodenum must be documented in both frontal and lateral projections, because the lateral view is essential to document the retroperitoneal position of both ascending and descending portions.37,54 The duodenojejunal junction, recognized by an acute flexure as the duodenum returns to the peritoneal cavity, should be located to the left of the left pedicle at the same craniocaudal level as the duodenal bulb. The lateral view should show ascending and descending portions entirely superimposed anterior to the spine; anterior deviation of the descending limb is abnormal. The technique is discussed in Chapter 85. When malrotation with volvulus is suspected, we typically will use a low-osmolality water-soluble agent and position an enteric tube distally within the stomach to improve control over the first bolus. The fluoro-grab function of most current fluoroscopes allows movielike documentation of the progress of the first bolus through the duodenum without additional radiation dose, and it can be very helpful in confirming normal rotation, as well as in sorting out difficult cases and normal duodenal variants. The radiologist should be familiar with normal variants such as duodenum inversum, in which the duodenum shows a parallel ascending and descending course to the right of the spine, before crossing to the left and emerging into the peritoneal cavity at a normally positioned ligament of Treitz (Fig. 103-16, Video 103-1).55 Conversely, it is imperative that an abnormal course of the duodenum in patients without volvulus be recognized, including abnormal location of the duodenojejunal junction on the frontal image (see Fig. 103-16, C) and an abnormally anterior course of all or part of the duodenum on the lateral view. Abnormal location of the duodenojejunal junction—“excessive redundancy of the duodenum to the right of the spine”—may be indicative of malrotation55 and other findings, such as position of the duodenum on the lateral view, position of the cecum, and cross-sectional imaging may need to be considered.
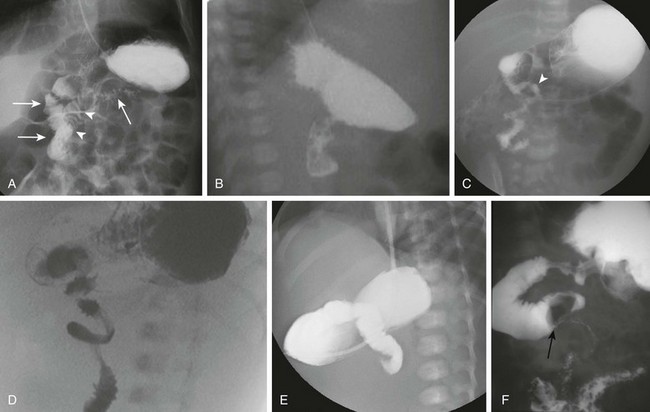
Figure 103-16 Upper gastrointestinal imaging (UGI) of the duodenum.
A and B, Normal variant of duodenum inversum. A frontal image (A) of an infant with duodenum inversum. Note that after the vertical course of the duodenum on the right (left, parallel arrows), it ascends (arrowheads) before crossing the midline to a normally positioned duodenojejunal junction at the ligament of Treitz (right arrow). A lateral view (B) in a patient with duodenum inversum shows normal posterior position of the duodenal course. C, Malrotation in an infant without volvulus. A frontal image demonstrates that the presumed duodenojejunal junction (arrowhead) is low and to the right of the midline. D, A fluoro-grab image obtained during UGI in the same 7-day-old patient illustrated in Figure 103-15, B and C. Note the classic corkscrew configuration of the duodenum as it wraps around the twisted mesenteric pedicle. E, A UGI on another 7-day-old infant with a 1-day history of bilious vomiting and dehydration. The duodenum was completely obstructed, terminating in a configuration resembling a beak. F, Ladd bands in a 2-month-old girl with malrotation. A left posterior oblique spot UGI from a series demonstrates a dilated proximal duodenum as a result of crossing bands at the level of the third portion of the duodenum. The presumed duodenojejunal junction is abnormal. Note the kinking of the duodenum (arrow).
In symptomatic patients with malrotation, the upper GI examination may reveal the volvulus itself, presenting a spiral twisting of the duodenum (classically described as a “corkscrew”) as it wraps around the axis of the superior mesenteric artery (see Fig. 103-16, D). When complete obstruction is present, the contrast column may terminate in a beaklike configuration as the contrast is propelled into the entrance of the spiral but cannot proceed further (see Fig. 103-16, E).
In patients with obstruction resulting from Ladd bands, the duodenum may demonstrate a kinked configuration (see Fig. 103-16, F) that at times resembles a Z-shape instead of the normal C-loop. The Z-shape is the result of tacking down and kinking of the duodenum by the abnormal attempts at fixation and at times may be very difficult to differentiate from volvulus.
Contrast Enema: Barium enema had been advocated by surgeons and radiologists in the investigation of patients suspected of malrotation and volvulus, in the belief that cecal position would define the presence or absence of malrotation while avoiding introduction of barium contrast proximal to the obstructed duodenum.20,56 However, with the understanding that up to 30% of patients with malrotation and its complications could have a normally rotated cecum, and with the realization that a high-riding cecum in a neonate may in fact be normally rotated,20,57,58 the contrast enema is no longer considered the main fluoroscopic diagnostic tool in the investigation of these patients. Follow-through small bowel to evaluate the cecum when the upper GI series shows confusing findings has been advocated and may be helpful in some cases, but the previous problems are not obviated. The cecum itself may be very difficult to define if the appendix does not fill, and a colonic segment in the right lower quadrant may be mistaken for the cecum. However, a clearly abnormal cecal position, especially in children with an equivocal location of the duodenal-jejunal flexure, is diagnostic of malrotation.
Overview: A clearly abnormal course of the duodenum renders the diagnosis of malrotation straightforward (see Fig. 103-16, C). Unfortunately, subtle cases exist that lead to false-negative interpretations, and as previously discussed, normal variants may lead to false-positive results. In a retrospective review of 163 patients with surgically proven malrotation, upper GI examination had a sensitivity of 96%, with seven false-negative examinations.59 On the other hand, a 15% false-positive diagnosis by upper GI examination, as demonstrated on subsequent surgical findings, also has been reported.60 The frontal course of the duodenum may be misleading if the duodenojejunal flexure occurs over rather than to the left of the pedicle of the spine, if it occurs below the plane of the duodenal bulb, or if abnormal redundancy of the course of the duodenum occurs. The duodenojejunal junction also can be displaced by postoperative changes after liver transplantation,61 peritoneal masses, or abnormal, dilated bowel loops.62 Limitations of the lateral view include the fact that the aorta and the superior mesenteric artery are not visualized, and therefore true retroperitoneal position of the duodenum cannot be ascertained unequivocally, despite the parallel course of its ascending and descending portions anterior to the spine.22
Interpretative Errors: False-positive interpretations usually result from failure to recognize normal variants or from displacement of a normal ligament of Treitz. Position of the proximal small bowel in the right upper quadrant, as an isolated finding, is not indicative of malrotation.55,59 We know that the neonatal duodenum is mobile.58 Therefore in patients in whom there is internal traction upon the duodenum, such as infants with dilatation of bowel, the duodenojejunal junction may appear abnormally located on the upper GI examination.62 Normal variants of duodenal course, such as duodenum inversum (see Fig. 103-16, A and B, and Video 103-1) or redundant duodenum, also may lead to false-positive interpretations, although marked redundancy of the duodenum also can be a sign of malrotation.55 Analysis of the true lateral view, or correlation with cross-sectional information, may be needed in some of these cases. A high position of the cecum or a mobile cecum, particularly in a neonate, also are typically normal findings but make evaluation for malrotation particularly challenging in individual patients.
Potential false-negative outcomes may result from suboptimal technique, such as failing to delineate the course of the duodenum on the first bolus of contrast from the stomach or from misinterpretation of the subtle finding of malrotation, such as kinking of the duodenum along its course or location of the duodenojejunal junction abnormally low or to the right of the left pedicle of the spine.21,37,54,58,59 A proposed scoring system whereby the presence of three of nine potential findings would indicate malrotation, whereas one of nine was considered normal and two of nine was considered indeterminate, has not met with universal success.55,58
Cross-Sectional Imaging: Although cross-sectional imaging is not usually the first type of examination performed in patients in whom malrotation is a concern, it nevertheless may be the first examination performed for a given patient. The radiologist needs to be familiar with the findings of malrotation, particularly if it is associated with volvulus. The findings can be categorized as follows: (1) abnormal relationship of the superior mesenteric artery and vein; (2) volvulus; and (3) abnormal course of the duodenum.
Ultrasound: On ultrasound, as with other cross-sectional imaging, the relationship of the superior mesenteric artery and vein is readily evaluated. The superior mesenteric artery has a smaller size and surrounding hyperechoic halo and is located posterior and to the left of the superior mesenteric vein; identification can be confirmed with brief Doppler interrogation. This relationship was noted to be abnormal in patients with malrotation63 but it is neither highly sensitive nor highly specific for malrotation with or without volvulus.64
The “whirlpool” sign in patients with volvulus refers to a whirlpool-like appearance of the duodenum and superior mesenteric vein wrapped clockwise around the axis of the superior mesenteric artery.65 In patients with this condition, a dilated duodenum can be followed as it enters into the volvulus (Fig. 103-17, A and E, and Video 103-2). It should be noted that this sign encompasses rotation of the duodenum, as well as the mesenteric vein. The sensitivity and specificity of the sonographic whirlpool sign has not been studied in large numbers of patients, but reports on smaller cohorts suggest that it is sensitive and specific for malrotation with volvulus.66,67 It should be noted that this sign is not present in patients with malrotation without volvulus at the time of the examination and that it can be relatively subtle (Fig. 103-17, C and D, and Video 103-3). In older patients presenting with chronic volvulus, the sonographic findings are similar, although the duodenum may be considerably larger, because it has had a longer time to become dilated (Fig. 103-17, E and F, and Video 103-4).
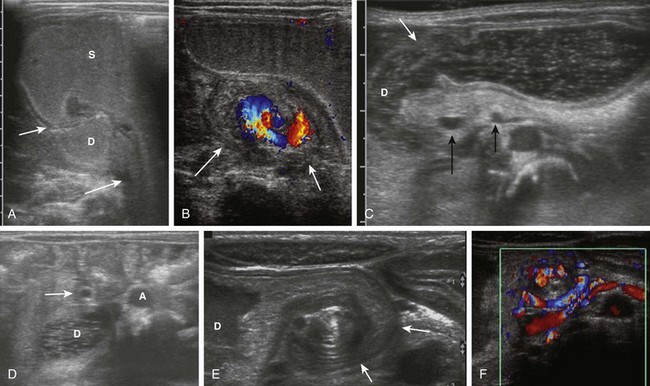
Figure 103-17 Acute and chronic midgut volvulus.
A and B, An 18-day-old boy with a history of vomiting since birth, which had recently become “projectile” and was “occasionally yellow.” Ultrasound was requested to assess for pyloric stenosis (see Video 103-2). A, An ultrasound image shows a dilated stomach (S) and duodenum (D), with the latter deviated medially as it enters into the volvulus (long arrow). The pylorus (short arrow) is widely open. B, A subsequent image shows the duodenum at the outer edge of the twist (arrows), with the twisting superior mesenteric vein and the centrally located superior mesenteric artery. The patient subsequently had surgical detorsion and lysis of Ladd bands. C and D, A 7-day-old girl presenting with history of 36 hours of vomiting (see Video 103-3). C, A transverse sonogram demonstrates a distended pylorus (white arrow) leading to a dilated duodenum (D), which curves medially around the superior mesenteric artery (SMA) (short black arrow) and superior mesenteric vein (long black arrow). D, A slightly more caudal image shows the dilated duodenum (D) terminating in a beak between the aorta (A) and the SMA (arrow). E and F, A 5-year-old boy who weighed 15 kg at presentation with a long history of vomiting, a 14-lb weight loss during the previous 3 weeks, and on a gluten-free diet for a recent diagnosis of gluten enteropathy (see Video 103-4). E, A transverse sonogram of the upper abdomen shows a markedly dilated duodenum (D) narrowing as it enters into the volvulus (arrows). F, Color Doppler imaging at a similar level as E shows the mesenteric vein around the twist. Detorsion of a 720-degree twist and division of Ladd bands was accomplished at surgery.
The normal retroperitoneal course of the duodenum posterior to the superior mesenteric artery is an indication of normal rotation of the bowel; this course can be seen in cross-sectional imaging and has been underscored recently as an important finding on abdominal ultrasound.22
CT is not the procedure of choice in evaluation of malrotation, but if it is performed for other reasons, the course of the duodenum and the position of the cecum should be assessed, and volvulus should be recognized if it is present (Fig. 103-18).
Treatment: The treatment for malrotation with volvulus consists of surgical detorsion, identification and lysis of Ladd bands, straightening of the duodenum along the right abdomen, and placement of the cecum in the left lower quadrant, thus broadening the base of the mesentery and creating a separation between the duodenojejunal junction and the cecum resembling nonrotation, with incidental appendectomy. This procedure, which bears his name, was described by Dr. Ladd, and remains the standard for patients with malrotation.23,68 A laparoscopic Ladd procedure can be performed, but it may be very difficult to perform in an acutely ill neonate with volvulus; it has also been suggeted that fewer postoperative adhesions in laparoscopic surgery may predispose to recurrent volvulus, although insufficient comparative data are available at this time.69,70 When accompanied by volvulus, the surgery is emergent, and correction of existing metabolic and electrolyte derangements must be undertaken. When malrotation in the absence of volvulus is found in the older child or adult, surgery is indicated if the patient is symptomatic. The role of surgery versus conservative management in asymptomatic older patients with malrotation without volvulus, such as patients with heterotaxy,71 remains controversial.72
Preduodenal Portal Vein
Overview: The portal vein forms at the junction of the superior mesenteric and splenic veins, and its normal course is retropancreatic and retroduodenal. The preduodenal portal vein, first reported in 1921, courses anterior to the pancreas and duodenum; it is important because of its associated anomalies, but its major significance is surgical.73
Etiology: A preduodenal portal vein is the result of abnormal resorption of segments of the paired embryonic vitelline veins and their connections (e-Fig. 103-19). Normally the upper communicating branch between the left and right vitelline veins persists, with resorption of the cephalad segment of the left vitelline vein, the caudal segment of the right vitelline vein, and of the lower communicating branch, resulting in the duodenum coursing ventral to the portal vein. The preduodenal portal vein results when the upper communicating branch is resorbed, with persistence of the lower communicating branch, which courses ventral to the duodenum; there is resorption of the left vitelline vein and persistence of the cranial portion of the right vitelline vein.74,75
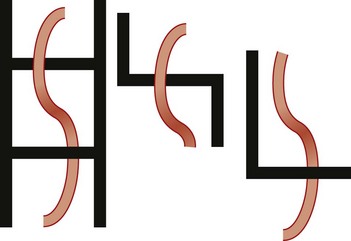
e-Figure 103-19 Schematic illustration of formation of the preduodenal portal vein.
The illustration on the left shows the paired vitelline veins with embryonic connections coursing dorsal (upper rung) and ventral (lower rung) to the duodenum. Normally (middle figure), there is persistence of the upper communicating branch, with resorption of the cephalad segment of the left vitelline vein, and of the caudal segment of the right. The preduodenal portal vein (illustration on the right) results from resorption of the upper communicating branch and persistence of the lower one, with resorption of the left vitelline vein and persistence of the cranial portion of the right vitelline vein.
Clinical Presentation: The preduodenal portal vein is associated with conditions that cause duodenal obstruction, including duodenal web, annular pancreas, and malrotation. Therefore the major presenting symptom of patients with a preduodenal portal vein is duodenal obstruction, as a result of the associated anomaly in most cases.76,77 A preduodenal portal vein is highly associated with heterotaxy, particularly polysplenia.53,78 Besides obstruction, the most important concern is inadvertent injury during surgery.77,79 A preduodenal portal vein should be identified before surgery whenever possible, particularly in patients at risk for this anatomy, such as patients with polysplenia and biliary atresia.51,53,80
Imaging: The preduodenal portal vein is well visualized with cross-sectional imaging. On ultrasound, the sagittal course of the superior mesenteric vein can be followed as it moves ventrally, over the pancreas, to enter the liver, ventral to both pancreas and duodenum (Fig. 103-20).53,81 On CT and MRI, the findings are similar (Fig. 103-21).82
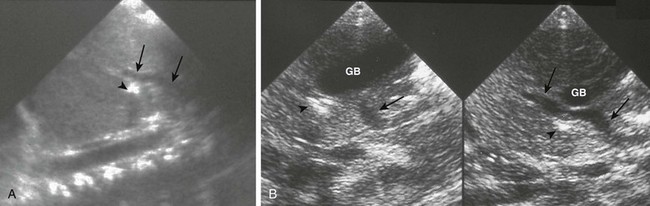
Figure 103-20 A preduodenal portal vein.
A, A sagittal sonogram in a child with asplenia shows the superior mesenteric vein (SMV)/portal vein continuation (arrows) into the liver passing anterior to the duodenum, outlined by a small amount of gas (arrowhead). B, Sequential sagittal sonograms in a child with polysplenia demonstrate the course of the SMV (arrows) coursing over the pancreas and the duodenal bulb (arrowheads), again outlined by a small amount of air. GB indicates a midline gallbladder.
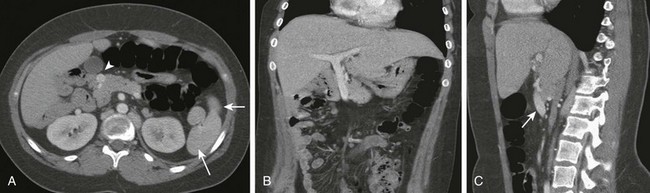
Figure 103-21 A preduodenal portal vein.
A, A computed tomography scan in a child with polysplenia (arrows) shows a preduodenal portal vein (arrowhead). The superior mesenteric artery is in normal position anterior to the aorta. The inferior vena cava was duplicated below the level of the renal hila. B, Coronal reformatting shows the abnormal course of the portal vein as it courses to the liver. C, Sagittal reformat shows the anterior course of the portal vein (arrow).
Small Bowel
Congenital/Neonatal Anomalies of the Small Bowel
Small Bowel Atresia and Stenosis
Overview: Atresia and stenosis affect the jejunum and ileum more commonly than any other portion of the abdominal gastrointestinal tract: approximately 51% involve the jejunum and ileum, compared with the duodenum in 40% and the colon in approximately 9%.84 The incidence of jejunoileal atresia ranges between one and three cases per 10,000 live births, depending on geographic location, with the incidence being greater in persons of European and African-American descent than in persons from Latin America. Small bowel atresias in the ileum occur distally in nearly two thirds of cases. In the jejunum this ratio is reversed, with nearly two thirds occurring proximally and one third distally. Atresia is much more common than stenosis, which accounts for only approximately 5% of cases,85 particularly in the ileum.
Jejunoileal atresias have been subdivided into five types and subtypes84,86 (Fig. 103-22). Type I represents mucosal or membranous atresia, which is limited to luminal discontinuity. In type II, the blind ends of the bowel are connected by a fibrous cord, with continuity of the underlying mesentery. Type IIIa indicates discontinuity between the bowel ends with an adjacent mesenteric defect, whereas IIIb is long-segment atresia with a wide mesenteric defect, typically described as “apple peel,” “Christmas tree,” and “maypole” mesentery because of the appearance of the residual distal small bowel coiled around its tenuous vascular supply through retrograde flow via the ileocolic, right colic, or inferior mesenteric arteries.87 Type IV refers to multiple atresias (e-Fig. 103-23).
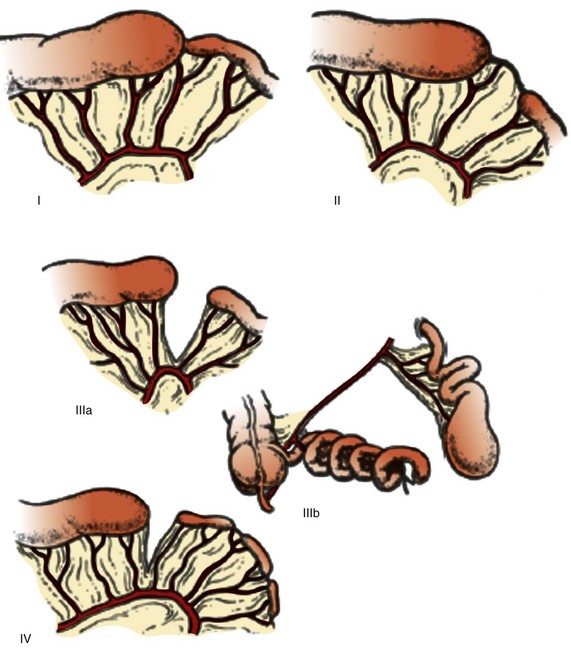
Figure 103-22 Classification of jejunoileal atresias.
Type I is a mucosal or membranous atresia, in which only the lumen is atretic, with continuity of outer wall and mesentery. In type II atresia, the atretic segment is connected to the distal bowel by a fibrous cord, with an intact mesentery. Type IIIa indicates discontinuity between the atretic segment and the distal bowel, with an associated mesenteric defect. In type IIIb the mesenteric defect is large, with absence of a portion of the bowel; the remaining distal bowel is wrapped in “Christmas tree” or “apple-peel” fashion around a remnant arterial supply. Type IV refers to multiple atresias. (From Grosfeld JL, Ballantine TV, Shoemaker R. Operative mangement of intestinal atresia and stenosis based on pathologic findings. J Pediatr Surg. 1979;14(3):368-375.)
Etiology: Unlike the duodenum, in which the etiology of atresia and stenosis is believed to be failure of recanalization, the most likely and accepted etiology in the jejunum, ileum, and colon is a vascular accident, the location and extent of which governs the location and severity of the resultant defect. Experimental data in multiple species undergoing prenatal ligation of mesenteric branches resulting in corresponding bowel atresias gives credence to this theory.85,88 More circumstantial evidence in humans includes the findings of lanugo and bile pigments distal to the point of atresia, indicating patency of the intestinal lumen beyond the point when recanalization of the gut had been completed.89
Conditions that can result in prenatal gut ischemia include gastroschisis, intrauterine volvulus, intussusception, and internal hernias.90 Atresias are found in patients who have undergone significant compression of bowel segments, such as incarceration in omphalocele or in tight gastroschisis defects. Signs of peritonitis have been found in up to 48% of patients with atresia.85 Extensive “apple-peel” small bowel atresia is postulated to occur as a result of occlusion of the superior mesenteric artery distal to the origin of the right colic and ileocolic arteries (Fig. 103-24).
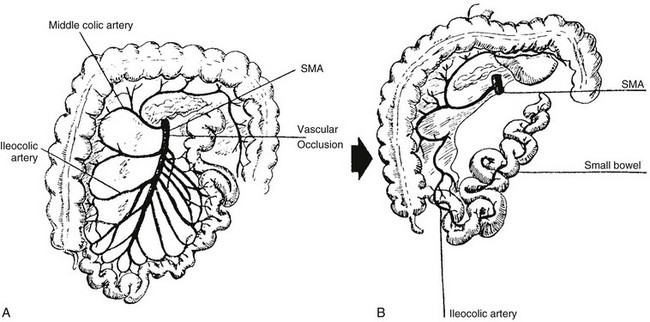
Figure 103-24 Apple-peel variant of intestinal atresia.
A, A schematic drawing of the normal distribution of the superior mesenteric artery (SMA) and its branches. If there is prenatal occlusion at the point indicated, part of the jejunum and the accompanying dorsal mesentery will involute. B, Sequelae of A, with collateral supply of the residual distal small bowel via ileocolic branches of the distal superior mesenteric artery that are supplied by connections to the inferior mesenteric artery. The residual distal ileal loops wrap around their collateral supply, resembling an apple peel.
Clinical Presentation: Patients with intestinal atresia may present prenatally with polyhydramnios or postnatally with bilious vomiting, upper or generalized abdominal distension, jaundice, and abnormalities in passage of meconium.
The percentage of infants with polyhydramnios increases with more proximal atresia, because less bowel surface area is present to absorb swallowed amniotic fluid, and polyhydramnios is present in approximately 38% of patients with jejunal atresia. The percentage of patients presenting with bilious emesis postnatally also increases with more proximal atresia, seen in approximately 84% of patients with jejunal atresia. Jaundice occurs more often in patients with jejunal (32%) than ileal (20%) atresia. Most patients, though not all, fail to pass meconium in the first 24 hours of life. Patients with more proximal atresias may show some distension of the upper abdomen, but abdominal distension due to dilatation of multiple loops of bowel is the rule in patients with distal atresias. Infants with the apple-peel variant of intestinal atresia tend to be premature and have a low birth weight, and more than half are shown to have malrotation of the midgut.85
Other anomalies outside of the gastrointestinal system may be present in nearly one third of patients, more often in patients with jejunal than with ileal atresia.91 Although duodenal atresia shows a high association with trisomy 21, this is not the case with jejunoileal atresia, which is seen in approximately 0.55% to 3% of patients with Down syndrome. Multiple atresias and the apple-peel variant of intestinal atresia can show a genetic pattern in some families, and apple-peel atresias have been reported with a constellation of other anomalies, including ocular abnormalities and microcephaly.95
Imaging: Plain radiographs in patients with bowel obstruction will differ from the normal appearance of the bowel (Fig. 103-25). Patients with proximal atresias will show dilatation of the stomach, duodenum, and jejunum to the point of atresia, although the degree of dilatation may be decreased if decompression occurs via the enteric tube (Fig. 103-26). In patients with ileal atresia (Fig. 103-27), abdominal radiographs will reveal dilatation of multiple gas-filled loops of bowel, often with protuberance of the flanks and elevation of the hemidiaphragms. Unlike older infants and adults, in neonates it is not possible to distinguish small from large bowel, and dilated loops of small bowel distributed along the expected location of the colon can mimic a dilated colon.92 However, gas will not be found in the rectum (Fig. 103-27), a finding that can be optimized by obtaining a prone cross-table lateral radiograph. Patients with jejunal apple-peel atresia will have findings of proximal atresia (e-Fig. 103-28). In patients with jejunal stenosis, the findings will be similar, with distal gas and proximal dilatation dependent upon the degree of stenosis (e-Fig. 103-29). Ileal stenosis is much less common.
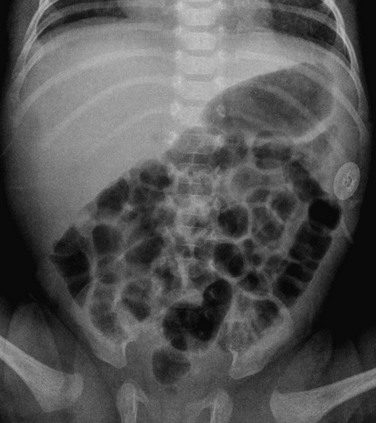
Figure 103-25 Normal neonatal bowel gas pattern.
A supine abdominal radiograph in a female neonate outlining a normal bowel gas pattern, with multiple rounded and polyhedral gas bubbles of similar size distributed throughout the abdominal cavity, and gas in the rectum.
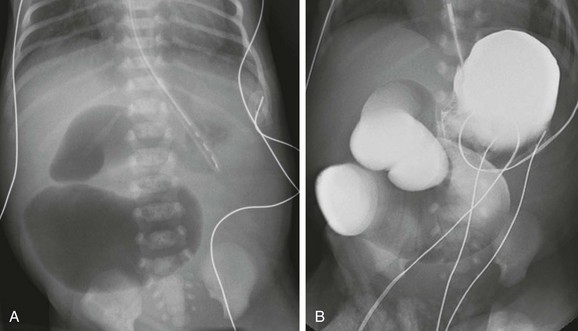
Figure 103-26 Jejunal atresia.
A, Abdominal radiograph in an infant boy on his first day of life. An orogastric tube has decompressed the stomach, but markedly dilated proximal loops are identified, with no distal gas. Note that despite the marked distension of the obstructed bowel, the flanks are not protuberant, and there is no elevation of the hemidiaphragms, confirming the decompressed state of the more numerous distal bowel loops. B, An upper gastrointestinal examination, with water-soluble iso-osmolal contrast material in the same infant. Contrast fills the dilated proximal loops to the point of atresia. Notice the correlation with the abdominal radiograph in A.
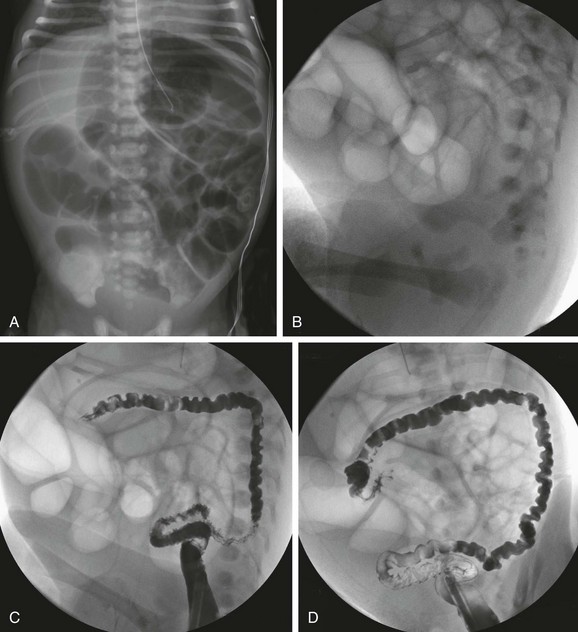
Figure 103-27 Ileal atresia.
A, An abdominal radiograph in an infant boy demonstrates multiple dilated loops of bowel filling the abdomen. Some distension of the flanks and elevation of the diaphragms is seen, despite orogastric tube decompression. B, A lateral radiograph prior to a contrast enema demonstrates absence of distal gas. A catheter is present in the distal rectum. C and D, Consecutive images from a contrast enema demonstrate a microcolon, outlined by introduced contrast and air; the colon is otherwise empty, without evidence of meconium pellets.
e-Figure 103-28 Apple-peel small bowel.
An abdominal radiograph in a child with apple-peel atresia shows dilated loops of bowel to the point of first atresia, with collapsed distal bowel.
e-Figure 103-29 Jejunal stenosis.
A, An abdominal radiograph in an infant girl with bilious gastric aspirates on day 1 of life shows several dilated loops of proximal bowel, with distal gas. B, An abdominal radiograph on day 2 of life in another infant shows several dilated upper abdominal loops with a normal distal bowel gas pattern. C, An upper gastrointestinal examination on the same infant shown in part B confirms dilatation of the proximal jejunum, with some passage of contrast into distal loops beyond the point of stenosis.
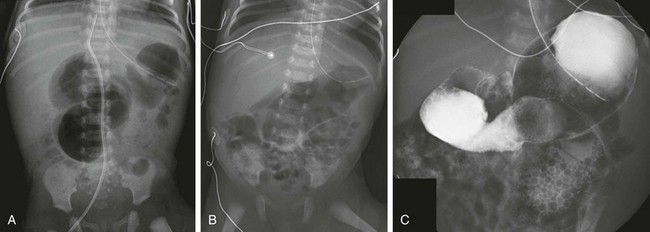
In patients with proximal jejunal atresia, plain films with the aforementioned findings are usually diagnostic. Upper GI examination will demonstrate the dilated stomach, duodenum, and small bowel to the point of first atresia (see Fig. 103-26). In patients with plain film findings of distal obstruction, fluoroscopic diagnosis is accomplished with contrast enema. In patients with distal obstruction, use water-soluble contrast material diluted to a near iso-osmolal concentration (see Chapter 85 for additional information on technique). In patients with ileal atresia, the contrast enema will reveal a very small, unused colon, known as a microcolon, whose lumen has not been distended by swallowed material or succus entericus. The differential diagnosis of microcolon is that of distal small bowel obstruction, ileal atresia, or meconium ileus. The differential overlaps with some cases of long-segment Hirschsprung disease. Meconium pellets typically are absent from the microcolon in patients with ileal atresia (see Fig. 103-27).
Treatment: The treatment and management of patients with jejunal and ileal atresia or stenosis is surgical. Although considerable morbidity still plagues the survivors of these abnormalities,90 the prognosis of these infants has undergone revolutionary improvement. Survival has improved from approximately 1% to 10% in the first half of the twentieth century to 95% currently, related to improvement in surgical techniques, perioperative care, and nutritional support.90
Meconium Ileus
Overview: Meconium ileus is responsible for approximately 20% of neonatal intestinal obstruction and denotes an obstruction in the terminal ileum as a result of inspissated meconium.93 Meconium ileus is the initial presentation in approximately 15% to 20% of patients with cystic fibrosis (CF). Although most patients who present with meconium ileus will be diagnosed with CF, exceptions have been reported, including patients with pancreatic insufficiency and patients with very long segment Hirschsprung disease encompassing the small bowel.94–97 More recent reports suggest that a greater percentage of infants with meconium ileus might not have CF, but the underlying cause of distal small bowel obstruction in such infants is unknown.95,98
Etiology: Patients with CF have a defect in the gene sited in locus q31.2 of chromosome 7, which encodes for the CF transmembrane conductance regulator (CTFR) protein and renders it ineffective in the regulation of chloride transport across cell membranes in lungs, liver, pancreas, skin, digestive, and reproductive tracts. In the fetal gut, abnormal secretory products of the intestinal epithelium result in meconium with an abnormal electrolyte environment and increased concentration of protein (particularly albumin), which interacts with other components of meconium, such as mucopolysaccharides, to form abnormally viscid and tenacious meconium. Degradation of this material is impeded by abnormal concentration of pancreatic enzymes, which also is the result of a defective CTFR protein.99,100 The meconium inspissates within the distal small bowel lumen, causing high-grade small bowel obstruction (Fig. 103-30).
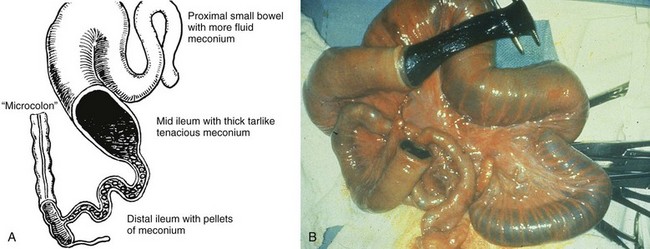
Figure 103-30 Meconium ileus.
A, A schematic drawing of an uncomplicated meconium ileus. Pellets of inspissated meconium fill the terminal ileum proximal to a microcolon. Several loops of more proximal ileum contain thick, tenacious meconium. B, An intraoperative radiograph shows an enterotomy of proximal bowel and the nature of the thick and tenacious meconium. Note the dilated proximal loops of bowel filled with meconium and the progressively small caliber of the distal bowel leading to the microcolon. (A, Leonidas JC, et al. Meconium ileus and its complications. A reappraisal of plain film roentgen diagnostic criteria. Am J Roentgenol Radium Ther Nucl Med. 1970;108(3):598-609. B, Courtesy Dr. Wallace W. Neblett III, Nashville, TN.)
Clinical Presentation: The presentation of infants with meconium ileus is one of distal bowel obstruction, which may be complicated by an in utero event leading to prenatal perforation. Patients with uncomplicated or simple meconium ileus may have been identified prenatally with abdominal distension, dilatation of bowel loops, and hyperechoic bowel.101–103 Approximately 20% of the infants demonstrate polyhydramnios in utero, more frequently in the subgroup with complicated meconium ileus. Postnatally they will present with abdominal distension, failure to pass meconium, and bilious vomiting.
Complicated meconium ileus occurs in approximately 40% to 50% of cases95,104 and results from an in utero event: the weight of the tenacious meconium can lead to a segmental volvulus, which in turn can lead to perforation with meconium peritonitis, resulting in a loculated meconium cyst or a small bowel atresia. The prenatal perforation may seal over in utero or may persist postnatally. By the age of 6 months, prognosis is similar to patients who did not present with meconium ileus.104
Patients with CTFR G542X mutations have been reported to have a higher incidence of meconium ileus than do patients with other mutations, whereas complicated meconium ileus has been reported more frequently in patients homozygous for the classic ΔF508 mutation. Modifier genes located on chromosomes 4q35.1, 8p23.1, 11q25, and 19q13 also have been reported to affect the clinical presentation of infants with CF and the development of meconium ileus.98
Meconium peritonitis is not specific to meconium ileus; it occurs in patients in whom an intrauterine perforation is present for any reason. Occasionally a child is born with intraperitoneal calcifications consistent with meconium peritonitis, in whom the perforation has sealed over, and who is otherwise well, with no definable clinical sequelae (Fig. 103-31, A).
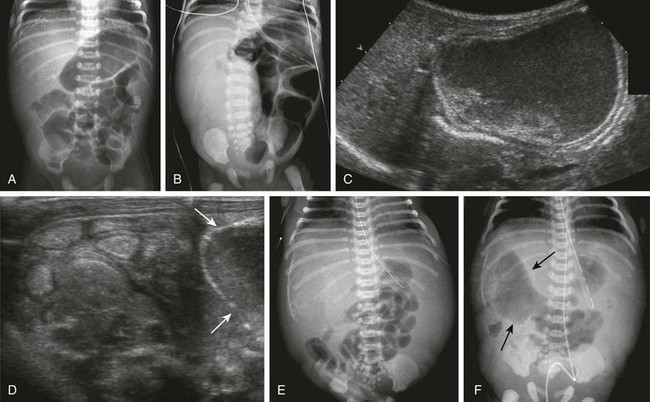
Figure 103-31 Spectrum of meconium peritonitis and meconium cyst.
A, An abdominal radiograph of a 2-day-old infant with extensive intraperitoneal calcifications consistent with in utero perforation and meconium peritonitis. The child did well, without evidence of persistent perforation or obstruction, and after appropriate observation, was discharged home. B, A 2-day-old female infant with abdominal distension and bilious aspirates. Abdominal radiograph shows markedly dilated loops of bowel, absence of bowel gas in the right abdomen, and a partly calcified mass. C, Ultrasound images from the same infant in part B demonstrate the subhepatic, partly calcified mass with internal debris and a fluid-fluid level. D, An additional ultrasound image from the same infant in part B showing a portion of the cyst wall (arrows) and multiple, abnormal, hyperechoic loops of bowel. E, Another infant boy with bilious aspirates and abdominal distension on day 1 of life. Abdominal radiographs demonstrate marked distension of the flanks and localized calcification in the right upper quadrant consistent with a meconium cyst; this diagnosis was confirmed upon subsequent ultrasound imaging. F, An abdominal radiograph on the same patient as shown in part E, obtained a few hours later. The radiograph shows a persistent perforation, with extraluminal air (arrows) extending into the site of the meconium cyst.
Imaging: Radiographs of infants with complicated meconium ileus, in addition to signs of distal bowel obstruction, may show intraperitoneal calcifications consistent with meconium peritonitis and/or mass effect consistent with a meconium cyst (see Fig. 103-31, B to F). Patients with such signs of complicated meconium ileus are not candidates for contrast fluoroscopic evaluation of either the upper or lower GI tracts. However, ultrasound is useful in some infants with complicated meconium ileus to document the location and size of a suspected meconium cyst. Hyperechoic bowel loops, described in the fetus,101–103 also may be seen postnatally (see Fig. 103-31, D).
In infants with no evidence of complicated meconium ileus, abdominal radiographs will show abdominal distension and multiple dilated loops of bowel, consistent with a distal obstruction (Fig. 103-32). In patients with meconium ileus, the ileal loops are packed with meconium, which often renders a characteristic “soap bubble” appearance, originally described by Neuhauser in 1946.105 Air-fluid levels tend to be less conspicuous than in patients with ileal atresia,106 although their presence does not negate the diagnosis.
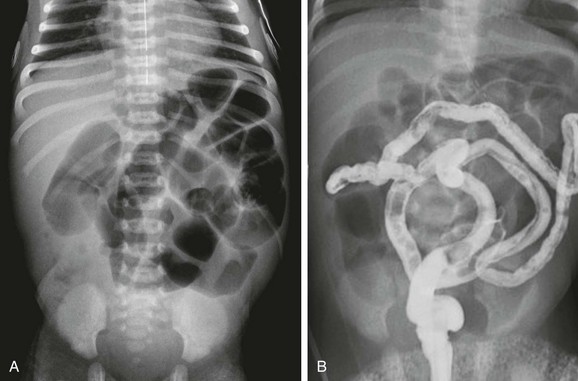
Figure 103-32 A meconium ileus.
A, An abdominal radiograph in a 3-day-old infant with abdominal distension and bilious aspirates shows dilatation of multiple loops of bowel. No calcifications are seen on the radiograph. B, Contrast enema on the same infant as shown in part A demonstrates a microcolon with multiple tiny meconium plugs, consistent with the diagnosis of meconium ileus. Compare to Fig. 103-27, C and D.
Similar to patients with ileal atresia, diagnostic imaging evaluation starts from below with contrast enema. As previously discussed, the diagnostic examination begins with water-soluble contrast material diluted to a near iso-osmolal concentration. Barium is not recommended because barium inspissated within the tarry meconium is unlikely to prove helpful, and it is not necessary for diagnosis. Hyperosmolal material likewise will not be helpful if the diagnosis proves to be Hirschsprung disease (see Chapter 85 for additional information on technique). The examination will reveal a small colon, known as a microcolon, similar to that seen in patients with ileal atresia. However, in patients with meconium ileus, in whom the distal ileum is impacted with tarry, tenacious meconium, small meconium pellets may be seen within the microcolon (see Fig. 103-32, B). If contrast material refluxes into the distal ileum, meconium pellets may be identified; if contrast material reaches into the dilated loops of bowel, ileal atresia is excluded. However, contrast material may not reflux readily into the dilated bowel loops in patients with meconium ileus because of the inability to traverse the segments containing inspissated meconium; in such cases, final diagnosis is made at surgical intervention.
Treatment: Patients with complicated meconium ileus as revealed on clinical evaluation, findings of calcifications on abdominal radiographs or free intraperitoneal air, are managed surgically. An enema procedure has no role in these patients.
The mainstay of treatment for patients with simple meconium ileus initially was surgery, with enterotomy and intraoperative irrigation of the bowel to solubilize, disimpact, and remove the inspissated obstructing meconium.107 Since the initial description by Noblett in 1969, the therapeutic Gastrograffin contrast enema often is the initial treatment in patients with uncomplicated meconium ileus. Its success has been attributed to the hyperosmolarity of the medium (1900 mOsm/L), as well as to the surfactant properties of polysorbate 80 (Tween 80).108 Expected complications related to hyperosmolarity of the agent and subsequent fluid shifts can be managed with careful fluid and electrolyte monitoring and replacement after the enema procedure. Before the procedure, the patient likewise should be prepared for the enema with adequate fluid and electrolyte replacement, and surgical consultation and standby are imperative.
Subsequent investigators have reported problems with bowel necrosis after administration of the Gastrografin enema,41 ascribed to the polysorbate component, which has been found experimentally to cause mucosal damage and necrosis in experiments on animals.109 For these reasons, many pediatric radiologists avoid use of Gastrografin and instead use other moderately hyperosmolar media. A survey of pediatric radiologists in North America, published in 1995, showed a higher success rate when an additive such as N-acetylcysteine (Mucomyst) was used but showed no correlation of success rate with osmolarity of the contrast medium used; the reported perforation rate was 2.75%, with a higher risk of perforation in cases in which balloon occlusion of the rectum was used.110
A therapeutic enema may need to be repeated for definitive resolution of the obstruction. Success may be dependent at least partly on the perseverance of the operator, because the contrast material does not easily advance beyond the obstructing meconium into the dilated loops of bowel. The overall success can vary between 0 and 100% in specific institutions110 but is reported to be between 50% and 65%.104
Meckel Diverticulum
Overview: Meckel diverticulum is named after Johann Friedrich Meckel the younger, who was the first to report extensively on its embryology, anatomy, and clinical manifestations in the early nineteenth century. Meckel diverticulum is found in approximately 1% to 4% of the population and is located within 100 cm from the ileocecal junction.111
Etiology: The Meckel diverticulum is a remnant of the vitelline or omphalomesenteric duct, the communication between the embryonic gut and the yolk sac. This duct normally obliterates by the fifth to seventh week of gestation. Approximately 25% of diverticula may have a persistent attachment to the abdominal wall, considered to represent a remnant of the left omphalomesenteric artery. In addition to a Meckel diverticulum with or without persistent attachment to the abdominal wall, remnants of the omphalomesenteric duct include patency of the duct presenting as an umbilicoileal fistula and fibrous vitelline cords connecting the ileum to the umbilicus, with or without a vitelline cyst along the length of the cord.112,113
Clinical Presentation: Most Meckel diverticula remain asymptomatic, with complication rates estimated at 4% to 6%, decreasing with age.113,114 Although the prevalence of diverticula is similar in both genders, symptoms are more likely to occur in males and in younger patients.113,114 The most common clinical presentation is that of painless rectal bleeding. Meckel diverticulum accounts for approximately 50% of lower GI bleeding in infants and toddlers, typically occurring as a complication of gastric mucosa within the diverticulum. The incidence of gastric mucosa within bleeding Meckel diverticula is estimated at 23% to 80%.112,113 Heterotopic pancreatic tissue is found in 5% to 16% of cases.113 In addition to bleeding, gastric and pancreatic mucosa can lead to inflammation of the diverticulum, with abdominal pain mimicking appendicitis, and which similarly can evolve into perforation and/or obstruction.112,115,116
Meckel diverticulum can present as distal small bowel obstruction through a variety of other mechanisms. The diverticulum may invert into the ileal lumen and serve as a lead point for intussusception. If the diverticulum remains attached to the umbilicus, it can serve as an anchor for a segmental volvulus, or the fibrous cord can promote a bowel obstruction as an adhesion or as a closed loop obstruction. Volvulus also can occur with very large or “giant” diverticula. The Meckel diverticulum can become incarcerated in an inguinal hernia, known as a Littre hernia.113 Patients with obstruction present with abdominal pain and vomiting, which is complicated by bleeding in cases of intussusception.
Meckel diverticula also can present with neoplasia, typically in adult patients; the tumors are most commonly carcinoids, and complications include luminal obstruction and intussusception. Meckel diverticula may contain enteroliths and also may present with parasitic infections, such as ascariasis, and lodged ingested foreign bodies.112
Imaging: Plain films are not particularly helpful in the diagnosis of Meckel diverticula except as indicators of some of its complications, such as obstruction. Fluoroscopic examination such as small bowel follow-through requires very careful technique and may be able to demonstrate the antimesenteric saccular outpouch from the distal ileum, particularly when enteroclysis is performed, or when the diverticula are larger, such as the “giant” diverticula that measure greater than 5 to 6 cm in diameter and up to 15 cm in length.113 However, small diverticula may be inconspicuous and difficult to subtract from the multiple bowel loops in the background.
The most well-known imaging examination for detection of Meckel diverticula is the technetium pertechnetate abdominal scintigram (see Chapter 85).117 This examination is dependent upon the presence of gastric mucosa, with a reported sensitivity of 80% to 90%, a specificity of 95%, and accuracy of 90% in pediatric patients,118 although accuracy may be lower in patients who present with a hemoglobin <11 g/dL.117 Use of single photon emission CT has been reported to identify the Meckel diverticulum in some patients in whom conventional planar imaging is unsuccessful.113 Duplication cysts with gastric mucosa are part of the differential diagnosis of a positive scan but often can be differentiated clinically or with other cross-sectional imaging modalities.
In patients with inflamed diverticula, ultrasound and CT can detect the inflamed diverticulum, which appears as a blind-ending structure, surrounded by inflammation. On cross-sectional imaging the findings may be very difficult to distinguish from appendicitis in some cases; however, in other cases, a much larger size and periumbilical location may suggest the correct diagnosis preoperatively (Fig. 103-33). In patients in whom intussusception is present, the intussuscepted diverticulum is outlined by air during air enema reduction.119 Both ultrasound and CT may identify the intussuscepted, thick-walled, fluid-filled diverticulum within the intussusceptum complex.113,120,121
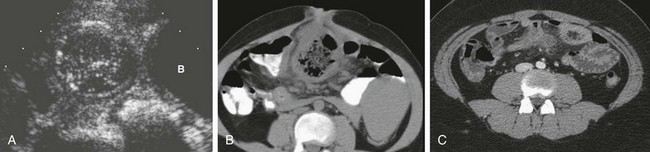
Figure 103-33 Meckel diverticulum.
A, A sagittal ultrasound image along the midline in a 2-year-old girl outlines a round structure with intestinal contents and a gut signature located above the bladder (B) fundus, with surrounding echogenicity; an inflamed Meckel diverticulum was identified at subsequent surgery. B, A computed tomography (CT) image of an adolescent boy with abdominal pain demonstrates a large, inflamed loop of bowel in close association with the umbilicus, representing an inflamed Meckel diverticulum. C, A CT image in another adolescent shows a smaller Meckel diverticulum in the periumbilical region, with associated inflammation demonstrated by fat stranding.
Treatment: The treatment of complicated Meckel diverticula is surgical resection. However, the treatment of diverticula that are asymptomatic and incidentally discovered during surgery for another reason is less clear; the potential for the development of complications needs to be weighed against the potential for postsurgical complications, reported as 1% to 2% for patients with incidental resection, with a mortality rate of 0.001%.112 Some authors advocate a judicious approach to this problem, taking into consideration higher risk factors for complications, such as younger age and male gender.122 Laparoscopic diagnosis and resection of symptomatic diverticula is currently advocated, with some investigators suggesting that laparoscopy can replace the Meckel technetium scan in patients with a high clinical suspicion of Meckel diverticulum as the bleeding source.112,123
Duplication Cysts
Overview: Duplication cysts of the GI tract are characterized by contiguity with and adherence to a segment of the alimentary tract, by a smooth muscle coat and mucosal lining including one or more types of cells found within any portion of the alimentary canal, and by a shared a blood supply with native GI structures.124 They can occur anywhere along the length of the entire GI tract from the mouth to the anus and are most common near the terminal ileum.125 Duplication cysts occur along the mesenteric border of the involved bowel loop, and therefore in the duodenum they are found medial to the descending limb or along its third portion; duodenal duplications account for approximately 6% of the total and thus are uncommon.126 Most duplication cysts do not communicate with the intestinal lumen.
Etiology: The etiology of alimentary tract duplications is not certain, and no one single theory satisfactorily explains all instances of duplication anomalies. One theory holds that duplication cysts are the result of incomplete attempts at twinning, a theory that also suggests an explanation for duplications of other organs, such as the bladder and urethra. The split notochord theory suggests that there is herniation of a diverticulum of the foregut through a gap in the notochord; this theory is helpful in understanding duplications that are associated with anomalies of the spine. A third possibility theorizes that these anomalies are the result of diverticula that form during the recanalization stage of the bowel. A fourth hypothesis suggests that environmental factors, such as trauma or hypoxia, can induce the events that can result in an enteric duplication.126
Clinical Presentation: The presentation of duplication cysts is usually related to their size, thus presenting with mass effect, palpable mass, obstruction, or localized volvulus, or to the type of mucosa in its lining because cysts containing gastric mucosa can ulcerate, bleed, and lead to inflammatory changes. Patients present with abdominal pain and vomiting. Mass effect by duplication cysts can lead to bilious or nonbilious vomiting, or the patients may present with weight loss and failure to thrive. Duodenal duplication cysts can lead to pancreatitis, possibly related to compression of the pancreatic duct,127 and also have been reported to cause fluid accumulation within the lesser sac.128 Duplication cysts may act as a fulcrum for segmental volvulus.125 Those located in the terminal ileum and ileocecal valve may act as a lead point in intussusception and should be suspected when intussusception occurs in infants within the first 3 months of life.
Imaging: Plain radiographs are usually noncontributory but may demonstrate a soft tissue mass or evidence of obstruction if the cyst is large, particularly if it is located along the distal aspect of the stomach or the duodenum.
An upper GI series could be the first study obtained in a child presenting with vomiting; in cases of duplication cysts of the duodenum, the examination reveals a filling defect along the mesenteric wall of the duodenum or extrinsic compression (Fig. 103-34). Compression of the gastric outlet or of the first portion of the duodenum by the cyst has been described as a “beak” sign.129 If focal communication occurs between the cyst and the duodenum, contrast material will enter the duplication.
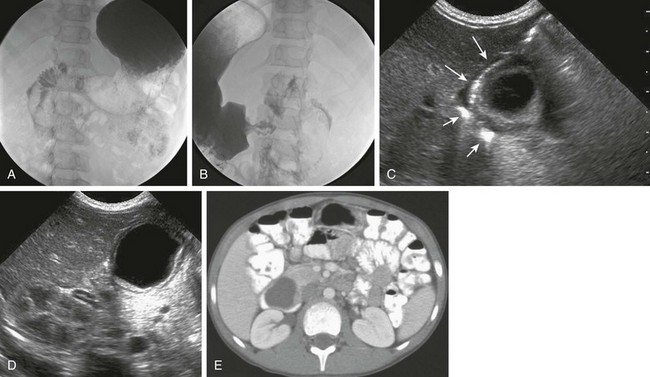
Figure 103-34 A duodenal duplication cyst.
A and B, Two images from an upper gastrointestinal examination in an 11-year-old boy demonstrate intramural mass effect upon the mesenteric border of the duodenum. C and D, A 1-day-old infant with a prenatal diagnosis of an abdominal cyst resected at 2 weeks of age. C, The sonogram shows the cyst to be intimately related to the distal antrum (long arrows) and the descending duodenum (short arrows). D, An additional image shows the typical double layer of a duplication cyst, indicative of gut signature. E, A computed tomography image on the same patient as in parts A and B illustrates clearly the location of the mass along the medial border of the duodenum.
Small bowel duplication cysts beyond the duodenum may present with obstruction, inflammation, intussusception, or a combination (e-Fig. 103-35; also see Chapter 108). Cross-sectional imaging can confirm the diagnosis of a duplication cyst suspected from a prior study, or it may be the initial imaging modality used. Ultrasound typically reveals an anechoic cyst, but the fluid may contain debris if there has been infection or hemorrhage from associated ectopic gastric mucosa and ulceration. The typical “bowel wall signature” of an enteric duplication cyst is usually present.130–132 The presence of bowel wall signature may help to differentiate a duodenal duplication cyst from a pancreatic pseudocyst. However, the gut signature is not completely sensitive or specific to duplication cysts; the gut signature may be obliterated by superimposed inflammatory changes and may be seen in other conditions, such as mesenteric cyst, cystic teratoma,133 and ovarian cysts.
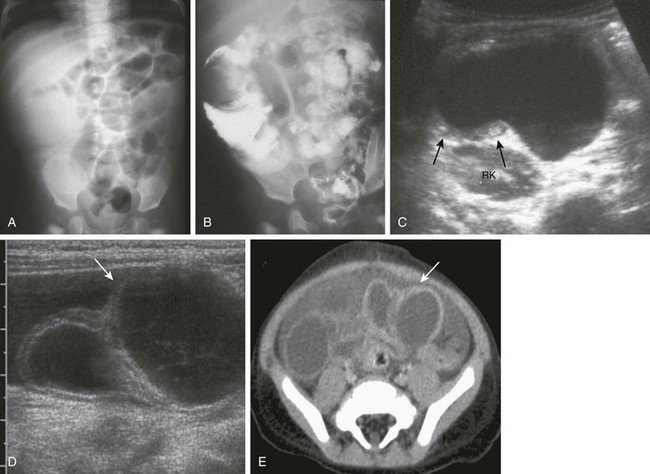
e-Figure 103-35 Ileal duplication cysts.
A, A plain abdominal radiograph of a 4-month-old infant presenting with vomiting demonstrates dilated loops of small bowel and mass effect along the right flank. B, An upper gastrointestinal examination performed at an outside institution shows mass effect upon the ascending colon that was mistaken for an intussusception, and the child was referred for further evaluation and reduction. C, A sonogram demonstrates a large cystic mass in the right flank, with mass effect upon the adjacent, empty colon (arrows). At surgery, a duplication cyst attached to the terminal ileum was identified and resected. RK, right kidney. D, An ultrasound image of the lower abdomen in a 3-year-old girl presenting with vomiting, abdominal pain, and abdominal distension reveals a duplication cyst (arrow) containing echogenic debris, sharing a common wall with the distal ileum. E, A computed tomography scan with intravenous contrast material of the same patient in part D demonstrates enhancement of both the duplication cyst (arrow) and the adjacent ileal loop with which it shares a common wall. Ascites and findings of bowel obstruction with dilated loops of proximal bowel are seen. At surgery, an ileal duplication cyst was resected.
CT demonstrates a discrete fluid-filled cyst on the mesenteric border of the involved bowel segment, often with contrast enhancement of the cyst wall. On MRI, hemorrhage or infection can alter the signal characteristics of the cyst on water-sensitive sequences. MRI is particularly helpful in cases of duodenal duplication cyst, where MR cholangiopancreatography can demonstrate the relationship of the cyst to the duodenal wall, the pancreatic head, and the pancreatic and biliary ductal systems, which is important preoperative information for the surgeon.126
Lymphatic Malformation, Mesenteric, and Omental Cysts
Overview: Abdominal lymphatic malformations can be located in the mesentery or omentum as mesenteric or omental cysts. Lymphatic malformations have only an endothelial lining, are usually multiloculated, and may contain chyle. Most lymphatic malformations occur in the mesentery rather than the omentum because of its richer lymphatic content, most commonly in the ileal mesentery.134,135 Such cysts of lymphatic origin are lined by flat endothelial cells and contain lymphoid tissue within their walls.
Mesothelial cysts, on the other hand, occur in the small bowel, the mesentery, and the mesocolon and are the result of accumulation of fluid between mesothelial layers. They are lined by mesothelium, without mural muscle fibers or lymphatic structures.136 Nonpancreatic pseudocysts also may occur in the mesentery, or more often in the omentum, and are believed to result from prior trauma or infection, with no inner cellular lining on histology.
Etiology: The etiology of lymphatic malformations and lymphatic mesenteric and omental cysts is not known; hypotheses include failure of embryonic lymphatic spaces to develop normal connections and drainage into the venous system, and benign proliferation of ectopic lymphatics sequestered from the venous system.134
Clinical Presentation: Abdominal lymphatic malformations are rare, representing approximately 5% of all such malformations, and with a quoted prevalence of approximately 1 per 20,000 admissions at a children’s hospital, with a lower incidence in African Americans.134,137 In children, presentation is more common in boys, although in adults the presentation is more common in women, through a putative estrogenic effect.138 These lesions may be asymptomatic and discovered incidentally, or they may present as a result of size or complications of the lesion. Mass effect can result in the development of partial obstruction over time or acutely as a result of acute enlargement of the cyst secondary to hemorrhage. Abdominal pain can occur through torsion of the cyst or one of its components (Fig. 103-36) or torsion of adjacent bowel loops with the cyst acting as a fulcrum. Cysts can become infected, producing fever, abdominal pain, or sepsis.134,135
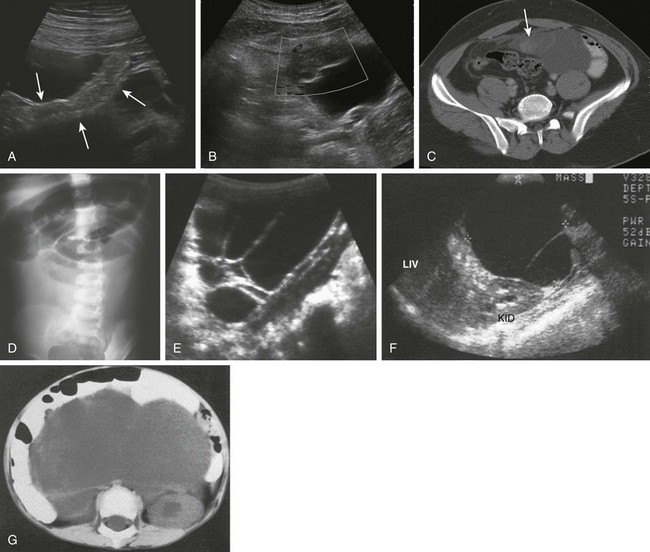
Figure 103-36 Mesenteric lymphatic malformations.
A, A 14-year-old boy presented with abdominal pain and dysuria. An ultrasound image of the left midabdomen reveals a multiseptated cystic mass with an intervening dumbbell loop of small bowel (arrows). B, A color Doppler image obtained more inferiorly in the supravesical area shows an avascular solid-appearing component to the cystic lesions, with surrounding hyperechogenicity consistent with inflammation and edema. C, A computed tomography (CT) an shows the solid-appearing component (arrow) corresponding to the avascular structure noted in part B and surrounding inflammation with fat stranding in the supravesical area. At surgery, a loculated mesenteric cyst was removed, with volvulus and infarction of a supravesical component, leading to the presenting symptoms and imaging findings. D, An abdominal radiograph from a different patient presenting with vomiting with clinical concern for intussusception. The radiograph demonstrates dilated loops of bowel, displaced into the upper abdomen. E, A longitudinal sonogram along the right flank in the same patient as shown in part D demonstrates a multiseptated cystic mass occupying the lower portion of the abdomen, representing a cystic lymphangioma. F, A longitudinal ultrasound scan through the upper abdomen in another patient shows a septated, hypoechoic mass abutting the liver (LIV) and right kidney (KID). G, A CT scan of shows the same patient as shown in part F confirms the massive fluid-filled structure displacing contrast-filled loops of bowel.
Imaging: Plain radiographs may show a large mass displacing intestinal loops. Dilated loops may be seen in patients with obstruction. Upper GI contrast studies show displacement of bowel with no communication. Ultrasound demonstrates the multilocular nature of the mass with fine septations (see Fig. 103-36). Most of the mass is anechoic, but some of the loculated spaces may have internal echogenicity, depending on the chyle or blood content.139 On CT the attenuation values of the fluid range from near fat to near water, depending on its composition. On CT, septa and loculations are usually demonstrated, unless their attenuation value merges with that of the surrounding fluid in scans performed without contrast material; septa may enhance with injection of intravenous contrast material. Fine calcifications may be present in some cases.134 MRI of lymphatic malformations shows characteristics of the cyst ranging from fluid with low-intensity signal on T1-weighted images to fat with high-intensity signal.140
Treatment: Traditional treatment is surgical resection with complete extirpation of the cyst, although percutaneous drainage and sclerotherapy is a reasonable alternative in some cases. In children with mesenteric cysts, treatment is more likely to require segmental bowel resection than in adults (50% to 60% vs. 33%).134 If complete resection is not possible, marsupialization of the cyst with sclerosis of the lining can be performed. A surgical classification of mesenteric cysts, based on operative management, includes four types: type 1 is on a pedicle, and easily removed, although more likely to undergo torsion. Type 2 is sessile and restricted to the mesenteric boundaries, whereas type 3 has retroperitoneal extension, representing an increased surgical challenge. Type 4 refers to multicentric cysts.137
Laparoscopic resection is possible; however, this technique is best approached with caution because of relatively limited experience, if the diagnosis is not certain preoperatively, or if the procedure will necessitate segmental bowel resection.141–143
Applegate, KE, Anderson, JM, Klatte, EC. Intestinal malrotation in children: problem-solving approach to the upper gastrointestinal series. Radiographics. 2006;26(5):1485–1500.
Cheng, G, et al. Sonographic pitfalls in the diagnosis of enteric duplication cysts. AJR Am J Roentgenol. 2005;184(2):521–525.
Gorter, RR, et al. Clinical and genetic characteristics of meconium ileus in newborns with and without cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;50(5):569–572.
Lampl, B, et al. Malrotation and midgut volvulus: a historical review and current controversies in diagnosis and management. Pediatr Radiol. 2009;39(4):359–366.
Losanoff, JE, et al. Mesenteric cystic lymphangioma. J Am Coll Surg. 2003;196(4):598–603.
Sagar, J, Kumar, V, Shah, DK. Meckel’s diverticulum: a systematic review. J R Soc Med. 2006;99(10):501–505.
Stollman, TH, et al. Decreased mortality but increased morbidity in neonates with jejunoileal atresia; a study of 114 cases over a 34-year period. J Pediatr Surg. 2009;44(1):217–221.
Strouse, PJ. Disorders of intestinal rotation and fixation (“malrotation”). Pediatr Radiol. 2004;34(11):837–851.
Yousefzadeh, DK. The position of the duodenojejunal junction: the wrong horse to bet on in diagnosing or excluding malrotation. Pediatr Radiol. 2009;39(suppl 2):S172–S177.
References
1. Cragan, JD, et al. Descriptive epidemiology of small intestinal atresia, Atlanta, Georgia. Teratology. 1993;48(5):441–450.
2. Magnuson, D, Schwartz, M. Stomach and duodenum. In: Oldham K, et al, eds. Principles and practice of pediatric surgery. Philadelphia: Lippincott Williams & Wilkins, 2005.
3. Applebaum, H, Sydorak, R. Duodenal atresia and stenosis—annular pancreas. In: Coran A, et al, eds. Coran: pediatric surgery. Philadelphia: Mosby, 2012.
4. Bailey, PV, et al. Congenital duodenal obstruction: a 32-year review. J Pediatr Surg. 1993;28(1):92–95.
5. Dalla Vecchia, LK, et al. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg. 1998;133(5):490–496. [discussion 496-497].
6. Fonkalsrud, EW, DeLorimier, AA, Hays, DM. Congenital atresia and stenosis of the duodenum. A review compiled from the members of the Surgical Section of the American Academy of Pediatrics. Pediatrics. 1969;43(1):79–83.
7. Tashjian, DB, Moriarty, KP. Duodenal atresia with an anomalous common bile duct masquerading as a midgut volvulus. J Pediatr Surg. 2001;36(6):956–957.
8. Lundstedt, C, Lyttkens, K, Andren, S. Intraluminal duodenal diverticulum causing pancreatitis in a patient with a polysplenia syndrome. Eur Radiol. 1998;8(3):454–457.
9. Tasu, JP, et al. Intraluminal duodenal diverticulum: radiological and endoscopic ultrasonography findings of an unusual cause of acute pancreatitis. Eur Radiol. 1999;9(9):1898–1900.
10. Kassner, EG, et al. Retention of small foreign objects in the stomach and duodenum. A sign of partial obstruction caused by duodenal anomalies. Radiology. 1975;114(3):683–686.
11. Fujiwara, T, et al. Intraluminal duodenal diverticulum in a child: incidental onset possibly associated with the ingestion of a foreign body. Eur J Pediatr. 1999;158(2):108–110.
12. Freeman, SB, et al. Congenital gastrointestinal defects in Down syndrome: a report from the Atlanta and National Down Syndrome Projects. Clin Genet. 2009;75(2):180–184.
13. Kimble, RM, Harding, J, Kolbe, A. Additional congenital anomalies in babies with gut atresia or stenosis: when to investigate, and which investigation. Pediatr Surg Int. 1997;12(8):565–570.
14. Sekmenli, T, et al. Duodenal atresia and Hirschsprung disease in a patient with Down syndrome. Eur J Gen Med. 2011;8(2):157–159.
15. Ein, SH, Palder, SB, Filler, RM. Babies with esophageal and duodenal atresia: a 30-year review of a multifaceted problem. J Pediatr Surg. 2006;41(3):530–532.
16. Kim, JH, et al. Heterotaxia syndrome with intraluminal duodenal diverticulum. J Korean Surg Soc. 2010;79(1):75–78.
17. Rathaus, V, et al. The bubble sign in the gasless abdomen of the newborn. Pediatr Radiol. 1992;22(2):106–109.
18. Traubici, J. The double bubble sign. Radiology. 2001;220(2):463–464.
19. Knechtle, SJ, Filston, HC. Anomalous biliary ducts associated with duodenal atresia. J Pediatr Surg. 1990;25(12):1266–1269.
20. Berdon, WE, et al. Midgut malrotation and volvulus. Which films are most helpful? Radiology. 1970;96(2):375–384.
21. Applegate, KE, Anderson, JM, Klatte, EC. Intestinal malrotation in children: a problem-solving approach to the upper gastrointestinal series. Radiographics. 2006;26(5):1485–1500.
22. Yousefzadeh, DK. The position of the duodenojejunal junction: the wrong horse to bet on in diagnosing or excluding malrotation. Pediatr Radiol. 2009;39(suppl 2):S172–S177.
23. Ladd, W. Congenital obstruction of the duodenum in children. N Engl J Med. 1932;206(6):277–283.
24. Kay, S, Yoder, S, Rothenberg, S. Laparoscopic duodenoduodenostomy in the neonate. J Pediatr Surg. 2009;44(5):906–908.
25. Kimura, K, et al. Diamond-shaped anastomosis for duodenal atresia: an experience with 44 patients over 15 years. J Pediatr Surg. 1990;25(9):977–979.
26. Spilde, TL, et al. Open vs laparoscopic repair of congenital duodenal obstructions: a concurrent series. J Pediatr Surg. 2008;43(6):1002–1005.
27. Sandrasegaran, K, et al. Annular pancreas in adults. AJR Am J Roentgenol. 2009;193(2):455–460.
28. Zyromski, NJ, et al. Annular pancreas: dramatic differences between children and adults. J Am Coll Surg. 2008;206(5):1019–1025. [discussion 1025-1027].
29. Lainakis, N, et al. Annular pancreas in two consecutive siblings: an extremely rare case. Eur J Pediatr Surg. 2005;15(5):364–368.
30. Kamisawa, T, et al. A new embryologic hypothesis of annular pancreas. Hepatogastroenterology. 2001;48(37):277–278.
31. Shan, YS, Sy, ED, Lin, PW. Annular pancreas with obstructive jaundice: beware of underlying neoplasm. Pancreas. 2002;25(3):314–316.
32. Ruben, GD, Templeton, JM, Jr., Ziegler, MM. Situs inversus: the complex inducing neonatal intestinal obstruction. J Pediatr Surg. 1983;18(6):751–756.
33. Torres, AM, Ziegler, MM. Malrotation of the intestine. World J Surg. 1993;17(3):326–331.
34. Dassinger, M, Smith, S. Disorders of intestinal rotation and fixation. In: Coran A, et al, eds. Coran: pediatric surgery. Philadelphia: Mosby, 2012.
35. Lampl, B, et al. Malrotation and midgut volvulus: a historical review and current controversies in diagnosis and management. Pediatr Radiol. 2009;39(4):359–366.
36. Sadler, T. Digestive system. In: Langman’s medical embryology. Philadelphia: Williams & Wilkins; 2010.
37. Strouse, PJ. Disorders of intestinal rotation and fixation (“malrotation”). Pediatr Radiol. 2004;34(11):837–851.
38. Filston, HC, Kirks, DR. Malrotation—the ubiquitous anomaly. J Pediatr Surg. 1981;16(4 suppl 1):614–620.
39. DePrima, SJ, Hardy, DC, Brant, WE. Reversed intestinal rotation. Radiology. 1985;157(3):603–604.
40. Estrada, RL, Gurd, FN. Surgical correction of reversed rotation of the midgut loop. Surg Gynecol Obstet. 1962;114:707–717.
41. Leonidas, JC, et al. Possible adverse effect of methylglucamine diatrizoate compounds on the bowel of newborn infants with meconium ileus. Radiology. 1976;121(3 pt. 1):693–696.
42. Seashore, JH, et al. Familial apple peel jejunal atresia: surgical, genetic, and radiographic aspects. Pediatrics. 1987;80(4):540–544.
43. Potts, SR, et al. The duodenal triangle: a plain film sign of midgut malrotation and volvulus in the neonate. Clin Radiol. 1985;36(1):47–49.
44. Stewart, D, Colodny, A. Malrotation of the bowel in infants and children: a 15 year review. Surgery. 1976;79(6):716–720.
45. Imamoglu, M, et al. Rare clinical presentation mode of intestinal malrotation after neonatal period: malabsorption-like symptoms due to chronic midgut volvulus. Pediatr Int. 2004;46(2):167–170.
46. Zellos, A, et al. Malrotation of the intestine and chronic volvulus as a cause of protein-losing enteropathy in infancy. Pediatrics. 2012;129(2):e515–e518.
47. Spigland, N, Brandt, ML, Yazbeck, S. Malrotation presenting beyond the neonatal period. J Pediatr Surg. 1990;25(11):1139–1142.
48. Berdon, WE. The diagnosis of malrotation and volvulus in the older child and adult: a trap for radiologists. Pediatr Radiol. 1995;25(2):101–103.
49. Berdon, WE, et al. Megacystis-microcolon-intestinal hypoperistalsis syndrome: a new cause of intestinal obstruction in the newborn. Report of radiologic findings in five newborn girls. AJR Am J Roentgenol. 1976;126(5):957–964.
50. Torfs, CP, Christianson, RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. 1998;77(5):431–438.
51. Applegate, KE, et al. Situs revisited: imaging of the heterotaxy syndrome. Radiographics. 1999;19(4):837–852. [discussion 853-854].
52. Chang, J, Brueckner, M, Touloukian, RJ. Intestinal rotation and fixation abnormalities in heterotaxia: early detection and management. J Pediatr Surg. 1993;28(10):1281–1284. [discussion 1285].
53. Hernanz-Schulman, M, et al. Current evaluation of the patient with abnormal visceroatrial situs. AJR Am J Roentgenol. 1990;154(4):797–802.
54. Koplewitz, BZ, Daneman, A. The lateral view: a useful adjunct in the diagnosis of malrotation. Pediatr Radiol. 1999;29(2):144–145.
55. Long, FR, et al. Intestinal malrotation in children: tutorial on radiographic diagnosis in difficult cases. Radiology. 1996;198(3):775–780.
56. Kiesewetter, W, Smith, J. Malrotation of the midgut in infancy and childhood. AMA Arch Surg. 1958;77:483–491.
57. Slovis, TL, Klein, MD, Watts, FB. Incomplete rotation of the intestine with a normal cecal position. Surgery. 1987;87:325–330.
58. Katz, ME, et al. The position and mobility of the duodenum in children. AJR Am J Roentgenol. 1987;148(5):947–951.
59. Sizemore, AW, et al. Diagnostic performance of the upper gastrointestinal series in the evaluation of children with clinically suspected malrotation. Pediatr Radiol. 2008;38(5):518–528.
60. Dilley, AV, et al. The radiologist says malrotation: does the surgeon operate? Pediatr Surg Int. 2000;16(1-2):45–49.
61. Benya, EC, et al. Duodenum and duodenal-jejunal junction in children: position and appearance after liver transplantation. Radiology. 1998;207(1):233–236.
62. Taylor, GA, Teele, RL. Chronic intestinal obstruction mimicking malrotation in children. Pediatr Radiol. 1985;15(6):392–394.
63. Weinberger, E, et al. Sonographic diagnosis of intestinal malrotation in infants: importance of the relative positions of the superior mesenteric vein and artery. AJR Am J Roentgenol. 1992;159(4):825–828.
64. Zerin, JM, DiPietro, MA. Superior mesenteric vascular anatomy at US in patients with surgically proved malrotation of the midgut. Radiology. 1992;183(3):693–694.
65. Pracros, JP, et al. Ultrasound diagnosis of midgut volvulus: the “whirlpool” sign. Pediatr Radiol. 1992;22(1):18–20.
66. Patino, MO, Munden, MM. Utility of the sonographic whirlpool sign in diagnosing midgut volvulus in patients with atypical clinical presentations. J Ultrasound Med. 2004;23(3):397–401.
67. Chao, HC, et al. Sonographic features related to volvulus in neonatal intestinal malrotation. J Ultrasound Med. 2000;19(6):371–376.
68. Ladd, W. Surgical diseases of the alimentary tract. N Engl J Med. 1936;216(16):705–708.
69. Georgeson, KE, Robertson, DJ. Minimally invasive surgery in the neonate: review of current evidence. Semin Perinatol. 2004;28(3):212–220.
70. Kuebler, JF, Ure, BM. Minimally invasive surgery in the neonate. Semin Fetal Neonatal Med. 2011;16(3):151–156.
71. Chang, R, Andreoli, S, Ziegler, MM. Malrotation. In: Johnson L, ed. Encyclopedia of gastroenterology. Waltham, MA: Academic Press, 2004.
72. McVay, MR, et al. Jack Barney Award. The changing spectrum of intestinal malrotation: diagnosis and management. Am J Surg. 2007;194(6):712–717. [discussion 718-719].
73. Knight, HO. An anomalous portal vein with its surgical dangers. Ann Surg. 1921;74(6):697–699.
74. Bower, RJ, Ternberg, JL. Preduodenal portal vein. J Pediatr Surg. 1972;7(5):579–584.
75. De Wailly, P, et al. Pre-duodenal portal vein in polysplenia syndrome: clinical effects and surgical application. Surg Radiol Anat. 2011;33(5):451–454.
76. Esscher, T. Preduodenal portal vein—a cause of intestinal obstruction? J Pediatr Surg. 1980;15(5):609–612.
77. Mordehai, J, et al. Preduodenal portal vein causing duodenal obstruction associated with situs inversus, intestinal malrotation, and polysplenia: a case report. J Pediatr Surg. 2002;37(4):E5.
78. Ohno, K, et al. Evaluation of the portal vein after duodenoduodenostomy for congenital duodenal stenosis associated with the preduodenal superior mesenteric vein, situs inversus, polysplenia, and malrotation. J Pediatr Surg. 2007;42(2):436–439.
79. Bhorat, N, Thomson, SR, Anderson, F. Preduodenal portal vein: a potential laparoscopic cholecystectomy nightmare. S Afr J Surg. 2009;47(1):17–18.
80. Abramson, SJ, et al. Biliary atresia and noncardiac polysplenic syndrome: US and surgical considerations. Radiology. 1987;163(2):377–379.
81. McCarten, K, Littlewood Teele, R. Preduodenal portal vein: venography, ultrasonography and review of the literature. Ann Radiol. 1978;21:155–160.
82. Tsuda, Y, et al. Preduodenal portal vein and anomalous continuation of inferior vena cava: CT findings. J Comput Assist Tomogr. 1991;15(4):585–588.
83. Fernandes, ET, et al. Preduodenal portal vein: surgery and radiographic appearance. J Pediatr Surg. 1990;25(12):1270–1272.
84. Grosfeld, JL, Ballantine, TV, Shoemaker, R. Operative management of intestinal atresia and stenosis based on pathologic findings. J Pediatr Surg. 1979;14(3):368–375.
85. Frischer, J, Azizkhan, R. Jejunoileal atresia and stenosis. In: Coran A, et al, eds. Coran: pediatric surgery. Philadelphia: Mosby, 2012.
86. Martin, L, Zerella, J. Jejuno-ileal atresia: a proposed classification. J Pediatr Surg. 1976;11:399–403.
87. Dickson, JA. Apple peel small bowel: an uncommon variant of duodenal and jejunal atresia. J Pediatr Surg. 1970;5(6):595–600.
88. Louw, J, Barnard, C. Congenital intestinal atresia observations on its origin. Lancet. 1955;266(6899):1065–1067.
89. Santulli, TV, Blanc, WA. Congenital atresia of the intestine: pathogenesis and treatment. Ann Surg. 1961;154:939–948.
90. Stollman, TH, et al. Decreased mortality but increased morbidity in neonates with jejunoileal atresia; a study of 114 cases over a 34-year period. J Pediatr Surg. 2009;44(1):217–221.
91. Harris, J, Kallen, B, Robert, E. Descriptive epidemiology of alimentary tract atresia. Teratology. 1995;52(1):15–29.
92. Leonidas, JC, et al. Meconium ileus and its complications. A reappraisal of plain film roentgen diagnostic criteria. Am J Roentgenol Radium Ther Nucl Med. 1970;108(3):598–609.
93. DeLorimier, AA, Fonkalsrud, EW, Hays, DM. Congenital atresia and stenosis of the jejunum and ileum. Surgery. 1969;65(5):819–827.
94. Shigemoto, H, et al. Neonatal meconium obstruction in the ileum without mucoviscidosis. J Pediatr Surg. 1978;13(6):475–479.
95. Fakhoury, K, et al. Meconium ileus in the absence of cystic fibrosis. Arch Dis Child. 1992;67(10 spec No.):1204–1206.
96. Tal, A, et al. Familial meconium ileus with normal sweat electrolytes. Clin Pediatr (Phila). 1985;24(8):460–462.
97. Stringer, MD, et al. Meconium ileus due to extensive intestinal aganglionosis. J Pediatr Surg. 1994;29(4):501–503.
98. Gorter, RR, et al. Clinical and genetic characteristics of meconium ileus in newborns with and without cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;50(5):569–572.
99. Hernanz-Schulman, M. CT as an outcome surrogate in patients with cystic fibrosis: does the effort justify the risks? Radiology. 2012;262(3):746–749.
100. Ziegler, MM. Meconium ileus. In: Coran A, et al, eds. Coran: pediatric surgery. Philadelphia: Mosby, 2012.
101. Benacerraf, BR, Chaudhury, AK. Echogenic fetal bowel in the third trimester associated with meconium ileus secondary to cystic fibrosis. A case report. J Reprod Med. 1989;34(4):299–300.
102. De Oronzo, MA. Hyperechogenic fetal bowel: an ultrasonographic marker for adverse fetal and neonatal outcome? J Prenat Med. 2011;5(1):9–13.
103. Yaron, Y, et al. Evaluation of fetal echogenic bowel in the second trimester. Fetal Diagn Ther. 1999;14(3):176–180.
104. Murshed, R, et al. Meconium ileus: a ten-year review of thirty-six patients. Eur J Pediatr Surg. 1997;7(5):275–277.
105. Neuhauser, EB. Roentgen changes associated with pancreatic insufficiency in early life. Radiology. 1946;46:319–328.
106. White, H. Meconium ileus: a new roentgen sign. Radiology. 1956;66(4):567–571.
107. Donnison, AB, Shwachman, H, Gross, RE. A review of 164 children with meconium ileus seen at the Children’s Hospital Medical Center, Boston. Pediatrics. 1966;37(5):833–850.
108. Noblett, HR. Treatment of uncomplicated meconium ileus by Gastrografin enema: a preliminary report. J Pediatr Surg. 1969;4(2):190–197.
109. Lutzger, LG, Factor, SM. Effects of some water-soluble contrast media on the colonic mucosa. Radiology. 1976;118(3):545–548.
110. Kao, SC, Franken, EA, Jr. Nonoperative treatment of simple meconium ileus: a survey of the Society for Pediatric Radiology. Pediatr Radiol. 1995;25(2):97–100.
111. Matsagas, MI, et al. Incidence, complications, and management of Meckel’s diverticulum. Arch Surg. 1995;130(2):143–146.
112. Snyder, C. Meckel diverticulum. In: Coran A, et al, eds. Coran: pediatric surgery. Philadelphia: Mosby, 2012.
113. Levy, AD, Hobbs, CM. From the archives of the AFIP. Meckel diverticulum: radiologic features with pathologic correlation. Radiographics. 2004;24(2):565–587.
114. Bani-Hani, KE, Shatnawi, NJ. Meckel’s diverticulum: comparison of incidental and symptomatic cases. World J Surg. 2004;28(9):917–920.
115. Sung, T, Callahan, MJ, Taylor, GA. Clinical and imaging mimickers of acute appendicitis in the pediatric population. AJR Am J Roentgenol. 2006;186(1):67–74.
116. Baldisserotto, M, Maffazzoni, DR, Dora, MD. Sonographic findings of Meckel’s diverticulitis in children. AJR Am J Roentgenol. 2003;180(2):425–428.
117. Swaniker, F, Soldes, O, Hirschl, RB. The utility of technetium 99m pertechnetate scintigraphy in the evaluation of patients with Meckel’s diverticulum. J Pediatr Surg. 1999;34(5):760–764. [discussion 765].
118. Sagar, J, Kumar, V, Shah, DK. Meckel’s diverticulum: a systematic review. J R Soc Med. 2006;99(10):501–505.
119. Kim, G, et al. The appearance of inverted Meckel diverticulum with intussusception on air enema. Pediatr Radiol. 1997;27(8):647–650.
120. Daneman, A, et al. Sonographic appearances of inverted Meckel diverticulum with intussusception. Pediatr Radiol. 1997;27(4):295–298.
121. Bennett, GL, Birnbaum, BA, Balthazar, EJ. CT of Meckel’s diverticulitis in 11 patients. AJR Am J Roentgenol. 2004;182(3):625–629.
122. Sai Prasad, TR, et al. Meckel’s diverticular complications in children: is laparoscopy the order of the day? Pediatr Surg Int. 2007;23(2):141–147.
123. Chan, KW, et al. Laparoscopic management of complicated Meckel’s diverticulum in children: a 10-year review. Surg Endosc. 2008;22(6):1509–1512.
124. Ladd, W. Duplications of the alimentary tract. Southern Med J. 1937;30(4):363–371.
125. Puligandla, PS, et al. Gastrointestinal duplications. J Pediatr Surg. 2003;38(5):740–744.
126. Lund, D. Alimentary tract duplications. In: Coran A, et al, eds. Coran: pediatric surgery. Philadelphia: Mosby, 2012.
127. Keller, MS, et al. Duodenal duplication cysts: A rare cause of acute pancreatitis in children. Surgery. 2001;130(1):112–115.
128. Cauchi, JA, Buick, RG. Duodenal duplication cyst: beware of the lesser sac collection. Pediatr Surg Int. 2006;22(5):456–458.
129. Blake, NS. Beak sign in duodenal duplication cyst. Pediatr Radiol. 1984;14(4):232–233.
130. Barr, LL, et al. Enteric duplication cysts in children: are their ultrasonographic wall characteristics diagnostic? Pediatr Radiol. 1990;20(5):326–328.
131. Segal, SR, et al. Ultrasonographic features of gastrointestinal duplications. J Ultrasound Med. 1994;13(11):863–870.
132. Narlawar, RS, et al. Sonographic findings in a duodenal duplication cyst. J Clin Ultrasound. 2002;30(9):566–568.
133. Cheng, G, et al. Sonographic pitfalls in the diagnosis of enteric duplication cysts. AJR Am J Roentgenol. 2005;184(2):521–525.
134. Ricketts, R. Mesenteric and omental cysts. In: Coran A, et al, eds. Coran: pediatric surgery. Philadelphia: Mosby, 2012.
135. Tan, JJ, Tan, KK, Chew, SP. Mesenteric cysts: an institution experience over 14 years and review of literature. World J Surg. 2009;33(9):1961–1965.
136. de Perrot, M, et al. Mesenteric cysts. Toward less confusion? Dig Surg. 2000;17(4):323–328.
137. Losanoff, JE, et al. Mesenteric cystic lymphangioma. J Am Coll Surg. 2003;196(4):598–603.
138. Goh, BK, et al. Intra-abdominal and retroperitoneal lymphangiomas in pediatric and adult patients. World J Surg. 2005;29(7):837–840.
139. Khong, PL, et al. Ultrasonography of intra-abdominal cystic lesions in the newborn. Clin Radiol. 2003;58(6):449–454.
140. Levy, AD, Cantisani, V, Miettinen, M. Abdominal lymphangiomas: imaging features with pathologic correlation. AJR Am J Roentgenol. 2004;182(6):1485–1491.
141. Conzo, G, et al. Laparoscopic treatment of an omental cyst: a case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2005;15(1):33–35.
142. Trompetas, V, Varsamidakis, N. Laparoscopic management of mesenteric cysts. Surg Endosc. 2003;17(12):2036.
143. Jain, V, et al. A case of laparoscopic mesenteric cyst excision. Case Rep Surg. 2012;2012:594095.