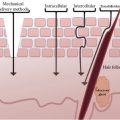
FIGURE 10.1 Principle of measurement of CRS. (a) Monochromatic laser light is focused in the SC in a volume of about 5 μm length (axial resolution) and 1 μm width (dimension of the laser spot). Photons interact with the molecules, releasing some of their energy. Of the photons that exit the skin, only the ones coming from the focus region are detected thanks to the presence of a confocal pinhole. (b) The photons which underwent frequency shifts due to the release of energy to molecules during interaction are used to obtain Raman spectra. The position and the intensity of each peak are representative of the different molecules and their amounts, respectively. Raman spectra can be obtained in a low-(fingerprint) as well as high-energetic region; each region contains different information about the molecular composition of the SC. (c) By focusing the laser light at different depths, concentration profiles of molecules are obtained. The minimum measurement time for acquiring a spectrum ranges from 1 to 3 s according to the energetic region. (Modified from Falcone et al. (12).)
Technical Implementations
The first commercially available confocal Raman instrumentation for in vivo measurements on human subjects was introduced on the market in 2004 (model 3510 skin composition analyzer, RiverD International B.V., the Netherlands) (13). The device consists of an inverted microscope coupled to a Raman microspectrometer assembled on a top-table configuration (10). Laser light is transmitted from the source to the sample through a flat fused silica window. The skin of the volunteer has to be placed in contact with the window, and laser light is focused at different depths by means of a precision translation table. While this top-table implementation allows obtaining an excellent refractive index matching, leading to maintenance of a good depth resolution and of a high signal-to-noise ratio, it limits the body locations that can be measured (14).
An alternative technical implementation overcoming this inconvenience is based on coupling the Raman microspectrometer with fiber optic probes for sample irradiation and scattered light collection (15). While this design allows easier handling of the probe with access to more body locations, technical issues linked to the fiber optic design arise, such as lower depth-resolving power in the SC and strong background signal in the fingerprint region given by the fused silica used in the fiber construction (14).
In vivo confocal Raman instruments may typically employ one or two laser sources in the visible or near infrared range (10,11); the choice of the wavelength depends on the application, since shorter wavelengths allow faster collection of Raman spectra but generate higher tissue autofluorescence in the fingerprint region, while longer wavelengths generate less autofluorescence but require longer exposure times (13).
Characterization of SC Molecular Composition and Structure
Water
Water content measured with CRS is usually expressed as the ratio of the Raman signal intensity of water band (due to OH-stretching vibrations) integrated from 3350 to 3550 cm−1 to that of protein (due to CH3 symmetric stretching vibrations) integrated from 2910 to 2965 cm−1 (10). Normalization by the protein band serves to compensate for signal losses taking place at increasing depths. A typical water concentration profile obtained with CRS is shown in Figure 10.2. The validity of this method of water measurement was demonstrated by comparison with the Karl Fisher titration method in an ex vivo study (16). Decreased capacitance values, as well as subjective assessment of dry skin, have been reported for sensitive skin in experimental and epidemiological studies, respectively (4,17). As CRS is validated and gives water concentration as a function of depth in the skin, we recommend benchmarking of subjective assessments as well as of traditional biophysical measurements with the direct measurement of the water content in the SC by means of CRS. Besides measuring water concentration at baseline, the water holding properties of the SC could be evaluated dynamically following, for example, exogenous water application (18,19). Changes in water holding properties could be due to an interplay of factors linked to barrier function structure and composition (such as corneocyte maturity, lipid amount and organization, surface path length (19)), water binding to hygroscopic substances (natural moisturizing factor [NMF]) (20), and water diffusion in SC due to different mobilities of water molecules. Many of these factors can be measured by CRS, as described in the following paragraphs. For what concerns water diffusion in SC, a study showed that CRS can distinguish the contributions of three different water-binding states (unbound, partially bound, and totally bound), determined by the strength of hydrogen bonds between the water molecules and other SC components (21).
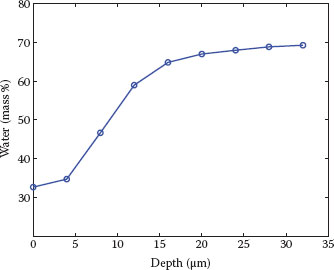
FIGURE 10.2 Water concentration profile measured on the volar forearm of a healthy volunteer. Water concentration shows a steep gradient across the SC and reaches a plateau in the upper epidermis. The determination of water concentration is quantitative (i.e., expressed in grams of water per 100 g of wet tissue or mass percentage) because of the addition of a calibration factor based on a solution of protein in water (see data in Caspers et al. (10)).
As a last remark, one study reported that two distinct groups of subjects could be identified based on the difference in water profiles measured with CRS before and after repetitive tape stripping: one group with almost no changes in the water profile and a group in which considerable changes were present (22). The difference in water influx from the viable epidermis following tape stripping could reflect differences in SC composition and be possibly linked to sensitive skin.
Natural Moisturizing Factor
NMF is a hygroscopic mixture of several substances, including amino acids and their derivatives, mainly derived from the degradation of the epidermal protein filaggrin (FLG), as well as components such as urea, lactate, sodium, and potassium, derived from eccrine sweat (23). NMF is an efficient humectant, helping to bind water within the cells and to maintain skin hydration and flexibility (24). It could therefore be another parameter of interest in the evaluation of water-handling properties in the SC of subjects with sensitive skin. The relation between the amount of (bound) water and the NMF content across the SC could also be explored (25). NMF information in Raman spectra can be extracted from the fingerprint region, and a semiquantitative method based on least square fitting with good agreement with in vitro results has been described (10). A typical NMF concentration profile obtained with CRS is shown in Figure 10.3.
Less clear is the role of NMF content on the impairment of skin barrier. Previous studies have shown that patients with atopic dermatitis (AD) carrying mutations in the FLG gene have significantly lower NMF content with respect to wild-type AD patients; at the same time, no significant differences could be found in the impairment of the skin barrier evaluated with TEWL (26,27). Similarly, sensitive skin could be characterized by a decrease in NMF, explaining the frequent association with dryness symptoms, and yet no detectable difference at the level of skin barrier impairment measured by TEWL.
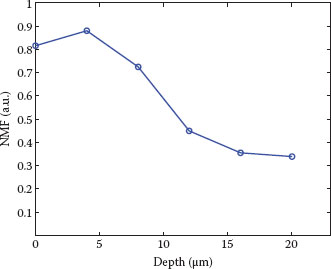
FIGURE 10.3 NMF concentration profile measured on the volar forearm of a healthy volunteer. Increase of NMF from the bottom of the SC is in correspondence with the degradation process of FLG. The characteristic depletion near the skin surface has been associated with washing-out processes, for example, due to daily cleansing (24). NMF is expressed here as the weighted sum of the dominant constituents pyrrolidone carboxylic acid, ornithine, serine, proline, glycine, histidine, and alanine. The determination of NMF concentration is semiquantitative (i.e., expressed in arbitrary units, relative to the concentration of keratin) (see data in Caspers et al. (10)).
Intercellular Lipids
The lipid matrix surrounding the corneocytes in the SC plays an essential role in the skin barrier function by preventing loss of water and other electrolytes and by blocking the entry of exogenous compounds (28). The lipid matrix is composed of a mixture of ceramides, cholesterol, and fatty acids arranged in parallel layers (lamellae) between the corneocytes; within the lamellae, lipids are present in three different lateral organizations, ranging from a very dense to a disordered liquid phase (29). An imbalance of the intercellular lipids has been suggested among the mechanisms leading to an impaired barrier function in sensitive skin (3). In addition, a study demonstrated decreased ceramide levels in the face of subjects with sensitive skin (30). Several spectral features providing direct information on the lateral organization and the conformational order of the lipid chains have been identified in both the fingerprint and high wave number regions (31). In addition, the same semiquantitative method developed for NMF can be used to differentiate cholesterol from ceramides/fatty acid contribution (10). Finally, lipid content can be expressed as the ratio of the Raman signal of lipids (due to CH2 asymmetric stretching) integrated from 2866 to 2900 cm−1 to that of protein (due to CH3 symmetric stretching vibrations) integrated from 2910 to 2965 cm−1 (32).
Besides the diagnosis of sensitive skin, lipid measurement with CRS could be beneficial for studies of the adverse effects of psychological stress on sensitive skin and on other skin disorders. In fact, stress resulted as one of the main triggering stimuli of sensitive skin in an epidemiological study (33) and an association with the exacerbation of several skin disorders is recognized (34). Epidermal lipid synthesis was shown to decrease following acute psychological stress, possibly due to increased glucocorticoid levels; this, in turn, would decrease the production and secretion of lamellar bodies, necessary for the recovery of barrier function following perturbation (34). Such mechanism would explain the delayed recovery of barrier function following tape stripping in the presence of various types of stress (35,36).
Stratum Corneum Thickness
The high end of the steep water concentration profile measured with CRS has been identified as the boundary between the SC and the stratum granulosum (10,37). Several algorithms to determine the SC thickness have been developed and validated against other techniques with similar spatial resolution (38–40).
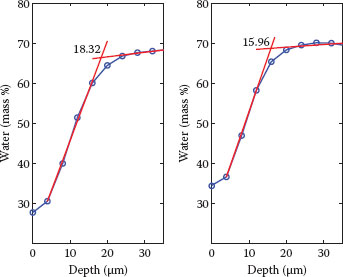
FIGURE 10.4 Examples of SC thickness calculated from the water concentration profile as the intersection of two straight lines in the SC and upper epidermis (38). The fit with a linear model in the SC follows the assumption of validity of Fick’s first law of diffusion (38). Both water concentration profiles represent the average profile measured on the volar forearm of six volunteers, where subjects with sensitive skin and NSS were included. Left, baseline (SC thickness = 18.32 μm); right, following a mild skin MDA treatment using a Philips VisaCare home use device (SC thickness = 15.96 μm). The sensitivity of measurements with CRS allows for the monitoring of small changes in thickness of the superficial layers of the SC, such as those occurring after a mild abrasion. Hydration in the deeper layers stays constant, indicating that the skin barrier function remains intact.
A thinner SC had been hypothesized in sensitive skin (3). Also, it is evident that daily activities as well as personal care treatments subject the skin to mild mechanical trauma (e.g., shaving, exfoliating, scratching, rubbing, clothing). The high spatial resolution afforded by CRS might help determine whether changes between the SC thickness of subjects with sensitive skin and NSS are present and how these subjects respond to mild mechanical trauma. An example of how CRS allows for the monitoring of small changes in thickness of the SC is shown in Figure 10.4. Here, we compared the SC thickness calculated at baseline and following a mild skin microdermabrasion (MDA) treatment using a Philips VisaCare* home use device, which shows gentle removal of the outmost cells composing the SC. Further evaluation of the water profile with CRS also confirms that hydration in the deeper layers stays constant, indicating that the skin barrier function stays intact after MDA. This is an important parameter to consider when evaluating the impact of personal care treatments on the skin and, in particular, on subjects with sensitive skin.
Transcutaneous Penetration of Substances
CRS can be used to investigate the transcutaneous penetration of topically applied substances, provided that these have a distinct Raman signature and are applied in sufficient amounts to be detected by currently available spectrophotometers (41). In addition to the kinetics of penetration, the effects of different vehicles on the delivery of active ingredients into the skin can be distinguished (42). Good agreement between in vivo measurement with CRS and in vitro measurements with Franz-type diffusion cells has been found for different substances (43,44).
Sensory testing methods based on the report of sensations induced by topically applied substances have been utilized in the diagnosis of sensitive skin for years (3,5). Despite being quick, easy, and inexpensive to perform, these methods lack objective criteria (5). The concomitant measurement of the topically applied substance with CRS would allow correlation of the subjective assessments of intensity and time to onset of the sensation with the objective measurements of amount and penetration kinetics of the substance in the skin. With CRS, transcutaneous penetration of substances can also be evaluated in light of the biomolecular composition and structure of the skin barrier. It would thus be possible to determine whether the hyperreactivity of sensitive skin to exogenous substances is more linked to an impairment of the barrier function, to an acceleration of the neurosensory system, or to a concomitant effect of both.
Evaluation of Treatments for Barrier Function Repair and Improvement
As no consensus on definition and pathophysiology of sensitive skin has been reached at this point in time, no standardized and validated treatments for sensitive skin have been proposed so far (3). Yet as the current evidence suggests an impairment of the skin barrier function, solutions aimed at its improvement and, if necessary, repair, might be the first ones to consider as remedies for sensitive skin treatment.
Previous studies have elucidated the repair mechanisms that lead to the restoration of the skin barrier following damage due to mechanical (tape stripping) or chemical (solvent, detergent) perturbations (45). Within the first minutes after damage, preformed lamellar bodies containing epidermal lipid precursors and enzymes involved in the extracellular processing of lipids are released from keratinocytes in the stratum granulosum, situated at the boundary with the SC. Subsequently, new lamellar bodies are formed and secreted in an accelerated fashion sustained by an increased activity of enzymes responsible for de novo lipid synthesis. The precursors of epidermal lipids are processed by the enzymes in the extracellular space of the SC, leading to the formation of ceramides, fatty acids, and cholesterol, ultimately contributing to the restoration of the barrier function.
Interestingly, several physical modalities with the ability to accelerate or delay this barrier repair process have been found, among which temperature, electric potential and visible light (46). For example, exposure of tape-stripped human and murine skins at temperature between 36°C and 40°C accelerated barrier recovery, while exposure at 42°C delayed recovery. These effects were attributed to the cation-permeable channels TRPV4 and TRPV1, present in epidermal keratinocytes and activated by temperatures at and above 35°C and 42°C, respectively. The influx of calcium ions into epidermal keratinocytes, previously shown to delay lamellar body secretion, could explain the delaying effects of TRPV1 but not the accelerating effects of TRPV4 (47). Other signaling systems regulating lamellar body secretion might play a role here and remain to be elucidated. As another example, exposure of tape-stripped murine skin to red light (550–670 nm) accelerated barrier recovery, while exposure to blue light (430–510 nm) delayed recovery. These results suggest the presence of a sensory system for visible radiation in the epidermis. This hypothesis is supported by the discovery of the expression of an opsin-like protein, a photosensitive protein found in the retina, in human epidermal cells (46). Next to this, the application of blue light to human epidermal keratinocytes and fibroblasts resulted in a dose-dependent reduction in proliferation due to an increased differentiation, as shown in in vitro studies (48,49). This effect laid a base for the treatment of hyperproliferative skin conditions; promising results in clinical trials involving patients with psoriasis vulgaris and atopic eczema have already been reported (50,51).
Temperature, electrical potential, visible light, and other physical factors shown to accelerate barrier function recovery could constitute innovative treatments for the restoration of skin barrier homeostasis in sensitive skin following acute perturbations. Their long-term use might also determine an overall improvement of the barrier function, leading to decreased transcutaneous penetration of irritants and consequent soothed sensory hyperreactivity (4). CRS, with its biomolecular discrimination capacity, high spatial and temporal resolution, and nondestructive nature, could provide valuable insights into such mechanisms, when applied during skin barrier studies. This is supported by a recent study where acute increase of ceramide levels attributed to increased lamellar body secretion was measured with CRS following skin barrier perturbation with sodium lauryl sulfate (SLS) (52).
Summary and Conclusion
Although sensitive skin has been investigated for years, a clear and consistent picture of the pathomechanisms involved in this condition is still lacking. Indirect assessments of barrier function and hydration by means of TEWL and electrical methods such as capacitance lack specificity and sensitivity if barrier function impairment in sensitive skin is subtle. CRS, being able to detect differences at the molecular level, could provide a breakthrough in the evaluation of barrier function involvement in sensitive skin. The underlying causes of impaired skin barrier, such as changes in molecular composition, could be directly measured in a depth-resolved, in vivo, and noninvasive way. The subjective assessment of cutaneous hyperreactivity involved in sensitive skin could be linked to the objective measurement of increased transcutaneous penetration of a topically applied substance. In addition to sensitive skin diagnosis, CRS could start playing a leading role in the evaluation of innovative treatments for barrier function restoration, not only for sensitive skin but also for hyperproliferative skin conditions such as psoriasis and atopic dermatitis.
In conclusion, CRS could open the way to more focused diagnosis of skin conditions involving the barrier function, helping to pave the path toward personalized treatments based on differences in molecular composition of individual subjects.
REFERENCES
1. Primavera G, Berardesca E. Sensitive skin: Mechanisms and diagnosis. Int J Cosmet Sci. 2005;27:1–10.
2. Richters R, Falcone D, Uzunbajakava N, Verkruysse W, van Erp P, van de Kerkhof P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Physiol. 2015;28:75–83.
3. Berardesca E, Farage M, Maibach H. Sensitive skin: An overview. Int J Cosmet Sci. 2013;35:2–8.
4. Seidenari S, Francomano M, Mantovani L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermatitis. 1998;38:311–315.
5. Farage M, Katsarou A, Maibach H. Sensory, clinical and physiological factors in sensitive skin: A review. Contact Dermatitis. 2006;55:1–14.
6. Nilsson GE. Measurement of water exchange through skin. Med Biol Eng Comput. 1977;15:209–218.
7. Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol. 1980;175:500–507.
8. Diogo L, Papoila AL. Is it possible to characterize objectively sensitive skin? Skin Res Technol. 2010;16:30–37.
9. Distante F, Rigano L, D’Agostino R, Bonfigli A, Berardesca E. Intra- and inter-individual differences in sensitive skin. Cosmet Toiletries. 2002;117:39–46.
10. Caspers PJ, Lucassen GW, Carter EA, Bruining HA, Puppels GJ. In vivo confocal Raman microspectroscopy of the skin: Noninvasive determination of molecular concentration profiles. J Invest Dermatol. 2001;116:434–442.
11. Chrit L, Hadjur C, Morel S, Sockalingum G, Lebourdon G, Leroy F et al. In vivo chemical investigation of human skin using a confocal Raman fiber optic microprobe. J Biomed Opt. 2005;10:44007.
12. Falcone D, Uzunbajakava NE, Varghese B, de Aquino Santos GR, Richters RJ, van de Kerkhof PC et al. Micro-spectroscopic confocal Raman and macroscopic biophysical measurements in the in vivo assessment of the skin barrier: Perspective for dermatology and cosmetic sciences, Skin Pharmacol Physiol. 2015;28(6):307–317.
13. Sieg A. Raman Spectroscopy. In: Berardesca E, Maibach HI, Wilhelm K-P, editors. Non Invasive Diagnostic Techniques in Clinical Dermatology: Springer, Berlin–Heidelberg; 2014. 217–223.
14. Pudney PD, Bonnist EY, Caspers PJ, Gorce JP, Marriot C, Puppels GJ et al. A new in vivo Raman probe for enhanced applicability to the body. Appl Spectrosc. 2012;66:882–891.
15. Santos L, Wolthuis R, Koljenovic S, Almeida R, Puppels G. Fiber-optic probes for in vivo Raman spectroscopy in the high wavenumber region. Anal Chem. 2005;77:6747–6752.
16. Wu J, Polefka T. Confocal Raman microspectroscopy of stratum corneum: A pre-clinical validation study. Int J Cosmet Sci. 2008;30:47–56.
17. Willis C, Shaw S, De Lacharriere O, Baverel M, Reiche L, Jourdain R et al. Sensitive skin: An epidemiological study. Br J Dermatol. 2001;145:258–263.
18. Egawa M, Kajikawa T. Changes in the depth profile of water in the stratum corneum treated with water. Skin Res Technol. 2009;15:242–249.
19. Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J Invest Dermatol. 2008;128:1728–1736.
20. Rawlings AV, Arding CRH. Moisturization and skin barrier function. Dermatol Ther. 2004;17:43–48.
21. Vyumvuhore R, Tfayli A, Duplan H, Delalleau A, Manfait M, Baillet-Guffroy A. Effects of atmospheric relative humidity on stratum corneum structure at the molecular level: Ex vivo Raman spectroscopy analysis. Analyst. 2013;138:4103–4111.
22. Boncheva M, de Sterke J, Caspers PJ, Puppels GJ. Depth profiling of stratum corneum hydration in vivo: A comparison between conductance and confocal Raman spectroscopic measurements. Exp Dermatol. 2009;18:870–876.
23. Watabe A, Sugawara T, Kikuchi K, Yamasaki K, Sakai S, Aiba S. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177–182.
24. Crowther JM, Matts PJ, Kaczvinsky JR. Changes in stratum corneum thickness, water gradients and hydration by moisturizers. In: Loden M, Maibach HI, editors. Treatment of Dry Skin Syndrome: Springer, Berlin–Heidelberg; 2012. 545–560.
25. Boireau-Adamezyk E, Baillet-Guffroy A, Stamatas GN. Mobility of water molecules in the stratum corneum: Effects of age and chronic exposure to the environment. J Invest Dermatol. 2014;134: 2046–2049.
26. Kezic S, O’Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031–1039.e1.
27. O’Regan GM, Kemperman PM, Sandilands A, Chen H, Campbell LE, Kroboth K et al. Raman profiles of the stratum corneum define 3 filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol. 2010;126:574–580.e1.
28. Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280–294.
29. van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841:295–313.
30. Cho HJ, Chung BY, Lee HB, Kim HO, Park CW, Lee CH. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. J Dermatol. 2012;39:295–300.
31. Tfayli A, Guillard E, Manfait M, Baillet-Guffroy A. Raman spectroscopy: Feasibility of in vivo survey of stratum corneum lipids, effect of natural aging. Eur J Dermatol. 2012;22:36–41.
32. Janssens M, van Smeden J, Puppels GJ, Lavrijsen AP, Caspers PJ, Bouwstra JA. Lipid to protein ratio plays an important role in the skin barrier function in patients with atopic eczema. Br J Dermatol. 2014;170:1248–1255.
33. Saint-Martory C, Roguedas-Contios AM, Sibaud V, Degouy A, Schmitt AM, Misery L. Sensitive skin is not limited to the face. Br J Dermatol. 2008;158:130–133.
34. Choi E, Brown B, Crumrine D, Chang S, Man M, Elias P et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol 2005;124:587–595.
35. Altemus M, Rao B, Dhabhar F, Ding W, Granstein R. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol 2001;117:309–317.
36. Muizzuddin N, Matsui M, Marenus K, Maes D. Impact of stress of marital dissolution on skin barrier recovery: Tape stripping and measurement of trans-epidermal water loss (TEWL). Skin Res Technol. 2003;9:34–38.
37. Caspers P, Lucassen GW, Puppels GJ. Combined in vivo confocal Raman spectroscopy and confocal microscopy of human skin. Biophys J. 2003;85:572–580.
38. Bohling A, Bielfeldt S, Himmelmann A, Keskin M, Wilhelm KP. Comparison of the stratum corneum thickness measured in vivo with confocal Raman spectroscopy and confocal reflectance microscopy. Skin Res Technol. 2014;20:50–57.
39. Crowther JM, Sieg A, Blenkiron P, Marcott C, Matts PJ, Kaczvinsky JR et al. Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br J Dermatol. 2008;159:567–577.
40. Egawa M, Hirao T, Takahashi M. In vivo estimation of stratum corneum thickness from water concentration profiles obtained with Raman spectroscopy. Acta Derm Venereol. 2007;87:4–8.
41. Lademann J, Meinke MC, Schanzer S, Richter H, Darvin ME, Haag SF et al. In vivo methods for the analysis of the penetration of topically applied substances in and through the skin barrier. Int J Cosmet Sci. 2012;34:551–559.
42. Melot M, Pudney PD, Williamson AM, Caspers PJ, Van Der Pol A, Puppels GJ. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J Control Release. 2009;138:32–39.
43. Mateus R, Moore DJ, Hadgraft J, Lane ME. Percutaneous absorption of salicylic acid—In vitro and in vivo studies. Int J Pharm. 2014;475:471–474.
44. Mohammed D, Matts PJ, Hadgraft J, Lane ME. In vitro-in vivo correlation in skin permeation. Pharm Res. 2014;31:394–400.
45. Feingold KR, Denda M. Regulation of permeability barrier homeostasis. Clin Dermatol. 2012;30:263–268.
46. Denda M. Physical and chemical factors that improve epidermal permeability barrier homeostasis. In: Esparza-Gordillo J, editor. Atopic Dermatitis—Disease Etiology and Clinical Management: Available from http://www.intechopen.com/books/atopic-dermatitis-disease-etiology-and-clinical-management/chemical-and-physical-factors-that-improve-epidermal-permeability-barrier-homeostasis; 2012.
47. Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007;127:654–659.
48. Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol. 2010;130:259–269.
49. Oplander C, Deck A, Volkmar CM, Kirsch M, Liebmann J, Born M et al. Mechanism and biological relevance of blue-light (420–453 nm)-induced nonenzymatic nitric oxide generation from photolabile nitric oxide derivates in human skin in vitro and in vivo. Free Radic Biol Med. 2013;65:1363–1377.
50. Becker D, Langer E, Seemann M, Seemann G, Fell I, Saloga J et al. Clinical efficacy of blue light full body irradiation as treatment option for severe atopic dermatitis. PLoS One. 2011;6:e20566.
51. Weinstabl A, Hoff-Lesch S, Merk HF, von Felbert V. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology 2011;223:251–259.
52. Hoffman DR, Kroll LM, Basehoar A, Reece B, Cunningham CT, Koenig DW. Immediate and extended effects of sodium lauryl sulphate exposure on stratum corneum natural moisturizing factor. Int J Cosmet Sci. 2014;36:93–101.
* Philips and VisaCare are registered trademarks in the name of Koninklijke Philips N.V.