CHAPTER 7 Conditions of the Reproductive Organs
UTERINE FIBROIDS
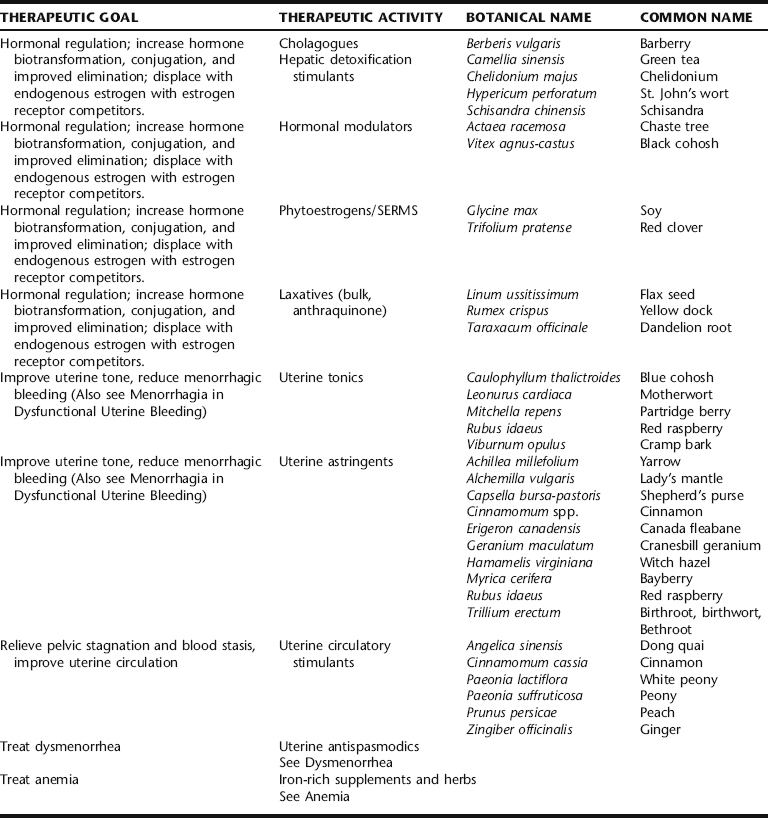
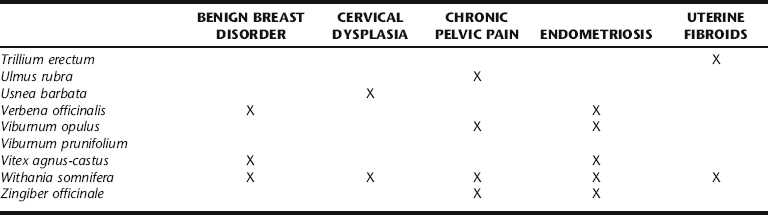
Uterine fibroids (properly called leiomyomata or myomas) are solid, well-defined benign monoclonal tumors of the smooth muscle cells of the uterus (Fig. 7-1).1,2 They range in size from microscopic to many pounds in weight, and may be singular or clustered. Multiple myomas in the same uterus are not clonally related.3 Fibroid size is described in comparison to a pregnant uterus (i.e., a fibroid the size of a 16-week pregnancy). As many as 20% to 40% of all women develop fibroids by age 40.1 Approximately 17% of all hysterectomies performed in the United States are for uterine myomas, with a peak incidence of surgery occurring for women around age 45, making fibroids the primary annual cause of premenopausal hysterectomy in the United States.3,4 They are rare in a premenarchal young women and shrinkage typically occurs in post-menopausal women with the natural decline in estrogen levels, unless stimulated by exogenous estrogen (foreign estrogens usually a result of environmental exposure, for example, from pesticides or plastics).1 For unknown reasons, fibroids are two to three times more common in black women than white, Asian, and Hispanic women.1,3 Fibroids are classified according to their site of growth in the uterine or surrounding tissue as submucosal, intramural, and subserous (see Figure 7-1). They also may occur in the cervix (cervical fibroids), between the uterine broad ligaments (interligamentous fibroids), or they may be attached to a stalk (pedunculated fibroids) and protrude into the uterine cavity (pedunculated submucosal fibroids) or through the cervix.1,3
Figure 7-1 Uterine fibroids.
Salvo S: Mosby’s Pathology for Massage Therapists, St. Louis, 2004, Mosby.
The exact etiology of uterine fibroids remains undetermined.5 Leiomyomas are hormone dependent. This is evidenced by the fact that they develop during hormonally active years and decline during menopause, fibroid tissue has an increased number of estrogen and progesterone receptors, fibroid tissue is hyperestrogenic, hypersensitive to estrogen, and does not possess the normal regulatory mechanism that limits estrogen response, the peak mitotic activity occurs during the luteal phase, and they respond to treatment with gonadotropin-releasing hormone (GnRH) agonists.3, 6 7 8 Growth factor also plays a role in leiomyomata development.3,9 As estrogen and progesterone levels rise, insulin is released causing the transient hypoglycemia commonly experienced premenstrually. When plasma glucose levels fall, pituitary growth hormone is released, exerting bodywide effects. Its action on hepatocytes causes the release of insulin-like growth factors (IGFs). In a study by Vollenhoven et al., it is postulated that the net effect of these changes increases the bioavailability of free (bioactive) IGF, which may play a major role in promoting fibroid growth.9 A further study by De Leo and Morgante states that concentrations of epidermal growth factor, insulin-like growth factor 1 (IGF 1), and platelet-derived growth factor (PDGF AB) are present in myomatous tissues together with their receptors.6 Prolactin also may be a factor. Leiomyomata express a number of hormones, including parathyroid hormone–related protein (a growth factor), prolactin, and IGF.3
Factors that might increase fibroid development and growth include:
The use of oral contraceptives is not associated with any changes in fibroid size, and may even be protective; however, one study reported a slight increase in risk with a history of OC use beginning in the early teenage years. 2 3 4
PATHOPHYSIOLOGY
Myoma risk is inversely related to increasing parity and age at last pregnancy, and is decreased by smoking (due to its inhibition of estrogen) and increased by obesity (likely due to increased estrogen levels) and hypertension. 1 2 3,10 Fibroids occur in 1% to 2% of pregnancies. However, it is uncertain whether this relationship is entirely causal. Infertility, as well as early pregnancy loss, may be due to mechanical obstruction of implantation or distortion of the cervix or endometrium. Once a pregnancy is established, it is rare for myomata to interfere with its progress, and most proceed uncomplicated. However, a higher rate of cesarean section has been noted, and premature labor may result from very large myomata.1 Degeneration of fibroids, caused by hemorrhagic infarction, may rarely occur during late pregnancy and is marked by pain, and also may be accompanied by rebound tenderness, fever, nausea, vomiting, and leukocytosis.3 Treatment consists of rest and analgesia; surgery is a last resort.
Anemia and fatigue can be caused by excessive blood loss associated with fibroids. Pressure on the bowel or bladder can cause constipation, urinary frequency, and dyspareunia. Large fibroids may mask the diagnosis of serious gynecologic neoplasm. Rapidly growing fibroids may indicate a more serious pathology such as leiosarcoma and should be investigated. Malignancy is rarely associated with uterine fibroids; however, they occur with increased frequency in endometrial hyperplasia and are associated with a fourfold increased risk of developing endometrial cancer.1
SYMPTOMS
Most women with myomas are asymptomatic, never knowing that they have them unless informed of such by gynecologic examination. This was actually discovered based on ultrasound and autopsy results revealing that many more women had fibroids than had ever been diagnosed or treated for symptoms.2 The most common symptoms are menorrhagia and the physical effects caused by large myomata such as increased pelvic pressure, frequent urination, difficulty with defecation, and dyspareunia with deep penetration.1,3,5 Abnormal uterine bleeding is present in about 30% of all patients, and periods are typically heavy and prolonged, often with premenstrual and postmenstrual spotting.2,4 Uterine bleeding caused by myomas can be associated with significant social, emotional, financial, and medical difficulties; women’s concerns should be addressed. Some women experience dysmenorrhea.2 Metrorrhagia, may occur, but should be evaluated with an endometrial biopsy to rule out other endometrial disease.4 About 2% to 10% of women experience infertility as a result of fibroids, ostensibly due to abnormal uterine of tubal motility, interference with sperm movements, or abnormal uterine blood flow.3,11 Fibroid degeneration, torsion, or compression of a nerve against the pelvis caused by encroachment by a fibroid can lead to significant pain.4,11
DIAGNOSIS
Diagnosis can be determined by:
CONVENTIONAL TREATMENT
Unless fibroids are symptomatic, observation is the most prudent form of treatment and no other intervention is necessary.1,4 GnRH agonists (e.g., Leuprolide) have been used effectively to control symptoms and reduce myoma size through suppression of estrogen and progesterone production.3,12 Mean uterine size decreases 30% to 64% after 3 to 6 months of treatment, and symptoms associated with fibroids are alleviated as a result. Possible side effects include hot flashes, headache, vaginal dryness and vaginitis, decreased libido, joint and muscle stiffness, and depression, and 30% of patients continue to have light, irregular vaginal bleeding.3,12 Local allergic reaction occurs in about 10% of patients.3 Bone loss occurs but is reversible, and a small number of women (2%) experience major vaginal hemorrhage 5 to 10 weeks after treatment commences. Steroid add-back therapy has been investigated to prevent bone loss in women requiring long-term GnRH therapy; however, because of the risk of osteoporosis, long-term therapy is inadvisable.3,12
Surgery should be reserved for women who are past childbearing, who are heavily symptomatic and not responsive to drug therapy, or who have suspected malignancies. Women wanting to preserve childbearing ability should be given the option of conservative therapy. GnRH therapy may be prescribed as pretreatment for surgical procedures to reduce fibroid size and bleeding. Myomectomy may be performed vaginally, hysteroscopically, or laparoscopically, and when performed skillfully, improves symptoms in 80% to 90% of patients. Between 15% and 30% experience fibroid regrowth after 5 years. Uterine scarring may occur from the procedure and affect fertility.12 Endometrial ablation and uterine artery embolization (UAE) are additional options.13,14 UAE is increasingly popular, and appears generally safe, but it is uncertain how long the treatment lasts and whether future fertility may be affected. The procedure involves injection of polyvinyl or gelatin particles into the uterine arteries to cut off blood supply to the fibroids, which leads to shrinkage over the next 3 to 12 months. Approximately 85% of patients gain relief from the procedure, which has been performed since 1995. However, it is not risk free. Adverse outcomes include infection, bleeding, and formation of emboli, as well as future fertility problems. For women intending to become pregnant in the future, myomectomy may still be the most certain conventional surgical intervention.14 Hysterectomy is generally recommended when women are past childbearing age, are symptomatic, malignancy is suspected, or if other therapies are ineffective.1,3,12
BOTANICAL TREATMENT
Among Western herbalists specializing in gynecologic complaints, there is a common perception that although symptoms of uterine fibroids are not difficult to control with botanical medicines, and their growth can be arrested, they are difficult to eliminate entirely unless the fibroid is small at the onset of treatment (smaller than 12-week size). Many women are content to have symptom control over pharmaceutical or surgical intervention, as long as the fibroids present no mechanical problems.15 Traditional Chinese medicine (TCM) has clearly defined diagnostic constructs, many herbal formulae, and well-developed adjunctive treatment protocols (e.g., acupuncture, moxibustion) for treating uterine fibroids and has claimed success in entirely eliminating uterine fibroids.
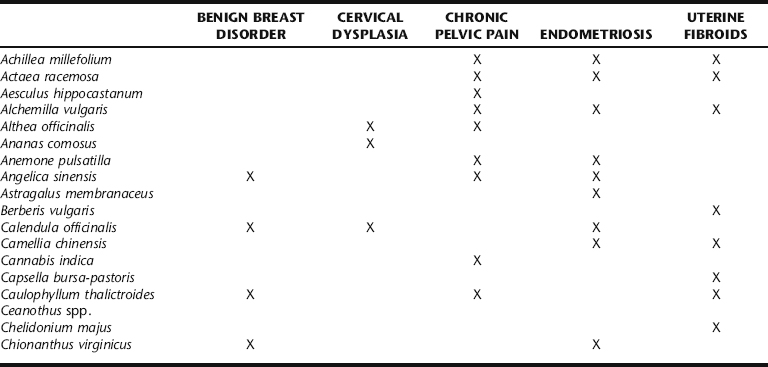
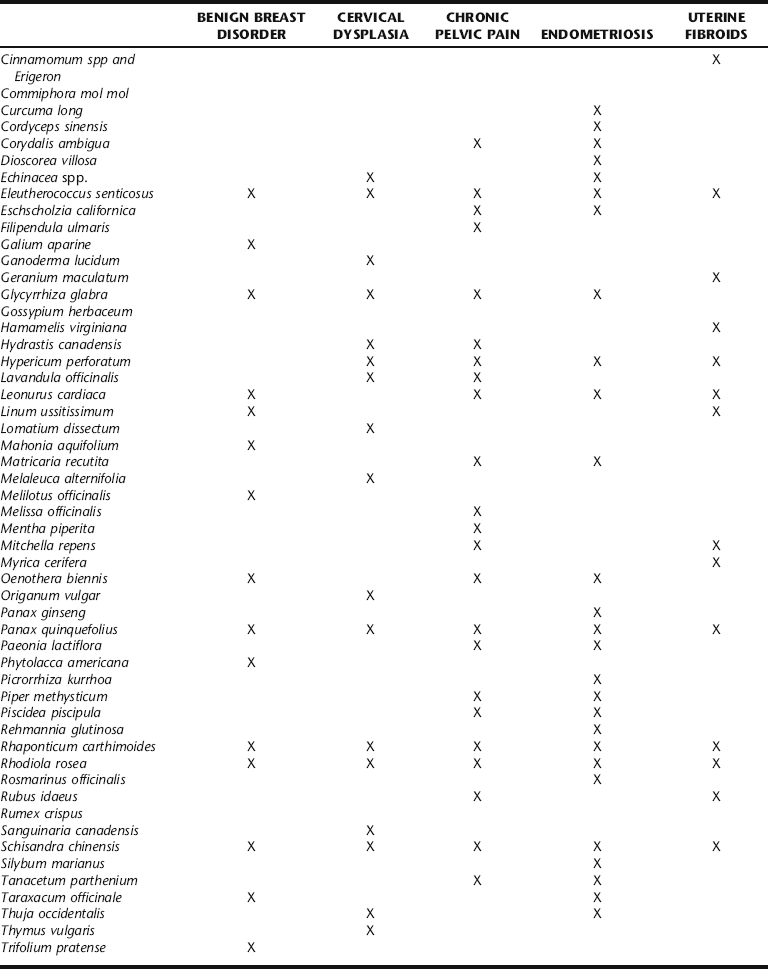
Western herbal treatment protocols include a variety of strategies (Table 7-1). These include weight reduction, promoting hormonal balance, specifically through the elimination of estrogens by enhancing liver detoxification mechanisms, promoting pelvic circulation while simultaneously controlling bleeding if necessary, and general improvement of uterine tone. These are integrated with the general recommendation to avoid excess exposure to xenoestrogens (environmental estrogens) and reduce overall estrogen levels, exposure to both being a risk factor for the development of uterine fibroids. Women with fibroids report greater frequency of red meat and pork intake, and less frequent green vegetable, fruit, and fish consumption.4 Although there is little correlation between the development of uterine fibroids and cancer, numerous studies have demonstrated a connection between diet, estrogen levels, and hormone-dependent cancers, as well as a protective effect of fruit and vegetables against cancer.4 No studies have evaluated the effects of US dairy consumption and the development of uterine fibroids. However, an association between dairy intake and increased risk of ovarian cancer has been reported.16,17 Herbalists recommend that patients avoid foods that increase risk, and emphasize intake of those shown to facilitate estrogen biotransformation, for example, by increasing dietary fiber, and regular intake of complex carbohydrates as found in vegetables and grains.11,18,19 Botanical strategies are aimed at reducing the estrogen burden through liver detoxification and improved elimination, promoting gynecologic health in general by improving pelvic circulation, reducing symptoms, and controlling fibroid size.
TCM treatment for fibroids has been evaluated through several preliminary studies, which are presented in the following section. Western botanical protocol for the treatment of uterine fibroids has not been subjected to controlled trials.4 The Western botanical information presented in this chapter reflects the opinions of herbalists practicing in the United States, United Kingdom, Canada, Australia, and New Zealand, regarding the efficacy and safety of the primary herbs used to treat myomas. Given the general safety of the botanicals being discussed, and the lack of noninvasive long-term effective medical treatments for fibroids, it seems that investigation of the primary Western herbal protocols cited in Table 7-1 is warranted. Nervines, laxatives, adaptogens, and other herbs included in fibroid protocol are discussed elsewhere throughout this text. Stress reduction should not be overlooked as part of the treatment protocol for women with symptomatic fibroids, as chronic uterine bleeding can cause emotional, social, financial, and medical consequences.2
Traditional Chinese Medicine Treatment
Cinnamon and Peony
Traditional Chinese medicine has numerous well-developed treatment protocols and formulations, some of which have been used for several centuries for promoting gynecologic health in general and for treating uterine fibroids specifically. For a more comprehensive review of the Chinese treatments for gynecologic problems, readers are referred to the primary TCM literature. Generally speaking, TCM views uterine myomas as a result of poor circulation of chi (energy) and blood through the pelvic region. Many formulas are designed to dispel pelvic stagnation and increase the flow of blood to uterine and ovarian tissues and facilitate the smooth flow of blood via menses. A classic TCM formula used for relieving blood stagnation is Cinnamon Twig and Poria Pill (gui zhi fu ling wan) consisting of: Cinnamomum aromaticum twigs, Poria cocos, Paeonia lactiflora root, Paeonia suffruticosa root, and Prunus persica seed. It should also be noted that in TCM, each herbal formula has specific diagnostic criteria for which it is used as well as clear contraindications and cautions. For maximum efficacy in using TCM protocols, a qualified herbal TCM practitioner should be consulted. In addition to herbal protocol for promoting gynecologic health and specifically treating uterine fibroids, TCM also employs numerous other modalities, which may include walking to promote circulation in general and abdominal circulation specifically, moxibustion, acupuncture, external application of compresses, and other such adjunctive therapies. Specific lifestyle recommendations also can be given such as the avoidance of cold foods and drink (in TCM coldness is said to cause congealment and stagnation) and constrictive clothing.20
Several studies have looked at the efficacy of the Cinnamon Twig and Poria Pill formula noted in the preceding section for the treatment of uterine fibroids. Specifically, the studies investigated the effectiveness of the Japanese version of this formula (Keishi-bukuryo-gan, KBG) in an open study on 110 premenopausal women with symptomatic uterine fibroids measuring less than 10 cm in diameter. They were treated with 22.5 g/day of a freeze-dried decoction of the herbs for 12 weeks. Twenty-one women were considered “normal” and 47 women much improved by the end of the trial. This herbal formula is frequently used to treat a range of gynecologic disorders including dysmenorrhea, cervical erosion, ovarian cysts, chronic salpingitis, and endometriosis, to name a few conditions.15 There is research to suggest that the Paeonia species in this formula may act as an LH-releasing hormone (LH-RH) antagonist with weakly antiestrogenic effects in the presence of estrogen.21 In another study, the authors applied individualized TCM formulations and treatments to treat 223 cases of uterine fibroids with a reported 72% reduction of menorrhagia in 160 women complaining of this symptom, 58% improvement in backache, and an overall effectiveness rate of 92.4%. Myomas were eliminated in 29 of 223 patients and markedly diminished in 42 patients. In 32 patients, no changes were seen and there were no positive results in 12.5% of patients.15 If the TCM treatments were tailored to the individual patients, then this can be kept with the addition I made. If the treatment was specific, a similar level of detail as the previously reported study should be given for consistency of presentation.
In an interesting study by Mehl-Madrona et al., an integrated TCM-Western medicine pilot study was conducted to compare the cost and efficacy of a set of therapies typically used by CAM practitioners and conventional medicine on ability to reduce uterine fibroid size. All patients were premenopausal and age 24 to 45 years, educated, employed, and from a socioeconomic bracket that allowed them to pay cash for all treatments. None were on pharmaceutical treatment or hormonal contraceptives at the time of the study and all received a pelvic ultrasound before and again 6 months after treatment. Sonograms were obtained on patients who dropped out of the study as well, so sonograms were available on all patients. Uterine fibroids measured at least 6- to 8-week pregnancy size, with palpable fibroids 2 to 3 cm in diameter. Inclusion in the study required hemoglobin greater than 8 g/dL, with fibroid growth of less than 6 cm/year. CAM treatment included a combination of nutritional, herbal, acupuncture, bodywork, and psychological interventions. Acupuncture and herbal protocols were selected individually for the patient, using formulae and points traditionally indicated for the patient’s patterns: symptoms, constitution (based on TCM pulse and tongue diagnosis), and condition. The comparison group used progestational agents, oral contraceptives, and NSAIDs. The results of this study demonstrated no statistically significant difference in change of symptoms between the two groups when measured after 6 months of treatment. Both experienced improvement in symptoms and fibroid size. Patients in the treatment group considered the pilot study a success because they were able to achieve results equivalent to pharmaceutical interventions using nonconventional methods.15
Hormonal Modulators
Chaste Berry
Chaste berry is the primary herb employed by herbalists and integrative medicine practitioners for hormonal modulation in the botanical treatment of fibroids.4,22 It acts as a dopamine agonist, resulting in a reduction in prolactin release.23 Prolactin may play a role in fibroid growth. No scientific evidence in the literature has been found for the use of chaste berry specifically in the treatment of fibroids, and although its use may result in reduction of apparent estrogen excess due to relative progesterone deficiency, increased progesterone levels have been shown to result in increased mitotic division in fibroid tissue. Wuttke et al. studied the putative estrogenic effects of a chaste berry extract and found it contained substances that replaced radiolabeled estradiol from a cytosolic estrogen receptor preparation, and appeared to be agonistic to ERβ. However, because the uterus expresses ERα, no effects on the uterine expression of estrogen were expected or have been experimentally observed.23
Phytoestrogens and Selective Estrogen Receptor Modules
Phytoestrogens are plant compounds with a similar molecular shape and structure to endogenous estrogen molecules, and which can bind competitively to estrogen receptors, preventing the binding of more potent estrogen and estrogen metabolites (see Part IV).24 They appear to behave similarly to selective estrogen receptor modulators (SERMs). Low Dog explains their potential clinical application in conditions of estrogen excess, in relationship to the role of phytoestrogens in breast cancer treatment:
By binding to estrogen receptors in the premenopausal woman, phytoestrogens “turn down” estrogen production through negative feedback at the level of the hypothalamus and pituitary gland…when endogenous estrogen levels are high, phytoestrogens may have an antiestrogenic activity by preventing estrogen from binding to the estrogen receptor through competitive inhibition.25
Legumes, including soybeans and red clover, are rich in phytoestrogens.26 In a study by Liu et al. methanol extracts of red clover (Trifolium pratense), chaste berry, and hops (Humulus lupulus) showed significant competitive binging to both ERα and ERβ. In the same study, dong quai (Angelica sinensis) and licorice (Glycyrrhiza uralensis) showed weak ER binding, whereas black cohosh did not exhibit any competitive binding. Controversy abounds as to the mechanisms of action of black cohosh, which do not appear to be directly phytoestrogenic.27 Current research is suggesting a dopaminergic or serotonergic effect for this botanical.25,27 The application of phytoestrogens may be a promising area for further investigation for the botanical treatment of fibroids, and should be considered in the development of botanical protocols.
Hormone Excretion and Biotransformation
Greater than 50% of all estrogen metabolism and conjugation occurs in the liver, suggesting a basis for the belief among herbalists that herbs that improve liver function may increase estrogen excretion and either treat or lower the risk for uterine fibroids.4,22 Herbalists commonly include liver-specific herbs in formulae for treating fibroids. Several herbs actively effect phase 1 and phase 2 liver detoxification systems and CYP450, an enzyme system partially involved in the metabolism of estrogen. These effects and their relationship to uterine fibroid treatment, if any, have not been formally investigated but are often applied by modern herbal practitioners in putatively reducing estrogen burdens. Cholagogues, herbs which stimulate the release of bile from the gallbladder, also may be useful for clearing estrogen through increased bowel clearance resulting from their indirect laxative action. Examples of cholagogues include bayberry and chelidonium.22
Uterine Tonics, Astringents, and Hemostatics
Because bleeding is a common symptom of uterine fibroids, numerous antihemorrhagic herbs are used in botanical medicine protocols (see Menorrhagia in Dysfunctional Uterine Bleeding).22 Yarrow dried plant infusion is perhaps one of the most widely used uterine antihemorrhagics, reliably reducing acute uterine bleeding, but conversely promoting menstrual flow when suppressed. It has been used since ancient times as a styptic.25 Either dry or fresh plant can be used as a tea or tincture. Many herbalists believe that yarrow herb taken as tea is more quickly effective for stopping acute uterine bleeding than other preparations. Other traditionally used uterine antihemorrhagic herbs include lady’s mantle, shepherd’s purse (fresh only), cranesbill geranium, witch hazel, bayberry, red raspberry, and bethroot. These are all generally
| Yarrow | (Achillea millefolium) | 40 mL |
| Lady’s mantle | (Alchemilla vulgaris) | 20 mL |
| Bayberry bark | (Myrica cerifera) | 15 mL |
| Shepherd’s purse | (Capsella bursa-pastoris) | 15 mL |
| Cinnamon | (Cinnamomum cassia) | 10 mL |
| Total: 100 mL | ||
taken in tincture form in 2- to 4-mL doses repeated every 15 minutes as needed until bleeding subsides, or combined into larger formulae for the treatment or prevention of chronic menorrhagia. Shepherd’s purse in particular has been used traditionally as a uterine antihemorrhagic. The 1986 Commission E monograph recommends daily oral doses of 10 to 15 g of crude herb (or equivalent in extract) for mild gynecologic bleeding.28 Extracts of the drug contain a hemostyptic action, likely owing to the presence of a peptide that has demonstrated oxytocin-like activity in vitro.28,29 Many modern Western herbalists believe that it is imperative to prepare Shepherd’s purse from fresh, not dry, plant material. Lady’s mantle’s mechanism of action lies in its high tannin content, indicating it for bleeding, diarrhea, and wound healing, a likely mechanism for many of the other herbs used as uterine antihemorrhagics.29 The combination of Cinnamomum and Erigeron was relied upon by the Eclectics for uterine hemorrhage, and is still employed by midwives today for the treatment of nonemergency postpartum bleeding, and by herbalists for the treatment of menorrhagia.30,31 Red raspberry leaf is typically used more as a long-term uterine tonic than to arrest acute bleeding. Blue cohosh has been used historically for its utero-tonic actions. It is listed in the 1918 US Dispensatory for the treatment of menorrhagia and dysmenorrhea, and is still widely used by herbalists for these conditions.32
Relieving Uterine Stasis: Circulatory Stimulants
Improving pelvic circulation and relieving stasis is a common approach to fibroid treatment in both Western and traditional Chinese herbal medicine, based on the belief that relieving stagnation and congestion in the pelvis will facilitate the removal of “blockages” and growths (e.g., fibroid tissues), remove wastes, and promote greater health and nourishment of the pelvic organs in general.11,18,20 Decreasing pelvic stagnation is also thought to help reduce uterine hemorrhage. Ginger and cinnamon are both traditionally used to increase circulation to the reproductive organs. Further, cinnamon has been used historically to reduce uterine bleeding, making it specific for the treatment of uterine fibroids with menorrhagia or metrorrhagia.30,31 White peony, an ingredient in Keishi-bukuryo-gan, discussed in the preceding, is a common herb used in TCM for the treatment of women’s disorders, including menstrual dysfunction and uterine bleeding.33 Red peony is often combined with white peony and peach seed to dispel blood stasis, and conditions associated with it, including excessive uterine bleeding, particularly with the presence of thick, purple clots.33
Treatment Protocol
| Lady’s mantle | (Alchemilla vulgaris) | 25 mL |
| Raspberry | (Rubus idaeus) | 30 mL |
| Nettles | (Urtica dioica) | 20 mL |
| White peony | (Paeonia lactiflora) | 15 mL |
| Ginger | (Zingiber officinalis) | 10 mL |
| Total: 100 mL |
After 3 months the above protocol was modified to:
| Red raspberry leaf | (Rubus idaeus) | 40 mL |
| White peony | (Paeonia lactiflora) | 40 mL |
| False unicorn | (Chamaelirium luteum) | 15 mL |
| Ginger | (Zingiber officinalis) | 5 mL |
| Total: 100 mL |
| Ashwagandha | (Withania somnifera) | 40 mL |
| Lemon Balm | (Melissa officinalis) | 30 mL |
| Hops | (Humulus lupulus) | 15 mL |
| Valerian | (Valeriana officinalis) | 15 mL |
| Total: 100 mL |
NUTRITIONAL CONSIDERATIONS
Xenoestrogens/Endocrine Disruptors
Avoid xenoestrogen ingestion from pesticide and herbicide residue by eating organically cultivated foods and avoiding foods in plastic containers. Xenoestrogens are found most concentrated in the fat of meat, farmed fish, and nonorganic dairy products.12 Eating primarily organic meat, dairy, and produce, washing fruits and vegetables thoroughly before eating, and minimizing the use of soft plastics, such as for food storage, can help reduce xenoestrogen intake.
Estrogen Biotransformation and Diet
Metabolism and detoxification of estrogen in the body ultimately determines its biological effects. Estrogen biotransformation occurs mainly in the liver through phase I hydroxylation and phase II methylation and glucuronidation, allowing estrogen to become a water-soluble, excretable compound.11 This is predominantly excreted by the liver in bile (see Dietary Fiber). Phase I detoxification yields three estrogen metabolites with highly variable biological activity: 2-hydroxyestrone (2-HE), 16-alpha-hydroxyestrone (16α-HE), and 4-hydroxyestrone (4-HE). 2-HE is a beneficial estrogen metabolite in that among its effects, it competitively binds estrogen sites, blocking more potent estrogens. Conversely, 4-HE and 16α-HE are potent estrogens that may promote the growth of estrogen-sensitive tissue.11 Dietary consumption of cruciferous vegetables, such as broccoli and cabbage, as well as green tea, garlic, and rosemary can increase the amount of 2-HE by modifying P450 activity in phase I, and have antioxidant effects as well.11,34
Dietary Fiber
Once estrogen metabolites are excreted by the liver in bile, the metabolites are soaked up by fiber in the small intestines and excreted via defecation. If the diet lacks fiber, bile, along with the estrogen metabolites are reabsorbed, adding an unnecessary estrogen burden to the body. Soluble fiber such as the lignins found in flax seeds also increases sex hormone binding globulin (SHBG), decreasing the amount of available active estrogen, as estrogen bound to SHBG is rendered inactive.11 Brassicae vegetables such as cabbage and broccoli contain indole glucosinolates, which when chewed, are degraded by a plant enzyme into a variety of indole structures. When degraded in the body, these structures induce cytochrome P450 expression (CY1A1) in hepatic and extrahepatic tissue, leading to greater conversion of 2-hydroxyestrone (2-HE), and decreasing the availability of E1 for conversion to 16-HE, thereby reducing the estrogen burden overall.25 This is partly associated with the anticancer effects associated with these foods.
ADDITIONAL THERAPIES
Treatment Summary: Uterine Fibroids
What to expect with treatment
Eclectic Specific Condition Review: Uterine Fibroids—a Historical Perspective
stress. Herbalists often recommend specific yoga postures or Kegel exercises to assist in improving pelvic circulation particularly.35 Vigorous walking, hip circling, pelvic thrusts, and belly dancing can all be useful to improve pelvic circulation.
FIBROCYSTIC BREASTS AND BREAST PAIN
Benign breast conditions are a common finding in clinical practice, with fibrocystic breast changes and fibroadenomas occurring in 60% to 90% of all women.36 The hallmark of fibrocystic breast changes is that the cysts fluctuate in size and shape, may entirely disappear and reappear cyclically, and are associated with hormonal changes in the menstrual cycle. Women with this condition describe their breasts as feeling lumpy, “ropey,” and tender. The changes occur bilaterally. Fibroadenomas are mobile, solid, firm, rubbery masses that typically occur singly, and are not usually painful (Fig. 7-2). They are second only to fibrocystic changes as the most common of the benign breast conditions, and are commonly found in women in their 20s. Breast tenderness that accompanies the menstrual cycle is known as cystic mastalgia.38,39 Cyclic mastalgia may be associated with other premenstrual complaints. The terms benign breast disorder and benign breast disease are unfortunate misnomers, as they are neither a disorder nor disease. In only a small percentage of cases are the atypical ductal and lobular hyperplasias associated with increased risk of breast carcinoma. Practitioners consulting with women for fibrocystic breast changes and other findings must be sensitive to a patient’s increased anxiety about finding a breast lump, and provide clear information and calming reassurance both during the exam and while the patient awaits tests results if any were deemed necessary.
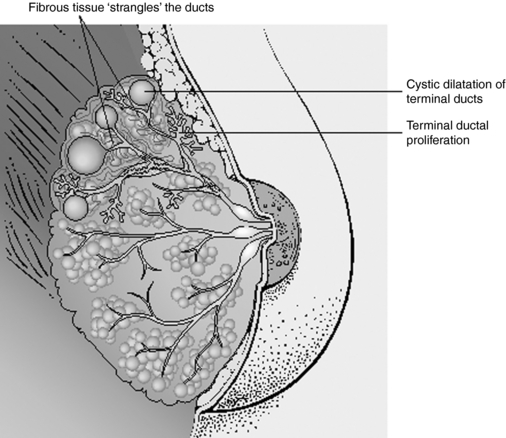
Figure 7-2 Comparison of normal and fibrocystic breast tissue.
Salvo S: Mosby’s Pathology for Massage Therapists, St. Louis, 2004, Mosby.
PATHOPHYSIOLOGY
Fibrocystic breast changes are an exaggerated response to cyclic ovarian hormones.39 The etiology of fibroadenomas and cyclic mastalgia may also be hormonal, though in some cases, the cause of a fibroadenoma may be unclear. When this occurs in women over 30, removal of the mass is generally recommended.
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
There are two primary aims when arriving at a diagnosis of fibrocystic breasts. The first is to rule out breast cancer, and the second is to determine if benign breast symptoms warrant treatment. A careful history, physical exam, and cancer risk assessment are indicated (Box 7-1 and Table 7-2).36 A thorough breast exam is best performed after the menses, as examination prior to menses (when the pain is actually likely to be most acute) can obscure problematic lumps caused by normal breast tissue proliferation and nodularity from normal hormonal changes. If a suspicion of breast cancer remains after the history and physical, further diagnostic tests should be performed as appropriate.36 Diagnosis of fibrocystic breasts can be made on the basis of cancer exclusion. For women experiencing symptoms including pain, tenderness, swelling, inflammation, or nipple discharge, the comprehensive history and physical can be used to determine if the problem is cyclic or noncyclic in nature and whether it is associated with other signs and symptoms, including fever or premenstrual mood swings. It is also important to gently move aside the breast tissue and examine the chest wall and muscle to determine whether breast pain or muscle pain is the proper diagnosis.40 Depending on the associated signs and symptoms, a diagnosis, including breast infection, muscle sprain/strain, premenstrual syndrome, or noncyclic mastalgia can be determined.
TABLE 7-2 Risk for Development of Breast Cancer by Type of Benign Breast Disease
| HISTOLOGIC PATTERN | APPROXIMATE RELATIVE RISK OF DEVELOPING BREAST CARCINOMA | PROPORTION OF BENIGN LESIONS* |
|---|---|---|
| Nonproliferative changes | No increased risk | 70% |
| Proliferative disease without atypical hyperplasia | Twofold increased risk | 27% |
** Family history limited to mother, daughter, or sister with breast cancer
CONVENTIONAL TREATMENT
Conventional treatment for fibrocystic breasts includes encouraging women to wear loose fitting brassieres, decreased caffeine consumption, and smoking cessation, and a pharmacologic focus on hormonal modulation, including oral contraceptives (OCs), prolactin antagonists, and antiestrogen agents as well as diuretics for moderate premenstrual mastalgia; and analgesics such as ibuprofen, salicylates, and acetaminophen for pain.36,38 Hormonal therapies often carry unwanted side effects, including weight gain, lipid profile changes, depression, and abnormal bleeding.36 Although OCs reduce symptoms in up to 90% of women, symptoms return upon discontinuation.36 Danazol, which suppresses the pituitary ovarian axis by inhibiting the output of both FSH and LH from the pituitary gland, is also used for mastalgia. Its side effects include virilization, muscle cramps, CPK elevations, and liver damage. Bromocriptine is also used for breast pain and nodularity but has several common side effects, including nausea, giddiness, and postural hypotension.36 Reduction in dietary fat intake has been shown to reduce cyclic mastalgia.
BOTANICAL TREATMENT
Botanical treatment for fibrocystic breasts has not been widely subject to scientific evaluation, in spite of this being a commonly treated condition in the herbal clinic. Treatment aims primarily at hormonal regulation through direct (i.e., HPA and HPO axes) and indirect (i.e., improved hormonal biotransformation and excretion) actions, and reduction of local congestion and symptomatic pain relief through topical applications (Table 7-3). The liver plays a central role in metabolizing and detoxifying sex hormones.34 Consequently, herbal practitioners typically include herbs that are known or thought to enhance hepatic detoxification functions in formulae for treatment of fibrocystic breasts.34 Such herbs, many of them considered “bitters,” include dandelion root, burdock, root, licorice root, Oregon grape root, fringe tree, motherwort, blue vervain, and celandine. These botanicals are usually included in ranges of 5% to 20% of formulae, in tincture or decoction forms. Although there has been little investigation of such herbs to establish their pharmacologic or physiologic action for such use, they are nonetheless a common part of the protocol for many gynecologic concerns, including benign breast complaints, and their role in formulae should be considered and further evaluated.
Chaste Berry
Chaste berry is extensively recommended by herbal practitioners for cyclic breast pain and fibrocystic breasts, both when it presents independently and when associated with PMS. This traditional use is supported by clinical trials. Chaste berry may be used singly or in combination with other herbs that enhance hormonal regulation and hormone metabolism (e.g., herbs that promote liver function and hormonal conjugation and elimination). There have been three placebo-controlled, double-blind, randomized clinical trials (RCT) examining the effects and safety of a proprietary chaste berry extract–containing solution (VAC) on cyclic mastalgia. VAC is sold as Mastodynon®, and manufactured by Bionorica Arzneimittel GmbH (Neumark/Opf. Germany).41 It contains 32.4 mg of chaste berry fruit extract/60 drops as well as a mixture of homeopathic ingredients, including Caulophyllum thalictroides, Cyclamen, Ignatia, Iris, and Lilium. This product is available as both a tablet (MR 1025 E1) and a liquid extract in Germany. German drug indications for the product include menstrual disorders based on a temporary or permanent corpus luteum insufficiency, infertility resulting from corpus luteum insufficiency, and menstrually related complaints, including mastodynia.42 All three of the studies used the liquid solution, which contained 53% (v/v) alcohol, although the study by Wuttke et al. also used the tablets.41,43 All three studies defined cyclic mastalgia as having at least 5 days of breast pain the previous cycle and treated women for three menstrual cycles with 30 drops two times daily of VAC (1.8 mL/equivalent to 32.4 mg extract of chaste berry drug). Researchers found that both the severity (assessed on a 1- to 100-mm visual analog scale [VAS]) and presence of breast pain (as measured by women’s diaries) were significantly improved in the women who were assigned to the chaste berry groups compared with placebo after the first month of treatment. Although pain intensity was reduced by 30% in the chaste berry group compared with 11% in placebo after one cycle, pain intensity was even more reduced at the end of the second month of treatment, with 53% of women receiving chaste berry having decreased severity of breast pain compared with 25% of the placebo group (p = 0.006).41 No further improvement was obtained with longer treatment periods. However, to reduce the number of days with severe pain women needed to receive VECS for three to four cycles before they had significantly fewer days with breast pain compared with the placebo group (p = 0.21).41,44 Two of the studies also measured serum hormone levels including estradiol, progesterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and basal prolactin levels at baseline and in the premenstrual weeks of cycles 1, 2, and 3.43,45 One study found a significant rise in prolactin levels and a decrease in progesterone levels,45 whereas another study found no effect on FSH, LH, and progesterone, but did see a decrease in estradiol levels and a significant decrease in basal prolactin levels (3.7 ng/mL tablets and 4.35 ng/mL liquid extracts) compared with placebo.43 Adverse events were rare and did not differ from placebo in any of the RCTs.
Dong Quai and Blue Cohosh
Along with chaste berry, dong quai and blue cohosh are commonly employed by herbal clinicians to modulate hormone levels. Blue cohosh, as part of the German herbal formulation Mastodyn® (reviewed in the preceding), may provide some of the therapeutic benefit of that formulation. However, to date, no studies have demonstrated that blue cohosh has any effect on hormonal levels. Dong quai in vitro can weakly bind to estrogenic receptors and induce progesterone receptors. However, it did not stimulate vaginal cells or increase endometrial thickness and had no estrogenic effect showing no transactivation of either alpha- or beta-estrogen receptors.27,46,47 Additionally, dong quai showed no significant effect on either hormonal levels or symptoms in an RCT in menopausal women.48,49 Consequently, dong quai appears to have a limited if any effect on hormone levels. In a recent study, dong quai was found to have significant anti-inflammatory effects because of one of its constituents, ferulic acid.50 Although this study is very preliminary, it may offer an alternative mechanism through which dong quai could be helping to decrease mastalgia in women with fibrocystic breasts. According to TCM theory, dong quai dissolves blockages and relieves blood stagnation, and is thus a common ingredient in formulae for mastalgia.
Flax Seed and Evening Primrose Oil
Flax seeds are the richest source of plant-based omega-3 fatty acids, with a-linolenic acid (ALA) being the primary fatty acid (18:3n-3).51 These fatty acids are considered strongly anti-inflammatory, being precursors for the anti-inflammatory series prostaglandins (PGE3). Flax seeds are also rich in fibers called lignins. Like isoflavones in soy and other foods, lignins and their associated phenolic compounds are classified as phytoestrogens.52,53 Flax seeds are an especially rich source of dietary lignins, with 75 to 800 times more than any other food source.51,54 Research has shown lignins to be a promising agent for binding excess sex hormones, including testosterone and estrogens. Through both its anti-inflammatory and possible anti-estrogenic effects researchers believed that flax may prove a beneficial treatment for fibrocystic breasts. One study examined the effect of eating one muffin daily supplemented with 25 g of ground flax seeds in 127 women with mastalgia.55 Women experienced a significant reduction of symptoms; however, the full results of this study were never published and thus it is unknown how long women needed to take the flax seed–enriched muffins, what symptoms were reduced, what the degree of symptom reduction was, or if there was any placebo control to assess for the considerable level of spontaneous remission (60% to 80%) of symptoms in women with breast pain over time.55 Evening primrose oil (EPO), is a rich source of omega-6 essential fatty acids (EFAs). EFAs are precursors to either series 1 or 2 prostaglandins, depending on substrate availability. The more omega-6 EFAs there are in the diet, the more likely it is for the inflammatory series prostaglandins to be made; conversely, the less omega 6 EFAs there are in the diet, the more likely it is for anti-inflammatory series 1 prostaglandins to be produced. Because of EPO’s potential anti-inflammatory properties, two randomized placebo-controlled, double-blind clinical trials and one open-labeled trial of EPO in both cyclic and noncyclic mastalgia have been conducted. One double-blind, placebo-controlled randomized study in 73 women with either cyclic or noncyclic mastalgia found that 1000 mg of EPO or placebo three times daily over 3 months significantly reduced symptoms of pain and tenderness in the women who received EPO compared with placebo.56 In a similar study, 291 women with severe persistent breast pain given either placebo or 1000 mg EPO three times daily for 3 to 6 months found that 45% of women with cyclic pain improved. Further, 27% of women with noncyclic breast pain improved compared with 9% in the placebo group.57 In a nonrandomized open-labeled study, 94 women with cyclic and 32 women with noncyclic mastalgia received 3 g of EPO for at least 4 months. Severity of pain was diminished in a “clinically useful”40 manner in 58%49 of the women with cyclic mastalgia and 38%12 of the women with noncyclic mastalgia taking EP. Unfortunately, all three of these studies are difficult to assess because of lack of reporting of how pain and symptoms were measured and how great the effect, and thus how clinically relevant the effect of EPO is in women with breast pain.40 The recommended dose of flax oil to treat and prevent mastalgia and nodularity is two to four 500-mg capsules twice daily or 1 to 2 tablespoons of the oil daily. One to three grams daily is the recommended dose of ground seeds.58 A typical dose of EPO is 1500 mg daily.
Red Clover
Red clover is rich in isoflavones, especially genistein and daidzein. Genistein and daidzein have weak estrogenic effects, which have led researchers to hypothesize that genistein may compete with stronger endogenous estrogens such as estradiol for estrogen receptors; although what effects this may have on breast tissue is unclear at this time. One unpublished study found that a red clover extract had a significant effect on improving mastalgia. No further information is available concerning this trial.59
Topical Applications
Poke Root
Poke root has traditionally been used to stimulate the immune system, relieve lymph congestion, and resolve
Botanical Protocol for the Treatment of Fibrocystic Breasts
I. Prepare the Following Tincture:
| Calendula | (Calendula officinalis) | 20 mL |
| Chaste berry | (Vitex agnus-castus) | 20 mL |
| Burdock root | (Arctium lappa) | 20 mL |
| Sarsaparilla | (Smilax ornata) | 20 mL |
| Dandelion root | (Taraxacum officinale) | 20 mL |
| Total: 100 mL | ||
lumps and cysts, and by extension, has been widely applied topically for the treatment of fibrocystic breasts. Poke root oil is applied topically by rubbing in a small amount (1 tsp) of the oil throughout the affected breast(s) for at least 5 nights per week for 1 to 2 months. The addition of 5 to 7 drops each of rose geranium and sandalwood essential oils makes the oil slightly more stimulating to the local circulation and also adds a pleasant scent to the oil. All parts of the plant are toxic and can lead to contact dermatitis or even toxicity from handling large amounts. Internal use is not recommended without the supervision of a qualified practitioner.
CASE HISTORY: CYCLIC MASTALGIA
She was prescribed the following tea for her to take after supper to help her sleep:
| Passionflower | (Passiflora incarnata) | 2 parts |
| Chamomile | (Matricaria chamomilla) | 2 parts |
| Skullcap | (Scutellaria lateriflora) | 1 part |
For her mastalgia and menstrual irregularities, she was prescribed the following tincture:
| Chaste berry | (Vitex agnus-castus) | 20 mL |
| Burdock root | (Arctium lappa) | 20 mL |
| Cleavers | (Galium aparine) | 20 mL |
| Dong quai | (Angelica sinensis) | 40 mL |
| Total: 100 mL |
NUTRITIONAL CONSIDERATIONS
Elimination of Coffee, Tea, and Other Caffeinated Products
An association between caffeine, or methylxanthines, and fibrocystic breast disease has been reported but remains controversial. In one study of a group of 102 women who had mammograms performed to measure the level of fibrocystic breast disease, a strong correlation was found with both caffeine and total methylxanthine ingestion and fibrocystic breasts as determined by a series of questionnaires.60 Similar results were found in a large case control study of 634 women.61 Other studies, however, have found only weak associations. Normal fluctuations in hormonal effects on breast tissue and difficulty in consistently measuring caffeine or methylxanthine intake make it difficult to conclusively demonstrate a causal relationship.62,63 In a review of the literature presented on AltMedex, the following studies are cited: A controlled clinical trial showed no clinically or statistically significant effects of alcohol- or methylxanthine-free diets on signs and symptoms of fibrocystic breast disease. One hundred sixty-two women with clinical and thermographic diagnoses of fibrocystic breast disease completed the study with evaluation at 6 months. It was concluded that abstinence from alcohol or methylxanthine-containing beverages is not likely to substantially reduce severity of fibrocystic breast disease within a few months.64 A case control study examined the relationship between coffee consumption and the development of benign breast disease involving the analysis of 854 cases of histologically diagnosed benign breast disease and 1748 control subjects. No association between coffee consumption and benign breast disease was found; neither was a dose–response relationship between methylxanthine consumption and benign breast disease development noted. These results suggest no association between caffeine intake and the development of benign breast disease.65 In a randomized study, 158 women with breast concerns were divided into two groups; one group abstained from consumption of methylxanthine-containing foods and beverages. The second group (controls) had no dietary restrictions. The patients were re-examined at 4 months for palpable breast findings. One hundred forty patients completed the study. There was a statistically significant decrease in clinically palpable breast findings in the abstaining group compared with controls, but the absolute change was minor and may be of little clinical significance. This study offered little support for the claim that caffeine-free diets are associated with clinically significant improvement in benign breast disease.66 In a study of 66 patients, restriction of dietary caffeine ingestion can cause improvement in fibrocystic breast disease. Graphic stress telethermometry (GST) was performed as an objective monitor for fibrocystic breast. At baseline, an average score of 83.5 on GST was observed in these women. Following dietary methylxanthine restriction, these scores were observed to be an average of 69.5 at 2 months and 55.5 at 6 months. Forty-two of the 66 patients had decreases in GST scores of more than 20 points at 6 months. Eighty-five percent of the patients showed improvement in GST patterns at 6 months, 15% of patients showed no change, and none showed worsening in GST patterns. Subjectively, at 6 months, 22 of 66 patients reported marked improvement, 30 of 66 moderate improvement, 6 of 66 mild improvement, and 8 of 66 no change in symptoms of fibrocystic breast disease. At pretreatment, 78% of patients had 2+ or 3+ nodularity on palpation. At the 6-month examination, 89% of patients had no or 1+ nodularity on palpation (91% had improvement, 9% had no change, and none had worsening). In 85 US women with clinical and mammographically confirmed fibrocystic disease, complete abstention from methylxanthine consumption resulted in complete resolution of fibrocystic breast disease in 82.5% and significant improvement in 15% of the patients studied.67
Vitamin E and B6 Supplementation
Supplementation with vitamin E (400 to 800 IU) may be beneficial for reducing mastalgia and nodularity of fibrocystic breasts and pyridoxine (vitamin B6/50 to 100 mg) to reduce breast tenderness and pain.68,69 Women are also encouraged to increase dietary fiber and complex carbohydrates, reduce dietary fat to 15% to 20% of their diet, and move toward a more plant-based diet, rich in phytoestrogens. A recent review examined various dietary therapies, and their potential effects in treating fibrocystic breasts.38 The review found that some dietary therapies, including vitamin E and B6, do not have adequate evidence to support their use in fibrocystic breasts. Studies were either of poor quality or had too few study participants to make definitive conclusions. Because of the dynamic nature and very high placebo response (20%) in fibrocystic breast complaints, only well-designed studies with large numbers of participants can address the efficacy of these treatments. Indeed, better-designed studies of vitamin E have all showed no significant effect on any parameter of fibrocystic breast. Studies on reducing caffeinated products from the diet have been variable, some showing positive outcome, others showing no benefit at all. This review found no studies that had examined the effect on fibrocystic breasts of low-fat diets, increased dietary fibers, soy isoflavones, or a more plant-based diet. However, there are considerable mechanistic data, including increasing unabsorbable estrogen conjugates for excretion, reducing the recirculation of estrogen, and positively affecting bowel microflora populations that support the use of these dietary strategies. The general health benefits of adjunct therapies such as adding vitamin E, reducing poor-quality fat intake, or reducing caffeine consumption suggest that these may be worthwhile strategies to try.38
ADDITIONAL THERAPIES
Exercise
It is important to evaluate whether women with breast pain are exercising appropriately and properly. Some women who believe that they are experiencing breast pain or tenderness are instead having chest wall pain, often resulting from inappropriate or overexercise, especially strength-building exercises that emphasize the pectoral muscles.39 No studies have examined the impact of any type of exercise on the symptoms or nodularity of fibrocystic breasts. Consequently, it is difficult to know what duration, type, or frequency of exercise would most benefit women with fibrocystic breasts.
Clothing
Inadequate support of the breasts is thought to lead to suspensory ligament strain, which may cause or contribute to pain and tenderness.55 No randomized controlled studies have examined breast support and its relationship to breast pain.
Stress Reduction
No studies have examined the effect of stress, anxiety, depression, or sleep disturbances on fibrocystic symptoms. However, many other pain syndromes, including fibromyalgia and vulvar pain, are closely associated with levels of “distress” in a woman’s life.70 No randomized clinical trials have been conducted using acupuncture for fibrocystic breast symptoms; however, several open label trials have found that up to 95% of women’s mastalgia was improved after acupuncture treatment.
TREATMENT SUMMARY FOR FIBROCYSTIC BREASTS AND BREAST PAIN
ENDOMETRIOSIS
Amanda McQuade Crawford, Aviva Romm
Endometriosis is one of the most common gynecologic problems in the United States and a leading gynecologic cause of both hospitalization and hysterectomy.25,71,72 Women with symptomatic endometriosis face chronic and sometimes debilitating pain; asymptomatic and symptomatic women alike may experience significant fertility problems due to this condition. The least-biased estimate for the overall prevalence of endometriosis in reproductive-age women is about 10%.73 Endometriosis is defined as the presence and growth of endometrial tissue in locations outside of the uterus. These cells may appear on the ovaries, fallopian tubes, bowel, bladder, peritoneal tissue, ligaments, or other structures in the abdominal cavity, and rarely may occur at other sites, including the nasal and respiratory passages leading to nosebleeds or pink frothy sputum at the time of the menses. Displaced endometrial tissue responds to cyclic hormonal changes, proliferating and shedding outside of the uterus. The bleeding is accompanied by inflammation caused by irritation of local tissue, such as, the peritoneum. Recurrent inflammation can cause scarring and adhesions that can cause pain and dysfunction of other affected sites. Endometriosis is common in women between menarche and menopause, and is associated with as many as 25% of cases of infertility; however, causality has not been definitively established.3,73
Endometriosis occurs across all socioeconomic and ethnic populations, is more common in women who experience early menstruation and fewer than two pregnancies, is associated with menstrual cycle length greater than 30 days, and is more prevalent in women with IUD use greater than 2 years (Box 7-2). Studies demonstrate that women who have experienced repeated vaginal and uterine infections have higher rates of endometriosis than the general population.3 Women with a mother or sister with endometriosis are more likely to suffer from severe endometriosis, suggesting a genetic predisposition; however, milder forms do not always have familial association. The literature is conflicting on the relationship between oral contraceptive (OC) use and the risk of endometriosis. A 1993 review by Vercellini et al. showed that four prospective investigations found a nonsignificant reduction in risk of up to 20%.74 Of three case control studies, two suggested an increased risk and one indicated a reduced risk of developing endometriosis with OC use. The 1994 analysis of the Oxford Family Planning Association OC study found a significantly reduced risk of endometriosis in current OC users. The researchers found that OCs were associated with a 60% reduction in endometriosis. The risk of endometriosis was significantly related to age with the highest risk occurring at ages 40 to 44 years when compared with women ages 25 to 29 years. On the other hand, the risk of endometriosis was elevated among women who formerly used the pill by almost twice the rate of women who had never used OCs. 75 76 77
Multiple theories exist on the etiology of this condition, including retrograde menstrual flow, lymphatic flow theory, and de novo origin. In fact, Konickx et al. propose that mild endometriotic lesions are common and to some extent normal at varying times in all women, and that it is symptomatic, aggressive, or deeply infiltrating endometriosis that should be considered a disease.78 Retrograde menstrual flow theory describes menstrual or endometrial tissue flowing backward through the fallopian tubes and into the abdominal cavity. Lymphatic flow theory suggests the spread of endometrial tissue throughout the body via the lymphatic system. Some researchers postulate that coelomic metaplasia, a de novo origin, might be induced by pathologic processes as a result of chemical exposure. A role for oxidative stress has also been suggested as one of the contributing factors for the development of endometriosis, possibly as part of a conglomeration of factors that pair immunologic and inflammatory factors in its etiology.79,80
There is substantial evidence that immunologic factors play a role in the pathogenesis of endometriosis and endometriosis-associated infertility, and that there is a bidirectional relationship between the endocrine and immune systems.81 In early endometriosis, elevated levels of inflammatory mediators such as cytokines, lymphocytes, and macrophages can be identified in the peritoneal fluid. Immune alterations include increased number and activation of peritoneal macrophages, decreased T-cell reactivity and natural killer cell cytotoxicity, increased circulating antibodies, the presence of autoantibodies, and changes in the cytokine network. Decreased natural killer cell cytotoxicity leads to an increased likelihood of implantation of endometriotic tissue. In addition, macrophages and a complex network of locally produced cytokines modulate the growth and inflammatory behavior of ectopic endometrial implants.79, 82 83 84 There also may be a positive correlation between immunosuppression and disease progression in the presence of established disease.85,86 Further, women with endometriosis appear to have higher rates of atopic conditions and susceptibility to opportunistic infections (e.g., candidiasis) than women who do not have endometriosis.87
SYMPTOMS
The following symptoms (Box 7-3), alone or in constellations, should alert a woman and her practitioner to the possibility of endometriosis: premenstrual pain, dysmenorrhea, dyspareunia, generalized pelvic pain throughout the month without other explanation, atypical periods, nausea, vomiting, exhaustion, bladder problems, frequent infections, dizziness, painful defecation, rectal pain, low backache, irritable bowels, or infertility. The far-reaching nature of these symptoms and their possible association with other conditions helps to explain why this condition is difficult to diagnose. Dysmenorrhea and painful intercourse become even more suggestive of endometriosis if they begin after a history of relatively pain-free menstruation and intercourse. Severity of pain is not indicative of the severity of the condition, with the exception of severe pain, which is associated with extensive endometriosis and adenomyosis (deeply infiltrating endometriosis.)3,78 Other causes of pelvic and abdominal pain or bleeding must be ruled out.
DIAGNOSIS
Endometriosis is most commonly seen in women 30 to 40 years old and is rarely found in postmenopausal women. Endometriosis has been thought not to occur prior to menarche; however, the rates of this condition are increasing among teenagers.3 The site of lesions, although widely variable, is generally the posterior cul de sac or ovaries. Diagnosis is based on pelvic examination, diagnostic ultrasound, or laparoscopy, with definitive diagnosis based on laparoscopy. CA-125 is a serum antigen found in endothelial cervical cancer that can also be found to be elevated in women with endometriosis. The diagnostic importance of the test for endometriosis is still uncertain; however, there appears to be some predictive value demonstrating which women might benefit from specific treatments on the basis of CA-125 levels, and CA-125 levels may indicate whether improvement is occurring.88 Endometriosis is staged based on the location(s) of the endometrial tissue as follows:
CONVENTIONAL TREATMENT APPROACHES
Medical treatment of endometriosis includes both pharmaceutical and surgical approaches. Pharmaceutical treatments provide only suppression of the disease; they do not exact a cure.3 Decisions regarding treatment are based on endometriosis severity and staging, symptom picture, and ultimately, the woman’s needs and goals, for example, desire for children in the future.89 For women experiencing mild symptoms (or none) and for women who are close to menopause, the appropriate treatment may be to do nothing.78 For women with mild to moderate symptoms, and those who desire pregnancy, the appropriate pharmacologic therapy should be considered, and if necessary, can be combined with conservative surgery. It should be noted that, in spite of medical treatment, endometriosis has a high recurrence rate of 5% to 20% unless total hysterectomy and bilateral oophorectomy are performed. With pharmacologic interventions, pain typically resumes upon cessation of medications, although initially with pain that is less intense than prior to treatment. Pain relief, pregnancy rates, and recurrence rates are similar with all treatment methods. The goal of pharmaceutical treatment is to interrupt patterns of endometrial stimulation and bleeding.89 Pharmaceutical options include nonsteroidal anti-inflammatory drugs (NSAIDs) and hormonal therapies (progestins, GnRH analogs, Danazol, and oral contraceptives). NSAIDs (i.e., ibuprofen, naproxen) may be prescribed for mild to moderate pain. They are relatively safe for short-term symptomatic relief; however, long-term use can lead to health consequences, including gastrointestinal bleeding, and should be avoided in patients with a history of peptic ulcer disease or renal failure. It should be noted that none of these therapies demonstrates significant benefit over the others, and all are associated with a high recurrence rate (20%–50%) upon discontinuation of the therapy.89 Progestins (medroxyprogesterone acetate, i.e., Depo-Provera) suppress the response of endometrial tissue to cyclic hormones, leading to atrophy of this tissue and decreased pain. They are typically better tolerated than oral contraceptives (OCs), with fewer side effects, and are less costly than Danazol and GnRH analogs. This is often considered the pharmaceutical treatment of choice for endometriosis, although the FDA no longer supports the use of Depo-Provera for this purpose. Side effects include weight gain, fluid retention, and breakthrough bleeding. Depression is a common significant side effect of medroxyprogesterone use. In high doses, medroxyprogesterone can adversely affect lipid profiles, and may lead to a state of hypoestrogenism, with subsequent potential for bone loss. Oral contraceptives are used to control pain in women not desiring pregnancy. The combined effect of estrogen and progesterone is to induce a state of “pseudopregnancy,” and appears to lead to a 90% rate of improved symptoms with long-term use. Side effects include all those typically associated with general OC use. Danazol, a synthetic testosterone, is used for the treatment of mild to moderate endometriosis in women who desire fertility but not necessarily in the immediate future. It induces a state of “pseudomenopause,” eliminating mid-cycle FSH and LH surges. Once considered the optimal treatment for endometriosis, it is now considered no more effective than any of the other pharmaceutical treatments. Possible side effects include weight gain, fluid retention, fatigue, decrease in breast size, hirsutism, atrophic vaginitis, hot flashes, muscle cramps, emotional fluctuations, voice changes, spotting, and decreased HDL cholesterol levels. In some patients it may cause hepatocellular damage; thus is contraindicated in patients with liver disease, and liver enzymes should be monitored in all patients during treatment. It is also contraindicated in patients with a history of hypertension, hyperlipidemia, congestive heart failure, renal impairment, and pregnancy.3,25,89,90 Gonadotropin-releasing hormone analogues (GnRH-a, e.g., Leuprolide) also cause a suppression of endometriosis via a “pseudomenopausal” state. GnRH treatment does not carry the same risks of negative impact on serum lipids and lipoproteins compared with Danazol; however, it does interfere with calcium metabolism via stimulation of a hypoestrogenic state, and thus can cause osteoporosis. Even after only 6 months of use, a 6% to 8% loss in trabecular bone has been observed. It can take up to 2 years after cessation of treatment to replace this bone loss; thus a treatment is usually restricted to 6-month durations.3,91
Surgical options include conservative surgery (destruction and removal of endometriomas while maintaining reproductive function) and radical surgery (hysterectomy, generally accompanied by bilateral salpingo-oophorectomy). Conservative treatment involves the removal of endometriotic lesions and restoration of normal anatomical relationships via removal of adhesions to the greatest extent possible, with the goal of pain relief, and possible restoration of fertility when achieving pregnancy is desired and has been impaired by the condition. In approximately one-fourth of women treated surgically, however, there is no improvement in fertility even if the disease was considered mild. Procedures include knife excision, laser surgery, electrocautery, curettage, and laparotomy. The worse the disease, the worse the statistics for conceiving after surgery.92,93 Recurrence of endometriosis after surgery is dependent on the skill of the surgeon and extent of disease, and as with other endometriosis treatments, rates may be as high as 20%. Hysterectomy will not remove lesions outside the uterus and is not considered a successful treatment when used primarily to reduce the symptom of chronic pelvic pain. Surgery carries the risk of complications, especially adhesion formation and continuing pain. The total rate at which symptoms of chronic pelvic pain returned after drug treatment is estimated to be 5% to 15% at the end of 1 year, or up to 50% at the end of 5 years. Nonetheless, the risks of surgery or medication may be justifiable for severe pain that does not respond to other methods, especially if menopause is not expected for some time. There is a concern that diagnostic microsurgery may aggravate or cause the transfer of viable endometrial cells into general or lymphatic circulation, thereby causing the very condition being identified. Because of this, some wary clients choose natural treatment approaches for the condition without a certain diagnosis, believing that if the signs and symptoms respond, the holistic prescription was correct for the presumed diagnosis.
BOTANICAL TREATMENT
Herbalists share the conventional medical perspective that endometriosis has multifactorial causes. The botanical approach, however, takes into consideration immune dysregulation, inflammation, hormonal dysregulation, diet and nutritional status, lifestyle, exposure to exogenous estrogens, and the woman’s emotional and psychological mechanisms for coping with this condition as components of a whole picture. Given that nonradical medical treatments for endometriosis are purely suppressive rather than curative, the high recurrence rate of endometriosis upon cessation of pharmaceutical treatment, and the potential for drug-related or surgical side effects, botanical medicines may provide women with a safe alternative for symptomatic pain relief, reduction of inflammation, prevention and reduction of recurrent vaginal and pelvic infections, stress reduction, and improvement of overall immunologic health (Table 7-4). By applying a comprehensive natural health care protocol, many cases of endometriosis can also be resolved. The herbal approach should also include as part of the protocol, herbs that address concomitant discomforts arising from the condition, such as irritable bowel complaints or depression.
DISCUSSION OF BOTANICALS
For many women with endometriosis pain is the single most debilitating aspect of this condition (other than chronic fertility problems in women desiring pregnancy). Therefore, pain management should be an important focus in the care of women with this condition. Herbalists reliably employ a number of herbs for the treatment of pelvic and abdominal pain, many of which have a long history of traditional use for painful gynecologic conditions. These herbs can be used singly but are generally used in various combinations with other herbs in these categories, or as part of a larger protocol. Analgesic herbs are used for generalized or local pain of an aching or sharp quality and include black cohosh, black haw and cramp bark, chamomile, corydalis, pulsatilla, dong quai, ginger, and Jamaican dogwood. Corydalis, Jamaican dogwood, and pulsatilla are especially dependable for moderate to serious pain. Pulsatilla is considered specific for ovarian pain.22 Antispasmodics are typically used for cramping pain, but also may be used for sharp or dull pain, aching, and drawing pains in the lower back and thighs, and include, such as wild yam, the viburnums (cramp bark and black haw), black cohosh, chamomile, and ginger. Dong quai’s traditional TCM uses for gynecologic conditions, specifically for conditions of blood vacuity and stasis, the latter of which endometriosis may be considered among, along with its antispasmodic, anti-inflammatory, and immunomodulatory qualities, make it an important herb to consider.25,50,94 Many antispasmodics and anti-inflammatories, such as wild yam, the viburnums, ginger, and chamomile are specific not only for uterine pain, but also for intestinal, bowel, and urinary pain and irritability, making them uniquely suitable for endometrial pain and accompanying bowel and bladder discomforts. This is important to keep in mind, because the pain of endometriosis is related to irritation of tissue by endometrium outside of its normal site in the uterus. Sedatives are useful when there is the need to induce deep rest or sleep to obtain pain relief, and include
Formula for Treatment of Mild to Moderate Pain Associated with Dysmenorrhea
| Black cohosh | (Actaea racemosa) | 20 mL |
| Cramp bark | (Viburnum opulus) | 20 mL |
| Chamomile | (Matricaria recutita) | 15 mL |
| Dong quai | (Angelica sinensis) | 15 mL |
| Wild yam | (Dioscorea villosa) | 15 mL |
| Licorice | (Glycyrrhiza glabra) | 10 mL |
| Ginger | (Zingiber officinale) | 5 mL |
| Total: 100 mL |
Formula for Treatment of Moderate to Severe Pain Associated with Dysmenorrhea
| Black cohosh | (Actaea racemosa) | 25 mL |
| Cramp bark | (Viburnum opulus) | 25 mL |
| Wild yam | (Dioscorea villosa) | 20 mL |
| Corydalis | (Corydalis ambigua) | 15 mL |
| Jamaican dogwood | (Piscidea piscipula) | 15 mL |
| Total: 100 mL | ||
California poppy, a combination of black cohosh and cramp bark, or a combination of cramp bark and Jamaican dogwood. Valerian and hops are also useful sedatives. The successful use of these herbs for pain depends largely upon adequate dosing and frequency of administration.
Immunomodulation
The exact immunologic underpinnings of endometriosis remain uncertain. There appears to be a complex interplay of hyperimmune, autoimmune, and hypoimmune function at work, either variably or concurrently-leading only to the clear understanding that there is some level of immune dysregulation that accompanies this condition. The most appropriate response seems to be twofold: (1) to look at the unique constellation of symptoms presented by each individual woman, for example, whether she is depleted and susceptible to frequent colds and repeated vaginal infections or chronic atopic conditions—and to treat accordingly, and (2) to provide botanicals which support immune regulation—notably, the adaptogens. For women who evidence a state of immune depletion in combination with endometriosis some amount of immunostimulation may be appropriate to bolster the overall immune response, and may be provided through the use of herbs such as echinacea, astragalus, or Picrorrhiza kurrhoa in combination with adaptogens such as ashwagandha, American ginseng, rhaponticum, or rhodiola. These women also may benefit greatly from medicinal mushrooms such as Reishi and Cordyceps for immune support. For women with signs of hyperimmunity, atopic conditions such as eczema or chronic rhinitis, or autoimmunity, the use of immunosupportive anti-inflammatory adaptogens may be most appropriate, such as licorice, ashwagandha, and American ginseng. It is unknown and a matter of great debate as to whether immunostimulating herbs such as echinacea and astragalus are safe and appropriate for use when there is autoimmunity.22 Using adaptogens for treating endometriosis makes sense in that their actions simultaneously influence and restore normalcy to the functions of the immune system and the HPA axis, both of which appear to have dysregulated function in this condition. The uterine endometrium is a complex structure of interspersed glandular tissue and endometrial stroma, closely associated with lymphoid tissue.81 The inclusion of herbs that are traditionally thought to improve lymphatic circulation, such as calendula, echinacea, cleavers, and pokeroot, are commonly included in botanical protocol for endometriosis.
Anti-Inflammatories and Antioxidants
Inflammation is a hallmark of endometriosis, and as discussed, free radical damage may be part of the etiology of this disorder. It has been suggested that growth factors and inflammatory mediators produced by activated peritoneal leukocytes participate in the pathogenesis of endometriosis by facilitating endometrial cells growth at ectopic sites.81 Elevated levels of inflammatory cells and mediators such as peritoneal macrophages, prostaglandins, proteolytic enzymes, complement fragments, IL-1, and tumor necrosis factor (TNF) have been identified in the peritoneal fluid of patients with endometriosis.81 Numerous herbs that have been used traditionally for inflammatory types of conditions demonstrate significant anti-inflammatory and antioxidant effects and should be considered for use in formulations for treatment and symptomatic relief, along with herbs whose use for inflammation is only recently being discovered. These are discussed in the following.
Dong Quai
Dong quai has antispasmodic, analgesic, and tonic effects, and has demonstrated significant antioxidant and free radical scavenging actions, partially through inhibition of anion radical formation. Limited animal and in vitro studies have reported on the specific immunomodulatory effects of dong quai, including a stimulation of phagocytic activity and interleukin-2 (IL-2) production, and an anti-inflammatory effect. There is evidence to suggest that the polysaccharide fraction of dong quai may contribute to these effects. Immunostimulatory and anti-inflammatory effects have also been documented for isolated ferulic acid. Dong quai has been traditionally used in Chinese medicine for the treatment of “blood stasis,” which encompasses a diagnosis of endometriosis.
Echinacea
Echinacea is widely used by herbalists for its immunostimulatory and anti-inflammatory effects, to support and promote the body’s natural immune responses.25,95,96 Antioxidant effects appear to include free radical scavenging mechanisms and transition metal chelating, whereas immunostimulating effects include enhanced phagocytosis, and the stimulation of cytokine and immunoglobulin production.29,97
Feverfew
Feverfew has been used for the treatment of menstrual complaints since at least the time of the ancient Greeks. In fact, its botanical name may reflect such use—parthenos means “virgin” in Greek.98 It is mentioned by the Eclectic physicians for use in the treatment of menstrual irregularity.99 Feverfew also has been used for the treatment of other inflammatory conditions, including headache, fever, psoriasis, and arthritis. Although studies have not been done on the use of this herb in the treatment of endometriosis, and indeed, it is not widely discussed for such use even in the herbal literature, its pharmacology and actions as an antinociceptive and anti-inflammatory suggest that consideration of such use is warranted.100 Feverfew has exhibited inhibition of prostaglandin synthetase, which inhibits the conversion of arachidonic acid to inflammatory prostaglandins, inhibits mast cell degranulation and subsequent histamine and serotonin release, and has shown inhibition of other inflammatory cytokines such as TNF-α, IL-1, NFκB, and IFN-γ, as well as inhibiting peritoneal cyclooxygenase in animal models.97
Ginger
Herbalists use ginger root as an anti-inflammatory and antispasmodic herb for the treatment of numerous painful inflammatory conditions from arthritis to dysmenorrhea.101,102 No studies have been identified for its use for painful gynecologic complaints. One trial of 120 women reported ginger to be an effective antiemetic for the treatment of postoperative nausea, with specific trials demonstrating its efficacy in reducing postlaparoscopic gynecologic procedures. However, two other trials demonstrated either no effect compared to placebo, or negative effect (increased nausea) with increased doses of ginger.97 Ginger remains popular among Western and TCM herbalists as an antispasmodic treatment for dysmenorrhea.25 It is taken in tincture form in combination with other herbs, in infusions, and also used externally as a poultice and in baths for pelvic discomfort.
Gotu Kola
Numerous studies support the traditional uses of the popular Ayurvedic herb gotu kola to promote wound healing, and as an anti-inflammatory and antimicrobial herb. Additionally, it has been demonstrated to have antiproliferative and antioxidant effects, and to prevent the formation of keloid scar tissue. 103 104 105 106 107 Its use as a neurogenerative and neurotrophoretorative adaptogen makes it particularly useful in the treatment of stress disorders, and may play some role in its mediation of other effects.108 Given the association of these actions with the clinical and etiologic picture of endometriosis, this herb deserves consideration as part of a protocol for this condition both when botanical treatment is the primary modality, or to heal from surgical intervention and reduce adhesion and scar formation. Gotu kola is typically used in combination with other herbs as part of a comprehensive formula.
Green Tea
Green tea is rich in polyphenolic compounds, with catechins as its major component. Studies have shown that catechins possess diverse pharmacologic properties that include antioxidative, anti-inflammatory, anticarcinogenic, and antibacterial effects. Tea catechins are well absorbed in the gastrointestinal tract; thus drinking unfractionated green tea is the most simple and beneficial way to consume this herb.109,110
Licorice, Calendula, and St. John’s Wort
Licorice root (Glycyrrhiza glabra) and calendula blossoms (Calendula officinalis) are used by herbalists as anti-inflammatory herbs, and may frequently be included in formulae for treating endometriosis. The aim of one recent study was to investigate whether standardized hydroalcoholic plant extracts such as calendula, St. John’s wort, plantain (Plantago lanceolata), and licorice can suppress the activities of 5-lipoxygenase (5-LO) and cyclooxygenase-2 (COX-2), key enzymes in the formation of proinflammatory eicosanoids from arachidonic acid (AA). The researchers concluded that licorice extract might be a potential drug possessing anti-inflammatory activity devoid of the most troublesome (gastric) side effects seen for drugs used as COX-2 and 5-LO inhibitors. They purport that St. John’s wort, plantain, and licorice extracts can be added to an already impressive list of botanicals with anti-inflammatory activity.111
Peony and Rehmannia
Two herbs commonly used in TCM formulae, peony and rehmannia, have demonstrated significant anti-inflammatory and antispasmodic activity. Both are specifically recommended by Mills and Bone for endometriosis, whereas Low Dog discusses the use of peony in the traditional Japanese medicine Shakuyaku-kanzo-to (which also contains the anti-inflammatory Glycyrrhiza uralensis).22,25,108 Studies using the latter formula have demonstrated prostaglandin production inhibition in the uterine myometrium via phospholipase A2 inhibition, whereas other studies have demonstrated arachadonic acid inhibition, PAF inhibition, reduction in free radical formation, and smooth muscle relaxation. Note that nearly all of the studies use these herbs in traditional formulae rather than in isolation, and that studies are conducted in animal models, and have focused on arthritis, ulcers, and other chronic inflammatory conditions. Licorice, a potent anti-inflammatory, is frequently included in TCM formulae that also contain peony and rehmannia, as is dong quai when these herbs are used for gynecologic conditions. It must be remembered that in TCM, herbs are not prescribed on the basis of a disease entity or a pharmacologic expectation of efficacy, but rather on an individual diagnostic approach using traditional parameters and categories.
Sour Cherries and Raspberry Fruit
Although not part of a traditional botanical approach to gynecologic problems, interesting new data suggests that sour cherry anthocyanins may have a beneficial role in the treatment of inflammatory pain. The antihyperalgesic effect may be related to the anti-inflammatory and antioxidant properties of anthocyanins and was found comparable to the commercial antioxidants and superior to vitamin E, at a test concentration of 125 µg/mL.112 Anthocyanins from raspberries (Rubus idaeus) and sweet cherries (Prunus avium) demonstrated cyclooxygenase-I and cyclooxygenase-II inhibitory activities comparable to those of ibuprofen and naproxen at 10 μM concentrations.113 The value of these findings in endometriosis is not known, but perhaps this is a worthy reminder that a diet rich in deep-colored berries and other fruits and vegetables may hold a key to improving health and preventing disease.
Hormonal Modulation
Predicated on the belief that steroid hormones are the primary regulators of the growth and activity of ectopic endometrial tissue, therapies aimed at hormonal modulation have been the foundation of conventional therapy for endometriosis, and have also featured prominently in botanical protocol.81 Unfortunately, even less is known about the effects of botanical therapies on the endocrine system than about pharmaceutical medications. The goal of pharmaceutical therapies is to create an acyclic, low-estrogen environment that prevents bleeding, leads to atrophy of ectopic implants, and possibly minimizes retrograde bleeding. However, endometrial tissue may be histologically different than normal uterine endometrial tissue, and may respond differently to hormonal stimulation. Again, much remains unknown about this enigmatic condition.81 Several botanicals are frequently used as hormonal modulators, in conjunction with other herbs discussed in this chapter. Little research is available on their application in endometriosis. But as part of a comprehensive protocol, many herbalists and naturopathic physicians report positive outcomes for achieving the goals established earlier in this paragraph. There is also some small discrepancy in the herbal literature as to which herbs should be avoided due to potential exacerbating hormonal effects. For example, Mills and Bone caution against the use of what they refer to as “estrogen promoting herbs” such as false unicorn (Chamaelirium luteum), the use of which should primarily be avoided in clinical practice due to its endangered status) and wild yam (Dioscorea villosa); however, wild yam is used widely for abdominal and pelvic cramping pain associated with the condition, whereas the late Silena Heron included these in endometriosis protocol and authors Hobbs and Keville, in Women’s Herbs, Women’s Health, mention wild yam as an antispasmodic specifically for endometriosis.22 There would actually potentially be numerous plants in this category, ranging from fennel and hops to common foods such as legumes, most of which are rich in phytoestrogens, which would need to be avoided on this presupposition. One study on the estrogenic contents and activity of commonly used herbs found that soy, red clover, licorice, hops, and fo-ti have a large amount of measurable estrogen bioactivity not previously reported. Chaste tree berry, black cohosh, and dong quai did not have measurable activity with the methods used in the study.114 Confusion stems largely from the fact that so much remains unknown about the endocrine effects of botanicals, and until more information is available, rational conclusion are hard to draw, suggesting that caution and observation of clinical response are required. Women with a predisposition to estrogen-dependent cancers are probably wise to avoid unnecessary and excess consumption of herbs with estrogenic effects. However, it should also be considered that herbs that competitively bind with estrogen receptors might actually displace endogenous estrogen with weaker, plant-based estrogens, actually decreasing a woman’s overall estrogen response.
Blue Vervain
Blue vervain (Fig. 7-3) has a long history of use in traditional European herbalism as an emmenagogue and galactagogue, and a contemporary popularity among herbalists experienced in women’s reproductive care for its regulating effects on gynecologic complaints, particularly for irritability associated with PMS. A BIOSIS database search and extensive review of the herbal literature references yields very little data on the medicinal uses of this plant, although historical references to its use as a treatment for rheumatism were identified.29,115 A single study from 1974 on the effect of this herb on the uterus and its interactions with prostaglandins was identified but not obtainable. According to studies cited in Wichtl et al., hot water extracts of European verbena stimulates luteinizing hormone (LH) and FSH secretion. Other noted endocrine effects include antithyrotropic and abortifacient effects via inhibition of human chronic gonadotropin (hCG). Verbena has also demonstrated immunomodulatory effects, primarily through inhibition of phagocytosis by human granulocytes.29 The German Commission E cites its uses, among other things, for irregular menstruation, nervous disorders and exhaustions, and complaints of the lower urinary tract; however, the efficacy for these claims remains unsubstantiated.29 Many herbalists consider it an excellent herb for “sluggishness of the liver,” and attribute its hormonal action to stimulated liver function and subsequent actions on hormonal metabolism and elimination. It is typically used as a small part of a larger general formula aimed at treating underlying causes of endometriosis.
Chaste Berry
Chaste berry has a reputation for its ability to regulate female menstrual cycles and relieve complaints and complications stemming from dysregulation of sex hormones. Clinical trials support the use of Vitex for menstrual irregularities (secondary amenorrhea, oligomenorrhea, polymenorrhea), relief of PMS symptoms, mastalgia, latent hyperprolactinemia, and infertility due to luteal phase dysfunction.116 The effects of Vitex on estrogen levels remains uncertain, with one study (the full details of which were undisclosed) demonstrating its ability to elicit estrogen-like effects (increased uterine growth) in ovariectomized rats, and another reporting decreased estradiol levels, whereas other studies have reported no effects or were inconclusive.116 Its efficacy in the treatment of endometriosis, for which is it widely used by herbalists, is supported by clinical observation, with no research identified for its use for this condition.
Cotton Root
Cotton is predominantly used as a uterine tonic and to stimulate uterine contractions. Gossypol, the active ingredient in the roots and seeds of cotton, has been used in the treatment of gynecologic disorders ranging from uterine myomas to menopausal bleeding, based on the discovery that regular cooking with cottonseed oil over long periods of time leads to amenorrhea and endometrial atrophy in females. Several studies over a 15-year period have demonstrated short-term efficacy of up to almost 90% in the treatment of endometriosis, and long-term effectiveness after 1 to 3 years of 54% to 63%. Treatment is typically accompanied by amenorrhea persisting for up to 6 months in 80% of women, and up to 1 year in 16% of women, with 4% experiencing amenorrhea lasting longer than 1 year.117 Gossypol is reported to antagonize the actions of estrogen and progesterone, and may mimic a pseudomenopausal state.117 A frequent side effect of gossypol treatment is hypokalemia, which is treated with administration of slow-releasing potassium salts. High-dose programs can lead to elevated liver enzymes, nausea, edema, and palpitations, as well as possible rash, reduced appetite, fatigue, and possible inhibition of thyroid function and mitochondrial energy metabolism. This compound is not available in the West, and would be considered a pharmaceutical drug rather than a botanical product were it made so. Studies on the use of cotton root bark, used, as a partus-preparator, emmenagogue, and abortifacient by Western herbalists, as an herbal extract for endometriosis have not been conducted.
St. John’s Wort
According to modern clinical research, St. John’s wort is commonly used for the treatment of mild to moderate depression and additionally has been shown to exhibit antiviral activity. In traditional herbal medicine currently and historically, it is used internally for anxiety and as a general nervous system tonic, whereas externally it is used as a primary application for scrapes and burns. Recent concerns regarding the interaction between St. John’s wort and numerous pharmaceutical drugs have led to a host of contraindications for use of this herb. One such contraindication is the use of oral contraceptives, as it has been shown to induce the activity of cytochrome P450 3A4 (CYP3A4) and increase the clearance of numerous drugs and steroids, such as cortisol and ethinyl estradiol.118 This interaction suggests the potential for use of St. John’s wort to positively interfere with estrogen binding in states of estrogen excess, for example, in endometriosis. A limited number of studies have evaluated the estrogen-binding capacity of St. John’s wort extracts. One study by Simmen et al. found that estrogen binding was 50% inhibited by the bioflavonoid I3,II8-biapigenin at micromolar concentration in the CNS.119 Use of St. John’s wort to deliberately modulate estrogen levels represents a potentially novel application for this botanical. This herb should also be considered in endometrioses treatment for its beneficial role in the treatment of mild to moderate depression, which may accompany this condition in women who suffer with it chronically.
Uterotonics and Emmenagogues
Endometriosis Patient 1
| Chaste berry | (Vitex agnus-castus) | 12.5 mL |
| Black haw | (Viburnum prunifolium) | 25.5 mL |
| Blue vervain | (Verbena officinalis) | 25.5 mL |
| Yarrow | (Achillea millefolium) | 12.5 mL |
| Milky oats | (Avena sativa) | 12.5 mL |
| Corydalis | (Corydalis ambigua) | 12.5 mL |
| Total: 100 mL |
Tincture Formula II (end of menses to ovulation)
| Chaste berry | (Vitex agnus-castus) | 25 mL |
| Calendula | (Calendula officinalis) | 12.5 mL |
| White peony | (Paeonia lactiflora) | 20 mL |
| St. John’s wort | (Hypericum perforatum) | 30 mL |
| Partridge Berry | (Mitchella repens) | 12.5 mL |
| Total: 100 mL |
Endometriosis Patient 2
| Chaste berry | (Vitex agnus-castus) | 12.5 mL |
| Cramp bark | (Viburnum opulus) | 25 mL |
| Blue vervain | (Verbena officinalis) | 25.5 mL |
| Ashwagandha | (Withania somnifera) | 25 mL |
| Black cohosh | (Actaea racemosa) | 12.5 mL |
| Total: 100 mL |
Tincture Formula II (end of menses to ovulation)
| Chaste berry | (Vitex agnus-castus) | 20 mL |
| Blue Cohosh | (Caulophyllum thalictroides) | 10 mL |
| Sarsaparilla | (Smilax ornate) | 10 mL |
| Milk Thistle | (Carduus marianus) | 20 mL |
| Partridge Berry | (Mitchella repens) | 10 mL |
| Wild Yam | (Dioscorea villosa) | 20 mL |
| Valerian | (Valeriana officinalis) | 10 mL |
| Total: 100 mL |
NUTRITIONAL CONSIDERATIONS
In addition to a balanced whole foods diet, use high-quality oils, and minimize consumption of caffeine, sugar, alcohol, red meat, and large amounts of dairy. Also avoid excess refined carbohydrates, and address hypoglycemia with frequent small meals and snacks with high protein and complex carbohydrates. Adequate consumption of essential fatty acids is important; encourage two to three servings of salmon or other high-quality cold-water fish per week. Fatty acid–mediated mechanisms have demonstrated decreased cytokine-induced adhesion molecule expression, thereby reducing inflammatory leukocyte–endothelium interactions and modified lipid mediator synthesis, thus influencing the transendothelial migration of leukocytes and leukocyte trafficking in general. Even the metabolic repertoire of specific immunocompetent cells such as cytokine release or proliferation is modified by n-3 fatty acids. Beyond this these fatty acids regulate lipid homeostasis shifting the metabolic pathways toward energy supply, thus optimizing the function of immune cells. Because of the regulatory impact on different processes of inflammatory and immune cell activation n-3 fatty acids provide positive effects on various states of immune deficiencies and diseases with a hyperinflammatory character, among which selected examples are presented.120
CERVICAL DYSPLASIA: BOTANICAL AND NATUROPATHIC APPROACHES
Cervical dysplasia describes cervical cells with an atypical appearance, loss of uniformity in cell structure, and loss of their normal architectural orientation. Each year between 250,000 and 1 million women in the United States are diagnosed with cervical dysplasia. It can occur at any age, but the mean ages are 25 to 35 years old. Atypia and dysplasia can be caused by inflammation, cervical intraepithelial neoplasia (CIN), or carcinoma in situ (CIS) (see Staging). Atypical cervical cells can be a precursor to invasive cervical cancer. Mild dysplasia is the most common form of cervical dysplasia, and up to 70% of these cases regress on their own, the cervical tissue returning to normal without treatment. Moderate and severe dysplasias are less likely to resolve spontaneously and have a higher rate of progression to cancer. The greater the abnormality of the cells as determined by staging, the higher the risk for developing cervical cancer. Cervical cancer is the third most common gynecological malignancy in US women (see Chapter 11). Cervical dysplasia is inversely related to the age of first intercourse; it is directly related to the number of sexual partners in the woman’s lifetime, and the risk increases
CONVENTIONAL TREATMENT APPROACHES
cervical cerclage, in which a suture is inserted into the cervix to allow the woman to maintain pregnancy and prevent premature labor.
BOTANICAL AND NATUROPATHIC TREATMENT
Intrinsic to both herbal and naturopathic treatment of cervical dysplasia is the belief that conventional therapy alone does not address a woman’s underlying propensity to dysplasia, nor does it address preventable causes. This is significant as the 5-year return rate, with conventional therapies is as high as 75%. In contrast to conventional approaches, botanical and naturopathic treatments attempt to addresses multifactorial causes, treating the woman, not just her cervix. A disadvantage is that natural medicine protocols are demanding, inconvenient, and potentially costly, requiring multiple office visits. Naturopathic and botanical medicine practitioners emphasize a number of therapies including the use of immune enhancing, anti-inflammatory, hormone-regulating, and antiviral botanicals both for internal and topical treatments.25,121 Adaptogens often feature prominently in a botanical program to address immune and endocrine function. Stress can depress immune response, which can increase viral activation. Recent lifestyle changes or stressors, even as seemingly benign as exposure to increased amounts of sunlight, may suppress the immune system sufficiently to cause viral activation.122 Environmental causes of gynecologic disease—ranging from exposure to excess exogenous estrogens to stress—must be addressed in the long-run for the benefit of all women. (See Endometriosis for a brief discussion of the role of exogenous estrogens on gynecologic health.)
An Herbalist’s Approach to Cervical Dysplasia
Because mild cervical dysplasia has a high rate of spontaneous regression one approach of botanical practitioners is to encourage watchful waiting while using antiviral and immune supportive herbs internally to boost immunity and topically to reduce viral proliferation and inflammation and promote tissue healing. For persistent or more than mild cervical dysplasia, experienced gynecologic botanical practitioners favor the use of the LEEP procedure in combination with the postprocedural use of herbal suppositories and internal therapies to enhance immunity, reduce inflammation, and promote healing. Because stress may play both a contributory role in immune and endocrine dysregulation, and is often a consequence of a diagnosis of cervical dysplasia, long-term use (3 to 6 months) of adaptogens is also commonly recommended. Topical herbal applications consist of insertion of a medicated suppository 5 nights per week for as many as 12 weeks, with a repeat PAP at the end of 12 weeks. Several companies sell preformulated suppositories (see Resources at the end of this chapter). Alternatively, practitioners can make suppositories for patients using a suppository mold, or make a mold available to patients who wish to prepare their own. General instructions for preparing suppositories can be found in Chapter 3, with specific recipes for dysplasia treatment in the following pages. They can be prepared in large batches and kept refrigerated for the duration of treatment for use as needed. If after 3 months of botanical treatment the dysplasia has not improved or has progressed, further medical treatment should be pursued.
Although there are many similarities between botanical and naturopathic treatment of cervical dysplasia, the two approaches diverge over the use of escharotic treatments, a popular naturopathic approach. Escharotics can be caustic and irritating, and are much less controllable and reliable than the LEEP. The direct experience of herbalists with escharotic treatments does not endorse their use. There is no evidence for the efficacy of escharotic treatments for cervical dysplasia, and side effects seen with the LEEP, such as cramping and abdominal pain, may also be seen with escharotics. Additionally, escharotic treatment used topically on other tissue (e.g., for the treatment of breast tumors or skin cancers) has been associated with significant tissue damage in some cases. One observational study reports on dermatologic cases in which four patients had used escharotics in the treatment of basal cell carcinomas (skin cancer) in lieu of the recommended conventional treatment. One patient had a complete clinical response but had a residual tumor on follow-up biopsy. A second patient successfully eradicated all tumors but experienced severe scarring. A third patient disagreed with the physicians regarding her care and was lost to follow-up. One patient presented with a basal cell carcinoma that “healed” for several years following treatment with an escharotic agent but recurred deeply and required extensive resection. The lesion eventually metastasized. The researchers concluded that physicians should advise their patients against the use of escharotics. Low Dog states that although at this time there is only anecdotal evidence of the efficacy of escharotic treatments for cervical dysplasia, and because several of the herbs typically used possess antiviral and anti-inflammatory effects, further research is warranted.25 Nonetheless, their use is popular among naturopathic physicians, and women may choose this option when looking for alternatives to conventional medical treatments. Thus, practitioners should be aware of their use.
Many licensed naturopaths specializing in the treatment of gynecologic complaints report excellent results and a high level of safety when using these preparations. No evidence for efficacy or safety for cervical dysplasia was identified in the literautre.25 Naturopathic protocols, including the use of escharotics are described in detail in Women’s Encyclopedia of Naturopathic Medicine by Tori Hudson. It remains a popular alternative that women may seek or be offered through their naturopathic care provider. It is strongly urged that only licensed NDs with adequate training and experience in the use of escharotic treatment for cervical dysplasia be consulted for this procedure.
Herbs commonly used internally and topically, both for herbal and naturopathic treatments are listed in Table 7-5. Evidence and discussion of adaptogens is found elsewhere in this volume. Readers are also referred to the chapter on cervical cancer for relevant botanical information as well as to Chapter 9.
Botanical Treatment Program for Cervical Dysplasia
Internal Formula for Immune Support: Antiviral, Anti-inflammatory, and Adaptogenic Effects
| Reishi mushroom | (Ganoderma lucidum) | 30 mL |
| Echinacea | (Echinacea spp.) | 25 mL |
| St. John’s wort | (Hypericum perforatum) | 15 mL |
| Ginseng | (Panax ginseng) | 15 mL |
| Licorice | (Glycyrrhiza spp.) | 15 mL |
| Total: 100 mL |
Suppository for Cervical Dysplasia/HPV Infection
Low Dog states that a multivitamin with folate and B vitamins may be especially indicated for women with cervical abnormalities, citing one study evaluating the relationship between individual nutrients and persistent HPV infection, which showed that circulating levels of vitamin B12 were inversely correlated with persistent HPV infection after adjusting for numerous factors, and another study demonstrating that low serum homocysteine levels were highly predictive of invasive cervical cancer risk, possibly suggesting folate, B12, or B6 insufficiency.25 Also see Cervical Cancer.
DISCUSSION OF BOTANICALS
Blood Root
The blood-red color of the sap from the roots of blood root led to its traditional use as a blood purifier. It was used as an emmenagogue, in the treatment of respiratory conditions, as a strong emetic, and for the treatment of fungal infections and ulcers.124 By the eighteenth century, blood root was used topically to treat indolent chancres and tumors as an ingredient in the popular “black salve,” an escharotic treatment that was used topically for the treatment of tumors.25 Extracts of sanguinarine, an alkaloid from the herb, have been shown to possess anti-inflammatory, antimicrobial, antioxidant, antiviral, antiproliferative, and apoptotic activities, and are under active research for the treatment of cancer.125,126 Sanguinarine, an alkaloid compound fund in blood root, is a potent inhibitor of NF-kappa B activation.25,125,126 Sanguinarine is an ingredient in dental hygiene products, for example, toothpaste, used for its antiplaque activity and in the treatment of gingivitis. There is controversy over the safety of its use in dental products, with contradictory research over whether it may cause malignant cell change and lead to the development of leukoplakia. 127 128 129 130 131 132 133 Most studies have concluded that the extract is safe for dental use; however, at least one study concludes that it should not be used until safety can be established. One study on reproductive and developmental toxicology conducted by orally administering blood root extract to rats and rabbits concluded that the oral intake of blood root extract has no selective effect on fertility or reproduction of fetal and neonatal development in either group.134 The question of safety and effects of the herb on the oral mucosa remains relevant as the application to the cervix is similar in terms of direct treatment of epithelial tissue. The form used in black salve is the whole plant extract rather than isolated alkaloid for which cautions have been raised. At this time, evidence regarding the internal use of this herb for cervical dysplasia treatment is lacking, and serious caution is suggested regarding its topical use.
Bromelain
Bromelain is a complex mixture of proteinases derived from pineapple stems and fruit. Beneficial therapeutic effects of bromelain have been demonstrated in vitro, and in animal and human inflammatory disease models, including treatment of arthritis and inflammatory bowel disease, among others.135,136 Bromelain inhibits plasma exudation through inhibiting the generation of bradykinin at the inflammatory site via depletion of the plasma kallikrein system, and possibly through other mechanisms, such as inhibition of the arachidonic acid pathway. 137 138 139 Beneficial anti-inflammatory effects have also been observed in patients suffering from HIV and cancer. 140 141 142 143 In one randomized study, 36 patients with Chlamydia infections were assigned either to a tetracycline-HCl plus bromelain (250 and 40 mg, respectively, four times per day) or a doxycycline (100 mg, twice daily) treatment for a period of 14 days. After 7 days, the pathogen was eliminated in 66.7% of the patients treated with tetracycline plus bromelain and in 55.6% of the patients receiving doxycycline. After the completion of the course of therapy, an infection with Chlamydia was no longer detectable in any patient of the two groups. The clinical effectiveness of the two therapies was considered to be good or very good in all cases. Adverse effects occurred in 11% (tetracycline + bromelain) and 16% (doxycycline) of the patients. Treatment of the sexual partner (with antibiotics) was also considered essential to the success of the study.144 Bromelain is an important proteolytic ingredient in the treatment of cervical dysplasia.
Calendula
Calendula flowers are indicated for the topical treatment of minor inflammations of the skin and mucosa, to assist in the healing of minor wounds, and for the treatment of burns.95,97 The most common topical applications include infusions used as washes, oil-based extractions, ointments, and the succus, or juice, which is high in enzymatic activity.97,195 Hydroalcoholic extracts have demonstrated antibacterial and antifungal activity, as well as high virucidal action.26,29 Calendula extracts have shown specific activity against HSV, HIV, and Trichomonas.97,146 Anti-inflammatory and wound-healing effects have been demonstrated in vitro and in vivo, with topical anti-inflammatory effects attributed to the effects of the polysaccharide fractions of the plant. 149 150 151 Other important compounds are thought to be the major anti-inflammatory triterpenoid esters in the flower heads faradiol 3-O-laurate, palmitate and myristate. 152 153 154 In one study, freeze-dried extracts of St. John’s wort, calendula, chamomile, and plantain were found to suppress both inflammatory effects and leukocyte infiltration in animal models.155 Wound-healing effects also have been attributed to the angiogenic activity of the herb.28 Calendula succus is used as a wash in the escharotic treatment, and as an ingredient in other topical applications for the treatment of cervical dysplasia, particularly in suppositories for vulnerary and anti-inflammatory effects after invasive gynecologic procedures (e.g., biopsy, LEEP). Calendula is used topically to hasten healing by reducing inflammation through an increase in granulation. No studies were identified using calendula for the treatment of HPV infection. Some concern exists as to whether use of calendula can lead to sensitization and potential for developing contact dermatitis; however, this risk appears to be insignificant, and in fact, the herb has been found to be highly effective for the prevention of acute dermatitis of grade 2 or higher in patients undergoing postoperative irradiation for breast cancer.156 Known sensitivity to the Composita family can theoretically pose this risk; however, adverse effects from topical use have not been widely observed despite its widespread use.97
Goldenseal
Goldenseal is one of the five top-selling herbs in the United States, yet little scientific evidence is available regarding its efficacy.157 Many herbalists consider goldenseal an indispensable antimicrobial herb, in addition to it being anti-inflammatory, immune enhancing, and antiproliferative, effects largely attributed the herb’s berberine content.158 These actions form the basis for its topical use in the treatment of cervical dysplasia. Although no research has been done specifically on the treatment of HPV with goldenseal, the herb has shown broad antimicrobial effects, with specific effects against Chlamydia, S. aureus, E. coli, V. cholera, Trichomonas vaginalis, Giardia lamblia, and H. pylori, as well as other organisms. 159 160 161 162 It has also demonstrated antifungal effects against numerous organisms, including Candida albicans.157 Its anti-inflammatory effects are attributed to its ability to interfere with the arachidonic acid pathway and cyclooxygenase generation, particularly COX-2 regulation and inhibition of phospholipase enzymes.25,157 Berberine was demonstrated in vitro to have antiproliferative effects via inhibition of protein, DNA, RNA, and lipid synthesis in specific tumor cell lines; however, these effects were not borne out in vivo. Berberine extracts were able to induce apoptosis during S-phase of the cell cycle, and have demonstrated the ability to activate antitumor macrophages, in addition to several other anticancer in vitro effects.157 In a study of the immunomodulatory effects of 6 weeks of orally administered goldenseal, the treated group showed an increase in the primary IgM response during the first 2 weeks of treatment, suggesting that goldenseal may enhance immune function by increasing antigen-specific immunoglobulin production.163 Although direct effects against HPV are unknown, use of this herb in suppositories may be effective for reducing comorbid infection, allowing the body to direct its immune activity against the HPV, and through eliminating overgrowth of pathogenic microorganisms, allow the body to restore a healthy vaginal environment that may be less likely to support the growth of HPV. As with other herbs, goldenseal’s anti-inflammatory effects may be beneficial in reducing cervical irritation or inflammation that might contribute to the development of dysplasia.
Licorice
Licorice is used in the treatment of cervical dysplasia, both topically and orally, for its antimicrobial, anti-inflammatory, immunomodulating, and antitumorigenic effects. It has been shown to inhibit prostaglandin and leukotriene synthesis in a similar way to corticosteroids such as prednisone.164 It has also demonstrated specific antiviral activity against a wide range of viruses associated with chronic illness and latent infection. In one study, treatment of cells latently infected with Kaposi’s sarcoma–associated herpes virus (KSHV) with glycyrrhizic acid (GL), a component of licorice, reduced synthesis of a viral latency protein and induced apoptosis of infected cells. This finding suggests a novel way to interrupt latency.165 GL demonstrated activity against EBV replication in superinfected cells in a dose-dependent fashion in a novel way that differed that of the nucleoside analogs that inhibit viral DNA polymerase.166 The mechanism underlying licorice’s antiviral and antitumorigenic effects is poorly understood. One study looking at mechanisms was able to demonstrate that glycyrrhetinic acid (GA), an aglycone of GL, stimulates NO production and is able to upregulate iNOS expression through NF-κB transactivation in macrophages.167 In vitro studies have demonstrated activity against HIV virus.168 Licorice and its extracts have been shown to improve immune function in HIV patients by stabilizing helper and T-lymphocyte counts in comparison with the control groups in one study.169 In another study, it increased T-helper cell levels, improved helper/suppressor cell ratios and improved liver function, and stopped the progression of HIV-positive patients to AIDS in comparison with the control group that did progress on to AIDS.170,171 In yet another study, it showed a reduction of P24 antigen, an indicator of viral load.172 The antiviral properties of these compounds have been found to be effective in hepatitis B and C where IV preparation has resulted in up to 40% going into complete remission.173 Topical use of licorice extract on herpes reduces the healing time and pain associated with both genital herpes and cold sores.174 Another component of licorice, deoxoglycyrrhetol (DG), also showed a remarkable improvement in anti-inflammatory, antiallergic, and antiulcer activities in animal experiments. Immunomodulating effects of GL, GA, and DG derivatives, which induce interferon-gamma and some other cytokines, have been demonstrated in relation with their antiviral activities.175 Glycyrrhizin has been used for the treatment of chronic viral hepatitis. One study evaluated the mechanism by which glycyrrhizin inhibits complement. Glycyrrhizin inhibited the cytolytic activity of complement via the activation of both the classical and alternative pathways, whereas it had no effect on immune adherence, suggesting that it blocks C5 or a later stage of the complement cascade. Further analysis revealed that glycyrrhizin inhibits the lytic pathway in which the membrane attack complex (MAC) is formed. This mechanism suggests that glycyrrhizin may prevent tissue injury caused by MAC not only in chronic hepatitis, but in many autoimmune and inflammatory diseases as well.176 Topical treatment of herpes simplex virus blisters with licorice extract may improve healing and prevent recurrence.157 Although no studies were identified on the treatment of HPV with licorice or its extracts, other viral studies, as well as the herb’s traditional uses, suggest that investigation into such use may be promising. See Plant Profiles: Licorice for warnings and contraindications to regular internal use of this herb.
Lomatium
Lomatium has been used historically by Native Americans, mostly as a treatment for respiratory illness.177 It is considered antiviral, antibacterial, and antiseptic and is commonly used by naturopathic physicians and taken internally, for the treatment of cervical dysplasia. Lomatium has demonstrated in vivo and in vitro efficacy against HPV and HSV and has been investigated for its effects against HIV. 178 179 180 Its use has been described for the treatment of “slow” viruses with accompanying immune depression, and may commonly be combined with other herbs with immune-building effects.181 Lomatium is also used topically for gum and mouth inflammations and as a douche for vaginal infections.182
Marshmallow
Marshmallow root (Fig. 7-4) is a polysaccharide-rich herb, loved by herbalists for its soothing, demulcent properties.25 The mucilaginous quality of aqueous extracts and moistened powdered herb provides a protective, soothing coating to mucosa; thus, it is commonly included in preparations for throat, GI, and vaginal mucosal irritation.97 Several studies have found the herb efficacious, in combination with other specific herbs, for the treatment of cough, and the herb is approved for use by the German Commission E for the treatment of irritation of the oral and pharyngeal mucosa and mild inflammation of the gastric mucosa.95 The root also exerts immune-enhancing and antibacterial effects.25
Myrrh
The tincture and powdered forms of this herb are used topically for the treatment of inflammatory mucosal conditions, usually of the oral and pharyngeal mucosa but also as an ingredient in vaginal suppositories. Local anesthetic, antibacterial, and antifungal activities also have been ascribed to the sesquiterpene fraction of the herb.29 It is a common ingredient in oral hygiene preparations, for example, ointments, dentifrices, and toothpastes.29 It is approved by the German Commission E for the topical treatment of mild inflammations of the oral and pharyngeal mucosa. Low Dog states that “No data have been found to document antiviral activity [of myrrh], but in light of the antiseptic, cytoprotective, and anti-inflammatory effects of the herb it may offer some benefit” in the treatment of cervical dysplasia.25
Oregano and Thyme
Both oregano and thyme essential oils are regularly included in vaginal suppositories for the treatment of vaginal infections, including HPV infection. They are also used topically as antimicrobials against numerous bacterial and fungal infections, for which they are considered highly effective ingredients. 183 184 185 One study reports on the efficacy of thyme as an antibacterial, and in another study oregano and clove oils were diluted and examined for their activity against enveloped and nonenveloped RNA and DNA viruses. Olive oil was also included as a control. Viruses were incubated with oil dilutions and enumerated by plaque assay. Antiviral activity of oregano and clove oils was demonstrated on two enveloped viruses of both the DNA and RNA types and the disintegration of virus envelope was visualized by negative staining using transmission electron microscopy.185,186 Care should be taken in the use of essential oils topically; used undiluted (neat) they can be irritating to sensitive tissues such as cervical or vaginal mucosa.
Reishi
Reishi (Fig. 7-5) is a medicinal fungus with a long history of use as a Chinese folk medicine for promotion of health and longevity. Numerous in vitro and animal studies have demonstrated antitumor and immunomodulatory effects of Reishi mushrooms.187 An OVID search for this herb yielded over 900 papers reporting on in vivo and in vitro effects. A wide range of antitumor and immunomodulatory mechanisms have been purported and observed, with the water extract and the polysaccharide fraction, as well as the alcohol extract or the triterpene fraction, and include enhanced function of antigen-presenting cells, the mononuclear phagocyte system, humoral immunity, and cellular immunity.188,189 Reishi polysaccharide peptide (Gl-PP) has demonstrated antitumor effects in mice and potential antiangiogenesis, a reduction of Bcl-2 antiapoptotic protein expression and an increase of Bax proapoptotic protein expression; therefore, inducing cell apoptosis might be one of the mechanisms of action in inhibition of human carcinoma cells. High doses of Gl-PP resulted in a decrease in the secreted vascular endothelial growth factor (VEGF). Taken together, these findings support the hypothesis that the key attribute of the antiangiogenic potential of Gl-PP is that it may directly inhibit vascular endothelial cell proliferation or indirectly decrease growth factor expression of tumor cells.190 It has been demonstrated that G. lucidum induces apoptosis, inhibits cell proliferation, and suppresses cell migration of highly invasive human prostate cancer cells PC-3.191 Experimental results on cell-mediated immunity showed that G. lucidum could increase the percentage of CD5+, CD4+, and CD8+ T lymphocytes. Experimental results on humoral immunity in horses showed that G. lucidum could help horses to produce a significantly higher quantity of specific antibodies in a shorter time.192 Although the pharmacology and clinical application of water extracts of G. lucidum have been extensively documented, little is known regarding its alcohol extract. In the present study, the antitumor effect of an alcohol extract was investigated using MCF-7 breast cancer cells. The extract inhibited cell proliferation in a dose- and time-dependent manner, which might be mediated through upregulation of p21/Waf1 and downregulation of cyclin D1. Furthermore, this compound can directly induce apoptosis in MCF-7 cells, which might be mediated through upregulation of a proapoptotic Bax protein and not by the immune system. There are likely multiple mechanisms underlying the antitumor effects of G. lucidum.193 G. lucidum also demonstrated antioxidant activity, free-radical scavenging, and chelating abilities.194 No specific studies were identified on the use of G. lucidum for the treatment of HPV infection or cervical dysplasia; however, given the mechanisms of action of this herb, this may be a promising area of research, and certainly merits consideration of this herb in an immune-enhancing protocol.
Thuja
Thuja is used by many herbalists and naturopathic physicians for the treatment of genital and anal warts, and is commonly recommended in the naturopathic treatment of cervical dysplasia for its antiviral activity.195 The main constituent is an essential oil consisting of α- and β-thujone, the content of which varies proportionally with the amount of ethanol used in producing the plant extract. If consumed internally, thujone can be neurotoxic, convulsant, and hallucinogenic. Long-term or excessive use of thujone-rich products can cause restlessness, vomiting, vertigo, tremors, renal damage, and convulsions.196 Internal use of thuja decoctions and even very small doses of thuja oil (i.e., 20 drops per day for 5 days) as an abortifacient has been associated with neurotoxicity, convulsions, and death.195 Additionally, thuja is associated with a substantial risk of inducing fetal malformation, and is absolutely contraindicated for use in pregnancy.195 No research on the short- or long-term topical use of this herb was identified. Ingestion of thuja cannot be recommended because of potential for toxicity.
CHRONIC PELVIC PAIN
Chronic pelvic pain (CPP) is defined as pelvic pain lasting more than 6 months. Some authors add the additional criteria that the pain be noncyclic.197 It is one of the most common presenting complaints in gynecologic practice, affecting as many as one in seven American women. CPP comprises up to 10% of outpatient gynecologic visits, accounts for 20% of laparoscopies, and results in 12% (75,000/year) of all hysterectomies performed annually in the United States.198 Estimated annual direct medical costs for outpatient visits for CPP in the United States among women 18 to 50 years old is estimated to be $881.5 million. It is often an extremely frustrating condition for both patient and care provider because in many cases an etiology cannot be identified and there is no apparent pathology. Treatment of presumed underlying conditions is frequently ineffective, and the “pain itself becomes the illness.”198 Because the cause often cannot be identified, CPP is frequently attributed to psychogenic causes. Although these may play a role in CPP for some women with lack of an identifiable cause, this does not necessarily equate with a psychosomatic origin for this complaint.199
Common causes of chronic pelvic pain include endometriosis, pelvic inflammatory disease (PID), adhesions, ovarian remnant syndrome, pelvic congestion syndrome, and cyclic uterine pain, which may be caused by primary or secondary dysmenorrhea, uterine myomata, and adenomyosis. History of psychosexual trauma is common in women diagnosed with CPP.200 Chronic pelvic pain is frequently associated with systemic inflammation, including autoimmune diseases. Peritoneal chronic inflammation is sometimes also associated. A study of chronic pain reveals that the immune system is intimately involved in the production, conduction, and exacerbation of pain and of its clinical features, such as hyperalgesia and allodynia.201
Not all pelvic pain is of gynecologic origin; other conditions must be ruled out. Genitourinary pain (e.g., due to interstitial cystitis, urethral syndrome, or overactive bladder), gastrointestinal pain (e.g., irritable bowel syndrome, bowel obstruction, or bowel neoplasm), and neuromuscular pain are also common causes of CPP. CPP may be intermittent or continual. Pain is affected by physical and mental fatigue, as well as stress. It may lead to depression and anxiety, dyspareunia (painful sex/intercourse), and difficulties with sleep, decreased ability to work and enjoy normal activities, and may be a contributing factor in job loss, relationship dysfunction and divorce. 202 203 204
SYMPTOMS
Symptoms associated with CPP include:
DIFFERENTIAL DIAGNOSIS
Differential diagnosis in CPP is really a matter of identifying the possible causes of pain (Table 7-6) and treating the etiology while addressing the pain and concomitant symptoms. In patients under 30, the most common causes of pelvic pain include endometriosis and pelvic inflammatory disease; in older women, causes most likely include uterine myoma, adenomyosis, or pelvic relaxation. It is critical to rule out any serious or life-threatening causes, as well as to assess for depression, anxiety, and serious mental health disorders.
| CLASSIFICATION | CONDITIONS |
|---|---|
| Gynecologic |
Data from Forrest D: Common Gynecologic Pelvic Disorders. In Youngkin E, Davis M, eds. Women’s Health: A Primary Care Clinical Guide, Stamford, Appleton and Lange,1998, pp. 313-362; Ryder R: Chronic pelvic pain, Am Fam Physician 54(7):2225-2232, 1995; Ostrzens A: Gynecology: Integrating Conventional, Complementary, and Natural Alternative Therapy, Philadelphia, Lippincott Williams & Wilkins, 2002.
CONVENTIONAL TREATMENT APPROACHES
The choice of medical treatment for CPP depends on the etiology of the pain, thus necessitating careful diagnosis. Treatment of underlying conditions is the primary treatment strategy. However, in one-third of cases, no etiology is identified. Sympathetic and supportive care is critical, with reassurance and validation of the woman’s symptoms essential, especially in the absence of an identifiable cause.199 The pain should be treated as a real problem. Multidisciplinary team management of CPP may be the most productive strategy, including the expertise of a gynecologist, a psychologist with expertise in sexual and relationship counseling, and also possibly an acupuncturist for pain management, in addition to the appropriate specialists for the underlying cause.199,205 Treatment with medication includes the use of NSAIDs, antidepressants for depression and sleep disorders, and hormonal therapies (i.e., oral contraceptives for management of cyclic pain or GnRH analogs for pain associated with endometriosis or uterine fibroids). Trigger point injections of local anesthetics has proved helpful for prolonged pain relief in some patients, as has TENS therapy.206 Acupuncture has been used with good results in the treatment of dysmenorrhea, and may be beneficial in pain reduction for CPP.206 Immune modification using steroids and disease-modifying antirheumatic drugs, such as hydroxychloroquine, are known to inhibit inflammatory cells and cytokines, such as interleukin-1, interleukin-6, and tumor necrosis factor, which are responsible for pain and tissue damage. These drugs are found to be effective in the treatment of chronic pelvic pain of an inflammatory nature and for symptomatic chronic inflammation of the vagina.201,206 Surgical interventions include laparoscopy for the lysis of pelvic adhesions or removal of endometrial tissue, or hysterectomy. Although hysterectomy without an associated pathology has not proved effective, it is nonetheless indicated as a reason for hysterectomy in 10% to 15% of those performed in the United States.199 According to one study, 25% of hysterectomy patients reported persistent pain 1 year after surgery.198
BOTANICAL TREATMENT
In the absence of a clearly identified pathology, the practitioner can approach treatment symptomatically via specific botanical treatments for pain reduction, and attempt to address mechanisms that may be associated with CPP, for example, inflammation. One theory of CPP that was popular among physicians in the early-and mid-twentieth century, and that is still considered a possibility, is that of “pelvic congestion syndrome.”8,198,206,207 Women with this syndrome, which is poorly defined, are thought to exhibit many of the symptoms associated with CPP, including aching and dragging sensations in the lower back, lower abdomen, and pelvis, dysmenorrhea, and dyspareunia. The theory of pelvic congestion parallels Chinese medical theory regarding various forms of gynecologic pain. Pelvic vascular congestion is thought to be a dynamic vascular process, similar to migraine headache, with drug inducible [dihydroergotamine (DHE) injection] reversibility of vascular dilatation.206 As with CPP, symptoms are commonly accompanied by depression, fatigue, and insomnia. Upon pelvic exam or laparoscopy, the uterus may be found to be enlarged and tender and the pelvic vessels engorged. However, there is no direct correlation between vessel engorgement and pain; some women have either pain without engorgement or vice versa.8 Herbalists may include herbs in a formulae to tonify and astringe the uterus and pelvic vessels, ostensibly to reduce pelvic congestion. Psychogenic causes may contribute to CPP. Although this should not be overemphasized, it should also not be overlooked. Chronic pain can affect nearly every aspect of a patient’s life: physically, mentally, emotionally, socially, and even economically. Because chronic pain can lead to depression and anxiety, as well as to sleep disturbance, which can create a vicious cycle of psychoemotional upset and increased pain, care should be taken to approach pain holistically, including in protocol herbs that are restorative to the nervous system, for example, adaptogens and nervines, and when needed, anxiolytics or antidepressants.
IBS and inflammatory bowel syndromes are highly associated with CPP. Herbs commonly used for the treatment of CPP are listed in Table 7-7. Many of these herbs are discussed elsewhere in this book or in Plant Profiles.
Analgesia
The history of botanical medicine reveals many herbs that have been used for the treatment of a variety of types of pain.208 Many traditional medicines have actions such as inhibition of platelet-activating factor, cyclooxygenase, prostaglandin formation, or arachidonic acid pathways.209 Although not typically as fast-acting as conventional medications, repeated appropriate dosing over a short period of time, such as 1 to 2 hours, and continued as needed, often leads to satisfactory temporary alleviation of pain. Several herbs are reputed for their efficacy in the treatment of pain of gynecologic origin, as well as more generally (see Dysmenorrhea).
Black Cohosh
Black cohosh has historically been used by Northeast Native American tribes as an analgesic and as an emmenagogue.210 The Eclectics used a resin of black cohosh specifically as a uterine tonic and in the treatment of dysmenorrhea and a number of other painful spasmodic or cramping gynecologic complaints.211 It was also used in the treatment of deep muscle drawing in the legs, loins, and back, dull aching of the bowels, ovarian pains of a dull, aching quality, dragging uterine pain, and delayed menses with dull pain and muscle soreness. Felter specifically describes a condition called “rheumatism of the uterus” for which this herb was prescribed.99 The plant’s anti-inflammatory and analgesic properties are attributed to its aromatic acids, which appear to inhibit prostaglandin production. The herb is approved for use in Germany for the treatment of premenstrual discomfort and menstrual cycle pain.25
California Poppy
California poppy (Fig. 7-6) traditionally has been prescribed for reducing pain and producing calm sleep without the potential dangers of conventional opiate drugs. It may be useful for painful conditions in which there is irritation or stimulation of afferent pain fibers, in disturbed sleep, and for anxiety.108 Its medical use as an analgesic and sedative in the United States dates as far back as the late nineteenth century, even being included in the Parke-Davis catalog for these purposes, and as an excellent alternative to morphine without its side effects.108,211 Today, California poppy is widely used by herbalists in tincture form. Pharmacologic data demonstrate sedative activity in vivo, as well as GABAergic activity, sedative and anxiolytic action, and dose-dependent analgesia (when administered by injection). Two controlled clinical trials, the herb, combined with Corydalis cava, both standardized extracts, demonstrated normalization of disturbed sleep without carryover effects or addiction.108
Corydalis
The Chinese botanical corydalis, is a strong and reliable analgesic. It is commonly used for headache, lumbar pain, abdominal pain, joint pain, menstrual pain, and other neurologic pain, making it specific for the symptoms associated with CPP. Alcohol and acetic acid extractions are the strongest, although powdered herb is considered effective as well. The mechanism of action of analgesia is thought to be inhibition of the reticular-activating system in the brainstem. Corydalis can increase the pain threshold significantly. Continuous use of corydalis results in tolerance and may theoretically lead to a cross-tolerance to morphine.212,213 However, from a Chinese medical perspective the effects of corydalis are more than palliative as it is used to help promote pelvic circulation and therefore may treat underlying pelvic congestion. The alkaloids in this herb have sedative and hypnotic effects and act synergistically with barbiturates.212 Chinese pharmaceutical companies have produced several preparations from corydalis alkaloids for use as analgesics. The available preparations include a 30-mg tablet containing all alkaloids and a 10% tincture used in doses of 5 mL three times daily.212 Overdose leads to muscle relaxation and CNS depression. Corydalis is contraindicated in pregnancy.32,212,213
Cramp Bark and Black Haw
Cramp bark and black haw were similarly used for the treatment of pelvic pain, particularly of a spasmodic nature, and specifically when accompanied by a sensation of dragging pressure in the groin and drawing pain in the legs.31,211
Jamaican Dogwood
Jamaican dogwood is a reliable analgesic and spasmolytic herb with mild sedative properties. It was prescribed by the Eclectics for neuralgias, spasmodic complaints, migraines, dysmenorrhea, nervous tension, insomnia, and nervous excitability, although Felter cautioned about potential toxic effects (including convulsions) in large doses.99 Ellingwood elaborated on its effects in quieting uterine pains of labor, promoting rest, and having a specifically relaxing influence, in addition to its general analgesic effects. He stated that the herb “acts in close harmony with the vegetable uterine remedies, promoting the influence of Macrotys [Actaea racemosa-black cohosh], the viburnums…pulsatilla and dioscorea among others.”30 The spasmolytic activity of Jamaican dogwood may be attributable to its isoflavone constituents; however, this plant has been only minimally studied.154 Combined in equal parts with cramp bark or black cohosh, this author has found it a highly effective treatment for gynecologic and pelvic pain of neuromuscular origin, for dysmenorrhea, endometrial pain, urinary tract infection, and other pelvic pain. It also may be used postsurgically as an alternative to conventional pain medications. Regarding its toxicity, it is advisable that the recommended dosage range not be exceeded and that the herb not be used by pregnant women, or patients with bradycardia or cardiac insufficiency.108
Kava kava
Kava kava has been used traditionally as a muscle relaxant to reduce anxiety and may be considered for the treatment of muscle spasms associated with CPP. Both aqueous and lipid soluble extracts of kava have demonstrated antinociceptive activity through nonopiate receptor mechanisms.158 It is commonly used by herbalists for the treatment of pain as well as anxiety. (See Plant Profiles: Kava Kava for safety considerations.)
Pulsatilla
Pulsatilla (Fig. 7-7), also called pasque flower, has analgesic and sedative properties. It is listed in the British Herbal Compendium for the treatment of painful spasmodic conditions of the female reproductive systems and dysmenorrhea. It is generally used in tincture form. Fresh herb contains potentially irritant and toxic compounds; therefore, only dried plant should be used, and the herb should not be used during pregnancy. Overdose can lead to gastric irritation, coma, and convulsions; thus, it is essential that patients stay within the proper dosage range, and use be monitored by an experienced practitioner.25,108 This herb is more commonly prescribed by naturopathic practitioners than herbalists in the United States, although it is also used by European herbalists.
Dong Quai and Peony
Dong quai and peony, in addition to their significant analgesic and spasmolytic actions, are considered herbs that “move blood” and relieve stasis or stagnation in TCM.92, 214 215 216 The TCM concept of uterine stasis is consistent with the Western concept of pelvic congestion syndrome described in the preceding. Additionally these herbs, often used together in combination, and often with the addition of licorice (Glycyrrhiza glabra or G. uralensis) are considered effective for the treatment of a number of gynecologic conditions that may be involved in the etiology of CPP, such as dysmenorrhea, polycystic ovarian syndrome (PCOS), and uterine fibroids. The Japanese traditional formula TJ-68, Shakuyaku-kanzo-to (Chinese: shao-yao-gan-cao-tang), which contains concentrated white peony root and licorice, has been approved by the Japanese government for clinical use in the treatment of pain and acute muscle spasm, including dysmenorrhea.108
Marijuana
One herb, not available widely (or at least, legally available) for clinical use that has clinically demonstrated significant uterine antispasmodic and analgesic effects is Cannabis indica, more commonly referred to as marijuana (Fig. 7-8). This controversial medicinal plant and recreationally used herb has a long history of use for relief of uterine spasms and dysmenorrhea, considered by the Eclectics to be a “soothing uterine tonic.”30 In fact, its use is ancient, with references and artifacts of its use found widely in Middle Eastern, Ayurvedic, and Semitic writings, continuing through to its medical use in Europe well into the late nineteenth century for the treatment of a variety of gynecologic and obstetric conditions, not limited to but including dysmenorrhea. A pharmaceutical product from the late nineteenth century, Dysmenine Compound, produced by the Keysall Pharmical Company, Kansas City, MO, contained Cannabis, Cypripedium, Scutellaria, Pulsatilla, Viburnum prunifolium, Caulophyllum, Viburnum opulus, and Capsicum. The compound was indicated for dysmenorrhea, menstrual colic, and cramps.217 Indeed, this formula is not very different from one that might be prescribed by herbalists today (see sample formulae in the following); however, minus the now illegal cannabis and the ecologically endangered lady’s slipper orchid (Cypripedium). Although it is not possible given the current legal–medical climate surrounding the use of Cannabis to prescribe this herb clinically, it is worthwhile to note its use and possible beneficial effects, as these have likely not escaped those who manage to procure it for self-medication for the treatment of chronic or cyclic pelvic pain. Russo et al., in Women and Cannabis: Medicine, Science, and Sociology, provide substantial evidence of its use. They cite Grinspoon and Bakalar in their 1993 book Marihuana, the forbidden medicine, who discuss numerous case studies of women using cannabis effectively to treat PMS, menstrual cramps, and labor pain, and when used at low doses, without cognitive impairments. They also cite an Australian study of the uses of cannabis for obstetric and gynecologic complaints in which 51% of respondents indicated use for PMS or dysmenorrhea. Discussing this herb’s appropriate use with patients, outside the context of prescribing or condoning its use, is therefore possibly important and appropriate. The mechanisms of action appear to be primarily through anti-inflammatory activities. An interesting approach for inflammation-mediated pelvic pain is the use of the seeds of the hemp plants, which are notably rich in gamma-linolenic acid, in which women with PMS and dysmenorrhea have found to be low. In one study, a daily dose of 150 to 200 mg of over 12 weeks greatly improved PMS related symptoms; this dose could be provided by a 5-mL daily dose of hemp seed oil.217
Motherwort
Motherwort (Leonurus cardiaca) is a classic herb for the treatment of pelvic pain. Its actions appear to modulate both relaxant and contractile activity of the uterus, perhaps with an overall effect of regulating a balance between the two for effective uterine muscle activity. The commonly used Western species L. cardiaca has barely been evaluated for its effects in gynecology, whereas Chinese species have been evaluated in several investigations and have been found to have stimulating effects on the myometrium in vivo. The effect on the uterine smooth muscle may be related to alteration of the ion concentration in relation to myoelectric activity, resulting in the increase of myoelectric activity of pace setter cells as well as in the acceleration of depolarization of spike activity.218 Leonurine, a plant alkaloid present in Chinese motherwort, has demonstrated some efficacy as a vascular smooth tone inhibitor, possibly through inhibition of Ca2+ influx and the release of intracellular Ca2+.219 It is uncertain whether these findings and effects can be extrapolated to effects on uterine vascular tone. Other studies have demonstrated interesting effects on mediators of the inflammatory and coagulation pathways in relationship to coronary blood flow and alleviation of stasis that may have some correlation to the use of this herb in both TCM and Western herbal medicine to alleviate pelvic congestion (in TCM “blood stasis” or “stagnation”). In one study of 105 patients, 94.5% showed improvements in reduction in blood viscosity and fibrinogen content, important both for healthy blood flow but also in the prevention of release of inflammatory compounds associated with clot formation.220 A Russian study reported on the soporific activity of a combination of equal parts of valerian, motherwort, and hawthorn (Crataegus spp.) in tincture form. This combination prolonged the soporific effect of sodium ethaminal.221 The effects of motherwort (L. cardiaca) for the treatment of spasmodic uterine pain and pelvic congestion are predicated on historical and contemporary clinical use, for which it remains a popular choice in gynecologic formulae.
Wild Yam and Ginger
Wild yam and ginger are considered important herbs to include in the treatment of CPP, especially when it is associated with irritable bowel–type complaints, as they are both effective not only for treating spasmodic uterine complaints and, in the case of ginger, inflammation, they exert these actions in the digestive system, thus addressing what may be causal associations, or concomitant conditions that are mutually exacerbating.25,108 These herbs may be used in combination in capsule or tincture form, and may be included in formulae with other herbs.
Yarrow
Yarrow, a favorite herb of many herbalists, has the interesting characteristic of being considered an effective antispasmodic for painful, cramp-like conditions of psychosomatic origin in the lower pelvis in women when used as a sitz bath.95 It is also used for dyspeptic complaints, including mild, spastic discomforts of the gastrointestinal tract. This combination of qualities makes it a particularly interesting herb to consider for the treatment of CPP, especially when of psychogenic origin and/or when occurring in conjunction or as a result of irritable bowel disorders.
Adaptogens
The use of adaptogens in the treatment of CPP is primarily for the reduction of stress and anxiety, modulation of inflammation, and improvement of sleep disorders. They are part of a long-term treatment plan rather than quick-acting for specific symptoms (see Chapter 6 and Plant Profiles). Ashwagandha has specific analgesic activity, and is among the most specific of choices for CPP.
Anti-inflammatories
Dong Quai
Dong quai possesses antispasmodic, analgesic, anti-inflammatory antioxidant, uterine tonic, as well as specific immunomodulatory effects (see Plant Profiles). Immunostimulatory and anti-inflammatory effects have been attributed to isolated ferulic acid. It has been used traditionally in Chinese medicine for the treatment of “blood vacuity” and “blood stasis,” which may be considered related to CPP.94
Evening Primrose Oil
It is thought that the use of evening primrose oil (EPO), with its high gamma linoleic acid content, may preferentially promote the synthesis of anti-inflammatory prostaglandin series over inflammatory prostaglandins. One critical review of the effects of EPO for the treatment of PMS concluded that there was no benefit. However, in a study of women (n = 40) who experienced symptoms of irritable bowel syndrome (IBS) just prior to and at the onset of menstruation, 53% reported an improvement in symptoms, whereas no improvement was seen in the placebo group. Improvement generally took 2 to 3 months to become apparent. Blood analysis at the beginning and end of treatment revealed significant improvement in fatty acid imbalances in the EPO-treated group.158
Feverfew
Feverfew has exhibited inhibition of prostaglandin synthetase preventing the conversion of arachidonic acid to prostaglandins, inhibits mast cell degranulation and subsequent histamine and serotonin release, and has shown inhibition of other inflammatory cytokines such as TNF-α, IL-1, NFκB, and IFN-γ, as well as inhibiting peritoneal cyclooxygenase in animal models.97 These effects suggest possible application of this herb to treat pain related to inflammation in CPP.
Ginger
Herbalists commonly use ginger root as an anti-inflammatory and antispasmodic herb for the treatment of pelvic pain and congestion, as an infusion, and also in hip baths and hot compresses over the affected area. No studies have been identified for its use for gynecologic complaints. Ginger remains popular among Western and TCM herbalists as an antispasmodic treatment for dysmenorrhea; however, no clinical trials have been done to evaluate its efficacy.25 Ginger’s historical use for treatment of digestive disorders may be applicable for women with concurrent abdominal discomfort resulting from digestive complaints.
Licorice
Licorice root is commonly included in formulae when an anti-inflammatory herb is indicated. It may be considered an effective anti-inflammatory activity without many of the most troubling side effects seen for drugs used as COX-2 and 5-LO inhibitors.111 However, high doses of licorice may exacerbate hypertension (see Plant Profiles).
Peony and Rehmannia
Two herbs commonly used in TCM formulae, peony and rehmannia, have demonstrated significant anti-inflammatory and antispasmodic activity.22,25,108 Studies using a traditional formula containing both herbs have demonstrated prostaglandin production inhibition in the uterine myometrium via phospholipase A2 inhibition, whereas other studies have demonstrated arachidonic acid inhibition, PAF inhibition, reduction in free radical formation, and smooth muscle relaxation. Note that nearly all of the studies use these herbs in traditional formulae rather than in isolation, and that studies are conducted in animal models, and have focused on arthritis, ulcers, and other chronic inflammatory conditions. Licorice is frequently included in TCM formulae that also contain peony and Rehmannia, as is dong quai when these herbs are used for gynecologic conditions.
Uterine Tonics: Venotonics
Treatment of pelvic congestion syndrome incorporates a combination of therapeutic actions, including anti-inflammatory, uterine tonics, and herbs used as vascular tonics. Uterine tonics, which historically have included herbs such as blue cohosh, goldenseal, lady’s mantle, motherwort, partridge berry, red raspberry leaf, and cramp bark and black haw, are thought to exert their efforts by improving the overall tone of the uterine smooth musculature and vasculature. Goldenseal, for example, typically regarded for its antimicrobial effects, was used extensively by the Eclectics for the treatment of uterine bleeding resulting from a variety of conditions, including endometriosis, fibroids, and changes associated with menopause.211 Although no clinical studies have been conducted using whole herb, in vitro trials using berberine, one of the primary alkaloids in goldenseal, have demonstrated both uterine smooth muscles stimulant and inhibitory activity.22 Aqueous extracts of red raspberry leaf also have demonstrated both stimulatory and inhibitory effects on uterine smooth muscle.108 In fact, this paradoxic effect is seen with several of the herbs commonly used as both uterine tonics and spasmolytics, for example, cramp bark and black haw. It is thought that the effect of these dual activities is a normalization of uterine activity, and the promotion of smooth, nonspasmodic uterine muscle activity, thus improving tone and reducing pain.222,223
Several herbs with venotonic activity should be considered for the treatment of pelvic congestion in CPP. Most notable are blue cohosh and horse chestnut. Blue cohosh has demonstrated uterine tonic, vasoconstrictive activity, and continues to be used for the treatment of many gynecologic formulae in which a uterine tonic is required. Historically, it has been used for labor induction, amenorrhea, dysmenorrhea, menorrhagia, and to induce abortion.195,224 Blue cohosh is listed in the British Herbal Pharmacopoeia (1983) as a spasmolytic and emmenagogue.225 It also may be used as an ovarian tonic and for the treatment of a variety of menstrual complaints, including menorrhagia, amenorrhea, dysmenorrhea, and pelvic congestion syndrome.8 Horse chestnut is used to improve circulation through vascular tonification, to improve venous tone in venous insufficiency, and for the relief of aching discomfort in the lower limbs associated with varicosities and for complaints associated with chronic venous insufficiency (CVI).22,100,157 Traditionally, it was used in the treatment of neuralgia and “conditions of venous congestion particularly with dull, aching pain and fullness.”22 One studied demonstrated safe use for 56 months without harmful effects. Horse chestnut extract is the third most widely sold herbal product in Germany, where it is used long-term in clinical practice apparently without adverse effects.100 There appears to be very low risk associated with proper administration, although it is recommended that only product standardized to its presumed active ingredient, escin (aescin) be used, and not to exceed 12 weeks at recommended doses.157 Adverse effects from use of horse chestnut seed extract have included GI upset and calf spasm most commonly, with headache, nausea, and pruritus occurring less commonly. Overall, adverse effects are extremely rare, in an observational study occurring at a rate of less than 0.6% in more than 5000 subjects.
FORMULAE FOR CPP TREATMENT
Formulae for Chronic Pelvic Pain
General Tincture for CPP: Uterine Tonic/Antispasmodic
| Blue cohosh | (Caulophyllum thalictroides) | 20 mL |
| Cramp bark | (Viburnum opulus) | 20 mL |
| Peony | (Paeonia lactiflora) | 20 mL |
| Motherwort | (Leonurus cardiaca) | 15 mL |
| Horse chestnut | (Aesculus hippocastanum) | 15 mL |
| Yarrow | (Achillea millefolium) | 10 mL |
| Total: 100 mL | ||
Pelvic Analgesic and Antispasmodic Tincture: Moderate to Strong Pain
| Cramp bark | (Viburnum opulus) | 40 mL |
| Wild yam | (Dioscorea villosa) | 20 mL |
| Jamaican dogwood | (Piscidea piscipula) | 15 mL |
| Corydalis | (Corydalis ambigua) | 15 mL |
| Yarrow | (Achillea millefolium) | 10 mL |
| Total: 100 mL | ||
Immune Support and Stress Reduction Tincture
| Ashwagandha | (Withania somnifera) | 30 mL |
| Milky oats | (Avena sativa) | 20 mL |
| Blue vervain | (Verbena officinalis) | 20 mL |
| Licorice | (Glycyrrhiza glabra) | 15 mL |
| Lemon balm | (Melissa officinalis) | 15 mL |
| Total: 100 mL | ||
treatment options, for example, dysmenorrhea or interstitial cystitis.
DIETARY CONSIDERATIONS
Dietary changes are indicated when the client suffers from digestive complaints such as constipation, bloating, flatulence, overweight, lethargy, excessive fatigue, or irritability accompanying CPP.198 Achieving an optimal weight and stable blood sugar may lead to improvements in digestion and mood, and increasing dietary fiber and fluids can lead to reduction in constipation and bloating.198 Additionally, a Mediterranean-type diet with the addition of high quality essential fatty acids can reduce the production of inflammatory mediators, and thus be beneficial in chronic pain reduction. Consider calcium and magnesium supplementation for relief of muscle spasm.
ADDITIONAL THERAPIES
Muscle Relaxation and Re-education, Biofeedback, and Electrical Stimulation
External Treatments for Chronic Pelvic Pain
Ginger-Yarrow Sitz Bath
and relax tension, become aware of and adjust her body mechanics and standing and sitting posture, and wear appropriate shoes to minimize postural problems can help to reduce pain caused by structural imbalances. Pelvic relaxation training techniques should be taught and practiced regularly. Much of this can be done at home, but physical therapy can be helpful if there is limited joint movement or muscular problems. Prolonged sitting or standing can aggravate CPP, so patients may need suggestions and supportive counseling for modifying jobs or activities that require positions that exacerbate the problem. Exercises such as running or high-impact aerobics also may be aggravating, and should be replaced with gentler, relaxing forms of exercise, for example, walking, tai chi, yoga, or dance.226 Physical therapy for the treatment of musculoskeletal problems or postural problems can be beneficial for women with CPP.227
Electrical stimulation using vaginal, rectal, or surface electrodes is used to produce rhythmic contraction and relaxation of the pelvic floor muscles. Electrical stimulation may give immediate reduction in the level of pain early in treatment, restore more normal muscle activity patterns over time, and also may help to disperse inflammatory mediators caused by chronic muscle spasm. 228 229 230
Uterine Displacement–Mayan Uterine Massage
It has been suggested that uterine retrodisplacement can lead to symptoms of CPP.198 Although the role of pelvic tension and improper posture in the etiology of CPP is accepted, conventional medicine does not address the potential for uterine displacement, other than prolapse associated with pelvic relaxation as an etiologic factor. Mayan uterine massage is a practice introduced into the United States by Rosita Arviga, after dedicated study with a Belizean shaman who specialized in this technique. Ms. Arviga trains and certifies people in this technique and it has grown in popularity because of many anecdotal reports of success for the treatment of vague but sometimes debilitating complaints such as CPP, as well as for many other gynecologic problems. The treatment is predicated on the belief that uterine displacement, which may occur as a result of childbearing, poor posture, sedentary lifestyle, improper carrying and work habits, etc., can lead to significant pelvic congestion, gynecologic, nervous, circulatory, and digestive problems. No studies have been done to objectively demonstrate efficacy. The practice appears generally noninvasive (it is an intervention); however, it should not be used for pregnant women.