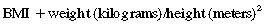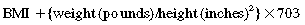Other conditions
A Allergic reactions and hypersensitivity
Definition
In some cases, the immune response to antigen is greatly exaggerated, a situation referred to as hypersensitivity. Anaphylaxis is a life-threatening response that a sensitized person develops within minutes after administration of a specific antigen. Hypersensitivity reactions are classified as types I, II, III, and IV.
Type I hypersensitivity pathophysiology
Type I hypersensitivity is a rapidly developing reaction that results from antigen–antibody interaction in an individual who has been previously exposed and sensitized to the antigen. The responsible antigen, referred to as an allergen, reacts with specific IgE antibodies on tissue mast cells and circulating basophils to trigger mediator release and an allergic response. A key mediator of allergic symptoms is histamine, which is described in the following section. Chemically, allergens are usually proteins, and a multitude of environmental factors, including grass, pollen, dust, mites, molds, and animal dander, can generate type I hypersensitivity reactions.
Histamine
Histamine is a basic amine stored in granules within mast cells and basophils and secreted when allergen interacts with membrane-bound IgE or when complement components C3a and C5a interact with specific membrane receptors. Histamine produces symptoms of allergic reactions by acting on H1– or H2-receptors on target cells. The main actions of histamine in humans (via the receptors involved) are:
• Vascular permeability (H1 and possibly H2)
• Contraction of most smooth muscle other than that of blood vessels (H1)
Histamine causes the cutaneous “triple response,” which includes erythema from local vasodilatation, wheal from increased vascular permeability and protein and fluid extravasation, and flare from an “axon” reflex in sensory nerves that releases a peptide mediator. The pathophysiologic effects of histamine can be blocked by H1 receptor antagonists (diphenhydramine, hydroxyzine, cyclizine, loratadine) and H2 receptor antagonists (cimetidine, ranitidine, famotidine).
Clinical manifestations
Allergic reactions present with symptoms such as rhinitis, conjunctivitis, urticaria, pruritus, and possibly anaphylaxis. The term anaphylaxis refers to a severe, generalized, immediate hypersensitivity reaction that includes pruritus, urticaria, angioedema (especially laryngeal edema), hypotension, wheezing and bronchospasm, and direct cardiac effects (including arrhythmias). A shocklike state can develop from hypotension secondary to systemic vasodilatation and extravasation of protein and fluid. Clinical manifestations of an allergic reaction can occur in various combinations and usually occur within minutes of exposure to the precipitating antigen(s). In some cases, though, the onset of signs and symptoms may be delayed for 1 hour or longer. Signs and symptoms can be protracted and variably responsive to treatment. Biphasic anaphylaxis can also occur, in which early signs and symptoms clear, either spontaneously or after acute therapy, and symptoms reoccur several or many hours later. Generally, the severity of an anaphylactic event relates to the suddenness of its onset and to the magnitude of the challenge (i.e., the greater the provocative stimulus, the more severe is the reaction). However, anaphylaxis can occur after exposure to minute amounts of allergen in highly sensitive individuals.
Anaphylactoid reactions are caused by mediator release from basophils (but not from mast cells) in response to a non–immunoglobulin E (IgE)-mediated triggering event. Such reactions present with similar clinical manifestations as those with anaphylaxis; however, it has been reported that cutaneous symptoms are more frequent and cardiovascular collapse is less frequent in patients experiencing anaphylactoid reactions versus those experiencing anaphylactic reactions.
Tryptase is a marker for mechanistic delineation of an allergic response. It is an enzyme that is released from mast cells along with histamine and other inflammatory mediators during an allergic response. A significantly elevated tryptase level (>25 mcg/L) strongly suggests an allergic mechanism. The presence of a normal tryptase level, however, does not exclude an immunologic reaction because elevated tryptase levels are not found in almost one-third of anaphylactic cases. Although the diagnosis of anaphylaxis should not rely on a single test, the high positive predictive value of tryptase makes it useful medicolegally and for subsequent patient management.
Type II hypersensitivity pathophysiology
Type II hypersensitivity reactions result when IgG and IgM antibodies bind to antigens on cell surfaces or extracellular tissue components such as basement membrane. The antigen–antibody reaction activates the complement cascade, causing production of C3a and C5a, which attract polymorphonuclear leukocytes and macrophages, and production of the C5b5789 membrane attack complex that inserts into target cell membranes. Examples of type II hypersensitivity reactions include transfusion reactions, autoimmune hemolytic anemia, myasthenia gravis, and Goodpasture’s syndrome.
Type III hypersensitivity pathophysiology
Type III hypersensitivity represents immune complex disease in which antigen–antibody complexes deposit in tissues and cause injury. Normally, immune complexes are cleared by the mononuclear phagocyte system shortly after their formation. In some situations, however, immune complexes persist and deposit in tissues. Protracted infections or autoimmune processes can lead to type III reactions. The mechanism of tissue injury is similar to that in type II reactions, involving activation of complement and recruitment of phagocytes. SLE, rheumatoid arthritis, and glomerulonephritis are examples of immune complex diseases.
Type IV hypersensitivity pathophysiology
Type IV hypersensitivity is also referred to as delayed-type hypersensitivity. By strict definition, type IV reactions require at least 12 hours after contact with antigen. Migration of antigen-specific CD4+ lymphocytes to the reaction site is followed by cytokine release and a local inflammatory response. Contact hypersensitivity is one form of type IV reaction and occurs where skin has come into contact with antigen. Contact dermatitis and the response to poison ivy are examples of contact hypersensitivity. Another form of type IV hypersensitivity is granulomatous hypersensitivity, in which chronic infection leads to the formation of granulomas in tissues. Granulomatous diseases include tuberculosis, sarcoidosis, and Crohn’s disease.
Drug reactions
Predicting who will react adversely to a drug or combination of drugs is difficult. Fortunately, life-threatening adverse reactions to drugs and products used during anesthesia and surgery are very uncommon, with the overall incidence estimated to be one in every 5000 to 10,000 anesthetics.
Pathophysiology
Adverse reactions to anesthetic agents have been found to be two-thirds immune mediated (anaphylactic reactions); the other third was classified as anaphylactoid reactions. Of anesthetic drugs that triggered anaphylactic reactions, neuromuscular blocking agents (NMBAs) do so most frequently. Anaphylactic and anaphylactoid reactions occurred more frequently in female patients, which is thought to be because of chemical epitopes that NMBAs and many cosmetics have in common. This observation may also explain why many patients generate an allergic response to NMBAs on their first exposure to the drug.
Persons who have an increased allergic tendency are termed atopic and exhibit a genetic predisposition to such events. Atopic patients frequently present with some history of hay fever, rhinitis, asthma, or food or drug allergy. A generalized history of allergy does not necessarily predispose a patient to anaphylactic or anaphylactoid reactions to anesthetic drugs. If a patient has a history of sensitivity to a particular anesthetic drug, such as a muscle relaxant, that individual may well be at increased risk for allergic responses to other agents in that class.
Anaphylactic reactions to local anesthetics are uncommon; ester local anesthetics are more likely than amide agents to elicit an allergic response. Ester local anesthetic metabolites, such as para-aminobenzoic acid, have been identified to be responsible for this higher incidence of allergic response. Local anesthetic solutions containing methylparaben and propylparaben as preservatives may induce allergic responses in susceptible individuals. Thus, administration of preservative-free local anesthetic solutions may reduce the likelihood of an allergic response. Recent theories suggest that allergies to various antioxidants and certain sulfite components may be responsible for some degree of allergic reactions to local anesthetic preparations.
Avoiding known causal agents (particularly those that induce histamine release), combined with careful selection and application of additional drugs, can reduce risk of adverse reactions. The most common causal agents are antibiotics (cephazolin), NMBAs, latex, opioids, protamine, propofol, and contrast dyes. A thorough history and discussion with the patient or the patient’s guardian can usually reveal the potential for untoward drug effects and alert the anesthesia provider to avoid suspicious agents. Patients frequently mistake drug sensitivity or an unpleasant response for an allergy. This is especially true with local anesthetic solutions containing epinephrine or administered with opioids. Careful investigation and cautious interviewing techniques are usually beneficial in clarifying these questionable areas. Reviewing past procedural notes and anesthesia records and possible consultation with an allergist when appropriate can further help in determining situational specifics and facilitate appropriate planning. The administration of H1– and H2-receptor antagonists preemptively may prevent allergic reactions in many cases when a known or suspected sensitivity is present.
Treatment
Patients who do not appear to have life-threatening symptoms on initial presentation may nonetheless progress to life-threatening anaphylaxis. Early administration of medications may be beneficial in halting this progression. Standard therapy for non–life-threatening situations includes the following:
1. Epinephrine: The initial adult dose may range from 100 to 500 mcg subcutaneously or intramuscularly. This dose may be repeated every 10 to 15 minutes as needed up to a maximum of 1 mg per total dose. The dose in children is 10 mcg/kg up to a maximum of 500 mcg per total dose. The total dose can be repeated every 15 minutes for two doses and then every 4 hours as needed. Evidence indicates that more rapid systemic absorption and higher peak plasma levels occur after intramuscular administration than after subcutaneous administration.
2. Diphenhydramine: 1 to 2 mg/kg or 25 to 50 mg/dose (parenterally)
3. Steroids may also be administered. However, the efficacy of steroids in treating acute anaphylaxis or in reducing a late anaphylactic reaction has not been clearly established.
Life-threatening anaphylaxis requires immediate administration of epinephrine and may require other immediate measures for support of cardiorespiratory status. Cardiopulmonary resuscitation (CPR) should be instituted if there is loss of circulation or respiration. Oxygen (100%) should be administered and the airway secured. Hypotension should be addressed by administration of vasopressors and infusions of large volumes of intravenous fluids or colloids (or both) to compensate for peripheral vasodilation and intravascular fluid loss. Bronchospasm should be treated with inhaled bronchodilators, theophylline, or both.
Patients experiencing anaphylaxis may not always respond adequately to one injection of epinephrine. Epinephrine has a rapid onset but a short duration of action. At the same time, mediator release from mast cells and basophils may be prolonged, producing biphasic or protracted anaphylaxis. Moreover, patients who are taking β-adrenergic blocking agents may not respond to epinephrine and may require substantial fluid replacement. For patients with life-threatening anaphylaxis who are poorly responsive to initial doses of epinephrine, more frequent or higher doses may be required. If the patient does not respond to subcutaneous epinephrine, intravenous administration of epinephrine must be initiated. Bolus doses of 50 to 100 mcg should be titrated to effect. Epinephrine infusion should initially be administered at 1 mcg/min, which can be increased to 2 to 10 mcg/min. For refractory cardiorespiratory arrest in children, the initial intravenous epinephrine dose is 10 mcg/kg. Subsequent doses of 100 mcg/kg can be administered every 3 to 5 minutes, and if the patient is still refractory, the dose may be increased to 200 mcg/kg.
A good clinical response represents resolution of the allergic reaction. If there is partial resolution or concern about biphasic anaphylaxis, continuous monitoring is suggested. Additional history might reveal previous episodes of anaphylaxis or asthma. Antihistamines may be useful in the treatment of anaphylaxis, particularly for symptoms of urticaria and angioedema. An H1 receptor antagonist, alone or in combination with an H2 receptor antagonist, may be useful in reversing hypotension refractory to epinephrine and intravascular fluid replacement. Steroids, such as 200 mg of intravenous hydrocortisone, may reduce the risk of recurring or protracted anaphylaxis, although direct clinical evidence for this has not been clearly established.
Transfusion reactions
Because of advances in technological capabilities and quality-control practices, blood transfusion reactions are, fortunately, not a common occurrence. Whereas the relative risk of an allergic transfusion reaction of mild severity (urticaria and pruritus) is approximately one in 500, a fatal hemolytic reaction occurs in approximately one in 250,000 to 600,000 transfusions administered nationally.
Pathophysiology
The mechanism responsible for most transfusion reactions involves ABO incompatibility. Transfusion of incompatible blood type causes recipient antibodies to react with donor red blood cells, causing their destruction and the potential for significant consequences. Disseminated intravascular coagulation, renal failure, and death are not uncommon after this type of reaction. Because the most common cause for a major hemolytic transfusion reaction is human error, it should never be assumed that another person is solely responsible for checking blood that one is preparing to administer to a patient.
Transfusion reactions are frequently masked, or at least delayed appreciably, during anesthesia. Hallmark symptoms of cardiovascular instability, such as hypotension, as well as fever, hemoglobinuria, and bleeding diathesis are indicative of a transfusion incompatibility and should be immediately treated.
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related morbidity and mortality. Recipient risk factors include higher interleukin-8 levels, liver surgery, chronic alcohol abuse, shock, higher peak airway pressure while being mechanically ventilated, current smoking, and positive fluid balance. Transfusion risk factors were recipient of plasma or whole blood from female donors, volume of human leukocyte antigens (HLA) class II antibody with normalized background ratio (NBG) greater than 27.5, and volume of anti-human neutropil antigens (HNA).
Latex allergy
Allergies to latex-containing products continue to be a source of significant problems for specific populations. Health care workers and certain patients, particularly those with congenital neural tube defects and those who have undergone multiple surgical procedures, have shown particular sensitivity to latex-containing products.
It has been estimated that approximately 0.8% of the general population has some form of sensitivity to latex. Atopic persons who react with skin dermatitis and who are allergic to certain fruits (particularly kiwi and bananas) should be further evaluated for latex allergy. Health care workers and patients who experience frequent exposure to devices and products that contain latex also exhibit such allergic reactions. The incidence of health care worker allergy to latex-containing products ranges between 8% and 25%. The most frequent clinical manifestations of latex reactions include some form of contact dermatitis, type I hypersensitivity reaction with the potential for anaphylaxis, or type IV hypersensitivity reaction.
Preventive procedures and recommended protocols have been established for the management of latex allergies that can have significant anaphylactic consequences. The incidence of latex allergies has increased proportionately with the 10-fold increase in medical glove usage to accommodate universal precautions and barrier protection during anesthesia, surgery, and obstetric care. Using gloves that do not contain latex (e.g., gloves processed from polyvinyl or neoprene) can prevent this source of latex exposure. Although skin prick, patch testing, and radioallergosorbent tests for latex allergy are available, all present various challenges in qualifying a conclusive diagnosis.
B Geriatrics
Definition
Geriatrics is the branch of medicine that deals with the physiologic effects of aging and the diagnosis and treatment of persons who are 65 years of age or older. By 2030, it has been estimated that approximately one in five people in the United States will be older than 65 years of age. Persons reaching age 65 years have an average life expectancy of an additional 18.4 years (19.8 years for women and 16.8 years for men).
Pathophysiology
Human organ function shows a linear decline with age. The rate constant for this decline is slightly less than 1% per year of the functional capacity present at age 30 years. As a consequence, a 70-year-old geriatric patient may have a 40% decrease in the function of any specific organ compared with that present at the age of 30 years.
Clinical manifestations
Clinical manifestations include an increased prevalence of age-related concomitant disease (hypertension, renal disease, atherosclerosis, myocardial infarction, chronic obstructive pulmonary disease, cardiomegaly, diabetes, liver disease, congestive heart failure, angina, cerebrovascular accident). The commonly age-related anatomic and physiologic changes that occur are listed in the box on pg. 225.
Anesthetic considerations
The choice of anesthetic technique should be based on the changes in organ system function in the patient, the pharmacokinetic and pharmacodynamic effects anticipated, the surgical requirements, and the needs and predisposition of the patient. As a rule, geriatric patients are likely to be predisposed to hypotension as a result of reduced activity of the sympathetic nervous system and decreased intravascular volume. Decreased cardiac output and delayed drug clearance are likely to prolong the onset of drug effects and the duration of action (see the table below).
Age-Related Changes and Pharmacokinetics
| Change | Effect |
| Contracted vascular volume | High initial plasma concentration |
| Decreased protein binding | Increased availability of free drug |
| Increased total body lipid storage sites | Prolonged action of lipid-soluble drugs |
| Decreased renal and hepatic blood flow | Prolonged action of drugs dependent on kidney and liver elimination |
Regional, general, and monitored anesthesia care techniques are appropriate selections for geriatric patients. Each technique has its corresponding cadre of supporters. No conclusive study has demonstrated the superiority of any one specific anesthetic technique. With regional and local techniques, maintenance of consciousness during the surgical procedure may be associated with less confusion during the postoperative period. However, general anesthesia with endotracheal intubation may be advantageous for promoting bronchopulmonary toilet and facilitating surgical conditions. A progressive decrease in the reactivity of protective airway reflexes, such as coughing and swallowing, can be expected with age. Because elderly patients often are edentulous, a sealed fit with the anesthetic face mask may be difficult. These factors may increase the likelihood of regurgitation of gastric contents, with aspiration of vomitus into the lungs. The changes that accompany cervical arthritis and osteoarthritis, limiting extension and flexion of the neck, often make endotracheal intubation difficult.
It appears likely that the patient’s preoperative health status and events during the course of the anesthetic that precipitate such physiologic changes as hypotension, hypoxia, hypercarbia, and hypertension do more to affect patient outcome than does anesthetic technique.
Great care must be taken to prevent trauma to the skin and bony prominences when geriatric patients are positioned for surgery on the operating table. Collagen loss and decreased elasticity of tissue make the skin more sensitive to damage from tape, monitoring devices, and contact with hard table surfaces. Additionally, any invasive procedure, including insertion of intravenous, spinal, and epidural catheters, should be accomplished with the goal of protecting the integrity of the skin.
Postoperative implications
Postoperative complications in the elderly population are often related to cardiac and pulmonary dysfunction and decreased reserves. Monitoring with a pulse oximeter may permit detection of the need for supplemental oxygenation or ventilation during the postoperative period because ventilation–perfusion mismatch is common in geriatric patients. Elderly patients may be especially prone to regurgitation and aspiration from a reduction in airway reflexes. In addition, renal and hepatic dysfunction may prolong the duration of action of pharmacologic agents administered to the patient.
Elderly patients are also prone to postoperative heat loss. To encourage rewarming and prevent problems associated with shivering, the patient should be placed in a warmed environment. Geriatric patients may require special assistance in being oriented to time and place. Prolonged anesthetic effect may compound disorientation to an unfamiliar environment.
Postoperative delirium (PD) and cognitive dysfunction are higher in the elderly population. Postoperative delirium is a transient and fluctuating disturbance of consciousness that occurs shortly after surgery. Postoperative cognitive dysfunction is a more persistent change in cognitive performance diagnosed by neuropsychological tests.
Postoperative delirium in elderly adults leads to increased morbidity, delayed functional recovery, prolonged hospital stay, nursing home placement, and mortality. PD tends to occur between postoperative days 1 and 3 and usually resolves anywhere from hours to days. PD symptoms may persist for weeks to months. Patients at risk for PD are listed in the following box. Sedative–hypnotics, narcotics, benzodiazepines, and anticholinergics have been identified as the classes of drugs associated with PD. Protocols have been developed with effective interventions to decrease PD. Haloperidol has been successfully utilized in the treatment of immediate agitation. Benzodiazepines tend to worsen agitation unless the cause of delirium has been identified as alcohol withdrawal.
C Glaucoma or open globe
Definition and etiology
In glaucoma, intraocular pressure (IOP) is increased, resulting in impaired capillary flow to the optic nerve. If the condition is left untreated, loss of sight may result.
Pathophysiology
Types of glaucoma
• Open-angle glaucoma: This is characterized by elevated IOP with an anatomically open anterior chamber. Sclerosed trabecular tissue impairs aqueous filtration and drainage. As treatment, miosis and trabecular stretching should be produced medically (eyedrops, epinephrine, timolol).
• Closed-angle glaucoma: The peripheral iris moves in direct contact with the posterior corneal surface, mechanically obstructing aqueous flow. This is caused by a narrow angle between the iris and posterior cornea and produces swelling of the crystalline lens.
• Congenital glaucoma is associated with some eye diseases (retinopathy of prematurity, aniridia, mesodermal dysgenesis syndrome). Surgical goniotomy or trabeculotomy should be performed to route aqueous flow into Schlemm canal. Cyclocryotherapy decreases aqueous formation by destroying the ciliary body by freezing tissue with a probe.
Clinical manifestations
The clinical manifestations of acute glaucoma are a dilated, irregular pupil and pain in and around the eye.
Diagnostic and laboratory findings
Normal IOP is 10 to 25 mmHg; abnormal elevated IOP is greater than 25 mmHg. Pressure becomes atmospheric when the globe is opened. Any sudden rise in IOP at this time may lead to prolapse of the iris and lens, extrusion of the vitreous humor, and loss of vision. Coagulation studies should be evaluated before retrobulbar block is instituted.
Treatment
Intraocular pressure is increased by the following: external pressure on the eye, including venous congestion associated with coughing, vomiting, or the prone position; scleral rigidity, which is increased in elderly persons; and changes in the intraocular structure or fluids. Pilocarpine hydrochloride decreases resistance to improve drainage of aqueous humor. Acetazolamide reduces the rate at which aqueous humor is formed.
Anesthetic considerations
Drug therapy should be continued to maintain miosis. Anticholinergic drugs are acceptable in preoperative medication. Increases of IOP, hypercarbia, and central venous pressure should be avoided. Succinylcholine causes transient increases in IOP. Rapid-sequence induction generally is acceptable for patients with open globe and a full stomach. Awake intubations are not desirable because they may contribute to increases in IOP. Techniques and drugs associated with decreasing IOP include volatile anesthetics, intravenous anesthetics, hypocarbia, hypothermia, mannitol, glycerin, nondepolarizing muscle relaxants, and timolol. General anesthesia or retrobulbar blocks are acceptable for eye surgery. General anesthesia is typically used for open globe repair.
Drug interactions also must be considered. Echothiophate prolongs the effect of succinylcholine. Timolol may result in bradycardia. Etomidate may induce myoclonus.
Anesthetic goals for ophthalmic surgery
The goals for ophthalmic surgery are akinesia, profound analgesia, minimal bleeding, avoidance of the oculocardiac reflex, control of IOP, awareness of drug interactions, and smooth induction and emergence without vomiting or coughing or bucking. If succinylcholine is necessary, a defasciculating dose of nondepolarizing agent will decrease muscle fasciculations and may not dramatically increase IOP.
D Malnutrition
Definition
Malnutrition, or nutritional failure, is associated with protein depletion in the presence of adequate calories or with combined protein-calorie deficiency. Protein-calorie depletion is a frequent finding in surgical patients and in critically ill individuals.
Incidence and prevalence
Critically ill patients experience negative caloric intake because of the hypermetabolic state produced by their illness. Trauma, fever, sepsis, and wound healing result in a drastically increased metabolism.
Pathophysiology
Basic energy requirements are an intake of 1500 to 2000 calories/day. An increase in body temperature of 1° C increases daily caloric requirements by 15%. Multiple fractures increase energy needs by 25%. Major burns cause the greatest increase in energy requirements: 100%. In addition, patients with large tumors may also have greatly increased energy requirements.
Clinical manifestations
Protein depletion affects the protein content of all organs. The liver and the gastrointestinal tract are rapidly depleted; the brain is affected less than other organs. If protein depletion is severe, the gastrointestinal system will be unable to tolerate or digest food because protein is needed to produce digestive enzymes. Skeletal muscle is most affected and may lose as much as 70% of its protein. Patients with malnutrition are at increased risk of infections and complications in the postoperative period.
Diagnostic and laboratory findings
The lack of a specific test for protein-calorie malnutrition often makes diagnosis difficult. The best single index of malnutrition is evidence of weight loss from the patient’s normal level of weight. Plasma albumin levels lower than 3 g/dL and transferrin levels less than 200 mg/dL have also been used to diagnose malnutrition. Nitrogen balance, which requires the careful collection of all drainage and excretions over 24 hours, may be used to evaluate nutritional status. Nitrogen balance provides an estimate of net protein degradation or synthesis.
Treatment
The steps in planning a nutritional regimen are first to identify the need for intervention, then to determine the route of delivery, and finally to adjust the amounts of macronutrients and micronutrients the patient requires.
Anesthetic considerations
Enteral and parenteral are the two routes of choice. At times, these two routes are combined. Enteral supplements can be sipped or administered through a nasogastric feeding tube or gastrostomy tube. If the gastrointestinal tract is nonfunctional, intravenous (parenteral) nutrition is instituted. Isotonic solutions can be delivered through peripheral veins. However, if the solution is hypertonic because of a greater caloric need, a central line should be used.
Patients receiving exogenous nutritional support are prone to deficits or an overabundance of certain electrolytes. Careful evaluation of laboratory values preoperatively as well as of any function tests performed is imperative. Parenteral nutrition has the greatest potential for complications. Hypoglycemia and hyperglycemia are common. Increased carbon dioxide resulting from metabolism of large amounts of glucose may hinder early extubation postoperatively. Patients with compromised cardiac function are at risk of congestive heart failure related to fluid overload. Electrolyte abnormalities include hypokalemia, hypomagnesemia, hypocalcemia, and hypophosphatemia. If parenteral nutrition is continued intraoperatively, infusions of other fluids should be minimized. Malnutrition is a clinical finding that must be partially corrected preoperatively. Therapy must be maintained.
E Obesity
Definition
Obesity is a complex multifactorial chronic disease that develops from an interaction of genotype and the environment. Overweight is defined as a body mass index (BMI) of 25 to 29 kg/m2 and obesity as a BMI of 30 kg/m2, as shown in the table on pg. 230.
Classification of Overweight and Obesity by Body Mass Index
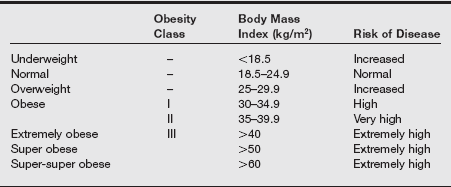
Data from National Institute of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: the Evidence Report. NIH publication no. 98-4083. Washington, DC: National Institutes of Health; September 1998; Klein S, Romijn J. Obesity. In Larsen PR, et al. Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders; 2003: 1619-1641; Flier JS. Obesity. In Braunwald E, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York: McGraw-Hill; 2001:479-486; Brodsky JB, Lemmens HJ. Is the super-obese patient different? Obes Surg 2004;14(10):1428.
Incidence and prevalence
Obesity is a disease that affects more than one-third of the adult U.S. population. It is the second leading cause of preventable death in the United States. Current estimates are that 65% of adults in the United States are classified as overweight or obese and more than 30% of adults are classified as obese; this means the prevalence of obesity has doubled over the past 20 years. There are an estimated 23 million persons in the United States with a BMI of no less than 35 kg/m2 and 8 million with a BMI of 40 kg/m2 or higher. Obesity in children and adolescents has also increased significantly since the mid-1990s. In the United States, 30% of this population is overweight, and 15% is obese.
Pathophysiology
Genetic predisposition, believed to be a primary factor in the development of obesity, explains only 40% of the variance in body mass. The significant increase in the prevalence of obesity has resulted from environmental factors that increase food intake and reduce physical activity. Other factors such as socialization, age, sex, race, and economic status affect its progression. In the United States, food consumption has risen as a result of the supersizing of portions and the availability of fast food and snacks with high fat content. Physical activity has been reduced as a result of modernization (television and computers), a sedentary lifestyle, and work activities. Cultural and lifestyle variations play an important role in the development of obesity.
Clinical manifestations
Manifestations include increased cardiac output, blood volume, oxygen consumption, minute ventilation, work of breathing, and carbon dioxide production, as listed in the following box. Obesity is associated with an increase in the incidence of more than 30 medical conditions. The risk of cardiovascular disease, certain cancers, diabetes, and disease overall is linearly related to weight gain. Type 2 diabetes, coronary heart disease, hypertension, and hypercholesterolemia are prominent conditions in overweight and obese patients. With increasing weight gain and increased adiposity, glucose tolerance deteriorates, blood pressure rises, and the lipid profile becomes more atherogenic. Hormonal and nonhormonal mechanisms contribute to the greater risk of breast, gastrointestinal, endometrial, and renal cell cancers. Psychological health risks often stem from social ostracism, discrimination, and an impaired ability to participate fully in activities of daily living. The physiologic changes associated with obesity are listed in the following box.
Diagnostic and laboratory findings
Findings include hypercholesterolemia, hypertriglyceridemia, and altered pulmonary function test results. Baseline arterial blood gases, chest radiography, electrocardiography, and echocardiography are used for diagnosis.
Treatment
A multimodal approach in the treatment of obesity includes dietary intervention, increased exercise, behavior modification, drug therapy, and surgery. Weight loss programs are individualized to each patient based on the degree of obesity and coexisting conditions. Drug therapy is initiated in patients with a BMI greater than 30 kg/m2 or a BMI between 27 and 29.9 kg/m2 with a coexisting medical condition. Medications that promote weight loss have limited efficacy. Despite the enormous potential market, efforts to develop effective drug therapies have been disappointing. The U.S. Food and Drug Administration’s guidance for long-term weight loss drugs recommends that a 5% weight reduction be maintained for 12 months after treatment initiation. Two drugs are available for use: orlistat (Xenical and Alli) and phentermine (Adipex-P, others). When used in combination with a comprehensive weight loss program, they can occasionally be effective in producing weight loss in the range of 4 to 5.5 kg.
Orlistat is a lipase inhibitor that decreases the absorption of fat in the gastrointestinal tract. It has recently been released as an over-the-counter medication. Side effects are minor and most related to gastrointestinal discomfort. Phentermine, a sympathomimetic agent, is approved for short term use (up to 12 weeks) as a weight loss management drug. Tolerance, dependence, abuse, and a relatively high number of side effects limit its usefulness. Several antidepressants, antiepileptic, and antidiabetic drugs may promote weight loss and are used off label for this indication.
Surgical approaches designed to treat obesity can be classified as malabsorptive or restrictive. Malabsorptive procedures, which include jejunoileal bypass and biliopancreatic bypass, are rarely used at the present time. Restrictive procedures include the vertical banded gastroplasty (VBG) and gastric banding, including adjustable gastric banding (AGB). Roux-en-Y gastric bypass (RYGB) combines gastric restriction with a minimal degree of malabsorption. VBG, AGB, and RYGB can all be performed laparoscopically. RYGB, the most commonly performed bariatric procedure in the United States, involves anastomosing the proximal gastric pouch to a segment of the proximal jejunum and bypassing most of the stomach and the entire duodenum. It is the most effective bariatric procedure to produce short- and long-term weight loss in severely obese patients. Advances in laparoscopic surgery have significantly improved surgical procedure times, morbidity, and mortality related to bariatric surgery.
Pharmacologic considerations
Obesity is associated with significant alterations in body composition and function that can alter the pharmacodynamics and pharmacokinetics of drugs. Alterations in the volume of distribution are related to the size of the fat organ, increased blood volume, increased cardiac output, increased total body weight, and alterations in protein binding and lipophilicity of the drug. Highly lipophilic drugs have an increased volume of distribution in obese persons compared with persons of normal weight. The increased volume of distribution requires higher doses of lipophilic drugs to produce the required pharmacologic effect and prolongs the elimination of certain drugs such as benzodiazepines. Factors such as protein binding and end-organ clearance affect volume of distribution.
There is no relationship for some highly lipophilic drugs (digoxin, remifentanil, and procainamide) between their solubility and distribution in obese patients. Dosing by ideal body weight is appropriate for these drugs. Drugs with weak or moderate lipophilicity are usually dosed based on ideal body weight or lean body mass. Recommendations for dosing commonly used anesthetics are listed in the table on pg. 233.
Dosing Guidelines for Intravenous Anesthetics
| Anesthetic Agent | Dosing | Guidelines |
| Midazolam (Versed) | TBW | Increased central Vd; increase initial dose to achieve therapeutic effect; prolonged sedation |
| Thiopental | TBW | Increased Vd; increase initial dose; prolonged time to awakening |
| Propofol | TBW: Initial and infusion | Increased Vd; increase initial dose; high affinity for fat; high hepatic extraction |
| Fentanyl | TBW | Increased Vd; increased elimination half-life |
| Sufentanil | TBW | Increased Vd; increased elimination half-life |
| Remifentanil | IBW | Consider age and lean body mass |
| Cisatracurium | TBW | No difference than those with normal weight |
| Vecuronium | IBW | Increased Vd; impaired hepatic clearance; prolonged duration of action |
| Rocuronium | IBW | Faster onset and similar duration of action |
| Succinylcholine | TBW | Increased plasma pseudocholinesterase activity; increase dose |
IBW, Ideal body weight; TBW, total body weight; Vd, volume of distribution.
Elimination of drugs in obese individuals is normal or increased in phase I reactions (oxidation, reduction, and hydrolysis) and increased in phase II reactions (metabolism). Renal clearance is increased by the augmented renal blood flow and glomerular filtration rate.
Anesthetic considerations
No demonstrable difference in emergence from inhalation versus narcotic technique has been discerned in obese patients. The use of short-acting water-soluble anesthetics facilitates smooth anesthetic induction, maintenance, and emergence from anesthesia. Objectives for maintenance of anesthesia in obese patients include strict maintenance of airway, adequate skeletal muscle relaxation, optimum oxygenation, avoidance of the residual effects of muscle relaxants, provision of appropriate intraoperative and postoperative tidal volume, and effective postoperative analgesia. Depending on the patient’s condition, these can be achieved by either general or regional anesthesia regimens. An epidural anesthetic with concomitant “light” general anesthesia is frequently chosen. A light general anesthetic can facilitate management of the airway, ventilation, and the patient’s level of consciousness, and the epidural anesthetic provides surgical analgesia and anesthesia. Combining these techniques accomplishes all of the objectives. The epidural catheter can be used for postoperative analgesic administration. This enhances earlier resumption of deep-breathing and coughing maneuvers.
Airway evaluation
A thorough airway evaluation is warranted to determine the optimal airway management technique in overweight and obese patients. Most practitioners use evaluation of multiple patient physical characteristics to identify potential airway problems indicative of the unanticipated difficult airway. These include measurement of interincisor distance, thyromental distance, head and neck extension, Mallampati classification, body weight, and a history of difficult airway. Evaluation of the length of upper incisors, visibility of the uvula, shape of the palate, compliance of the mandibular space, and length and thickness of the neck provides further assessment. Increasing neck circumference and the Mallampati classification higher than grade III have been identified as the two most important factors in morbidly obese patients.
Anatomic aberrations of the upper airway induced by severe obesity include reduced temporomandibular and atlanto-occipital joint movement. Unsatisfactory mouth opening, presence of neck or arm pain, or inability to place the head and neck into “sniffing position” may indicate the need for awake fiberoptic intubation. Extreme airway narrowing, in conjunction with shortened mandibular–hyoid distance (less than three fingerbreadths), can complicate mask ventilation and intubation. Presence of a short, thick neck; pendulous breasts; hypertrophied tonsils and adenoids; and beards can contribute to a difficult ventilation or intubation. Marginal room air pulse oximeter saturations, abnormal arterial blood gas results, and previous history of complicated airway management also indicate a potentially difficult intubation, which occurs in at least 13% of severely obese patients.
Aspiration prophylaxis
Of significance to the airway in anesthetized obese patients is the increased risk of regurgitation (passive and active) and subsequent pulmonary aspiration. Obese persons have greater volumes and more acidic gastric fluid than persons of normal weight. Gastroesophageal reflux and hiatus hernia, which are more prevalent in obese patients, also predispose them to esophagitis and pulmonary aspiration. Other conditions that cause delayed gastric emptying, such as diabetes mellitus or traumatic injury, further increase the risk of aspiration. For these reasons, obese patients are considered to have a “full stomach” even if the prescribed nothing-by-mouth intake restriction has been followed. Debate and controversy exist as to the relative risk of aspiration in obesity; most practitioners use techniques to attenuate this complication.
Timely preinduction administration of histamine-2 receptor and dopamine receptor antagonists coupled with oral administration of nonparticulate antacids decreases morbidity resulting from pulmonary aspiration and Mendelson syndrome. Head-up positioning of the patient, with application of the Sellick maneuver during rapid sequence induction, limits the volume of vomitus that enters the trachea if regurgitation occurs. Nasogastric or orogastric suctioning before emergence further reduces the amount of fluid available for aspiration.
Intubation
The obese patient should be positioned with the head elevated (reverse Trendelenburg position) on the operating room table. This position facilitates patient comfort, reduces gastric reflux, provides easier mask ventilation, improves respiratory mechanics, and helps to maintain functional residual capacity. The reduced functional residual capacity in obese patients contributes to the rapid desaturation that occurs with induction of general anesthesia. To attenuate the desaturation and to maximize oxygen content in the lungs, patients are preoxygenated with 100% mask oxygen for at least 3 to 5 minutes. The patient’s head, neck, and shoulders should be carefully moved into “sniffing position” by using pillows, “doughnuts,” or foam head supports.
Some practitioners advocate the use of an “awake look” to visualize the difficulty of the airway. Careful administration of sedative drugs, application of topical anesthesia to the oropharyngeal structures, and transtracheal and superior laryngeal nerve blocks are performed. Nasal oxygen is used as a supplement during awake laryngoscopy. If epiglottic and laryngeal architecture is easily visualized, successful asleep intubation can be done. If the airway structures cannot be visualized, airway management using a glidescope, intubating laryngeal mask airway, or awake fiberoptic intubation should be performed. The surgeon and another skilled anesthesia provider must also be in attendance during the induction. Muscle hypotonus in the floor of the mouth followed by rapid occurrence of soft tissue obstruction and hypoxia requires one person to support the mask and airway while another person bag ventilates the patient.
Volume replacement
The normal adult percentage of total body water is 60% to 65%. In a severely obese patient, it is reduced to 40%. Therefore, calculation of estimated blood volume should be 45 to 55 mL/kg actual body weight rather than the 70 mL/kg apportioned to a nonobese adult. Use of reduced parameters for volume replacement and avoidance of rapid rehydration lessen cardiopulmonary compromise. Fluid management is guided by blood pressure, heart rate, and urine output measurements. Volume expanders, such as hetastarch (Hespan), should not be administered at greater than recommended volumes per kilogram of ideal body weight (20 mL/kg). Dilutional coagulopathy, factor VIII inhibition, and decreased platelet aggregability can result from excessive administration. Albumin 5% and 25% should be used as indicated to support circulatory volume and oncotic pressure. When replacing blood loss with crystalloid, the 3:1 ratio (3 mL of crystalloid to 1 mL of blood loss) is applicable to severely obese patients.
Intraoperative positioning
Surgical positioning of morbidly obese patients necessitates extra precautions to prevent nerve, integumentary, and cardiorespiratory compromise. The type of surgery, combined with inordinate stretching or compression of nerve plexus, and prolonged immobility cause local tissue ischemia and damage that begins at the cellular level. Hypothermia, hypotension, table positioning, and the hydraulic pressure effect that the adipose patient places on orthopedic or cardiopulmonary structures potentiate impairment.
Regional anesthesia
Regional anesthesia can be used as the primary anesthetic technique in selected cases or as an accompaniment to postoperative pain and mobility management. Difficulties are frequently encountered, however, in severely obese patients. Anatomic landmarks used to guide conduction blockade are not easily visualized or palpable.
Extubation
The risk of airway obstruction after extubation is increased in obese patients. A decision to extubate depends on evaluation of the ease of mask ventilation and tracheal intubation; length and type of surgery; and presence of preexisting medical conditions, including obstructive sleep apnea. Criteria for extubation include an awake patient; tidal volume and respiratory rate at preoperative levels; sustained head lift or leg lift for at least 5 seconds; strong, constant hand grip; effective cough; adequate vital capacity of at least 15 mL/kg; and inspiratory force of at least 25 to 30 cm H2O negative. Patients must be placed with their heads up or in a sitting position. If doubt exists about the ability of the patient to breathe adequately, the endotracheal tube is left in place. Extubation over an airway exchange catheter or through a fiberoptic bronchoscope may be performed.
F Scleroderma
Definition
Widespread, symmetric lesions that cause induration of the skin and are followed by atrophy and pigmentation changes characterize scleroderma. It is a systemic disease that affects muscles, bones, the heart, and the lungs. Intestinal and pulmonary changes also occur. Lung volumes, vital capacity, compliance, and dead space all decrease. The respiratory rate increases, and diffusion capacity is impaired. Pulmonary hypertension can occur.
Anesthetic considerations
Tightening of the skin around the neck may limit mobility and mouth opening. Alternate methods to secure the airway (e.g., fiberoptic intubation) should be considered. Baseline pulmonary function tests and arterial blood gases may assist in optimizing these patients preoperatively, intraoperatively, and postoperatively. Postoperative ventilatory assistance may be needed. Be alert for vasospastic phenomena after induction of anesthesia; these should be treated with a plasma expander. Regional anesthesia is acceptable. Core temperature must be maintained. Gastrointestinal pretreatment (e.g., histamine blockers, metoclopramide) may be necessary because of poor gastric emptying.
G Systemic lupus erythematosus
Definition
Systemic lupus erythematosus (SLE) is a chronic inflammatory disorder of connective tissues that affects multiple organ systems with periods of remissions and exacerbations.
Incidence and prevalence
Systemic lupus erythematosus occurs eight to 15 times more often in women than in men and affects approximately 75 in 1,000,000 persons every year. It occurs most often in Asians and in African Americans. Exacerbations are more common in spring and summer and during stresses such as infection, pregnancy, and surgery.
Pathophysiology
The origin of SLE is unknown. One theory is that it is an antibody–antigen autoimmune response. Another theory deals with predisposing factors that promote susceptibility to SLE. These factors include stressors such as infection, exposure to ultraviolet light, immunizations, and pregnancy. A third theory suggests that drugs such as procainamide, hydralazine, penicillin, anticonvulsants, oral contraceptives, and sulfa drugs may trigger SLE.
Clinical manifestations
Clinical manifestations of SLE include arthritis of the upper and lower extremities as well as avascular necrosis of the femur. Systemically, SLE affects major organ systems (heart, lungs, kidneys, liver, neuromuscular system, skin). Pericarditis, myocarditis, tachycardia, arrhythmias, and congestive heart failure may develop. Left ventricular dysfunction and endocarditis have also been associated with SLE. About 50% of patients with SLE develop such cardiopulmonary abnormalities. Pneumonia, pleural effusions, cough, dyspnea, and hypoxemia are common. Glomerulonephritis and oliguric renal failure may result. Some patients develop lupoid hepatitis, which may be fatal. They may also incur intestinal ischemia. The neuromuscular system may be affected by myopathies. Psychological changes include schizophrenia and deterioration of the intellect. The skin may exhibit the typical lesion associated with SLE; this “butterfly rash” appears over the nose and is erythematous. Alopecia may also be seen clinically.
Diagnostic and laboratory findings
Complete blood count with differential may reveal anemia, a decreased white blood cell count, and a decreased platelet count. Specific tests for SLE include antinuclear antibodies, anti-DNA, and lupus erythematosus cell tests; urine analysis may reveal both red and white blood cells. Chest radiography may reveal pulmonary involvement, and electrocardiography may show conduction abnormalities.
Treatment
The usual treatment of SLE includes anti-inflammatory therapy with aspirin. Corticosteroids are often used to suppress adverse renal and cardiovascular system changes. For patients who do not respond well to steroids, immunosuppressive agents may be used. Antimalarial drugs, in small doses, have been found to be effective in treating arthritis and skin lesions.
Anesthetic considerations
Anesthesia management is based on medications used to treat the disorder as well as the organ involvement. Care must be taken in positioning the patient to avoid hyperextension of the neck. These patients may be difficult to intubate because of their inflammatory changes, and they frequently have restrictive lung disease and therefore may be difficult to ventilate. Rapid rates with smaller tidal volumes may be helpful. Overall, a thorough preoperative evaluation should be performed to establish organ system involvement.
Cutaneous lesions on the nose and mouth may make mask fit difficult. Cricoarytenoid arthritis rarely occurs. Patients with advanced disease may be debilitated, and chest radiography findings, complete blood cell count, and electrolytes should be checked. Anemia and thrombocytopenic purpura have been identified. The partial thromboplastin time may be falsely elevated because antibodies of SLE react with phospholipids used to determine partial thromboplastin time. If renal dysfunction is advanced, drugs dependent on renal elimination should be avoided. If the patient is currently receiving steroids or has taken them within 6 months, a steroid bolus should be used.