CHAPTER 270 Concepts of Disk Degeneration and Regeneration
Conceptually, disc degeneration is a product of lifelong degradation with synchronized remodeling of discs and neighboring vertebrae, including simultaneous adaptation of the disc structures to changes in physical loading and responses to the occasional injury.1
Early degenerative changes should refer to accelerated age-related changes.…Degenerative disc disease should be applied to a degenerate disc that is also painful.2
Much of the published literature concentrates on degeneration in the lumbar region, and it remains to be seen whether the same concepts apply to other regions. It should be emphasized that disk degeneration and disk herniation are closely related but not synonymous. Conceptually, degeneration is required to allow herniation to occur. It is well accepted that a herniated disk may cause pain and neurologic deficit. The role of disk degeneration in spine symptomatology is less clear. Although it is believed that disk degeneration may contribute to low back complaints, accurate identification of an abnormally or symptomatic degenerative disk has been difficult.3 The ability to do so will be essential for the successful application of potential regenerative technologies.
The financial burden of spinal disorders is considerable. The annual spine-related health care expenditure in the United States for 2005 was estimated to be $86 billion, in the same realm as cancer care ($89 billion) and accounting for 9% of all health care spending.4 However, the indirect costs associated with spine disorders may be much greater.5 If disk regeneration is possible and proves safe and efficacious, the potential impact on population health would therefore be significant.
Composition of a “Normal” Disk
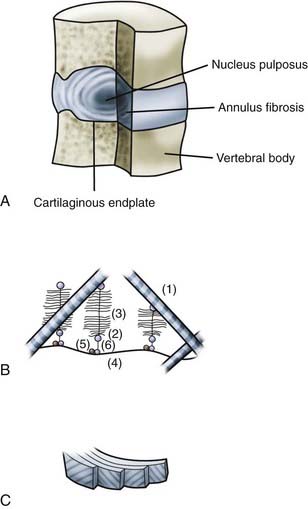
The intervertebral disk consists of three components. The central, gelatinous nucleus pulposus is contained by the multilaminated annulus fibrosus. The superior and inferior aspects of the disk are covered by cartilaginous vertebral end plates (Fig. 270-1). Significant changes in the anatomic structure and biochemical composition of these elements occur in development and continue throughout life. It is therefore difficult to define an archetypal normal disk. The generally accepted description of a healthy young adult disk is as follows.6 The nucleus pulposus consists of scattered cells and thin collagen fibrils dispersed in a proteoglycan-rich ground substance. The nucleus has a very high water content because of the hydrophilic nature of the proteoglycans. The annulus fibrosus consists of concentric lamellae of collagen fibers and a small amount of elastic fibers bound together by a lesser amount of proteoglycan gel. Within a lamella, the obliquely oriented collagen fibers are parallel to each other, but adjacent lamellae alternate the orientation of their fibers. The fibroblast-like cells of the annulus fibrosus are spindle-shaped and extend along the collagen fibrils, whereas the cells of the nucleus pulposus appear rounded and have been likened to chondrocytes.7 The outer lamellae of the annulus fibrosus insert into the circumferential bony ring apophysis of the upper and lower vertebral surfaces. The fibers of the inner lamellae are continuous with those of the vertebral end plate and form an envelope around the nucleus pulposus. The vertebral end plates are mostly fibrocartilage, with lesser amounts of hyaline cartilage found nearer the vertebral bodies.
Nerve fibers have been identified in the outer layers of the annulus fibrosus. The sources of these fibers in the anterior and lateral aspect of the annulus are direct branches from the ventral rami of the spinal nerve roots and the gray rami communicantes of the sympathetic trunk. The posterior annulus receives fibers from the sinuvertebral nerve, which forms from the union of an autonomic root from the gray rami communicantes and a somatic root from the ventral rami.8–10
The adult disk is avascular and relies on nutrients from adjacent vertebrae to maintain its living cellular constituents. Hence, the metabolic needs of the disk are met passively by diffusion through the adjacent bony and cartilaginous end plates, as well as through its own matrix. This leads to a marked concentration gradient in the disk, with lowest oxygen tension, glucose concentration, and pH in the center. Disk chondrocytes are well adapted to this environment, with maximal proteoglycan production occurring at 5% oxygen and a pH of 7.0.11
The dominant proteoglycan in disk matrix is aggrecan, which consists of a core protein conjugated with many keratan sulfate and chondroitin sulfate molecules. Keratin sulfate and chondroitin sulfate provide the high anionic charge densities needed for the osmotic properties of aggrecan. Through interaction with hyaluronan and link protein, aggrecan forms large aggregates and functions to retain water in the nucleus.12 Nonaggregating proteoglycans are also found in the disk matrix and include small leucine-rich repeat proteoglycans, fibromodulin, lumican, decorin, and biglycan. They may have a role in the mechanical behavior of the disk through their interaction with fibrillar collagen.13
Collagen in the disk is predominantly of the type I and type II varieties. Both are found in close to equal amounts in the annulus, whereas type II predominates in the nucleus.14 In progressing from the outer annulus inward, the ratio of type I to type II decreases. Multiple other collagen types are also found in disks, including types V and XI (as hybrid fibrils), types IX, XII, and XIV (noncovalently bonded to fibril surfaces), and types VI and III (pericellular).15
Disk Degeneration
Morphology
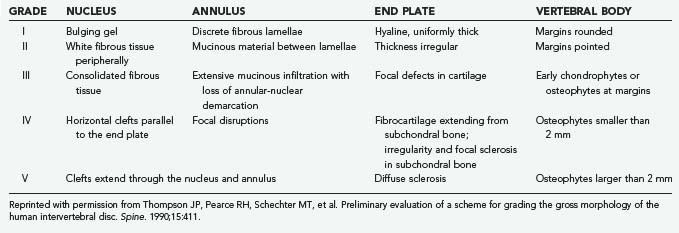
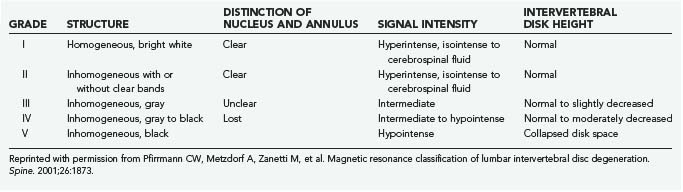
The macroscopic appearance of lumbar disk degeneration has been classified into stages defined by the three components of the disk (nucleus, annulus, and end plates) from the perspective of a midsagittal section by Thompson and coworkers.16 The mean grade of disk degeneration increases with age, but a wide variation in grades can be appreciated within any given age group. The stages are described in general terms as I, normal juvenile disk; II, normal adult disk; III, mild disk degeneration; IV, moderate disk degeneration; and V, severe disk degeneration (Table 270-1).17
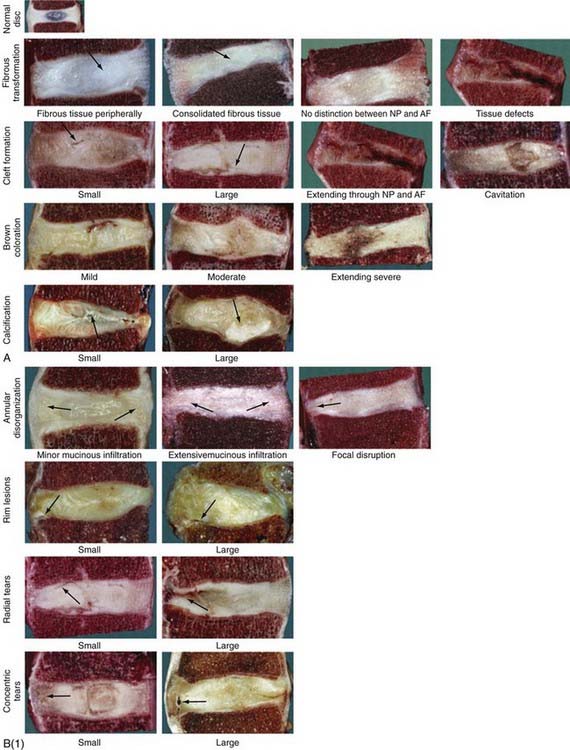
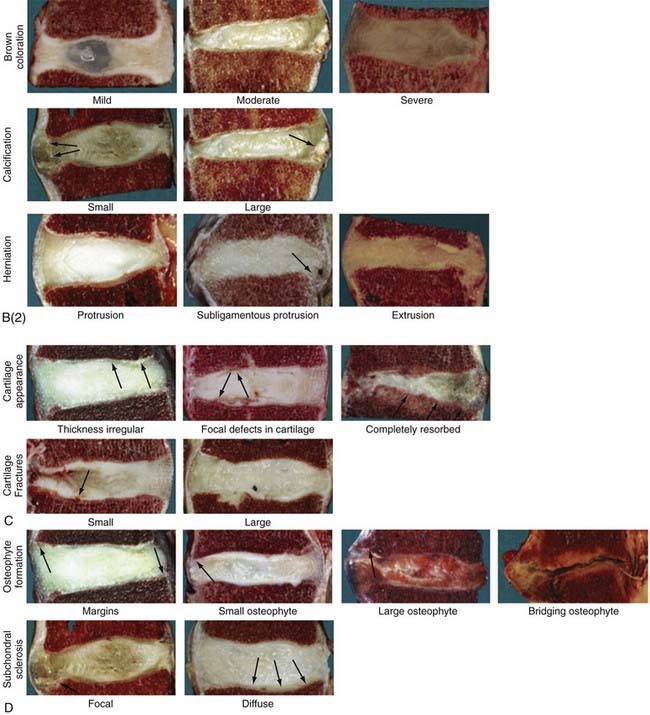
The temporospatial course of degeneration has been further detailed in cadaveric studies of lumbar spine specimens at the macroscopic18 and histologic17 levels (Fig. 270-2). The degenerative process begins in the first 2 decades of life, plateaus in the third and fourth decades, and then accelerates in the fifth to seventh decades and beyond; however, within the gross categories of age and Thompson macroscopic grade, substantial variation in the degree of histologic changes can be seen. The foci of abundant notochordal cells seen in fetal specimens disappear by the end of the first decade and are replaced by chondrocytes, whose proliferation rates, cell density, and death increase further with advancing age. True degeneration begins in the nucleus with fibrous transformation, mucous degeneration, and granular changes. The subsequent development of jagged empty spaces in the nucleus (called nuclear clefts) precedes the three types of annular tears—concentric, radial, and rim tears. In specimens of advanced age, the appearance of scar-like tissue formation and the development of large tissue defects impede appreciation of the disk’s morphology.
Underlying these macroscopic and histologic changes is deterioration of the biochemical composition of the disk. Three phases of matrix turnover have been described.19 The first phase (growth) is characterized by active synthesis of type II collagen and aggrecan. The second phase (maturation) consists of a reduction in the synthesis of both these constituents. In the third phase (degeneration and fibrosis), denaturation of type II collagen occurs and synthesis of type I collagen is increased. No change occurs in the low levels of aggrecan synthesis. Overall, the total proteoglycan and water content of the disk decreases with increasing age and morphologic grade,19 and the ratio of aggregating proteoglycans to total proteoglycans decreases. The relative increase in nonaggregating proteoglycan molecules is due to an accumulation of aggrecan fragments as a result of proteolysis.20 The proteolytic aggrecanases found in the intervertebral disk are produced by enzymes from both the matrix metalloproteinase (MMP) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) families, each with specific sites of action.21
There is evidence that aggrecan breakdown is under the influence of a variety of signaling molecules, including growth factors—insulin-like growth factor I (IGF-I), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β)—some bone morphogenic proteins (BMPs), and the cytokines interleukin-1 (IL-1) and IL-6, and tumor necrosis factor-α (TNF-α).21 Central to the interplay between growth factors and aggrecanases are the cells of the intervertebral disk. They control the balance between anabolic and catabolic processes in the matrix. As mentioned earlier, cells of the intervertebral disk rely on diffusion for delivery of nutrients and removal of metabolic waste. Interference or degradation of these pathways, particularly at the subchondral bone–disk junction, may lead to a decline in cell function, loss of growth factor production, and activation of aggrecanase, all likely to be key events in the pathogenesis of disk degeneration.22
Imaging
Macroscopic, histologic, or biochemical examination of the disk is limited to cadaveric specimens and is therefore not useful in the clinical or epidemiologic study of disk disease. Instead, medical imaging studies, including both plain radiography and magnetic resonance imaging (MRI), have been used as a surrogate measure of disk degeneration. On plain radiography, the intervertebral disk itself is radiolucent, and assessment of degeneration depends on measurement of loss of disk height, as well as the effect on the adjacent vertebrae in terms of osteophyte formation and end plate sclerosis. MRI allows direct assessment of the disk itself, including hydration and the degree of desiccation. Such factors have been combined and validated in several proposed grading scales.23,24
The most accepted MRI classification is the system described by Pfirrmann and colleagues (Table 270-2).25 It is based on the macroscopic Thompson grade and relies on the T2-weighted appearance of the nucleus and annulus, as well as disk space height. T2 disk signal characteristics on MRI have been correlated with both proteoglycan and water content.26,27 Changes in the appearance of the vertebral end plates were previously described by Modic’s group and are now synonymous with his name.28 Type I changes are hypointense on T1 sequences and hyperintense on T2. They correlate with histologic evidence of fibrovascular replacement of the subchondral marrow. Type II changes are T1 and T2 hyperintense and correlate with fatty deposition in the end plate zones. The later described type III changes are T1 and T2 hypointense and correlate with end plate sclerosis on plain radiographs.29
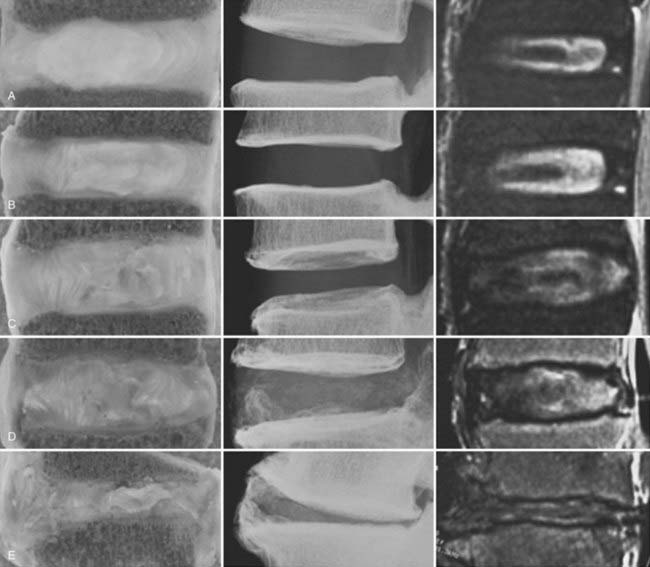
A combined imaging score incorporating elements from radiographic and MRI examination has been developed and correlated with morphologic and biochemical variables (Fig. 270-3).27 The score correlates well with macroscopic grade in advanced degeneration but fails to discriminate well between lower grades, thus suggesting that routine imaging techniques lack the sensitivity needed for early detection of changes. This lack of sensitivity has led to investigation of specialized MRI techniques. End plate diffusion patterns, assessed by the change in signal intensity of the disk space after gadolinium enhancement, have been used to identify end plate changes, but these sequences are time-consuming to obtain.30 High-resolution magnetic resonance spectroscopy has been used to examine the biochemical spectra of intervertebral disks from cadavers but has not been reported in the clinical setting.31,32 Showing great clinical promise is the quantitative MRI spin-lock technique T1ρ, which has been correlated with proteoglycan content and macroscopic grade in cadaveric studies33,34 and has been shown to be feasible for in vivo studies (Fig. 270-4).35,36
Epidemiology
Study of the epidemiology of disk degeneration is hampered by the overlap between normal aging and accelerated degeneration, the insensitivity of noninvasive imaging techniques, and the lack of consistent definitions of disk degeneration. As described previously, studies based on gross macroscopic assessment and imaging techniques have established a relationship between age and disk degeneration but at the same time noted much variability within age groups. Degeneration is also related to the spinal level, with degeneration occurring more frequently in some regions of the spine than in others.37
Given these observations, it is not unexpected that an extensive review of the published literature demonstrated wide-ranging estimates of the prevalence of MRI findings of disk degeneration.37 For the two components of the Pfirrmann assessment, signal intensity and disk space height, prevalence estimates in selected asymptomatic subjects range from 20% to 83% for decreased signal intensity and 3% to 56% for disk space narrowing (age range, 8 to 54 years). In unselected subjects, the range of prevalence estimates was 9% to 86% and 15% to 53%, respectively (age range, 12 to 58 years). Undoubtedly, such wide ranges are most likely due to inconsistencies in definition and the insensitivity of measurements.
Risk Factors
Unquestionably, repetitive or continuous axial overloading is the key determinant in the pathogenesis of lumbosacral degenerative disease.38
Physical loading … plays a relatively minor role in disc degeneration. Recent research indicates that heredity has a dominant role in disc degeneration.1
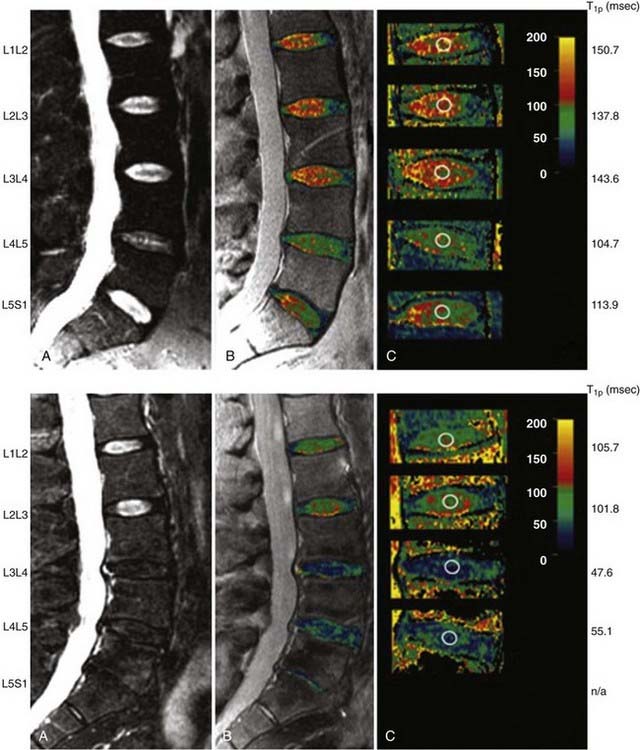
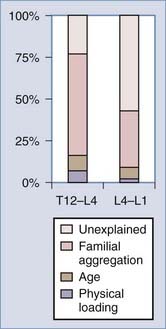
Our understanding of factors predisposing individuals to symptomatic degenerative disk disease has undergone a major paradigm shift over the last 2 decades. Mechanical loading has traditionally been believed to play a central role. However, a new appreciation of the importance of hereditary factors has evolved and superseded the reverence previously placed on loading. Much of this new information has been derived from a series of identical twin studies, many based on data from the Finnish twin cohort. An initial study of 40 male twins identified a remarkable degree of similarity in lumbar MRI appearance between the homozygous siblings, greater than that expected by chance (Fig. 270-5).39 Subsequently, the same authors examined 115 monozygotic twins from a national registry selected on the basis of highly discordant environmental predisposing exposures.40 Statistical models allowed factors predicting symptomatic disk degeneration to be weighted according to their importance through regression analysis. In the upper lumbar region (T12-L4), physical loading variables accounted for 7% of the predictive model, age explained a further 8%, and familial aggregation (hereditary and some shared environmental factors) accounted for more than 50%. In the lower lumbar region (L4-S1), physical loading variables accounted for just 2% of symptomatic disk degeneration, age explained 7%, and familial aggregation accounted for 34%. The remaining variability, 23% (upper) and 57% (lower), was unaccounted for by the factors studied (Fig. 270-6).
A classic twin study design that includes monozygotic twins with identical genomes and dizygotic twins with lesser homozygosity can be used to differentiate between genetic and environmental influences. Using an overall disk degeneration score, it was estimated that 74% of lumbar disease and 73% of cervical disease was attributable to heritability. In scores for the extent of disease or presence of severe disease, heritability estimates ranged from 63% to 79%. These findings confirm the importance of genetic susceptibility to disk degeneration.41
Twin studies have produced conflicting results regarding the role of cigarette smoking as a risk factor for disk degeneration. In a twin cohort with high mean discordance (32 pack-years), smoking accounted for 2% of degeneration.37,42 In a study with a lesser degree of discordance (14 pack-years), no contribution was detected.40 Nonetheless, we acknowledge in our own practices that the number of people with symptomatic degenerative disk disease seems to be disproportionately higher in smokers than nonsmokers. Occupational driving does not appear to contribute significantly to disk degeneration.43 Follow-up studies on the Finnish twins have demonstrated slow progression of degenerative changes in the majority of twins irrespective of environmental influences at 5-year follow-up.44,45
Genetics
Recognition of the dominant role played by genetic factors in the pathogenesis of degenerative disk disease has supported the search for candidate genes (Table 270-3).46–64 The predominant methodology used has been a candidate gene association approach, in which genes whose products are purported to play a role in disk degeneration are studied in a defined population. The probable polygenic nature of disk degeneration has made linkage analysis studies, in which candidate regions of the genome are identified through their segregation in families with the disease of interest, more difficult to use.
TABLE 270-3 Genes with Potential Association with Magnetic Resonance Imaging–Demonstrated Disk Degeneration in Humans*
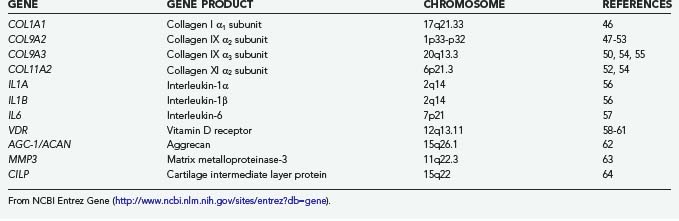
Among the most extensively studied genes is the vitamin D receptor gene (VDR on chromosome 12q13.11). Four studies in three different ethnic populations have established a relationship between two VDR restriction fragment length polymorphisms (Taq I and Fok I) and disk degeneration on MRI.58–61 In two studies, the association was strongest in younger patients, thus further suggesting a role in accelerated degeneration. The mode of predisposition is unclear because only the Fok I polymorphism results in a functionally altered receptor.65 Vitamin D has been reported to perform a role in proteoglycan synthesis,60 and receptor polymorphisms may affect the quality of the extracellular matrix of the disk. Alternatively, the close proximity of the collagen type II α1 subunit gene (COL2A1) and the IGF gene may be significant.
Also extensively studied are the collagen type IX genes for the α2 subunit (COL9A2 on chromosome 1p33-p33) and α3 subunit (COL9A3 on chromosome 20q13.3). The Trp 2 polymorphism of COL9A2 is particularly prevalent in Asian populations and has been associated with increased disk degeneration in Japanese and Chinese studies.47–49 A lower incidence in white populations has led to mixed associations in European studies. The Trp 3 polymorphism of COL9A3 has been associated with disk degeneration in Finnish populations.50,54,55 Disturbances in the structure of the disk secondary to altered collagen IX function is a plausible mechanism of disk failure.
Polymorphism of the aggrecan gene (AGC1 on chromosome 15q26.1) in the form of a variable number of tandem repeats potentially altering the length of the molecule has been associated with the degree of disk degeneration in one small study.62 Other genes with established associations but less well studied are listed in Table 270-3 and include those for other collagen subtypes, extracellular matrix components, cytokine signaling molecules, and cellular enzymes.
Disk Regeneration
Regeneration is characterized by the production of new, functionally active tissue with normal cytoarchitecture. Tissue engineering efforts to renew degenerated disk tissue also involve broader strategies of repair and replacement. Components of any given strategy may involve each of three elements of tissue engineering—cells, signals, and scaffolds.66 Research efforts toward regeneration and repair remain predominantly laboratory-based and focused on renewal of the nucleus pulposus.
Cells
Three groups of cells have been proposed as candidates for tissue-engineered disk renewal—differentiated nucleus pulposus cells, mesenchymal stem cells,67,68 and notochordal cells.69 In contrast to native disk cells, mesenchymal stem cells can be obtained from a variety of sources and may be easier than differentiated cells to manipulate toward a desired phenotype. Notochordal cells, by virtue of their role in regulating early disk development, may be useful in regeneration but are conceivably difficult to obtain.
Signals
Signaling molecules may be used either alone via direct implantation, impregnated in a scaffold for slow release, or with gene therapy to alter the expression of molecules by local cells.68,70 The candidate molecules can be categorized into anticatabolics (e.g., tissue inhibitor of MMP [TIMP-1]), mitogens (e.g., IGF, epidermal growth factor, FGF, PDGF, nerve growth factor), chondrogenic morphogens (e.g., BMPs, TGF-β, growth differentiation factors), and intracellular regulators (e.g., the LIM mineralization protein SOX-9).
Scaffolds
In early degeneration, molecular therapy to stimulate existing cells to alter the imbalance in matrix synthesis and degradation may suffice. As the degeneration progresses, it may become necessary to implant engineered tissue or a cell-seeded scaffold. Because of the perilous state of disk nutrition, a hostile microenvironment, incompetence of the annulus fibrosus, and disk space collapse, advanced degeneration may require the implantation of a hybrid disk prosthesis in which disk replacement technology is combined with developments in tissue engineering (Fig. 270-7).
A tissue engineering technique consisting of autologous disk-derived chondrocyte transplantation (ADCT) has been used in the clinical setting.71,72 The technique, used in patients undergoing discectomy, involves harvest of disk-derived chondrocytes at the time of surgery, in vitro culture, and percutaneous reimplantation at 12 weeks. After reported success in canine studies and a human pilot study, a randomized, nonblinded, non–placebo-controlled trial (EuroDISC) is currently under way. Although interim analysis of patient-reported outcomes in a nonblinded study must be interpreted with extreme caution, the group treated by discectomy and ADCT has demonstrated lower Oswestry Low Back Pain Questionnaire scores than the group undergoing discectomy alone. MRI studies suggest greater disk height and liquid content in the group treated by discectomy and ADCT. However, caution is still prudent. Among the obvious limitations of a nonrandomized, nonblinded, non–placebo-controlled study, other limitations exist, including unpublished methodology. Nonetheless, the final results of this and further trials are eagerly anticipated.
Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3.
Benneker LM, Heini PF, Anderson SE, et al. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27.
Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631.
Grunhagen T, Wilde G, Soukane DM, et al. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(suppl 2):30.
Haefeli M, Kalberer F, Saegesser D, et al. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31:1522.
Johannessen W, Auerbach JD, Wheaton AJ, et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253.
Kalichman L, Hunter DJ. The genetics of intervertebral disc degeneration. Associated genes. Joint Bone Spine. 2008;75:388-396.
Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652.
Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193.
O’Halloran DM, Pandit AS. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13:1927.
Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873.
Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691.
Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411.
1 Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3.
2 Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151.
3 Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16:1539.
4 Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656.
5 Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8.
6 Adams MA. The Biomechanics of Back Pain. New York: Churchill Livingstone; 2002.
7 Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(suppl 2):10.
8 Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286.
9 Bogduk N. Clinical Anatomy of the Lumbar Spine and Sacrum, 4th ed. New York: Churchill Livingstone; 2005.
10 Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39.
11 Grunhagen T, Wilde G, Soukane DM, et al. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(suppl 2):30.
12 Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691.
13 Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19:287.
14 Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29.
15 Eyre DR, Matsui Y, Wu JJ. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans. 2002;30:844.
16 Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411.
17 Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631.
18 Haefeli M, Kalberer F, Saegesser D, et al. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31:1522.
19 Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996.
20 Roughley P, Martens D, Rantakokko J, et al. The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater. 2006;11:1.
21 Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652.
22 Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700.
23 Lane NE, Nevitt MC, Genant HK, et al. Reliability of new indices of radiographic osteoarthritis of the hand and hip and lumbar disc degeneration. J Rheumatol. 1993;20:1911.
24 Wilke HJ, Rohlmann F, Neidlinger-Wilke C, et al. Validity and interobserver agreement of a new radiographic grading system for intervertebral disc degeneration: Part I. Lumbar spine. Eur Spine J. 2006;15:720.
25 Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873.
26 Panagiotacopulos ND, Pope MH, Krag MH, et al. Water content in human intervertebral discs. Part I. Measurement by magnetic resonance imaging. Spine. 1987;12:912.
27 Benneker LM, Heini PF, Anderson SE, et al. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27.
28 Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193.
29 Modic MT, Masaryk TJ, Ross JS, et al. Imaging of degenerative disk disease. Radiology. 1988;168:177.
30 Rajasekaran S, Babu JN, Arun R, et al. ISSLS prize winner: A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29:2654.
31 Keshari KR, Lotz JC, Kurhanewicz J, et al. Correlation of HR-MAS spectroscopy derived metabolite concentrations with collagen and proteoglycan levels and Thompson grade in the degenerative disc. Spine. 2005;30:2683.
32 Keshari KR, Zektzer AS, Swanson MG, et al. Characterization of intervertebral disc degeneration by high-resolution magic angle spinning (HR-MAS) spectroscopy. Magn Reson Med. 2005;53:519.
33 Johannessen W, Auerbach JD, Wheaton AJ, et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253.
34 Nguyen AM, Johannessen W, Yoder JH, et al. Noninvasive quantification of human nucleus pulposus pressure with use of T1rho-weighted magnetic resonance imaging. J Bone Joint Surg Am. 2008;90:796.
35 Blumenkrantz G, Li X, Han ET, et al. A feasibility study of in vivo T1rho imaging of the intervertebral disc. Magn Reson Imaging. 2006;24:1001.
36 Auerbach JD, Johannessen W, Borthakur A, et al. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15(suppl 3):S338.
37 Battie MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine. 2004;29:2679.
38 Rengachary SS, Balabhadra RS. Black disc disease: a commentary. Neurosurg Focus. 2002;13(2):E14.
39 Battie MC, Haynor DR, Fisher LD, et al. Similarities in degenerative findings on magnetic resonance images of the lumbar spines of identical twins. J Bone Joint Surg Am. 1995;77:1662.
40 Battie MC, Videman T, Gibbons LE, et al. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601.
41 Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366.
42 Battie MC, Videman T, Gill K, et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine. 1991;16:1015.
43 Battie MC, Videman T, Gibbons LE, et al. Occupational driving and lumbar disc degeneration: a case-control study. Lancet. 2002;360:1369.
44 Videman T, Battie MC, Parent E, et al. Progression and determinants of quantitative magnetic resonance imaging measures of lumbar disc degeneration: a five-year follow-up of adult male monozygotic twins. Spine. 2008;33:1484.
45 Videman T, Battie MC, Ripatti S, et al. Determinants of the progression in lumbar degeneration: a 5-year follow-up study of adult male monozygotic twins. Spine. 2006;31:671.
46 Tilkeridis C, Bei T, Garantziotis S, et al. Association of a COL1A1 polymorphism with lumbar disc disease in young military recruits. J Med Genet. 2005;42:e44.
47 Jim JJ, Noponen-Hietala N, Cheung KM, et al. The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine. 2005;30:2735.
48 Seki S, Kawaguchi Y, Mori M, et al. Association study of COL9A2 with lumbar disc disease in the Japanese population. J Hum Genet. 2006;51:1063.
49 Higashino K, Matsui Y, Yagi S, et al. The alpha2 type IX collagen tryptophan polymorphism is associated with the severity of disc degeneration in younger patients with herniated nucleus pulposus of the lumbar spine. Int Orthop. 2007;31:107.
50 Paassilta P, Lohiniva J, Goring HH, et al. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA. 2001;285:1843.
51 Karppinen J, Paakko E, Raina S, et al. Magnetic resonance imaging findings in relation to the COL9A2 tryptophan allele among patients with sciatica. Spine. 2002;27:78.
52 Noponen-Hietala N, Kyllonen E, Mannikko M, et al. Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis. 2003;62:1208.
53 Annunen S, Paassilta P, Lohiniva J, et al. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285:409.
54 Solovieva S, Lohiniva J, Leino-Arjas P, et al. Intervertebral disc degeneration in relation to the COL9A3 and the IL-1ss gene polymorphisms. Eur Spine J. 2006;15:613.
55 Solovieva S, Lohiniva J, Leino-Arjas P, et al. COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene-environment interaction. Spine. 2002;27:2691.
56 Solovieva S, Kouhia S, Leino-Arjas P, et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15:626.
57 Noponen-Hietala N, Virtanen I, Karttunen R, et al. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186.
58 Videman T, Gibbons LE, Battie MC, et al. The relative roles of intragenic polymorphisms of the vitamin D receptor gene in lumbar spine degeneration and bone density. Spine. 2001;26:E7.
59 Videman T, Leppavuori J, Kaprio J, et al. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine. 1998;23:2477.
60 Kawaguchi Y, Kanamori M, Ishihara H, et al. The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J Bone Joint Surg Am. 2002;84:2022.
61 Cheung KM, Chan D, Karppinen J, et al. Association of the Taq I allele in vitamin D receptor with degenerative disc disease and disc bulge in a Chinese population. Spine. 2006;31:1143.
62 Kawaguchi Y, Osada R, Kanamori M, et al. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24:2456.
63 Takahashi M, Haro H, Wakabayashi Y, et al. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83:491.
64 Seki S, Kawaguchi Y, Chiba K, et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet. 2005;37:607.
65 Uitterlinden AG, Fang Y, Van Meurs JB, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143.
66 O’Halloran DM, Pandit AS. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13:1927.
67 Jandial R, Aryan HE, Park J, et al. Stem cell–mediated regeneration of the intervertebral disc: cellular and molecular challenge. Neurosurg Focus. 2008;24(3k-4):E21.
68 Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J. 2007;16:447.
69 Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667.
70 Yoon ST, Patel NM. Molecular therapy of the intervertebral disc. Eur Spine J. 2006;15(suppl 3):S379.
71 Meisel HJ, Ganey T, Hutton WC, et al. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15(suppl 3):S397.
72 Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation: a treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5.