Components of the Immune System
I Types and Goals of Host Defense Mechanisms
A Nonspecific (innate) immunity
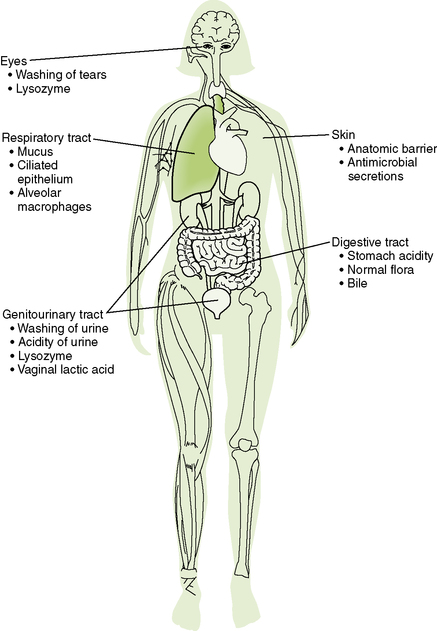
1. Anatomic and physiologic barriers exclude many microbes (Fig. 1-1).
2. Inflammation and the resulting increase in vascular permeability permit leakage of antibacterial serum proteins (acute phase proteins) and phagocytic cells into damaged or infected sites.
3. Phagocytosis, initially by neutrophils and later by macrophages, destroys whole microorganisms, especially bacteria.
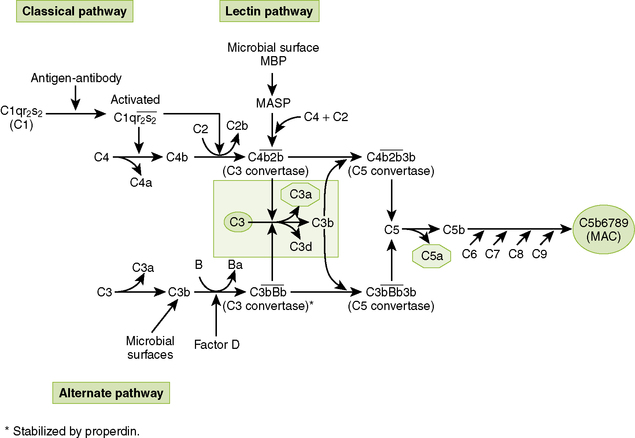
4. Complement system can be activated by microbial surfaces (alternate and lectin pathways) and by immune complexes (classical pathway).
B Specific (acquired) immunity
• Results from random recombination of immunoglobulin and T cell receptor genes within lymphocytes and selection by antigen-dependent activation, proliferation (clonal expansion), and differentiation of these cells to resolve or control infections.
1. Thymus is the site for maturation of T cells
2. Bone marrow and fetal liver are the sites for maturation of B cells
1. Lymph node (see Chapter 4, Fig. 4-1)
• Site where immune response is initiated
• Swollen lymph node denotes stimulation of immunity and cell growth.
• Dendritic cells and antigen from the periphery enter through the afferent lymphatic vessel into the medulla where the T cells reside.
• B cells wait in follicles for T cell activation, and antigenically stimulated B cells are in the germinal centers within the follicles.
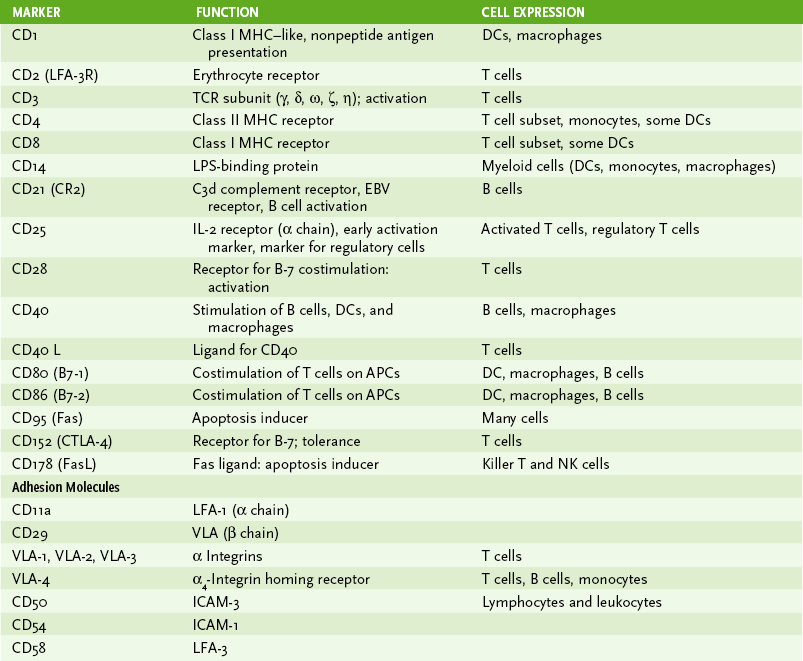
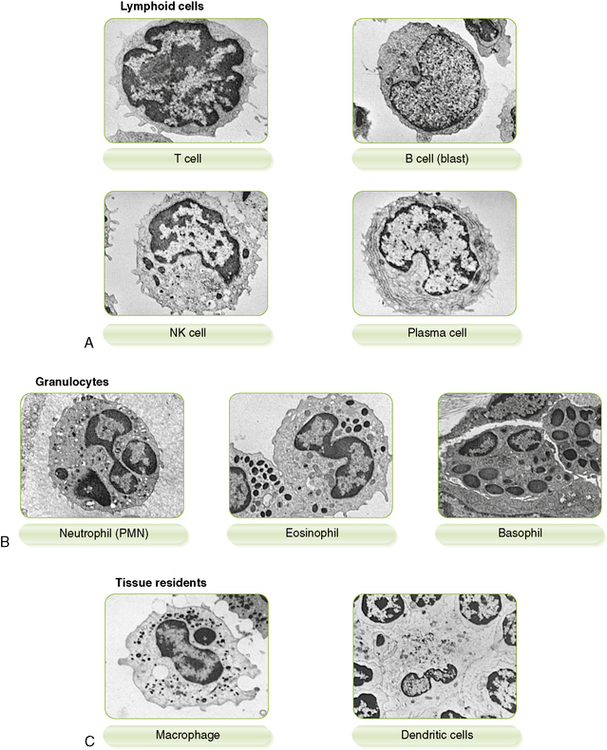
1. Immune cells can be distinguished by morphology, cell surface markers, and/or function (Box 1-1, Fig. 1-2, and Tables 1-1 and 1-2).
TABLE 1-1
Major Cells of the Immune System
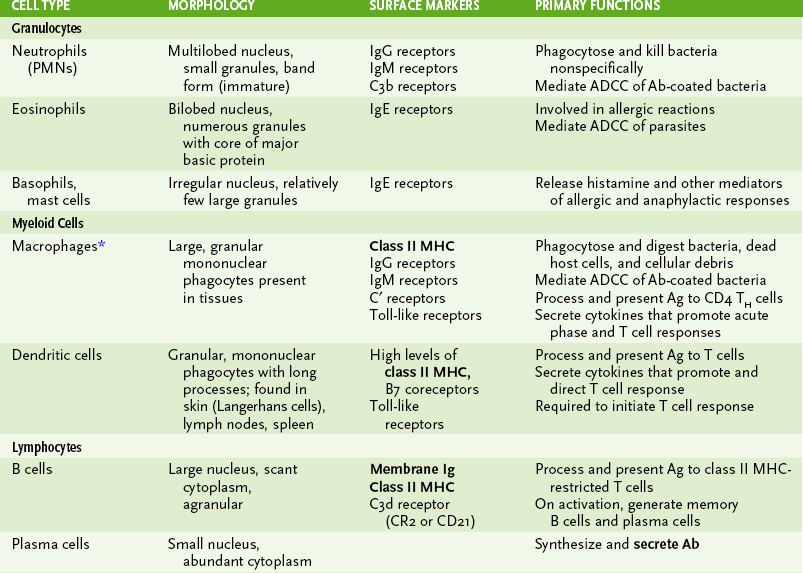
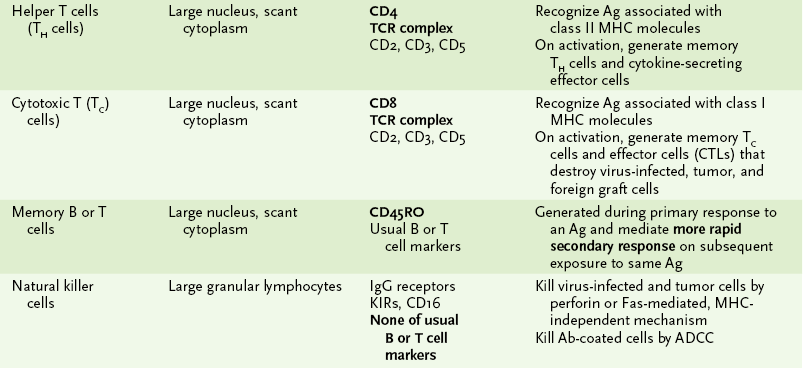
*Activation of macrophages, by interferon-γ or other cytokines, enhances all their activities and leads to secretion of cytotoxic substances with antiviral, antitumor, and antibacterial effects.
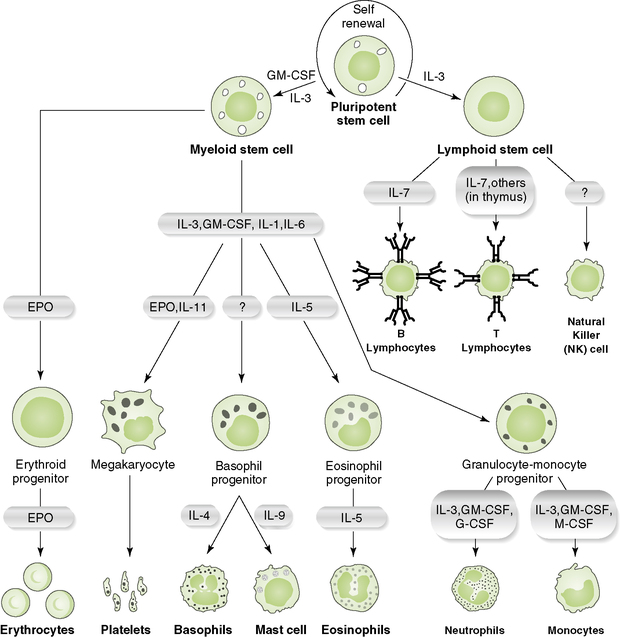
2. Development of the various cell lineages from stem cells in the bone marrow requires specific hematopoietic growth factors, cytokines, and/or cell-cell interactions (Fig. 1-3).
B Antigen-recognizing lymphoid cells
1. B lymphocytes express surface antibodies that recognize antigen.
2. T lymphocytes express T cell receptors (TCRs) that recognize antigenic peptides only when displayed on a major histocompatibility complex (MHC) molecule (Box 1-2).
3. Memory cells are generated during clonal expansion of antigen-stimulated lymphocytes.
• Monocytes are released from the bone marrow, circulate in the blood, and enter tissues where they mature into dendritic cells or macrophages.
• Have long arm-like processes
• Required to initiate an immune response and very efficient at presenting antigen to both CD4 TH and CD8 TC cells
• Secrete cytokines that direct the nature of the T cell response (e.g., IL-12 for TH1)
• Help to initiate early innate immune response (Table 1-3)
TABLE 1-3
Macrophages Versus Neutrophils
| Property | Neutrophils | Macrophages |
| First to arrive at local site of infection or tissue damage | Arrive later | |
| Phagocytic activity | Yes | Yes |
| Bacterial destruction | Very effective | Less effective unless activated |
| Oxidative burst | Yes | Only when activated |
| Antigen presentation on class II MHC molecules | No | Yes |
| Cytokine secretion | No | Yes (IL-1, IL-6, IL-12, TNF-α, etc) |
| Antibody-dependent cell-mediated cytotoxicity | Yes | Yes |
| Life span | Short | Long |
IL, interleukin; MHC, major histocompatibility complex; TNF-α, tumor necrosis factor-α.
• Secrete numerous cytokines that promote immune responses (Box 1-3)
• Secrete antibacterial substances, inflammatory mediators, and complement
• Phagocytose and inactivate microbes (see later in this chapter)
• Present antigen associated with class II MHC molecules to CD4 TH cells
3. Activated (“angry”) macrophages: larger and exhibit enhanced antibacterial, inflammatory, and antigen-presenting activity
• Specificity of NK cells for virus-infected and tumor cells may depend on reduced expression of class I MHC molecules and alterations in surface carbohydrates on these target cells.
2. Mechanism of NK cell killing
• Direct cytotoxicity involving contact with target cell and lysis by perforin-mediated mechanism similar to that used by TC cells
a. Perforin-mediated lysis by NK cells is antigen independent and not MHC restricted, whereas TC cells only attack cells bearing specific antigenic peptides bound to a class I MHC molecule.
• Fas (on target cell) and Fas ligand (on NK or T cell) killing of target cell through tumor necrosis factor receptor–like apoptosis pathway
1. The complement system consists of numerous serum and cell surface proteins that form an enzymatic cascade.
2. Cleavage of inactive components converts them into proteases that cleave and activate the next component in the cascade.
B Complement pathways (Fig. 1-4)
• The three complement pathways differ initially, but all form C3 and C5 convertases and ultimately generate a common membrane attack complex (MAC).
1. Alternate pathway (properdin system) most commonly is activated by microbial surfaces and cell surface components (e.g., lipopolysaccharide and teichoic acid).
2. Lectin pathway interacts with mannose on bacterial, viral, and fungal surfaces.
3. Classical pathway is activated primarily by antigen-antibody complexes containing IgM or IgG.
C Biologic activities of complement products
1. MAC acts as a molecular drill to puncture cell membranes.
• Formation of MAC begins with cleavage of C5 by C5 convertases formed in all pathways (see Fig. 1-4).
• Sequential addition of C6, C7, and C8 to C5b yields C5b678, a complex that inserts stably into cell membranes but has limited cytotoxic ability.
• Binding of multiple C9 molecules produces a highly cytotoxic MAC (C5b6789n) that forms holes in the cell membrane, killing the cell.
2. Complement cleavage products promote inflammatory responses, opsonization, and other effects summarized in Table 1-4.
TABLE 1-4
Major Biologic Activities of Complement Cleavage Products
| Activity | Mediators | Effect |
| Opsonization of antigen | C3b and C4b | Increased phagocytosis by macrophages and neutrophils |
| Chemotaxis | C3a and C5a | Attraction of neutrophils and monocytes to inflammatory site |
| Degranulation | C3a and C5a (anaphylotoxins) | Release of inflammatory mediators from mast cells and basophils |
| Clearance of immune complexes | C3b | Reduced buildup of potentially harmful antigen-antibody complexes |
| B cell activation | C3d | Promotion of humoral immune response |
• Various regulatory proteins, which bacteria do not produce, protect host cells from complement activity.
1. C1 esterase inhibitor prevents inappropriate activation of the classical pathway.
2. Inactivators of C3 and C5 convertases include decay-accelerating factor (DAF), factor H, and factor I.
3. Anaphylotoxin inhibitor blocks anaphylactic activity of C3a and C5a.
E Consequences of complement abnormalities
1. C1, C2, or C4 deficiency (classical pathway); examples include:
• Immune complex diseases such as glomerulonephritis, systemic lupus erythematosus (SLE), and vasculitis
2. C3, factor B, or factor D deficiency (alternate pathway); examples include:
3. C5 through C9 deficiency; examples include:
V Phagocytic Clearance of Infectious Agents
1. Attachment of phagocytic cells to microbes, dead cells, and large particles is enhanced by opsonins (Fig. 1-5A).
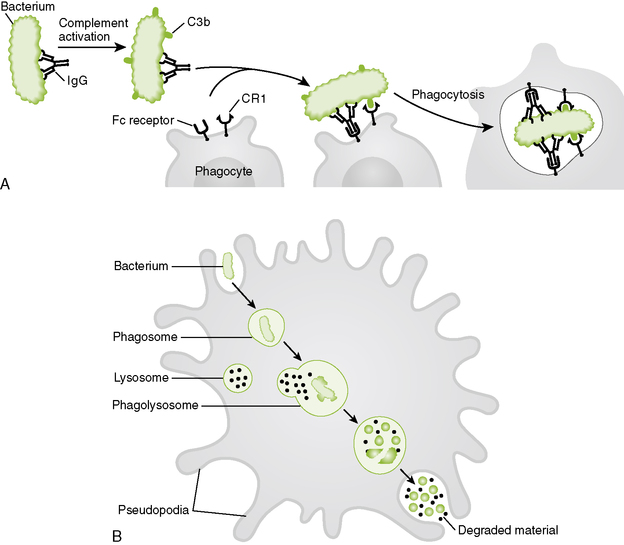
1-5 Phagocytic destruction of bacteria. A, Bacteria are opsonized by immunoglobulin M (IgM), IgG, C3b, and C4b, promoting their adherence and uptake by phagocytes. B, Hydrolytic enzymes, bactericides, and various reactive toxic compounds kill and degrade internalized bacteria (see Box 1-4). Some of these agents are also released from the cell surface in response to bacterial adherence and kill nearby bacteria.
• C3b and C4b coated bacteria bind to CR1 receptors on phagocytes.
• IgM and IgG bound to surface antigens on microbes interact with Fc receptors on phagocytes.
2. Internalization and formation of phagolysosome promote destruction of bacteria (Fig. 1-5B).
3. Destructive agents kill internalized bacteria and also are released to kill bacteria in the vicinity of the phagocyte surface (Box 1-4).
B Genetic defects in phagocytic activity
• Defects in phagocyte killing and digestion of pathogens increase the risk for bacterial and yeast infection (Table 1-5).
TABLE 1-5
Inherited Phagocytic Disorders
| Disease | Defect | Clinical features |
| Chédiak-Higashi syndrome | Reduced ability of phagocytes to store materials in lysosomes and/or release their contents | Recurrent pyogenic infections (e.g., Staphylococcus and Streptococcus species) |
| Chronic granulomatous disease | Reduced production of H2O2 and superoxide anion due to lack of NADPH oxidase (especially in neutrophils) | Increased susceptibility to catalase-producing bacteria (e.g., Staphylococcus species) and fungal infections |
| Job syndrome | Reduced chemotactic response by neutrophils and high immunoglobulin E levels | Recurrent cold staphylococcal abscesses; eczema; often associated with red hair and fair skin |
| Lazy leukocyte syndrome | Severe impairment of neutrophil chemotaxis and migration | Recurrent low-grade infections |
| Leukocyte adhesion deficiency | Defect in adhesion proteins reducing leukocyte migration into tissues and adherence to target cells | Recurrent bacterial and fungal infections; poor wound healing; delayed separation of umbilical cord |
| Myeloperoxidase deficiency | Decreased production of HOCl and other reactive intermediates | Delayed killing of staphylococci and Candida albicans |
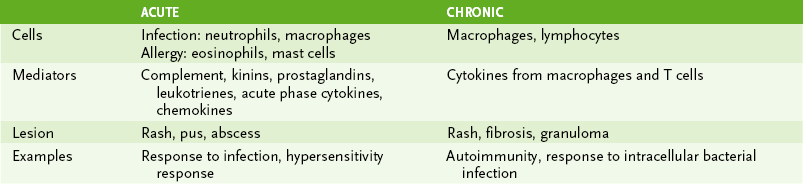
VI Inflammation: Induced by tissue damage due to trauma, injurious agents, or invasion of microbes; Mediated primarily by innate and immune cells, cytokines, and other small molecules (Table 1-6).
A Acute inflammation occurs in response to bacteria and physical injury.
1. Localized response is characterized by increased blood flow, vessel permeability, and phagocyte influx (redness, swelling, and warmth).
• Anaphylotoxins C3a and C5a stimulate mast cells to release histamine and serotonin (↑ vascular permeability) and prostaglandins (↑ vasodilation).
• Endothelial damage activates plasma enzymes, leading to production of bradykinin, a potent vasoactive mediator, and formation of fibrin clot, which helps prevent the spread of infection.
• Initially neutrophils and later macrophages migrate into the affected tissue and are chemotactically attracted to invading bacteria.
2. Systemic acute phase response accompanies localized response (see Box 1-3).