Chapter 7 Complications Related to Radiofrequency Procedures for the Treatment of Chronic Pain
 RFA can allow for reliable and discrete thermal lesioning in a desired specific neurologic location with prolonged pain relief.
RFA can allow for reliable and discrete thermal lesioning in a desired specific neurologic location with prolonged pain relief. Severe complications are rare but can be as catastrophic as heart block and permanent spinal cord or brain injury.
Severe complications are rare but can be as catastrophic as heart block and permanent spinal cord or brain injury. Adequate training and board certification of the interventional physician are ideal and could theoretically lead to greater safety measures and less chance of complications when RFA is used.
Adequate training and board certification of the interventional physician are ideal and could theoretically lead to greater safety measures and less chance of complications when RFA is used. The ability to implement lifesaving measures such as intubation, advanced cardiac life support, and management of local anesthetic toxicity as well as an extensive knowledge of the anatomy and proficiency at reading fluoroscopic images are mandatory.
The ability to implement lifesaving measures such as intubation, advanced cardiac life support, and management of local anesthetic toxicity as well as an extensive knowledge of the anatomy and proficiency at reading fluoroscopic images are mandatory. Consent; sterile technique; and a review of the patient’s allergies, use of anticoagulants, and medical history (especially infection risks and indwelling pacemakers, defibrillators, or spinal cord or peripheral nerve stimulators) are essential.
Consent; sterile technique; and a review of the patient’s allergies, use of anticoagulants, and medical history (especially infection risks and indwelling pacemakers, defibrillators, or spinal cord or peripheral nerve stimulators) are essential. General anesthesia should be avoided; light sedation via the guidelines set forth by the American Society of Anesthesiologists for conscious sedation and monitoring can be used by properly trained personnel, but it is recommended that the patient be able to maintain meaningful communication throughout the entire procedure.
General anesthesia should be avoided; light sedation via the guidelines set forth by the American Society of Anesthesiologists for conscious sedation and monitoring can be used by properly trained personnel, but it is recommended that the patient be able to maintain meaningful communication throughout the entire procedure. Use of negative aspiration; myelogram-compatible contrast under live fluoroscopy; and when indicated, a test dose with local anesthetic and dilute epinephrine can mitigate risks.
Use of negative aspiration; myelogram-compatible contrast under live fluoroscopy; and when indicated, a test dose with local anesthetic and dilute epinephrine can mitigate risks. Evaluation of specific motor stimulation and, when applicable, sensory stimulation with their respective relevant responses should always be used.
Evaluation of specific motor stimulation and, when applicable, sensory stimulation with their respective relevant responses should always be used. Severe complications associated with RFA of the cervical, thoracic, and lumbar medical branches include but are not limited to pneumothorax and permanent nerve or spinal cord injury.
Severe complications associated with RFA of the cervical, thoracic, and lumbar medical branches include but are not limited to pneumothorax and permanent nerve or spinal cord injury. Severe complications associated with RFA of the sympathetic ganglion and nerves include but are not limited to heart block, total spinal blockade, pneumothorax, meningitis, major vessel injury (vertebral artery, subclavian vessels, vena cava, or aorta), blindness, spinal cord injury, and brain injury.
Severe complications associated with RFA of the sympathetic ganglion and nerves include but are not limited to heart block, total spinal blockade, pneumothorax, meningitis, major vessel injury (vertebral artery, subclavian vessels, vena cava, or aorta), blindness, spinal cord injury, and brain injury.Introduction
Radiofrequency (RF) has been used clinically as a modality to treat chronic pain as early as the 1950s.1,2 It is unique in that it can allow for reliable and discrete thermal lesioning in a desired specific neurologic location. The end result can produce pain relief from a prolonged duration up to 6 months or longer. RF is a low-energy, high-frequency, alternating current of which an electrical field is created between the active electrode and the grounding pad. The term radio was implemented because the frequency field that is used for treatment is often within the same frequency range of AM radio (300 to 500 kHz).3 When the RF probe and dispersive grounding pad are properly placed, the body tissue completes the circuit. The uninsulated needle tip is the active portion of the electrode. The probe itself does not generate heat; rather, the frictional dissipation of current within the surrounding tissue heats the electrode.4
Radiofrequency can either be continuous at a temperature usually greater than 45°C or intermittently dispersed at 42°C; the latter is referred to as pulsed RF. Continuous RF ablation (RFA) is considered to be neurodestructive. Pulsed RF therapy is not fully understood but is thought to be a type of neuromodulation.5 The active tip of the RF probe is beneficial as it also can be used for monitoring electrical impedance, sensory and motor stimulation, and real-time temperature measurement and control. The RF technique virtually eliminates undesired results such as combustion, sticking and breaking of the probe, and charring of the adjacent tissue. Other neuroablative techniques such as chemical destruction and cryotherapy do not have this luxury and are less predictable with the lesioning size. Even the use of a laser for neurodestruction has apparent limitations in creating an effective lesion compared with RF therapy.6 Although conventional RFA for the treatment of chronic pain has proven to be an effective minimally invasive treatment option, it is not without its inherent risks and complications.
Closed Claims Cases in Pain Management and Ablative Procedures
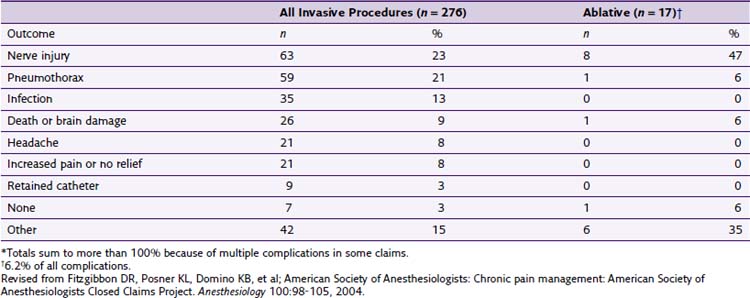
The American Society of Anesthesiologists (ASA) Closed Claims Project in 2004 by Fitzgibbon et al7 reported an increase in adverse chronic pain outcomes from 1979 to 1999. During the 1980s, there was a 3% increase in chronic pain claims. It increased to 10% in the 1990s. Fortunately, most of the chronic pain management claims were temporary or nondisabling injuries. Of the 276 claims related to chronic pain invasive procedures, 17 were associated with ablative procedures (chemical or thermal), making up 6.2% of the overall claims. Interestingly, only four cases were related to temperature (thermal RF or cryoablation) complications (Table 7-1).
Table 7-1 Procedures in Chronic Pain Management Claims, 1970-1999 (n = 276)
| Claims | Number | Percentage |
|---|---|---|
| Invasive procedures | 276 | 100 |
| Injections | 138 | 50 |
| Epidural steroids ± local anesthetic agents | 114 | |
| Trigger point | 17 | |
| Facet | 4 | |
| Other | 3 | |
| Blocks | 78 | 28.3 |
| Peripheral | 28 | |
| Stellate ganglion | 19 | |
| Other autonomic | 9 | |
| Neuraxial | 9 | |
| Upper or lower extremity | 7 | |
| Axial | 4 | |
| Head and neck | 2 | |
| Ablative Procedures | 17* | |
| Chemical (phenol, alcohol) | 13 | |
| Temperature (RF, cryoablation) | 4 | |
| Implantation or Removal of Devices | 12 | 4.3 |
| Implantable pump | 5 | |
| Nerve stimulator | 4 | |
| Catheter | 3 | |
| Device Maintenance | 20 | 7.2 |
| Other Interventions | 11 | 4 |
RF, radiofrequency.
Revised from Fitzgibbon DR, Posner KL, Domino KB, et al; American Society of Anesthesiologists: Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology 100:98-105, 2004.
One of the largest confounding variables of the Closed Claims Project is that it primarily selects for cases that are of a greater severity and injury. Pursuit of cases that will likely yield less than $50,000 is not typically characteristic of plaintiff attorneys.8 Therefore the amount of overall untoward events and complications are more than likely greater because they are not recorded from a legal standpoint or simply not reported by the pain practitioner. Thirteen of the 17 closed claims were related to chemical ablation. It is clear that chemical ablation seems to carry a higher risk of severe complications. Unfortunately, the ASA project did not further delineate between which specific complications were related to chemical versus temperature-related neurolysis (Table 7-2).
Avoidance of Radiofrequency Complications
Patient Selection Factors
Absolute contraindications include a lack of patient consent, infection at the site of RF probe placement, systemic infection, anticoagulants,* pacemaker or implantable cardioverter defibrillator (ICD),* and nonsystemic infection distant from the RF site.*
Relative contraindications include active comorbid disease, pregnancy,* poor hygiene, allergy to pertinent RF-related medication (local anesthetic, iodine, latex), anticoagulants,* pacemaker or ICD,* preexisting dorsal column or peripheral nerve stimulator,* and nonsystemic infection distant from the RF site.*
Sedation
Conscience sedation or monitored anesthetic care during chronic pain procedures is a topic of debate among experts in the field of pain medicine. Some believe that sedation should be avoided in procedures that have an inherently higher risk of catastrophic outcome if injury to vascular or neurologic structures were to occur (e.g., cervical epidural steroid injection, stellate ganglion [SG] block). It is thought that local anesthetic only would allow for the patient to communicate marked pain related to ensuing injury to vital structures.9 The opposing view is that if a patient has light sedation via the guidelines set forth by the ASA for conscious sedation and monitoring as well as implemented by the hands of an experienced anesthesia provider, he or she may have a more comfortable experience and is thus less likely to make sudden movements. Sudden patient movement during the procedure could significantly affect the outcome of the procedure and potentially increase the chance of an untoward event. The anesthetic goal for advocates of light sedation is to have a minimally anxious patient who is able to maintain meaningful communication throughout the entire procedure and stable vital signs. In either case, it is at the discretion of the pain and anesthesia providers to decide which patients are good candidates. Light sedation with agents that can be reversed are recommended (benzodiazepines: midazolam; short-acting opioids: fentanyl). A propofol anesthetic would be less than ideal because of the chance of (1) the patient becoming disinhibited during the procedure10 and (2) unintentional general anesthesia could prevent the patient from alerting the physician to complications. In the United States, general anesthesia for common percutaneous chronic pain procedures is thought to be excessive and risky by most pain specialists.
Infection
Although there have been no literature reports of infection complications with RF procedures, pain practitioners should minimize this risk by postponing RF treatment with the presence of active infection or concurrent antibiotic or antimicrobial use. Consultation with an infectious disease specialist should be considered if the patient has a history of osteomyelitis or methicillin-resistant Staphylococcus aureus. Studies have shown that smoking cessation promotes better healing in surgical patients.11 Although no studies have linked the abatement of smoking with patients undergoing percutaneous RFA, the practitioner may want to take this into consideration if the patient is at high risk for infection (e.g., history of infection, abscess). Providers may also want to instruct patients with poor hygiene to bathe with over-the-counter Hibiclens (4% chlorhexidine) the night before the procedure. This may decrease the chance of infection by the seeding of human skin flora via percutaneous technique.12
Cheng and Abdi13 describe infection complications from several different case reports with patients undergoing intraarticular zygapophyseal (facet) joint injections. These complications include epidural abscess, septic arthritis, spondylodiscitis, and chemical meningism (thought to be related to inadvertent dural puncture with a high-dose steroid injection). These authors also report no known case reports of infections related directly to RF neurolysis; however, one could reasonably conclude that the above complications could occur with RF if confirmation of proper needle placement and sterile precautions are not used.13
Presence of a Pacemaker or an Implantable Cardioverter-Defibrillator Device
Radiofrequency in patients with a cardiac pacemaker or ICD is a controversial topic. Some authors believe that it is adequate to turn off the pacemaker before proceeding with RF lesioning.14 However, the routine practice of placing of a magnet to set the pacer to an asynchronous mode should be reconsidered because of the ever-changing complexity of cardiac pacemakers. A comprehensive pre- and postprocedure evaluation should be performed by the device representative as well as intraoperative monitoring during the RF procedure itself.15 This practice is supported by the 2002 American College of Cardiology and American Heart Association (ACC/AHA) guideline update for perioperative cardiovascular evaluation for noncardiac surgery.16 Consulting the patient’s cardiologist is advised, but one should be aware that there has been inaccurate information about pacer function given by cardiologists;17 therefore the presence of the cardiac device representative is still advised. RFA procedures should never be done while the defibrillator function is still active, regardless of the location of the RF lesioning in the body. There are known medicolegal cases involving patients being defibrillated after RF treatment was started. There have been anecdotal reports of RF lesioning being done safely for pain patients with indwelling defibrillators, but this was done after the defibrillator function was deactivated and the ACC/AHA guidelines used; the formal literature is lacking. In the rare case that the pain practitioner decides to move forward with an RF procedure, it is advised to use extreme caution with meticulous attention to the cardiologist’s direction and complete compliance with the ACC/AHA guidelines.
Anticoagulation
The American Society of Regional Anesthesia Consensus Guidelines for the anticoagulated patient by Horlocker et al18 have become the gold standard when performing neuraxial procedures in anticoagulated patients. Interventionalists should have a thorough understanding of these guidelines, anticoagulant medications, and the nuances of pathology associated with a deviation from the normal clotting cascade. Adherence to these guidelines is not a fail-safe measure, and the pain specialist should always exercise vigilance when there is the possibility of an epidural hematoma.
A traumatic procedure seems to be the biggest risk factor in epidural hematoma formation.19 The incidence of a hematoma when entering the epidural space is estimated to be one in 20,000 (traumatic) to one in 220,000 (nontraumatic).18,20 Because of the nature of the common RF spine procedures, inadvertent needle placement in the epidural space is certainly possible. Because the epidural space is not accessed during most RF procedures, it is thought that the incidence of epidural hematoma would be much lower than with epidural steroid injections. No case reports in the literature have been associated with RFA and epidural hematoma formation. However, there have been epidural hematomas with surgical evacuation after intraarticular facet joint injections for pain.21 The larger gauge needles used in RF procedures could have a higher propensity to cause severe bleeding if cessation of anticoagulation medications is not done. As a general rule for outpatient elective chronic pain procedures, an international normalized ratio (INR) of less than 1.3 and a platelet count greater than 100,000 (assuming no qualitative platelet dysfunction such as in end-stage renal disease) is desired, although some practitioners use an INR of less than 1.5 and platelets greater than 80,000 if the patient is asymptomatic.
Physician-Dependent Factors
Infection or Abscess
Poor sterile technique by the pain physician or the procedural team can certainly increase the risk of infection or abscess formation. Surgical masks can contribute to a decrease in morbidity related to infection.12 A surgical scrub and prep with chlorhexidine with ethanol (CHE) has been shown in studies to be superior to iodine-based solutions.22 Viable microorganisms of the human skin flora were still present on the skin of patients after preparation with iodine-based products to a level of significance (P <.01) compared with CHE.23 Proper precautions by the procedural team as well as the above suggestions will decrease the likelihood of complications related to infection.
Faulty Equipment
A careful examination of the insulation of the RF needles should always be done. To date, there have been four reported cases of superficial burns secondary to disrupted insulation of the probe.24 Kornick and associates25 described two case reports of burns related to a malfunction of the adhesion site of the grounding pad.
Radiofrequency Probe Placement and Neurolysis
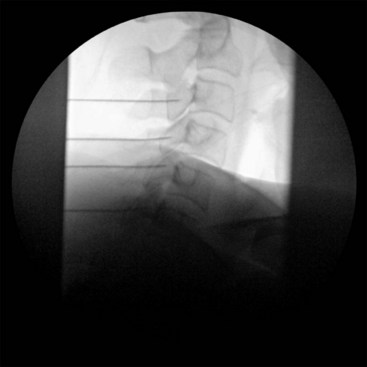
Fluoroscopic guidance when placing the RF needle is essential to ensure patient safety and mitigate complication risks. As mentioned above, neurologic injury was the most common severe complication related to neuroablative procedures in the ASA Closed Claims Project cases. Pneumothorax was also an issue. The application of an extensive knowledge of the anatomy along the trajectory of the needle will minimize risk to the nerves, viscera, and vascular structures. It is imperative that the fluoroscopic images have no parallax. Sharp alignment of the margins of the bilateral osseous target structures minimizes confusion of where exactly the needle is being placed. Fig. 7-1 is a lateral fluoroscopic image that demonstrates a malalignment or parallax of bilateral osseus structures (C5) and an optimized or crisp alignment of structures (C3) in the same image; parallax of the target area increases the risk of incorrect needle placement and possible neurologic injury.
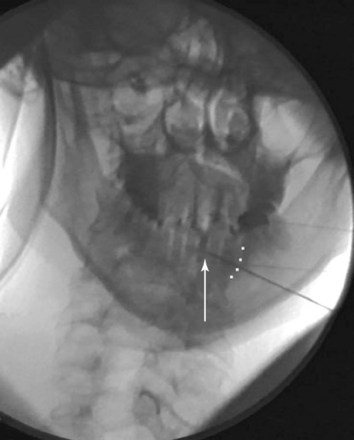
Contrast dye injected under live fluoroscopy should always be used if the needle tip is to be placed in the vicinity of a vascular structure or certain vital organs (e.g., lung, bowel). There have been no formal case reports related to RFA and nerve or viscera injury; however, Bogduk and associates26 report two medicolegal cases involving direct spinal cord trauma. The first case of spinal cord injury involves a patient undergoing a C3–C4 RFA. The case was done under general anesthesia. The proceduralist placed the active tip of the RF probe medial to the facet joint, traversed the lamina, and into the spinal cord. This was confirmed via the intraoperative images. The patient developed Brown-Séquard syndrome subsequent to the procedure. The two key points are that (1) general anesthesia rendered the patient helpless in being able to report symptomatology related to impending cord injury and (2) the radiographic images were not properly used to ensure correct needle placement. The second case involved a patient who underwent an attempted C3 (third occipital) RFA. The anteroposterior (AP) images showed the RF probe was placed at the wrong level (C3–C4). The tip was also too medial and anterior and thus inside the foramen (Fig. 7-2); the result was severe neurologic injury.
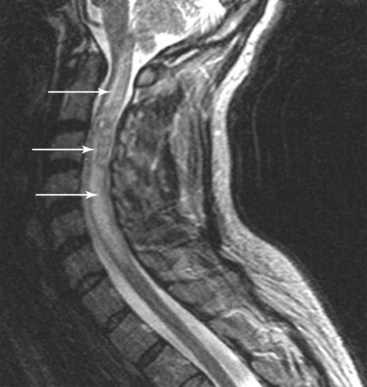
If the probe had been correctly placed, the active tip would be at the correct level (C2–C3) and would be flush against the lateral pillar just lateral to the lateral margin of the facet joint. It was later determined that a radicular artery was coagulated via RF, which led to an infarction of the anterior spinal artery (Fig. 7-3).26
Postprocedural Pain and Neuritis
These may be looked more as a part of the healing process of RF neurolysis rather than complications. Postprocedural pain is thought to be related to neuropathic pain with the ablation of the target nerve as well as injury to the adjacent soft tissue and periosteum. Symptoms associated with postprocedural pain usually resolve within 1 to 3 weeks. Neuritis typically abates in 2 to 6 weeks but may last for several months.27
Complications Associated with Continuous Radiofrequency Ablation
Zygapophyseal (Facet) Joint Medial Branch Radiofrequency Neurolysis
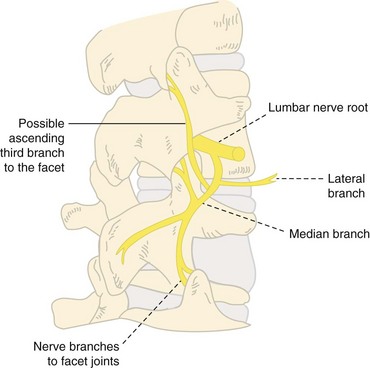
The treatment of cervical, thoracic, and lumbar facet–mediated pain with medial branch RF neurolysis has a low morbidity. The most common complaint was burning or neuropathic pain that was usually self-limited. Complaints of temporary sensory deficits consistent with the cutaneous branches of the posterior rami have been reported.28 As mentioned previously, cases involving treatment by intraarticular facet joint blocks leading to complications such as epidural hematoma and abscess with subsequent surgical evacuation have been reported.13,21 The proceduralist should always use vigilance when using the typically larger gauge RFA needles as well a proper sterile technique. Medicolegal cases of permanent spinal cord injury related to RFA have been described.26
Cervical Medial Branches
Cervical facet medical branch neurolysis is typically done via the posterolateral approach (Fig. 7-4). With this technique, there have been fewer complications reported.29 A prospective pilot study by van Suijlekom and colleagues30 reported a 30% incidence of postprocedural burning pain that usually resolved in 1 to 3 weeks.27 Lord and Bogduk31 reported observed or theoretical complications related to cervical RF neurolysis as outlined in Table 7-3.
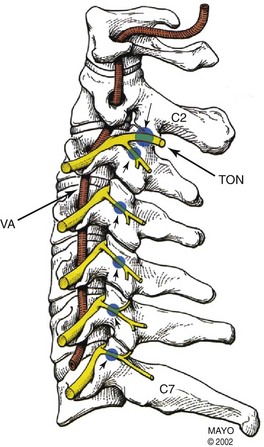
Fig. 7-4 Anatomic depiction of the usual location of the cervical medial branches (blue dots).
(Copyright © 2002 Mayo Clinic.)
Table 7-3 Reported and Theoretical Complications from Cervical Medial Branch Ablation
| Observed Frequency/Procedures Performed | Percentage | |
|---|---|---|
| Allergic reactions | 0/83 | 0 |
| Vasovagal syncope | 2/83 | 2 |
| Risks associated with implanted electrical devices (pacemakers) | 0/83 | 0 |
| Vascular injury | 0/83 | 0 |
| Nerve injury | 0/83 | 0 |
| Burn injury | 0/83 | 0 |
| Postprocedural pain | 81/83 | 97 |
| Ataxia, unsteadiness, spatial disorientation | 19/83 | 23 |
| Cutaneous Numbness | ||
| Third occipital procedures | 22/25 | 88 |
| C3–C4 procedures | 8/10 | 80 |
| Lower cervical level procedures | 9/48 | 19 |
| Dysesthesias | ||
| Third occipital procedures | 14/25 | 56 |
| C3–C4 procedures | 3/10 | 30 |
| Lower cervical level procedures | 8/48 | 17 |
| Neurologic Complications | ||
| Transient neuritis | 2/83 | 2 |
| Neuroma | 0/83 | 0 |
| Rare Complications | ||
| Infection | 0/83 | 0 |
| Hematoma | 0/83 | 0 |
| Dermoid cyst | 1/83 | 1 |
Adapted from Lord S, Bogduk N: Radiofrequency procedures in chronic pain, Best Pract Res Clin Anesth 16(4):597-617, 2002.
The symptom of dizziness can occur after medial branch blocks at the higher cervical levels. Ataxia and unsteadiness may also occur. It is thought that a blockade of the cervical proprioceptive afferents may be the primary contributing factor to these transient symptoms. No prolonged cases have been reported.32 Patients should be instructed to not drive or operate heavy or dangerous machinery subsequent to a cervical medial branch RFA, although a clear time frame has not been established. There was a higher percentage (13%) of patients reporting postprocedural pain than reported in other studies. The pain was burning in nature and lasted 2 to 6 weeks in duration. A 4% incidence of occipital hyperesthesia was also reported, lasting approximately 3 months and thought to be related to the lesioning of the third occipital nerve.32
Thoracic Medial Branches
Apart from post procedural pain, pneumothorax (Fig. 7-5) is anecdotally the most common complication. Fluoroscopic guidance is essential to minimize this risk. The patient should be informed about the increased risk of a pneumothorax. Before discharge, the patient should be instructed to go immediately to the emergency department if he or she has difficulty breathing or if pain with inspiration ensues. If there is any question by the provider about a possible pneumothorax, a radiograph should be obtained.29 A theoretical risk of nerve damage applies to the thoracic region as well as the other complications related to any RFA procedure.
Lumbar and Sacroiliac
Kornick et al25 did a retrospective analysis of 92 patients who had a total of 616 lumbar RF medial branch lesions (Fig. 7-6). There was a total 1% overall incidence of minor complications per each RF site with no major complications such as infection, bleeding, or new motor or sensory deficits. There were three cases (0.5%) of neuropathic pain lasting less than 2 weeks and three cases (0.5%) of prolonged pain lasting longer than 2 weeks.25 Coskun and colleagues33 reported a case of RFA nerve injury that led to lumbosacral radiculopathy. Gekht and associates34 described a case of a patient who developed a painful medial branch neuroma after numerous lumbar RFA procedures. The mechanism is unclear, and no other reports in the literature of this type of complication exist. The pain from the neuroma resolved completely after a surgical neurectomy. There have been no formal reports of complications related to the RF neurolysis of the sacroiliac joint innervation. There have been anecdotal findings of buttock discomfort usually lasting about 2 weeks. Numbness in the buttock region has also been reported but was typically self-limited to about 2 to 6 weeks’ duration.27
Observed and Theoretical Complications Associated with Radiofrequency Neurolysis of the Cervical, Thoracic, and Lumbar Medial Branches and Sacroiliac Denervation (Table 7-4)
These observed and theoretical complications include:
 All spinal levels: nerve injury, neuritis, radiculopathy, transient cutaneous numbness, postprocedural pain, neuroma and dermoid cyst formation (both extremely rare)
All spinal levels: nerve injury, neuritis, radiculopathy, transient cutaneous numbness, postprocedural pain, neuroma and dermoid cyst formation (both extremely rare)Table 7-4 Avoidance of Observed and Theoretical Complications Associated with Radiofrequency Neurolysis of the Cervical, Thoracic, and Lumbar Medial Branches and Sacroiliac Denervation
| Complication | Avoidance |
|---|---|
| Postprocedural pain, neuritis, transient cutaneous numbness | Intrinsic to thermal RFA |
| Ataxia, dizziness, dysesthesias | Consider RF at a lower temperature, especially at higher cervical levels involving the third occipital |
| Pneumothorax, nerve or spinal cord injury, radiculopathy | Fluoroscopically guided probe placement, especially if needles are readjusted; avoidance of placing the probe tip too anterior in the lateral view (avoid placing in the foramen), or too lateral in the AP view; motor stimulation >1.0-3.0 V with no motor response of the associated nerve root |
AP, anteroposterior; RF, radiofrequency; RFA, radiofrequency ablation.
Sympathetic Radiofrequency Ablation
Sympathetically mediated pain has been implicated in chronic pain syndromes of various origins. Blockade of the sympathetic nervous system has been associated with marked pain relief in syndromes such as complex regional pain syndrome (CRPS), cancer pain, vascular pain, and visceral pain.35 When the transmission of pain via the sympathetic nervous system has been interrupted or attenuated via local anesthetics or neurolysis, it has proven to decrease the use of opioids in these patient populations.36 Local anesthetic blockade of the sympathetic ganglia of the splanchnic and celiac plexus was first reported by Kappis in 1914, and evolved chemical neurolysis by Jones in 1957.37 Sympathetic neurolysis has evolved to include the sphenopalatine ganglion (SPG) for facial pain; SG for pain of the cervicothoracic region, upper extremities, and face; splanchnic nerves and celiac plexus for abdominal and visceral pain; lumbar plexus for lower extremity pain; superior hypogastric plexus for pelvic pain; and ganglion impar for pain of the perineum. Although some of these structures are not made up entirely of sympathetic nerve fibers, RF neurolysis of these structures has shown to have lasting relief in many patients with chronic sympathetically mediated pain. As with any percutaneous procedure, bleeding and infection are always concerns. Although rare, severe complications are thought to be more commonly associated with improper needle placement, but RF neurolysis can also have devastating outcomes if it is not executed properly.
Stellate Ganglion
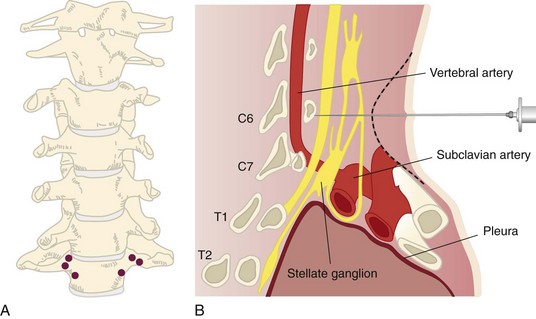
Radiofrequency ablation of the SG has been effective in treating patients with CRPS of the upper extremity, phantom limb pain, peripheral neuropathy, Raynaud phenomenon, postherpetic neuralgia, and atypical facial pain. Posttraumatic stress disorder has been shown to be treatable by SG blockade, although the mechanism for this is poorly understood.38 Most of the complications associated with SG RFA are related to incorrect needle placement or the spread of the local anesthetic into a precarious location. Fig. 7-7, A demonstrates the proper needle placement of the RF probes. Fig. 7-7, B demonstrates the close proximity of the vertebral artery and other vital structures close to the target area.
The most severe theoretical complication is complete heart block associated with an RFA of the SG if the patient is being treated for a bilateral sympathetic pain syndrome (Table 7-5). Bilateral continuous RFA, even done on separate occasions, is not recommended because of the risk of prolonged blockade of the sympathetic fibers to the sinoatrial node and cardiac accelerators. Pulsed RF treatment may be a viable option. There are case reports of even unilateral SG blockade leading to heart block. Saxena and associates39 reported a case in which a 29-year-old woman experienced sinus arrest after a right-sided SG block. The complication of total spinal anesthesia has been reported with intrathecal spread of local anesthetic.40 Cases of cerebral air embolisms have occurred and can easily be prevented by removal of the air from the syringe. Transient neuritis and persistent cough have been reported. A sudden death was reported after a SG block. The patient was found to have severe bleeding and bilateral pneumothorax. Paralysis related to spinal cord injury has been a debilitating outcome.37 One case of osteomyelitis of the cervical spine was reported in a patient who received multiple SG blocks. The patient did not appear to have any other pathology that would predispose her to develop such an infection. A complication of this nature is exceedingly rare.41
Table 7-5 Avoidance of Observed and Theoretical Complications Associated with Radiofrequency Neurolysis of the Stellate Sympathetic Ganglion
| Complication | Avoidance |
|---|---|
| Complete heart block | Avoid bilateral RF; 0.5-mL test dose |
| Total spinal or epidural injection leading to unconsciousness, apnea, and possibly bradycardia | Negative aspiration; myelogram-compatible contrast use indicating no epidural or subarachnoid spread; 0.5-mL test dose (although the patient could have this complication from < 0.5 mL of local anesthetic) |
| Intravascular uptake: seizure or local anesthetic toxicity from uptake into the vertebral artery or subclavian artery or vein | Negative aspiration; contrast use indicating no vascular uptake; 0.5-mL test dose (although the patient could have this complication from < 0.5 mL of local anesthetic); fluoroscopic image should be optimized; osseous contact should be made with extreme caution in not moving the needle posterolateral into the vertebral artery; needle placement at C7 or T1 may create better lesioning of the stellate ganglion, but there is a greater risk of subclavian puncture |
| Recurrent laryngeal nerve injury leading to hoarseness, loss of laryngeal reflexes, respiratory distress (bilateral injury) | Have the patient say “ee” while implementing motor stimulation; if there are no changes in the pronunciation, then the nerve should be preserved |
| Phrenic nerve injury leading to respiratory distress | Motor stimulation with no diaphragm movement |
| Persistent Horner syndrome | 1-mL test dose or negative Horner syndrome with the local anesthetic block (although this is not a complete fail-safe method to prevent this complication) |
| Pneumothorax or pneumochylothorax | Fluoroscopic guidance; avoid lateral placement of the needle; use vigilance if the block is to be done below C6 |
| Cerebral air embolism | Remove air from injectate |
| Paralysis from spinal cord injury | Confirm proper needle placement with fluoroscopy before RF lesioning |
| Retropharyngeal, cervicomediastinal, or posttracheal hematoma | Retract the carotid artery lateral if necessary; if vascular uptake is appreciated, especially if it appears arterial, close postprocedural observation is recommended |
RF, radiofrequency.
Splanchnic Nerves and Celiac Plexus
Saltzburg and Foley42 reported relief of a visceral abdominal pain and pancreatic cancer pain via splanchnic nerve and celiac plexus blocks and neurolysis to be at 45% to 100% (Fig. 7-8). These structures include innervations to the distal third of the esophagus to the transverse colon, mesentery, pancreas, spleen, kidneys, liver, adrenals, and biliary tract.36 Complications vary with these blocks. Transient hypotension and diarrhea are the more common complications. Cases of pneumothorax have occurred.36 Takahashi and associates43 reported a case of silent gastric perforation during a celiac plexus block. The use of a blunt needle may obviate the risk of visceral structures such as the stomach, liver, bowel, and kidneys as demonstrated by Heavener and Racz44 (Table 7-6).35,36 Cases of spinal cord injury and paraplegia have been reported with chemical neurolysis, but no major complications have been described with RFA.36
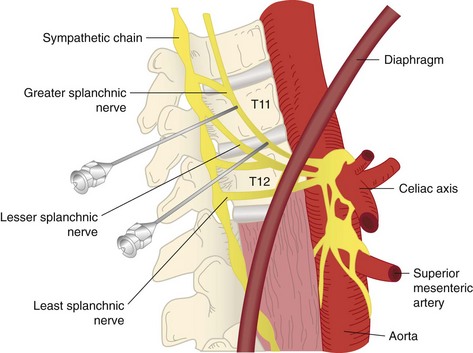
Fig. 7-8 Anatomic depiction of the location of the greater splanchnic nerve.
(Modified from Waldman SD (editor): Interventional pain management, 2nd ed, Philadelphia, 2001, Saunders.)
Table 7-6 Avoidance of Observed and Theoretical Complications Associated with Radiofrequency of the Sympathetic Celiac Plexus or Splanchnic Nerves
| Complication | Avoidance |
|---|---|
| Paraplegia, spinal cord injury, nerve damage | Negative motor stimulation ≤3.0 V with the associated nerve root; confirm proper needle placement with fluoroscopy before RF lesioning; avoid chemical neurolytic agents; avoid aortic injury to minimize risk of injury to the artery of Adamkiewicz |
| Intercostal neuritis | Negative motor stimulation ≤3.0 V with no response of intercostal muscle contraction; if this occurs, move the needle a few millimeters anterior |
| Bowel, gastric, liver, renal, ureter injury | Fluoroscopic guidance; use of a blunt needle |
| Pneumothorax, chylothorax | Avoid placing needle >4 cm lateral from the midline |
| Aortic or inferior vena cava injury | Careful needle placement not to go too anterior, but for celiac plexus block, it is an inherent risk of the procedure; use of a blunt needle |
| Seizure or local anesthetic toxicity from intravascular injection | Negative aspiration; myelogram-compatible contrast |
| Vascular embolism or plaque dislodgement | Avoid transaortic approach in high-risk patients |
| Retroperitoneal hematoma or abscess or peritonitis | Proper sterile technique; avoid bowel puncture by using a blunt needle |
| Discitis | Avoid transdiscal approach; use preprocedure antibiotics |
| Transient diarrhea | Common intrinsic result of block |
| Transient hypotension | Avoid bilateral block on the same day; fluid bolus before the procedure |
RF, radiofrequency.
From Raj P, Lou L, Erdine S, et al, editors: Interventional pain management: image-guided procedures, ed 2, Philadelphia, 2008, Saunders.
Lumbar Sympathetic Ganglion
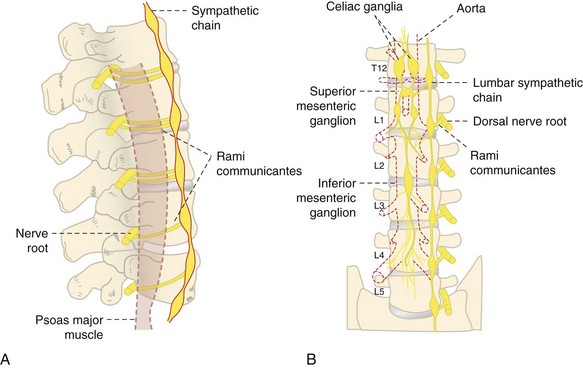
The lumbar sympathetic block was first described by Brunn and Mandl in 192437 (Fig. 7-9). It has been implicated in the treatment of chronic pain disorders such as CRPS, postsurgical neuropathic pain, peripheral neuropathy, phantom limb pain, postherpetic neuralgia, and pain associated with peripheral vascular disease.35,37 The concern for hypotension secondary to sympathectomy is more common with lumbar blockade; this is a transient effect, and no long-term cases are described in the literature. Intravascular injection and subarachnoid spread are always of concern, and contrast should always be used. The use of a blunt needle may decrease the risk of vital structure injury.44 The remaining complications are described in Table 7-7.
Table 7-7 Avoidance of Observed and Theoretical Complications Associated with Radiofrequency of the Lumbar Sympathetic Ganglion
| Complication | Avoidance |
|---|---|
| Seizure or local anesthetic toxicity from intravascular injection | Negative aspiration, myelogram-compatible contrast |
| Lumbar nerve root injury | Negative motor stimulation ≤3.0 V with the associated nerve root; confirm proper needle placement with fluoroscopy before RF lesioning; avoid chemical neurolytic agents |
| Lumbar plexus injury | Negative motor stimulation of the quadriceps ≤2.0 V; confirm proper needle placement with fluoroscopy before RF lesioning; avoid chemical neurolytic agents |
| Genitofemoral neuralgia | Negative sensory stimulation ≤3.0 V; confirm proper needle placement with fluoroscopy before RF lesioning; avoid chemical neurolytic agents |
| Aortic or inferior vena cava injury | Avoid needle placement going too anterior in the lateral view; use of a blunt needle |
| Transient hypotension (can be significant for patients who have severe aortic stenosis) | Avoid bilateral block on the same day; fluid bolus before the procedure |
| Renal injury | Confirm proper needle placement; use of a blunt needle |
| Sexual dysfunction in men (no ejaculation), especially if bilateral sympathetic blockade37 | Avoid bilateral RF ablation; consider pulsed RF treatment |
RF, radiofrequency.
Modified from Raj P, Lou L, Erdine S, et al, editors: Interventional pain management: image-guided procedures, ed 2, Philadelphia, 2008, Saunders.
Superior Hypogastric Plexus
The superior hypogastric plexus block has proven to be effective in treating visceral pelvic pain (Fig. 7-10).45 Chronic pain associated with pelvic pathology such as endometriosis, adhesions from surgery, interstitial cystitis, and malignancies, have been treated with RF neurolysis.35,37 The superior hypogastric plexus innervates the descending colon to the rectum and the urogenital tract and viscera.36 Complications such as inadvertant intravascular and subarachnoid injection should be considered as with all neuraxial procedures, and contrast should always be used. The transdiscal approach seems to be more effective with less complication risk than the classic approach.35 The risk of discitis is the concern with this approach (Table 7-8).
Fig. 7-10 Depiction of the superior hypogastric plexus in relation to L5 vertebral body and sacrum.
(Modified from Raj P, Lou L, Erdine S, et al (editors): Interventional pain management: image-guided procedures, 2nd ed, Philadelphia, 2008, Saunders.)
Table 7-8 Avoidance of Observed and Theoretical Complications Associated with Radiofrequency of the Superior Hypogastric Sympathetic Plexus
| Complication | Avoidance |
|---|---|
| Seizure or local anesthetic toxicity from intravascular injection | Negative aspiration, myelogram-compatible contrast |
| Lumbar nerve root injury | Negative motor stimulation ≤3.0 V with the associated nerve root; confirm proper needle placement with fluoroscopy before RF lesioning; avoid chemical neurolytic agents |
| Iliac vessel injury | Avoid needle placement going too anterior in the lateral view; use of a blunt needle |
| Transient hypotension (can be significant for patients that have severe aortic stenosis) | Avoid bilateral block on the same day; fluid bolus before procedure |
| Ureter injury | Use of a blunt needle |
| Sexual dysfunction in men (no ejaculation),37 especially if bilateral sympathetic blockade | Avoid bilateral RF ablation; consider pulsed RF treatment |
| Discitis | Avoid transdiscal approach or use preprocedural antibiotics |
| Bowel or bladder incontinence (rare) | Consider pulsed RF |
| Rectum injury | Use of a blunt needle |
RF, radiofrequency.
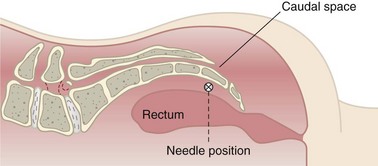
Ganglion Impar
The ganglion impar supplies the innervation to the perineum and rectum (Fig. 7-11). Patients with malignancies or coccydynia may receive prolonged relief from RFA of the terminal end of the sympathetic chain.37 There have been case reports showing up to 100% pain relief with this block.36 To date, there are no formal case reports on complications from RF neurolysis of the ganglion impar. Table 7-9 includes observed and theoretical complications.
Table 7-9 Avoidance of Observed and Theoretical Complications Associated with Radiofrequency of the Sympathetic Ganglion Impar
| Complication | Avoidance |
|---|---|
| Seizure or local anesthetic toxicity from intravascular injection | Negative aspiration; myelogram-compatible contrast |
| Caudal epidural or intrathecal spread | Confirm proper needle placement with fluoroscopy and contrast before RF lesioning; avoid chemical neurolytic agents when possible |
| Rectum injury | Use of a blunt needle |
| Infection | Proper sterile technique and preparation |
RF, radiofrequency.
Radiofrequency Treatment for Head and Facial Pain
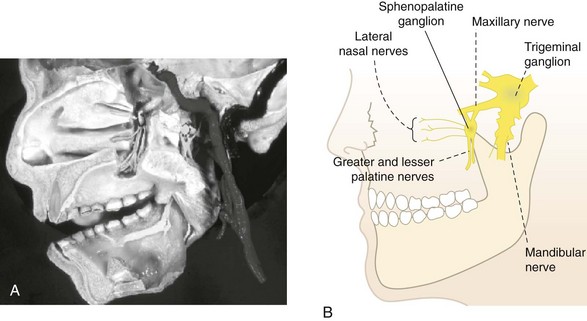
Sphenopalatine (Pterygopalatine) Ganglion
Radiofrequency ablation of the SPG (Fig. 7-12) has been used as a treatment modality to treat refractory headaches and atypical facial pain. Narouze and coworkers46 demonstrated this treatment as being an effective means to treat intractable cluster headaches. Pain practitioners have also used this method in the treatment of severe refractory headaches unresponsive to conservative therapies, migraines, and atypical facial pain.27,37 Sphenopalatine RF procedure complications primarily involved incorrect needle placement rather than with neurolysis issues. The complication of the needle entering the superior orbital fissure has been described but not reported.47 Careful attention to lateral and AP fluoroscopic images to confirm proper needle placement should be used (Table 7-10). Konen48 reported reflex bradycardia or the oculocardiac reflex being triggered during RFA of the SPG. Immediate procedure cessation and needle retraction and removal are recommended. Atropine or glycopyrrolate should be readily available to reverse bradycardia. Inadvertent local anesthetic vascular injection via the maxillary artery (see Fig. 7-12) may cause loss of consciousness or seizure. Appropriate supportive action should be taken.27 Dryness of the ipsilateral eye and numbness of the soft palate have been associated with RF neurolysis of the SPG. If numbness of the soft palate occurs, it is usually transient, typically resolving in 4 to 6 weeks. There has been anecdotal discussion of an extremely rare complication involving taste deficiencies.29
Table 7-10 Avoidance of Observed and Theoretical Complications Associated with Radiofrequency of the Sphenopalatine Ganglion
| Complication | Avoidance |
|---|---|
| Loss of consciousness or seizure: uptake of local anesthetic via the maxillary artery | Use of a blunt needle; test dose; confirm proper needle placement with fluoroscopy and myelogram-compatible contrast before RF lesioning |
| Oculocardiac reflex or severe bradycardia | Varies; if bradycardia occurs, stop the procedure immediately; give supportive cardiac medications if necessary |
| Globe injury secondary to the needle or probe entering the superior orbital fissure | Use of a blunt needle; test dose; confirm proper needle placement with fluoroscopy in the AP view, incrementally each time on advancing the needle |
| Perforation of the perpendicular palatine bone and nasal mucosa by probe being place too medial | Confirm needle placement with the AP view; do not force the needle through any areas of resistance |
| Ipsilateral eye desiccation, numbness of the soft palate (usually transient), taste deficiencies (extremely rare) | Inherent risks of the procedure |
AP, anteroposterior.
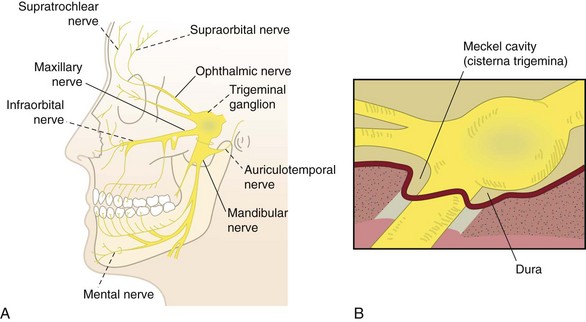
Trigeminal (Gasserian) Ganglion
Radiofrequency neurolysis of the trigeminal ganglion (Fig. 7-13) has been implicated in treatment of idiopathic trigeminal neuralgia (tic douloureux), atypical trigeminal neuralgia, atypical facial pain, intractable ocular pain, cluster headache, and cancer pain.27,29,37 As with all invasive procedures, bleeding and infection are of concern. Numerous complications have been reported in the literature as outlined in Table 7-11. Overall, there is a low morbidity and no mortality with percutaneous trigeminal RF neurolysis; however, because of the close proximity to cerebral matter and the cranial nerves, it should be performed only by experienced proceduralists.3,29
Table 7-11 Observed and Theoretical Complications Associated with Percutaneous Radiofrequency of the Trigeminal Ganglion
| Complications |
|---|
Data from Benzon H, Rathmell J, Turk D, Argoff C, editors: Raj’s practical management of pain, ed 4, Philadelphia, 2008, Mosby-Elsevier; Neal J, Rathmell J, editors: Complications in regional anesthesia and pain medicine, ed 4, Philadelphia, 2006, Elsevier.
Complications of Pulsed Radiofrequency Treatment
Pulsed RF treatment for chronic pain has been implemented as a minimally destructive means to attenuate the signals from nerve fibers that transmit pain. It is thought to be a form of neuromodulation. Small studies have demonstrated good to fair results in chronic pain relief. Vatansever and colleagues49 demonstrated at a microscopic level that pulsed RF of peripheral nerves appeared to be less neurodestructive compared with traditional RFA. Benzon and associates50 state that complications related to pulsed RF are lacking in the literature. Apart from similar complications related to continuous RF needle placement, there appear to be no additional anecdotal or theoretical complications associated with pulsed RF itself.
1 Hunsperger R, Wyss O. Production of localized lesions in the nervous tissue by coagulation with high frequency current. Helv Physiol Pharmacol Acta. 1953;11:283.
2 Rosomoff H, Carrol F, Brown J, Sheptak T. Percutaneous radiofrequency cervical cordotomy: technique. J Neurosurg. 1965;23:639.
3 Neal J, Rathmell J, editors. Complications in regional anesthesia and pain medicine, ed 4, Philadelphia: Elsevier, 2006.
4 Van Zundert J, Sluijter M, van Kleef M. Thermal and pulsed radiofrequency. In: Raj P, editor. Interventional pain management: image-guided procedures. Philadelphia: Saunders; 2008:58.
5 Sluijter M, Vosman E, Rittman W, van Kleef M. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion—a preliminary report. Pain Clin. 1998;11:109-117.
6 Young R. Clinical experience with radiofrequency and laser DREZ lesions. J Neurosurg. 1990;72:715.
7 Fitzgibbon DR, Posner KL, Domino KB, et al. American Society of Anesthesiologists: Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology. 2004;100:98-105.
8 Huycke L, Huycke M. Characteristics of potential plaintiffs in malpractice litigation. Ann Intern Med. 1994;120:792-798.
9 Hodges S, Castleberg R, Miller T, et al. Cervical epidural steroid injection with intrinsic spinal cord damage: two case reports. Spine. 1998;23(19):2137-2140.
10 Canaday B. Amorous, disinhibited behavior associated with propofol. Clin Pharm. 1993;12(6):449-451.
11 Møller A, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
12 Steffen P, Seeling W, Essig A, et al. Bacterial contamination of epidural catheters: microbiological examination of 502 epidural catheters used for postoperative analgesia. J Clin Anesth. 2004;16:92-97.
13 Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11(3):141-147.
14 Rosenthal R, Starley D, Austin C. Avoiding complications from interventional spine techniques. Pract Pain Manage. 2010;10(2):77-87.
15 Sun D, Martin L, Honey C. Percutaneous radiofrequency trigeminal rhizotomy in a patient with an implanted cardiac pacemaker. Anesth Analg. 2004;99:1585-1586.
16 Eagle K, Berger PB, Calkins H, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines: ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery. Circulation. 2002;105(10):1257-1267.
17 Rozner M. Pacemaker misinformation in the perioperative period: programming around the problem. Anesth Analg. 2004;99:1582-1584.
18 Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (The Second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28(3):172-197.
19 Abram S, O’Conner T. Complications associated with epidural injections. Reg Anesth. 1996;21:149-162.
20 Stafford-Smith M. Impaired haemostasis and regional anaesthesia. Can J Anaesth. 1996;43:R129-R141.
21 Nam K, Choi C, Yang M, Kang D. Spinal epidural hematoma after pain control procedure. J Korean Neurosurg Soc. 2010;48(3):281-284.
22 Sato S, Sakuragi T, Dan K. Human skin flora as a potential source of epidural abscess. Anesthesiology. 1996;85:1276-1282.
23 Kinirons B, Mimoz O, Lafendi L, et al. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children: a randomized, controlled trial. Anesthesiology. 2001;94:239-244.
24 Shealy C. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J Neurosurgery. 1975;43:448-451.
25 Kornick C, Kramarich S, Lamer T, Sitzman B. Complications of lumbar facet radiofrequency denervation. Spine. 2004;29(12):1352-1354.
26 Bogduk N, Dreyfuss P, Baker R, et al. Complications of spinal diagnostic and treatment procedures. Pain Med. 2008;9(suppl 1):S11-S34.
27 Waldman S, editor. Pain management. Philadelphia: Elsevier, 2007.
28 Tzaan W, Tasker R. Percutaneous radiofrequency facet rhizotomy: experience with 118 procedures and reappraisal of its value. Can J Neurol Sci. 2000;27(2):125-130.
29 Benzon H, Rathmell J, Turk D, Argoff C, editors. Raj’s practical management of pain, ed 4, Philadelphia: Mosby-Elsevier, 2008.
30 van Suijlekom JA, van Kleef M, Barendse G, et al. Radiofrequency cervical zygapophyseal joint neurotomy for cervicogenic headache: a prospective study in 15 patients. Funct Neurol. 1998;13:297-303.
31 Lord S, Bogduk N. Radiofrequency procedures in chronic pain. Best Pract Res Clin Anesth. 2002;16(4):597-617.
32 Vervest A, Stolker R. The treatment of cervical pain syndromes with radiofrequency procedures. Pain Clin. 1991;4:103-112.
33 Coskun D, Gilchrist J, Dupuy D. Lumbosacral radiculopathy following radiofrequency ablation therapy. Muscle Nerve. 2003;28:754-756.
34 Gekht G, Nottmeier E, Lamer T. Painful medial branch neuroma treated with minimally invasive medial branch neurectomy. Pain Med. 2010;11(8):1179-1182.
35 Day M. Sympathetic blocks: the evidence. Pain Pract. 2008;8(2):98-109.
36 Alshab A, Goldner J, Panchal S. Complications of sympathetic blocks for visceral pain. Tech Reg Anesth Pain Med. 2007;11:152-156.
37 Raj P, Lou L, Erdine S, et al, editors. Interventional pain management: image-guided procedures, ed 2, Philadelphia: Saunders, 2008.
38 Mulvaney S, McLean B, De Leeuw J. The use of stellate ganglion block in the treatment of panic/anxiety symptoms with combat-related post-traumatic stress disorder; preliminary results of long-term follow-up: a case series. Pain Pract. 2010;10(4):359-365.
39 Saxena A, Saxena N, Aggarwal B, Sethi A. An unusual complication of sinus arrest following right-sided stellate ganglion block: a case report. Pain Pract. 2004;4(3):245-248.
40 Forrest J. An unusual complication after stellate ganglion block by the paratracheal approach: a case report. Can Anaesth Soc J. 1976;23(4):435-439.
41 Maeda S, Murakawa K, Fu K. A case of pyogenic osteomyelitis of the cervical spine following stellate ganglion block. Masui. 2004;53:664-667.
42 Saltzburg D, Foley K. Management of pain in pancreatic cancer. Surg Clin North Am. 1989;69:629-649.
43 Takahashi M, Yoshida A, Ohara T, et al. Silent gastric perforation in pancreatic cancer patient treated with neurolytic celiac plexus block. J Anesth. 2003;17:196-198.
44 Heavner JE, Racz GB, Jenigiri B, et al. Sharp versus blunt needle: a comparative study of penetration of internal structures and bleeding in dogs. Pain Pract. 2003;3:226-231.
45 Bosscher H. Blockade of the superior hypogastric plexus for visceral pelvic pain. Pain Pract. 2001;1:167-170.
46 Narouze S, Kapural L, Casanova J, Mekhail N. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache. 2009;49(4):571-577.
47 Racz G, Day M: Needle probe entering superior orbital fissure during a sphenopalatine ganglion block, verbal communication, 2009.
48 Konen A. Unexpected effects due to radiofrequency thermocoagulation of the sphenopalatine ganglion: two case reports. Pain Digest. 2000;10:30.
49 Vatansever D, Tekin I, Tuglu I, et al. A comparison of the neuroablative effects of conventional and pulsed radiofrequency techniques. Clin J Pain. 2008;24(8):717-724.
50 Benzon H, Nader A, Avram M, Moore J. Pulsed radiofrequency ablation for chronic pain: a review of its effectiveness and complications [meeting abstract]. San Diego: Annual American Society of Anesthesiology; 2010.
51 Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25 years experience with 1600 patients. Neurosurgery. 2001;38(3):524-534.
52 Kanpolat Y, Savas A, Berk C. Abducens nerve palsy after radiofrequency rhizolysis for trigeminal neuralgia: case report. Neurosurgery. 1999;44(6):1364.
53 Egan R, Pless M, Shults W. Monocular blindness as a complication of trigeminal radiofrequency. Am J Ophthalmol. 2001;131(2):237-240.