Chapter 6 Complications of Therapeutic Minimally Invasive Intradiscal Procedures
 Spinal pain that originates from the disc is thought to be a major cause of severe and chronic back pain.
Spinal pain that originates from the disc is thought to be a major cause of severe and chronic back pain. Discogenic pain may be caused by herniated and bulging discs, as well as internal disc disruption (IDD), which may be caused by annular tears that irritate small nociceptive nerve fibers within the outer layer of the annulus fibrosis.
Discogenic pain may be caused by herniated and bulging discs, as well as internal disc disruption (IDD), which may be caused by annular tears that irritate small nociceptive nerve fibers within the outer layer of the annulus fibrosis. People presenting with discogenic pain are typically treated with conservative modalities, including physical therapy, chiropractic care, proper activity performance, and antiinflammatory and over-the-counter medications.
People presenting with discogenic pain are typically treated with conservative modalities, including physical therapy, chiropractic care, proper activity performance, and antiinflammatory and over-the-counter medications. If conservative care fails to provide relief, other diagnostic and therapeutic modalities may be performed, including epidural steroid injections, selective nerve root blocks, discography, and facet-related interventions.
If conservative care fails to provide relief, other diagnostic and therapeutic modalities may be performed, including epidural steroid injections, selective nerve root blocks, discography, and facet-related interventions. However, if conservative diagnostic and therapeutic interventions do not alleviate discogenic pain, minimally invasive intradiscal procedures are often considered.
However, if conservative diagnostic and therapeutic interventions do not alleviate discogenic pain, minimally invasive intradiscal procedures are often considered. Some of these minimally invasive intradiscal treatments include chemonucleolysis, IDET, biacuplasty, Disc Dekompressor, nucleoplasty, percutaneous laser disc decompression, and injectable treatments such as methylene blue and tumor necrosis factor α. Each of these procedures addresses a key element in disc pathology.
Some of these minimally invasive intradiscal treatments include chemonucleolysis, IDET, biacuplasty, Disc Dekompressor, nucleoplasty, percutaneous laser disc decompression, and injectable treatments such as methylene blue and tumor necrosis factor α. Each of these procedures addresses a key element in disc pathology. Although some of these minimally invasive treatments are new and research is limited, they typically involve fewer risks and quicker recovery times compared with traditional open surgical practices.
Although some of these minimally invasive treatments are new and research is limited, they typically involve fewer risks and quicker recovery times compared with traditional open surgical practices. Minimally invasive intradiscal procedures are designed to lower risks, but as in any spine-related procedure, specific and sometimes catastrophic sequelae do occur.
Minimally invasive intradiscal procedures are designed to lower risks, but as in any spine-related procedure, specific and sometimes catastrophic sequelae do occur. Because these procedures penetrate the protective skin barrier and are placed near critical spinal structures, complications may include bleeding; nerve trauma; spinal cord injury; infection; and damage to vital structures, including vessels, nerve roots, vertebral endplates, the spinal cord, and organs.
Because these procedures penetrate the protective skin barrier and are placed near critical spinal structures, complications may include bleeding; nerve trauma; spinal cord injury; infection; and damage to vital structures, including vessels, nerve roots, vertebral endplates, the spinal cord, and organs. A potentially catastrophic type of infection from intradiscal procedure is discitis, or infection within the disc.
A potentially catastrophic type of infection from intradiscal procedure is discitis, or infection within the disc. Another potentially serious complication that may be related to infection is transverse myelitis, which involves inflammation of the spinal cord. It has been reported to occur in conjunction with chemonucleolysis.
Another potentially serious complication that may be related to infection is transverse myelitis, which involves inflammation of the spinal cord. It has been reported to occur in conjunction with chemonucleolysis. Allergic reaction can be caused by preoperative skin preparations, antibiotics, local anesthesia, and latex.
Allergic reaction can be caused by preoperative skin preparations, antibiotics, local anesthesia, and latex. Another complication of intradiscal procedures is bleeding in and around the spine, which can lead to epidural, subdural, muscular, and superficial hematomas.
Another complication of intradiscal procedures is bleeding in and around the spine, which can lead to epidural, subdural, muscular, and superficial hematomas. A common type of structural trauma associated with intradiscal procedures is a dural tear, or tearing of the outermost of the three meninges surrounding the spinal cord.
A common type of structural trauma associated with intradiscal procedures is a dural tear, or tearing of the outermost of the three meninges surrounding the spinal cord. Various intradiscal procedures use heat, leading to risk of heat damage to surrounding structures. One potentially serious complication that has been reported in conjunction with these high-heat intradiscal procedures is osteonecrosis, which may result in a painful fracture that requires surgery or percutaneous vertebral augmentation.
Various intradiscal procedures use heat, leading to risk of heat damage to surrounding structures. One potentially serious complication that has been reported in conjunction with these high-heat intradiscal procedures is osteonecrosis, which may result in a painful fracture that requires surgery or percutaneous vertebral augmentation.Introduction
Chronic spinal pain is one of the most common reasons why people seek out medical attention. Between 70% and 90% of the population will experience back pain at some point in their lives,1,2 but thankfully, 80% to 90% of these people will recover from acute episodes with conservative therapy within 3 months.3,4 But for those who continue to suffer with chronic pain, performing activities of daily living can be excruciating. This ongoing turmoil leads to frequent absence from work, loss of employment, opioid dependence, and ever-increasing disability. Spinal pain that originates from the disc, or intradiscal pain, is thought to be a major cause of severe and chronic pain in this population. Over the past 50 years, researchers have been attempting to identify and treat the various sources of spinal pain. In fact, many procedures have been developed to identify the source of one’s pain. Some of the more commonly performed diagnostic procedures include medial branch blocks, selective nerve root blocks, and discography. After the source of one’s pain is located, disc-related procedures may be considered to relieve the pain.
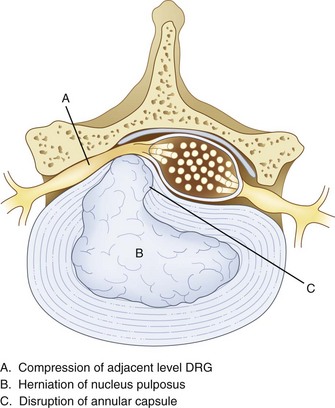
Surgery, typically involving laminectomy and microdiscectomy, has been shown to produce excellent clinical outcomes in patients with disc extrusion and neurologic deficits. However, patients with disc herniation smaller than 6 mm have fair or poor surgical outcomes.5 In addition, conventional open disc surgery can be associated with complications from general anesthesia, nerve damage, epidural fibrosis, chronic postoperative pain syndrome, and adjacent spinal instability. Intradiscal procedures aim to address key issues in disc pathology. Bulging, herniation, prolapse, and disc tears (internal disc disruption [IDD]) are some of the most common causes of spine pain. Disc bulging or herniation can cause pain, numbness, or weakness by mechanical irritation and compression of an adjacent nerve root. When this occurs, pain presents typically in a radicular pattern. Herniation is caused when fluid from the nucleus does not leak out of the annulus but bulges against it (Fig. 6-1). This can cause compression of nerve roots as well as stretching of annular fibers.
Various studies have shown that disc herniation disrupts the capsule around the dorsal root ganglion (DRG) (Fig. 6-1), allowing permeation of large molecules such as albumin, subsequently followed by edema, scarring, and inflammation.6 When an inflammatory reaction occurs, chemical irritation of an adjacent DRG or nerve root can occur, often causing axial or radicular pain.
Conversely, discogenic pain can occur without a disc bulge or prolapse. Such pain may originate from within the disc itself and is thought to be caused by tears and fissures in the annular layer of the disc.7 This type of pain is often called IDD or simply “discogenic pain” and is diagnosed by concordant pain on a provocative discography. IDD is believed to be caused by the lamellar fibers of the posterior annulus that are weaker than the anterior. This allows more flexion and extension of the lower lumbar spine but also predisposes the area to tears and herniations.8 Current thought suggests that discogenic pain may stem from irritation of small nociceptive nerve fibers within the outer third of the annulus fibrosis. The annulus is innervated by gray rami laterally, lateral ventral rami anteriorly, and sinuvertebral nerves posteriorly.9,10 People with nerve penetration deeper than the initial third of the annulus may be at higher risk for IDD pain problems.11 Additionally, as people age, the discs between the vertebrae lose water content and become brittle (degenerative disc disease).12 This can cause small tears in the annulus fibrosis, allowing some of the nucleus pulposus to leak out and causing irritation of the nociceptive fibers, local sympathetic nerves, and afferent nerve roots. Many irritating fluids are found within this space, including glycosaminoglycans and lactic acid. Pain signals also release irritating chemical mediators such as phospholipase A2, prostaglandin E, tumor necrosis factor α (TNF-α), nitric oxide (NO), substance P, and many others.13–15
Disc-related pain from a disc herniation or IDD can take many forms. Pain can be sharp and shooting with radiation along a dermatomal distribution with corresponding magnetic resonance imaging (MRI) disc findings, or it can be more complex with radiation into other structures (e.g., groin pain from L5–S1). As we learn about pain pathways from nerve roots, the adjacent DRGs, the spinal cord, and the brain, our understanding of the complex pain patterns experienced by patients is growing. For instance, research has shown that pain signals from the lower lumbar discs travel from lower DRGs along the sympathetic nerves of the gray rami communicans to higher DRGs. There may also be a convergence of lower level lumbar disc-related pain signals to the L2 DRG.16,17 This may explain the nondermatomal pain patterns that some patients experience. Some treatments are now being directed at the DRG as opposed to intradiscal for discogenic pain from IDD.18–20 Another treatment consideration is use of an investigational device from Spinal Modulation, Inc., which offers hardware that applies DRG neurostimulation for pain relief.21
Disc pathology can also cause localized pain; for instance, discogenic low back pain is often described as “a belt of pain across my low back.” People presenting with these types of pain symptoms are typically treated with conservative care, including physical therapy, chiropractic care, proper activity performance, over-the-counter medications, and antiinflammatory medications.22 If conservative care fails to provide relief, imaging is typically performed at this stage if it has not already been done. If the disc is believed to be responsible for the pain symptoms, epidural steroid injections are often performed based on the imaging and patient’s pain pattern. Other diagnostic and therapeutic modalities may be performed based on the patient’s pain complaints. These treatments may include epidural steroid injections, selective nerve root blocks, discography, facet-related interventions, and others. If a patient fails to respond to conservative therapy and pain interventions, minimally invasive intradiscal procedures or surgery is often considered.
Review of Intradiscal Procedures
Chemonucleolysis
A study by Hoogland and Scheckenback23 supporting the efficacy of low-dose chemonucleolysis showed good or excellent results in 19 of 22 patients with obvious preoperative cervical disc herniation with predominantly radicular pain over a follow-up period of at least 1 year. In one patient, a fair result was obtained, and in two patients, the symptoms were unchanged; one of these patients subsequently underwent discectomy and anterior cervical spine fusion. No intra- or postoperative complications were noted. Furthermore, the authors of a review of 105 consecutive cases of chymopapain chemonucleolysis for single-level lumbar disc herniation concluded “the advantages of chemonucleolysis are well recognized.”24 Of the studies included in the review, mean follow-up was 12.2 years (range, 10 to 15.3 years). Eighty-seven patients were assessed using the Oswestry Disability Questionnaire during follow-up. An excellent or good response occurred in 58 patients (67%); four patients (4.5%) had a moderate response but were only minimally disabled. The treatment failed in 25 patients (28.5%), and 21 of them went on to surgery within a mean of 5.2 months (range, 3 weeks to 12 months). In 15 patients (71%), disc sequestration or lateral recess stenosis was found. Five of the remaining six patients had a large disc herniation at surgery. Surgery resulted in a significant improvement in nine cases. Discitis after chemonucleolysis occurred in six patients (5.7%). The authors of the review further concluded that chemonucleolysis is a safe and effective alternative to surgery in the treatment of herniated lumbar intervertebral discs in appropriately selected patients. The authors also noted chemonucleolysis appears to be a minimally invasive technique that has little traumatic effect on surrounding structures and is safer than surgery. They also remarked chemonucleolysis requires less operative time and postoperative hospital stay than discectomy and can be performed under local anesthesia, although all of the patients in this series had general anesthesia.
Intradiscal Electrothermal Therapy (Annuloplasty)
Intradiscal electrothermal therapy was first developed by Saal and Saal9 in 2000. The underlying theory is that intradiscal pain is caused by irritation of small nerves within the annulus fibrosis and that destroying those nerves will relieve the patient’s pain.9,25 IDET is designed to address the pain from annular tears, something very few procedures are designed to do. This procedure was meant to be a less invasive and safer alternative to spinal fusion for discogenic pain.
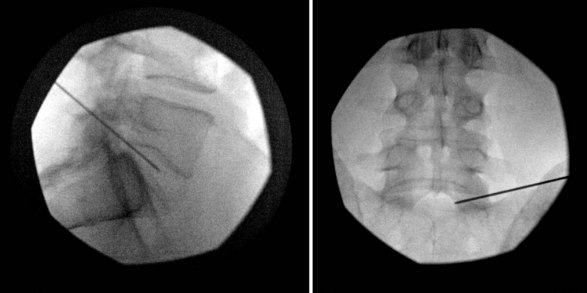
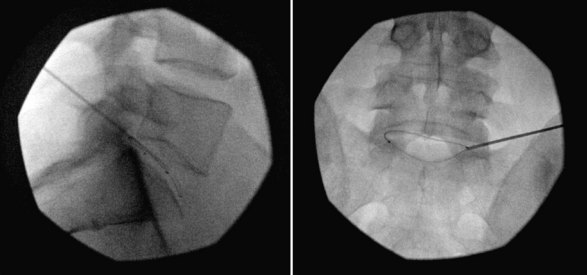
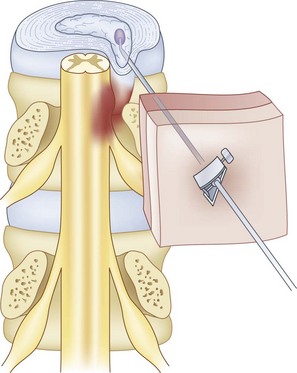
Intradiscal electrothermal therapy is performed under fluoroscopy and conscious sedation. It consists of a needle being inserted into the posterior lateral disc (Fig. 6-2) and a catheter being threaded into the disc (Fig. 6-3). This catheter has a special thermal coil that converts radiofrequency energy to heat. This coil is threaded through the disc in a circular fashion and is positioned adjacent to the tear or nerve distribution area in the posterior lateral aspect of the disc.3 Proper positioning is the key to a good result. The catheter should span the entire lesion and should be positioned near the pedicles on each side of the posterior annulus.26,27 Subsequently, the tip is heated, through radiofrequency, to 90°C, modifying the collagen matrix, and breaking the triple helix bonds, causing thickening, contraction, and a 10% decrease in disc volume.27 Heat is transferred through the coil to the surrounding tissues by conduction. The surrounding vascular areas act as a heat sink, preventing damage to tissues beyond the desired region.9,27 The heating seals the tear if present and causes thermal injury to all intradiscal nerve fibers in close proximity, effectively destroying pain transmission.27,28
The heating, thickening, and contraction process in IDET is believed to enhance the structural integrity of the disc, stabilizing intradiscal fissures and decreasing new nerve growth, although as of yet there are no studies to support this thought.9,26 However, studies using an electron microscope have been able to show visible shrinking of collagen molecules, giving credibility to this theory.27
Biacuplasty
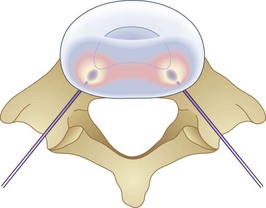
Biacuplasty is a newer technique developed by Baylis Medical to address intradiscal back pain produced by irritation of intradiscal nerve fibers.28,29 This procedure is considered by many to be safer than IDET because its bipolar design system allows for internal cooling of the radiofrequency transfer of heat between electrodes. The bipolar design pinpoints the area of treatment and prevents outside structures from being heated. Cooling is accomplished by running cool sterile water through an adjacent channel inside the probe. This prevents tissue closest to the probes from being scorched and protects spinal nerves outside the desired region from being heated by conduction through the hot probe. If needed, this system can treat an area of up to 4 cm.28,29 Unlike IDET, biacuplasty uses a lower temperature, at 40° to 50°C. This range is sufficient for thermoregulating nerves but may be insufficient for repairing annular tears, thus limiting its scope. The procedure consists of two radiofrequency probes placed under fluoroscopy posterior laterally into the annulus fibrosis with one on each side.29,30 Because the probes only need to be placed opposite each other, another advantage to this procedure is how easy it is to position the probes into the appropriate place. Whereas IDET requires threading a catheter and using fluoroscopy to make sure it is near the desired area, biacuplasty uses two adjacent probes coming from opposite sides of the vertebrae toward each other (Fig. 6-4), allowing for much easier placement.29,31
Subsequently, current is passed between the two probes, causing them to warm. The heat destroys the surrounding annular nerve fibers, and the probes are removed. Karaman et al30 and other physicians performing this technique recommend that patients wear an external stabilizing back brace for 6 to 8 weeks after the procedure to prevent injury to the area before stabilization of the disc can occur.29
Nucleoplasty
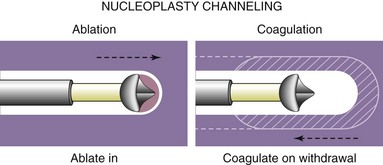
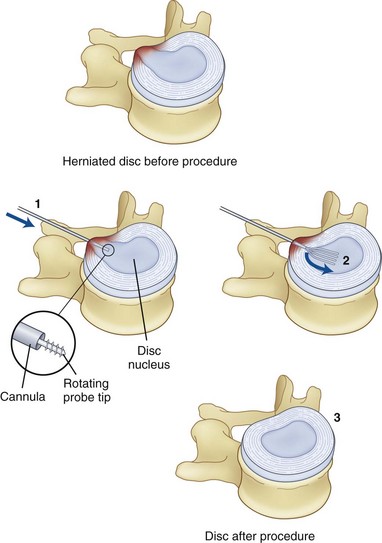
Another intradiscal technique is nucleoplasty. This procedure is fundamentally different than IDET and biacuplasty in that it treats disc herniation. It was first used in 2000 and is effective in mild to moderate herniation of discs.3,32 Herniation is problematic in that it not only causes stretching of intradiscal fibers but also can compress adjacent nerve roots, causing radicular pain. Nucleoplasty may be the most commonly used form of disc decompression. The procedure differs from IDET and biacuplasty in that the wand is placed into the nucleus pulposus and not into the annulus. It also uses radiofrequency waves. Heating the gel-like matrix of the nucleus to about 40° to 70°C, it breaks the bonds and vaporizes the matrix. The vaporized matrix gases are then removed through the probe. The wand is moved back and forth throughout the gel, creating channels that allow for a creation of space (Fig. 6-5). The vaporization and retraction of matrix causes a vacuum in those channels that produces subsequent contraction in the rest of the disc.32 This central vacuum is believed to allow space for the herniated disc section to contract back into the disc, consequentially relieving compression on the adjacent nerve roots. In total, about 1 mL of gel is removed (Fig. 6-6).32,33 Because the probe acts within the nucleus pulposus, it is relatively unlikely to cause injury to surrounding structures. Although existing studies are limited and are mostly observational, level II evidence exists for pain relief and improved functional outcomes.33,34 Available studies show nucleoplasty to be an encouraging procedure with few complications, decrease in pain, and rapid recovery time postoperatively.34 Li et al35 presented a study done in England with 126 patients undergoing cervical nucleoplasty, finding favorable results in 83% of patients. The only reported complication was a broken wand.
Disc Dekompressor
The Disc Dekompressor, created in 2002, is a handheld device also designed to reduce herniated discs. It consists of a disposable helical probe with an outer shell and an inner rotating probe (Fig. 6-7). The Dekompressor is placed posterior laterally into the nucleus pulposus under fluoroscopic guidance (Fig. 6-8). The rotating probe mines out a designated amount of matrix, creating channels similar to those created in the nucleoplasty procedure (Figs. 6-9 and 6-10).36,37 These channels retract, causing contraction of the disc and effectively shrinking the herniated portion. This releases compression of the nerve root and adjacent annular nerves as in nucleoplasty. Studies are limited with this new technique. A review of four studies done in 2009 by Singh et al38 found that all of the studies produced favorable results and were able to achieve level III evidence. It was thought that the studies could have been more conclusive if they were nonrandomized and nonblinded and entailed longer follow-up times. Alò et al37 studied 50 patients and found 88% satisfaction at 1-year follow-up with a 78.6% decrease in analgesic intake. No complications were reported in their study. Although the existing literature on the Disc Dekompressor is promising, much more research needs to be completed.
Percutaneous Laser Disc Decompression
Percutaneous laser disc decompression was used by Choy and Ascher in 198639 and has made a tumultuous entry into the medical field. Similar to many other intradiscal procedures, data are lacking in prospective randomized controlled studies.38,40 However, despite the paucity of studies, the procedure has been consistently performed with success for more than 2 decades. Since its inception, a variety of lasers have been used, including yttrium aluminium garnet (YAG), potassium-titanyl-phosphate, holmium, argon, and CO2. However, most physicians prefer the Nd:YAG (neodymium-doped YAG) laser because of its optimal wavelength for PLDD and applicability to other types of commonly performed laser procedures.40,41
Percutaneous laser disc decompression is similar in approach to other intradiscal procedures and some use the procedure for disc herniations, but others use the procedure to perform laser annuloplasty for discogenic pain and disc tears. It starts with sterile technique, and a needle is introduced under direct fluoroscopy into the nucleus pulposus posterior laterally. A laser fiber is advanced through the needle, extending 1 cm beyond the tip (Figs. 6-11 and 6-12).41 Laser pulses of 20 to 40 W deliver 1000 to 2000 J of energy into the nucleus, converting light into heat, vaporizing the water, and relieving intradiscal pressure.41,42 In addition to the vaporization, the laser also destroys the chemical bonds within the matrix, causing protein denaturation and a definitive change that will not allow the nucleus to absorb further water in the future. This prevents the disc from swelling back to its previous size and again causing the herniation that the procedure sought to eliminate39,43 After the procedure is finished, the patient is monitored for 30 minutes and released.
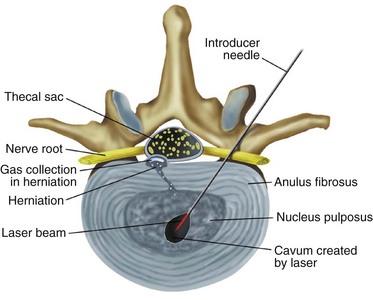
Fig. 6-11 Percutaneous laser disc decompression.
(From Brouwer P, Schenk B: Percutaneous intradiscal therapies. In Pope TL Jr, editor. Imaging of the musculoskeletal system, Philadelphia, 2008, Elsevier.)
A problem with this procedure is that laser thermal energy is difficult to control. Temperatures can reach up to 250°C and have the potential to burn adjacent tissues, causing scarring and inflammatory reactions.42,43 Conversely, problems also seem to arise when studies fail to use laser intensity strong enough to denature the matrix, succeeding only in vaporizing the water. If the matrix is not changed, then the nucleus over time will reabsorb the water volume lost in the procedure, and the herniation will return.
Initial studies in PLDD by Choy39 reported a 91% success rate at 1-year follow up, and Gupta et al42 reported an 85% success rate as far as a 7-year follow up for their study of 40 patients. Despite favorable clinical trials, however, strong evidence of the efficacy of this procedure is still missing.43 Gibson and Waddell44 performed one of the most extensive reviews of the literature available and found that the evidence for the efficacy of surgical correction for disc prolapse is still unresolved. Through their review of more than 40 articles, only level II evidence could be demonstrated.
Methylene Blue
Methylene blue, used widely as a histologic stain, is considered by some to have a viable role in the treatment of discogenic pain. This is attributed to its weak neurolytic effects and ability to block synthesis of NO, which has been implicated in the inflammatory processes associated with disc pain and degeneration.45,46
A 72-patient Chinese study in the injection of methylene blue to relieve discogenic pain called the treatment “revolutionary”46 and “extraordinary”47 and yielded highly encouraging results. According to the authors of this trial, methylene blue proved to be a safe, effective, and minimally invasive method with significantly better outcomes over placebo for the treatment of intractable and incapacitating discogenic low back pain.48 Patients in a methylene injection group showed a mean reduction in pain measured by numerical rating scale of 52.50, a mean reduction in Oswestry Disability Index (ODI) scores of 35.58, and satisfaction rates of 91.6% compared with 0.70%, 1.68%, and 14.3%, respectively, in a placebo treatment group (P <.001, P <.001, and P <.001, respectively). No adverse effects or complications were found in the group of patients treated with intradiscal methylene blue injection.
The efficacy of methylene blue for discogenic back pain is likewise supported by a preliminary report of a clinical trial.49 According to the authors, 24 patients with chronic discogenic low back pain who met criteria for lumbar interbody fusion surgery were treated instead with an intradiscal injection of methylene blue for pain relief; of these patients, 21 (87%) reported a disappearance or marked alleviation of low back pain and experienced a definite improvement in physical function. A statistically significant and clinically meaningful improvement in the changes in ODI and the Visual Analog Scale scores were obtained in the patients with chronic discogenic low back pain after the treatment.
Tumor Necrosis Factor α Inhibitors
Although anti-TNF-α medications are frequently prescribed for low back pain, very few randomized controlled clinical trials back their effectiveness in this capacity. For example, a double-blind, placebo-controlled pilot study found a single low dose of intradiscal etanercept did not seem to be an effective treatment for chronic radicular or discogenic low back pain.50 Six patients received 0.1, 0.25, 0.5, 0.75, 1.0, or 1.5 mg etanercept intradiscally in pain-generating discs, and in each escalating dose group of six patients, one patient received placebo. Neurologic examinations and postprocedure leukocyte counts were performed in all patients at 1-month follow-up visits. In patients who experienced significant improvement in pain scores and function, follow-up visits were conducted 3 and 6 months after the procedure. At 1-month follow-up, no differences were found for pain scores or disability scores between or within groups for any dose range or subgroup of patients. Furthermore, only eight patients remained in the study after 1 month and elected to forego further treatment. No complications were reported, and no differences were noted between pre- and postprocedure leukocyte counts. Although the study failed to find efficacy in TNF-α–blocking medications, no adverse effects were reported.
However, a study in pigs found these two anti-TNF-α medications prevented the reduction of nerve conduction velocity and also seemed to limit nerve fiber injury, intracapillary thrombus formation, and intraneural edema formation in the animals’ spines.51 The authors of the study further concluded that TNF-α is clearly involved in the basic pathophysiologic events leading to structural and functional changes in the nerve root.
Contraindications
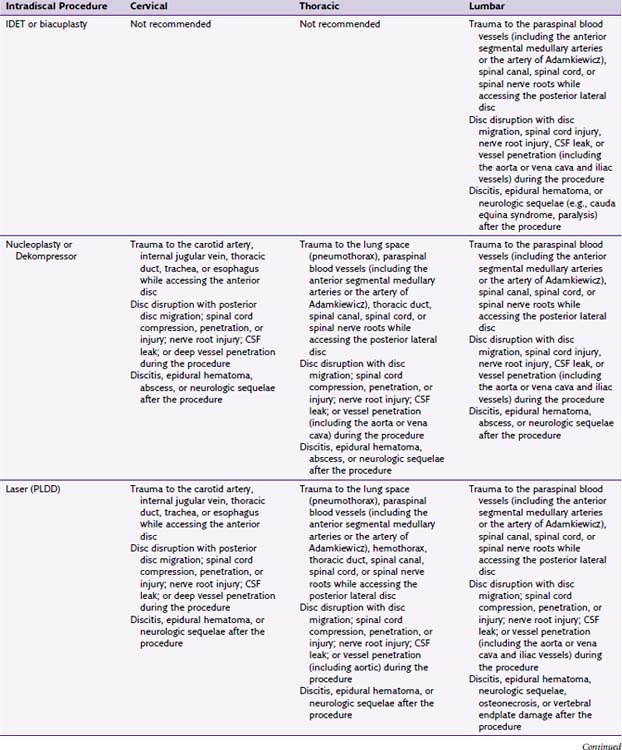
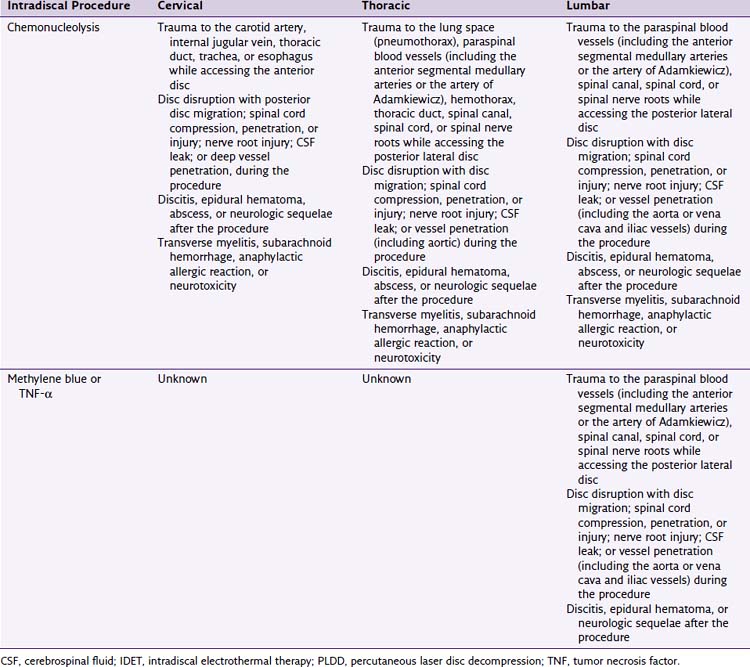
Intradiscal procedures are similar to most other invasive procedures in that they are absolutely contraindicated in patients who are pregnant, cannot follow instructions, have an active infection, or have a bleeding disorder that prevents surgery.4
Relative contraindications include the presence of other sources of pain such as spinal stenosis, spinal fracture, spondylolisthesis, or spinal tumors. Patients may also be excluded if they have problems at more than two levels or have significant comorbidities or psychiatric problems.37–39 Morbid obesity can also be a relative contraindication because it is an impediment to accomplishing the procedure. Instrumentation comes in specific sizes and may not be of adequate length to place probes in the desired area. Additionally, IDET and biacuplasty are contraindicated in cervical and thoracic disc spaces because the discs are not large enough to accommodate the catheters used in theses procedures.52 Nucleoplasty and Disc Dekompressor are contraindicated in patients with greater than 50% loss of vertebral height or severe disc degeneration because further reduction of the disc risks more harm than benefit.38,53
Selected Complications
Infection
Because infection is a known risk of intradiscal procedures, the practitioner should endeavor to lower the incidence of topical and systemic infection in the patient. Prophylactic antibiotics may be given intravenously (e.g., 1 g of cefazolin), particularly in intradiscal procedures that involve injection or insertion of foreign materials into the body.54 Beyond administration of antibiotics, screening patients for topical infections, immunocompromised status, and systemic conditions, the most important measure against infection is meticulous surgical technique. To achieve this, iodine or chlorhexidine should be used as a surgical preparation with a wide area of the back prepared because a variety of angles of approach may be used. In many intradiscal procedures, sterile drapes should be placed over the patient, and measures such as a surgical scrub and use of masks and gowns should be incorporated when appropriate. One must remember that the disc is the largest structure in the body without its own circulation and therefore has an increased risk of severe and catastrophic infections.
Discitis
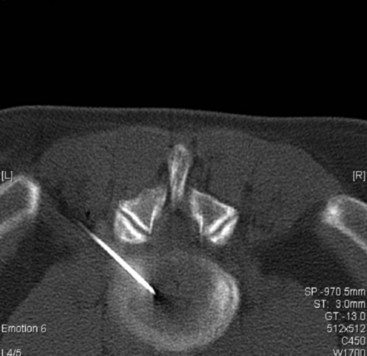
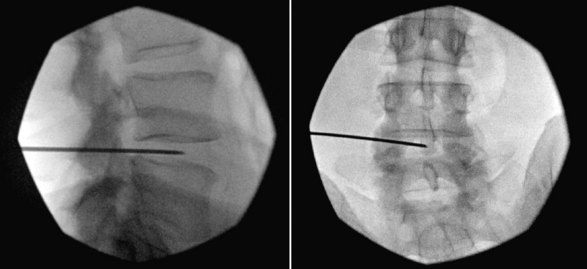
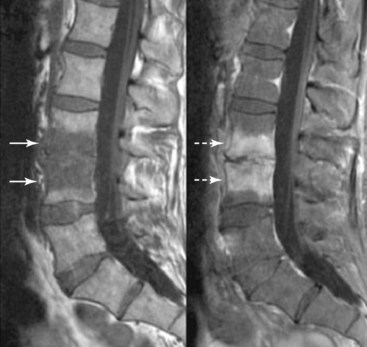
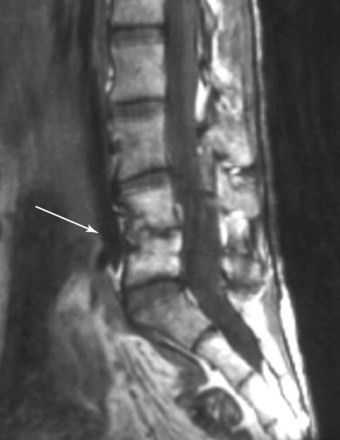
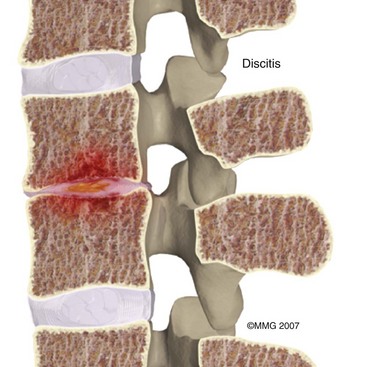
A potentially catastrophic type of infection from intradiscal procedure is discitis (Figs. 6-13 to 6-15), or infection within the disc. It presents as worsening back pain after an intradiscal procedure and is most commonly caused by Staphylococcus aureus.55,56 Discitis can also present with nausea, vomiting, fever, chills, malaise, or any other complaints commonly associated with infections. The possibility of intradiscal infection is why administration of prophylactic preoperative antibiotics is currently the standard of care and why discography is typically performed with intradiscal antibiotic administration.56,57 Classen et al58 prospectively monitored timing of antibiotic prophylaxis and development of surgical wound infections in 2847 patients undergoing surgical procedures. Among patients who received antibiotics up to 24 hours before surgery, 2 hours before surgery, 3 hours after surgery, and more than 3 hours after surgery, those who received antibiotics 2 hours before surgery had the lowest rates of subsequent surgical wound infections. Some studies recommend intradiscal injection of antibiotics after procedures, but this recommendation is controversial.4 Before the commencement of preoperative and intradiscal antibiotics, vertebral osteonecrosis and epidural abscesses were reported. Thankfully, most infectious complications can be avoided with meticulous use of sterile surgical technique. Unfortunately, discitis can also be aseptic, the result of inflammatory changes in the disc secondary to heat damage. This consequence may be prevented with precision of location of procedure and is also better controlled in techniques such as biacuplasty in which there is an internal cooling system.
Transverse Myelitis
Another potentially serious complication that may be related to infection is transverse myelitis, which has been reported to occur in conjunction with chemonucleolysis. Transverse myelitis, which involves inflammation of the spinal cord caused by a range of etiologies, may result in diminished or absent sensation below the injury. It may arise as a complication from an intradiscal procedure as a result of infection, autoimmune reaction, or thrombosis of spinal arteries. A case report describes mild paraplegia secondary to transverse myelitis in a 48-year-old male patient who underwent chemonucleolysis for disc degeneration and radiculopathy.59 However, no specific source of infection was found in his case, which suggested that the patient’s transverse myelitis may have been caused by an autoimmune reaction or a thrombosis of the anterior spinal artery.
Allergic Reactions
Most intradiscal procedures are done under conscious sedation, minimizing risks for sedation-related side effects. Allergic reaction can be caused by preoperative skin preparations, antibiotics, local anesthesia, and latex. Because the patient may be unaware of any allergies surrounding these products, the physician should remain vigilant for development of allergic sequelae during the procedure. For example, local anesthetics or antibiotics are common elicitors of adverse reactions with clinical symptoms such as anaphylaxis with tachycardia; hypotension; and subjective feelings of weakness, heat, or vertigo.60 Latex can also produce allergic reactions as serious as anaphylaxis.60,61 Physicians and staff should always be vigilant about monitoring the patient for deleterious events. Local pain is frequently reported at the catheter or probe insertion site, but most patients remain comfortable throughout the entire procedure. Occasionally, muscle spasm can also occur but is usually short lived.
Bleeding or Spinal Hematoma
Another feared but infrequently reported complication of intradiscal procedures is bleeding in and around the spine, which can lead to epidural, subdural, muscular, and superficial hematomas. Although rare, spinal hematomas can occur because needles pass precariously close to spinal nerve roots and blood vessels. Subarachnoid hemorrhage is an infrequent and unexplained finding that has been reported with a chemonucleolysis patient. A report of clinical and postmortem findings in a 42-year-old man who died 5 days after chemonucleolysis at the L4–5 and L5–S1 disc spaces found the predominant histologic abnormality was a severe inflammatory arteritis of a medium-sized artery at the upper cervical level with disruption of the vessel wall. The potential causative role of chymopapain in this situation and the correlation of a vascular basis for many of the complications found after inadvertent intrathecal chymopapain injection remain topics of discussion.62
Dural Puncture and Spinal Cord or Nerve Root Damage
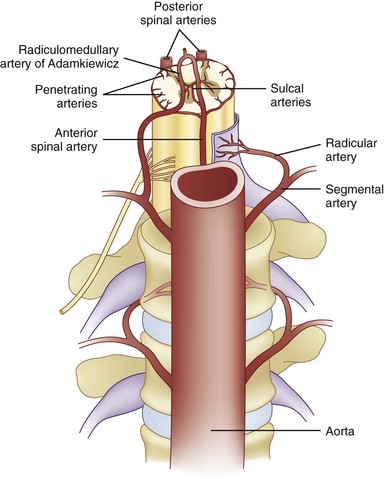
Because of the location of the procedure, the risk of puncturing the dura and damage to the thecal sac is also possible. One case of cauda equina syndrome was reported with IDET caused by improper needle and catheter placement by the physician.63 With PLDD, there was one reported case of subacute cauda equina syndrome from an internist performing PLDD on a patient with lumbar spinal stenosis.64 Cauda equina syndrome was resolved after the patient underwent a hemilaminectomy and discectomy 2 months later by a neurosurgeon. All of these problems are usually avoided by use of fluoroscopy during the procedure and being performed by one well versed in interventional techniques, spinal anatomy, and spinal fluoroscopy. A thorough knowledge of vertebral anatomy and proper technique drastically minimize these risks. All five procedures use a posterolateral spinal approach in the lumbar spine, and many believe this is the best angle to use to minimize injury. However, there is still a risk of injuring nerve roots and vessel damage, including the radiculomedullary artery of Adamkiewicz (Fig. 6-16). In addition, patients are also kept under light sedation so they can tell the physician if they experience any radicular pain. Finally, because of the location of the procedure, the potential for intrathecal placement of the device is possible along with spinal headache, but as of yet, no studies have reported patients with this complication. If any of the procedures are attempted in the cervical or thoracic region in which IDET is contraindicated, new risks are associated with the placement of the surgical instrumentation via the typically used paramedian anterior approach.
Damage to Surrounding Structures
A more common complication of intradiscal procedures is structural damage to tissues, such as damage to the endplate, a structure that plays a crucial role in nutritional supply to the intervertebral disc. When structural damage from an intradiscal procedure does occur, it is normally caused by complications from the instruments used in these procedures. One common instrument-related complication is catheter or probe breakage. Each intradiscal procedure uses instrumentation with fine-tipped devices. Poor positioning or difficult anatomy can force these tips against the edges of the vertebrae or the posterior or anterior longitudinal ligaments, causing trauma. Most commonly, this results in the need to surgically remove the probe from the disc. Li et al36 presented one case in which a probe fractured off during nucleoplasty. The probe was unable to be retrieved, and it was therefore left inside the patient. No subsequent complications were reported from the patient during follow-up. However, current guidelines do not recommend leaving the probe tip unless removal proves to be more dangerous than leaving the tip in place. Additionally, the IDET catheter is subject to damage of its insulation with frequent repositioning. Therefore, if the catheter must be repeatedly moved, caution should be taken, and at any sign of kinking, the catheter should be removed and replaced. Three studies reported breakage of the Disc Dekompressor during a procedure; however, in each instance, the part was able to be successfully removed surgically with no additional complications.4 Physicians performing these procedures should be trained by manufacturers to correctly use the device. Likewise, performance of the technique should be done as directed to minimize the potential for damage to the probes and subsequent patient injury. Multiplanar fluoroscopic views should be used throughout the procedure to ensure positioning and advancement.
Osteonecrosis and Heating Complications
Biacuplasty, IDET, nucleoplasty, and PLDD all use heat as part of the procedure, leading to a risk of heat damage to surrounding structures. Osteonecrosis is a potentially serious complication that has been reported in conjunction with these high-heat intradiscal procedures. Osteonecrosis of the spine may result in a painful fracture that requires further intervention in the form of surgery or percutaneous vertebral augmentation to stabilize the injury and relieve the pain.65
Intradiscal electrothermal therapy and PLDD in particular have been reported to expose cortical and cancellous bone to temperatures well within the range reported to induce necrosis.66,67 IDET presents the greatest risk of producing this outcome if the physician is not careful with exact catheter placement. Because the heating element in IDET extends 5 cm, improper placement can position this heating element near spinal nerve roots. Proper orientation on radiography is crucial. Furthermore, in IDET, nucleoplasty, and PLDD, the needle used to place the catheter is not insulated. Therefore, care must be taken to ensure that the probe is not touching the needle when the heating element is activated or the needle will heat risking damage to structures outside the desired area along the needle path. Biacuplasty and the injectable therapies may be the safest of the techniques with regard to this problem. Biacuplasty’s bipolar water cooling system prevents elements outside the desired area from being heated by conduction. Fortunately, in all heat-generating procedures, the nucleus of the disc itself has high water content and acts as a heat sink, absorbing much of the heat. If done properly, the heat is absorbed and presents minimal risk to adjacent structures external to the disc.
Nucleoplasty carries with it an added risk of wand failure because it vaporizes the matrix and removes the gases. Rarely, chunks of the matrix may occlude the probe, causing blockage and leading to failed completion of the procedure.13 Another nucleoplasty complication reported by Cohen et al68 was an incidence (15%) of minor new-onset neurologic symptoms occurring in patients, including numbness and twitching in the distal lower extremities. A total of 4% of patients reported new-onset low back pain different than previous, but all new low back pain resolved within 2 weeks.
Biacuplasty studies reported minimal complications of 1 to 2 days of low back pain after the procedure that resolved spontaneously.29,30 Many believe that the increased pain is related to inflammatory changes secondary to thermal injury, and pain resolves with the passing of the inflammation. One incidence of vasovagal reaction was also noted. In this instance, the patient was treated with fluid support and released the same day.30 Some studies have reported recurrence of pain over time.
Percutaneous laser disc decompression (PLDD) carries with it a unique set of complications, including safety risks to the physician and staff performing the procedure. Safety goggles must be worn at all times by the physician, patient, and other staff. If pointed toward the eyes, the laser can cause significant retinal damage. Many facilities and hospitals using this procedure have a designated laser safety technician who is in charge of ensuring that safety procedures are observed.40,43 Likewise, all physicians using these lasers should be trained by the manufacturers on how to avoid errors that can be costly to patients and staff. All lasers use high levels of heat, and care must be taken to prevent adjacent nerve root injury. One study reported four cases of thermal nerve root damage because of accidental heating of the cannula by CO2 laser.69
Complication Prevention
Recommendations to avoid potential complications in intradiscal procedures (Table 6-1) include meticulous observation of aseptic technique and administration of preprocedural antibiotics to lower the incidence of postprocedural infection and extensive knowledge of relevant anatomy to promote accurate placement of instruments and injected medications and to avoid structural damage of the spine and nervous tissues. If a patient appears to be experiencing a complication after an intradiscal procedure, the practitioner should quickly consider its possible etiologies and in particular rule out neurologic insult and infection. A thorough neurologic examination should be performed and documented. Typically, an MRI or computed tomography scan with contrast is used to assess structural damage and may be beneficial in identifying edema or infection in late cases. If neurologic insult is suspected, surgical consultation should be obtained immediately. If an infection is suspected, hospitalization is recommended with an infectious disease and surgical consultation. If a complication is recognized early in its course, more serious insult may be avoided by early recognition and intervention when appropriate.
1 Kennedy M. IDET: A new approach to treating lower back pain. WMJ. 1999;98:18-20.
2 Andersson GBJ. Epidemiological features of chromic low back pain. Lancet. 1999;354:581-585.
3 Raj PP, Lou L, Erdine S, et al. Percutaneous therapeutic procedures for disc lesions. In: Ruiz-Lopez R, Pichot C, editors. Interventional pain management: image guided procedures. Philadelphia: Saunders Elsevier; 2008:539-558.
4 Fenton D, Czervionke L. Intradiscal electrothermal therapy (IDET). In: Fenton D, Czervionke L, editors. Image guided spine intervention. Philadelphia: Saunders; 2003:257-284.
5 Carragee EJ, Kim DH. A prospective analysis of magnetic resonance imaging findings in patients with sciatica and lumbar disc herniation. Correlation of outcomes with disc fragment and canal morphology. Spine. 1997;22(14):1650-1660.
6 Murata Y, Rydevik B, Takahashi K, et al. Incision of the intervertebral disc induces permeability of the dorsal root ganglion capsule. Spine. 2005;30(15):1712.
7 Maroon J. Current concepts in minimally invasive discectomy. Neurosurgery. 2002;51(5):137-145.
8 Peng B, Hou S, Wu W, et al. The pathogenesis and clinical significance of a high intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J. 2006;15(5):583-587.
9 Saal JS, Saal JA. Management of chronic discogenic low back pain with a thermal intradiscal catheter: a preliminary report. Spine. 2000;25(3):382-388.
10 Cohen SP, Larkin TM, Barna SA, et al. Lumbar discography: a comprehensive review of outcome studies, diagnostic accuracy, and principles. Reg Anesth Pain Med. 2005;30:163-184.
11 Deramond H, Debussche C, Pruvo JP, Galibert P. La vertebroplastie. Feuillets Radiol. 1990;30:262-268.
12 Nachemson A. Measurement of intradiscal pressure. Acta Orthop Scand. 1959;28:269-289.
13 Singh V, Derby R. Percutaneous lumbar disc decompression. Pain Physician. 2006;9(2):139-146.
14 Freemont A, Peacock T, Goupille P, et al. Nerve in-growth into diseased intervertebral disc in chronic low back pain. Lancet. 1997;350:178-181.
15 Jimbo K, Park JS, Yokosuka K, et al. Positive feedback loop of interluken-1 beta upregulation production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine. 2005;2(5):589.
16 Morinaga T, Takahashi Y, Yamagata M, et al. Sensory innervation of the anterior portion of the lumbar intervertebral disk in rats. Spine. 1996;21:1848-1851.
17 Ohtori S, Takahashi Y, Takahashi K, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24:2295-2299.
18 Oh WS, Shim JC. A randomized controlled trial of radiofrequency denervation fo the ramus communicans nerve for chronic discogenic low back pain. Clin J Pain. 2004;20(1):55-60.
19 Simopoulos T, Malik A, Sial K, et al. Radiofrequency lesioning of the L2 ramus communicans in managing discogenic low back pain. Pain Physician J. 2005;8:61-65.
20 Menedez R, Bailey S, Paine G, et al. Evaluation of the L2 spinal nerve root infiltration as a diagnostic tool for discogenic low back pain. Pain Physician J. 2005;8:55-59.
21 Caraway D, Deer T, Levy R: Results from a prospective, multicenter dorsal root ganglion (DRG) stimulation clinical trial. Presentation at the North American Neuromodulation Society meeting, Las Vegas, Nevada, December 2010.
22 Benzon HT, Rathmell J, Wu C, et al. Intradiskal procedures for the management of low back pain. In: Gretter B, Mekhail N, editors. Raj’s practical management of pain. ed 4. Philadelphia: Mosby Elsevier; 2008:1112-1117.
23 Hoogland T, Scheckenbach C. Low-dose Chemonucleolysis combined with percutaneous nucleotomy in herniated cervical disks. J Spinal Disord. 1995;8(3):228-232.
24 Poynton A, O’Farrell D, Mulcahy N, et al. Chymopapain chemonucleolysis: a review of 105 cases. J R Coll Surg Edinb. 1998;43:407-409.
25 Broadkey JS, Miyazaki Y, Ervin FR, et al. Reversible heat lesions with radiofrequency current. J Neurosurg. 1964;21:49-53.
26 Waldman S. Intradiscal electrothermal annuloplasty, percutaneous laser diskectomy. In: Whitworth M, editor. Pain management. Philadelphia: Saunders; 2007:1484-1499.
27 Intradiscal electrothermal (IDET) therapy training course syllabus. Menlo Park, CA: Oratec Interventions Inc., 2006.
28 Deen HG, Fenton DS, Lamer TJ. Minimally invasive procedures for disorders of the lumbar spine. Mayo Clinic Proc. 2003;78(10):1249-1256.
29 Kapural L, Cata J, Narouze S. Successful treatment of lumbar discogenic pain using intradiscal biacuplasty in previously discectomized disc. Pain Pract. 2009;9(2):130-134.
30 Karaman H, Tufek A, Kavak GO, et al. 6 month results of transdiscal biacuplasty on patients with discogenic low back pain: preliminary findings. Int J Med Sci. 2011;8(1):1-8.
31 Benzon H, Raja S, Molloy R, et al. Intradiscal techniques: intradiscal electrothermal therapy and nucleoplasty. In: Walega D, editor. Essentials of pain medicine and regional anesthesia. ed 2. Philadelphia: Elsevier Churchill Livingstone; 2005:485-493.
32 Singh V, Piryani C, Liao K, Nieschultz S. Percutaneous disc decompression using Coblation (Nucleoplasty) in the treatment of chronic discogenic pain. Pain Physician. 2002;5:250-259.
33 Gerges F, Lipsitz S, Nedeljkovic S. A systematic review of the effectiveness of nucleoplasty procedure for discogenic pain. Pain Physician. 2010;13(1):117-132.
34 Yakovlev A, Tamimi M, Liang H, Eristavi M. Outcomes of percutaneous disc decompression utilizing nucleoplasty for the treatment of chronic discogenic pain. Pain Physician. 2007;10(1):319-327.
35 Li J, Yan D, Zhang Z. Percutaneous cervical nucleoplasty in the treatment of cervical disc herniation. Eur Spine J. 2008;17(1):1664-1669.
36 Stryker Corp: Dekompressor for clinicians. Interventional Pain, Kalamazoo, MI, Stryker Corp, 2004.
37 Alò KM, Wright RE, Sutcliffe J, Brandt S. Percutaneous lumbar discectomy: one year follow-up in an initial cohort of fifty consecutive patients with chronic radicular pain. Pain Pract. 2005;5(2):116-124.
38 Singh V, Manchikanti L, Benyamin R, et al. Percutaneous lumbar laser disc decompression: a systematic review of current evidence. Pain Physician. 2009;12:573-588.
39 Choy DS, Ascher PW, et al. Percutaneous laser disc decompression: a new therapeutic modality. Spine. 1992;17:949-956.
40 Schenk B, Brouwer PA, Van Buchem MA. Experimental basis of percutaneous laser disc decompression (PLDD): a review of literature. Lasers Med Sci. 2006;21:245-249.
41 Patel J, Singh M. Laser discectomy. eMedicine J. 3(6), 2002.
42 Gupta AK, Bodhey NK, Jayasree RS, et al. Percutaneous laser disc decompression: clinical experience at SCTIMST and long term follow up. Neurol India. 2006;54(2):164-167.
43 Brouwer PA, Peul WC, Brand R, et al. Effectiveness of percutaneous laser disc decompression versus conventional open discectomy in the treatment of lumbar disc herniation; design of a prospective randomized control trial. BMC Musculoskelet Disord. 2009;10:49.
44 Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane review. Spine. 2007;32:1735-1747.
45 Ballantyne J, Fishman S, Rathmell J. Bonica’s management of pain. Philadelphia: Lippincott Williams & Wilkins; 2009.
46 Mangrum S. A cure for back pain? Review of intradiscal methylene blue study. Back Exercise Doctor. http://www.backexercisedoctor.com/journal/2011/1/17/a-cure-for-back-pain-review-of-intradiscal-methylene-blue-st.html, 2011. Available at
47 Ballantyne J, Fishman S, Rathmell J. Bonica’s management of pain. Philadelphia: Lippincott Williams & Wilkins; 2009.
48 Peng B, Pang X, Wu Y, et al. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain. 2010;149(1):124-129.
49 Peng B, Zhang Y, Hou S, et al. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16(1):33-38.
50 Cohen S, Wenzell D, Hurley R, et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology. 2007;107(1):99-105.
51 Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine (Phila Pa 1976). 2001;26(8):863-869.
52 Derby R, Eek B, Chen Y, et al. Intradiscal electrothermal annuloplasty (IDET): A novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:82-88.
53 ArthoCare Corporation DISC nucleoplasty overview. Available at http://www.Nucleoplasty.com/dphy.aspx?s=0201
54 Ruiz-Lopez R, Pichot C. Vertebroplasty. In Interventional pain management, ed 2, Philadelphia: Saunders; 2008:560.
55 Guyer RD, Collier R, Stith WJ, et al. Discitis after discography. Spine. 1998;13:1352-1354.
56 Silber JS, Anderson DG, Vaccaro AR, et al. Management of postprocedural discitis. Spine. 2002;2:279-287.
57 Rathmell JP, Lake T, Ramundo MB. Infectious risks of chronic pain treatments: injection therapy, surgical implants and intradiscal techniques. Reg Anesth Pain Med. 2006;31:346-352.
58 Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281-286.
59 Eguro H. Transverse myelitis following chemonucleolysis. Report of a case. J Bone Joint Surg Am. 1983;65(9):1328-1330.
60 Ring J. Anaphylactic reactions to local anesthetics. Chem Immunol Allergy. 2010;95:190-200.
61 Heitz JW, Bader SO. An evidence-based approach to medication preparation for the surgical patient at risk for latex allergy: is it time to stop being stopper poppers? J Clin Anesth. 2010;22(6):477-483.
62 Cusick J, Khang-Cheng H, Schamberg J. Subarachnoid hemorrhage following chymopapain chemonucleolysis. J Neurosurg. 1987;5(66):775.
63 Hisa A, Isaac K, Katz J. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2000;55:320-322.
64 Epstein NE. Laser-assisted diskectomy performed by an internist resulting in cauda equine syndrome. J Spinal Discord. 1999;12(1):77.
65 Ma R, Chow R, Shen FH. Kummell’s disease: delayed post-traumatic osteonecrosis of the vertebral body. Eur Spine J. 2010;19(7):1065-1070.
66 Scholl B, Theiss S, Lopez-Ben R, Kraft M. Vertebral osteonecrosis related to intradiscal electrothermal therapy: a case report. Spine (Phila Pa 1976). 2003;28(9):E161-E164.
67 Djurasovic M, Glassman S, et al. Vertebral osteonecrosis associated with the use of intradiscal electrothermal therapy: a case report. Spine (Phila Pa 1976). 2002;27(13):E325-E328.
68 Cohen S, Williams S, Kurihara C, et al. Nucleoplasty with or without intradiscal electrothermal therapy (IDET) as a treatment for lumbar herniated disc. J Spinal Disorder Tech. 2005;18:119-124.
69 Nerubay J, Caspi L, Levinkopf M. Percutaneous carbon dioxide laser nucleolysis with 2- to 5-year followup. Clin Orthop Relat Res. 1997;337:45-48.