27 Complications of permanent fillers
Summary and Key Features
• Permanent fillers comprise mostly synthetic materials that cause collagen deposition via fibroplasia as their mechanism of action
• Permanent fillers are better at facial volumizing and deep structural augmentation than at ‘line filling’
• Silicones, polyalkylimides, polyacrylamides, polymethylmethacrylate, and acrylic hydrogels are the most common permanent fillers worldwide
• All cosmetic fillers, whether temporary or permanent, may induce adverse reactions
• Permanent filler complications may be due to the injection itself, injector-dependent variables, or host tissue and filler interactions
• Permanent filler complications are difficult to treat since the product will not dissipate, but rather remains in vivo
• Foreign-body granulomas and late-onset granulomas are the most challenging permanent filler complications to treat
• Late-onset granulomas are sometimes due to biofilms from bacteria introduced at the time of injection
• Effective treatments of granulomas must target bacterial etiologies and host immune response mechanisms
• Invasive and scarring treatments such as surgery should be reserved and used after more conservative treatments have failed
Products (Box 27.1, Table 27.1)
Table 27.1 Selected permanent fillers by category and composition
| Category | Branded product | Composition |
|---|---|---|
| Liquid injectable silicone | Silikon-1000® | Purified polydimethylsiloxane polymer |
| Adatosil-5000® | Purified polydimethylsiloxane polymer | |
| Other ‘silicones’ | Adulterated and unknown products | Variable and often unknown |
| Polyalkylimide gels (hydrophilic) | Bio-Alcamid® | 3% or 4% polyalkylimide gel in 97% or 96% sterile water |
| Polyacrylamide gels (hydrophilic) | Amazingel® | Polyacrylamide gel in sterile water |
| Aquamid® | 2.5% polyacrylamide gel in 97.5% sterile water | |
| Polymethylmethacrylate (hydrophobic) | Arteplast® (first generation) | 30–42 µm PMMA microspheres in a gelatin carrier |
| Artecoll® (second generation) | 30–50 µm PMMA microspheres in a bovine collagen carrier | |
| Artefill® (third generation) | 30–50 µm PMMA microspheres in a bovine collagen carrier | |
| Metacril® | PMMA in carboxyglutamate | |
| Acrylic hydrogel (hydroxyethyl-methacrylate / ethylmethacrylate) (hydrophobic) | Dermalive® | 45–65 µm polygonal fragments acrylic hydrogel (40%) in HA (60%) |
| Dermadeep® | 80–110 µm polygonal fragments acrylic hydrogel (40%) in HA (60%) |
HA, hyaluronic acid; PMMA, polymethylmetacrylate.
Polyalkylimide gel
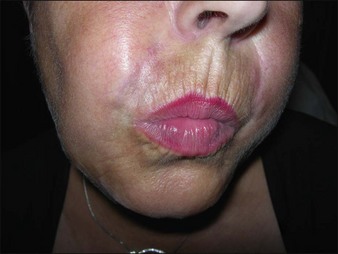
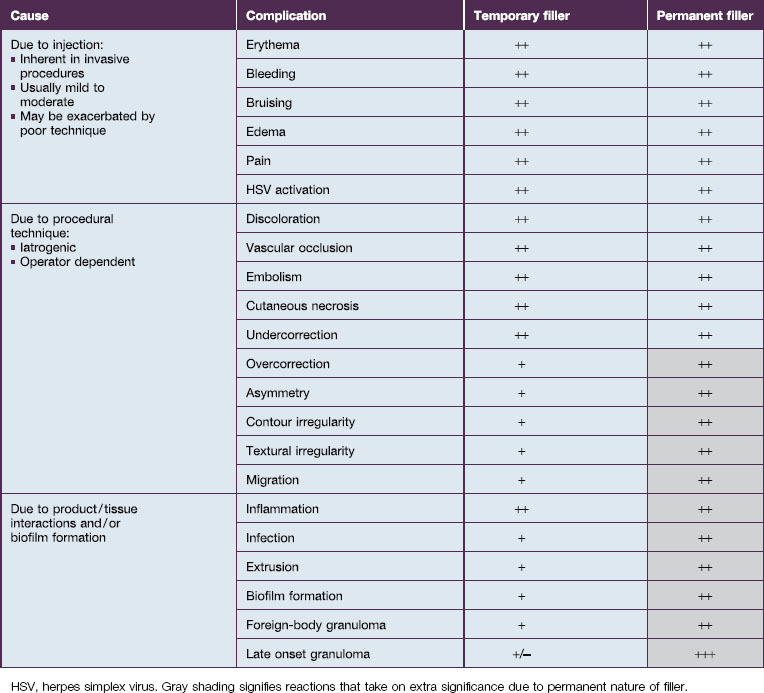
Bio-Alcamid® (Polymekon, Italy) was first launched in 2000 as an ‘endoprosthesis’ for the treatment of pectus excavatum, postoperative defects, and aesthetic use in the lips, cheeks, and nasolabial folds (Fig. 27.1). It contains a non-degradable, atoxic, non-immunogenic synthetic polyalkylimide cross-linked polymer suspended in water. Although it is approved in Europe, it is not currently approved by the FDA. Bio-Alcamid® contains a ratio of either 3% or 4% alkylimide polymer to 97% or 96% sterile water. The product is not a particulate one, but rather a gel that is injected as a large bolus. It is meant to be injected subcutaneously rather than into the dermis. It does not require allergy testing and has been histologically shown to induce fibroplasia once implanted.
Polyacrylamide gels
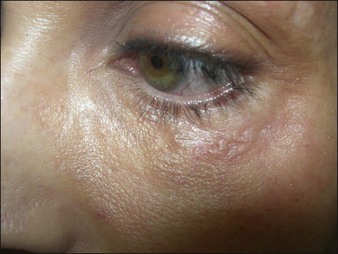
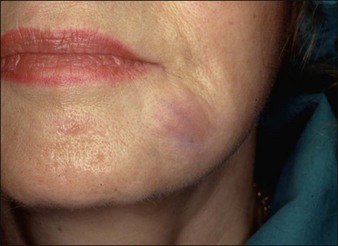
Aquamid® (Contura International, Denmark) contains a 2.5% cross-linked polyacrylamide gel in 97.5% sterile water. It has been in use in Europe and worldwide since 2001, primarily for augmentation of nasolabial folds and lips, facial contouring, and for correction of HIV facial lipoatrophy (Fig. 27. 2). It is also a viscoelastic gel without particles. On histology, Aquamid® appears as a multivacuolated non-birefringent material with a surrounding layer of thin fibrous connective tissue, macrophages, multinucleated giant cells, and lymphocytes.
Polymethylmethacrylate
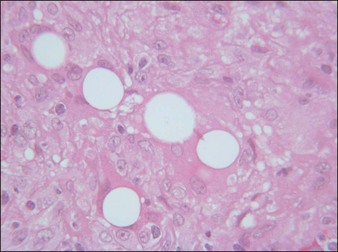
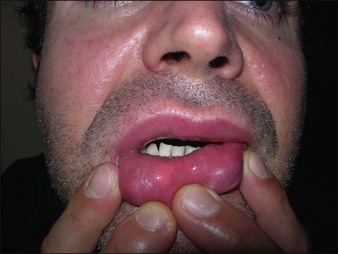
Artecoll® (Rofil Medical International, Breda, The Netherlands) (Fig. 27.3) was then introduced in 1992 as a second-generation product with an improved processing and washing method that reduced impurities. Significantly, the carrier was also reformulated, and the PMMA was suspended as a 20% product in 80% bovine collagen with 0.3% lidocaine for improved comfort. Because of the bovine collagen component, Artecoll® requires allergy testing prior to injection. As such Artecoll® is considered a biphasic implant, inducing augmentation through displacement with an early collagen component and later through a fibroplastic component caused by PMMA. In 1998, Artecoll® was approved in Canada, and processing methods were further improved to reduce particle size variability, resulting in fewer incidences of late granuloma formation compared with Arteplast®. Histology of skin biopsy specimens from areas treated with Artecoll® show round, sharply circumscribed, translucent, non-birefringent particles, epithelioid histiocytes, multinucleated giant cells, lymphocytes, and occasional eosinophils surrounding the microspheres.
Acrylic hydrogel plus hyaluronic acid
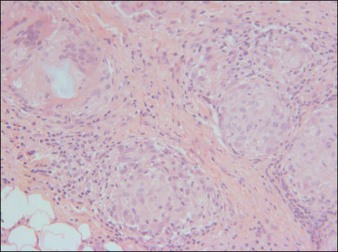
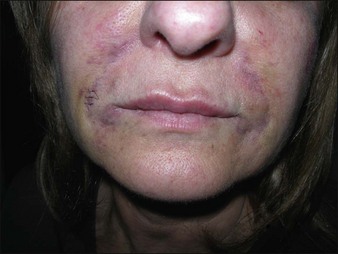
Dermalive® and Dermadeep® (Dermatech, Paris, France) are biphasic permanent fillers composed of a mixture of 40% hydroxyethylmethacrylate and ethylmethacrylate particles and 60% biodegradable, fluid cross-linked, bacterially derived HA (Fig. 27.4). The acrylic particles are hydrophobic and irregularly shaped in a polygonal fashion. Dermalive® contains acrylic particles 45–65 µm in diameter, whereas Dermadeep® contains particles 80–110 µm in diameter. Both products are intended for injection in the deep dermis and subcutaneous regions, with the Dermadeep product indicated for deep subcutaneous injection only. As with most permanent fillers, injections into the superficial and mid-reticular dermis are not recommended. Both products were CE approved and available in Europe in 1998, but were withdrawn several years ago owing to the high incidence of complications. Neither product has ever been FDA approved for use in the USA. Histology of acrylic hydrogels in tissue reveals pseudocystic structures of different sizes and shapes containing polygonal, pink, translucent, non-birefringent foreign bodies with a surrounding granulomatous reaction of epithelioid histiocytes, multinucleated giant cells, some lymphocytes, and occasional eosinophils surround the microstructures.
Complications
All cosmetic fillers, whether temporary or permanent, may induce adverse reactions (Table 27.2). Practically, it is useful to divide complications into early (0–14 days), late (14 days to 1 year), and delayed (beyond 1 year) reactions. Early reactions include erythema, pain, edema, bruising, and bleeding. Such reactions are inherently due to the invasive nature of the injection itself, are usually mild to moderate, and are typically self-limited to the first 14 days. They may be exacerbated, however, by poor technique.
HSV, herpes simplex virus. Gray shading signifies reactions that take on extra significance due to permanent nature of filler.
Overcorrection is an injector-dependent iatrogenic complication that is particularly troublesome with permanent fillers, and may occur with any of the permanent fillers (see Table 27.1). Overcorrection may be due to both too much product placed in a particular area as well as an underestimation of the degree of fibroplasia that will occur over time. With bovine collagens in the 1980s and 1990s, slight overcorrection was intentional at the time of treatment, and in fact was good practice, as a significant degree of the immediate correction achieved in the office would dissipate over the next few days. Gross volume displacement was the mechanism for achieving results with collagen alone, and further augmentation by fibroplasia was not expected. However, such a strategy does translate well into the realm of permanent fillers, as these depend heavily on augmentation by fibroplasia over time in addition to immediate gross volume displacement. To apply the overcorrection strategy of collagens to the permanent fillers is to invite disaster, as products that work by inducing collagenous deposition will continue to effect augmentation over several weeks to months afterwards. That is, tissue will be augmented beyond the immediate gross displacement due to product volume. For this reason, most permanent fillers should be placed in smaller amounts over multiple sessions spaced several weeks to even months apart in order to allow adequate time for tissue augmentation to occur between sessions. Otherwise, the physician runs the risk of injecting more product ahead of the oncoming augmentation curve – a set-up for overcorrection.
Complications in the final group are due to the host response to a foreign body, or product and host tissue interactions. Inflammation, foreign-body granulomas (Figs 27.5, 27.6), and late-onset granulomas (Fig. 27.7) may be due to the host immune response independent of infection, in which case the filler serves as the foreign body. However, they may also be due to bacterial biofilm formation, in which case both the filler and biofilm colony, or possibly bacteria that have been reactivated to a planktonic state, serve as a foreign-body nidus for pathological inflammation. Such complications have been seen, but poorly understood, for as long as permanent fillers have been utilized. Only in the last decade have theories regarding the etiology of such complications begun to coalesce and, although biofilms are now viewed as the likely culprit, more research is needed to fully elucidate all mechanisms involved.
Foreign-body granulomas and late-onset granulomas are the most challenging complications to treat, and are the archetypal permanent filler complications. Both are manifestations of the host’s natural immunologic response to a foreign body. Indeed, injecting a foreign body into the host, even a biocompatible, non-toxic, inert one as the permanent fillers are, naturally elicits a granulomatous foreign-body response, characterized by the appearance of macrophages and foreign-body giant cells that arrive to both phagocytose the foreign material and deposit a fibroplastic collagenous response in an effort to ‘wall-off’ or neutralize it. This is, after all, the main mechanism of action for fibroplastic tissue augmentation. Ideally, the foreign-body granulomatous reaction creates a controlled fibrous microscar around the surface of the implanted filler, and augmentation proceeds in a controlled, limited, and predictable manner. However, if the fibroplastic response does not cease, either because of an ongoing interaction with the foreign product itself or because of the synergistic presence of bacteria, possibly present in a bacterial biofilm, the fibroplastic response will manifest as a foreign-body granulomatous complication. Late-onset granulomas result from the same phenomena, but typically occur at least 1 year after injection. These may be explained by continued host–filler interactions, as there is evidence that the ‘permanent’ microparticles and gels can be further modified by the host immune response even years after injection. Alterations to the surface of Dermalive® particles have been documented by electron microscopy (EM) at 2 years, suggesting the product is altered in some fashion over time. This may be due to incomplete polymerization, where low-molecular-weight oligomers are left in the copolymer after final processing, or ongoing hydrolization. Most late-onset granulomas are likely explained, however, by the fact that bacteria that were introduced during injection had the opportunity to establish a biofilm around the injected product, and the biofilm has been reactivated or triggered to cause a host response, either by distant inflammation or infection or by further mechanical insult (Box 27.2). One aspect of the IFS Study evaluated the time elapsed until the appearance of adverse reactions after the injection of permanent fillers. Results showed that the mean time until the appearance of granulomatous reactions with polyacrylamide gels was 7.0 ± 10.6 months, with acrylic hydrogels was 12.3 ± 13.7 months, and with PMMA was 25.5 ± 37.1 months, demonstrating that late-onset granulomas are a significant potential complication with each of these products.
Evaluation methods
The first question is best addressed through histopathology rather than history. Histopathological study of the lesions is the ‘gold standard’ technique to identify the responsible filler. With modern products, the particles of each filler have specific microscopic characteristics that allow identification (Fig. 27.8). EM may be used if histopathological studies prove inconclusive or further research is warranted (Fig. 27.9). Recently, high-frequency ultrasound has also been reported to identify and quantify the presence of filler in vivo, as well as to detect inflammation, granulomas, and the presence of different fillers in the same area. Nevertheless, evaluation of the exact offending agent may prove difficult when a patient presents with a history of injection with an ‘unknown’ product, or an illicitly injected one. A history of ‘silicone’ injections most often eludes detection. Although silicone may be detected on histopathology, the clinician has no method of testing whether or not adulterants were also present in the injected material. Non-purified and unknown products are often lumped under ‘silicone’ injections by patient history, and these should be distinguished from modern purified products meant for human soft tissue augmentation and appropriately injected.
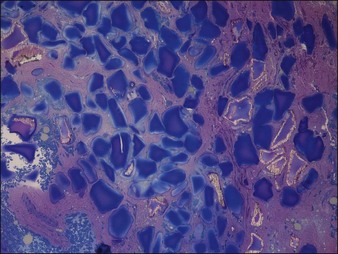
Figure 27.8 Histology of Dermalive® particles 4 years after injection, showing incomplete polymerization.
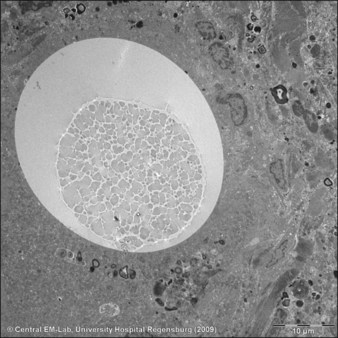
Figure 27.9 Electron microscopic photograph of an Artecoll® microsphere surrounded by a multinucleated giant cell.
Courtesy Dr Josef Schroeder, Central EM-Unit, Department of Pathology, University Hospital Regensburg, Germany.
Treatment
Treatment of transient, self-limited filler complications due to injection, including erythema, bleeding, bruising, edema, pain, and herpes simplex virus activation, has been well documented in the literature. However, treatment of permanent filler complications in the second and third categories (see Table 27.2) presents a greater challenge. Several treatment methods have been reported in the literature, including topical products, systemic medications, minimally invasive procedures and modalities, and surgical excision. Treatment options are dependent upon the specific product and patient reaction, and should be individualized. One tenet is to avoid surgical excisions that will result in scarring until all other treatment options have been exhausted.
Treatment of complications in the third category, which includes inflammation, infection, extrusion, and granuloma formation, are best geared toward the host immune response and infectious agents, or ultimately by product reduction and removal (Box 27.3). Treatment is difficult, and no one single method has reliably proven effective. Immunomodulators strike at the biological mechanisms underlying the granulomatous response. Topical immunomodulating treatments such as imiquimod have been reported effective for granulomas, likely through dampening of the local host immune response. Injected immunomodulating medications such as corticosteroids and 5-fluorouracil (5-FU) can also be effective for localized inflammation and granulomas (Fig. 27.10A,B). Oral steroids work by systemic, non-specific immunomodulation and may be required for severe reactions over short to medium durations. Systemic biological medications such as etanercept and infliximab have also been successful in altering the systemic immune response and decreasing granuloma activation and formation.
Box 27.3
Anecdotal treatments of granulomas
 Triamcinolone intralesional + cryotherapy
Triamcinolone intralesional + cryotherapy
 Bleomycin (1.5 IU/mL) intralesional
Bleomycin (1.5 IU/mL) intralesional
 Prednisone (60 mg/day) oral + ibuprofen (1800 mg/day)
Prednisone (60 mg/day) oral + ibuprofen (1800 mg/day)
 Cortivazol (3.75 mg/1.5 mL) intralesional
Cortivazol (3.75 mg/1.5 mL) intralesional
 Amoxicillin (1.5–3 g twice daily) oral (Christensen)
Amoxicillin (1.5–3 g twice daily) oral (Christensen)
 Ciprofloxacin (500 mg to 1 g twice daily) oral (Christensen)
Ciprofloxacin (500 mg to 1 g twice daily) oral (Christensen)
 Minocycline (2 × 100 mg/day) oral
Minocycline (2 × 100 mg/day) oral
 Minocycline (2 × 250 mg/day) + prednisolone (4 mg/day) oral
Minocycline (2 × 250 mg/day) + prednisolone (4 mg/day) oral
 Ciclosporin (5 mg/kg/day) oral
Ciclosporin (5 mg/kg/day) oral
 Allopurinol (200–600 mg/day) oral
Allopurinol (200–600 mg/day) oral
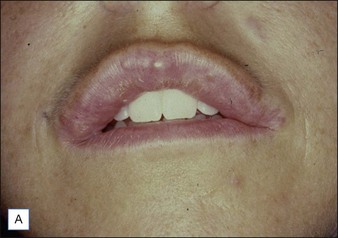
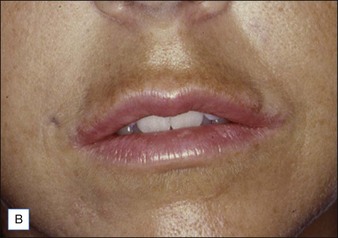
Figure 27.10 (A) Dermalive® granuloma; (B) with improvement after treatment with intralesional 5-fluorouracil and steroids.
If product reduction or removal is necessary, minimally invasive techniques should be attempted first when practical, including needle aspiration, extrusion after incision, and liposuction. Ultimately, laser destruction or surgical removal may be necessary, but these modalities should be reserved for cases that are particularly problematic or that have not responded to more conservative therapies. Cassuto et al have described a minimally invasive, minimally scarring technique for laser-assisted evacuation of infectious lesions after hydrogels using a lithium triborate laser at 532 nm as well as a method for intralesional treatment of granulomas caused by gels containing microparticles with an 808 nm diode laser to facilitate product evacuation (Fig. 27.11A,B).
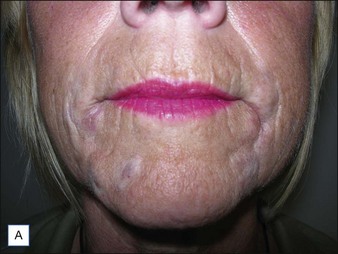
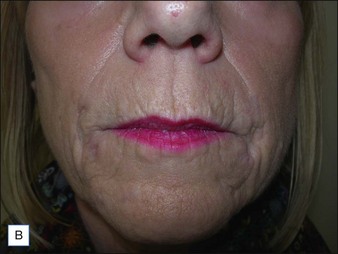
Figure 27.11 (A) Late-onset granulomas; (B) with improvement after treatment with the method described by Cassuto et al.
Al-Qattan MM. Complications related to Artecoll injections for soft tissue augmentation of the hand: 3 case reports. Journal of Hand Surgery of America. 2011;36(6):994–997.
American Society for Aesthetic Plastic Surgery. Survey. Online. Available http://www.surgery.org/sites/default/files/2010-top5.pdf, 2011. accessed 30 September
Cassuto D, Marangoni O, de Santis G, et al. Advanced laser techniques for filler-induced complications. Dermatologic Surgery. 2009;35(suppl 2):1689–1695.
Chrastil-LaTowsky B, Wesley NO, MacGregor JL, et al. Delayed inflammatory reaction to Bio-Alcamid polyacrylamide gel used for soft-tissue augmentation. Archives of Dermatology. 2009;145(11):1309–1312.
Christensen LH. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatologic Surgery. 2009;35(suppl 2):1612–1619.
Christensen L, Breiting V, Janssen M, et al. Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plastic Surgery. 2005;29(1):34–48.
Cohen SR, Rubin MG. Artefill. In: Sadick NS, ed. Augmentation fillers. New York: Cambridge University Press; 2010:53–67.
Epstein RE, Spencer JM. Correction of atrophic scars with artefill: an open-label pilot study. Journal of Drugs in Dermatology. 2010;9(9):1062–1064.
Furmanczyk PS, Wolgamot GM, Argenyi ZB, et al. Extensive granulomatous reaction occurring 15 years after DermaLive injection. Dermatologic Surgery. 2009;35(suppl 1):385–388.
Goldberg DJ. Bioalcamid. In: Sadick NS, ed. Augmentation fillers. New York: Cambridge University Press; 2010:113–116.
Grippaudo FR, Mattei M. The utility of high-frequency ultrasound in dermal fillers evaluation. Annals of Plastic Surgery. 2011;67(5):469–473.
Jones DH. Semipermanent and permanent injectable fillers. Dermatologic Clinics. 2009;27(4):433–444. vi
Khan I, Shokrollahi K, Bisarya K, et al. A liposuction technique for extraction of Bio-Alcamid and other permanent fillers. Aesthetic Surgery Journal. 2011;31(3):344–346.
Monheit GD, Rohrich RJ. The nature of long-term fillers and the risk of complications. Dermatologic Surgery. 2009;35(suppl 2):1598–1604.
Narins RS, Coleman WP, 3rd., Rohrich R, et al. 12-Month controlled study in the United States of the safety and efficacy of a permanent 25% polyacrylamide hydrogel soft-tissue filler. Dermatologic Surgery. 2010;36(suppl 3):1819–1829.
Ono S, Ogawa R, Hyakusoku H. Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plastic and Reconstructive Surgery. 2010;126(4):1349–1357.
Pacini S, Ruggiero M, Morucci G, et al. Bio-Alcamid: a novelty for reconstructive and cosmetic surgery. Italian Journal of Anatomy and Embryology. 2002;107(3):209–214.
Pallua N, Wolter TP. A 5-year assessment of safety and aesthetic results after facial soft-tissue augmentation with polyacrylamide hydrogel (Aquamid): a prospective multicenter study of 251 patients. Plastic and Reconstructive Surgery. 2010;125(6):1797–1804.
Prather CL, Jones DH. Liquid injectable silicone for soft tissue augmentation. Dermatologic Therapy. 2006;19(3):159–168.
Rauso R, Freda N, Parlato V, et al. Polyacrylamide gel injection for treatment of human immunodeficiency virus-associated facial lipoatrophy: 18 months follow-up. Dermatologic Surgery. 2011;37(11):1584–1589.
Requena L, Requena C, Christensen L, et al. Adverse reactions to injectable soft tissue fillers. Journal of the American Academy of Dermatology. 2011;64(1):1–34. quiz 35-36
Rossner M, Rossner F, Bachmann F, et al. Risk of severe adverse reactions to an injectable filler based on a fixed combination of hydroxyethylmethacrylate and ethylmethacrylate with hyaluronic acid. Dermatologic Surgery. 2009;35(suppl 1):367–374.
Sachdev M, Anantheswar Y, Ashok B, et al. Facial granulomas secondary to injection of semi-permanent cosmetic dermal filler containing acrylic hydrogel particles. Journal of Cutaneous and Aesthetic Surgery. 2010;3(3):162–166.
Schelke LW, Van Den Elzen HJ, Erkamp PP, et al. Use of ultrasound to provide overall information on facial fillers and surrounding tissue. Dermatologic Surgery. 2010;36(suppl 3):1843–1851.
Schuller-Petrović S, Pavlović MD, Schuller SS, et al. Early granulomatous foreign body reactions to a novel alginate dermal filler: the system’s failure? Journal of the European Academy of Dermatology and Venereology. 2011. doi: 10.1111/j.1468-3083.2011.04264.x [Epub ahead of print]
Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatologic Surgery. 2009;35(suppl 2):1672–1680.
Wiest LG, Stolz W, Schroeder JA. Electron microscopic documentation of late changes in permanent fillers and clinical management of granulomas in affected patients. Dermatologic Surgery. 2009;35(suppl 2):1681–1688.
Wolter TP, Pallua N. Removal of the permanent filler polyacrylamide hydrogel (Aquamid) is possible and easy even after several years. Plastic and Reconstructive Surgery. 2010;126(3):138e–139e.
Zielke H, Wölber L, Wiest L, et al. Risk profiles of different injectable fillers: results from the Injectable Filler Safety Study (IFS Study). Dermatologic Surgery. 2008;34(3):326–335. discussion 335