Chapter 2 Complications of Peripheral Nerve Stimulation
Open Technique, Percutaneous Technique, and Peripheral Nerve Field Stimulation
 PNS is a neuromodulation technique involving application of an electrical current adjacent to peripheral nerves to treat chronic, intractable, debilitating neuropathic pain conditions that are refractory to less invasive treatments.
PNS is a neuromodulation technique involving application of an electrical current adjacent to peripheral nerves to treat chronic, intractable, debilitating neuropathic pain conditions that are refractory to less invasive treatments. There are three main variants of PNS: PNS performed with an open surgical technique (PNS:OT); PNS executed with a percutaneous technique (PNS:PT), and a subcutaneous; nonperineural technique called peripheral nerve field stimulation (PNfS).
There are three main variants of PNS: PNS performed with an open surgical technique (PNS:OT); PNS executed with a percutaneous technique (PNS:PT), and a subcutaneous; nonperineural technique called peripheral nerve field stimulation (PNfS). PNS techniques, which are reversible, cost effective, nonaddictive, and nonpharmacologic, are based on delivery of low-level electric impulses to pain-generating nerves via an implantable system, consisting of a programmable generator connected to electric transmission leads.
PNS techniques, which are reversible, cost effective, nonaddictive, and nonpharmacologic, are based on delivery of low-level electric impulses to pain-generating nerves via an implantable system, consisting of a programmable generator connected to electric transmission leads. Common neural targets amenable to PNS include cranial nerves, occipital nerves, segmental truncal nerves, and upper and lower extremity plexus and peripheral nerves or areas not accessible or effectively treated by spinal cord or spinal nerve root stimulation.
Common neural targets amenable to PNS include cranial nerves, occipital nerves, segmental truncal nerves, and upper and lower extremity plexus and peripheral nerves or areas not accessible or effectively treated by spinal cord or spinal nerve root stimulation. The risk of serious problems resulting from PNS therapies appears to be relatively low in clinical practice, especially for PNfS. However, PNS therapy is not without risk, and lead placement along specific target areas may be more challenging technically than in other implantable therapies.
The risk of serious problems resulting from PNS therapies appears to be relatively low in clinical practice, especially for PNfS. However, PNS therapy is not without risk, and lead placement along specific target areas may be more challenging technically than in other implantable therapies. The most common complication is infection. Other reported complications include peripheral nerve trauma and damage to other tissues, pain over the device, and seroma.
The most common complication is infection. Other reported complications include peripheral nerve trauma and damage to other tissues, pain over the device, and seroma. Technical complications generally involve equipment failure, including lead migration and lead fracture, and less commonly, battery malfunction.
Technical complications generally involve equipment failure, including lead migration and lead fracture, and less commonly, battery malfunction. Devastating infectious and technical complications, although rare, have been reported and deserve special attention.
Devastating infectious and technical complications, although rare, have been reported and deserve special attention. Biologic complications commonly occur within 3 months of implant, although infections can rarely present much later. Technical complications typically occur within 2 years of implant.
Biologic complications commonly occur within 3 months of implant, although infections can rarely present much later. Technical complications typically occur within 2 years of implant. Meticulous attention to surgical technique is the best measure against the development of infection, the most common PNS complication. In addition, adequate patient screening, use of high-quality imaging, practitioner expertise, and familiarity with the different types of available hardware are recommended to preclude adverse outcomes.
Meticulous attention to surgical technique is the best measure against the development of infection, the most common PNS complication. In addition, adequate patient screening, use of high-quality imaging, practitioner expertise, and familiarity with the different types of available hardware are recommended to preclude adverse outcomes.Introduction
Using PNS:OT, a lead, often a paddle lead, is surgically placed directly adjacent to the target nerve. An example is placement of a paddle lead along the sciatic nerve for a person with neuropathic sciatica. Using PNS:PT, a percutaneous lead is placed through a needle that is usually guided via a nerve stimulator or by ultrasonography. An example is placement of a percutaneous lead through a needle under ultrasound guidance along nerves of the brachial plexus for a painful brachial plexopathy. Using PNfS, which is also called subcutaneous field stimulation, a lead is typically placed through a needle into the subcutaneous tissue in the direct area of pain experienced by the patient, remote to named peripheral nerves. An example is a lead placed in the subcutaneous tissues for axial low back pain in a patient with postlaminectomy syndrome.1
In contemporary times, PNS techniques, which are reversible, cost effective, nonaddictive, and nonpharmacologic, are based on delivery of low-level electric impulses to pain-generating nerves via an implantable system, consisting of a programmable generator connected to electric transmission leads. Over the past decade, they have been used increasingly to treat a wide range of conditions involving pain in peripheral or cranial neural distributions. In particular, they may be an effective treatment for neuropathic pain that is not accessible or effectively treated by spinal cord or spinal nerve root stimulation. Common neural targets amenable to PNS include cranial nerves (e.g., trigeminal peripheral terminal branches), occipital nerves, segmental truncal nerves (e.g., nerve root, intercostal, ilioinguinal, iliohypogastric, genitofemoral), and upper and lower extremity plexus and peripheral nerves (e.g., ulnar, median, radial, lateral femoral cutaneous, sciatic, anterior and posterior tibial nerves).2
Another reason PNS therapies have increased in popularity may be their lower incidence of complications. In spite of reported complications including infection, lead migration, and device failure, the risk of serious problems resulting from PNS or PfNS therapies appears to be relatively low in clinical practice. Although there has been no formal comparison of PNS versus spinal cord stimulation (SCS) complications, when compared with intrathecal drug delivery, electrical neuromodulation techniques rarely impact morbidity or mortality significantly.3
Selected Complications
Perhaps one of the most significant advantages of PNS is its relatively low rate of complications (Table 2-1). Mobbs et al4 mention relatively minor complications in their retrospective study (currently the largest in the literature), which examines the role of the implantable PNS device in the chronic pain patient. In 38 patients who received implanted PNS devices, six stimulators were removed after implantation (15%). Two were removed due to infection, representing a 5% infection rate. One of these patients had hemophilia despite factor VIII cover, and an episode of bleeding that was further complicated by infection, necessitating stimulator removal. Despite a positive result during the trial period, one stimulator was removed after one month because of minimal effect post-implantation. This patient subsequently improved again after his workers’ compensation issues were resolved. One stimulator was removed at 4 years post-implantation since the patient maintained it was no longer needed. Two stimulators in one patient had an initially positive effect, lasting 3 months, followed by a rapid decline in effect. The patient did not wish to have the stimulators re-trialed or re-implanted. A single lead had to be replaced as it was fractured following a fall from a tree. The stimulator continued to function following revision of the lead. During the follow-up period, two battery generators were replaced because of battery failure and a further two generator/lead combinations were repositioned as they were uncomfortable and restricted arm movement. One electrode was relocated during the trial period due to a substantial, uncomfortable motor effect in an adjacent muscle. A further 8 electrodes were resutured during the second operation due to electrode lead migration.
| Complication | Reported Rate (If Reported) |
|---|---|
| Overall revision rate | 27%32 |
| Requiring explant | 15%4 |
| Procedural | |
| Tissue trauma | Theoretical |
| Allergic reactions | Case reports,33* 0.8%34 |
| Specific anesthesia-related complications | Anecdotal evidence |
| Hemorrhage | Theoretical |
| Peripheral nerve trauma | 60%4 |
| Organ trauma | Theoretical |
| Post-Procedural | |
| Infection | 5%,4 3%-5%,17 4.5%,18 1%35 |
| Seroma | 2.5%36 |
| Lead migration | 27%-33%,34,37 2%35 |
| Skin erosion | 12.5%,21 7%35 |
| Pain at generator site | 0.9%-5.8%38,39* |
| Excessive bleeding | Theoretical |
| Sepsis | Theoretical, unpublished case report at the Cleveland Clinic |
| Battery failure/hardware failure | 1.6%,40* 2%35 |
| Lead migration | 33%,27 24%30 |
* Extrapolated from spinal cord stimulation devices.
Before undertaking a PNS procedure, several factors should be considered, according to a review of surgical procedures pertaining to implantable neuromodulation technology.5 These factors include the incidence, severity, and time to resolution of complications, as well as the net impact on the patient given that complications may detract from the beneficial effect of the procedure.
Procedural Complications
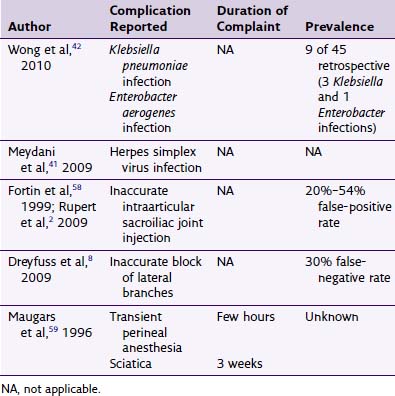
Harm caused to tissues during PNS procedures may consist of bleeding, peripheral nerve trauma, and damage to vital structures (e.g., vessels and organs). Because vital internal structures are vulnerable in PNS, the use of high-quality fluoroscopy is indicated; for example, pneumothorax, a potential organ-related complication of PNS device installation in the thoracic region, is best circumvented by high-quality imaging.6 In deeper tissues, damage to vessels can be evaded by using an open rather than percutaneous technique, which may help to prevent blind injury of vasculature, embolism, and other negative sequelae.
The use of PNS therapy was commonly used to treat pain after previous nerve damage as described by Mobbs et al4 in their retrospective study (currently the largest in the literature) of 38 patients implanted with 41 nerve stimulators. The previous nerve damage included blunt and or sharp nerve trauma (in 14 of 38 patients) and inadvertent injection of a nerve (in nine of 38 patients). The incidence of nerve damage from PNS therapy itself is unknown and believed to be rare. To avoid nerve damage, practitioners should maintain excellent knowledge of relevant anatomy and watch for patient neuralgia and radicular pain in the postoperative period. Treatment of suspected nerve injury may include steroid protocol, anticonvulsants, and referral for neurologic consult.
Taking a thorough patient history is beneficial in determining whether the patient is allergic to any of the agents used in PNS therapies. Because the patient may be unaware of any allergies surrounding these products, the physician should remain vigilant for development of allergic sequelae during PNS. For example, local anesthetics are common elicitors of adverse reactions with clinical symptoms such as anaphylaxis with tachycardia; hypotension; and subjective feelings of weakness, heat, or vertigo.7 Furthermore, during general anesthesia or sedation, anaphylactic response to IV hypnotics and other drugs can occur; cardiovascular collapse and bronchospasm are frequent in immunoglobulin E–dependent reactions.8 In addition, latex can produce allergic reactions as serious as anaphylaxis.9
Although the incidence of allergic or toxic reactions to skin preparations is unusual, practitioners should remain aware that iodine tincture and chlorhexidine can produce adverse outcomes in some patients. It is advisable to take a thorough patient history to avoid cutaneous manifestations, particularly in patients with skin sensitivity or other drug allergies. Iodine is associated with adverse effects ranging from minor skin irritation to anaphylaxis, with symptoms occurring within minutes and up to 8 hours after contact.10 In addition, the incidence of contact dermatitis to chlorhexidine in atopic patients is approximately 2.5% to 5.4%, and acute hypersensitivity reactions to chlorhexidine are often not recognized and therefore may be underreported.11
Skin preparations are a topic of concern not only because of their allergic potential but also because of choice of agent (e.g., iodine tincture vs. chlorhexidine). According to a 2010 study published in the New England Journal of Medicine,12 preoperative cleansing of the patient’s skin with chlorhexidine–alcohol was superior to cleansing with povidone–iodine for preventing surgical site infection (SSI) after clean-contaminated surgery. Chlorhexidine–alcohol was significantly more protective than povidone–iodine against both superficial incisional infections (4.2% vs. 8.6%; P = 0.008) and deep incisional infections (1% vs. 3%, P = .05), although it was not effective against organ space infections (4.4% vs. 4.5%). Furthermore, according to Barenfanger et al,13 in choosing a skin preparation for surgical site antisepsis in PNS, it should be noted that although iodine tincture has been called the “gold standard” in preoperative skin preparation, it does not provide statistically greater utility than chlorhexidine in terms of contamination rates, and chlorhexidine may be safer, less expensive, and preferred by staff members. Furthermore, iodine tincture has the disadvantage of being toxic when used repeatedly, but toxicity or sensitization caused by chlorhexidine is very uncommon. However, Barenfanger et al13 found that the average contamination rate with chlorhexidine was found to be slightly greater than with iodine (3.13%, or 186 contaminants in 5936 cultures, vs. 2.72%, or 158 contaminants in 5802 cultures).
Numerous randomized, controlled trials in the literature underscore the benefits of giving prophylactic antibiotics to the patient immediately before surgical procedures such as PNS to inhibit development of infection, although the risk of allergic reaction to antibiotics exists. Classen et al14 prospectively monitored the timing of antibiotic prophylaxis and development of surgical wound infections in 2847 patients undergoing surgical procedures. Among patients who received antibiotics up to 24 hours before surgery, 2 hours before surgery, 3 hours after surgery, and more than 3 hours after surgery, those who received antibiotics 2 hours before surgery had the lowest rates of subsequent surgical wound infections. Furthermore, according to a surgeon’s perspective by Nichols,15 it is generally recommended in elective clean surgical procedures using a foreign body and in clean-contaminated procedures that IV antibiotics should be administered in the operative suite immediately before incision.
Although innumerable clinical trials demonstrate the efficacy of preoperative administration of antibiotics against subsequent infection, physicians should remain mindful of the possibility of unexpected antibiotic allergy in these patients. Although allergic reactions to antibiotics account for only a small proportion of reported adverse drug reactions and estimates of their prevalence vary widely, they are associated with substantial morbidity and mortality and increased health care costs.16
Post-Procedural Complications
The most common complication after a PNS procedure is infection, which is estimated at the approximate rate of 3% to 5%,17 and is most likely to occur at the site of implantation. Infection may include wound cellulitis, gross infection at the generator and lead sites, and sepsis. Close adherence to specific guidelines to prevent infection and meticulous attention to sterile technique during the procedure and throughout wound closure are considered to be the best measures against infection.
Wound infections involving the generator, tunneled area, or lead incision site can occur in up to 4.5% of patients based on reported incidences.18 To avoid development of wound infections, meticulous surgical technique is necessary to prevent primary contamination of the implanted equipment even from common skin flora. In infected patients, subsequent wound dehiscence with external exposure of any of the implant requires explantation of the total device, although a previously infected area can be successfully reimplanted after successful treatment of the affected area.
To lower the incidence of infection, some experts recommend swabbing of the wound for microbiologic analysis in the stage subsequent to the trial period and before permanent implantation. According to Rudiger et al,19 swabbing should function as a prerequisite for permanent implantation, with the anchoring site wound opened and inspected for visible signs of infection and swabbed for microbiologic analysis, including sensitivities for positive results. Furthermore, Rudiger et al19 noted a lowered incidence of infection in patients given a double-layer hydrocolloid dressing and noted that this type of dressing reduced movement and prevented dislocation of temporary leads at the wound exit site. In addition, another study noted silver-impregnated wound covers lowered infection rates in 786 patients implanted with a neurosurgical device, including spinal cord stimulators.20
Although it is recommended that pocket size be minimized to inhibit seroma formation, an excessively small pocket can promote inadequate wound closure as well as pressure on the tissue with gradual skin erosion over the hardware components. For PNS specifically, lead migration can occur at the skin when the leads are placed too superficially. For example, Slavin et al21 reported one of eight patients who received PNS of trigeminal nerve branches for infraorbital pain developed skin erosion over an electrode requiring removal and eventual reimplantation. Eruption of device components through the skin can be caused by poor tissue health from chronic disease, weight loss, and excessively superficial placement of hardware. Skin erosion can occur at any place along the device whether it is the implanted pulse generator, the electrodes, the leads, or the anchoring devices. It occurs most frequently at the generator site, requiring surgical revision to preclude system failure, which warrants complete removal of the system. If an anchoring method is used to secure the leads, the tissue must be closed in multiple planes to protect the anchor from erosion. Alternatively, some experts choose to use nonabsorbable sutures and secure the lead without creating a formal anchor. This technique is not well studied and is not recommended in most clinical scenarios.
Development of sepsis from PNS, although uncommon, is still a cause for concern, particularly because bacteria tend to thrive on the surface of implanted devices.22 Recent data indicate that Escherichia coli and Staphylococcus aureus continue to be the most frequent pathogens isolated in bloodstream infection;23 growth of these and other bacteria on the biomaterial surface of PNS hardware may multiply and physiologically transform into a “biofilm” community, which bolsters their resistance to antibiotic therapy and host immunity. Because of the hardiness of the resulting biofilm, treating sepsis without removing all foreign bodies and necrotic bone fragments is often ineffective.
Infection
According to Centers for Disease Control and Prevention (CDC) guidelines24 for prevention of SSIs:
 Whenever possible, identify and treat all infections remote to the surgical site before elective operation and postpone elective operations on patients with remote site infections until the infection has resolved.
Whenever possible, identify and treat all infections remote to the surgical site before elective operation and postpone elective operations on patients with remote site infections until the infection has resolved. Do not remove hair preoperatively unless the hair at or around the incision site will interfere with the operation.
Do not remove hair preoperatively unless the hair at or around the incision site will interfere with the operation. Adequately control serum blood glucose levels in all patients with diabetes and particularly avoid hyperglycemia perioperatively.
Adequately control serum blood glucose levels in all patients with diabetes and particularly avoid hyperglycemia perioperatively. Encourage tobacco cessation. At minimum, instruct patients to abstain for at least 30 days before elective operation from smoking cigarettes, cigars, pipes, or any other form of tobacco consumption (e.g., chewing or dipping).
Encourage tobacco cessation. At minimum, instruct patients to abstain for at least 30 days before elective operation from smoking cigarettes, cigars, pipes, or any other form of tobacco consumption (e.g., chewing or dipping). Require patients to shower or bathe with an antiseptic agent on at least the night before the operative day.
Require patients to shower or bathe with an antiseptic agent on at least the night before the operative day. Thoroughly wash and clean at and around the incision site to remove gross contamination before performing antiseptic skin preparation.
Thoroughly wash and clean at and around the incision site to remove gross contamination before performing antiseptic skin preparation. Apply preoperative antiseptic skin preparation in concentric circles moving toward the periphery. The prepared area must be large enough to extend the incision or create new incisions or drain sites if necessary.
Apply preoperative antiseptic skin preparation in concentric circles moving toward the periphery. The prepared area must be large enough to extend the incision or create new incisions or drain sites if necessary. Keep preoperative hospital stay as short as possible while allowing for adequate preoperative preparation of the patient.
Keep preoperative hospital stay as short as possible while allowing for adequate preoperative preparation of the patient. There is no recommendation to taper or discontinue systemic steroid use (when medically permissible) before elective operation.
There is no recommendation to taper or discontinue systemic steroid use (when medically permissible) before elective operation. There is no recommendation to enhance nutritional support for surgical patients solely as a means to prevent SSI.
There is no recommendation to enhance nutritional support for surgical patients solely as a means to prevent SSI.Furthermore, for optimal asepsis and surgical technique, the CDC recommends that the practitioner:
 Handle tissue gently, maintain effective hemostasis, minimize devitalized tissue and foreign bodies (i.e., sutures, charred tissues, necrotic debris), and eradicate dead space at the surgical site.
Handle tissue gently, maintain effective hemostasis, minimize devitalized tissue and foreign bodies (i.e., sutures, charred tissues, necrotic debris), and eradicate dead space at the surgical site.And for postoperative incision care and surveillance:
 Protect the wound with a sterile dressing for 24 to 48 hours postoperatively for an incision that has been closed primarily.
Protect the wound with a sterile dressing for 24 to 48 hours postoperatively for an incision that has been closed primarily. Educate the patient and family regarding proper incision care, symptoms of SSI, and the need to report such symptoms.
Educate the patient and family regarding proper incision care, symptoms of SSI, and the need to report such symptoms. There is no recommendation to cover an incision closed primarily beyond 48 hours, nor on the appropriate time to shower or bathe with an uncovered incision.
There is no recommendation to cover an incision closed primarily beyond 48 hours, nor on the appropriate time to shower or bathe with an uncovered incision. Assign the surgical wound classification upon completion of an operation. A surgical team member should make the assignment.
Assign the surgical wound classification upon completion of an operation. A surgical team member should make the assignment. For each patient undergoing an operation chosen for surveillance, record variables shown to be associated with increased SSI risk (e.g., surgical wound class, American Society of Anesthesiologists class, and duration of operation).
For each patient undergoing an operation chosen for surveillance, record variables shown to be associated with increased SSI risk (e.g., surgical wound class, American Society of Anesthesiologists class, and duration of operation).An additional means of reducing the number of SSIs is rapid screening and decolonizing of nasal carriers of S. aureus, according to a study by Bode et al.25 A total of 1270 nasal swabs from 1251 patients were positive for S. aureus; 917 of these patients were enrolled in an intention-to-treat analysis, and 808 (88.1%) underwent a surgical procedure. All S. aureus strains identified on polymerase chain reaction assay were susceptible to methicillin and mupirocin. The rate of S. aureus infection was 3.4% (17 of 504 patients) in the mupirocin–chlorhexidine group compared with 7.7% (32 of 413 patients) in the placebo group, and the authors noted that the effect of mupirocin–chlorhexidine treatment was most pronounced for deep SSIs.
Another study suggesting the importance of eradication of S. aureus concerned a group of patients with SCS implants.26 Of the 158 patients who participated in a weeklong trial of SCS, six (4%) developed infections. Of the 68 patients who received the implant after participating in the trial, eight (12%) developed infections. In five of the 14 total infected individuals, the site of infection was cultured. In each case, S. aureus was the only isolated pathogen.
Device-Related Complications
Although recent technologic improvements have allowed for improved quality and complexity of stimulators and better lead extensions, PNS therapies still carry a risk of complications pertaining to implanted components, particularly within the first 2 years after implantation of the device.5
Although percutaneous implantation of electrodes should be a relatively straightforward and simple technique, the procedure can still produce undesirable sequelae, leading to low patient satisfaction and overall increase in costs associated with the procedure.5 Lead migration felt as a change in the stimulation by the patient may be diagnosed by an inability to obtain coverage over the painful area and comparison of imaging studies of current lead position with those taken at baseline. In fact, lead migration is probably the most common problem related to PNS cases and may be responsible for 33% of reoperations, according to a retrospective analysis performed by Ishizuka et al.27
Correlates can be drawn to literature of SCS migration and its prevention. Studies in SCS show lead migration in 22% of patients, although the percentage of patients that needed surgical intervention has not been disclosed.28 In another case, 14.8% of patients required surgical intervention because of SCS lead migration or fracture.29 And in occipital nerve stimulation, lead migration occurred in 12 of 51 (24%) subjects in a multicenter, randomized, blinded, controlled feasibility study.30
To examine how mechanical failures such as lead breakage and migration can undermine the efficacy of implantable technologies, a panel of experienced implanters interpreted a systematic analysis of surgical techniques coupled with extensive in vivo and in vitro biomechanical testing of system components and related them to clinical observations.31 A computer model based on morphometric data was used to predict movement in a standard SCS system between an anchored lead and pulse generator placed in various locations, and these displacements were then used to determine a realistic range of forces exerted on components of the SCS system. Leads and anchors were subjected to repetitive stresses until failure occurred. In addition, an in vivo sheep model was used to determine system compliances and failure thresholds in a biologically realistic setting. According to panel consensus, use of a soft Silastic anchor pushed through the fascia to provide a larger bend radius for the lead was associated with a time to failure 65 times longer than an anchored but unsupported lead. In addition, whereas failures of surgical paddle leads occurred when used with an anchor, without an anchor, no failures occurred to 1 million cycles. Based on these findings, the panel recommended a paramedian approach, abdominal pulse generator placement, maximizing bend radius by pushing the anchor through the fascia and anchoring of the extension connector near the lead anchor.
Although no formal study has been performed specifically for PNS because the hardware is largely the same, surgical guidance to mitigate lead migration may be extrapolated. According to an expert panel, the optimal material for anchoring percutaneous leads for SCS is 0 black braided nylon, if possible.5 Some practitioners prefer 2.0 silk sutures; however, according to the same panel, these should not be tied too tightly, and thin sutures may cut through the anchor or insulation. Furthermore, the lead should be anchored to deep fascia, and the nose of the Silastic anchor should be pushed through it; if this is not performed properly, a kink in the lead may result, which can be responsible for fracturing of the lead.5 Another consideration is that a strain relief loop should be used after anchoring the lead and before connection to an extension cable, if used; even though fibrous tubular casing may develop around the loop, the extension cord moves within this fibrous casing, which does not promote extra strain.5 In addition, generator placement close to the area of stimulation and mini-generators may help to reduce migration problems.
Battery failure is another hardware-related complication in PNS. Battery failure may be directly associated with high energy use; battery conservation may be promoted by use of a cycling mode, low frequencies, and a limited number of active electrodes.5 Furthermore, a rechargeable system may be optimal when a patient requires continuous high-voltage stimulation that would otherwise shorten battery life of nonrechargeable systems.5
A general article published by the New York School of Regional Anesthesia (NYSORA)41 points out that the use of nerve stimulators does not exclude the possibility of nerve damage42,43 and recommends caution when stimulation is obtained with currents of less than 0.02 mA. The article further states that stimulation with such low-intensity current is often associated with paresthesia on injection, perhaps suggesting an intraneural placement of the needle. In this scenario, the NYSORA recommends that the practitioner routinely withdraw the needle until the motor response is obtained at a current of 0.2 to 0.5 mA. The NYSORA also states that nerve stimulators used for peripheral nerve blockade can vary greatly in their features, stimulating frequency, maximum voltage output, stimulus duration, and accuracy, and although most modern units it studied performed adequately within a clinically relevant range of currents and impedance loads, some older models may be grossly inaccurate. For that reason, the NYSORA states the recommendations on the current intensity in older books may not be applicable with all nerve stimulators.
1 Paicius RM, Bernstein CA, Cheryl Lempert-Cohen C. Peripheral nerve field stimulation for the treatment of chronic low back pain: preliminary results of long-term follow-up: a case series. Neuromodulation. 2007;10(3):279-290.
2 Stanton-Hicks M. Neuromodulation. London: Elsevier; 2009. pp 2:400
3 Coffey RJ, Woens ML, Broste SK, et al. Medical practice perspective: identification and mitigation of risk factors for mortality associated with intrathecal opioids for non-cancer pain. Pain Med. 2010;11:1001-1009.
4 Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14(3):216-221. discussion 222-223
5 Kumar K, Buchser E, Linderoth B, et al. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007;10(1):24-33.
6 Liu SS, Gordon MA, Shaw PM, et al. A prospective clinical registry of ultrasound-guided regional anesthesia for ambulatory shoulder surgery. Anesth Analg. 2010;111(3):617-623.
7 Ring J. Anaphylactic reactions to local anesthetics. Chem Immunol Allergy. 2010;95:190-200.
8 Moneret-Vautrin DA, Mertes PM. Anaphylaxis to general anesthetics. Chem Immunol Allergy. 2010;26(8-9):719-723.
9 Heitz JW, Bader SO. An evidence-based approach to medication preparation for the surgical patient at risk for latex allergy: is it time to stop being stopper poppers? J Clin Anesth. 2010;22(6):477-483.
10 Rahimi S, Lazarou G. Late-onset allergic reaction to povidone-iodine resulting in vulvar edema and urinary retention. Obstet Gynecol. 2010;116(suppl 2):562-564.
11 Lim KS, Kam PC. Chlorhexidine—pharmacology and clinical applications. Anaesth Intensive Care. 2008;36(4):502-512.
12 Darouiche RO, Wall MJJr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
13 Barenfanger J, Drake C, Lawhorn J, Verhulst SJ. Comparison of chlorhexidine and tincture of iodine for skin antisepsis in preparation for blood sample collection. J Clin Microbiol. 2004;42(5):2216-2217.
14 Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281-286.
15 Nichols RL. Preventing surgical site infections: a surgeon’s perspective. Emerg Infect Dis. 2001;7(2):220-224.
16 Gruchalla RS, Pirmohamed M. Antibiotic allergy. N Engl J Med. 2006;354(6):601-609.
17 De Leon-Casasola O. Spinal cord and peripheral nerve stimulation techniques for neuropathic pain. J Pain Symptom Manage. 2009;38(2 suppl):S28-S38.
18 Deer T. Atlas of implantable therapies for pain management. New York: Springer; 2011.
19 Rudiger J. Thomson S: Infection rate of spinal cord stimulators after a screening trial period. A 53-month third party follow-up. Neuromodulation. 2011;14(2):136-141.
20 Turner MS, Flint KJ, Davis KE. Infection rates and use of silver-impregnated wound covers when implanting neurosurgical devices. AANS Neurosurgeon. 19(3), 2010.
21 Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5(1):100-106.
22 Gallo J, Kolár M, Novotný R, et al. Pathogenesis of prosthesis-related infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147(1):27-35.
23 Kern WV. [Bacteraemia and sepsis]. Dtsch Med Wochenschr. 2011;136(5):182-185.
24 Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97-132. quiz 133-1334; discussion 96
25 Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
26 Halwani M: LD-30: Infection spinal cord stimulator placement: a retrospective cohort study. Infectious Diseases Society of America. Presented Saturday, October 23, 2010; Vancouver, British Columbia.
27 Ishizuka K, Oaklander AL, Chiocca EA. A retrospective analysis of reasons for reoperation following initially successful peripheral nerve stimulation. J Neurosurg. 2007;106(3):388-390.
28 North RB, Kidd DH, Zahurak M, et al. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery. 1993;32(3):384-394. discussion 394-395
29 Ubbink DT, Vermeulen H, Spincemaille GH, et al. Systematic review and meta-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg. 2004;91(8):948-955.
30 Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31(3):271-285.
31 Henderson J, Schade C, Sasaki J, et al. Prevention of mechanical failures in implanted spinal cord stimulation systems. Neuromodulation. 2006;9(3):183-191.
32 Hassenusch SJ, Stanton-Hicks M, Schoppa D, Walsh JG, Covington EC. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996 Mar;84(3):415-423.
33 Ochani TD Almirante J, Siddiqui A, Kaplan R. Allergic reaction to spinal cord Stimulator. Clin J Pain. 2000;16:178-180.
34 Schwedt TJ, Dodick D, Hentz J, Trentman TL, Zimmerman RS. Occipital nerve stimulation for chronic headache-long-term safety and efficacy. Cephalalgia. 2007;27:153-157.
35 Verrillis P, Vivian D, Mitchell B, Barnard A. Peripheral Nerve Stimulation for Chronic Pain: 100 cases and Review of the Literature. Pain Medicine. 2011;12:1395-1405.
36 Beer GM, Wallner H. Prevention of Seroma after Abdominoplasty. Aesthetic Surgery Journal. 2010;30(3):414-417.
37 Jasper J, Hayek S. Implanted Occipital Nerve Stimulator. Pain Physician. 2008;11:187-200.
38 Kumar K, Buchser E, Linderoth B, Meglio M, Van Buyten JP. Avoiding Complications From Spinal Cord Stimulation: Practical Management Recommendations an International Panel of Experts. Neuromodulaton. 2007;10:24-33.
39 Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal Cord Stimulation for with Failed back Surgery Syndrome or complex Regional Pain Syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-147.
40 Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254-267.
41 New York School of Regional Anesthesia. Complications of peripheral nerve blocks. http://www.nysora.com/regional_anesthesia/other_topics/3132-compliations_of_regional_anesthesia.html, 2009. Retrieved January 31, 2010 from
42 Auroy Y, Narchi P, Messiah A, et al. Serious complications related to regional anesthesia: Results of a prospective survey in France. Anesthesiology. 1997;87(3):479-486.
43 Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service. Anesthesiology. 2002;97(5):1274-1280.