Chapter 17 Complications of Percutaneous Vertebral Augmentation
Vertebroplasty and Kyphoplasty
 Painful VCFs caused by osteoporosis and malignancy are seen more frequently as the U.S. population ages and life expectancies increase.
Painful VCFs caused by osteoporosis and malignancy are seen more frequently as the U.S. population ages and life expectancies increase. Because VCFs can cause debilitating problems, including spinal deformity, impairment of locomotion, decreased lung function, heightened risk of subsequent fracture, and increased mortality, treatment is essential to inhibit the development of deleterious sequelae.
Because VCFs can cause debilitating problems, including spinal deformity, impairment of locomotion, decreased lung function, heightened risk of subsequent fracture, and increased mortality, treatment is essential to inhibit the development of deleterious sequelae. PVA collectively refers to vertebroplasty and kyphoplasty, two minimally invasive procedures used to treat VCFs.
PVA collectively refers to vertebroplasty and kyphoplasty, two minimally invasive procedures used to treat VCFs. Vertebroplasty and kyphoplasty involve injection of medical-grade orthopedic cement into damaged, painful vertebral bodies. Whereas kyphoplasty incorporates the use of a balloon to expand the injection site before introduction of the orthopedic cement, vertebroplasty involves injection of cement directly into the collapsed vertebrae without balloon pretreatment.
Vertebroplasty and kyphoplasty involve injection of medical-grade orthopedic cement into damaged, painful vertebral bodies. Whereas kyphoplasty incorporates the use of a balloon to expand the injection site before introduction of the orthopedic cement, vertebroplasty involves injection of cement directly into the collapsed vertebrae without balloon pretreatment. The primary goal of PVA is to reduce the pain associated with VCFs. Secondary goals may include stabilization of crumbling vertebrae and prevention of further vertebral collapse, reversal of height loss (height restoration), and correction of kyphotic deformities that result from VCFs.
The primary goal of PVA is to reduce the pain associated with VCFs. Secondary goals may include stabilization of crumbling vertebrae and prevention of further vertebral collapse, reversal of height loss (height restoration), and correction of kyphotic deformities that result from VCFs. Because of their minimally invasive nature, PVA procedures carry a lower risk of complications than more invasive interventions such as open surgery. However, PVA procedures are associated with serious risks, including errant needle placement, cement extravasation, side effects related to the procedure (e.g., adjacent level fractures), infection, and risks from anesthesia.
Because of their minimally invasive nature, PVA procedures carry a lower risk of complications than more invasive interventions such as open surgery. However, PVA procedures are associated with serious risks, including errant needle placement, cement extravasation, side effects related to the procedure (e.g., adjacent level fractures), infection, and risks from anesthesia.Introduction
Worldwide approximately 1.4 million persons currently have vertebral compression fractures (VCFs),1 which are caused predominantly by osteoporosis and malignancy. The incidence of painful VCFs is increasing as age and life expectancy increase.
Osteoporosis, the leading cause of VCFs, is the most common metabolic bone disorder in the United States, affecting 25 million people and leading to approximately 750,000 new osteoporotic vertebral fractures each year.2,3 Of these, only one-third receive treatment,4 and more than one-third become chronically painful.2 Annualized direct care expenditures for osteoporotic fractures in the United States were estimated to range from $12 billion to $18 billion in 2002.5
Bone malignancy, another leading cause of VCFs, is a consequence of cancer, the second leading cause of death in the United States. Roughly two-thirds of cancer patients develop metastases,6,7 and vertebral body metastases occur frequently in systemic malignancy. In fact, the skeletal system is the third most common site of metastasis, and metastases favor the spine.8
Vertebral compression fractures can cause debilitating spinal deformity with possible impairments in locomotion,9,10 decreased lung function,11 a heightened risk of subsequent fracture,12 and increased mortality.13,14 Therefore many believe expert and efficacious treatment of VCFs is essential to inhibit the development of deleterious sequelae.
Although vertebroplasty and kyphoplasty are similar procedures with largely analogous methodologies and objectives, the chief distinction between them is that kyphoplasty incorporates a balloon to expand the injection site before introduction of orthopedic cement, while vertebroplasty involves injection of cement directly into the collapsed vertebrae without balloon pretreatment. The addition of balloon pretreatment may permit greater gains in vertebral height and kyphosis correction than are achievable with vertebroplasty.15 Because of the publication of numerous studies that suggest kyphoplasty may have greater efficacy than vertebroplasty, some practitioners consider kyphoplasty to be superior to vertebroplasty for the correction of painful VCFs.16 However, there exists a comparable number of studies that suggest vertebroplasty is as efficacious as kyphoplasty in stabilizing vertebral fractures and correcting attendant pain and other related consequences. Furthermore, the higher cost associated with kyphoplasty and the outpatient nature of vertebroplasty have made vertebroplasty a more attractive treatment option in many cases.17
Vertebroplasty
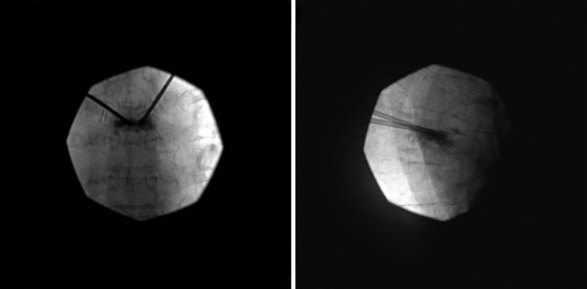
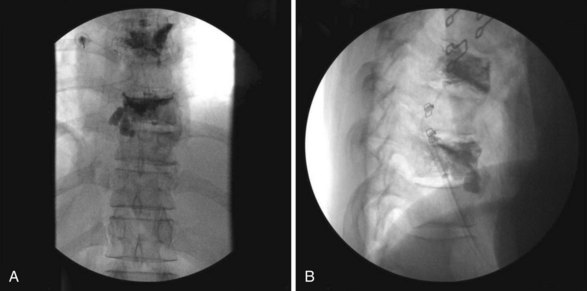
Bone cement may be mixed with antibiotic and barium or tantalum to reduce the risk of infection and facilitate fluoroscopic visibility. Normally, a transpedicular approach is used to access the central portion of the vertebral body in the thoracic and lumbar spine because this spares the spinal canal or segmental arteries flanking the vertebral body. The procedure can be done with an extrapedicular approach as well but may not have as robust of a safety profile. Vertebroplasty can be done unilaterally or bilaterally as desired by the performing surgeon (Figs. 17-1 and 17-2). Some experts do recommend a bilateral approach because they believe that this provides increased safety through redundancy and ensures central placement of cement. No current prospective studies have clearly defined the most efficacious method of these two approaches.
Kyphoplasty
Kyphoplasty is a newer therapy indicated for the treatment of painful VCFs. Kyphoplasty parallels vertebroplasty in that it is highly effective in relieving pain associated with VCFs. However, several studies suggest that kyphoplasty can also restore height and reduce deformity compared with vertebroplasty.18 The data are not completely clear, however, because one study found that kyphoplasty and vertebroplasty produce the same degree of height restoration,19 although a later cadaver study by the same group20 found the increase in vertebral height was greater with kyphoplasty than with vertebroplasty (5.1 mm vs. 2.3 mm, respectively).
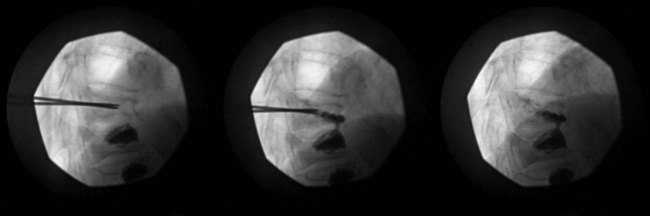
Normally, kyphoplasty is performed under intravenous (IV) sedation or monitored anesthesia care, but some practitioners prefer to do the procedure with general anesthesia. As in vertebroplasty, fluoroscopy (or CT scan) is used to guide the procedure. Typically, a small incision is made, and the trocars are advanced bilaterally into the vertebral body using a transpedicular approach (Fig. 17-3). Kyphoplasty can also be done via an extrapedicular approach, and some systems advocate a unilateral approach (Fig. 17-4). When the trocars are in the correct position, a cannulated drill is placed through the trocar, and the bone is drilled by hand creating space within the vertebral bodies for the balloons. The drill is then removed, and the balloons (inflatable bone tamps) are inserted through the trocars. The balloons are filled with contrast medium and carefully inflated under live radiography for easy visualization. Subsequently, the balloons are inflated until they expand to the desired height and then removed, leaving prearranged cavities for the cement within the fractured vertebral body. This process is what is thought to provide the height restoration for the procedure. The cavities are then filled with the same type of orthopedic cement used in vertebroplasty, and the trocars removed. Because of its widely promoted height-restoration effects, many physicians recommend kyphoplasty over vertebroplasty. It should be noted, however, that kyphoplasty is far more expensive a procedure, potentially costing significantly more than vertebroplasty. Clinicians are in need of prospective comparative studies that match these two modalities of treatment to specific types of fractures.
Indications and Contraindications
The vertebral compression associated with osteoporosis results from lowered bone strength, a consequence of accelerated loss of bone minerals. Furthermore, kyphosis secondary to osteoporotic VCF shifts the center of gravity anteriorly, increasing the risk of falls and additional fractures. In conjunction with physiologic decreases in lung capacity and appetite, a significant increase in 1-year mortality is associated with a patient’s first VCF.14
Absolute contraindications to vertebroplasty and kyphoplasty are rarely seen but include:
Benefits
In the literature, vertebroplasty and kyphoplasty have been described as bringing significant pain relief to patients with vertebral fractures secondary to osteoporosis and solid or marrow-based malignancy (80% to 89% in most reports). For most, relief is usually immediate after treatment, with maximal effect obtained as soon as 2 weeks after treatment. According to one systematic review of the literature, there is fair evidence (level II to III) that 2 years after the intervention, vertebroplasty provides a similar degree of pain control and physical function as optimal medical management. There is also fair evidence (level II to III) that kyphoplasty results in greater improvement in daily activity, physical function, and pain relief compared with optimal medical management for osteoporotic VCFs 6 months after the intervention.21 Furthermore, a meta-analysis of the literature examining pain relief and complications in vertebroplasty and kyphoplasty patients in 1036 studies found that both vertebroplasty and kyphoplasty provided significant pain improvement as measured by visual analog scores (VAS); vertebroplasty produced greater improvement in VAS scores than kyphoplasty but also carried a statistically greater risk of cement leakage and new fracture.22
In addition, the pain relief from both procedures has been noted as long lasting as demonstrated in studies with multiyear follow-ups.23,24 One such multiyear study, a retrospective single-center consecutive case series with 2-year follow-up, found kyphoplasty markedly improved clinical outcome and produced significant vertebral height restoration and normalization of morphologic shape indices that remained stable for at least 2 years after treatment.25 Pain scores, patient ability to ambulate independently and without difficulty, and need for prescription pain medications improved significantly after kyphoplasty and remained unchanged or improved at 2 years; furthermore, vertebral heights significantly increased at all postoperative intervals, with greater than or equal to 10% height increases in 84% of fractures. Asymptomatic cement extravasation occurred in 11.3% of fractures, and during the follow-up period, additional fractures occurred in previously untreated levels at a rate of 4.5% per year. There were no kyphoplasty-related complications.
Numerous studies have examined the specific benefits of kyphoplasty. For example, a randomized, controlled trial of 300 patients at 21 sites in eight countries noted significant improvement in quality of life in kyphoplasty patients over the control group as assessed by short form physical component summary.26 Additionally, a study in 314 kyphoplasty recipients with VCFs as a result of osteoporosis or multiple myeloma found that all patients tolerated the kyphoplasty procedure well, the average Owestry Disability Index score decreased by 12.6 points, and there was no statistically significant difference with regard to functional outcome in the osteoporosis and multiple myeloma subgroups.27 And a study in 555 patients with a total of 1150 vertebral fractures treated with kyphoplasty noted no cement-related complications as described in the literature and concluded by careful interdisciplinary indication setting and a standardized treatment model that kyphoplasty presented a safe and effective procedure for the treatment of various vertebral fractures.28
Despite numerous studies in the literature that support the efficacy of PVA, two controversial original articles questioning the worth of vertebroplasty were published in the August 6, 2009, issue of the New England Journal of Medicine. These included reports by Kallmes et al29 on the Investigational Vertebroplasty Safety and Efficacy Trial (ClinicalTrials.gov number, NCT00068822) and Buchbinder et al30 on a randomized trial of vertebroplasty for painful osteoporotic vertebral fractures (Australian New Zealand Clinical Trials Registry number, ACTRN012605000079640). In both studies, the authors concluded that vertebroplasty has no greater benefit than placebo in the treatment of painful osteoporotic compression fractures.
Kallmes et al29 reported, “Improvements in pain and pain-related disability associated with osteoporotic compression fractures in patients treated with vertebroplasty were similar to the improvements in a control group.” In this multicenter trial, 131 patients with one to three painful osteoporotic VCFs underwent either vertebroplasty or a simulated procedure without cement (control group). The primary outcomes were scores on the modified Roland–Morris Disability Questionnaire (RDQ) (on a scale of 0 to 23, with higher scores indicating greater disability), and patients’ ratings of average pain intensity during the preceding 24 hours at 1 month (on a scale of 0 to 10, with higher scores indicating more severe pain). Patients were permitted to cross over to the other study group after 1 month. All patients underwent the assigned intervention (68 vertebroplasties and 63 simulated procedures). The baseline characteristics were similar in the two groups. At 1 month, it was reported that there was no significant difference between the vertebroplasty group and the control group in either the RDQ score or the pain rating. Both groups had immediate improvement in disability and pain scores after the intervention. Although the two groups did not differ significantly on any secondary outcome measure at 1 month, there was a trend toward a higher rate of clinically meaningful improvement in pain (a 30% decrease from baseline) in the vertebroplasty group (64% vs. 48%; P = .06). At 3 months, there was a higher crossover rate in the control group than in the vertebroplasty group (43% vs. 12%; P < .001), according to the report.
The Buchbinder et al30 report described a randomized, double-blind, placebo-controlled trial in which participants (n = 78) had one or two painful osteoporotic vertebral fractures that were of less than 12 months’ duration and unhealed, as confirmed by magnetic resonance imaging (MRI), and randomly assigned to undergo vertebroplasty or a sham procedure. Participants were stratified according to treatment center, sex, and duration of symptoms (<6 weeks or ≥6 weeks). Outcomes were assessed at 1 week and at 1, 3, and 6 months. The primary outcome was overall pain (on a scale of 0 to 10, with 10 being the maximum imaginable pain) at 3 months. Buchbinder et al30 reported they found no beneficial effect of vertebroplasty compared with a sham procedure in patients with painful osteoporotic vertebral fractures at 1 week or at 1, 3, or 6 months after treatment. They noted that vertebroplasty did not result in a significant advantage in any measured outcome at any time point, although there were significant reductions in overall pain in both study groups at each follow-up assessment. Similar improvements were seen in both groups with respect to pain at night and at rest, physical functioning, quality of life, and perceived improvement.
In response to these original articles, proponents of vertebroplasty submitted correspondence that critiqued the studies. One group of respondents31 expressed “serious concerns” over both trials, noting that the study by Kallmes et al29 involved outpatients exclusively and excluded inpatients hospitalized with acute fracture pain. The same group of commentators noted that the study protocol mandated 4 weeks of medical therapy before enrollment, which they maintained resulted in a study on healed rather than subacute fractures, and noted a more appropriate selection criterion would have been uncontrolled pain for fewer than 6 weeks. They also noted that the Buchbinder et al30 trial included an inadequate number of patients for a subgroup analysis, substantially limiting statistical power, and that although the study is described as a multicenter trial, two of the four hospitals withdrew early from the study after enrolling five patients each. The authors of the correspondence also argued that 68% of the procedures were performed in one hospital by one radiologist, and the rates of eligible patients who declined to participate were 64% in the Buchbinder trial30 and 70% in the Kallmes trial29 (85% in the United States), raising further concerns regarding patient selection.
Another group of correspondents32 noted that patients with maximal back pain, who tend to have the greatest improvement in pain score after vertebroplasty, would also be the least likely to participate in such studies because they would risk being randomly assigned to the placebo group, as evidenced by the preprocedural pain scores among patients who were enrolled. The correspondents also commented that neither study was sufficiently powered to perform subanalyses, particularly on patients with certain types of fractures that would be more likely to have improved pain scores after vertebroplasty (e.g., those with gas-filled clefts and pathologic fractures).
Also, a singleton respondent33 questioned whether a group of patients who receive an injection of an anesthetic should be considered a true control because delivery of a local anesthetic can have beneficial effects for a period exceeding the activity of the drug. He also suggested that there may be a significant difference in crossover rates between the treatment and control groups (12% vs. 43%) in the study by Kallmes et al,29 which could suggest patient dissatisfaction with the sham procedure that was not fully captured by pain scales.
Certainly, these criticisms provide compelling reasons to question the results of the controversial Kallmes et al29 and Buchbinder et al30 studies. Furthermore, it should be noted that these two studies compete with a vast pool of published evidence that supports the therapeutic legitimacy of PVA.
Complications
Because of their minimally invasive nature, PVA procedures carry a lower risk of complications than more invasive interventions such as open surgery. For example, a level III therapeutic study found that kyphoplasty had a complication rate equivalent to nonoperative treatment (1.7% vs. 1.0%), and a lower rate of in-hospital mortality (0.3% vs. 1.6%).34
Errant Needle Placement
A potentially devastating error in PVA is errant needle placement, which can lead to adverse sequelae such as spinal cord injury; damage to nerves, vessels and organs; and excessive bleeding and pneumothorax.35
Cement Extravasation
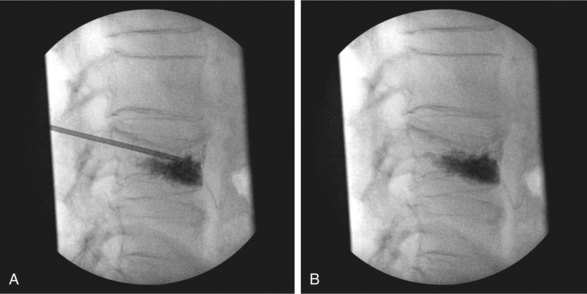
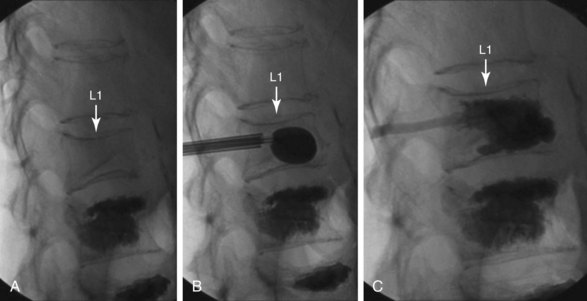
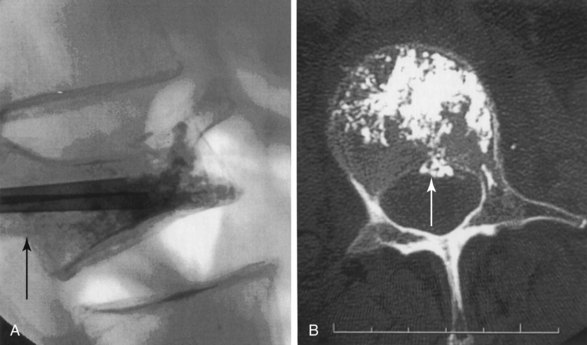
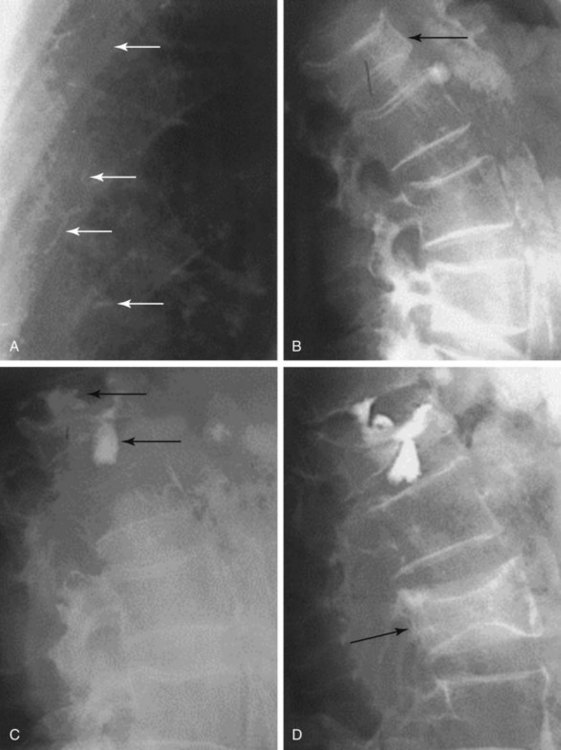
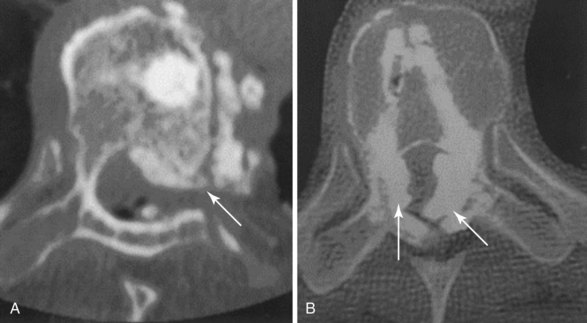
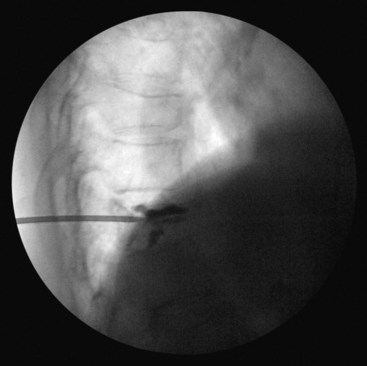
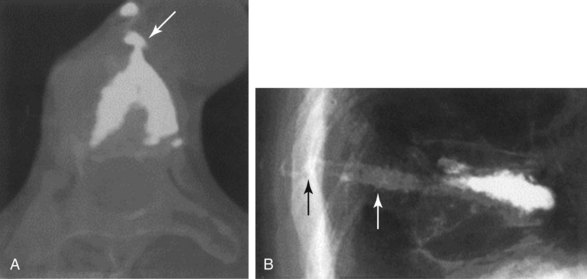
The chief complication reported in the literature for both procedures is cement leakage, or extravasation (Figs. 17-5 and 17-6). Normally, this complication is asymptomatic. However, patients with symptomatic cement leakage along the spinal nerve roots may experience dysesthesia, culminating in radicular pain or paraplegia (Fig. 17-7). Furthermore, cement leakage into vessels can lead to embolism into any vessels, including the pulmonary arteries, depending on the amount leaked (Fig. 17-8). Leakage into the spinal canal can lead to tremendous neurologic complications; a study documenting a series of cases of neurologic deficit after vertebroplasty and kyphoplasty found that catastrophic neurologic injury required open surgical intervention in 12 of 14 patients who developed neurologic deficit after undergoing vertebroplasty or kyphoplasty (Fig. 17-9).36
Despite cement leakage, however, vertebroplasty and kyphoplasty have proven to be safe and effective in many studies in the literature. For example, a 4-year study of 28 patients who underwent either of the two procedures found the only complication was cement extravasation, but no clinical symptom occurred23 (Fig. 17-10). Furthermore, there was no significant difference between the incidences of cement leakage between the two procedures. In addition, there was significant change in preoperative and postoperative VAS and Oswestry scores in both groups.
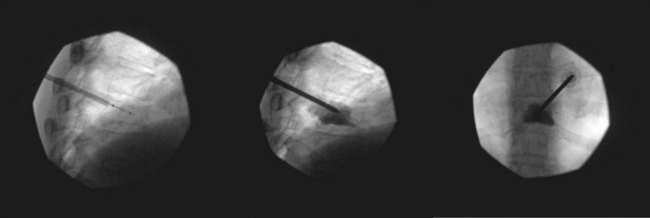
Because bone cement is a relatively low-viscosity medium when first injected, it carries a relatively high risk of extravasation (Fig. 17-11). According to one study,37 in almost all cases, cement leakage occurs because of premature application of cement before it reaches its optimum thickness. Furthermore, according to the authors of this study, the literature shows a percentage rate of about 9% for cement leakage from balloon kyphoplasty versus leakage rates of up to 41% for vertebroplasty, with some studies reporting cement leakage ratios of 4% to 10% for kyphoplasty versus 20% to 70% for vertebroplasty. Cement extravasation in vertebroplasty, which can result in spinal cord and nerve root compression, cardiac tamponade, and pulmonary and other emboli,38–40 has been shown to be a direct consequence of the amount of cement injected; this complication is mitigated by the fact that pain relief is not related to the volume of injection and appears to occur with relatively small amounts of injected cement.41
Side Effects from the Procedure
One side effect of PVA often reported in the literature is adjacent level fractures. Independent studies have demonstrated a recurrent fracture rate of 12.4% to 21%, with the majority of these new fracture levels occurring adjacent to the PV level.42,43 However, the evidence for increased risk of adjacent level fracture after PVA compared with conservative treatment remains inconclusive.40 A widely posed reason to reject this correlation is that new adjacent level fractures occur in patients with a natural history of osteoporosis regardless of whether they undergo PVA.44
One study45 showed an incidence of 6.16% (18 of 292) of adjacent level fractures after kyphoplasty in patients with a mean age of 72.7 years over a mean follow-up interval of 25.6 months, although predictors for new vertebral fracture failed to identify statistically significant risk factors despite a large sample size. Another retrospective study correlating PVA with a subsequent heightened adjacent fracture risk reported that of 38 patients, 10 patients sustained 17 subsequent fractures over the follow-up period, with nine at adjacent-above levels, four at adjacent-below levels, and four at remote levels.46 Most of those fractures at adjacent levels occurred within 2 months of the procedure. The authors concluded that the data indicated a higher rate of subsequent fracture after kyphoplasty compared with natural history data for untreated fractures and speculated that because there were only occasional subsequent fractures at remote levels after the 2-month postprocedural period, it confirmed biomechanical studies showing that cement augmentation places additional stress on adjacent levels.
One possible cause of new adjacent VCFs noted in the literature is cement leakage into the intervertebral disc, which may necessitate further PVA for pain relief. A retrospective study assessing new adjacent level VCFs after percutaneous vertebroplasty found VCFs occurred in 20 (18.9%) of 106 patients at 22 adjacent vertebral bodies after vertebroplasty during at least 24 months of follow-up, with the difference in number of new adjacent fractures between patients with cement leakage into the disc and those without leakage statistically significant and the risk of adjacent vertebral fracture higher if cement leaked into the disc.47
It is important to note that although numerous studies find an association between PVA and adjacent fracture, a relatively equivalent number of reports do not propose a correlation between these events. One study with this viewpoint, involving 188 patients (163 women, 25 men; mean age, 70.9 years; range, 42 to 92 years) who underwent 214 PVA sessions at 351 levels for osteoporotic VCFs, evaluated the effect of intradiscal cement leakage on new adjacent vertebral fracture formation after PVA and concluded that intradiscal cement leakage does not seem to be related to subsequent adjacent VCF in patients who underwent PVA for treatment of an osteoporotic compression fracture.48 The validity of the study is strengthened because additional risk factors were also analyzed using univariate and multivariate methods, including age, gender, mean bone mineral density (BMD), the vertebral level treated, the presence of an intravertebral cleft or cyst before treatment, the kyphosis angle, the wedge angle, and the injected cement volumes. The authors noted that the only factor correlated with an adjacent vertebral fracture after PVA was thoracolumbar location of the initial compression fracture. In addition, a prospective observational cohort study of consecutive osteoporotic VCFs in patients who were 90 years old and older evaluated at a multidisciplinary, university spine center found vertebroplasty for VCFs in the very elderly appears effective and safe without increased risk of adjacent level fracture.45 The patient sample, which included consecutive osteoporotic patients with symptomatic VCFs electing to enter the study, were evaluated on a baseline VAS rating, analgesic usage, and duration of symptoms. Subsequent VAS ratings, analgesic utilization, and new fractures were assessed at planned intervals. The mean VAS score was 7.6 at baseline, 3.1 at 30 minutes after the procedure, and 2.3, 1.2, 1.1, 0.9, 0.8, and 0.5 at 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years, respectively. Improvement over time was statistically significant, and no complications were encountered during the follow-up intervals. Thirteen new fractures were observed (10.6%) at a mean 20.8 weeks (1 to 52 weeks) after PVA with six new fractures (4.9%) involving an adjacent level in five patients (4.1%). Another study comparing the strength of polymethylmethacrylate and calcium phosphate cement in 24 spinal columns (T10–L2) from human cadavers found that the strength of bone filler materials (not cement leakage or other factors) is considered a risk factor in developing adjacent vertebral body fractures after PVA.49
A key argument is that subsequent fractures after PVA may be a natural consequence of osteoporosis. A study in 73 consecutive patients with painful VCFs found that more patients with a BMD higher or equal to 3.0 experienced a new fracture than those with a BMD less than 3.0 (odds ratio [OR], 13.00; 95% confidence interval [CI], 1.35 to 124.81), and the risk for adjacent level fractures decreased significantly when the postoperative kyphotic angle was less than 9 degrees compared with that of higher or equal to 9 degrees (OR, 12.00; 95% CI, 1.25 to 114.88).50 The authors concluded that balloon kyphoplasty and vertebroplasty are methods with a low risk of adjacent level fractures, and the most important factors for new VCFs after a PVA procedure are the degree of osteoporosis and altered biomechanics in the treated area of the spine caused by resistant kyphosis. They also noted that the results suggest that the adjacent vertebrae would fracture eventually even without the procedure and that PVA offers a comparable rate of pain relief.
Low body mass index (BMI) may be another risk factor for VCF in patients with osteoporosis, irrespective of whether PVA is performed. A study found that new VCFs were common in patients with low BMIs, suggesting osteoporosis as a mechanism of fracture, although it should be noted that the new fractures were also associated with proximity to the treated vertebra and greater kyphosis correction.51 Another study found that lower baseline BMD, older age, and more preexisting VCFs were demonstrated in patients who experienced VCFs two or more times, although it is noteworthy that in 852 patients (1131 vertebrae), 58.8% to 63.8% of new compression fractures after vertebroplasty were adjacent compression fractures, and these occurred much sooner than nonadjacent fractures (71.9 ± 71.8 days vs. 286.8 ± 232.8 days; P < .001).52 Given the risk of subsequent fracture in patients with osteoporosis, postoperative use of anti-osteoporotic medication seems to be an excellent measure for the prevention of new VCFs.53
As with any surgical procedure, infection represents a probable adverse outcome of PVA, although the incidence is rare.54 Signs and symptoms post-PVA infection may include pain, swelling, rubor, purulent drainage, fever, nausea, vomiting, and chills. Of particular concern are signs of advanced infection, including elevated white blood cell, C-reactive protein, and sedimentation rate counts. Because infection is a definite risk of PVA, practitioners should endeavor to lower the incidence of topical and systemic infection in their patients. Prophylactic antibiotics are given IV (e.g., 1 g cefazolin) because PVA involves injection of foreign material into the body.55 Beyond administration of antibiotics, screening patients for topical infections, immunocompromised status, and systemic conditions, the most important measure against infection is meticulous surgical technique. To achieve this, iodine or chlorhexidine should be used as a surgical preparation, with a wide area of the back prepared because a variety of angles of approach may be used. Sterile drapes are placed over the patient, and measures such as a surgical scrub and use of masks and gloves should be incorporated.
Percutaneous vertebral augmentation can be performed under mild sedation with local or general anesthesia, but the operation must be convertible in a short time frame to an open emergency operation in cases of severe bone-cement leakage into the spinal canal.55 Sedative analgesics in the form of fentanyl, midazolam, or propofol can be administered.56
Particularly in elderly osteoporosis patients, a notable side effect of surgery is rib fracture from prone positioning on the operating table. Physicians should remain mindful of this possibility and may opt to pad the voids between the patient and the table to achieve maximum extension of the spine, protect pressure points covered by thin skin, avoid rib fracture, and promote patient comfort; it should also be noted that in this largely elderly patient population, rib fracture can occur simply from mobilizing the patient.57
Other general side effects of PVA reported in the literature have included cerebrospinal fluid leakage,58 bleeding,59 new fracture,51 nerve damage,60 and epidural and subdural hematoma.61 Additional complications have included recurrent back pain,62 vena cava syndrome,63 osteitis,64 epidural abscess,65 and aseptic osteonecrosis.66 Development of local metastases along the tract of the needle (needle tract seeding) in a lung cancer patient has also been reported.67 Additionally, a recent report describes a rare case of anterior spinal cord syndrome caused by a cement embolism in the anterior spinal artery after vertebroplasty.68 And one group of authors postulates that local infiltration anesthesia is not sufficient for “a substantial proportion of patients” based on the results of a study in 44 vertebroplasty patients.69
Bleeding complications also represent a possible adverse effect in PVA. In general, the cement seals most of the bony needle tract from the vertebral body to the pedicle, which should alleviate most of the problem. Still, fracture of a pedicle or tearing of a vein proximal to the spinal canal could lead to a large hematoma in a patient with bleeding diathesis. This can be avoided by verifying that coagulation studies and platelet levels are normal before the procedure.35
Reducing Complications
One of the most crucial ways to reduce complications in PVA is to seek adequate training. The best route to achieving this is to obtain adequate supervision initially while performing the procedure. Guidance, repetition, and mentorship may be gained through residency and fellowship experience, cadaver courses, and “real-world” supervision during professional practice. In addition, better results may be expected when there is limited compression of the vertebral body, the fracture is less than 12 months old, or the radionuclide bone scan or T2-weighted MRI indicates the fracture contains active marrow edema.70
One of the fundamentals in complication avoidance in PVA is safe needle placement. Beyond practice and skill, mastering this technique requires advanced understanding of vertebral anatomy and the ability to skillfully interpret both normal and abnormal fluoroscopic images. This also requires a fully functional procedure suite with biplane or single-plane high-quality fluoroscopy capabilities. Poor visualization is associated with migration of cement. One study recommended over conventional CT and biplane fluoroscopy the use of isocentric C-arm fluoroscopic cone beam CT (CBCT), a new technique for near real time 3-dimensional volume imaging guidance of percutaneous interventional procedures of the spine. The CBCT has the capability to produce near real time high-resolution image reconstructions in any plane and may be superior to conventional fluoroscopy, but it is not widely used.71 Failure to master anatomic landmarks, interpret fluoroscopic images, and use high-quality fluoroscopic equipment could result in adverse outcomes such as leakage of cement into the epidural canal, which could result in partial or complete paralysis, neuritis, or embolism. Clearly, development of expertise with fluoroscopy is important in precluding disastrous consequences.
To ensure optimal needle placement, the site of entry should be carefully identified. In the thoracic and lumbar regions, a transpedicular approach is ideal. In contrast, in the cervical spine an anteromedial approach is optimal because the pedicles are too small to accommodate the needles, and a posterolateral approach threatens the vertebral artery.35 Initially, the fractured vertebral body should be identified with proper counting and visualized in both anteroposterior (AP) and lateral projections. There are many techniques to access the vertebral body in the transpedicular fashion, and most courses teach a systematic way to perform the procedure. Syed and Shaikh72 also have a technique review that many physicians find helpful. In general after the fractured vertebral body is identified, the endplates are then squared in an AP projection. Through craniocaudad tilting of the fluoroscopic image under live fluoroscopy, the pedicles are then placed in the center of the target area so that the needle traverses the pedicles into the correct portion of the vertebral body. The skin and subcutaneous tissue are then anesthetized (the authors of this chapter prefer lidocaine 1% with epinephrine) and the tracts are anesthetized down to the pedicles and the pedicles themselves are anesthetized. With the anesthetic needles left in place, one can then visualize the angles of needle trajectory on both AP and lateral views to ensure correct trocar placement in similar fashion. Some will then make a small incision with a scalpel, and the large-bore trocars (typically between 10 and 13 gauge) are inserted through the anesthetized tissue. The trocars are then advanced toward the upper outer quadrant of the pedicles and seated into the bone. The trocars are then advanced with frequent fluoroscopic images monitoring the needle trajectory and position. The trocar should follow a path that is parallel to the superior and inferior edges of the pedicle. In fractured vertebral bodies with endplate depression, a different angle may be required to avoid traversing the endplate. Care must be taken to visualize the medial cortex of the pedicle at all times, and the trocars must not violate this boundary as they traverse the vertebral body. Typically, frequent AP and lateral images are monitored as the trocars are being advanced through the pedicles. When the trocars are seen on lateral view to pass the posterior margin of the vertebral body, the spinal canal has been cleared safely. Up to this point, the medial cortex of the pedicle should not have been violated. The trocars can then be advanced to their correct depth within the vertebral body for either the vertebroplasty or kyphoplasty procedure. With vertebroplasty in a lateral fluoroscopic projection, at the posterior vertebral body, the trocars are advanced into the anterior third portion of the vertebral body because this area is mostly devoid of venous plexuses. In kyphoplasty, the trocars are advanced into the posterior third of the vertebral body, and the bone drills are then advanced through the trocars to make room for the inflatable balloons.
Although not commonly performed, some advocate that venography may be performed before cement injection to facilitate accuracy.73 This is achieved by highlighting the anastomosis of the epidural canal, central veins, and inferior vena cava, which may illustrate an outline of the venous drainage, vertebral cortex fractures, and possible leakage sites. Furthermore, accurate needle placement within the bony trabeculae may be confirmed. Corrections in needle placement should be made early in the procedure when the needle is still in the pedicle.74 It should be noted there is a risk of fracturing the pedicle because of the large size of the biopsy needle, and CT scan before the procedure can help assess the degree of this risk.75 Although this technique may highlight the venous anatomy, most practitioners do not use this technique and instead choose to inject thickened contrast-enhanced bone cement into the fractured vertebral body with live fluoroscopy.
Safe cement placement techniques are also important in evading complications in PVA. These techniques include adequate barium radio-opacification of cement; viscous low-pressure delivery of cement; lower volumes of cement; and cement delivery under high-quality, direct fluoroscopic visualization.76
Cement should be mixed sufficiently to achieve the optimal, “toothpaste-like” consistency important in precluding extravasation; however, superfluous mixing can result in excessive emulsification of the barium, which lowers fluoroscopic efficacy. Cement must be viscous enough to avoid excessive pressure during placement because this is associated with extravasation. In kyphoplasty, cavities are created before cement is injected. Care must still be taken when injecting the cement into the fractured vertebral body because extravasation can still occur. In vertebroplasty, a small amount of cement should be placed into the anterior two-thirds of the vertebral body. After 30 seconds, the spread of cement may be visualized in AP and lateral views. Pausing during injection to allow curing of the cement can sometimes halt extravasation and allow further filling of the vertebral body.77 This initial “test dose” of cement should be performed in both vertebroplasty and kyphoplasty to prevent extravasation of cement.
To lower the likelihood of cement extravasation, it is important to observe the spread of cement under live fluoroscopy. This is achieved optimally in the lateral view.56 Cement extravasation can be promoted by high cement volume because use of excessive amounts of cement invites migration into unwanted areas. In fact, one study finds that the rate of complications in PVA is directly related to the volume of cement injected,76 but another study asserts that pain relief is not related to volume of cement injected and appears to occur with relatively low amounts of injected cement.78 High viscosity of cement is another way to mitigate migration; some experts recommend the use of a new high-viscosity cement (Confidence Spinal Cement System) to inhibit cement leakage.78 This is based on published results of vertebroplasties performed with Confidence cement; the author concluded that highly viscous cements may increase the safety of vertebral augmentation techniques when compared with less viscous cements.
Tips for Avoidance of Complications from Percutaneous Vertebral Augmentation
 Safe needle placement: do not violate the medial cortex of the pedicle until the posterior aspect of the vertebral body has been entered as per the lateral view
Safe needle placement: do not violate the medial cortex of the pedicle until the posterior aspect of the vertebral body has been entered as per the lateral view Take a lateral view of the fractured vertebral body and surrounding anatomy before cement is injected as a reference point
Take a lateral view of the fractured vertebral body and surrounding anatomy before cement is injected as a reference point Test dose: after the cement is visualized exiting the trocar and entering the vertebral body stop, watch and wait for 30 to 60 seconds
Test dose: after the cement is visualized exiting the trocar and entering the vertebral body stop, watch and wait for 30 to 60 seconds If the cement seems to be spreading as expected, proceed slowly with continued cement placed under live radiography in the lateral view
If the cement seems to be spreading as expected, proceed slowly with continued cement placed under live radiography in the lateral viewConclusion
Despite the limited number of randomized, controlled trials in the literature, vertebroplasty and kyphoplasty are believed to significantly improve quality of life for patients with compression fractures, and evidence of their safety and efficacy is increasing, suggesting that both are more effective than conservative management and carry a low risk of complications.79 The majority of available evidence supports kyphoplasty and vertebroplasty as effective therapies in the management of patients with symptomatic VCFs refractory to conventional medical therapy.
1 HoiKee N. Kyphoplasty for osteoporotic vertebral compression fractures. JAAPA. 2008;21(7):28-31.
2 Riggs BL, Melton LJIII. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17(suppl 5):S505-S511.
3 Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12:429-437.
4 Carmona RH. Office of the Surgeon General. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: Department of Health and Human Services; 2004.
5 Carmona RH. Office of the Surgeon General. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: Department of Health and Human Services; 1984.
6 Silverberg E. Cancer statistics. J Clin Cancer. 1984;34:7-23.
7 Jaffe W. Tumors and tumorous conditions of the bones and joints. Philadelphia: Lea & Febiger; 1958.
8 Gokaslan Z. Spine surgery for cancer. Curr Opin Oncol. 1996;8:178-181.
9 Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(suppl 3):S185-S189.
10 Sinaki M. Falls, fractures, and hip pads. Curr Osteoporos Rep. 2004;2:131-137.
11 Leech JA, Dulberg C, Kellie S, et al. Relationship of lung function to severity of oseteoporosis in women. Am Rev Respir Dis. 1990;14:68-71.
12 Kado DM, Duong T, Stone KL, et al. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int. 2003;14:589-594.
13 Kado DM, Huan MH, Karlamangla AS, et al. Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J Am Geriatr Soc. 2004;52:1662-1667.
14 Kado DM, Browner WS, Palermo L. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215-1220.
15 Theodorou DJ, Theodorou SJ, Duncan TD, et al. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging. 2002;26(1):1-5.
16 Garfin S, Reilley MA. Minimally invasive treatment of osteoporotic vertebral body compression fractures. Spine J. 2002;2(1):76-80.
17 Cloft HJ, Jensen ME. Kyphoplasty: an assessment of a new technology. AJNR Am J Neuroradiol. 2007;28(2):200-203.
18 Gan M, Yang H, Zhou F, et al. Kyphoplasty for the treatment of painful osteoporotic thoracolumbar burst fractures. Orthopedics. 2010;33(2):88.
19 Hiwatashi A, Westesson PL, Yoshiura T, et al. Kyphoplasty and vertebroplasty produce the same degree of height restoration. AJNR Am J Neuroradiol. 2009;30(4):669-673.
20 Hiwatashi A, Sidhu R, Lee RK, et al. Kyphoplasty versus vertebroplasty to increase vertebral body height: a cadaveric study. Radiology. 2005;237(3):1115-1119.
21 Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976). 2006;31(17):1983-2001.
22 Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8(3):488-497.
23 Sun ZG, Miao XG, Yuan H, et al. [Assessment of percutaneous vertebroplasty and percutaneous kyphoplasty for treatment of senile osteoporotic vertebral compression fractures]. Zhongguo Gu Shang. 2010;23(10):734-738.
24 Dong Y, Wang DY. [Treatment of osteoporotic vertebral compression fractures by balloon kyphoplasty]. Zhongguo Gu Shang. 2010;23(6):466-467.
25 Ledlie J, Renfro M. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine (Phila Pa 1976). 2006;31(1):57-64.
26 Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373(9668):1016-1024.
27 Khanna AJ, Reinhardt MK, Togawa D, Lieberman IH. Functional outcomes of kyphoplasty for the treatment of osteoporotic and osteolytic vertebral compression fractures. Osteoporos Int. 2006;17(6):817-826.
28 McArthur N, Kasperk C, Baier M, et al. 1,150 kyphoplasties over 7 years: indications, techniques, and intraoperative complications. Orthopedics. 2009;32(2):90.
29 Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569-579.
30 Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557-568.
31 Clark W, Lyon S, Burnes J. Trials of vertebroplasty for vertebral fractures. N Engl J Med. 2009;361:2097-2100.
32 Baerlocher M, Munk PL, Liu DM. Trials of vertebroplasty for vertebral fractures. N Engl J Med. 2009;361:2098.
33 Lotz J. Trials of vertebroplasty for vertebral fractures. N Engl J Med. 2009;361:2098.
34 Zampini JM, White AP, McGuire KJ. Comparison of 5766 vertebral compression fractures treated with or without kyphoplasty. Clin Orthop Relat Res. 2010;468(7):1773-1780.
35 Miller D, Fenton D, Dion J. Vertebroplasty. In: Image-guided spine intervention. Philadelphia: Saunders; 2003:190.
36 Patel AA, Vaccaro AR, Martyak GG, et al. Neurologic deficit following percutaneous vertebral stabilization. Spine (Phila Pa 1976). 2007;32(16):1728-1734.
37 Bula P, Lein T, Strassberger C, Bonnaire F. [Balloon kyphoplasty in the treatment of osteoporotic vertebral fractures: indications—treatment strategy—complications]. Z Orthop Unfall. 2010;148(6):646-656.
38 Cohen J, Lane T. Right intra-atrial and ventricular polymethylmethacrylate embolus after balloon kyphoplasty. Am J Med. 2010;123(10):e5-e6.
39 Caynak B, Onan B, Sagbas E, et al. Cardiac tamponade and pulmonary embolism as a complication of percutaneous vertebroplasty. Ann Thorac Surg. 2009;87(1):299-301.
40 Röllinghoff M, Zarghooni K, Dargel J, et al. The present role of vertebroplasty and kyphoplasty in the treatment of fresh vertebral compression fractures. Minerva Chir. 2010;65(4):429-437.
41 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 suppl):21-30.
42 Syed M, Jan S, Patel NA, et al. Vertebroplasty: the alternative treatment of osteoporotic vertebral compression fractures in the elderly. Clin Geriatr. 2006;14:20-23.
43 Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119-124.
44 DePalma MJ, Ketchum JM, Frankel BM, Frey ME. Percutaneous vertebroplasty for osteoporotic vertebral compression fractures in the nonagenarians: a prospective study evaluating pain reduction and new symptomatic fracture rate. Spine (Phila Pa 1976). 2011;36(4):277-282.
45 Chen WJ, Kao YH, Yang SC, et al. Impact of cement leakage into disks on the development of adjacent vertebral compression fractures. J Spinal Disord Tech. 2010;23(1):35-39.
46 Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine (Phila Pa 1976). 2004;29(20):2270-2277.
47 Lo YP, Chen WJ, Chen LH, Lai PL. New vertebral fracture after vertebroplasty. J Trauma. 2008;65(6):1439-1445.
48 Lee KA, Hong SJ, Lee S, et al. Analysis of adjacent fracture after percutaneous vertebroplasty: does intradiscal cement leakage really increase the risk of adjacent vertebral fracture? Skeletal Radiol. 2011. in press
49 Nouda S, Tomita S, Kin A, et al. Adjacent vertebral body fracture following vertebroplasty with polymethylmethacrylate or calcium phosphate cement: biomechanical evaluation of the cadaveric spine. Spine (Phila Pa 1976). 2009;34(24):2613-2618.
50 Movrin I, Vengust R, Komadina R. Adjacent vertebral fractures after percutaneous vertebral augmentation of osteoporotic vertebral compression fracture: a comparison of balloon kyphoplasty and vertebroplasty. Arch Orthop Trauma Surg. 2010;130(9):1157-1166.
51 Lin WC, Cheng TT, Lee YC, et al. New vertebral osteoporotic compression fractures after percutaneous vertebroplasty: retrospective analysis of risk factors. J Vasc Interv Radiol. 2008;19(2 Pt 1):225-231.
52 Tseng YY, Yang TC, Tu PH, et al. Repeated and multiple new vertebral compression fractures after percutaneous transpedicular vertebroplasty. Spine (Phila Pa 1976). 2009;34(18):1917-1922.
53 Moon ES, Kim HS, Park JO, et al. The incidence of new vertebral compression fractures in women after kyphoplasty and factors involved. Yonsei Med J. 2007;48(4):645-652.
54 Waldman D. Vertebroplasty. In: Fenton D, Czervionke L, editors. Image-guided spine intervention. Philadelphia: Saunders, 2003.
55 Ruiz-Lopez R, Pichot C. Vertebroplasty. In Interventional pain management, ed 2, Philadelphia: Saunders; 2008:560.
56 Raj P, Lou L, Erdine S, Staats P. Radiographic imaging for regional anesthesia and pain management. Philadelphia: Churchill Livingstone; 2003. p 216
57 Ruiz-Lopez R, Pichot C. Vertebroplasty. In Interventional pain management, ed 2, Philadelphia: Saunders; 2008:561.
58 Jankowitz BT, Atteberry DS, Gerszten PC, et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J. 2009;18(8):1169-1174.
59 Kwak HJ, Lee JK, Kim YS, et al. Aortic aneurysm complicated with pyogenic spondylitis following vertebroplasty. J Clin Neurosci. 2008;15(1):89-93.
60 Stoffel M, Wolf I, Ringel F, et al. Treatment of painful osteoporotic compression and burst fractures using kyphoplasty: a prospective observational design. J Neurosurg Spine. 2007;6(4):313-319.
61 Cosar M, Sasani M, Oktenoglu T, et al. The major complications of transpedicular vertebroplasty. J Neurosurg Spine. 2009;11(5):607-613.
62 Lin CC, Shen WC, Lo YC, et al. Recurrent pain after percutaneous vertebroplasty. AJR Am J Roentgenol. 2010;194(5):1323-1329.
63 Kao FC, Tu YK, Lai PL, et al. Inferior vena cava syndrome following percutaneous vertebroplasty with polymethylmethacrylate. Spine (Phila Pa 1976). 2008;33(10):E329-E333.
64 Wendling D, Runge M, Toussirot E, et al. Vertebral osteitis adjacent to kyphoplasty. Joint Bone Spine. 2010;77(1):67-69.
65 Söyüncü Y, Ozdemir H, Söyüncü S, et al. Posterior spinal epidural abscess: an unusual complication of vertebroplasty. Joint Bone Spine. 2006;73(6):753-755.
66 Mueller M, Daniels-Wredenhagen M, Besch L, et al. Postoperative aseptic osteonecrosis in a case of kyphoplasty. Eur Spine J. 2009;18(suppl 2):213-216.
67 Chen YJ, Chang GC, Chen WH, et al. Local metastases along the tract of needle: a rare complication of vertebroplasty in treating spinal metastases. Spine (Phila Pa 1976). 2007;32(21):E615-E618.
68 Tsai YD, Liliang PC, Chen HJ, et al. Anterior spinal artery syndrome following vertebroplasty: a case report. Spine (Phila Pa 1976). 2010;35(4):E134-E136.
69 Venmans A, Klazen CA, Lohle PN, van Rooij WJ. Percutaneous vertebroplasty and procedural pain. AJNR Am J Neuroradiol.. 2010;31(5):830-831.
70 Waldman S. Atlas of interventional pain management, ed 2. Philadelphia: Saunders (Elsevier); 2004. pp 587-590
71 Powell MF, DiNobile D, Reddy AS. C-arm fluoroscopic cone beam CT for guidance of minimally invasive spine interventions. Pain Physician. 2010;13(1):51-59.
72 Syed MI, Shaikh A. Vertebroplasty: a systematic approach. Pain Physician. 2007;10(2):367-380.
73 Liu J, Bendok B. Osteoporosis and percutaneous vertebroplasty. In: Essentials of pain medicine and regional anesthesia. Philadelphia: Elsevier; 2005:506.
74 Miller D, Fenton D, Dion J. Vertebroplasty. In: Image-guided spine intervention. Philadelphia: Saunders; 2003:202.
75 Ruiz-Lopez R, Pichot C. Vertebroplasty. In Interventional pain management, ed 2, Philadelphia: Saunders; 2008:561-562.
76 Moreland DB, Landi MK, Grand W. Vertebroplasty: techniques to avoid complications. Spine J. 2001;1(1):66-71.
77 Miller D, Fenton D, Dion J. Vertebroplasty. In: Image-guided spine intervention. Philadelphia: Saunders; 2003:208.
78 Georgy B. Clinical experience with high-viscosity cements for percutaneous vertebral body augmentation: occurrence, degree, and location of cement leakage compared with kyphoplasty. AJNR Am J Neuroradiol. 2010;31(3):504-508.
79 Hamady M, Sheard S. Role of cementoplasty in the management of compression vertebral body fractures. Postgrad Med J. 2009;85(1004):293-298.