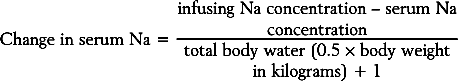Chapter 45 Complications of Laparoscopic and Hysteroscopic Surgery
INTRODUCTION
Unfortunately, many laparoscopic advances were developed by trial and error accompanied by significant morbidity and mortality.1 The application of electrosurgery, automated stapling devices, patient positioning, and trocar use produced unique mechanisms for surgical injury, many of which were not predicted. When can new technologies and procedures be considered safe? How many laparoscopic procedures should be performed before a surgeon is deemed proficient? A recent study of the learning curve associated with laparoscopically assisted vaginal hysterectomies (LAVH) suggests that a gynecologist must perform more than 30 cases before morbidity rates are significantly reduced.1
LAPAROSCOPY
Nerve Injury
Categories of Nerve Injuries
Fortunately, most neurologic injuries associated with patient positioning during laparoscopic surgery do not result in nerve separation and resolve spontaneously with time.2 Those associated with radical surgical dissection may be more likely to be serious and permanent.
Specific Nerve Injuries
Femoral Nerve
The femoral nerve is the largest nerve of the lumbosacral plexus and is derived from the posterior divisions of the L2, L3, and L4 nerve roots (Fig. 45-1). It pierces the psoas muscle and runs inferolaterally within the muscle to emerge between the iliacus and psoas muscles. It then courses under the inguinal ligament to enter the femoral sheath lateral to the femoral artery.
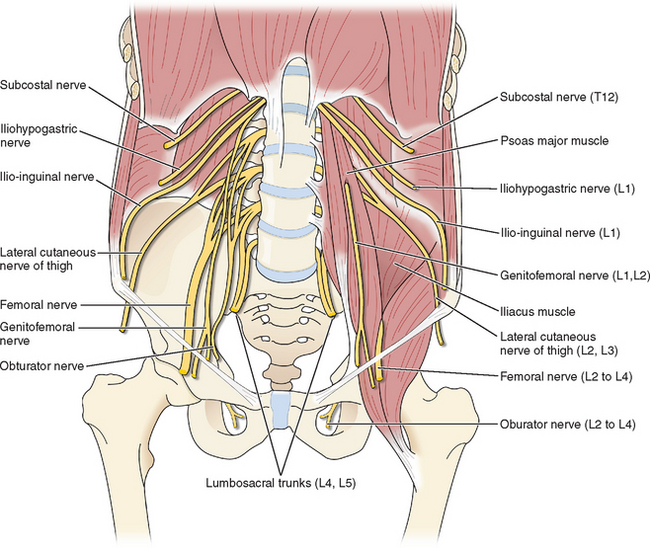
Figure 45-1 Schematic representation of nerves that can be injured during laparoscopy.
(Reproduced from Drake R, Vogl W, Mitchell AWM: Gray’s Anatomy for Students. Philadelphia, Elsevier, 2005.)
Femoral neuropathy during laparoscopy usually results from excessive hip flexion or abduction or from long operating times.2 Patients undergoing vaginal or laparoscopic surgery should be placed in a lithotomy position such that the thigh is flexed no greater than 90 degrees and abducted no greater than 45 degrees. If positioning is changed intraoperatively, these relationships should be maintained.
Obturator Nerve
The obturator nerve arises from the anterior nerve roots of L2, L3, and L4 (see Fig. 45-1). It descends through the psoas muscle, emerging medially at the pelvic brim, and passes through the obturator foramen into the thigh. Its main function is to allow thigh adduction; it supplies motor function to the gracilis, obturator, adductor longus, brevis, and magnus muscles.
Obturator neuropathy can also occur as a result of excessive hip flexion.3 The mechanism whereby excessive hip flexion can cause obturator nerve injury is anatomic. As the obturator nerve leaves the obturator foramen, it lies directly against bone and can become acutely angulated and deformed if the hips are excessively flexed, particularly during prolonged surgery. Injury to the obturator nerve usually manifests as weakness in the hip adductors and sensory loss in the upper medial thigh.
Nerves of the Anterior Abdominal Wall and Inguinal Region: Ilioinguinal, Iliohypogastric, and Genitofemoral
The ilioinguinal, iliohypogastric, and genitofemoral nerves have an overlapping sensory distribution in terms of their cutaneous manifestations, often making it difficult to distinguish between them. The course of these nerves is quite variable. The iliohypogastric and ilioinguinal nerves originate from the T12 and L1 nerve roots of the lumbosacral plexus (see Fig. 45-1). They cross over the quadratus lumborum muscles and pierce the transversus abdominis muscle in parallel at about the level of the anterior superior iliac spine.4 The genitofemoral nerve originates from the L1 and L2 nerve roots to pass anterior to the psoas muscles (see Fig. 45-1). The iliohypogastric nerve supplies sensation to the suprapubic region, and the ilioinguinal nerve supplies sensation to the inguinal canal. The genitofemoral nerve provides sensory innervation to the labial skin (genital branch) and the superior thigh (femoral branch).
The most common injury to these nerves is due to abdominal and pelvic incisions with subsequent suture ligature or fibrotic entrapment. With a Pfannenstiel incision, this injury is most likely to occur when the incision extends beyond the fascial aponeurosis to include the medial edge of the internal oblique muscle.5 During laparoscopic trocar insertion, the risk of injuring these nerves increases as the trocars are placed inferior to the anterior superior iliac spine.4,6 Palpation of the anterior superior iliac spine before trocar placement is useful for gaining lateral bearings.
Sciatic Neuropathy
The most common mechanism of injury to the sciatic nerve during gynecologic surgery is prolonged hip flexion resulting in nerve tension and stretching. Tension is increased with hip flexion when the knee joint becomes straightened or externally rotated. The sciatic nerve can stretch approximately 1.5 inches when the hip is flexed and the leg extended. Therefore, it is important to be cautious not to use excessive hip flexion when placing patients in stirrups, particularly the hanging type. When free-hanging stirrups are used for gynecologic cases, this injury has been reported in procedures lasting as short as 35 minutes.7
Effect of Carbon Dioxide Insufflation and Venous Gas Embolism
Effect of Carbon Dioxide Absorption
Capnography (ETCO2) and pulse oximetry are reliable markers of arterial gases in healthy patients. This may not apply to American Society of Anesthesiologists (ASA) class II and III. Increases in ETCO2 are usually due to absorption of CO2 from the peritoneal cavity. However, this may also be due to a variety of clinical circumstances that cause a ventilation/perfusion mismatch (V/Q) that increases the physiologic dead space. A steep Trendelenburg position and increased intra-abdominal pressures can accentuate this mismatch, especially in obese patients. A pneumoperitoneum will decrease pulmonary compliance. Obesity will decrease compliance further8 and increase the airway pressure required to ventilate the patient. Decreased cardiac output will also increase the V/Q mismatch and lead to increase in ETCO2.
Vascular Injuries during Laparoscopy
Veress Needle Injury
Avoidance
To avoid injury, laparoscopic surgeons must make every effort to place the Veress needle in the proper angle and direction (discussed in the section Retroperitoneal Vessel Injury). Once the needle is placed, the surgeon should attempt to demonstrate the intraperitoneal location of the needle tip before insufflation.
Retroperitoneal Vessel Injury
Injury of major abdominal blood vessels is a rare but treatable life-threatening complication of laparoscopy, which occurs in approximately 3 per 10,000 laparoscopies.11 These injuries most commonly occur during insertion of the Veress needle or the primary trocar.
Prevention
Injury to aorta and inferior vena cava can almost always be avoided by using the appropriate direction and angle for the insertion of both the Veress needle and primary trocar. It is common sense to direct the Veress needle and primary trocar toward the midline because the major retroperitoneal vessels bifurcate near the level of the umbilicus.12 However, because the exact midline is often difficult to gauge after the patient has been draped, the proper angle of insertion becomes especially important.
The best angle of insertion appears to change according to the patient’s body mass index (BMI=kg/m2) (see Chapter 44). In patients who are either in the ideal weight or overweight groups (corresponding BMI = 25kg/m2 and 25 to 30kg/m2, respectively), the Veress needle and primary trocar should be inserted through the umbilicus at 45 degrees from horizontal.13 A greater angle of insertion in these patients increases the risk of retroperitoneal vessel injury because the bifurcation is beneath the umbilicus in many cases, and the left common iliac vein is beneath the umbilicus in most patients.12 This is particularly important in the thinnest patients, in whom the distance from the umbilicus to the retroperitoneal vessels may be as little as 2 to 3 cm.13
Treatment
When a major vascular injury is suspected, the following steps should be taken without delay:
If either the surgeon or facility are not fully prepared to effectively treat a major vascular injury, thought should be given to using only an open laparoscopic technique or performing laparoscopy with a closed technique elsewhere.
Abdominal Wall Vessel Injury
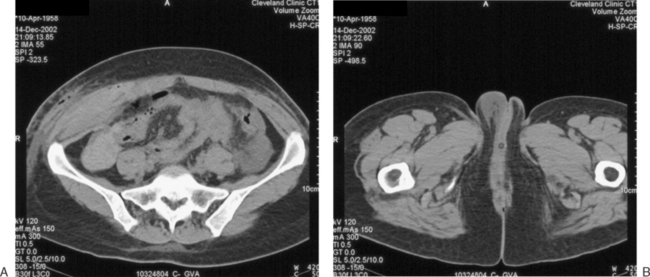
Damage to the deep vessels usually leads to immediate and rapid blood loss, whereas damage to the superficial vessels most often results in a persistent external trickle after the port is removed. Postoperatively, a superficial vessel injury (Fig. 45-2) typically presents with pain at the trocar site and a palpable mass. Postoperative deep vessel injury presents with increasing pain and decreasing hematocrit without a palpable mass because the vessels are located behind the rectus abdominis muscle or the bleeding occurs into the peritoneal cavity. A computed tomography scan will demonstrate the correct location of the hematoma.
Prevention
The primary method for avoiding injury to any of these vessels is to visualize the vessels before lateral trocar insertion. Two techniques have been used for this purpose: transillumination and direct laparoscopic visualization. Transillumination of the anterior abdominal wall with the laparoscopic light source is an effective way to visualize the superficial vessels in almost 90% of patients.14 The inferior epigastric vessels cannot be seen by transillumination because they lie beneath the rectus abdominis muscle and fascia. However, the inferior epigastric vessels can be directly visualized laparoscopically immediately beneath the peritoneum in 60% of patients where they lie between the insertion of the round ligament at the inguinal canal and the medial umbilical fold (see Chapter 7).
Treatment
Injury to an inferior epigatric vessel is almost always accompanied by immediate and brisk bleeding at the port site. Treatment consists of the following steps:
Gastrointestinal Injury
Despite the continued development of both laparoscopic instruments and techniques, gastrointestinal injury continues to be a common, yet potentially avoidable, complication of laparoscopy. In the past three decades, the risk of this complication appears to have increased from approximately 3 per 10,000 procedures to as high as 13 per 10,000 procedures.11,15 Most bowel injuries occur during placement of the Veress needle or primary trocar when bowel is adherent to the anterior abdominal wall from previous surgery.16 Other gastrointestinal injuries result from operative procedures, including adhesiolysis, tissue dissection, devascularization injury, and thermal injury.
It is essential to minimize morbidity related to gastrointestinal injuries both by prevention and early recognition. Despite an increasing awareness of these risks, gastrointestinal injuries continue to be one of the most lethal types of injuries associated with laparoscopy, with a mortality rate reported as high as 3.6%.15 Severe morbidity and mortality is seen with delayed diagnosis of an unrecognized bowel injury. Gastrointestinal injury should be suspected in any patient who presents postoperatively with increasing nausea, abdominal pain, distension, or fever. Rebound tenderness with a white blood cell (WBC) count that is elevated or depressed with a left shift and X-ray findings demonstrating an ileus or persistent air under the diaphragm are very suggestive of injury. CO2 gas is rapidly absorbed. Air seen under the diaphragm more than 36 hours after surgery, especially in the context of clinical symptoms, warrants further investigation for a gastrointestinal tract injury.
Preventive Measures
No method has yet to be discovered that completely prevents gastrointestinal injuries during laparoscopic port placement.17 However, it is well-established that patients with previous abdominal surgery are at increased risk of gastrointestinal injury during laparoscopy because adhesions to the anterior abdominal wall occur in approximately 25% of these patients. For this reason, certain measures have been used in an effort to decrease the risk of gastrointestinal injuries in these patients.
Two commonly used techniques for high-risk patients are open laparoscopy, as first described by Hasson, and a left upper quadrant closed technique utilizing Palmer’s point.17–20 Unfortunately, intestinal injury can occur with these approaches as well.17,21,22
Another alternative approach is the use of an optical-access trocar. These devices are designed to increase safety by visualizing each layer of the abdominal wall during port placement. Unfortunately, these devices too have been associated with gastrointestinal injuries, although the actual incidence associated with their use remains uncertain.23
Veress Needle Injuries
The standard closed technique for laparoscopic port placement begins with the blind insertion of a 14-gauge needle with a spring-loaded tip, commonly referred to as a Veress needle. Although the tip of this instrument might decrease the risk of perforating freely mobile bowel, it does not prevent perforation of adherent bowel or bowel with limited excursion related to physiologic attachments (e.g., transverse colon).24 As a general rule, bowel perforation with the Veress needle does not need to be repaired as long as the puncture is not actively bleeding or leaking bowel contents.
Stomach Injuries
Injury to the stomach during laparoscopy is relatively uncommon and was reported to occur in less than 3 in 10,000 cases in the earlier days of laparoscopy.25 Risk factors include a history of upper abdominal surgery and difficult induction of anesthesia, because a gas-distended stomach can be below the level of the umbilicus. Routine decompression of the stomach with a nasogastric or orogastric tube before Veress needle or trocar placement has virtually eliminated this risk, especially when a left upper quadrant approach is used.
Trocar injury to the stomach requires surgical repair. Although this is routinely performed by laparotomy, a laparoscopic approach has been reported.26 The defect should be repaired in layers with a delayed absorbable suture, preferably by a surgeon experienced in gastric surgery. The abdominal cavity should be irrigated, being careful to remove all food particles as well as gastric juices. Nasogastric suction is maintained postoperatively until normal bowel peristalsis resumes.
Small Intestine Injuries
A full-thickness injury to the small intestine of 5 mm or greater should be repaired in two layers, sewing perpendicular to the long axis of the intestine to avoid stricture formation. This can be accomplished with an initial interrupted layer of 3-0 delayed absorbable suture to approximate the mucosa and muscularis. A serosal layer of 3-0 delayed absorbable suture is commonly placed in an interrupted fashion. This is usually performed by laparotomy or by minilaparotomy at the umbilical site, where the injured bowel loop is pulled through to the skin surface and repaired. Laparoscopic repair has also been reported by surgeons with advanced gastrointestinal surgical skills.27 If the laceration to the small bowel exceeds one-half the diameter of the bowel lumen, segmental resection is recommended.
Large Intestine Injuries
Trocar injuries to the large intestines are reported to occur with a frequency of approximately 1 per 1000 cases.28 Due to the high concentration of coliform bacteria in the large intestine, unrecognized injuries can result in serious intra-abdominal infections that can quickly become life-threatening. Intraoperative detection and appropriate treatment of these injuries can greatly reduce subsequent morbidity.
Whenever a large intestine injury is suspected, the area should be carefully inspected using atraumatic bowel graspers. If adhesions or anatomy make laparoscopic inspection difficult, laparotomy should be performed. An occult injury to the rectosigmoid colon may be detected using the “flat tire test,” in which the posterior cul-de-sac is filled with normal saline solution and air is injected into the rectum using a proctosigmoidoscope or a catheter-tipped bulb syringe.29 Visible bubbles indicate a large intestine injury.
Thermal Bowel Injuries
In the early days of laparoscopy, thermal bowel injuries were associated with the use of electrosurgical equipment secondary to engineering and technical limitations. Modern advances in the use of these and alternative laparoscopic power sources has decreased but not totally eliminated thermal bowel injuries.30
Thermal bowel injuries are pathologically different from traumatic injuries and therefore must be treated differently.31 Thermal injuries are differentiated histologically from traumatic injuries by the presence of coagulation necrosis and the absence of both capillary proliferation and white cell infiltration. Due to this coagulation necrosis, it may take days for the extent of the injury to become grossly visible. As a result, thermal injuries require wide resection of normal-appearing bowel wall adjacent to the visible injury.
Port Site Hernia
Midline Ports
Several actions have been suggested that might reduce the risk of umbilical site herniation, but none have ever been proven to be effective. These include allowing all the carbon dioxide to escape before removal of the umbilical port, avoiding negative pressure within the port during removal by removing the laparoscope and port together or opening the valve, and preventing reversal of anesthesia (with resultant increases in intra-abdominal pressure) at the time of umbilical port removal.32
Lateral Ports
The use of lateral ports for more complex operative laparoscopy has resulted in a dramatic increase in the risk of port site herniation. In one retrospective review, port site hernias occurred in 5 of 3500 (0.17%) procedures, with all hernias occurring where ports with diameters of 10 mm or greater were placed lateral to the midline.33 Bowel herniation can occur between fascial layers in what has been called a Spigelian hernia.
To minimize the risk of lateral port site herniation, both the anterior and posterior fascial sheaths should be closed after removal of all ports 10 mm and larger. This closure is usually performed with the aid of one of a number of commercially available devices or needles that incorporate the peritoneum as well as both fascial layers. Unfortunately, port site herniation is not completely prevented by careful fascial closure.34
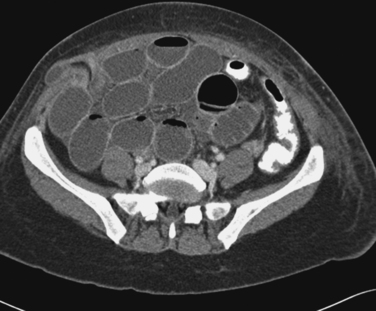
Trocar site hernias usually present as a palpable mass beneath a lateral trocar site skin incision that manifests during a Valsalva maneuver. A persistent mass associated with pain indicates an incarcerated hernia and represents a surgical emergency (Fig. 45-3). Standard treatment includes careful surgical exploration of the site and its contents. Any herniated bowel must be inspected carefully. Although simple repair of the peritoneal and fascial defects is all that is required in most healthy patients, in some cases synthetic mesh may be needed.
Urinary Tract Injuries
Bladder Injury
Trocar Injuries
Injury to the bladder during laparoscopic port placement is relatively uncommon and is usually related to insertion of the primary trocar in the presence of a distended bladder or insertion of a suprapubic midline trocar in a patient whose bladder dome had extended cephalad secondary to previous surgery.35
Draining the bladder with a catheter before primary trocar placement can decrease the risk of trocar injuries to the bladder. In patients with prior lower abdominal surgery, it seems prudent to place the suprapubic trocar above any previous transverse skin incisions. In all patients, an attempt should be made to visualize the superior bladder margin laparoscopically before suprapubic trocar placement.14 In cases in which the superior margin of the bladder cannot be seen, the bladder can be filled with 300 mL of sterile saline to better define its margin. An alternative approach is to use a lateral port site rather than a midline suprapubic site, although the decreased risk of bladder injury may be offset by an increased risk of vessel injury.
Operative Injuries
The rate of bladder injury associated with operative laparoscopy has increased dramatically to as high as 1 in 300 cases. The most common cause of bladder injury appears to be sharp electrosurgical dissection near the bladder.36 Most of these injuries occur during LAVH or bladder neck suspensions, with a risk of bladder injury reported as 2.8% and 1.9%, respectively.37–39 Resection of endometriosis that obliterates the anterior cul-de-sac is also a risk factor.
Recognition
Laparoscopic bladder injuries are often difficult to recognize intraoperatively. Visible leakage of urine at the time of injury is unlikely in patients with a Foley catheter in place. A common sign of bladder injury is significant bleeding from a suprapubic port site placed in the relatively avascular midline. Frank hematuria suggests a full-thickness injury. An uncommon, but pathognomonic, sign of bladder injury during laparoscopy is insufflation of the Foley catheter bag with carbon dioxide.40
Postoperative recognition of a bladder injury can likewise be difficult. Whenever a patient returns within days of laparoscopy with significant abdominal findings, the possibility of an occult bladder injury should be considered.35 Bladder injury should be included in the differential diagnosis in the presence of painful urination and microscopic hematuria (Table 45-1). Elevation of blood urea nitrogen and serum creatinine level suggests intra-abdominal spill of urine with transperitoneal reabsorption. Drainage from a suprapubic incision can be evaluated further by instillation of a dilute indigo carmine solution into the bladder.
Table 45-1 Signs of Bladder Injury Presenting in the Postoperative Period
| Hematuria |
| Oliguria |
| Elevated blood urea nitrogen and creatinine levels |
| Lower abdominal pain/distension |
| Peritonitis/sepsis |
| Fistula |
Treatment
Small, uncomplicated, and isolated injuries of the superior portion of the bladder can be treated with catheter drainage alone.41 A retrograde cystogram should be performed after 10 days of continuous drainage and will document spontaneous healing in 85% of patients with small injuries. Surgical repair may be necessary if the Foley catheter is unable to provide adequate bladder drainage due to blood clots and in cases of persistent extravasation.
Ureteral Injury
Ureteral injury occurs in approximately 1% of laparoscopies.42 The risk is greatest during LAVH, which has become the most common laparoscopic procedure causing ureteral injury, usually related to electrosurgery.36
Laparoscopic ureteral injuries are usually not diagnosed intraoperatively.43 Diagnosis is most commonly delayed until 2 to 7 days after surgery, but has been reported as late as 33 days after surgery. The most common presenting symptoms and findings are abdominal pain, fever, hematuria, flank pain, peritonitis, and leukocytosis.
Management of ureteral injury should be undertaken in collaboration with a urologic surgeon. In the majority of cases, percutaneous or cystoscopic stenting techniques can be successfully used. In severe cases, laparotomy is required for ureteral end-to-end anastomosis or bladder reimplantation. Laparoscopic ureteral repair has been reported.44
Electrosurgical Injuries
The use of electrosurgery during laparoscopy has resulted in a unique assortment of complications, many of which are difficult to recognize intraoperatively. Although our understanding of electrophysics as it applies to laparoscopic electrosurgery has improved, complications associated with electrosurgery continue to occur.45 Formal electrosurgical training for laparoscopists is uncommon. Unlike the medical laser setup, few institutions require electrosurgery safety courses or credentialing.46 This section provides a basic review of electrophysics as it relates to safety in gynecologic electrosurgery. Table 45-2 defines some of the most commonly used terms.
| Ampere (A) – the rate at which current flows |
| Bipolar system – current that flows from an active electrode, through tissue having a cutting or coagulating effect, and returning through a return electrode within the same instrument directly back to the electrosurgical unit, without the need for a return electrode plate on the patient |
| Capacitive coupling – transference of electrical energy from an insulated active electrode to nearby conductive material |
| Cautery — searing through the application of a heated element |
| Coagulating current (interrupted or damped current) — bursts of rapidly increasing current interrupted at intervals such that peak polarity alternates with zero polarity |
| Current (I) – electron flow measured in coulombs per second or the steady current produced by one volt applied across the resistance of one ohm. |
| Current density — the amount of current flow per cross-sectional area normal to the direction of current flow, described in amps per meters squared |
| Cutting current (continuous or undamped current) — continuous high-frequency, low-voltage flow of current from one peak of polarity to the opposite peak without pausing at the zero polarity |
| Desiccation – coagulation of targeted vessels through the process of dehydration |
| Fulguration – coagulation of surface bleeding through spraying long electrical sparks |
| Hertz (Hz) – a unit of frequency expressed in cycles per second |
| Ohm (Ω) – tissue resistance to current |
| Power (p) – work per unit time |
| Radiofrequency – a term given to the current frequency of electrosurgery because it is found within the AM radiofrequency range (500,000 to 4,00,000 Hz) |
| Unipolar (monopolar) system – current that flows from an active electrode, through tissue having a cutting or coagulating effect, and traveling through the patient, exiting by way of return electrode plate, usually on the patient’s thigh, to the electrosurgical unit |
| Vaporization – exploding cells at their boiling point (100°C) as a result of applied energy |
| Voltage (V) – electric potential expressed in volts |
| Watt (W) – the amount of work produced or work done at a rate of one joule per second |
Tissue Effects
Waveforms: Cutting versus Coagulation
Multiple factors are involved in the tissue effects of electrosurgery.
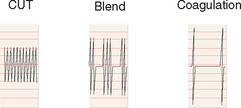
Electrosurgical generators can produce two distinct waveforms that differ in their rate of heat production. The rate of heat production determines whether tissue will be vaporized or coagulated: high heat causes vaporization, whereas low heat results in coagulation. A continuous waveform, referred to as cutting current, produces heat rapidly and thus vaporizes and cuts tissue (Fig. 45-4). The disadvantage of this type of current is that it does not seal vessels very effectively. Cutting current causes cells to vaporize at their boiling point (100°C) without elevating tissue temperatures to high levels, thus minimizing lateral thermal spread.
An intermittent waveform, referred to as coagulation current, produces less heat. The on time (duty cycle) is reduced, but the peak voltage is increased. Coagulation current seals vessels by producing a coagulum and is thus used for hemostasis.
Incidental Causes of Electrical Injury
Insulation Failure
In addition to capacitive coupling, insulation failure can also be a cause of inadvertent electrical injury despite using appropriate trocar sleeve systems. Damaged instruments have been demonstrated to cause stray current that can cause injuries during laparoscopy. Insulation failure can take place anywhere along the shaft of the unipolar instrument or within the electrosurgical cable where a breech of insulation has occurred. All reusable electrosurgical instruments should be regularly inspected for deterioration and tested by a qualified biomedical technician.
HYSTEROSCOPY
As with most complications, accurate data on complications of hysteroscopy are not available, and prevalence rates from the literature have a tendency to underestimate the occurrence. Table 45-3 lists the most frequently reported complications. Probably the most frequently seen complication is inability to complete the procedure because of inexperience or inadequate visibility of the uterine cavity.
| Short-Term | |
Uterine perforation usually occurs at the time of insertion of the cervical dilators or the operative hysteroscope. Perforation is more common in postmenopausal women, patients with cesarean section scars, and those with adenocarcinoma. The procedures that are most commonly associated with perforation are resection of septa or adhesions and myomectomy. Most uterine perforations are small, fundal, and require no specific treatment. If they occur laterally, laparoscopy is mandatory to assess potential uterine artery laceration. If they occur on the fundus, laparoscopy should be considered. At laparoscopy, a small fundal perforation that is not actively bleeding requires no further treatment other than a discussion with the patient that she has an increased risk of uterine rupture in a subsequent pregnancy. If a perforation occurred with an energy form such as the electrical loop, then laparoscopy or laparotomy to assess potential bowel burn is necessary. Persistent or worsening postoperative pain and nausea or fever are symptoms that suggest a complication such as bowel injury. The signs that uterine perforation has occurred during the operative procedure are the dramatic increase in fluid deficit as the fluid leaks into the peritoneal cavity and difficulties in maintaining proper uterine distension. Uterine rupture in a term pregnancy has been reported after hysteroscopic resction of a uterine septum without perforation.47
Distension Media
The choice and proper use of the distension media is critical for a safe operative procedure. CO2 is often used for diagnostic hysteroscopies but not for operative procedures. The CO2 is insufflated with a special device that delivers the gas at a maximum flow of 100 mL/minute and a maximum pressure of 100 mm Hg. Embolism of CO2 gas has been reported as a potential complication of the use of this gas. CO2 embolism has been especially described with the use of this gas when performing hysteroscopic surgery with the Nd:Yag laser.
One of the earliest distension media used is 32% dextran in 10% dextrose. It is a high-viscosity branched polysaccharide. Although visibility is excellent, the fluid crystallizes easily on instruments. The fluid is usually infused under pressure. Fluid overload can occur with this agent even with volumes of 500 mL due to the hydrophilic nature of this agent. The plasma volume is expanded by 860 mL for every 100 mL absorbed. Although at higher doses pulmonary edema is due to fluid overload, noncardiogenic pulmonary edema has also been reported. Coagulopathy and mild electrolyte disturbances can occur. It is recommended that volumes less than 500 mL be used. Hypersensitivity reactions have been reported with low doses.48
The intraoperative signs of hyponatremia are often subtle but include tremulousness, hypothermia, and hypoxemia. Postoperatively the patient can complain of mild symptoms that are similar to those seen in many postoperative patients, such as headache and nausea (Table 45-4). Intracerebral fluid shifts cause cerebral edema, increased intracranial pressure, and possible herniation of the brainstem. The intracerebral water gain will cause a decrease in brain osmolality. The brain will rapidly adapt with loss of sodium, potassium, and chloride and slowly adapt by loss of organic osmolytes that lead to loss of cerebral water.
| Headache |
| Nausea and emesis |
| Lethargy, confusion, change in mental status |
| Seizures |
For example, if the serum sodium level is 124mmol/L and the infusing solution is 0.9% sodium chloride (154mmol/L) and the total body water is 30 L (0.5 × 58 kg), then 1L would increase the serum sodium level by 1mmol/L. The fluid volume required would be too high. A 3% sodium chloride solution (513mmol/L) would require far less volume to raise the concentration by 1mmol/L per hour. Raising the serum sodium level too quickly can cause an osmotic demyelination syndrome. Demyelination of pontine neurons can lead to quadraplegia and other neurologic disorders. Severe symptoms in patients that received glycine may be due to ammonia toxicity, and this should be treated with hemodialysis.49
Other Acute and Chronic Complications
Pregnancies in patients after endometrial ablation can be associated with significant pregnancy loss or placental complications. Patients should be counseled that endometrial ablation does not remove the requirement for proper contraception. Tables 45-5 and 45-6 list the general principles of prevention and recognition of hysteroscopic complications.
Table 45-6 Recognizing Complications of Hysteroscopy
1 Altgassen C, Michels W, Schneider A. Learning laparoscopic-assisted hysterectomy. Obstet Gynecol. 2004;104:308-313.
2 Irvin W, Andersen W, Taylor P, Rice L. Minimizing the risk of neurologic injury in gynecologic surgery. Obstet Gynecol. 2004;103:374-382.
3 Pellegrino MJ, Johnson EW. Bilateral obturator nerve injuries during urologic surgery. Arch Phys Med Rehabil. 1988;69:46-47.
4 Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
5 Sippo WC, Burghardt A, Gomez AC. Nerve entrapment after Pfannenstiel incision. Am J Obstet Gynecol. 1992;80:420-421.
6 El-Minawi AM, Howard FM. Iliohypogastric nerve entrapment following gynecologic operative laparoscopy. Obstet Gynecol. 1998;91:871.
7 Batres F, Barclay DL. Sciatic nerve injury during gynecologic procedures using the lithotomy position. Obstet Gynecol. 1983;62:92S-94S.
8 Sprung J, Whalley DG, Falcone T, et al. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg. 2002;94:1345-1350.
9 Haroun-Bizri S, ElRassi T. Successful resuscitation after catastrophic carbon dioxide embolism during laparoscopic cholecystectomy. Eur J Anaesthesiol. 2001;18:118-121.
10 Cottin V, Delafosse B, Viale JP. Gas embolism during laparoscopy: A report of seven cases in patients with previous abdominal surgical history. Surg Endosc. 1996;10:166-169.
11 Mintz M. Risks and prophylaxis in laparoscopy: A survey of 100,000 cases. J Reprod Med. 1977;18:269-272.
12 Hurd WW, Bude RO, DeLancey JOL, Pearl ML. The relationship of the umbilicus to the aortic bifurcation: Implications for laparoscopic technique. Obstet Gynecol. 1992;80:48-51.
13 Hurd WW, Bude RO, DeLancey JOL, et al. Abdominal wall characterization by magnetic resonance imaging and computed tomography: The effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473-476.
14 Hurd WW, Amesse LS, Gruber JS, et al. Visualization of the bladder and epigastric vessels prior to trocar placement in diagnostic and operative laparoscopy. Fertil Steril. 2003;80:209-212.
15 Van Der Voort M, Heijnsdijk EA, Gouma DJ. Bowel injury as a complication of laparoscopy. Br J Surg. 2004;91:1253-1258.
16 Bateman BG, Kolp LA, Hoeger K. Complications of laparoscopy—operative and diagnostic. Fertil Steril. 1996;66:30-35.
17 Jansen FW, Kolkman W, Bakkum EA, et al. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190:634-638.
18 Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110:886-887.
19 Tulikangas PK, Nicklas A, Falcone T, Price LL. Anatomy of the left upper quadrant for trocar insertion. J Am Assoc Gynecol Laparosc. 2000;7:211-214.
20 Tulikangas PK, Robinson DS, Falcone T. Left upper quadrant cannula insertion. Fertil Steril. 2003;79:411-412.
21 Vilos GA. Laparoscopic bowel injuries: Forty litigated gynaecological cases in Canada. J Obstet Gynaecol Can. 2002;24:224-230.
22 Chapron C, Cravello L, Chopin N, et al. Complications during set-up procedures for laparoscopy: Open laparoscopy does not reduce the risk of major complications. Acta Obstet Gynecol Scand. 2003;82:1125-1129.
23 Sharp HT, Dodson MK, Watts DA, et al. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
24 Chee SS, Godfrey CD, Hurteau JA, et al. Location of the transverse colon in relationship to the umbilicus: Implications for laparoscopic techniques. J Am Assoc Gynecol Laparosc. 1998;5:385-388.
25 Loffer F, Pent D. Indications, contraindications and complications of laparoscopy. Obstet Gynecol Surv. 1975;30:407-427.
26 Spinelli P, Di Felice G, Pizzetti P, Oriana R. Laparoscopic repair of full-thickness stomach injury. Surg Endosc. 1991;5:156-157.
27 Nezhat C, Nezhat F, Ambrose W, Pennington E. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
28 Krebs HB. Intestinal injury in gynecologic surgery: A ten-year experience. Am J Obstet Gynecol. 1986;155:509-514.
29 Nezhat C, Seidman D, Nezhat F, Nezhat C. The role of intraoperative proctosigmoidoscopy in laparoscopic pelvic surgery. J Am Assoc Gynecol Laparosc. 2004;11:47-49.
30 Chapron C, Pierre F, Harchaoui Y, et al. Gastrointestinal injuries during gynaecological laparoscopy. Hum Reprod. 1999;14:333-337.
31 Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy. J Reprod Med. 1985;30:660-663.
32 Leung TY, Yuen PM. Small bowel herniation through subumbilical port site following laparoscopic surgery at the time of reversal of anesthesia. Gynecol Obstet Invest. 2000;49:209-210.
33 Kadar N, Reich H, Liu CY, et al. Incisional hernias after major laparoscopic gynecologic procedures. Am J Obstet Gynecol. 1993;168:1493-1495.
34 Montz FJ, Holschneider CH, Munro MG. Incisional hernia following laparoscopy: A survey of the American Association of Gynecologic Laparoscopists. Obstet Gynecol. 1994;84:881-884.
35 Godfrey CD, Schilder JM, Rothenberg JM, et al. Occult injuries to the bladder during laparoscopy: report of two cases. J Laparoendosc Surg. 1999;9:341-345.
36 Ostrzenski A, Radolinski B, Ostrzenska KM. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
37 Gill IS, Clayman RV, Albala DM, et al. Retroperitoneal and pelvic extraperitoneal laparoscopy: An international perspective. Urology. 1998;52:566-571.
38 Wang PH, Lee WL, Yuan CC, et al. Major complications of operative and diagnostic laparoscopy for gynecologic disease. J Am Assoc Gynecol Laparosc. 2001;8:68-73.
39 Soulie M. Multi-institutional study of complications in 1085 laparoscopic urologic procedures. Urology. 2001;58:899-903.
40 Classi R, Sloan PA. Intraoperative detection of laparoscopic bladder injury. Can J Anaesth. 1995;42:415-416.
41 Gomez RG, Ceballos L, Coburn M, et al. Consensus statement on bladder injuries. BJU Int. 2004;94:27-32.
42 Harkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: A follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
43 Oh BR, Kwon DD, Park KS, et al. Late presentation of ureteral injury after laparoscopic surgery. Obstet Gynecol. 2000;95:337-339.
44 Tulikangas PK, Gill IS, Falcone T. Laparoscopic repair of ureteral injuries. J Am Assoc Gynecol Laparosc. 2001;8:259-262.
45 Shen CC, Wu MP, Lu CH, et al. Small intestine injury in laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 2003;10:350-355.
46 Lo KW, Yuen P. Mortality following laparoscopic surgery. Gynecol Obstet Invest. 1999;48:203-204.
47 Kerimis P, Zolti M, Sinwany G, et al. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
48 Ahmed N, Falcone T, Tulandi T, Houle G. Anaphylactic reaction because of intrauterine 32% dextran-70 instillation. Fertil Steril. 1991;55:1014-1016.
49 Adrogue HJ, Madias NE. Hyponatremia. NEJM. 2000;342:1581-1589.
50 Drake R, Vogl W, Mitchell AWM. Gray’s Anatomy for Students. Philadelphia: Elsevier, 2005.