CHAPTER 61 Complications of Elbow Replacement Arthroplasty
INTRODUCTION
The majority of these complications neither require surgery nor adversely influence the ultimate result. Thus, these problems might best be discussed according to their management and significance: (1) those that increase the morbidity but do not influence the outcome by requiring additional surgery, removal, or replacement of the implant; and (2) those requiring additional surgery, including revision of the implant. Gschwend and colleagues,21 in their systematic review of the world’s literature from 1986 to 1992, discussed 828 procedures. Of these, 43% had complications (Table 61-1).
| Complication | Incidence (%)* |
|---|---|
| Aseptic loosening | |
| Radiologic | 17.2 |
| Clinical | 6.4 |
| Infections | 8.1 |
| Ulnar nerve lesions | 10.4 |
| Instability | 7-19 |
| Disassembly | — |
| Dislocation | 4.3 |
| Subluxation | 2.2–6.5 |
| Intraoperative fractures | 3.2 |
| Fractures of prosthesis | 0.6 |
| Ectopic bone formation | — |
* Total number of cases = 828.
In the intervening years, there have been many advances in prosthetic design and surgical technique. For example, constrained devices have been eliminated owing to their high rate of loosening. Semiconstrained and unconstrained devices are being used now, and recently, hybrid designs that are unlinked but can be converted to a linked design have been developed. Variations in techniques such as triceps tendon dissection and repair, better cementation techniques, routine antibiotic prophylaxis, and others have emerged. Despite advances in prosthetic design and surgical technique over the past decade, there had been no systematic review of the literature on the complications of TEA since the 1996 report by Gschwend et al.21 We recently conducted a comprehensive review of the literature to determine the complications associated with modern-day TEA.56 The review comprised 38 studies that reported on 1981 total elbow replacements. The overall significant complication rate (requiring another surgical procedure or resulting in permanent clinical sequalae) after primary TEA was 27.9% ± 13.3%. The main complications included 4.8% clinical loosening (including symptomatic loosening or revision), 3.8% instability including dislocation and symptomatic subluxation, 2.5% deep infection, and 2.5% ulnar nerve complications56 (Table 61-2). In this edition, the treatment of infection is discussed in detail in Chapter 62, and loosening and periprosthetic fractures are dealt with in the chapters on revision (see Chapters 65 and 66). A detailed summary of the current literature regarding complications is given in Table 61-2.
TABLE 61-2 Complications of Elbow Replacement, 1993-200456
| Complication | Incidence (%) |
|---|---|
| Aseptic loosening (clinical) | 4.81 |
| Aseptic loosening (clinical and radiographic) | |
| Linked Designs | 15.92 |
| Unlinked Designs | 18.53 |
| Infections | 2.51 |
| Ulnar nerve lesions | 2.51 |
| Disassembly | 4.21 |
| Dislocation/Subluxation | 3.81 |
| Intraoperative fractures | 1.81 |
| Fractures of prosthesis | 1.11 |
| Ectopic bone formation | — |
1 Total number of cases = 1981.
COMPLICATIONS NOT USUALLY REQUIRING SURGERY
MOTION RESTRICTION
The goal of elbow replacement surgery is to obtain the functional arc of 30 to 130 degrees of flexion.39 Those rheumatoid arthritic patients who have the ankylosing type of disease tend not to obtain the typical 30 to 130 degrees of flexion-extension after surgery.7 Therefore, we attempt to treat this motion restriction with an aggressive capsular resection at the time of the implantation. If the anterior capsule is contracted, sufficient depth of insertion is most important. A trial reduction is essential to identify this potential problem so that it can be avoided. Often, a static adjustable splint is used to gain or maintain motion. Furthermore, a slight but consistently greater flexion contracture is observed with resurfacing designs (see Chapter 52).
WOUNDS
Wounds are much less a problem today19 than the 5% incidence previously reported.9,13,23,34,46
Management
Wound healing problems are best avoided. At the Mayo Clinic, we avoid the use of the steri-drape after skin preparation with iodinated solutions, especially in patients with rheumatoid arthritis. I also use a straight incision just medial to the tip of the olecranon and carefully cauterize vessels during surgery. The elbow is placed in full extension with a compression/cryotherapy (Cryocuff Aircast, DJO, Vista, CA) device, which is now used routinely.1 If the wound remains tenuous at 1 week or 10 days, we do not hesitate to place the patient in a cast or anterior splint for 10 to 14 days and then reassess.33 If severe wound necrosis occurs, surgical treatment may involve special soft tissue coverage, which is discussed in Chapter 36.
NEURITIS
In patients with rheumatoid arthritis or in a joint that has been subjected to previous surgery, the ulnar nerve is particularly vulnerable. The incidence of ulnar nerve involvement has been reported in 2%3 to 26%13,54 of patients and varies in severity from profound neuropathy in less than 5%9,31,49,52 to transient paresthesias in as many as 25%.21 Implicated causes are excessive traction, perineural or epineural hematoma, direct mechanical pressure during the procedure, and irritation by the bandage or from swelling. The possibility of thermal damage from juxtaposed methylmethacrylate may be considered, as well as devitalization during the translocation procedure. Gschwend’s review of the experience in the literature from 1986 to 1992 reveals an incidence of approximately 10% after almost 900 procedures.21 We have found 3% subjective symptoms after 700 procedures at the Mayo Clinic. No patient had motor weakness.
In our review of literature in the last decade, the rate of ulnar neuropathy was found to be 2.5%.56 In light of the association between TEA and ulnar neuropathy, controversy persists regarding the need for routine ulnar nerve transposition during surgery. Some of the cited advantages of routine nerve transposition include protection of the nerve in a safe location during the exposure and elbow manipulation, decreasing the stretching forces on the nerve with elbow motion postoperatively, and reducing the compression on the nerve in the cubital tunnel. Some of the disadvantages include injury to the blood supply to the nerve and mechanical injury during the dissection. In our meta-analysis,56 the rates of significant ulnar neuropathy were compared between the studies that routinely transposed and the ones that selectively transposed the nerve. The results showed that routine ulnar nerve transposition resulted in lower rates of significant ulnar neuropathy (2.1% ± 3.5%) compared with the studies in which the nerve was not mobilized routinely (4.3% ± 5.2%) (P = 0.17). Even though this difference was not statistically significant, it is believed to be clinically significant. The overall rate of significant ulnar neuropathy was only 2.5%, making it very difficult to detect a statistically significant difference between routine and nonroutine ulnar nerve transposition.
TRICEPS INSUFFICIENCY
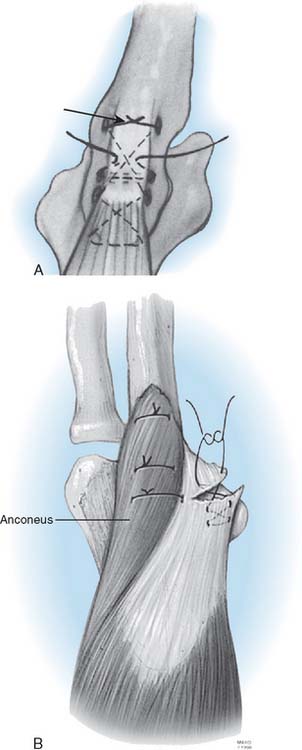
Triceps insufficiency is probably common, but it is not reported very often. The poor quality of the triceps tendon in patients with rheumatoid arthritis is well recognized, but only about 4% of patients with rheumatoid arthritis have been recognized as having significant triceps insufficiency.35,38 The Mayo approach and its modification57 was developed because of this problem.5 We have documented 13 of 700 (2%) since 1981. In our literature review of the last decade,56 triceps-related complications were analyzed after the three most common triceps dissection techniques. No triceps-related complications were found with the V-shaped tongue approach at the musculotendinous junction. Triceps reflection, with or without extra-articular wafers of olecranon, was associated with a complication rate of 2.3% ± 4.6%, whereas the triceps split, either with or without extra-articular wafers of olecranon, was associated with a 6.2% ± 14.8% complication rate. The difference in complication rates between the three approaches was not statistically significant.56
ECTOPIC BONE
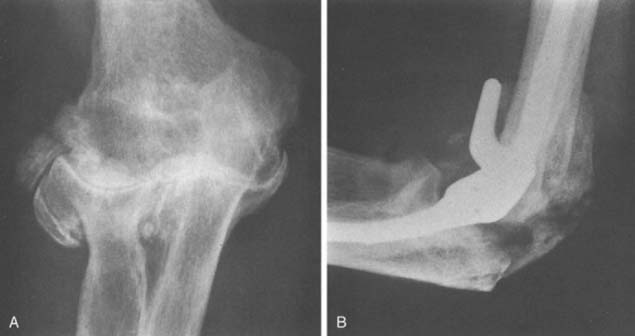
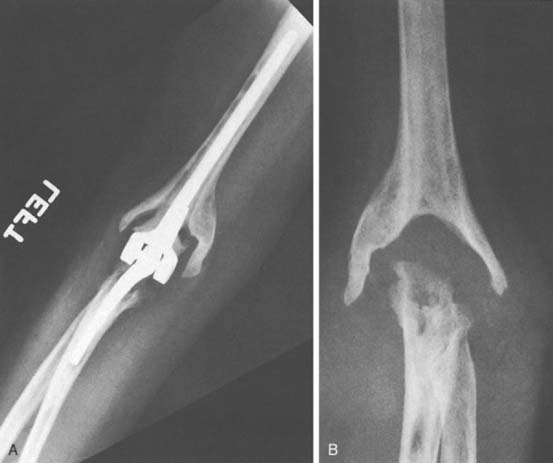
Ectopic bone has been reported after TEA.15 Although some heterotopic ossification may be seen following elbow replacement, in our experience and opinion, this is a very rare complication that occurs only under unusual circumstances, such as in patients with severe degenerative arthritis.26 We have observed only three cases of this problem after approximately 700 primary and revision total elbow replacements using several implant designs and exposures. In one patient, marked hypertrophic changes existed before surgery, extensive bleeding occurred after surgery, and moderate ectopic bone developed (Fig. 61-2).
COMPLICATIONS REQUIRING REOPERATION
As with all prostheses, reoperations may or may not require implant revision.
NONREIMPLANTATION REVISION PROCEDURES
Component Failure
Stem
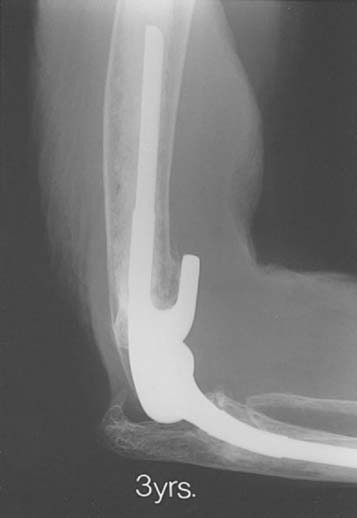
At the Mayo Clinic, we have observed an in-creasing frequency of ulnar component stem fracture, especially associated with traumatic arthritis in which near-normal activity is conducted after replacement (Fig. 61-3).51 An incidence of approximately 1.8% has been observed at the ulna and less than 0.5% at the humerus. The cause of these fractures is due to the success of theinitial replacement, which allows heavy use of the arm, and to the stress riser effect or to sintered titanium. Few cases of this kind of failure have been reported except with use of the Coonrad-Morrey component. Since 1994, this problem has not been observed, because the beaded surface finish has been removed from the ulnar component.
Articulation
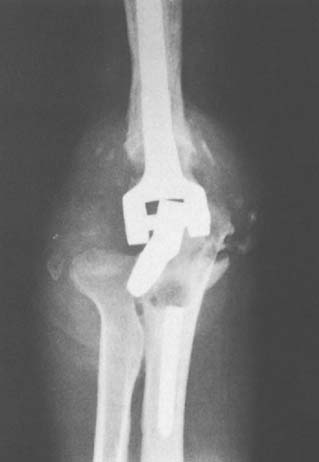
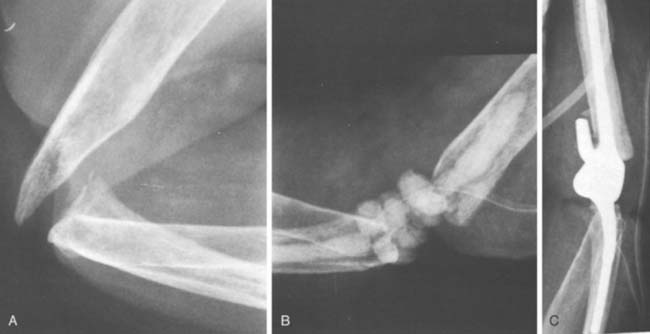
Bushing wear has been a major problem with the GSB and triaxial device,53 occurring in frequencies of 12% with the Pritchard II prosthesis,32 and in 5% of 173 GSB implants.21 This is attributed primarily to instability and less to osteolysis. Recent modifications in both designs are hoped to decrease or eliminate this problem. At Mayo, we have had an increasing incidence of displacement of the locking ring (Fig. 61-4), but it is unusual to have the pin back out. In our systematic review, semiconstrained devices had a disassembly rate of 4.2% ±0.8%.56 A modification of a pin within a pin (see Chapter 53), which has been approved by the Food and Drug Administration, should eliminate this problem.
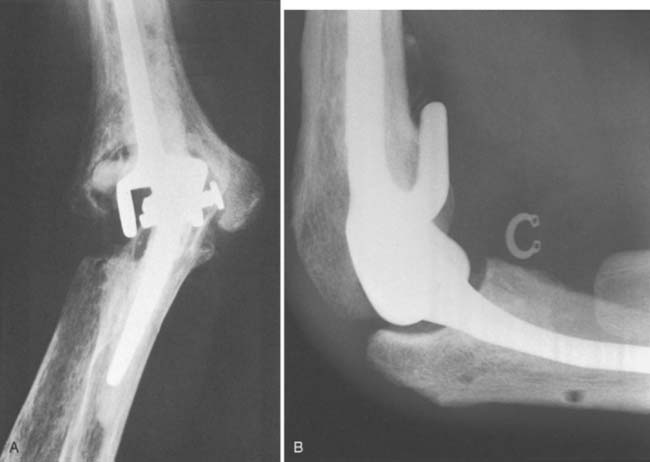
FIGURE 61-4 Pritchard Mark II device with pin failure (A); “C” ring displaced with Coonrad-Morrey device (B).
Inglis and Pellicci23 reported two of 36 articular bushing failures in the early design of the triaxial semiconstrained prosthesis. Pin backout had been a relatively common problem with the Pritchard-Walker implant in up to 5%.34,46 This is less a problem with the current Pritchard III design and has not occurred with the Coonrad device.
Component failure is rare in unlinked implants, usually occurring as deformity, wear, or subluxation of the polyethylene.27–2955
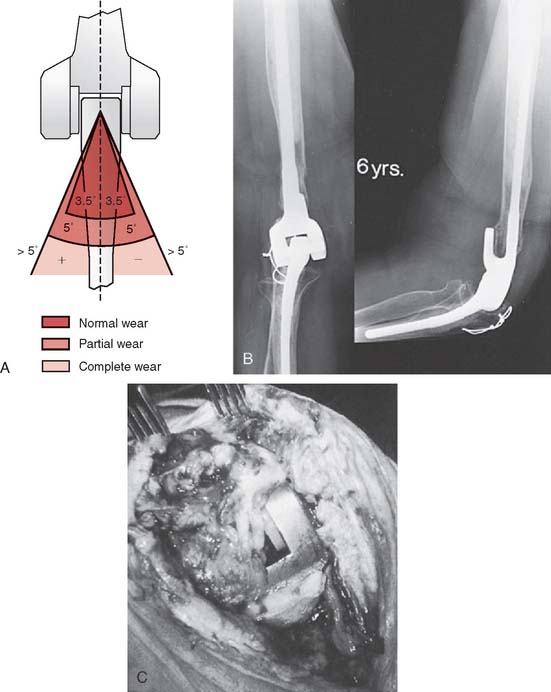
Wear
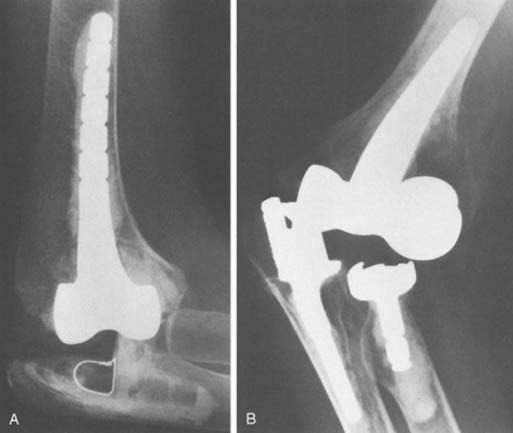
With increased longevity, an increased incidence of polyethylene wear was noted, at least for the Coonrad-Morrey device. This can be measured radiographically (Fig. 61-5). It has been observed in patients with survival in excess of 10 years19 and in those with greater stress of the articulation, as with arthritis and instability underlying the process.44,58
COMPLICATIONS TREATED BY IMPLANT REMOVAL
Infection
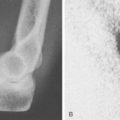
Sepsis after TEA is more common than after any other replacement procedure.40 A review of the literature suggests that infection with TEA occurs in about 7% of cases for both types of implant design. Thus, 22 of 332 (7%) resurfacing implants have been reported as infected9,10,13,31,45,49,52,54; a slightly lower frequency of semiconstrained implants have developed this complication.3,4,23,40,43 However, this is changing. Souter reported only one infection after more than 100 procedures using antibiotic-impregnated cement (Souter, personal communication, 1980). Our infection rate at the Mayo Clinic decreased from about 8%40 to less than 2% over the past 10 years. The systematic review of literature in the last decade demonstrated a 2.5% infection rate in modern literature.56 Although the diagnosis is not difficult (Fig. 61-6), the treatment is more challenging. This topic is a major one and is dealt with in a focused manner in Chapter 62.
Reimplantation
Reimplantation is required for loosening and implant fracture, and in some cases following deep sepsis.58 If a resection arthroplasty is painful or if resection causes significant functional insufficiency due to gross instability (Fig. 61-7), then reimplantation may be considered. If performed for infection, the technique used is similar to those recommended for reimplantation of the septic knee and hip implant. If an implant fractures, we make no effort to remove all cement, but rather leave the cement mantle, if intact, and enlarge it with a burr to remove the new device. Patients who have had resected joints may show significant shortening and must be prepared to accept this shortening. With the new longer flanged device, this should be less of an issue in the future. Also, patients should be advised that their extensor mechanism may not actively extend the elbow.
Instability
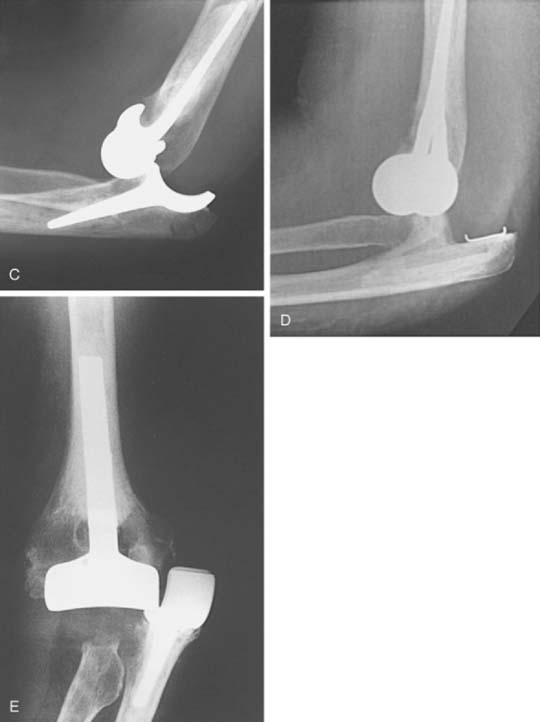
Instability after TEA is a problem that is basically limited to unlinked implants (see Chapter 52C). Elbow instability following the use of resurfacing implants, either as frank dislocation or subluxation, occurs in approximately 10% of cases.9,10,12,31,48,49,52,54 All designs are valuable for treating this problem (Fig. 61-8). Subluxation occurs about twice as frequently as frank dislocation. Only about 20% of patients with instability require surgical revision. Thus, instability requiring revision is seen in approximately 1% to 5% of the resurfacing devices. In our systematic review of modern literature, the instability rate was significantly higher with unlinked compared to linked designs, 3.4% ±0.7% and 0.4% ±0.9%, respectively (P = 0.014).56
Attempts have been made to improve the design and the surgical technique and, hence, to minimize this complication. It is disappointing to realize that even current reports continue to relate an instability rate of 7% to 8%.9,54 At the Mayo Clinic, 7 of 49 patients (14%) had instability with the capitellocondylar device (Johnson & Johnson, Warsaw, IN). Attaining the proper balance of the soft tissue envelope is technically very difficult, and it requires a thorough understanding of the anatomy and biomechanics25 and a meticulous surgical technique that preserves or restores the function and balance of both ligaments and muscles. Axial malrotation of either the humerus or the ulna appears to be a crucial pitfall of the surgical technique.25
Treatment
A period of immobilization to avoid full extension may help eliminate or decrease the frequency of this problem.45,52 If the elbow has dislocated, immobilization in flexion of 90 degrees or more for 3 to 6 weeks is the most common treatment. If this does not render the joint stable, translatory instability is generally well tolerated. If the patient still has symptoms, soft tissue revision is usually not successful, and revision to a semiconstrained device is required in my practice.
Loosening
Loosening after TEA is a well-publicized complication.2,4,18,20,24,41,47 Our initial experience at the Mayo Clinic reveals that approximately 25% of the constrained hinged total elbow arthroplasties will loosen within 5 years.41 In our systematic review of modern literature, the rate of clinically symptomatic nonseptic loosening was 4.8%.56
Biomechanics
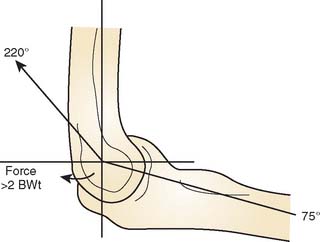
The resultant force vector of up to three times body weight is directed anteriorly during dynamic flexion and posteriorly during extension of the joint.22,42 A cyclic compression-distraction loading pattern occurs up to 1 million cycles per year8 (Fig. 61-9).
Prosthetic Design
Today, all devices are low-friction metal and polyethylene. In addition, the semiconstrained design includes some play or laxity at the bushing, which has dramatically lessened the rate of loosening of TEA.3,14,32,35,43,46,50 The anterior flange of the Mayo-modified Coonrad device absorbs the load applied to the humerus (Fig. 61-10).
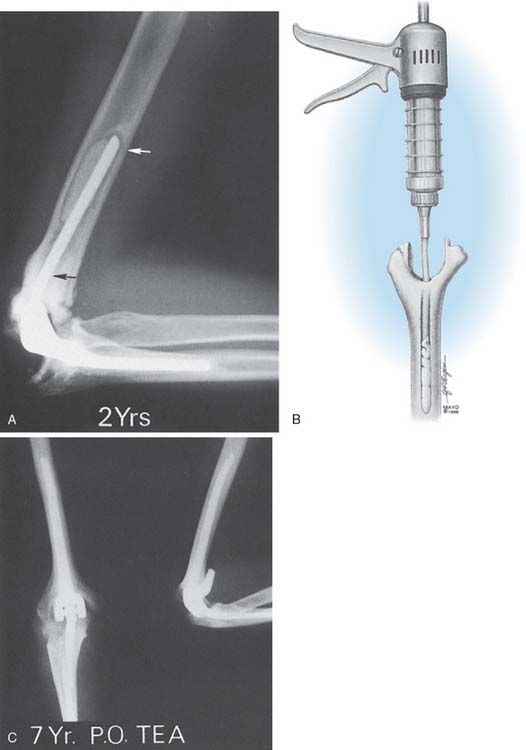
Surgical Technique
From the early experience, it is known that the cementing technique is directly correlated to the incidence of radiolucent lines. A stable interface is now reliably attained with intramedullary injecting systems (Fig. 61-11).
Current Data
As noted earlier, Gschwend and colleagues21 reviewed the literature from 1986 to 1992, collecting 828 cases. From this sample, a loosening rate was defined as radiographic: 17%, clinical: 6%. Our own experience at the Mayo Clinic constitutes a group of approximately 500 semiconstrained implants, of which 60% were for rheumatoid arthritis. The loosening rate at 5 years is, surprisingly, less than 2%. Others have reported similar and improved loosening rates as well.2,3,20,23,32,43,46 In our recent systematic review of modern literature, 28 studies from the list of 38 reported on the rate of clinical and radiographic loosening. Aseptic loosening, both clinical and radiographic, was correlated with the prosthetic design. Linked prosthesis and unlinked prosthesis were associated with 15.9% ± 22.1% and 18.5% ± 17.8% of aseptic loosening, respectively. There was no significant difference between these rates (P > 0.5). There is little question that loosening does not appear to be a major problem with the current generation of semiconstrained or resurfacing procedures in the rheumatoid population.21
Following trauma, we have reported a rate of loosening of less than 2% after 101 procedures followed for 5 years.6,36,51 Gschwend and colleagues21 note a loosening rate of 3% for rheumatoid arthritis and a radiographic rate of 6% for post-traumatic arthritis.
Treatment
Loosening is less a problem, but the treatment remains a challenge (see Chapter 65). With aseptic loosening and bone resorption, revision should be offered. Options include removal of the implant, leaving a resection arthroplasty; revision to a different type of prosthetic replacement; fusion of the resected joint; and possibly, cadaveric replacement of the resected elbow (see Chapter 67).
1 Adams, R., and Morrey, B. F.: The effect of cold compressive dressings after elbow surgery. AAOS Annual Meeting, Anaheim, CA, Feb. 4-8, 1999.
2 Bayley J.I.L. Elbow replacement in rheumatoid arthritis. Reconstr. Surg. Traumatol. 1981;18:70.
3 Bell S., Gschwend N., Steiger U. Arthroplasty of the elbow. Experience with the Mark III GSB prosthesis. Aust. N. Z. J. Surg. 1986;56:823.
4 Brumfield R.H., Volz R.G., Green J.F. Total elbow arthroplasty: a clinical review of 30 cases employing the Mayo and AHSC prostheses. Clin. Orthop. Relat. Res. 1981;158:137.
5 Bryan R.S., Morrey B.F. Extensive posterior exposure of the elbow: a triceps-sparing approach. Clin. Orthop. Relat. Res. 1982;166:188.
6 Cobb T.K., Morrey B.F. Use of distraction arthroplasty in unstable fracture dislocations of the elbow. Clin. Orthop. Relat. Res. 1995;312:201.
7 Conner P.M., Morrey B.F. Total elbow arthroplasty in patients who have juvenile rheumatoid arthritis. J. Bone Joint Surg. 1998;80A:678.
8 Davis P.R. Some Significant Aspects of Normal Upper Limb Functions. Conference on Joint Replacement of the Upper Extremity. London: Institute of Mechanical Engineers, 1977.
9 Davis R.F., Weiland A.J., Hungerford D.S., Moore J.R., Dowling S.V. Nonconstrained total elbow arthroplasty. Clin. Orthop. Relat. Res. 1982;171:156.
10 Dennis D.A., Clayton M.L., Ferlic D.C., Stringer E.A., Bramlett K.W. Capitello-condylar total elbow arthroplasty for rheumatoid arthritis. J. Arthroplasty. 1990;5(suppl):S83.
11 Dee R. Total replacement arthroplasty of the elbow for rheumatoid arthritis. J. Bone Joint Surg. 1972;54B:88.
12 Ewald F.C., Jacobs M.A. Total elbow arthroplasty. Clin. Orthop. Relat. Res. 1984;182:137.
13 Ewald F.C., Scheinberg R.D., Poss R., Thomas W.H., Scott R.D., Sledge C.B. Capitellocondylar total elbow arthroplasty: two- to five-year follow-up in rheumatoid arthritis. J. Bone Joint Surg. 1980;62A:125.
14 Figgie H.E.III, Inglis A.E., Mow C. Total elbow arthroplasty in the face of significant bone stock or soft tissue losses. J. Arthroplasty. 1986;1:71.
15 Figgie M.P., Inglis A.E., Mow C.S., Figgie H.E.III. Salvage of nonunion of supracondylar fracture of the humerus by total elbow arthroplasty. J. Bone Joint Surg. 1989;71A:1058.
16 Fitzgerald R.H.Jr., Nolan D.R., Ilstrup D.M., Van Scoy R.E., Washington J.A.II, Coventry M.B. Deep wound sepsis following total hip arthroplasty. J. Bone Joint Surg. 1977;59A:847.
17 Friedman R.J., Lee D.E., Ewald F.C. Nonconstrained total elbow arthroplasty. Development and results in patients with functional class IV rheumatoid arthritis. J. Arthroplasty. 1989;4:31.
18 Garrett J.C., Ewald F.C., Thomas W.H., Sledge C.B. Loosening associated with GSB hinge total elbow replacement in patients with rheumatoid arthritis. Clin. Orthop. Relat. Res. 1977;127:170.
19 Gill D.R.J., Morrey B.F. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis. A ten to fifteen year follow-up study. J. Bone Joint Surg. 1998;80A:1327.
20 Goldberg V.M., Figgie H.E.III, Inglis A.F., Figgie M.P. Total elbow arthroplasty. J. Bone Joint Surg. 1988;70A:778.
21 Gschwend N., Simmen B.R., Matejovsky Z. Late complications in elbow arthroplasty. J. Shoulder Elbow Surg. 1996;5((2) 1):86.
22 Hui F.C., Chao E.Y., An K.N. Muscle and joint forces at the elbow during isometric lifting. [Abstract.]. Orthop. Trans. 1978;2:169.
23 Inglis A.E., Pellicci P.M. Total elbow replacement. J. Bone Joint Surg. 1980;62A:1252.
24 Johnsson J.R., Getty C.J.M., Lettin A.W.F., Glasgow M.M.S. The Stanmore total elbow replacement for rheumatoid arthritis. J. Bone Joint Surg. 1984;66B:732.
25 King G., Itoi E., Niebur G.L., Morrey B.F., An K.N. Motion and laxity of the capitellocondylar total elbow prosthesis. J. Bone Joint Surg. 1994;76A:1000.
26 Kozak T.K.W. Total elbow arthroplasty in primary osteoarthritis of the elbow: a brief report. J. Arthroplasty. 1998;13:837.
27 Kudo H., Iwano K., Watanabe S. Total replacement of the rheumatoid elbow with a hingeless prosthesis. J. Bone Joint Surg. 1980;62A:277.
28 Linscheid R.L. Resurfacing elbow joint replacement. In: Morrey B.F., editor. Joint Replacement Arthroplasty. New York: Churchill Livingstone; 1991:293.
29 Ljung P., Lidgren L., Rydholm U. Failure of the Wadsworth elbow: 19 cases of rheumatoid arthritis followed for 5 years. Acta Orthop. Scand. 1989;60:254.
30 London, J. T.: Resurfacing Total Elbow Arthroplasty. Presentation. AAOS Annual Meeting, Atlanta, Georgia, February, 1980.
31 Lowe L.W., Miller A.J., Allum R.L., Higginson D.W. The development of an unconstrained elbow arthroplasty: a clinical review. J. Bone Joint Surg. 1984;66B:243.
32 Madsen F., Gudmundson G.H., Sojbjerg J.O., Sneppen O. The Pritchard Mark II elbow prosthesis in rheumatoid arthritis. Acta Orthop. Scand. 1989;60:249.
33 Maloney W.J., Schurman D.J. Cast immobilization after total elbow arthroplasty. A safe cost-effective method of initial postoperative care. Clin. Orthop. Relat. Res. 1989;245:117.
34 Morrey B.F. Complications of total elbow arthroplasty. In: Morrey B.B., editor. The Elbow and Its Disorders. 2nd ed. Philadelphia: W. B. Saunders Co.; 1993:665.
35 Morrey B.F. The Elbow and Its Disorders. Philadelphia: W. B. Saunders Co., 1985.
36 Morrey B.F., Adams R.A. Semiconstrained joint replacement arthroplasty for distal humeral nonunion. J. Bone Joint Surg. 1995;77B:67.
37 Morrey B.F., Adams R. Semiconstrained total elbow arthroplasty for rheumatoid arthritis. J. Bone Joint Surg. 1992;74A:479.
38 Morrey B.F., Askew L.J., An K.N. Strength function after total elbow arthroplasty. Clin. Orthop. 1988;234:43.
39 Morrey B.F., Askew L.J., An K.N., Chao E.Y. A biomechanical study of normal functional elbow motion. J. Bone Joint Surg. 1981;63A:872.
40 Morrey B.F., Bryan R.S. Infection after total elbow arthroplasty. J. Bone Joint Surg. 1983;65A:330.
41 Morrey B.F., Bryan R.S., Dobyns J.H., Linscheid R.L. Total elbow arthroplasty: a five-year experience at the Mayo Clinic. J. Bone Joint Surg. 1981;63A:1050.
42 Pearson J.R., McGinley D.R., Butzel L.M. A dynamic analysis of the upper extremity: planar motions. Hum. Factors. 1963;5:59.
43 Pritchard R.W. Long-term follow-up study: semiconstrained elbow prosthesis. Orthopedics. 1981;4:151.
44 Ramsey M.L., Morrey B.F. Instability of the elbow treated with semiconstrained total elbow arthroplasty. J. Bone Joint Surg. 1998;81A:38.
45 Roper B.A., Tuke M., O’Riordan S.M., Bulstrode C.J. A new constrained elbow. A prospective review of. 60 replacements. J. Bone Joint Surg. 1986;68B:566.
46 Rosenfeld S.R., Ansel S.H. Evaluation of the Pritchard total elbow arthroplasty. Orthopedics. 1982;5:713.
47 Ross A.C., Sneath R.S., Scales J.T. Endoprosthetic replacement of the humerus and elbow joint. J. Bone Joint Surg. 1987;69B:652.
48 Rozing P.M., Poll R.G. Use of the Souter-Strathclyde total elbow prosthesis in patients who have rheumatoid arthritis. J. Bone Joint Surg. 1991;73A:1227.
49 Rydholm U., Tjornstrand B., Pettersson H., Lidgren L. Surface replacement of the elbow in rheumatoid arthritis. J. Bone Joint Surg. 1984;66B:737.
50 Schlein A.P. Semiconstrained total elbow arthroplasty. Clin. Orthop. 1976;121:222.
51 Schneeberger A.G., Adams R., Morrey B.F. Semiconstrained total elbow replacement for the treatment of posttraumatic arthritis and dysfunction. J. Bone Joint Surg. 1997;79A:1211.
52 Soni R.K., Cavendish M.E. A review of the Liverpool elbow prosthesis. J. Bone Joint Surg. 1984;66B:248.
53 Souter W.A. Arthroplasty of the elbow: with particular reference to metallic hinge arthroplasty in rheumatoid patients. Orthop. Clin. North Am. 1973;4:395.
54 Trancik T., Wilde A.H., Borden L.S. Capitellocondylar total elbow arthroplasty. Two- to eight-year experience. Clin. Orthop. Relat. Res. 1987;223:175.
55 Trepman E., Vella I.M., Ewald F.C. Radial head replacement in capitellocondylar total elbow arthroplasty. Two- to six-year follow-up evaluation in rheumatoid arthritis. J. Arthroplasty. 1991;6:67.
56 Voloshin, I., Kakar, S., Krall Kaye, E., and Morrey, B. F.: Complications of total elbow replacement: Systematic review of literature in the last decade. [in press.]
57 Wolfe S.W., Ranawat C.S. The osteoanconeus flap. An approach for total elbow arthroplasty. J. Bone Joint Surg. 1990;72A:684.
58 Yamaguchi K.K., Adams R.A., Morrey B.F. Infection after total elbow arthroplasty. J. Bone Joint Surg. 1998;80A:481.