Complications During Anaesthesia
CAUSES OF COMPLICATIONS
AVOIDANCE OF COMPLICATIONS
 preoperative assessment, investigation and counselling of the patient
preoperative assessment, investigation and counselling of the patient
 preoperative checking of equipment and the assurance of backup equipment
preoperative checking of equipment and the assurance of backup equipment
 the availability of an appropriately trained assistant
the availability of an appropriately trained assistant
 preoperative consultation with more experienced personnel, where necessary, regarding the most appropriate anaesthetic technique
preoperative consultation with more experienced personnel, where necessary, regarding the most appropriate anaesthetic technique
Monitoring
Monitoring systems have been designed to detect and prevent complications during anaesthesia. Aspects of the patient should be monitored that are likely to deviate from the norm, or that are dangerous if they deviate from the norm. The Association of Anaesthetists of Great Britain and Ireland (AAGBI) has produced guidelines stipulating the acceptable minimum level of intraoperative monitoring (see Ch 16).
MANAGEMENT OF COMPLICATIONS
Generic Management of Complications
In general, complications should be dealt with through a sequence of:
1. Continual vigilance and monitoring
2. Recognition of the evolution of a problem
3. Creation of a list of differential diagnoses
4. Choice of a working diagnosis, which is either the most likely or the most dangerous possibility
5. Treatment of the working diagnosis
6. Assessment of the response of the problem to the treatment administered
7. Refinement of the list of differential diagnoses, especially if the response has not been as expected
8. Confirmation or elimination of the choice of working diagnosis; if the response to treatment has been unexpected then replacement with a more likely working diagnosis is indicated
Respiratory System
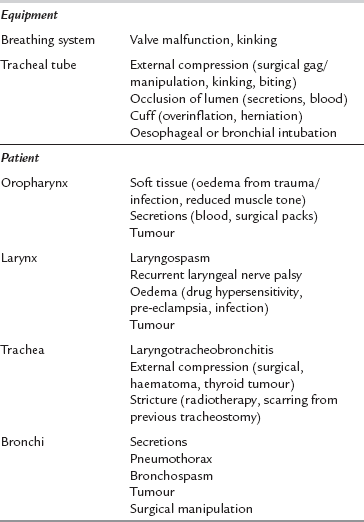
Table 43.1 details the common causes of respiratory obstruction during anaesthesia. Obstruction may occur at any point from the gas delivery system through the patient’s airway and bronchi to the expiratory parts of the breathing system and the scavenging tubing. It is common and potentially very dangerous. The commonest sites for obstruction are the larynx (e.g. laryngospasm), the tracheal tube (e.g. biting or secretions) and the bronchi (e.g. bronchospasm). Respiratory obstruction causes inadequate ventilation and impaired gas exchange. This causes hypercapnia, hypoxaemia and reduced uptake of volatile anaesthetic agent. Respiratory obstruction prevents the mass inflow of ambient gas which occurs during apnoea and thus produces hypoxaemia more rapidly than does apnoea with an open airway.
Management: Any significant airway obstruction should be treated by gentle, manual ventilation of the patient’s lungs with oxygen. Location of the site of obstruction should be sought urgently. In the absence of a laryngeal mask airway (LMA) or tracheal tube, apposition of the tongue and pharyngeal soft tissue is a common cause of upper airway obstruction. This may be overcome by a jaw lift or neck extension. It may require the use of an oro- or nasopharyngeal airway, although these devices may themselves provoke laryngospasm or pharyngeal abrasion. Absolute obstruction suggests an equipment problem. Easy passage of a suction catheter through the tracheal tube confirms its patency. If obstruction persists and no obvious cause is identified, then the tracheal tube or laryngeal mask may be the site of obstruction and should be replaced.
The most common airway complication is partial respiratory obstruction during spontaneous ventilation or during assisted manual ventilation in the absence of a formal (equipment) airway. A gentle jaw thrust, head tilt and chin lift usually clear a partially obstructed airway, and insertion of an oropharyngeal or nasopharyngeal airway resolves almost all of the remainder. In the rare situation of complete inability to ventilate the patient’s lungs manually, and after equipment failure has been excluded, the insertion of a laryngeal mask airway or tracheal tube may be necessary. Allowing the patient to awaken is prudent if there is no urgency to proceed with the operation. If it is necessary to continue the operation or if the patient cannot be wakened, and insertion of a laryngeal mask airway and tracheal tube have proved impossible, then it becomes necessary to establish a surgical airway. This may take the form of a cricothyroid cannula, a surgical cricothyrotomy or an emergency tracheostomy. These procedures must be learned and practised before the occurrence of the incident. They are technically demanding and are themselves potentially life-threatening to the patient if performed inexpertly (see Ch 22).
Laryngospasm
Management: Where possible, airway and surgical stimulation should be avoided during light anaesthesia and the lateral position should be used for control of secretions during extubation and transfer. Surgical stimuli should be anticipated and anaesthetic depth should be adjusted accordingly. The anaesthetist should remove the stimulus to laryngospasm, administer 100% oxygen and provide a clear airway. Gentle pharyngeal suction should be applied. Where appropriate, anaesthetic depth may be increased by administration of an intravenous anaesthetic agent and the lungs ventilated manually, applying continuous positive airway pressure (CPAP). Most episodes of laryngospasm respond to this treatment. If laryngospasm persists and hypoxaemia ensues, a small dose of succinylcholine (e.g. 25 mg in adults) relaxes the vocal cords and allows manual ventilation and oxygenation. A full dose of succinylcholine may be given if tracheal intubation is indicated, but this is usually unnecessary. Doxapram, an analeptic and respiratory stimulant, has also been used successfully in the treatment of laryngospasm.
Bronchospasm
Management: Bronchospasm during anaesthesia results in hypercapnia, hypoxaemia and pulmonary gas trapping, which may cause hypotension (through reduction in left ventricular preload). Management is aimed at preventing hypoxaemia, and resolving the bronchospasm. Initially, 100% oxygen should be given, anaesthesia deepened if appropriate and any aggravating factors removed (e.g. the tracheal tube should be repositioned and surgery stopped). If further treatment is necessary, a bronchodilator should be given in increments according to the response. Recommended drugs include intravenous aminophylline (up to 6 mg kg−1) or salbutamol (up to 3 μg kg−1). Volatile anaesthetic agents and ketamine are also effective bronchodilators. Adrenaline is indicated in life-threatening situations and may be given via the tracheal tube. Steroids and H1-receptor antagonists have no immediate effect but may be indicated in the later management of severe cases of bronchospasm.
Complications Associated with Tracheal Intubation
Poor management of difficult intubation is a significant cause of morbidity and mortality during anaesthesia. Sequelae include dental and airway trauma, pulmonary aspiration, hypoxaemia, brain damage and death. Table 43.2 shows the commonest causes of difficulty in intubation. The single most important cause is an inexperienced or inadequately prepared anaesthetist and the difficulty is often compounded by equipment malfunction. The anatomical features associated with difficult laryngoscopy are listed in Table 43.3. Of these, the atlanto-occipital distance is the best predictor of difficulty but requires a lateral cervical X-ray. Many of these factors are normal anatomical variations, but extreme abnormalities do occur. A cluster of normal variations in an apparently healthy patient may be sufficient to cause major difficulties in laryngoscopy.
TABLE 43.3
Anatomical Factors Associated with Difficult Laryngoscopy
Short, wide, muscular neck
Protruding incisors
High, arched palate
Receding lower jaw
Poor mobility of the mandible
Increased anterior depth of mandible
Increased posterior depth of mandible (reduces jaw opening, requires X-ray)
Decreased atlanto-occipital distance (reduces neck extension, requires X-ray)
Management: Preoperative examination of the airway (Table 43.4) is essential. Identification of patients with a potentially difficult airway (see Tables 43.2 and 43.3) before anaesthesia allows time to plan an appropriate anaesthetic technique. The Mallampati test is a widely used and simple classification of the pharyngeal view obtained during maximal mouth opening and tongue protrusion (see pp. 455–456). In practice, this test suggests a higher likelihood of difficult laryngoscopy if the posterior pharyngeal wall is not seen. The predictive value of this test may be strengthened if the thyromental distance (the distance between the thyroid cartilage prominence and the bony point of the chin during full head extension) is less than 6.5 cm.
TABLE 43.4
Preoperative Assessment of the Airway
General appearance of the neck, face, maxilla and mandible
Jaw movement
Head extension and neck movement
Teeth and oropharynx
Soft tissues of the neck
Recent chest and cervical spine X-rays
Previous anaesthetic records
Mallampati classification
Thyromental distance
Premedication with an antisialagogue reduces airway secretions. This is advantageous before inhalational induction and essential for awake fibreoptic laryngoscopy to maximize the effectiveness of topical local anaesthesia. An anxiolytic may also be given (but is contraindicated in patients with airway obstruction, e.g. caused by burns, trauma, tumour or infection affecting the larynx or pharynx). The presence of a trained assistant is essential and the availability of an experienced anaesthetist and a ‘difficult intubation’ trolley with a range of equipment such as bougies, a variety of laryngoscopes and tracheal tubes, and cricothyrotomy needles is desirable (see Ch 22).
A variety of options exists for the patient in whom a difficult laryngoscopy is anticipated. If the procedure can be carried out under local or regional anaesthesia, then this technique should be used as the first choice (see Ch 24). However, the patient, anaesthetist and equipment must be prepared for general anaesthesia in case a complication arises.
If general anaesthesia is necessary for the procedure, or if the patient refuses local or regional anaesthesia despite a frank discussion of the risks, then steps must be taken to secure the airway safely. Unless tracheal intubation is essential for airway protection or to enable muscle relaxation and ventilation, the use of an artificial airway such as the laryngeal mask with spontaneous ventilation is usually a safe technique. If intubation is essential, the appropriate anaesthetic technique depends on the anticipated degree of difficulty, the presence or absence of airway obstruction and the risk of regurgitation of gastric fluid. The management of the patient in whom difficulties with airway management are anticipated is detailed in Ch 22.
The safest anaesthetic technique may usually be chosen from the following clinical examples.
1. Patients with an increased risk of regurgitation and aspiration (e.g. full stomach, intra-abdominal pathology, pregnancy). An inhalational induction is inappropriate in these patients. Regional anaesthesia is preferable in the parturient (see Ch 35). Preoxygenation and a rapid sequence induction with succinylcholine can be used if there is little anticipated difficulty. If intubation is unsuccessful, no further doses of neuromuscular blocking drug should be used, the patient allowed to wake and further assistance sought. If there is a high degree of anticipated difficulty, an awake technique is recommended (see below).
2. Patients with little anticipated difficulty and no airway obstruction (e.g. mild reduction of jaw or neck movement). After a sleep dose of intravenous induction agent and confirmation of the ability to ventilate the lungs manually by mask, succinylcholine may be given to provide the best conditions for tracheal intubation. If difficulty is encountered, the patient is allowed to wake up and the procedure replanned. Where appropriate, anaesthesia is deepened by spontaneous ventilation using a volatile agent and alternative techniques to facilitate tracheal intubation used (see Ch 22).
3. Patients with severe anticipated difficulty and no airway obstruction (e.g. severe reduction of jaw or neck movement). Appropriate techniques include inhalational induction with sevoflurane or the use of fibreoptic laryngoscopy either in the awake patient or after inhalational induction. Neuromuscular blocking drugs must not be used until the ability to ventilate the lungs manually and view the vocal cords is confirmed.
4. Patients with airway obstruction (e.g. burns, infection, trauma). An inhalational induction may be used; otherwise an awake technique should be considered. Neuromuscular blocking drugs should not be used until tracheal intubation is confirmed.
5. Extreme clinical situations. Tracheostomy performed under local anaesthesia may be the safest technique.
Failed Intubation
Poor management of failed intubation is a significant cause of serious morbidity and mortality. The aims of management are to maintain oxygenation and prevent aspiration of gastric contents. The ‘failed intubation drill’ is now established as an important skill for safe anaesthetic practice. An early decision to use a failed intubation protocol and to call for assistance is essential, because continued attempts at tracheal intubation may result in trauma to the airway, pulmonary aspiration or hypoxaemia (see Ch 22). The obstetric patient is a special case and is considered in Chapter 35.
If the airway is obstructed and ventilation is inadequate during management of a failed intubation, then insertion of an LMA should be considered (see Figs 22.3–22.5). It has been used successfully to provide an airway and allow ventilation when attempts to intubate the trachea and ventilate the lungs by other means have failed. Alternatively, it may be possible to pass a small-diameter tracheal tube or a bougie through the LMA into the trachea; a variant of the LMA, the intubating LMA (ILMA) is designed specifically to facilitate tracheal intubation. The LMA should not be regarded as providing protection against pulmonary aspiration, although it is claimed that the ProSeal™ LMA, which has a rearward port for the downward passage of a gastric tube or the upward passage of gastric contents, is better in this regard. The oesophageal obturator airway and similar devices are alternatives in an emergency, but there are doubts about their efficacy and there have been reports of misplacement and oesophageal rupture associated with their use. A recent innovation is an ILMA which incorporates a video camera and LCD screen, allowing direct visualization of the introduction of a tracheal tube through the larynx.
Aspiration of Gastric Contents
Management: At-risk patients should be managed actively to prevent the aspiration of gastric contents. The volume and acidity of the gastric contents should be reduced as far as possible. Preoperative fasting, histamine H2-receptor blockers and a gastric prokinetic drug (e.g. metoclopramide) are recommended. If general anaesthesia is essential, then the trachea must be intubated. Most commonly, this is achieved using a rapid-sequence induction with cricoid pressure (see Ch 37), but awake intubation is advisable if difficulty in intubation is predicted. During emergence, the tracheal tube should not be removed until protective airway reflexes are regained when the patient is awake.
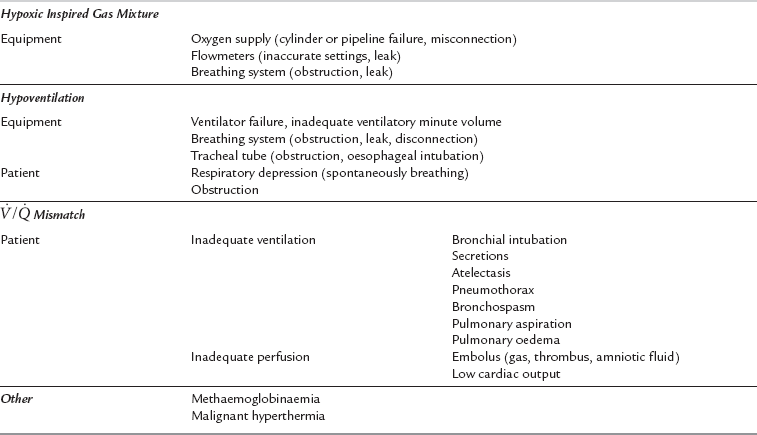
Hypoxaemia
Hypoxaemia is an inadequate partial pressure of oxygen in arterial blood. Hypoxia is oxygen deficiency at the tissue level. A practical classification of the causes of hypoxaemia is shown in Table 43.5. Hypoxaemia threatens tissues globally and, if allowed to persist, risks permanent damage to those organs most delicately dependent upon continued oxygen supply. The first organs to be damaged, most commonly, are the brain and heart, and any pathological impairment of their blood supply increases the risk of early and permanent damage. There is no categorically safe or unsafe level of arterial oxygen tension (PaO2). The risk presented by a level of hypoxaemia is dependent upon the patient’s haemoglobin concentration, cardiac output, state of hydration, concurrent disease processes (especially vasculopathic diseases) and the duration of exposure to the lowered PaO2. In general, few patients are harmed by arterial oxyhaemoglobin saturations of greater than 80%, but clearly, this low level provides very little margin for safety should any other complication occur. Most anaesthetists choose to set the arterial oxygen saturation alarm limits on the pulse oximeter at 92–94%.
Management: Hypoxaemia occurring during anaesthesia is almost invariably treatable and its complications are preventable. Cyanosis should seldom be witnessed by the vigilant anaesthetist because the routine use of pulse oximetry allows early detection and treatment of hypoxaemia. If hypoxaemia is detected, the following drill should be instituted.
1. A-B-C. Ensure an adequate airway, ensure adequate ventilation and check for an adequate cardiac output by feeling the carotid pulse.
2. Exclude delivery of a hypoxic gas mixture using an oxygen analyser. Increase the inspired oxygen concentration to 100%.
3. Test the integrity of the breathing system by manual ventilation of the lungs and confirm bilateral chest movement and breath sounds. Blow down the tracheal tube if necessary.
4. Confirm the position and patency of the tracheal tube by assessing the capnogram, passing a suction catheter through the tracheal tube and auscultating the chest.
5. Search for clinical evidence of the causes of ![]() mismatch with early exclusion of pneumothorax. If atelectasis or reduced functional residual capacity (FRC) is contributory, gentle hyperinflation of the lungs should improve oxygenation. Lung volume may be maintained by applying PEEP.
mismatch with early exclusion of pneumothorax. If atelectasis or reduced functional residual capacity (FRC) is contributory, gentle hyperinflation of the lungs should improve oxygenation. Lung volume may be maintained by applying PEEP.
6. If the diagnosis is difficult, measure core temperature, and consider arterial blood gas analysis and chest X-ray examination.
Apnoea
Management: Unless required for surgery (e.g. during cardiopulmonary bypass or delicate lung surgery), apnoea should not be allowed to persist untreated. Significant atelectasis, hypercapnia and acidaemia may develop silently. If atelectasis develops, then recruitment manoeuvres may be successful in reversing it (see p. 867).
Hypercapnia
Management: Mild degrees of hypercapnia are usually tolerated well by spontaneously breathing patients unless there is a contraindication such as head injury. If signs of significant hypercapnia occur, the anaesthetist should control ventilation and provide an appropriate ventilatory minute volume to normalize PaCO2.
Hypocapnia
Management: Decreasing the ventilatory minute volume or increasing the breathing system dead space reduce removal of carbon dioxide from the blood. It is important to note that the most accessible representation of PaCO2 currently available to anaesthetists is the end-tidal carbon dioxide tension (PE’CO2). This is usually approximately 0.5–1.0 kPa less than PaCO2 but this gap varies significantly with the site of sampling of expired gas and with variations in the alveolar dead space fraction. The latter is affected by alterations in arterial pressure, cardiac output, posture and tidal volume. The anaesthetist should not assume, therefore, that a low value of end-tidal carbon dioxide tension necessarily reflects a low PaCO2. Such an assumption could result in significant hypoventilation if the minute volume is reduced inappropriately.
Pneumothorax
Management: If pneumothorax is suspected prior to anaesthesia, then it should be excluded by chest X-ray. A chest X-ray should be performed preoperatively in all patients who have suffered recent chest trauma and in those in whom a central venous catheter has recently been inserted. Occasionally a chest X-ray fails to show a small pneumothorax which may expand rapidly with the use of nitrous oxide and positive-pressure ventilation. In patients with recent chest trauma, including rib fractures, regional analgesia may be the preferred technique. If tracheal intubation and mechanical ventilation are required in a patient with a pneumothorax, a chest drain should be inserted before induction of anaesthesia. If there is a chest drain in situ, its patency and position should be checked before induction of anaesthesia.
Atelectasis
Management: Atelectasis may be reduced through the use of mechanical ventilation during lengthy operations, the incorporation of nitrogen into the inspired gas mixture, the use of a head-up position where possible and the use of PEEP during mechanical ventilation or continuous positive airways pressure (CPAP) during spontaneous ventilation. If atelectasis is suspected (usually through observation of a gradual downward drift in arterial oxygen saturation or a gradual increase in peak inspiratory pressure during mechanical ventilation), several gentle manual hyperinflations of the lungs usually re-inflate the collapsed alveoli (alveolar recruitment) and result in an increase in arterial oxygen saturation. Inflation for up to 20 s at 40 cmH2O is often required for a successful recruitment manoeuvre. Such a recruitment manoeuvre is probably best performed using a mechanical ventilator.
Cardiovascular Complications
Table 43.6 shows the commonest causes of hypertension during anaesthesia. In the absence of pre-existing hypertension, the majority of instances of intraoperative hypertension are related to increased activity of the sympathetic nervous system. This may be associated with tachycardia and arrhythmias. The commonest causes of hypertension are inadequate analgesia, light anaesthesia, surgical stimulation and airway manipulation. Drug administration errors are probably under-recognised underlying causes. However, all instances of intraoperative hypertension must prompt exclusion of awareness and malignant hyperthermia as the cause.
TABLE 43.6
Causes of Hypertension During Anaesthesia
Pre-Existing
Undiagnosed or poorly controlled hypertension
Pregnancy-induced hypertension
Withdrawal of antihypertensive medication
Increased Sympathetic Tone
Inadequate analgesia
Inadequate anaesthesia
Hypoxaemia
Airway manipulation (laryngoscopy, extubation)
Hypercapnia
Drug Overdose
Vasoconstrictors (noradrenaline, phenylephrine)
Inotropes (dobutamine)
Mixed inotropes/vasoconstrictors (adrenaline, ephedrine)
Ketamine
Ergometrine
Other
Aortic cross-clamping
Phaeochromocytoma
Malignant hyperthermia
Management: Preoperative preparation reduces the incidence of unexpected intraoperative hypertension. Adequate pharmacological treatment of chronic hypertension is essential. Poorly controlled chronic hypertension may result in exaggerated vascular responses during anaesthesia and these patients may suffer greater intraoperative and postoperative morbidity and mortality from arrhythmias and myocardial ischaemia. A calm, relaxed patient is less likely to experience intraoperative hypertension, so consideration should be given to anxiolytic premedication. Where possible, surgery should be postponed until adequate control is achieved (e.g. arterial pressure less than 180/110 mmHg) and organ function (e.g. heart and kidneys) assessed. If end-organ damage is found, it should be investigated, treated and the perioperative risk re-assessed. In the absence of such damage, anaesthesia and surgery can usually proceed. There is some evidence that beta blockade, alpha2 agonists or thoracic epidural block may aid cardiovascular stability.
Stimulating events during anaesthesia and surgery cause surges in sympathetic tone, with significant increases in arterial pressure. These events may usually be anticipated and a short-acting opioid (e.g. alfentanil 10 μg kg−1), β-blocker (e.g. esmolol 0.5 mg kg−1), lidocaine (1 mg kg−1) or temporary deepening of the anaesthetic may be used to obtund potentially damaging hypertension. Such events include laryngoscopy, surgical incision, extubation and aortic cross-clamping. Hypertension which occurs despite normoxaemia, adequately deep anaesthesia and adequate analgesia should prompt the exclusion of the causes listed in Table 43.6. If no pathological cause is found, the use of an antihypertensive agent such as labetalol or hydralazine may be indicated. The effect of negatively inotropic and vasodilating drugs is potentiated by anaesthetic agents, so careful titration is required.
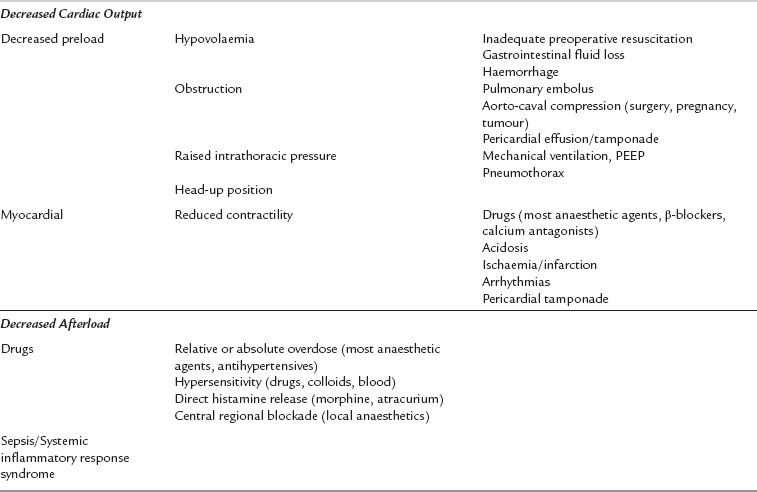
Hypotension
Hypotension is caused by decreases in cardiac output or systemic vascular resistance. Table 43.7 shows the common causes of intraoperative hypotension. Most anaesthetic agents cause vasodilatation and have a negative inotropic effect, and moderate hypotension is very common during anaesthesia, particularly before the start of surgery when there is no physical stimulation to counteract the effects of anaesthetic drugs. Concurrent hypovolaemia caused by preoperative fluid restriction, in combination with haemorrhage or concurrent antihypertensive drugs, may result in decreases in mean arterial pressure during anaesthesia and surgery. A mean arterial pressure of 50 mmHg or less is potentially damaging, even in healthy individuals, and should not be allowed to persist. The patients most at risk from the effects of hypotension are, unfortunately, often the patients most likely to develop it because of concurrent medications, poor myocardial reserve and atherosclerosis. Elderly or hypertensive patients should, therefore, be observed carefully for the development of hypotension, and it should be treated promptly.
Hypovolaemia
Hypovolaemia is a common intraoperative cause of hypotension. Its common aetiologies are listed in Table 43.8. The additive effects of anaesthesia, positive-pressure ventilation and hypovolaemia may cause sudden and severe hypotension, which may be life-threatening. All patients who are undergoing surgery, and in particular patients who require emergency surgery, should be assessed preoperatively with regard to intravascular fluid volume and fluid balance. Signs of hypovolaemia include thirst, dryness of mucous membranes, cool peripheries, oliguria (urine output < 0.5 mL kg−1 h−1), reduced tissue turgor, tachycardia and postural hypotension. Patients treated with a β-blocking drug may not develop a compensatory tachycardia despite being hypovolaemic. Fluid deficit and replacement are easily underestimated, especially in patients with intestinal obstruction or concealed haemorrhage. Surgery, unless immediately life-saving, should be delayed to allow adequate fluid resuscitation and restoration of intravascular volume. The response of central venous pressure (CVP) to fluid challenges is a useful guide when assessing and treating patients with significant hypovolaemia. Hypokalaemia and other electrolyte abnormalities are often associated with fluid deficits, particularly when there have been gastrointestinal losses.
TABLE 43.8
Causes of Hypovolaemia and Fluid Loss
Preoperative
Haemorrhage
Trauma
Obstetric
Gastrointestinal
Major vessel rupture (aortic aneurysm)
Gastrointestinal
Vomiting
Obstruction
Fistulae
Diarrhoea
Other
Fasting
Diuretics
Fever
Burns
Intraoperative
Haemorrhage
Insensible loss
Sweating
Expired water vapour
Third-space loss
Prolonged procedures
Extensive surgery
Prolonged retraction
Drainage of stomach, bowel, or ascites
Haemorrhage
Massive blood loss may require the administration of stored blood, fresh frozen plasma, clotting factors and electrolytes. The problems of massive transfusion are discussed in Chapter 13.
Disturbances of Heart Rate
Bradycardia: Bradycardia is commonly defined as a heart rate less than 60 beat min−1. Heart rate very commonly decreases during anaesthesia because much afferent input is lost and because many anaesthetic agents and opioids have parasympathomimetic actions. Surgical manipulations such as traction on the eye, cervical or anal dilatation and peritoneal traction may increase vagal tone, producing bradycardia and occasionally sinus arrest. Several drugs may cause bradycardia. Succinylcholine may produce a profound decrease in heart rate, especially following repeat doses. Bradycardia occurs in some patients after intravenous injection of rapidly acting opioid analgesics such as remifentanil, alfentanil and fentanyl. There have been several reports of profound bradycardia in association with the use of propofol. Finally, neostigmine and β-blockers may cause profound bradycardia. Anaesthesia-induced bradycardia often leads to an escape rhythm, with a wandering suprajunctional pacemaker. Hypothermia and hypothyroidism can also cause bradycardia.
Tachycardia: During anaesthesia, tachycardia may be defined as a heart rate greater than 100 beat min−1. Tachycardia is a normal sign of increased sympathetic nervous system activity. It is observed in most patients at some time during the perioperative period. Sympathetic nervous system activity is increased by hypoxaemia, hypercapnia, inadequate anaesthesia, inadequate analgesia, hypovolaemia, hypotension and noxious stimulation such as airway manipulations or surgical incision. Other signs of sympathetic nervous activity may be present, including hypertension. Tachycardia is associated also with an increase in metabolic rate (e.g. fever, sepsis, burns, hyperthyroidism, malignant hyperthermia), or the administration of vagolytic drugs (e.g. atropine, pancuronium) or sympathomimetic drugs (e.g. ephedrine, adrenaline). Isoflurane and desflurane may also increase heart rate, particularly if introduced rapidly in a high concentration. Tachycardia reduces diastolic coronary perfusion and simultaneously increases myocardial work. This may precipitate myocardial ischaemia in patients with coronary artery or hypertensive heart disease.
Arrhythmia: Arrhythmias are not infrequent during anaesthesia, and common causes are listed in Table 43.9. Extracellular potassium concentration has a profound effect on myocardial electrical activity. Hypokalaemia increases ventricular irritability and the risks of ventricular ectopics, tachycardia and fibrillation. This effect is potentiated in patients with ischaemic heart disease and in those receiving digoxin. Hyperventilation alters acid–base balance, with acute transmembrane redistribution of potassium. Serum potassium concentration decreases by approximately 1 mmol L−1 for every 2.5 kPa reduction in PaCO2. Life-threatening hyperkalaemia with atrioventricular conduction block or ventricular fibrillation may occur if succinylcholine is used in patients with burns or denervating injuries. Electrolyte disorders are discussed further in Chapter 12.
TABLE 43.9
Causes of Arrhythmias During Anaesthesia
Cardiorespiratory
Hypoxaemia
Hypotension
Hypocapnia
Hypercapnia
Myocardial ischaemia
Metabolic
Catecholamines:
Inadequate analgesia
Inadequate anaesthesia
Airway manipulation
Sympathomimetics
Hyperthyroidism
Electrolyte disturbance:
Hypokalaemia/hyperkalaemia
Hypercalcaemia/hypocalcaemia
Malignant hyperthermia
Surgical
Increased vagal tone (traction on eye, anus, peritoneum)
Direct cardiac stimulation (chest surgery, CVP cannulae)
Dental surgery
Drugs
Vagolytics (atropine, pancuronium)
Sympathomimetics (adrenaline, ephedrine)
Volatile anaesthetic agents (halothane, enflurane)
Digoxin
Management. Preoperative correction of fluid, electrolyte and acid–base imbalance is essential. Optimization of coronary artery disease and hypertension is also helpful in avoiding intraoperative arrhythmias (Ch 18).
Atrial Arrhythmias: These may reduce the atrial contribution to left ventricular filling, resulting in a decrease in cardiac output. Premature atrial contractions and wandering atrial pacemakers are common and of little consequence.
Ventricular Arrhythmias: Premature ventricular contractions (PVCs) are common in healthy patients and may be present preoperatively. If associated with a slow atrial rate (escape beats), increasing the sinus rate by administration of an anticholinergic drug should abolish them. In other situations, an underlying cause should be sought before antiarrhythmic agents are considered, as PVCs rarely progress to more serious arrhythmias unless they are multifocal and frequent or if there is underlying myocardial ischaemia or hypoxaemia. Halothane lowers the threshold for catecholamine-induced ventricular arrhythmias, and this effect is exacerbated by hypercapnia. Halothane should be used with care in patients receiving sympathomimetic drugs (including local anaesthetics containing adrenaline) and in patients taking aminophylline or drugs that block noradrenaline re-uptake, such as tricyclic or other antidepressants. The maximum recommended dose of adrenaline for infiltration in the presence of halothane is 100 μg (10 mL of 1 in 100 000) during any 10-min period, although the rate of absorption depends on the site of injection. The use of isoflurane carries a much lower risk of development of arrhythmias and sevoflurane has an even lower potential to cause myocardial sensitization to catecholamines.
Any arrhythmia which causes the loss of a palpable pulse should be treated as for cardiac arrest, with immediate external (or internal, if appropriate) cardiac massage, ventilation of the lungs with 100% oxygen and drug treatment as specified in the appropriate Advanced Life Support protocol (see Ch 47).
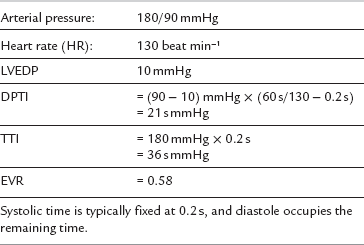
Myocardial Ischaemia
The ratio of DPTI/TTI is the endocardial viability ratio (EVR) and represents the myocardial oxygen supply–demand balance. The EVR is usually greater than one. A value of less than 0.7 is associated with subendocardial ischaemia. Such a value may be reached in a patient with the data shown in Table 43.10.
It is clear from the above that tachycardia is particularly dangerous in generating myocardial ischaemia, while systolic hypertension and diastolic hypotension may also contribute. Patients with coronary artery disease are most at risk. Intraoperative myocardial ischaemia may manifest clinically as arrhythmia, hypotension or pulmonary oedema. It is diagnosed by ECG ST-segment changes (usually depression), although these are not always detected reliably without computer-assisted analysis. The use of the V5 electrode is recommended for ECG monitoring in susceptible patients (e.g. the CM5 configuration; see Ch 16) because it is the most sensitive ECG lead for the detection of left ventricular ischaemia. When used alone, it may detect up to 85% of the ST abnormalities on a standard 12-lead ECG.
Management: Preoperative preparation of the at-risk patient includes optimization of anti-anginal and antihypertensive medication, suppression of anxiety and assurance of normal intravascular volume. During anaesthesia, the risks associated with ischaemia are minimized by the use of an appropriate anaesthetic technique and early detection by the use of appropriate monitoring in susceptible patients.
Embolus
Thromboembolus
Management: Patients with risks factors should be managed actively to prevent deep venous thrombosis. The oral contraceptive pill or hormone replacement therapy should be stopped at least 6 weeks before elective surgery in patients at risk. Prophylactic heparin, graduated compression stockings and intraoperative intermittent calf compression reduce the likelihood of new thrombosis. The use of subarachnoid or epidural anaesthesia reduces the risk of postoperative venous thromboembolism in some surgical groups.
Gas Embolus
Management: Prevention of intraoperative air embolus requires adjustment of the patient’s position and the site of the operative field with respect to the right atrium. If air embolism is detected, further air entry is prevented by flooding the operative site with saline. During head and neck procedures, the venous pressure at the surgical site may be increased by compressing the jugular veins. If possible, the operative site should be lowered relative to the right atrium. The application of PEEP increases venous pressure and reduces further ingress of air.
Neurological Complications
Management: If the anaesthetist suspects, intraoperatively, that a patient may be experiencing awareness, anaesthesia should be deepened immediately. If the arterial pressure is low despite an inadequate dose of anaesthetic agent, then the arterial pressure should be supported through the use of intravenous fluids, modification of ventilatory pattern or intravenous administration of a vasopressor, and anaesthesia deepened appropriately. Consideration should be given to the use of an intravenous benzodiazepine (e.g. midazolam 5 mg). Some retrograde amnesia may be gained and further recall is made less likely through the anterograde amnesic effect.
Neurological Injury
Ischaemia of the Central Nervous System
Management: Hypoxaemia and hypotension should not be allowed to persist, particularly in patients at risk of CNS ischaemic injury. Severe hypocapnia should be avoided because of its potential for global cerebral vasoconstriction. Deep anaesthesia results in a greatly lowered cerebral metabolic rate, and this confers some protection against cerebral ischaemia.
Temperature
Body temperature very commonly decreases during anaesthesia. A decrease to a core temperature below 36 °C represents hypothermia. Hypothermia causes physiological derangement and increases perioperative morbidity (see also Ch 11).
Management: Ambient temperature and humidity should be maintained as high as is comfortable for staff working in the operating theatre. The patient should be protected from the cool ambient environment during all phases of anaesthesia and during transfers. A convective warming blanket (e.g. Bair Hugger™), which surrounds the patient with a microenvironment of warm air, is particularly effective. The head and any exposed moist viscera lose heat particularly quickly and wrapping these is effective in preventing radiation and evaporative heat loss. Cold intravenous fluids should be warmed where possible, although this should not compromise the administration of adequate volumes. Inspired gases should be warmed and humidified. A heat and moisture exchanging device is very effective in this regard, and is often combined with a microbial filter.
Hyperthermia
Management: General measures include exposure of the body surface, application of ice packs, use of fans and administration of cold intravenous fluids. Specific measures depend on the cause. Paracetamol and non-steroidal anti-inflammatory agents may reduce core temperature if the cause is sepsis-related. The occurrence of any unexplained increase in temperature, especially if temperature is increasing rapidly, must prompt the urgent exclusion of malignant hyperthermia.
Malignant Hyperthermia
Management: Administration of a volatile anaesthetic agent should be discontinued immediately and the lungs hyperventilated with 100% oxygen. The anaesthetic breathing system should be replaced with an unused (and therefore uncontaminated) system. Anaesthesia should be maintained with an intravenous agent. The trachea should be intubated at the earliest opportunity if a tracheal tube is not already in place. Experienced help should be obtained in both the operating theatre and the laboratory, and the operation must be abandoned as soon as possible. Specific tasks should be allocated (e.g. preparation of dantrolene, monitoring, managing communication with lab etc.). Intravenous dantrolene should be given as an immediate bolus (2.5 mg/kg). Further boluses of 1 mg/kg should be given as required to a maximum of 10 mg/kg until the increase in PE’CO2 is controlled. Dantrolene is packaged as a powder which takes several minutes to reconstitute. For a 70 kg adult, the intial dose would be 9 vials of dantrolene (20 mg each), mixed with 60 mL sterile water. It is a skeletal muscle relaxant and causes muscle weakness in large doses. The massive increase in metabolic rate commonly produces severe acidosis, and large amounts of intravenous fluids and sodium bicarbonate may be required. Ice packs should be placed around the neck, axillae and groin, and chilled saline should be infused intravenously. If necessary, gastric, rectal and peritoneal lavage with iced saline may be life-saving if hyperthermia is severe. Cardiopulmonary bypass has been used in severe cases.
Drug Reactions
Management: Death may result from tissue hypoxia secondary to inadequate perfusion and/or hypoxaemia. Early recognition and treatment are essential. The aims of management are to obtund the effect of the anaphylaxis mediators and to prevent their further release. A management ‘drill’ should be used (Table 43.11). The early use of adrenaline is life-saving during severe anaphylactic reactions because it treats the symptoms (through peripheral vasoconstriction, increased cardiac output and bronchodilation) and the cause (through mast cell restabilization). Corticosteroids and histamine H1-receptor antagonists have a delayed onset of action, but have a role in later management.
TABLE 43.11
Drill for the Management of Major Anaphylaxis
Initial Therapy
Stop administration of drug(s) likely to have caused the anaphylaxis
Call for help and note the time
Maintain airway. Give 100% oxygen
Lay patient supine with feet elevated
Give adrenaline. This may be given intramuscularly in a dose of 0.5 (0.5 of 1:1000) and may be repeated after 5 min according to the arterial pressure and pulse until improvement occurs. Alternatively, adrenaline may be administered intravenously. The dose given depends upon the severity of the reaction. Minor symptoms may be treated with 50 μg boluses, while cardiovascular collapse requires a bolus dose of 1000 μg. Further doses are likely to be required
Start intravascular volume expansion with crystalloid or colloid
Secondary Therapy
Antihistamines (chlorpheniramine 10 mg i.v.)
Corticosteroids (hydrocortisone 200 mg i.v.)
Catecholamine infusions (starting doses:
adrenaline 0.05–0.1 μg kg−1 min−1 noradrenaline 0.05–0.1 μg kg−1 min−1)
Consider bicarbonate (0.5 mmol kg−1 i.v.) for acidosis, repeated as necessary
Airway evaluation (before extubation)
Bronchodilators (salbutamol 2.5 mg kg−1) may be required for persistent bronchospasm
Other Drug Reactions
Acute Intermittent Porphyria: Acute intermittent porphyria (AIP) is a rare but serious metabolic disorder caused by an inherited deficiency of an enzyme required for haem synthesis. Porphyrin precursors accumulate and cause acute neuropathy, abdominal pain (mimicking an acute abdomen), delirium and death. These precursors are produced in the liver by δ-amino laevulinic acid synthetase, and this enzyme may be induced by barbiturates, amongst other drugs. If an at-risk patient is identified, porphyrinogenic drugs (including barbiturates) must be avoided. Drugs considered safe include propofol, midazolam, succinylcholine, vecuronium, nitrous oxide, morphine, fentanyl, neostigmine and atropine.
Regional Anaesthesia
Hypotension: Arterial and venous dilatation caused by sympathetic pharmacological denervation reduce cardiac afterload and preload. Systemic hypotension results, and may be severe enough to compromise organ perfusion. Signs include tachycardia, low arterial pressure, confusion, nausea and dizziness. Fluid preloading may reduce the incidence of hypotension, and the left lateral position is helpful in pregnant patients. Intravenous titration of a vasopressor agent (e.g. bolus doses of ephedrine 5 mg or metaraminol 1 mg) usually restores arterial pressure rapidly. Directly acting arterial constrictors are more appropriate than indirectly acting sympathomimetics if tachycardia is present and in patients with severe ischaemic heart disease.
Nerve Injury: Nerve injury may result from direct trauma caused by the needle, or through chemical toxicity. It is widely held that epidural and subarachnoid block should be undertaken in the conscious patient to allow early identification of impingement upon a nerve or the spinal cord.
Infection and Haematoma: Spinal abscess presents as sudden, painless loss of motor function, usually several days after performance of the block, and is a devastating complication of central nervous blockade. Meticulous aseptic technique helps to reduce the incidence of this complication but some cases arise spontaneously, probably as a result of bacteraemia caused by surgery and inoculation of a small and otherwise asymptomatic epidural haematoma. Urgent magnetic resonance imaging and referral to a neurosurgeon are indicated if spinal abscess is suspected.
Peripheral Nerve Blocks: There is a risk of direct damage to nerves in association with any peripheral nerve block. Paraesthesia or pain in the distribution of a nerve are signs of the proximity of a nerve and should prompt needle withdrawal. Pain during injection of local anaesthetic, failure to abolish the muscular twitch from a nerve stimulator or the requirement for increased injection force may indicate intraneural positioning of the needle and should prompt immediate cessation of injection and withdrawal of the needle.
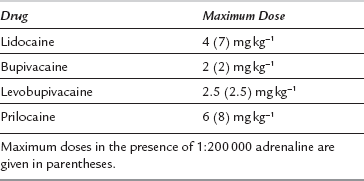
Local Anaesthetic Toxicity
Management: Intravenous access should be secured and adequate resuscitation equipment and drugs should be immediately available before a local anaesthetic block is undertaken. The patient should be adequately monitored. The lowest dose of the least toxic drug available should be used to achieve the effect required (Table 43.12). The total dose should be reduced in frail patients and in patients otherwise at risk of toxicity (e.g. patients with epilepsy or heart block). Local anaesthetics should always be injected slowly with repeated aspiration for blood, and with constant verbal contact with, and observation of, the patient. Any change in the patient’s apparent mental state should prompt immediate cessation of injection. Injection of a test dose of a local anaesthetic which contains adrenaline usually results in sudden tachycardia if intravascular injection has occurred.
If lipid has been given, its use should be reported to the international registry at www.lipidregistry.org.
Atlee J.L., ed. Complications in anesthesia, second ed., New York: Saunders, 2006.
Benumof J.L., Saidman L.J., eds. Anesthesia and perioperative complications, second ed., New York: Year Book Medical Publications, 1999.
Contractor, S., Hardman, J.G. Injury during anaesthesia. CEACCP. 2006;6:67.
Finucane B.T., ed. Complications of regional anesthesia. New York: Elsevier, 1999.
Gravenstein N., ed. Complications in Anesthesia. Philadelphia: Lippincott Williams & Wilkins, 2006.
Hardman, J.G., Aitkenhead, A.R. Awareness during anaesthesia. CEACCP. 2005;5:183.