Chapter 49 Complications After Hematopoietic Stem Cell Transplantation
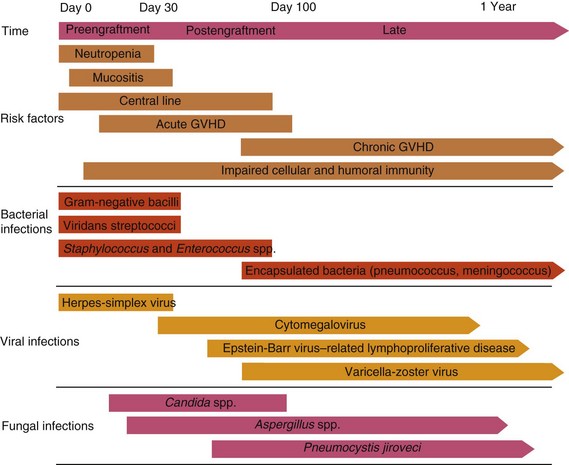
Table 49-1 Major Complications of Hematopoietic Cell Transplantation
Mo, Month; UCB, umbilical cord blood.
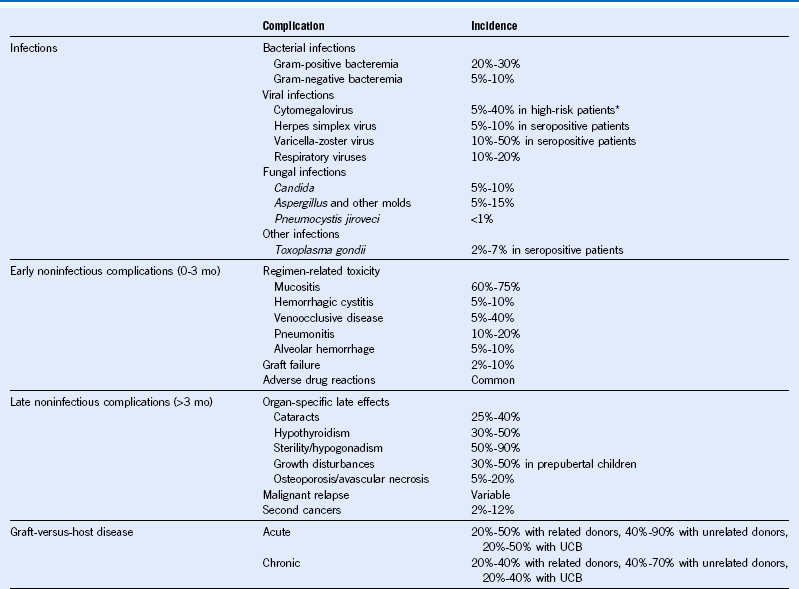
Table 49-2 Common Infections in Hematopoietic Cell Transplant Recipients
CMV, Cytomegalovirus; GVHD, graft-versus-host disease; GI, gastrointestinal; HCT, hematopoietic cell transplantation; HLA, human leukocyte antigen; PTLD, posttransplant lymphoproliferative disorder; TMP-SMX, trimethoprim-sulfamethoxazole; wk, week.
* Includes Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis.
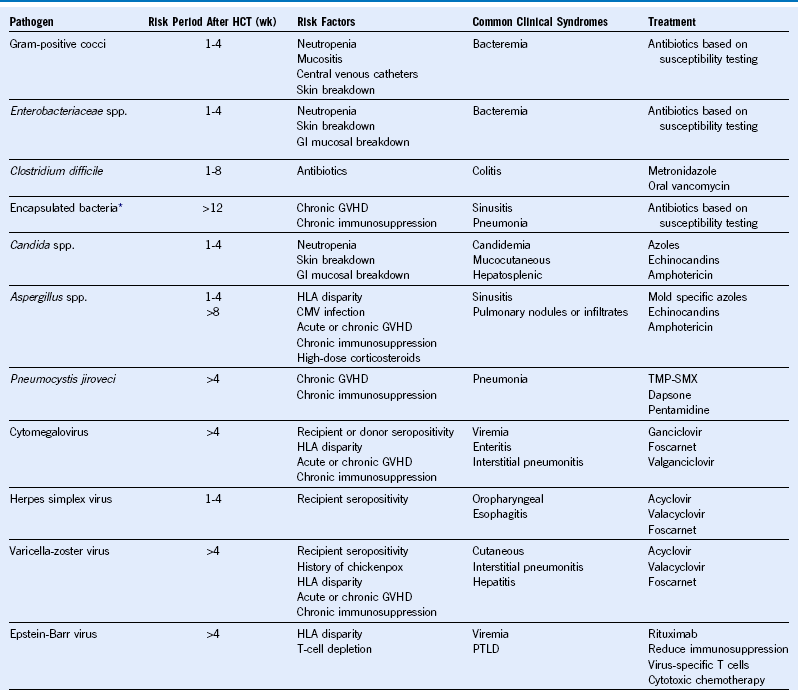
Table 49-3 Recommended Antimicrobial Prophylaxis Against Common Infections
| Pathogen | Preventing Early Disease (0-100 Days After HCT) | Preventing Late Disease (>100 Days After HCT) |
|---|---|---|
| Bacterial infections | No specific recommendations* | Antibiotics (based on local resistance patterns) to prevent infections due to encapsulated bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis) in patients on chronic immunosuppression |
| Cytomegalovirus | Prophylaxis or preemptive treatment with ganciclovir or valganciclovir in high-risk patients† | Preemptive treatment with ganciclovir or valganciclovir in high-risk patients† |
| Herpes simplex virus | Acyclovir in seropositive patients | Acyclovir in patients with recurrent HSV infections |
| Yeast infections | Fluconazole | Fluconazole in patients on chronic immunosuppression |
| Mold infections | No specific recommendations‡ | No specific recommendations* |
| Pneumocystis jiroveci | Trimethoprim-sulfamethoxazole (preferred) or dapsone or pentamidine | Trimethoprim-sulfamethoxazole (preferred) or dapsone or pentamidine in patients on chronic immunosuppression |
HCT, Hematopoietic cell transplantation; HSV, herpes simplex virus.
*Limited data exist favoring fluoroquinolones such as levofloxacin. No impact on infection-related mortality.
†Cytomegalovirus (CMV)-seropositive HCT recipients or CMV-seronegative recipients with a CMV-seropositive donor.
‡Limited data available. Prospective testing of voriconazole and posaconazole suggests possible benefit as prophylaxis. No impact on mold-related mortality.
Table 49-4 Recommended Vaccinations for Hematopoietic Cell Transplantation Recipients
| Vaccine* | Time After HCT to Initiate Vaccine | No. of Doses† |
|---|---|---|
| Pneumococcal conjugate | 3-6 mo | 2-3‡ |
| DTaP§ | 6-12 mo | 3 |
| Haemophilus influenzae type b conjugate | 6-12 mo | 3 |
| Inactivated poliovirus | 6-12 mo | 3 |
| Recombinant hepatitis B | 6-12 mo | 3 |
| Inactivated influenza | 4-6 mo | 1-2 yearly‖ |
| Measles, mumps, and rubella virus (live) | 24 mo | 1-2¶ |
| Varicella-zoster | 24 mo | 1¶ |
DTaP, Diphtheria and tetanus toxoids and acellular pertussis vaccine; HCT, hematopoietic cell transplantation.
*Vaccinations are deferred in patients with chronic graft-versus-host disease (GVHD) until discontinuation of immunosuppression.
†A minimum of 1-month interval between doses is suggested.
‡Following the primary series of three pneumococcal conjugate vaccine (PCV) doses, a dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) to broaden the immune response might be given. For patients with chronic GVHD who are likely to respond poorly to PPSV23, a fourth dose of the PCV should be considered instead of PPSV23.
§DTaP is preferred; however, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) can be used if DTaP is not available.
‖For children younger than 9 years of age, two doses are recommended yearly between transplant and 9 years of age.
¶Not recommended less than 24 months post-HCT, in patients with active GVHD, and in patients on immune suppression. In children, two doses of measles, mumps, and rubella virus vaccine live are favored. Lower viral-dose vaccines (varicella vaccine live [Varivax], not zoster vaccine live [Zostavax]) may be preferred as potentially safer.
Approach to Prevention and Treatment of Cytomegalovirus Infection
Prevention
1. Seronegative recipient with seronegative donor (allogeneic and autologous): Transfuse only cytomegalovirus (CMV)-safe blood products. Leukocyte depletion by filtration and blood from CMV-seronegative donors are clinically equivalent alternatives.
2. Seronegative recipient with seropositive donor (allogeneic): Deliver only CMV-safe blood products (seronegative or leukocyte depleted), but administer chemoprophylaxis as well to prevent reactivation of donor-derived endogenous virus.
3. Seropositive recipients: Prophylaxis with acyclovir appears to be somewhat useful. Use of immunoglobulin may be beneficial. There is no proven role for seronegative blood products. Ganciclovir is highly effective when given prophylactically but is myelosuppressive. Patients who must interrupt the course of ganciclovir because of leukopenia are at risk for development of CMV pneumonia. Ganciclovir (or valganciclovir) is the current best prophylaxis in high-risk patients but is not indicated for autologous recipients. Intensive surveillance and early preemptive therapy may be equivalently effective.
4. All patients need periodic (weekly) monitoring for CMV antigenemia or CMV deoxyribonucleic acid (DNA) polymerase chain reaction for 8 to 12 weeks posttransplantation. Longer-duration (beyond 12 weeks) surveillance is appropriate for allograft recipients, especially those with graft-versus-host disease.
Treatment
1. Asymptomatic infections (allogeneic and autologous): Ganciclovir or valganciclovir treatment of asymptomatic infection detected in blood or bronchioalveolar lavage (BAL), by either molecular detection or antigenic methods, is recommended to prevent the development of CMV pneumonia. Intensive induction treatment (2 weeks) followed by a maintenance phase of 5 days/wk therapy for an additional 4 to 8 weeks is necessary.
2. CMV pneumonia: Ganciclovir in combination with immunoglobulin is recommended. This should be instituted promptly. Once the disease has progressed to cause respiratory failure and ventilator dependence, survival is limited.
No Treatment
1. Empiric CMV therapy for interstitial pneumonitis is not indicated in seronegative recipients with seronegative graft and blood donors. Diagnostic evidence of CMV infection should be obtained.
2. Empiric CMV therapy for interstitial pneumonitis is also not indicated in patients whose BAL is negative for CMV by direct staining and molecular testing. However, BAL CMV studies have a small (<5%) false-negative rate, and close follow-up and monitoring is still required.
3. Asymptomatic CMV viruria does not require therapy but does need close follow-up and serial blood viral testing for surveillance of systemic disease.
Sinusoidal Obstruction Syndrome
Diagnostic Criteria for Sinusoidal Obstruction Syndrome
Bilirubin level of 2 mg/dL or above before day 21 after hematopoietic cell transplantation (HCT) and at least two of the following: (1) hepatomegaly or right upper quadrant pain, (2) ascites, or (3) weight gain of more than 5% over baseline.
Sinusoidal obstruction syndrome (SOS) is a clinical diagnosis; liver ultrasound with Doppler studies or liver biopsy can support or confirm the diagnosis.
Approach to Interstitial Pneumonitis
1. Timing: Within the first 3 weeks after HCT, interstitial pneumonitis is more likely to be idiopathic (including diffuse alveolar hemorrhage) or fungal than due to cytomegalovirus (CMV) infection. Beyond 6 weeks, idiopathic pneumonitis is unusual, and the cause is more likely infectious. Pneumocystis jiroveci pneumonia is rare beyond 1 year after transplantation except in patients with ongoing chronic graft-versus-host disease (GVHD). Respiratory syncytial virus (RSV) infections are seasonal (fall and winter), and community outbreaks can be prevalent. Influenza is also seasonal, whereas parainfluenza can occur year-round.
2. CMV serology and prophylaxis: If a seronegative recipient has received a seronegative graft and noninfective (seronegative or leukocyte-depleted) blood, CMV pneumonia is unusual. Seropositive recipients are at higher risk, although with ganciclovir or other antiviral prophylaxis, the risk is markedly reduced. Other prophylactic regimens for CMV, such as acyclovir or intravenous immunoglobulin, have still been associated with significant risk for serious CMV infection in the seropositive recipient. Serial negative testing for CMV antigenemia or deoxyribonucleic acid (DNA) polymerase chain reaction (PCR) makes CMV pneumonitis less likely.
3. Prolonged neutropenia: This factor is associated with infectious causes, particularly with fungal pneumonias.
4. Type of transplant: Diffuse alveolar hemorrhage is less frequently seen in patients undergoing autologous HCT. CMV pneumonia is unusual (2% to 3%) in autologous recipients, but it still has a high case fatality rate. All infectious causes are more common after allogeneic HCT. More-intensive conditioning regimens (e.g., higher total body irradiation, carmustine) are associated with more frequent pneumonitis.
5. Compliance and prophylaxis: A thorough assessment of what prophylaxis the patient has actually been receiving (e.g., trimethoprim-sulfamethoxazole, penicillin, CMV prophylaxis, transfusions outside the transplant center) is critical to assess risk.
6. Chest radiograph: The pattern and distribution of the infiltrate may narrow the differential diagnosis. Cardiac enlargement or pleural effusions may suggest pulmonary edema. A chest computed tomographic scan is useful, especially if nodularity, pleural involvement, or cavitary lesions (possibly fungal) are suspected.
7. Epidemiology: Identification of the causes of other recent cases can be most helpful with infections that are horizontally transmitted (e.g., RSV) or have common environmental risk factors (e.g., Aspergillus infection associated with construction).
8. Bronchoalveolar lavage (BAL): This can be extremely useful to establish a specific diagnosis or to exclude others. CMV rarely causes pneumonia without positive BAL findings (either direct staining of CMV-associated antigens in BAL cells or DNA PCR). BAL also usually detects RSV, Pneumocystis jiroveci, and other respiratory viruses, though not as rapidly, but is required to identify alveolar hemorrhage. It is less sensitive for diagnosis of fungal pneumonias.
9. Lung biopsy: Although this is the gold standard for definitive diagnosis of most of the possible causes of interstitial pneumonitis, it can often be avoided through the use of the clinical diagnostic measures listed. It may be necessary for the definitive diagnosis of fungal pneumonias, pulmonary changes associated with chronic GVHD (bronchiolitis obliterans), or idiopathic interstitial pneumonitis. Either bronchoscopic (transbronchial) biopsy, open surgical biopsy, or video-assisted thoracoscopic surgery can be performed. Transbronchial biopsies are insufficient for other than very diffuse processes and carry risks for bleeding and/or pneumothorax. The surgical approaches are more invasive, but more often definitive.
10. Ventilator therapy: Progressive respiratory failure after HCT is rarely reversible, especially in adults. Although aggressive diagnostic and therapeutic measures are essential, some centers offer patients and their families the option of foregoing mechanical ventilatory support if survival is not expected. Preliminary discussion of this possible complication in pretransplant patient counseling can facilitate decision making if respiratory failure does occur.
Table 49-5 Selected Late Complications of Hematopoietic Cell Transplantation
| Complication | Risk Factors | Monitoring and Prevention |
|---|---|---|
| Endocrine | ||
| Hypothyroidism | TBI/radiation | Periodic assessment of thyroid and gonadal function |
| Hypogonadism | Chronic GVHD | |
| Growth retardation | Chemotherapy | |
| Ocular | ||
| Cataracts | TBI/radiation | Periodic eye examination |
| Keratoconjunctivitis sicca | Corticosteroids | |
| Chronic GVHD | ||
| Oral | ||
| Dental caries | TBI/radiation | Periodic dental assessment |
| Dry mouth | Chronic GVHD | |
| Cardiovascular | ||
| Coronary artery disease | TBI/radiation | Periodic clinical evaluation |
| Cerebrovascular disease | Chemotherapy | Modification of risk factors |
| Respiratory | ||
| Bronchiolitis obliterans | TBI/radiation | Periodic clinical evaluation |
| Interstitial pneumonitis | Chronic GVHD | Smoking cessation |
| Infections | ||
| Hepatic | ||
| Cirrhosis | Hepatitis B or C | Periodic liver function tests |
| Iron overload | Transfusions | Serum ferritin level |
| Renal | ||
| Nephropathy | TBI/radiation | Periodic serum creatinine and urinalysis |
| Chemotherapy | Control hypertension | |
| Cyclosporine | ||
| Skeletal | ||
| Osteoporosis | TBI/radiation | Periodic bone densitometry |
| Avascular necrosis | Corticosteroids | |
| Second cancers | TBI/radiation | Periodic cancer screening |
| Chemotherapy | ||
| Chronic GVHD |
GVHD, Graft-versus-host disease; TBI, total body irradiation.
Table 49-6 Screening Guidelines for Common Cancers After Hematopoietic Cell Transplantation
| Site | Screening Recommendations |
|---|---|
| Breast | Mammogram annually starting at age 40; in women who have received ≥20 Gy to the chest region begin at age 25 or 8 years after radiation, whichever is later |
| Cervix | Papanicolaou test every year (for regular Papanicolaou test) or every 2 years (for liquid-based Papanicolaou test); may screen every 2 to 3 years after age 30 if patient has three consecutive normal tests |
| Colorectal | Beginning at age 50, fecal occult blood annually and/or flexible sigmoidoscopy every 5 years, or double-contrast barium enema every 5 years, or colonoscopy every 10 years; certain high-risk groups (e.g., patients with inflammatory bowel disease) may need earlier initiation and more frequent screening |
| Lung | Yearly pulmonary examination with imaging as appropriate |
| Oral | Yearly oral cavity examination |
| Thyroid | Yearly thyroid examination |
| Skin | Yearly skin examination |
Table 49-7 Common Clinical Manifestations of Chronic Graft-Versus-Host Disease
| Organ System | Clinical Manifestations |
|---|---|
| Cutaneous | Poikiloderma, lichen planus, dermal sclerosis, morphea-like features, hypopigmentation or hyperpigmentation, ichthyosis, nail dystrophy, onycholysis |
| Ocular | Keratoconjunctivitis sicca, conjunctivitis, corneal ulcerations |
| Oral | Lichen planus, hyperkeratotic plaques, xerostomia, mucosal atrophy, ulcers, restriction of mouth opening from sclerosis |
| Pulmonary | Bronchiolitis obliterans, bronchiolitis obliterans–organizing pneumonia |
| Gastrointestinal | Esophageal web and strictures, malabsorption syndrome, exocrine pancreatic insufficiency |
| Hepatic | Cholestasis |
| Genitourinary | Vaginal stenosis or scarring, lichen planus |
| Musculoskeletal | Fasciitis, joint contractures from sclerosis, myositis or polymyositis, arthritis |
| Hematopoietic | Thrombocytopenia, eosinophilia, lymphopenia, hemolytic anemia, hypogammaglobulinemia |