CHAPTER 24 Complex Regional Pain Syndrome after Distal Radius Fractures
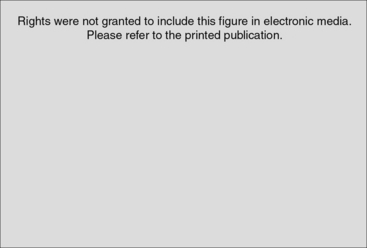
Complex regional pain syndrome (CRPS) occurring after distal radius fractures is a significant comorbid event. Symptoms and signs include delayed healing, pain, impaired recovery, persistent muscle stiffness, joint stiffness and contracture, severe cold sensitivity, and functional impairment. After trauma, persistent neuropathic pain of an inappropriate intensity with the absence of impending or ongoing tissue damage is defined as CRPS. In association with a distal radius fracture, CRPS may delay or prevent recovery (Fig. 24-1) and has a strong negative impact on health-related quality of life and function. In this chapter we will (1) provide a definition of CRPS, (2) detail clinical symptoms and signs to aid in the early recognition of this diagnosis, (3) provide an overview of treatment and referral that will improve outcomes, and (4) facilitate the practitioner’s understanding of questions related to standard of care.
Definitions
Synonyms for this syndrome include reflex sympathetic dystrophy, causalgia, and algodystrophy. CRPS type I includes pain, functional impairment, autonomic dysfunction, and dystrophic changes in the absence of an identifiable peripheral nerve lesion, injury, or irritation (traditionally reflex sympathetic dystrophy); CRPS type II is identical in the presence of an identifiable nerve lesion (traditionally causalgia).1,2 CRPS type III expands the concept to include all extremity pain dysfunction and is not discussed (Table 24-1). CRPS type I or II may be sympathetically maintained (SMP) or sympathetically independent (SIP) based on relief or persistence of pain after sympatholytic intervention.3,4 The SMP type may become SIP over time; this distinction recognizes the dynamic nature of dystrophic responses. In general, the SMP type has a better prognosis and different treatment approach.
| CRPS Type | Traditional Designation | Features |
|---|---|---|
| Type I | Reflex sympathetic dystrophy | Pain, functional deficit, autonomic signs of dysfunction, dystrophic changes with absence of any identifiable clinical peripheral nerve lesion/injury |
| Type II | Causalgia | Pain, functional deficit, autonomic signs of dysfunction, dystrophic changes in the presence of an identifiable peripheral nerve injury |
| Type III | Other pain dysfunction etiologies | Pain of myofascial origin |
Reproduced with permission from Koman LA, ed: Bowman Gray Orthopaedic Manual. Winston-Salem, NC: Orthopaedic Press, 2007.
Incidence and Prognosis
Fracture of the distal radius and ulna is one of the most common injuries producing CRPS.5–10 Tight casts,8 the presence of a nociceptive event such as compression of the median nerve, injury of the median or ulnar nerves, overdistraction, instability of the distal radioulnar joint (DRUJ), or ulnar fracture may contribute to CRPS after fractures of the distal radius.11,12
In prospective studies, CRPS type I or II is reported in 26% to 39% of patients,5,8,10,13 whereas, in retrospective studies, incidences of less than 2% are reported.14–20 This variation may be partly explained by the difference in study design, patient inclusion/exclusion criteria, and strictness of definition. CRPS after fracture of the distal radius is not linked statistically to patient age, sex, number of attempted fracture reductions, adequacy of reduction,5 side affected, or time from fracture to reduction and immobilization.13 Atkins and associates5 found no relationship to the severity of the fracture, whereas Bickerstaff and Kanis found CRPS was more likely to occur in patients who have sustained severe fractures.13
The importance of early recognition and management of CRPS cannot be overemphasized; this is the single most important predictor of functional recovery and pain relief.9,21 However, delay in diagnosis of 6 to 26 weeks is common.22 In general, for patients diagnosed within 1 year of onset, the overall prognosis is favorable in 80% of cases, whereas there is significant morbidity in 50% of patients for whom treatment is delayed for more than 1 year.23 Unfortunately, the prognosis of CRPS occurring after fracture of the distal radius varies from other entities and good outcomes are less common, with stiffness at 12 weeks correlating with residual CRPS at 10 years.24
Diagnosis and Evaluation
There is significant variation in the presentation of CRPS. The classic dystrophic progression from the acute phase (<3 months) to the dystrophic phase (3 to 6 months) to the chronic phase (>6 months) occurs infrequently; therefore, staging CRPS by the number of months is often inappropriate.23 In this chapter we discuss the use of objective instruments and tests that serve to stage CRPS physiologically and functionally.
Symptoms and Signs
Pain
CRPS does not exist in the absence of pain; however, different patients may have variable perceptions of pain, including allodynia, hyperalgesia, hyperesthesia or hypoesthesia, and hyperpathia. Pain descriptions also vary (“burning,” “throbbing,” “pressing,” “cutting,” “searing,” “shooting,” or “aching”). If present, nociceptive foci, including peripheral nerve lesions and mechanical derangements, must be identified. The nociceptive trigger may not be identified until the SMP is managed and the dystrophic manifestations are treated. An objective and reproducible analysis of pain symptoms is accomplished by the use of standardized and/or validated instruments,25 including the visual analog scale (VAS),26,27 the pain portion of the Rand Corporation Short Form 36 (SF-36),28 the McGill Short Form Pain Questionnaire,29 and/or self-administered questionnaires designed to assess upper extremity symptoms/function.30,31 Cold intolerance, or pain on exposure to cold, may be analyzed by using the McCabe Cold Sensitivity Severity Scale.32
Trophic Changes
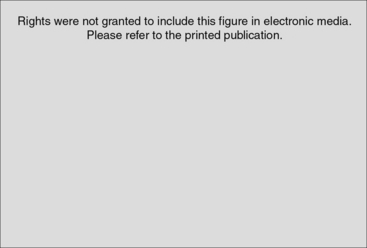
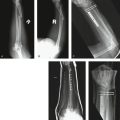
Upper extremity signs of CRPS include muscle and joint stiffness, edema, osteopenia, atrophy of the hair and nails, hypertrophy of skin, difficulty in fine finger manipulation, fixed posturing, and arthrofibrosis (Fig. 24-2). In 30% of cases, skin, hair, and/or nail changes may be apparent within 10 days of onset.33 Osteopenia is common and involves both cortical and cancellous bone (Fig. 24-3).6
Autonomic Dysfunction
Eighty percent of CRPS patients report a medical history of symptoms of autonomic dysfunction.34 Affected extremities may either be “hot” or “cold.” Sweating abnormalities (excessive or anhidrosis), vasomotor changes (redness or bluish discoloration of the extremity), and temperature (heat or cold) sensitivity/intolerance are the most common manifestations of autonomic dysfunction. During the painful stages of CRPS, autonomic dysfunction may be reported in up to 98% of patients.25,35,36
Diagnostic Testing
Evaluation of Pain
Although algometers,37 dolorimeters,38 computer-assisted stimuli,39 or thermal pain thresholds may be used to quantify pain,40 mechanical testing using Von Frey or Semmes-Weinstein monofilaments provides objective assessment of the quantity of pain and is a practical office procedure.23
Plain Radiographs
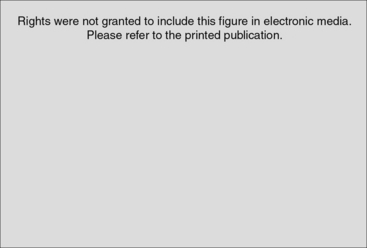
In approximately 80% of patients, regional osteopenia is evident on plain radiographs.5,38 These changes are visible only if there is significant demineralization, which may not be apparent until after a long period from onset of symptoms.41 The features of classic Sudeck’s atrophy include diffuse osteopenia with juxtacortical demineralization and subchondral erosions or cysts (see Fig. 24-3).23
Bone Scintigraphy
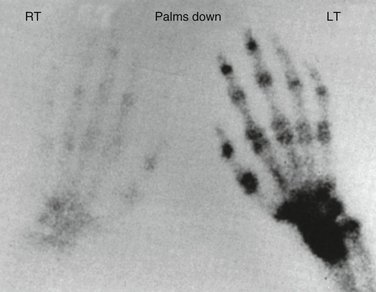
Three-phase technetium-99m bone scintigraphy (TPBS) (Fig. 24-4) has a significant role in the diagnosis of CRPS.36,42–45 The diagnostic accuracy of phase III of TPBS may equal that of the three-phase scan.43 TPBS has a high specificity but poor sensitivity.29,36,42,46 It provides objective support for the clinical diagnosis of CRPS, in combination with the other tests, but has no prognostic significance25 or any value in determining the appropriate management approach.21,36,47,48 The classic positive TPBS demonstrates increased periarticular uptake throughout the distal extremity.
Evaluation of Autonomic Control
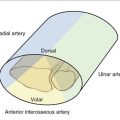
Total digital blood flow equals the sum of the thermoregulatory flow (normally, 80% to 95%), and nutritional flow (normally, 5% to 20%).23 In CRPS, inappropriate control of arteriovenous shunting leads to ischemia and deprivation of nutritional flow,25,35,49,50 which is the common denominator in either a “warm, swollen” hand with high total flow or a “cold, stiff” hand with low total flow (Fig. 24-5). These components may be assessed by measurements of the digital temperatures, laser Doppler fluxmetry (LDF), vital capillaroscopy, laser Doppler perfusion imaging (LDPI), and isolated cold stress testing (ICST).25,36,49,51–58 These measurements provide objective reproducible data, measure the effect of intervention, and guide treatment.25,59–61 Sweat function may be evaluated quantitatively, and combined with the measurements of ICST, provides objective evidence of autonomic function.9,25,62 Relief after sympatholytic intervention defines SMP.3,4 SMP may be diagnosed by nerve blocks of the stellate ganglion (most common), epidural space, brachial plexus, or peripheral nerves using short-acting or intermediate-acting local anesthetics.9 Stellate blocks are effective in only 70% to 75% of cases2; therefore, it is essential to confirm the sympatholysis by obtaining indirect evidence (i.e., Horner’s syndrome, nasal congestion, and/or facial anhidrosis) or by direct evidence (i.e., increase in temperature (3°C), venous engorgement of the hand veins, dry skin, changes in skin color, and characteristic laser Doppler changes).2,29,63 Thermography provides a large quantity of data and allows comparison with the contralateral extremity64; repeated measurements associated with stress over time are reproducible and correlate with symptoms.65 However, higher temperatures measured by thermography do not correlate with skin surface flow in CRPS.50 The use of a physiological stressor increases the predictive and diagnostic value of thermography.29,66
Management
General Principles
The two major pathophysiological stages associated with CRPS are (1) the “hot swollen” stage—increased total flow with decreased nutritional flow, and (2) the “cold stiff” stage—decreased total flow with decreased nutritional flow (Table 24-2, Fig. 24-5).67
TABLE 24-2 The Two States of Microvascular Perfusion Associated with Complex Regional Pain Syndrome
| Hot, Swollen | Cold, Stiff | |
|---|---|---|
| Symptoms |
Reproduced with permission from Koman LA, ed: Bowman Gray Orthopaedic Manual. Winston-Salem, NC: Orthopaedic Press, 1996.
Furthermore, after initial treatment, the response to sympatholytic intervention will classify the patient as having either SMP or SIP.3,68,69 SMP more frequently occurs than SIP and has a more favorable prognosis.
Management of CRPS, based on physiological staging, addresses the pathophysiology, and its goals are (1) to control pain, (2) to identify a “trigger” (nociceptive focus) and correct it, (3) to manage the patient based on the response to treatment, and (4) to manage any extremity deformities.
Hand Therapy and Adaptive Modalities
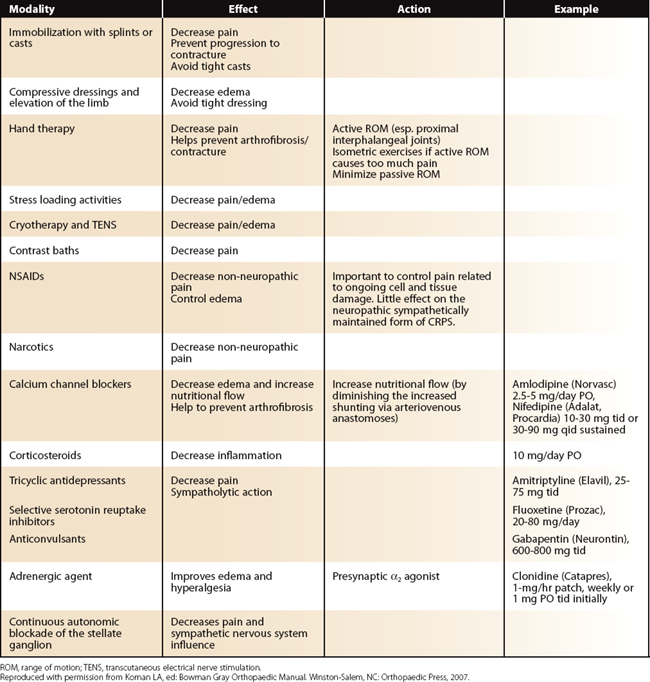
The benefits of hand therapy in the management of CRPS are well established.21,70,71 Hand therapy should address the entire limb; adhesive capsulitis to the shoulder may occur insidiously, and restoration of motion of the shoulder, elbow, and fingers is critical. Therapy should include active and passive range of motion, stress-loading activities,71 transcutaneous electrical nerve stimulation (TENS), desensitization techniques, and/or sensory re-education. Besides TENS, adaptive modalities include contrast baths, continuous passive motion, intermittent positive pressure with pneumatic pumps, and hydrotherapy.72–74
Medications
Oral Medications
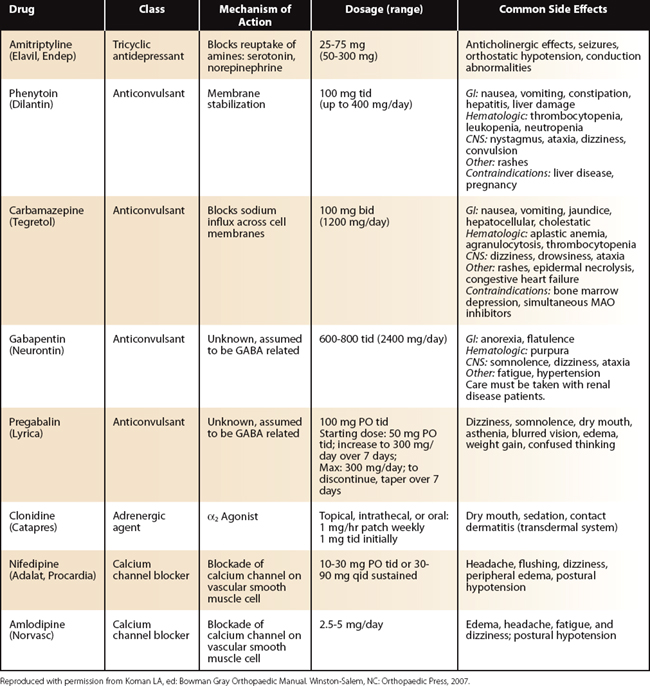
A myriad of oral medications with sympatholytic mechanisms are available; none is labeled by the U.S. Food and Drug Administration (FDA) for CRPS patients. Because they have no direct sympatholytic effect and are habit forming, narcotics are not ideal for CRPS; however, antidepressants, anticonvulsants, membrane stabilizers, adrenergic agents, calcium channel blockers, and neuromuscular blocking agents are beneficial. First-line drugs most frequently used include amitriptyline, phenytoin, carbamazepine, gabapentin, pregabalin, clonidine, nifedipine, and amlodipine (Table 24-3). Antidepressants may provide analgesia and modulate sympathetic hyperactivity in the peripheral and central nervous systems.4,75–77 One antidepressant may prove efficacious in one patient and not in another; thus, drug selection should be individualized. Often, the combination of two drugs belonging in different categories may be useful. For example, a tricyclic antidepressant combined with a selective serotonin reuptake inhibitor may be synergistic. Anticonvulsants have an incompletely delineated mechanism of action, including membrane stabilization. They are effective in the management of CRPS. Adrenergic agents include α1 antagonists, α2 antagonists, combined α1 and α2 antagonists, and α2 agonists.69,78,79 In patients with edema and hyperalgesia, administration of an α2 agonist, such as clonidine (Catapres), in three divided doses or as a continuous transcutaneous patch, may result in improvement.78,79 Calcium channel blockers often decrease pain, increase nutritional perfusion, and diminish abnormal arteriovenous shunting and have documented efficacy in CRPS.25,80,81 The most commonly used calcium channel blockers are nifedipine (Adalat, Procardia) and amlodipine (Norvasc). Corticosteroids have multiple mechanisms of action, including membrane stabilization; they relieve dystrophic pain effectively in selected patients.82–84 A high starting dose (e.g., 60 mg of prednisone) is rapidly tapered over 5 to 10 days.84 Long-term low dosing has also been employed.82,83 Currently, the use of corticosteroids is controversial because of their potential side effects, complications, and questionable benefit.
Parenteral Medications
Neuromuscular blocking agents such as botulinum toxins A and B result in relief of muscle spasm, dystonia, and skin hypersensitivity and have also been utilized in the management of pain.85 However, their mechanism of action in CRPS is unknown. Intravenous regional infusion, once the mainstay of most treatment regimens, is used less frequently. The use of intravenous corticosteroids, guanethidine, lidocaine, and bretylium tosylate (Bretylol) has been described.23,86 However, controlled studies do not support efficacy, and bretylium tosylate, which was the only drug labeled by the FDA for chronic pain, has been discontinued from the U.S. market. In CRPS patients requiring reconstructive procedures, or mobilization of stiff joints by manipulation under anesthesia, bretylium or corticosteroids have been used in conjunction with regional anesthesia.87
Epidural administration of corticosteroids and clonidine may be used with success in selected patients.88,89 A single block or a series of blocks of the stellate ganglion, brachial plexus, or spinal cord/nerve roots may be beneficial.23 Continuous infusion of local anesthetic over the area of the stellate ganglion or paravertebral ganglia, along the brachial plexus, or within the epidural space has been effective in controlling chronic sympathetic pain90,91 and is our modality of choice. Successful treatment occurs in 50% to 70% of patients.91,92
Sympathectomy, Implantable Devices, and Surgery
Implantable Devices: Peripheral Nerve Stimulators, Spinal Cord Stimulators
Placement of an implantable electrical stimulator in the gray matter, dorsal column, spinal cord, and peripheral nerves is possible to control pain.93–96 Spinal cord stimulation for CRPS provided successful pain relief for 50% of patients previously refractory to treatment97; however, these devices relieve pain without improving function.98
Management of the Nociceptive Focus
Neural nociceptive foci may include neuromas, neuromas-in-continuity, or compression neuropathies (e.g., median nerve at the carpal tunnel—the most frequent after distal radius fractures). Mechanical nociceptive foci include DRUJ or triangular fibrocartilage complex problems, joint subluxations or dislocations, and cartilage flaps in the wrist or metacarpal areas. After maximal conservative management has eliminated confounding factors due to the dystrophic process, clinical evaluation will confirm the suspicion of a neural nociceptive focus. Surgical correction of the neural or mechanical nociceptive foci should be performed only after maximal pharmacological intervention.9,23
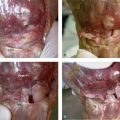
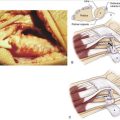
The following general principles refer to a complete nerve transection or to a neuroma-in-continuity. A tension-free nerve repair site may be accomplished by using a graft. Adhesions between the skin and nerve may be managed using a “Z” plasty, local flaps, or distant flaps. Modification of the neural bed using autologous fat, rotational muscle flaps, pedicled muscle or fascial flaps, free muscle transfer, or autologous or allograft venous wraps is indicated in cases of excessive scarring or adhesion development (Fig. 24-6).99–102 Internal neurolysis should be minimized so that further damage to the nerve is avoided. Appropriate hemostasis should control hematoma formation. Adequate postoperative care is essential to avoid a postoperative dystrophic flare and should include pharmacological interventions and hand therapy, including early active and passive range of motion. The use of nonocclusive dressings is proposed.
In the treatment of a nerve compression, careful evaluation is important. If symptoms are confirmed and warrant surgical intervention, nerve conduction studies should be considered. The nerve should be released, and if the neural bed is compromised it should be modified (see Fig. 24-6). Adequate postoperative care should be provided to prevent the recurrence of a dystrophic flare; it may include continuous blocks and/or oral medications.
Overview of Focused Approach
Early recognition and treatment of CRPS is the mainstay of management.9,21 Pain, swelling, and/or vasomotor changes that are out of proportion to the traumatic or surgical event or are significantly prolonged should raise a high index of suspicion. The diagnosis of CRPS is clinical, supported by imaging modalities and evaluation of autonomic function. Physiological staging serves as the basis of treatment. A “multidisciplinary” management approach is recommended, and the team leader is determined by the circumstances of the pain process. As a general rule, “simple” modalities and drugs (oral medication and therapy) should be used first before progressing to more complex modalities (e.g., autonomic blocks). Treatment should be individualized and altered based on the patient’s specific symptoms and his or her response to treatment. The goals of management are (1) to control the symptoms of pain, inflammation, and edema; (2) to improve nutritional flow; (3) to identify the presence of a nociceptive focus (if available); and (4) to prevent and/or manage deformity. The management of three possible clinical situations will be discussed.
Hot, Swollen Extremity
Cast or splint immobilization is used to decrease pain and prevent the development of contractures (Table 24-4). To decrease edema, the limb should be elevated above heart level and compressive dressings applied. Tight casts/dressings should be avoided. Early hand therapy is important and includes passive and active range of motion (ROM); it will prevent the development of arthrofibrosis, particularly in the proximal interphalangeal joints. Isometric muscle exercises may be used if active ROM is too painful; passive ROM should be minimal. Light activity or active motion is not enough, and a self-controlled, home program of stress loading activities may be helpful.71 Contrast baths (alternate hot and cold therapy) are useful. Adaptive modalities, including TENS may be beneficial.74,103 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be helpful in controlling pain and inflammation. Narcotics may be used for pain due to ongoing cell and tissue damage caused by the initial trauma but have minimal effect on the SMP component of CRPS. By contrast, calcium channel blockers, antidepressants, anticonvulsants, corticosteroids, and membrane stabilizers all exhibit direct or indirect sympatholytic effects. Calcium channel blockers (e.g., amlodipine, nifedipine) increase nutritional flow and prevent the development of deformity. Antidepressants (e.g., tricyclics [amitriptyline] or selective serotonin reuptake inhibitors [fluoxetine]) act at the amine pump level to decrease pain. Anticonvulsants (e.g., gabapentin) decrease pain and sympathetic tone. Corticosteroids limit inflammatory effects. Typically, an early use of a tricyclic antidepressant (e.g., amitriptyline) in combination with an anticonvulsant (e.g., gabapentin) is advised for a patient with a hot, swollen hand. If the patient is not responding well to treatment, continuous autonomic blockade of the stellate ganglion or the use of intravenous bretylium may be attempted. After favorable response to the blocks, hand therapy as described previously should be continued. If there is no response to sympatholytic intervention, or there is no response whereas there was one previously, the patient is classified as having SIP. The treatment of SIP is complex and includes specialized modalities, and its discussion is beyond the scope of this chapter.
1. Merskey H, Bogduk N, editors. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms Prepared by the Task Force on Taxonomy of the International Association for the Study of Pain, 2nd ed., Seattle, WA: IASP Press, 1994.
2. Stanton-Hicks M, Janig W, Hassenbusch S, et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain.. 1995;63:127-133.
3. Amadio PC, Mackinnon S, Merritt W, et al. Reflex sympathetic dystrophy syndrome: consensus report of an ad hoc committee of the American Association for Hand Surgery on the definition of reflex sympathetic dystrophy syndrome. Plast Reconstr Surg.. 1991;87:371-375.
4. Czop C, Smith TL, Koman LA. The pharmacologic approach to the painful hand. Hand Clin.. 1996;12:633-642.
5. Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after Colles’ fracture. J Bone Joint Surg Br.. 1990;72:105-110.
6. Bickerstaff DR, Charlesworth D, Kanis JA. Changes in cortical and trabecular bone in algodystrophy. Br J Rheumatol.. 1993;32:46-51.
7. Fernandez D, Jupiter J. Fractures of the Distal Radius: A Practical Approach to Management. New York: Springer-Verlag; 1996.
8. Field J, Protheroe DL, Atkins RM. Algodystrophy after Colles fractures is associated with secondary tightness of casts. J Bone Joint Surg Br. 1994;76:901-905.
9. Koman LA, Smith TL, Smith BP, Pollock DC. Reflex sympathetic and other dystrophies. In: Peimer CA, editor. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill; 1996:2295-2312.
10. Laulan J, Bismuth JP, Sicre G, Garaud P. The different types of algodystrophy after fracture of the distal radius. J Hand Surg [Br]. 1999;22(4):441-447.
11. Herron M, Faraj A, Craigen MA. Dorsal plating for displaced intra-articular fractures of the distal radius. Injury.. 2003;34(7):497-502.
12. Sennwald GR, Della SD. Unstable distal radial fractures treated by external fixation: an analytical review. Scand J Plast Reconstr Surg Hand Surg.. 2002;36:226-230.
13. Bickerstaff DR, Kanis JA. Algodystrophy: an under-recognized complication of minor trauma. Br J Rheumatol.. 1994;33:240-248.
14. Frykman G. Fractures of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. Acta Orthop Scand.. 1967;108:4-152.
15. Green JT, Gay FH. Colles’ fracture—residual disability. Am J Surg.. 1956;91:636-641.
16. Lidstrom A. Fractures of the distal end of the radius: a clinical and statistical study of end results. Acta Orthop Scand.. 1959;30:1-118.
17. Pool C. Colles’ fracture: a prospective study of treatment. J Bone Joint Surg Br.. 1973;55:540-544.
18. Bacorn RW, Kurtzke JF. Colles’ fracture: a study of two thousand cases from the New York State Workmen’s Compensation Board. J Bone Joint Surg Am.. 1953;35:643-658.
19. Plewes LW. Sudeck’s atrophy in the hand. J Bone Joint Surg Br.. 1956;38:195-203.
20. Stewart HD, Innes AR, Burke FD. The hand complications of Colles’ fractures. J Hand Surg [Br].. 1985;10:103-106.
21. Koman LA, Poehling GG. Reflex sympathetic dystrophy. In: Gelberman R, editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1990:1497-1524.
22. Li Z, Smith BP, Smith TL, Koman LA. Diagnosis and management of complex regional pain syndrome complicating upper extremity recovery. J Hand Ther.. 2005;18:270-276.
23. Koman LA, Poehling GG, Smith TL. Complex regional pain syndrome: reflex sympathetic dystrophy and causalgia. In Green’s Operative Hand Surgery, 4th ed., New York: Churchill Livingstone; 1999:636-666.
24. Field J, Warwick D, Bannister GC. Features of algodystrophy ten years after Colles’ fracture. J Hand Surg [Br].. 1992;17:318-320.
25. Koman LA, Smith BP, Smith TL. Stress testing in the evaluation of upper extremity perfusion. Hand Clin.. 1993;1:59-83.
26. Davidoff G, Morey K, Amann M, Stamps J. Pain measurement in reflex sympathetic dystrophy syndrome. Pain.. 1988;32:27-34.
27. Huskisson E. Measurement of pain. Lancet.. 1974:1127-1131.
28. Ware JEJr, Sherbourne CD. The MOS 36-item short-form health survey (SF0-36): I. Conceptual framework and item selection. Med Care.. 1992;30:473-481.
29. Koman LA, Smith TL, Poehling GG, Smith BP. Reflex sympathetic dystrophy. Curr Opin Orthop.. 1993;4(IV):85-88.
30. Levine D, Simmons B, Koris M, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am.. 1993;75:1585-1592.
31. McConnell S, Beaton DE, Bombardier C. The DASH Outcome Measure User’s Manual. Toronto, Canada: Institute for Work & Health; 1999.
32. McCabe SJ, Mizgala C, Glickman L. The measurement of cold sensitivity of the hand. J. Hand Surg [Am].. 1991;16:1037-1040.
33. Baron R, Maier C. Reflex sympathetic dystrophy: skin blood flow, sympathetic vasoconstrictor reflexes and pain before and after surgical sympathectomy. Pain.. 1996;67:317-326.
34. Cooke ED, Ward C. Vicious circles in reflex sympathetic dystrophy—a hypothesis: discussion paper. J R Soc Med.. 1990;83:96-99.
35. Kurvers HAJ, Jacobs M, Beuk R, et al. Reflex sympathetic dystrophy: evolution of microcirculatory disturbances in time. Pain.. 1995;60:333-340.
36. Pollock FEJr, Koman LA, Smith BP, Poehling GG. Patterns of microvascular response associated with reflex sympathetic dystrophy of the hand and wrist. J Hand Surg [Am].. 1993;18(5):847-852.
37. Bryan AS, Klenerman L, Bowsher D. The diagnosis of reflex sympathetic dystrophy using an algometer. J Bone Joint Surg Br.. 1991;73:644-646.
38. Atkins RM, Duckworth T, Kanis JA. Algodystrophy following Colles’ fracture. J Hand Surg [Br].. 1989;14:161-164.
39. Liu S, Kopacz D, Carpenter R. Quantitative assessment of differential sensory nerve block after lidocaine spinal anesthesia. Anesthesiology.. 1995;1:60-63.
40. Gracely R, Price D, Roberts W, Bennett G. Quantitative sensory testing in patients with complex regional pain syndrome (CRPS) I and II. In: Janig W, Stanton-Hicks M, editors. Reflex Sympathetic Dystrophy: A Reappraisal, Progress in Pain Research and Management. Seattle: IASP Press; 1996:151-170.
41. Arriagada M, Arinoviche R. X-ray bone densitometry in the diagnosis and followup of reflex sympathetic dystrophy syndrome. J Rheumatol.. 1994;21(3):498-500.
42. Davidoff G, Werner R, Cremer S, et al. Predictive value of the three-phase technetium bone scan in diagnosis of reflex sympathetic dystrophy syndrome. Arch Phys Med Rehabil.. 1989;70:135-137.
43. Mackinnon SE, Holder LE. The use of three-phase radionuclide bone scanning in the diagnosis of reflex sympathetic dystrophy. J Hand Surg [Am].. 1984;9:556-563.
44. Steinert H, Nickel O, Hahn K. Three-phase bone scanning in reflex sympathetic dystrophy. In: Stanton-Hicks M, Janig W, Boas RA, editors. Reflex Sympathetic Dystrophy: Current Management of Pain. Boston: Kluwer Academic Publishers; 1990:177-185.
45. Zyluk A. The usefulness of quantitative evaluation of three-phase scintigraphy in the diagnosis of post-traumatic reflex sympathetic dystrophy. J Hand Surg [Br].. 1999;24(1):16-21.
46. Kozin F, Soin J, Ryan L, et al. Bone scintigraphy in the reflex sympathetic dystrophy syndrome. Radiology.. 1981;138:437-443.
47. Hoffman J, Phillips W, Blum M, et al. Effect of sympathetic block demonstrated by triple-phase bone scan. J Hand Surg [Am].. 1993;18(5):860-864.
48. Koman LA, Barden A, Smith BP, et al. Reflex sympathetic dystrophy in an adolescent. Foot Ankle.. 1993;5:273-277.
49. Irazuzta J, Berde C, Sethna N. Laser Doppler measurements of skin blood flow before, during, and after lumbar sympathetic blockade in children and young adults with reflex sympathetic dystrophy syndrome. J Clin Monit.. 1992;8(1):16-19.
50. Matsumura H, Jimbo Y, Watanabe K. Haemodynamic changes in early phase reflex sympathetic dystrophy. Scand J Plast Reconstr Hand Surg.. 1996;30:133-138.
51. Bej M, Schwartzman R. Abnormalities of cutaneous blood flow regulation in patients with reflex sympathetic dystrophy as measured by laser Doppler fluxmetry. Arch Neurol.. 1991;48:912-915.
52. Fagrell B. Vital capillaroscopy. Angiology.. 1974;23:284-298.
53. Fagrell B. Microcirculation of the Skin, 2nd ed. Orlando, FL: Academic Press; 1984.
54. Fagrell B. Skin preparations: advantages and disadvantages. In: Baker CH, Nastuk WL, editors. Microcirculatory Technology. Orlando, FL: Academic Press; 1986:19-42.
55. Fagrell B. Clinical studies of skin microcirculation. Klin Wochenschr.. 1986;64:943-992.
56. Fagrell B. Microcirculatory methods for evaluating the effect of vasoactive drugs in clinical practice. Acta Pharmacol Toxicol.. 1986;59:103-107.
57. Ide J, Yamaga M, Kitamura T, Takagi K. Quantitative evaluation of sympathetic nervous system dysfunction in patients with reflex sympathetic dystrophy. J Hand Surg [Br].. 1997;22(1):102-106.
58. Ostergren J, Rosen L, Fagrell B, Stranden E. Skin microvascular circulation in the sympathetic dystrophies evaluated by videophotometric capillaroscopy and laser Doppler fluxmetry. Int J Microcirc Clin Exp.. 1988;7(3):289.
59. Cooke ED, Glick EN, Bowcock SA, et al. Reflex sympathetic dystrophy (algoneurodystrophy): temperature studies in the upper limb. Br J Rheumatol.. 1989;28:399-403.
60. Fagius J, Karhuvaara S, Sundlof G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand.. 1989;137:325-334.
61. Koman LA, Nunley J, Goldner J, et al. Isolated cold stress testing in the assessment of symptoms in the upper extremity: preliminary communication. J Hand Surg.. 1984;3:305-313.
62. Low PA, Caskey P, Tuck R, et al. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol.. 1983;14:573-580.
63. Malmqvist ELA, Bengtsson M, Sorensen J. Efficacy of stellate ganglion block: a clinical study with bupivacaine. Reg Anesth. 1992;17:340-347.
64. Ecker A. Contact thermography in diagnosis of reflex sympathetic dystrophy: a new look at pathogenesis. Thermology.. 1985;1:106-109.
65. Sherman RA, Barja RH, Bruno GM. Thermographic correlates of chronic pain: analysis of 125 patients incorporating evaluations by a blind panel. Arch Phys Med Rehabil.. 1987;68:273-279.
66. Gulevich SJ, Conwell TD, Lane J, et al. Stress infrared telethermography is useful in the diagnosis of complex regional pain syndrome, type I (formerly reflex sympathetic dystrophy). Clin J Pain.. 1997;13:50-59.
67. Koman LA, Smith TL, Smith BP, Li Z. The painful hand. Hand Clin.. 1996;12(4):757-764.
68. Raja SN, Meyer RA, Campbell JN. Peripheral mechanism of somatic pain. Anesthesiology.. 1988;68:571-590.
69. Raja SN, Davis KD, Campbell JN. The adrenergic pharmacology of sympathetically-maintained pain. J Reconstr Microsurg.. 1992;8:63-69.
70. Haines BL. Rehabilitation of the painful upper extremity. Hand Clin.. 1996;12(4):801-816.
71. Watson HK, Carlson L. Treatment of reflex sympathetic dystrophy of the hand with an active “stress loading” program. J Hand Surg [Am].. 1987;12(5):779-785.
72. Abram SE. Increased sympathetic tone associated with transcutaneous electrical stimulation. Anesthesiology.. 1976;45:575-577.
73. Long DM. Electrical stimulation for relief of pain from chronic nerve injury. J Neurosurg.. 1973;39:718-722.
74. Richlin DM, Carron H, Rowlingson JC, et al. Reflex sympathetic dystrophy: successful treatment by transcutaneous nerve stimulation. J Pediatr.. 1978;93:84-85.
75. Magni G. The use of antidepressants in the treatment of chronic pain. Drugs.. 1991;42(5):730-748.
76. Monks R. Psychotropic drugs. In Wall P, Melzack R, editors: The Textbook of Pain, 3rd ed., Edinburgh: Churchill Livingstone, 1994.
77. Watson CPN. Antidepressant drugs as adjuvant analgesics. J Pain Symptom Manage. 1994;9(6):392-405.
78. Davis KD, Treede RD, Raja SN, et al. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain.. 1991;47:309-317.
79. Kohn M, Chaprnka C, Caskey W, Ghignone M: Evaluation of transdermal clonidine in the treatment of reflex sympathetic dystrophy. ASSH Meeting, Toronto, Canada, September 27, 1990.
80. Raja SN, Turnquist JL, Meleka S, Campbell JN. Monitoring adequacy of alpha-adrenoceptor blockade following systemic phentolamine administration. Pain.. 1996;64:197-204.
81. Warfield C. Principles and practice of pain management. In: Warfield C, editor. Principles and Practice of Pain Management. New York: MacGraw-Hill, 1993.
82. Christensen K, Jensen E, Noer I. The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand.. 1982;148:653-655.
83. Glick EN. Reflex dystrophy (algoneurodystrophy): results of treatment by corticosteroids. Rheumatol Rehabil.. 1973;12:84-88.
84. Kozin F, Ryan L, Soin J, Wortmann R. The reflex sympathetic dystrophy syndrome (RSDS): III. Scintigraphic studies, further evidence for the therapeutic efficacy of systemic corticosteroids and proposed diagnostic criteria. Am J Med.. 1981;70:23-30.
85. Argoff CE. The use of botulinum toxins for chronic pain and headaches. Curr Treat Options Neurol.. 2003;5(6):483-492.
86. Ford SR, Forrest WH, Eltherington L. The treatment of reflex sympathetic dystrophy with intravenous regional bretylium. Anesthesiology.. 1988;68:137-140.
87. Duncan K, Lewis R, Racz G, Nordyke M. Treatment of upper extremity reflex sympathetic dystrophy with joint stiffness using sympatholytic Bier blocks and manipulation. Orthopedics.. 1988;11(6):883-886.
88. Dirksen R, Rutgers MJ, Coolen JMW. Cervical epidural steroids in reflex sympathetic dystrophy. Anesthesiology.. 1987;66:71-73.
89. Rauck RL, Eisenach J, Jackson K, et al. Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology.. 1993;79(6):1163-1169.
90. Betcher A, Bean G. Continuous procaine block of paravertebral sympathetic ganglions. JAMA.. 1953;151(4):288-292.
91. Linson MA, Leffert R, Todd D. The treatment of upper extremity reflex sympathetic dystrophy with prolonged continuous stellate ganglion blockade. J Hand Surg.. 1983;8:153-159.
92. Byas-Smith M. Management of acute exacerbations of chronic pain syndromes. In: Sinatra RS, editor. Acute Pain. St. Louis: Mosby-Yearbook; 1992:432-443.
93. Ballantine HT. Treatment of intractable psychiatric illness and chronic pain by sterotactic cingulotomy. In: Schmidek H, Sweet W, editors. Operative Neurosurgical Techniques: Indications, Methods, and Results. New York: Grune & Stratton; 1988:1069-1075.
94. Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. J Neurosurg.. 1986;64:543-553.
95. Kumar K, Nath R, Wyant G. Treatment of chronic pain by epidural spinal cord stimulation: a 10-year experience. J Neurosurg.. 1991;75:402-407.
96. Young RF, Chambi VI. Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter. J Neurosurg.. 1987;66:364-371.
97. Kemler MA, Schouten HJ, Gracely RH. Diagnosing sensory abnormalities with either normal values or values from contralateral skin: comparison of two approaches in complex regional pain syndrome I. Anesthesiology.. 2000;93(3):718-727.
98. Kemler MA, Barendse GA, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med.. 2000;343(9):618-624.
99. Jones NF. Treatment of chronic pain by “wrapping” intact nerves with pedicle and free flaps. Hand Clin.. 1996;12(4):765-772.
100. Koman LA, Ruch DS, Smith BP, et al. Reflex sympathetic dystrophy after wrist surgery. In: Levin L, editor. Problems in Plastic and Reconstructive Surgery. Philadelphia: JB Lippincott; 1992:300-322.
101. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin.. 1996;12(4):773-779.
102. Sotereanos DG, Giannakopoulos PN, Mitsionis GI, et al. Vein-graft wrapping for the treatment of recurrent compression of the median nerve. Microsurgery.. 1995;16:752-756.
103. Melzack R. Prolonged relief of pain by brief, intense transcutaneous somatic stimulation. Pain.. 1975;1:357-373.