Chapter 27 Complete Transposition of the Great Arteries
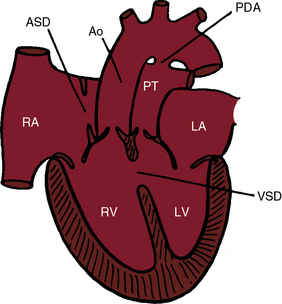
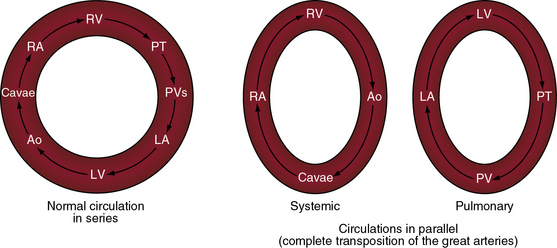
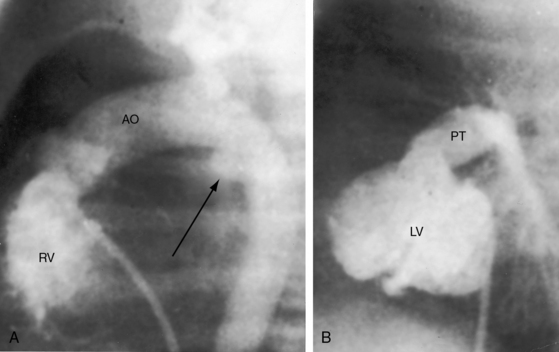
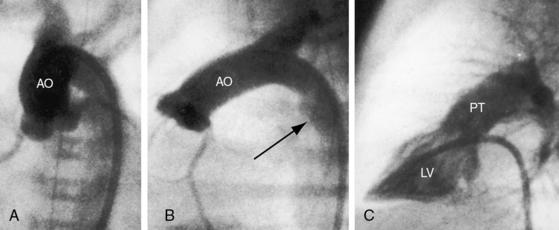
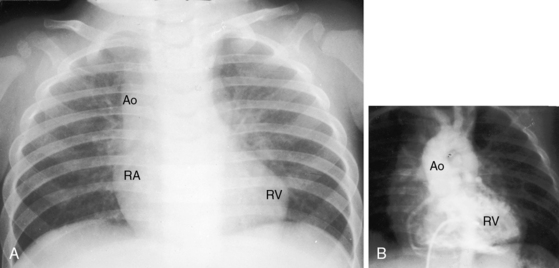
In 1797, Matthew Baillie called attention to “a singular malformation in which the pulmonary artery arises from the left ventricle and the aorta from the right ventricle.”1 Seventeen years later, John Farre2 used the term transposition to characterize Baillie’s singular malformation. Each great artery is placed across the ventricular septum: positio means placed, trans means across. The term transposition of the great arteries is used herein in its original sense to signify that the aorta arises from the morphologic right ventricle and the pulmonary trunk arises from the morphologic left ventricle—ventriculoarterial discordance (Figures 27-1 through 27-4).3–6 The designation “complete” means that ventriculoarterial discordance is associated with atrioventricular concordance. The single discordance between ventricles and great arteries makes the transposition physiologically complete. These alignments result in a unique extrauterine circulation in which two independent circulations function in parallel (Figure 27-5). Congenitally corrected transposition (see Chapter 6) refers to atrioventricular and ventriculoarterial discordance. The double discordance physiologically corrects the transposition, which is therefore not complete. Accordingly, there is one circulation in series, as in the normal heart. The term malposition of the great arteries refers to abnormal spatial relations but concordant ventricular alignments.7,8
d-Transposition and complete transposition are synonymous. Because d- refers to a dextro (rightward) bend in the bulboventricular loop, the designation is appropriate because virtually all examples of complete transposition of the great arteries are accompanied by a d-ventricular loop (see Figures 27-2A, and 27-4A). Segmental analysis identifies situs solitus (S), a dextro-ventricular loop (d), and a rightward (d) and anterior aorta (see Figures 27-2A, 27-3, and 27-4). In about one third of cases, the aorta is anterior and to the left, directly anterior, or rarely, posterior to the pulmonary trunk.3,5 The great arteries rise in parallel and do not cross, as in the normal heart (see Figures 27-1, 27-34, and 27-35).9 Anomalous origin of the left subclavian artery from the pulmonary artery has been reported in association with a right aortic arch.10
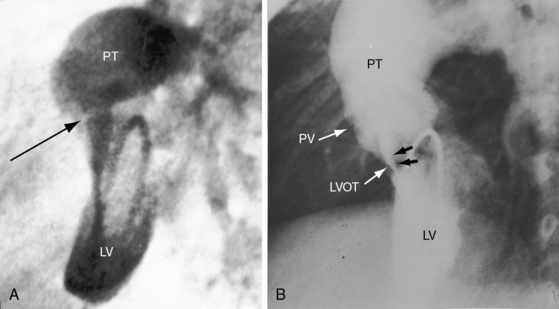
The origin and course of the coronary arteries are physiologically unimportant but anatomically pivotal because of the arterial switch operation (see Chapter 32).11–14 In complete transposition with situs solitus, the morphologic right coronary artery is concordant with the morphologic right ventricle and the morphologic left coronary artery is concordant with the morphologic left ventricle.15 Two aortic sinuses face the right ventricular outflow tract (Figure 27-6), irrespective of the spatial relations between the ascending aorta and the pulmonary artery.13,14 The two facing sinuses are the left sinus and the posterior sinus.13,14 The right aortic sinus does not face the right ventricular outflow tract (see Figure 27-6). Rarely, both coronary arteries are intramural.16 In complete transposition with situs inversus, the anatomy of the coronary arteries has not been well established.17
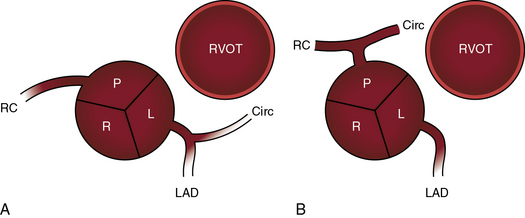
Dual sinus origin indicates that a coronary artery arises from each of the two facing sinuses (see Figure 27-6) and accounts for about 90% of cases; in most of these cases, the left main coronary artery arises from the left aortic sinus and gives rise to the anterior descending and circumflex coronary arteries, and the right coronary artery arises by a single ostium from the posterior aortic sinus (see Figure 27-6A). In the less common type of dual sinus origin, the left anterior descending coronary artery originates from the left aortic sinus, and the circumflex and right coronary arteries originate by a single ostium in the posterior aortic sinus (see Figure 27-6B.) Single sinus origin indicates that both coronary arteries arise from one of the two, but not both, facing sinuses by a single ostium or by multiple ostia. In a small minority of cases of single sinus origin, the left anterior descending, circumflex, and right coronary arteries arise by ostia in the posterior aortic sinus. The sinus node artery originates from the proximal right coronary artery and courses upward and to the right, partially embedded in the limbus of the atrial septum. An aberrant anterior descending coronary artery may pass intramurally between the aortic root and pulmonary trunk.18
The incidence rate of complete transposition of the great arteries is estimated at 1 in 2300 to 1 in 5100 live births.19 The malformation represents approximately 5% to 8% of congenital cardiac malformations but accounts for 25% of deaths from congenital heart disease in the first year of life. The morphogenesis of the ventriculoarterial discordance that characterizes complete transposition focuses on the conus as the crucial connection between the ventricles and the great arteries.20 The segmental components of the heart, the atria, the ventricles, and the great arteries, appear at different developmental stages.3 The straight heart tube is formed from primordia of the trabeculated portions of both ventricles. During looping, the atria are at the caudal end of the embryonic tube, and the conus is at the cephalic end of the tube. When the truncus appears, the developing heart has three segments: atrial, ventricular, and arterial.3 The ventral end of the arterial segment is continuous with the conus, and the dorsal end is continuous with the aortic arches. The spiral division that develops within the truncus progresses from the aortic arches toward the truncal ridges. Conal development then becomes pivotal.20 Rarely, the aortic arch is double.21 The subaortic portion of the conus persists in complete transposition of the great arteries, and the subpulmonary conus is absorbed (conal inversion). The aortic valve moves anteriorly, and the pulmonary valve moves inferoposteriorly into fibrous continuity with the mitral valve. The maldeveloped conus is inappropriate for the ventricular loop, so the great arteries are discordant relative to their ventricles of origin. Pulmonary/mitral continuity exists because a left-sided subpulmonary conus is absent, and aortic/tricuspid discontinuity exists because a right-sided subaortic conus is present. Aortic/tricuspid discontinuity causes the aortic valve to lie superior to the pulmonary valve and causes the ascending aorta to lie parallel to the pulmonary trunk.
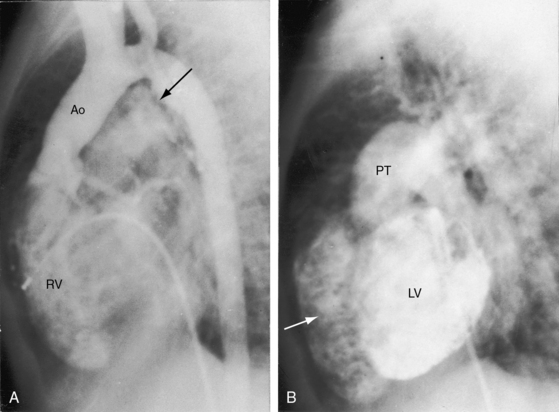
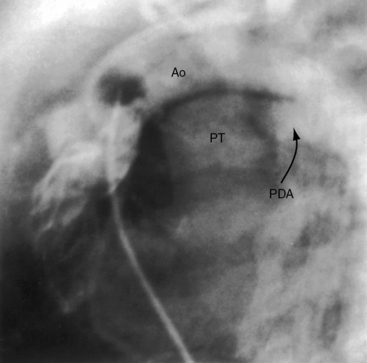
The ductus arteriosus is usually closed or insignificantly patent in complete transposition (Figures 27-3, 27-4, and 27-7)22 but is occasionally widely open (Figure 27-8).23 Interatrial communications take the form of a patent foramen ovale or an ostium secundum atrial septal defect.5,6,24 Rarely, the foramen ovale closes prematurely.25 Ventricular septal defects are inlet, muscular, perimembranous, or infundibular.26 Inlet defects caused by malalignment with the atrial septum result in straddling of the tricuspid valve. Perimembranous ventricular septal defects extend into the inlet septum and into the muscular septum. Infundibular septal defects result from malalignment of the infundibular septum, which is shifted leftward and posterior or rightward and anterior, and the malaligned defect can be subaortic, subpulmonary, or doubly committed.26
Pulmonary stenosis is represented by obstruction to left ventricular outflow and occurs in approximately 15% of cases of complete transposition.5,27 Fixed subpulmonary stenosis is characterized by a circumferential fibrous membrane or diaphragm (Figure 27-9A), a fibromuscular ridge, herniation of tricuspid leaflet tissue, anomalous septal attachments of the mitral valve, accessory mitral leaflet tissue, tissue tags from the membranous septum, or hypertrophy of the anterolateral muscle bundle.5,27 Leftward and posterior deviation of the infundibular septum causes tunnel or tubular subpulmonary stenosis.26 Pulmonary valve stenosis is uncommon.27
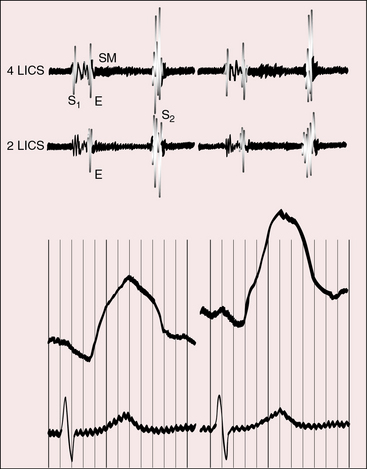
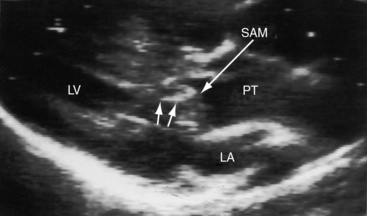
Dynamic subpulmonary stenosis develops during the first few weeks of life and is associated with systolic anterior motion of the anterior mitral leaflet (see Figures 27-9B, and 27-36).28 The obstruction is dynamic because the degree varies spontaneously and is augmented by isoproterenol and reduced by beta blockade.28 The development of dynamic subpulmonary stenosis coincides with a fall in pulmonary vascular resistance and a fall in left ventricular systolic pressure in the face of persistent systemic systolic pressure in the adjacent right ventricle. Systemic right ventricular pressure results in systolic movement of the base of the ventricular septum into the outflow tract of the adjacent low-pressure left ventricle. Systolic anterior motion of the anterior mitral leaflet is then caused by the Venturi effect that is generated by hyperkinetic ejection of a volume-overloaded left ventricle into a low-resistance pulmonary vascular bed (see Figure 27-9B).
Subaortic stenosis is the result of rightward and anterior deviation of a malaligned infundibular septum.26,29,30 Aortic arch anomalies are represented by hypoplasia, coarctation, and interruption and have been attributed to reduced flow during morphogenesis.31
Juxtaposition of the atrial appendages refers to an anomaly in which both atrial appendages or the left appendage and part of the right appendage are adjacent to each other (juxtaposed) on the same side of the heart. Juxtaposition is a rare anomaly that is strongly associated with complete transposition of the great arteries32–35 and occurs in 2% to 6% of cases of anatomically corrected malpositions.32 Juxtaposition reduces the size and volume of the right atrium. Left-sided juxtaposition is six times as frequent as right-sided juxtaposition. There is female preponderance with juxtaposition of the atrial appendages, in contrast to marked male preponderance in complete transposition of the great arteries without juxtaposition (see section History).34
Tricuspid valve abnormalities occur in about 30% of patients with complete transposition and inlet ventricular septal defects.36 These abnormalities include straddling or overriding of chordae, overriding of the tricuspid annulus, abnormal chordal attachments, and less commonly, tricuspid valve dysplasia, accessory tricuspid tissue, and double orifice tricuspid valve.36 Mitral valve abnormalities occur in 20% to 30% of necropsy specimens of complete transposition and include a cleft anterior leaflet, straddling of the mitral valve, abnormal size or position, anomalous mitral tissue strands, redundant mitral valve tissue, and abnormal papillary muscles.37,38
Matthew Baillie1 described the anatomic features of a singular malformation. The malformation is even more singular in physiologic terms. The normal heart is associated with a single circulation in series (see previous and Figure 27-5), with sequential flow from the right ventricle into the pulmonary artery, pulmonary veins, left atrium, left ventricle, aorta, systemic venous bed, venae cavae, and right atrium and back to the right ventricle. Blood at any given location must traverse both sides of the circulation before returning to that location (see Figure 27-5). In complete transposition of the great arteries, two circulations are in parallel (see Figure 27-5). The systemic circulation is characterized by flow from the right ventricle into the aorta, systemic venous bed, venae cavae, and right atrium and back to the right ventricle. The pulmonary circulation is characterized by flow from the left ventricle into the pulmonary artery, pulmonary capillary bed, pulmonary veins, and left atrium and back to the left ventricle. Blood within the systemic circulation recirculates within the systemic circulation, and blood within the pulmonary circulation recirculates within the pulmonary circulation. The two circulations do not cross unless they are joined by communications at the atrial, ventricular, or great arterial level, on which survival depends. These communications permit blood from the systemic circulation to enter the pulmonary circulation for oxygenation and permit oxygenated blood from the pulmonary circulation to enter the systemic circulation.
The net volume of blood exchanged between the systemic and pulmonary circulations must be isovolumetric over short periods of time or the donor circulation is rapidly depleted and the recipient circulation is rapidly overloaded. The amount of blood exchanged between the two parallel circulations is small relative to the volume that recirculates within each circulation. The volume of effective bidirectional mixing depends on the location and size of the communication that joins the two circulations and on the magnitude of pulmonary blood flow.39 Survival is tightly coupled to the delicate interplay between the intercirculatory communications and the pulmonary blood flow. In a neonate with complete transposition, a restrictive foramen ovale, and a nonpatent ductus arteriosus, the circulations are in parallel, as illustrated in Figure 27-5. The tendency for neonatal pulmonary vascular resistance to fall prompts an increase in pulmonary blood flow, but that advantage is lost because the oxygenated blood cannot enter the systemic vascular bed. The value of an adequate interatrial communication is dramatized by the immediate response to balloon atrial septostomy.40,41 An adequate interatrial communication permits quantitatively equal bidirectional shunting that is right-to-left during ventricular diastole and left-to-right during ventricular systole.39,42,43
An adequately sized ventricular septal defect permits bidirectional shunting that is determined by instantaneous pressure differences between the two ventricles. When pulmonary vascular resistance is low, there is preferential right-to-left systolic shunting into the low resistance pulmonary circulation and preferential left-to-right diastolic shunting away from the volume-loaded left ventricle. A large patent ductus arteriosus (see Figure 27-8) is initially accompanied by bidirectional flow that is replaced by virtually exclusive systemic-to-pulmonary flow as pulmonary vascular resistance falls.23 The temporary value of ductal patency is witnessed by the response to prostaglandin-induced ductal dilation as a pharmacologic bridge to balloon septostomy.44 Because ductal flow is essentially unidirectional, the pulmonary circulation becomes volume overloaded with no egress except a through restrictive foramen ovale.
Low pulmonary vascular resistance with an increase in pulmonary blood flow provides a large volume of oxygenated blood for intercirculatory mixing.39 Elevated pulmonary vascular resistance and pulmonary stenosis decrease pulmonary blood flow and result in a smaller volume of oxygenated blood for intercirculatory mixing. The increase in left ventricular afterload incurred by pulmonary vascular disease or pulmonary stenosis results in a reduction in left ventricular compliance that adversely affects intracardiac mixing.39,42 Hypoxemia provokes a fall in systemic vascular resistance and an increase in the volume of unsaturated blood recirculating in the systemic vascular bed.39,42
The geometry of the left ventricle in complete transposition is governed by the volume overload imposed by increased pulmonary blood flow and the pressure overload imposed by increased pulmonary vascular resistance or pulmonary stenosis. Right ventricular free wall thickness exceeds normal in the neonate and outstrips left ventricular wall thickness. Septal thickness and right ventricular free wall thickness then increase in parallel, so the septum becomes disproportionately thick relative to the left ventricular wall.45–49 When the ventricular septum bows into a low-pressure left ventricle, the right ventricle becomes spherical and the left ventricle resembles a prolate ellipsoid.50
When left ventricular pressure is elevated, the septum flattens or bows into the right ventricle, resulting in a more normal septal position and better left ventricular function.45 During the first few weeks of life, left ventricular function is normal, but mass does not increase in proportion to volume,51 a discrepancy that is responsible for decreased left ventricular function. The right ventricle performs normally at birth, but its contractility and ejection fraction then decline.51,52 A morphologic subaortic right ventricle is ill-equipped to support the systemic circulation because of the adverse effects of its mass, geometry, and the coronary circulation (see Chapter 6).
Pulmonary vascular disease is prevalent in patients with complete transposition of the great arteries, especially in the presence of a nonrestrictive ventricular septal defect53–55 or a large patent ductus arteriosus.23 Grade 3 or 4 Heath-Edwards changes of pulmonary vascular disease are found in 20% of infants before 2 months of age, and in about 80% after 1 year.56 A reduction in number of intra-acinar pulmonary arteries has been identified with quantitative morphomeric studies.57,58 Vasoconstriction of pulmonary arterioles induced by hypoxemic blood carried in the systemic arterial collaterals accelerates the pulmonary vascular disease.59 Early pulmonary vascular disease is more prevalent with a nonrestrictive ventricular septal defect and complete transposition than with an equivalent isolated ventricular septal defect.56,59,60 Even with an isolated atrial septal defect, the incidence rate of pulmonary vascular disease is about 6%, although progression is slower.53,61
History
Males outnumber females with a ratio as high as 4:1,5,62 unless there is juxtaposition of the atrial appendages.34 Complete transposition of the great arteries seldom occurs in firstborns; but in offspring of mothers who have had three or more pregnancies, a twofold increase in incidence rate has been reported. Familial recurrence of concordant cardiac defects within affected family members supports monogenic or oligogenic inheritance in selected pedigrees.63 Complete transposition and congenitally corrected transposition sometimes segregate in the same family, probably because of monogenic transmission, which supports a pathogenetic link between complete transposition and disorders of looping.63
Cyanosis begins as early as the first day of life in more than 90% of infants with an intact ventricular septum.19 Severe pulmonary stenosis or atresia results in intense neonatal cyanosis. Mild cyanosis with delayed onset is a feature of complete transposition with a nonrestrictive ventricular septal defect64 or a patent ductus arteriosus.23 A large isolated ductus (see Figure 27-8) is associated with severe congestive heart failure in the first few days of life, and spontaneous closure results in a sudden fall in systemic arterial oxygen saturation, rapid clinical deterioration, and death.23 Dynamic subpulmonary stenosis or a rise in pulmonary vascular resistance curtails pulmonary blood flow and alleviates congestive heart failure, but at the expense of increasing hypoxemia. Pulmonary stenosis is occasionally responsible for hypercyanotic spells characterized by intense cyanosis, tachypnea, extreme irritability, and hypothermia but seldom by loss of consciousness.28 Squatting is rare. Neonatal survival is tightly coupled to the delicate interplay between the intercirculatory communications and pulmonary blood flow.65 An older infant depends for survival on adequate pulmonary blood flow. Outcome is bleak, with an overall death rate of 30% in the first week, 50% in the first month, and 90% in the first year.24,66,67
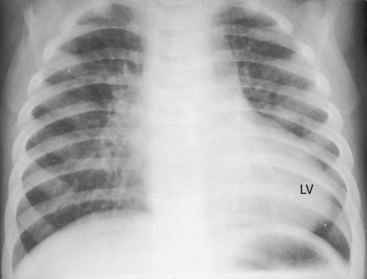
Survival is poorest when the foramen ovale is restrictive, the ventricular septum is intact, and the ductus is closed. The salutary effect of an adequately sized interatrial communication is underscored by the immediate response to balloon septostomy (see previous),41 after which 75% of patients survive for 6 months, 65% survive for 1 year, and many survive into their teens.40 A nonrestrictive ventricular septal defect with pulmonary vascular disease carries a 6-month survival rate of about 30% and a 1-year survival rate of about 20%. Moderate pulmonary stenosis improves longevity by regulating pulmonary blood flow, with three quarters of patients surviving for a year or more. Most of those who reach their teens have a nonrestrictive ventricular septal defect with pulmonary vascular disease or pulmonary stenosis. Sporadic examples of unusual longevity have been recorded in the third, fourth, and fifth decades (see Figure 27-7),31,68,69 and complete transposition was confirmed at necropsy in a 56-year-old patient.70 Brain abscess is rare in patients younger than 2 years of age but is believed to have a predilection for complete transposition of the great arteries and Fallot’s tetralogy.71
Physical appearance
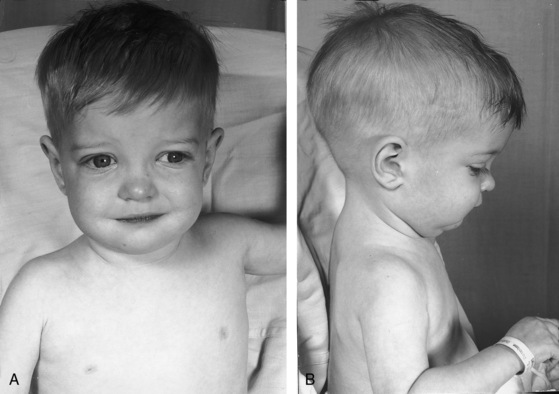
Birth weights in infants with complete transposition average greater than normal, with a substantial proportion above 8 pounds,72 in contrast to newborns with other forms of congenital heart disease whose birth weights average less than normal for gestational age. The illusion of robust health is soon dispelled by the catabolic effects of congestive heart failure (Figure 27-10). Increased anteroposterior chest dimensions are associated with hyperinflation of the lungs (see section X-Ray). Intense early cyanosis reflects poor intercirculatory mixing and low pulmonary blood flow. Cyanosis that is mild and delayed indicates good intercirculatory mixing and increased pulmonary blood flow. Complete transposition with a large patent ductus, pulmonary vascular disease, and an intact ventricular septum is associated with reversed differential cyanosis—the feet are less cyanotic than the hands—because oxygenated blood from the pulmonary artery enters the aorta distal to the left subclavian artery and flows selectively to the lower extremities (see Chapter 20).23 Deeply cyanotic patients with pulmonary vascular disease or severe pulmonary stenosis have varicosities of the scalp and arms because of the large volume of highly unsaturated blood in the systemic circulation.
Arterial pulse
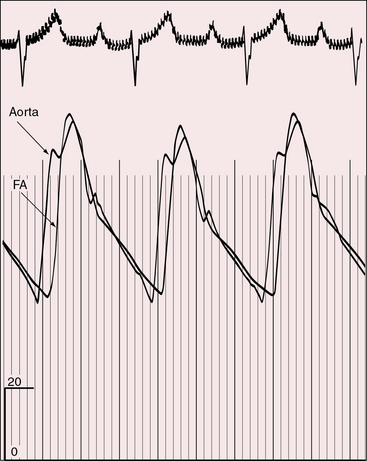
In 1957, Cleland and associates73 described peripheral arterial pulses of remarkably full volume (Figure 27-11) with visible pulsations of the dorsalis pedis and posterior tibial arteries. Shaher69 attributed the bounding pulses, the scalp varices, and the warm extremities to the large volume of highly unsaturated blood recirculating in the hyperkinetic low-resistance systemic vascular bed (see previous). Bounding pulses are not associated with patent ductus arteriosus because ductal flow is essentially systolic from aorta into pulmonary trunk and does not enter the right ventricle from which the aorta and systemic arteries arise.23 Diminished femoral pulses call attention to coexisting coarctation of the aorta with anterior and rightward deviation of the infundibular septum (subaortic stenosis).
Jugular venous pulse
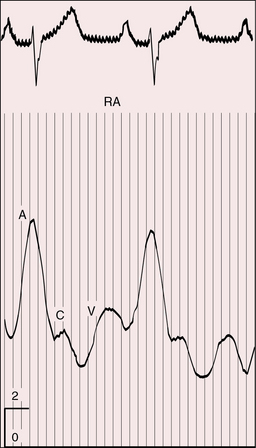
The jugular venous pulse is elevated under two widely divergent physiologic circumstances. The first and most common is congestive heart failure that accompanies a nonrestrictive ventricular septal defect with low pulmonary vascular resistance. The second circumstance is right ventricular failure in deeply cyanotic patients with a large volume of unsaturated blood recirculating through the right ventricle and right atrium (Figure 27-12).
Precordial movement and palpation
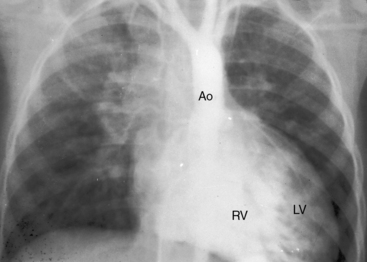
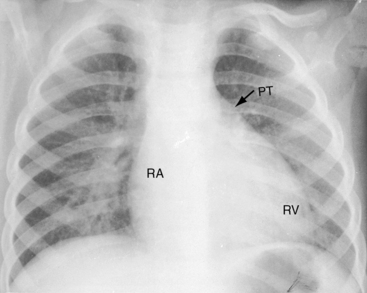
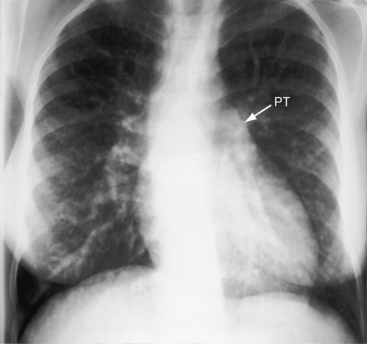
A loud palpable second heart sound at the left base originates in the aortic valve (Figure 27-13) because the transposed aorta is anterior. A dilated hypertensive posterior pulmonary trunk does not transmit its impulse or closure sound. A right aortic arch occurs in about 8% of patients with complete transposition and occasionally reveals itself as a right sternoclavicular impulse, especially when the aortic root is pulsatile and hyperkinetic in deeply cyanotic patients.74
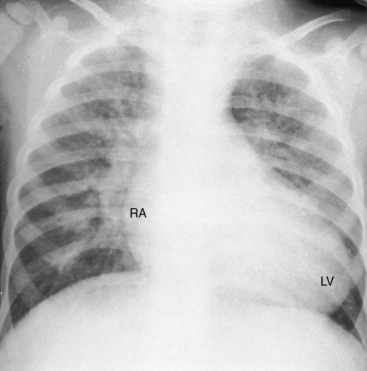
A left ventricular impulse is not identified in neonates and is overshadowed by the right ventricular impulse in older patients. The left ventricle is palpable when a nonrestrictive ventricular septal defect exists with low pulmonary vascular resistance because the left ventricle is both volume-overloaded and pressure-overloaded. The left ventricle is seldom palpable despite volume overload when there is a nonrestrictive atrial septal defect and an intact ventricular septum (see Figure 27-28) because ejection is at a low systolic pressure.
Auscultation
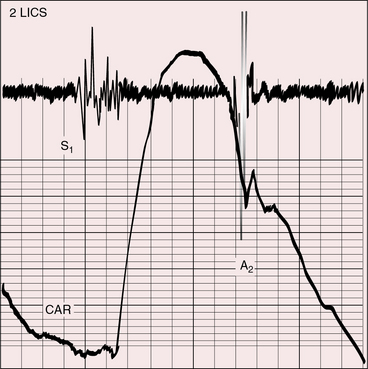
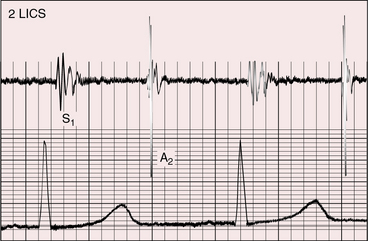
Pulmonary ejection sounds (Figure 27-14) originate in the dilated hypertensive posterior pulmonary trunk (Figure 27-15).75 The ejection sound does not selectively decrease during inspiration because the transposed pulmonary trunk arises from the left ventricle. Aortic ejection sounds occasionally occur when complete transposition is accompanied by a dilated aortic root with leftward and posterior malalignment of the infundibular septum (subaortic stenosis).
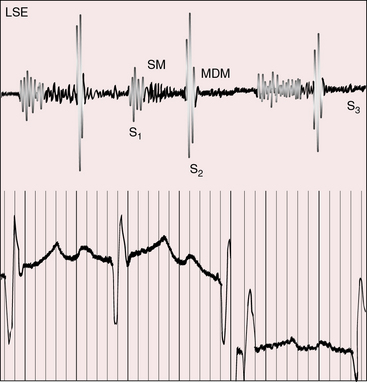
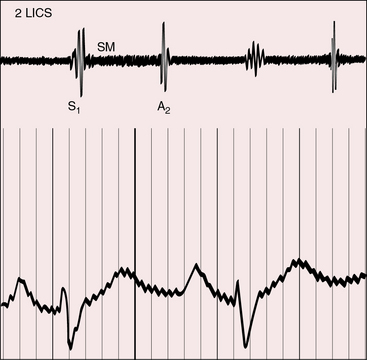
Midsystolic flow murmurs originate in the transposed anterior aorta, especially when the systemic circulation is hypervolemic and hyperkinetic. Midsystolic flow murmurs potentially originate in a dilated posterior pulmonary trunk (see Figure 27-15) but are inherently soft and are likely to be rendered inaudible by interposition of the anterior aorta (Figures 27-13 and 27-16).
A holosystolic murmur of ventricular septal defect awaits the neonatal fall in pulmonary vascular resistance (Figure 27-17; see Chapter 17).75 A subsequent rise in pulmonary resistance shortens and ultimately abolishes the murmur,76 as in patients with Eisenmenger’s syndrome (see Figure 27-14). A restrictive ventricular septal defect generates a holosystolic murmur or a soft early systolic murmur that disappears when the defect spontaneously closes.
The midsystolic murmur of fixed pulmonary stenosis is present at birth. The midsystolic murmur of dynamic obstruction to left ventricular outflow appears after the first few weeks of life and progressively increases in length and loudness. Pulmonary stenotic murmurs are heard best in the third left intercostal space at the sternal border because the stenosis is usually subvalvular. The murmur radiates upward and to the right because of the rightward course of the transposed pulmonary trunk (see Figure 27-2B). The midsystolic murmur of pulmonary stenosis with a nonrestrictive ventricular septal defect varies inversely in length and loudness with the degree of stenosis, as in Fallot’s tetralogy (see Chapter 18). A soft pulmonary stenotic murmur is rendered inaudible by the anterior aortic root (Figure 27-18).
The murmur of a nonrestrictive patent ductus arteriosus is confined to systole and not continuous because flow from the aorta into the pulmonary trunk is almost exclusively systolic.23 High neonatal pulmonary vascular resistance eliminates the diastolic portion of a continuous murmur by curtailing diastolic flow (see Chapter 20).76 A continuous murmur that could originate in a restrictive patent ductus is damped by the anterior aorta. The large systemic arterial collaterals that occasionally accompany complete transposition seldom cause continuous murmurs (see previous).
Mid-diastolic murmurs are generated across the mitral valve when pulmonary blood flow is increased (see Figure 27-17).75 Mid-diastolic murmurs across the tricuspid valve are generated in deeply cyanotic patients because the systemic circulation is hypervolemic and hyperkinetic.75 A high-frequency early diastolic Graham Steell murmur of pulmonary hypertensive pulmonary regurgitation is difficult to hear because of the posterior position of the pulmonary trunk.
A loud and single second heart sound is the aortic component because the aorta is anterior (see Figures 27-13, 27-14, 27-16, and 27-17). The sequence of semilunar valve closure is normal because the aortic component precedes the pulmonary component despite ventriculoarterial discordance. Low pulmonary vascular resistance and increased pulmonary capacitance result in normal timing or in only a slight delay of the pulmonary arterial dicrotic notch.77 Right bundle branch block causes paradoxical splitting of the second sound because the aortic component is delayed until after the pulmonary component.78 Pulmonary hypertension increases audibility of the pulmonary component, provided the pulmonary vascular resistance remains sufficiently low to permit inspiratory splitting. When pulmonary and systemic resistances equalize, the semilunar valves close simultaneously, the second sound is single, and its intensity is reinforced, as in Eisenmenger’s syndrome with a ventricular septal defect (see Figure 27-14; see Chapter 17). With pulmonary stenosis, splitting is not audible because the inherently soft pulmonary component is further attenuated by the posterior position of the pulmonary trunk.
A left ventricular third heart sound is generated in mildly cyanotic patients when increased pulmonary blood flow causes volume overload of the left ventricle, especially when the left ventricle fails (see Figure 27-17). A right ventricular third heart sound is generated in deeply cyanotic patients when increased systemic blood flow causes volume overload of the right ventricle, especially when the right ventricle fails.
Electrocardiogram
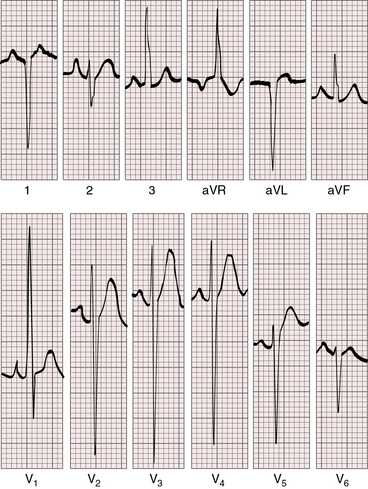
The electrocardiogram can be normal in the first few days of life (Figure 27-19).79,80 Tall peaked right atrial P waves soon emerge because mean right atrial pressure is increased (congestive heart failure) or because right atrial volume is increased (hypervolemic systemic circulation; Figures 27-20 and 27-21.)81,82 Left atrial P wave abnormalities are reserved for patients with a large atrial septal defect and increased pulmonary blood flow (Figure 27-22).
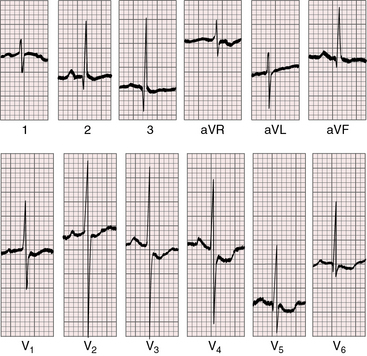
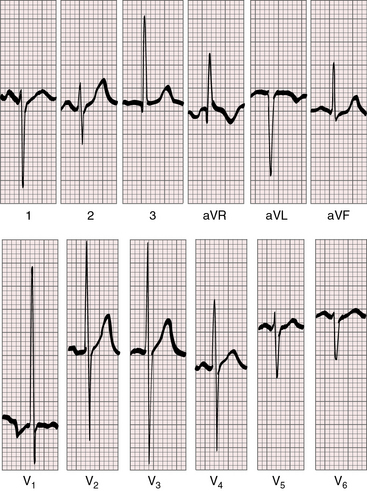
Figure 27-19 Electrocardiogram from a 7-day-old female with complete transposition of the great arteries and a nonrestrictive ventricular septal defect. For the first few days of life, the electrocardiogram was normal; and as shown, the electrocardiogram is virtually normal here except for nonspecific ST segment and T waves changes in leads V5-6. The QRS axis is +90 degrees. Compare with Figure 27-20, which is the electrocardiogram from the same patient at 4 years of age.
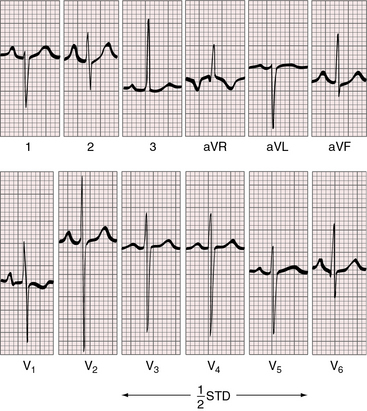
Figure 27-20 Electrocardiogram at 4 years of age from the patient whose normal neonatal electrocardiogram is shown in Figure 27-19. Tall peaked right atrial P waves are present in lead 2 and in leads V1-2. Precordial leads exhibit combined ventricular hypertrophy reflected in the large R/S complexes in leads V2-5, which are half standardized. Left ventricular volume overload is manifested by the Q wave and the well-developed R wave in lead V6. Right ventricular pressure overload is manifested by right axis deviation, a monophasic R wave in lead aVR, and a prominent S wave in lead V6.
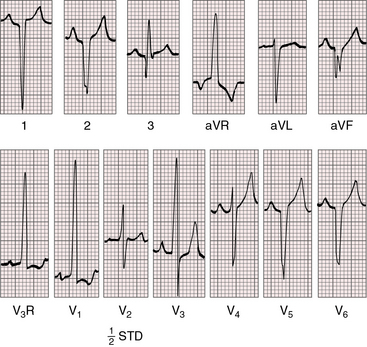
Right axis deviation is moderate or absent when the left ventricle is volume-overloaded in the presence of a nonrestrictive ventricular septal defect and increased pulmonary blood flow. Right axis deviation occurs when left ventricular volume overload is curtailed by pulmonary vascular disease or pulmonary stenosis (Figures 27-21 and 27-23). Right axis deviation is most striking when an atrial septal defect occurs with increased pulmonary blood flow, normal pulmonary artery pressure, and pure right ventricular hypertrophy (see Figure 27-22). Left precordial R waves are small, and Q waves are absent, despite left ventricular volume overload (see Figure 27-22). Pure right ventricular hypertrophy also occurs when pulmonary stenosis or a nonrestrictive ventricular septal defect with pulmonary vascular disease reduces left ventricular volume and when a hypervolemic systemic circulation increases right ventricular volume (see Figures 27-21 and 27-23). Biventricular hypertrophy is evidence of a nonrestrictive ventricular septal defect with low pulmonary vascular resistance and both volume and pressure overload of the left ventricle (see Figure 27-20).80 Right precordial T waves are seldom deeply inverted, even when the systemic right ventricle is volume-overloaded. Right precordial T waves often are not only positive but also tend to be distinctly taller than left precordial T waves (see Figures 27-21 and 27-22).
X-ray
An initially normal neonatal x-ray assumes typical features of complete transposition as pulmonary vascular resistance falls and pulmonary blood flow increases.19 Increased pulmonary vascularity is sometimes evident within the first few days of life (see Figures 27-24 and 27-25). The distribution of pulmonary blood flow favors the right lung because of the rightward direction of the pulmonary trunk (see Figures 27-2B, and 27-25B).9,83 A progressive increase in flow into the right lung may culminate in a substantial decrease in flow to the left lung.9 The crural portions of the hemidiaphragms are low when the lungs are hyperinflated.
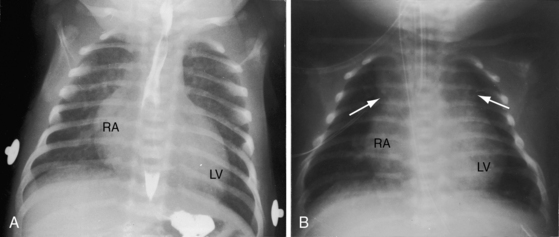
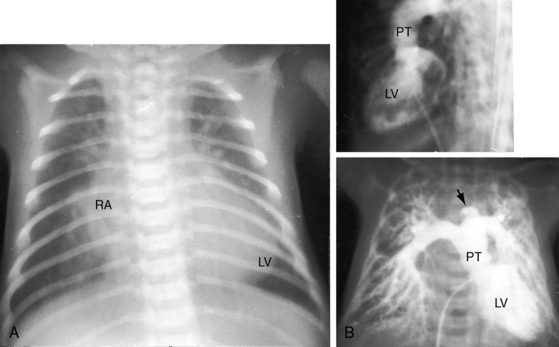
Thymic shadow is almost always absent after the first 12 hours of life (see Figure 27-24A), so the distinctive radiologic appearance of the narrow vascular pedicle is seldom obscured (Figures 27-24 through 27-27). The pulmonary trunk is posterior and medial (see Figures 27-2B, 27-3B, and 27-25B).84 The pedicle is narrowest when the ascending aorta courses vertically upward directly anterior to the pulmonary trunk (Figure 27-28). The aortic root is seldom sufficiently rightward to be border-forming (see Figures 27-2A, and 27-4), except when the ascending aorta enlarges in the presence of leftward and posterior malalignment of the infundibular septum (Figure 27-29). The vascular pedicle also widens when a dilated hypertensive posterior pulmonary trunk is convex to the left (Figure 27-30). When pulmonary stenosis and ventricular septal defect coexist, a right aortic arch is present in 11% to 16% cases.5,74,84–86
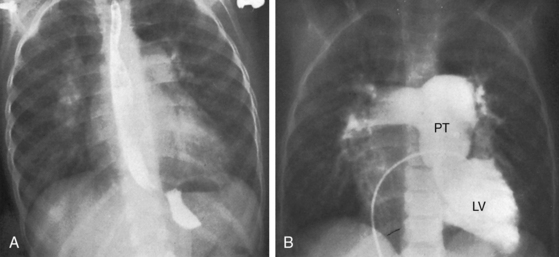
The size and shape of the heart are determined chiefly by the magnitude of pulmonary blood flow. When pulmonary vascular resistance is low and pulmonary blood flow is increased, the cardiac silhouette often has the distinctive appearance of a tilted egg lying on its side pointing downward and to the left (see Figures 27-26 and 27-27).84 The blunter right border of the egg consists of the right atrium, and the convex left border is the left ventricle. In the lateral projection, the heart assumes a circular appearance because an enlarged right ventricle merges with the anterior aorta, and an enlarged left ventricle merges with the dilated posterior left atrium (see Figure 27-3). Juxtaposition of the atrial appendages is recognized by a localized bulge along the mid left cardiac border that represents the contiguous mass of the two appendages.87
As pulmonary vascular resistance increases, lung vascularity decreases, the size of the left ventricle and left atrium decrease, and a dilated hypertensive posterior pulmonary trunk emerges at the left base (Figures 27-15, 27-30, and 27-31). Severe fixed pulmonary stenosis is associated with decreased pulmonary vascularity, a small left ventricle, a small left atrium, and enlargement of the right ventricle and right atrium because of the hypervolemic systemic circulation (see Figure 27-29).6
Echocardiogram
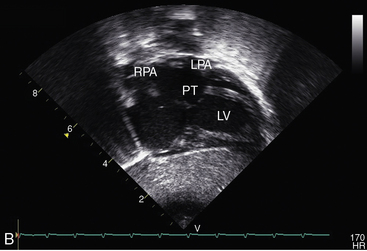
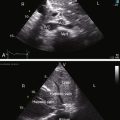
Echocardiography with color flow imaging and Doppler interrogation establishes the ventricular origins of the great arteries (ventriculoarterial discordance), their spatial relationships, the presence of a ventricular septal defect or an atrial septal defect, and the presence and degree of obstruction to ventricular outflow.88–90 The spiral relationship of the aorta and main pulmonary artery segment is normally represented in the short axis by an anterior “sausage,” which is the right ventricular outflow tract and main pulmonary artery segment transected tangentially, and a posterior circle, which is the aorta. In complete transposition, the great arteries appear as double circles, with the aorta anterior and to the right or side-by-side and to the right of the main pulmonary artery (Figure 27-32A, and Video 27-1). Identification of each great artery rests more securely on imaging the right and left branches of the main pulmonary artery (Figures 27-32B, and 27-33) and on imaging the brachiocephalic branches of the aortic arch with continuation as the descending thoracic aorta (Figure 27-34 and Video 27-2). The anterior aortic root and posterior pulmonary trunk run parallel to each other (see Figure 27-34 and Video 27-2) and do not cross as in the normal heart. The ventricle of origin of each discordant great artery can be properly assigned (see Figures 27-32, 27-33, and 27-34). An important role of echocardiography is in determining the origin and course of the coronary arteries.11 Commissural malalignment of the facing sinus can be established.91
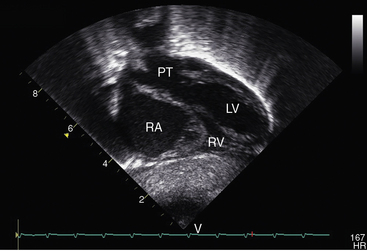
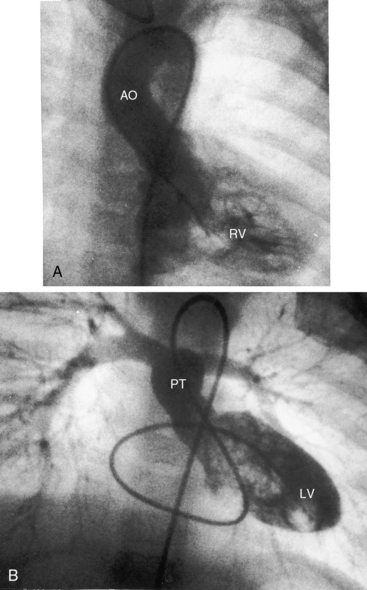
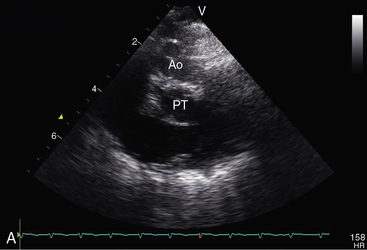
Figure 27-33 Echocardiogram (subcostal) from a male neonate with complete transposition of the great arteries, a patent foramen ovale, and an intact ventricular septum. The left ventricle (LV) gives rise to the pulmonary trunk (PT). A normal left ventricular outflow tract is delineated beneath delicate pulmonary valve echoes. Compare with the angiocardiogram in Figure 27-2B. (RA = right atrium; RV = right ventricle.)
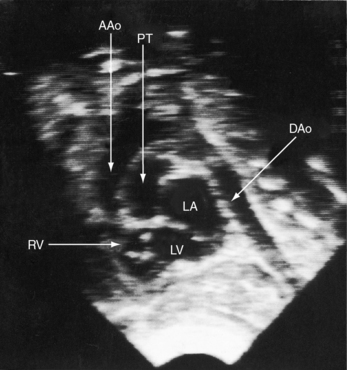
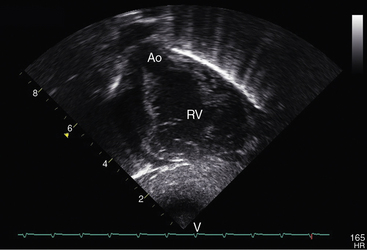
Figure 27-34 Echocardiogram (subcostal) from a male neonate with complete transposition of the great arteries, a patent foramen ovale, and an intact ventricular septum. The ascending aorta (AAo) is anterior, rising vertically from the right ventricle (RV) and giving off the brachiocephalic arteries before continuing as the descending aorta (DAo). Compare with the angiocardiogram in Figure 27-2A. The left ventricle (LV) gives rise to the pulmonary trunk (PT), which is posterior and parallel to the ascending aorta (Video 27-2). (LA = left atrium.)
Echocardiography with color flow imaging and Doppler interrogation establishes the presence of a ventricular septal defect or an atrial septal defect. Visualization of the left ventricular outflow tract determines the absence (see Figures 27-32 and 27-33), presence, and degree of obstruction as well as its morphologic type (Figures 27-35 and 27-36),89,90,92 and continuous wave Doppler interrogation establishes the magnitude of the gradient. Fixed obstruction to left ventricular outflow is represented by pulmonary valve stenosis (see Figure 27-35) or posterior and leftward malalignment of the infundibular septum.90,92 Dynamic obstruction to left ventricular outflow is characterized by systolic anterior movement of the anterior mitral leaflet that can be recognized with two-dimensional real time imaging (see Figure 27-36A).89 The combination of systolic anterior movement of the anterior mitral leaflet, abnormal systolic movement of the base of the ventricular septum, and a hyperkinetic left ventricular free wall conspire to produce the left ventricular outflow gradient of dynamic subpulmonary stenosis.89,90
Juxtaposition of the atrial appendages is recognized in the two-dimensional echocardiogram by malposition of the right atrial appendage with an abnormal plane of the atrial septum.93 Tricuspid valve anomalies can be identified, especially straddling or overriding.
1 Baillie M. Morbid anatomy of some of the most important parts of the human body, 2nd ed. London: Johnson and Nicol; 1797.
2 Farre J.R. On malformations of the human heart. London: Longman, Hurst, Rees, Orme, and Brown; 1814.
3 De La Cruz M.V., Arteaga M., Espino-Vela J., Quero-Jimenez M., Anderson R.H., Diaz G.F. Complete transposition of the great arteries: types and morphogenesis of ventriculoarterial discordance. Am Heart J. 1981;102:271-281.
4 De La Cruz M.V., Berrazueta J.R., Arteaga M., Attie F., Soni J. Rules for diagnosis of arterioventricular discordances and spatial identification of ventricles. Crossed great arteries and transposition of the great arteries. Br Heart J. 1976;38:341-354.
5 Elliott L.P., Neufeld H.N., Anderson R.C., Adams P., Edwards J.E. Complete transposition of the great vessels: I. An anatomic study of sixty cases. Circulation. 1963;27:1105.
6 Lev M., Rimoldi H.J., Paiva R., Arcilla R.A. The quantitative anatomy of simple complete transposition. Am J Cardiol. 1969;23:409-416.
7 Anderson R.H., Becker A.E., Losekoot T.G., Gerlis L.M. Anatomically corrected malposition of great arteries. Br Heart J. 1975;37:993-1013.
8 Van Praagh R., Durnin R.E., Jockin H., et al. Anatomically corrected malposition of the great arteries (S, D, L). Circulation. 1975;51:20-31.
9 Muster A.J., Paul M.H., Van Grondelle A., Conway J.J. Asymmetric distribution of the pulmonary blood flow between the right and left lungs in d-transposition of the great arteries. Am J Cardiol. 1976;38:352-361.
10 Mosieri J., Chintala K., Delius R.E., Walters H.L.3rd, Hakimi M. Abnormal origin of the right subclavian artery from the right pulmonary artery in a patient with D-transposition of the great vessels and left juxtaposition of the right atrial appendage: an unusual anatomical variant. J Card Surg. 2004;19:41-44.
11 Sim E.K., Van Son J.A., Edwards W.D., Julsrud P.R., Puga F.J. Coronary artery anatomy in complete transposition of the great arteries. Ann Thorac Surg. 1994;57:890-894.
12 Elliott L.P., Amplatz K., Edwards J.E. Coronary arterial patterns in transposition complexes. Anatomic and angiocardiographic studies. Am J Cardiol. 1966;17:362-378.
13 Gittenberger-De Groot A.C., Saucer U., Oppenheimer-Dekker A., Quaegebeur J. Coronary arterial anatomy in transposition of the great arteries: a morphological study. Pediatr Cardiol. 1983;4:15.
14 Rossi M.B., Ho S.Y., Anderson R.H., Rossi Filho R.I., Lincoln C. Coronary arteries in complete transposition: the significance of the sinus node artery. Ann Thorac Surg. 1986;42:573.
15 Li J., Tulloh R.M., Cook A., Schneider M., Ho S.Y., Anderson R.H. Coronary arterial origins in transposition of the great arteries: factors that affect outcome. A morphological and clinical study. Heart. 2000;83:320-325.
16 Padalino M.A., Ohye R.G., Devaney E.J., Bove E.L. Double intramural coronary arteries in D-transposition of the great arteries. Ann Thorac Surg. 2004;78:2181-2183.
17 Chiu I.-S., Wang J.-K., Wu M.-H. Coronary artery anatomy in complete transposition of the great arteries with situs inversus. Am J Cardiol. 2002;89:94-95.
18 Gittenberger-De Groot A.C., Sauer U., Quaegebeur J. Aortic intramural coronary artery in three hearts with transposition of the great arteries. J Thorac Cardiovasc Surg. 1986;91:566-571.
19 Levin D.L., Paul M.H., Muster A.J., Newfeld E.A., Waldman J.D. d-Transposition of the great vessels in the neonate. A clinical diagnosis. Arch Intern Med. 1977;137:1421-1425.
20 Chuaqui B. Doerr’s theory of morphogenesis of arterial transposition in light of recent research. Br Heart J. 1979;41:481-485.
21 Kondrachuk O., Yalynska T., Tammo R., Yemets I. D-transposition of the great arteries and double aortic arch. Eur J Cardiothorac Surg. 2009;35:729.
22 Al-Naami G.H., Al-Mesned A.A. Transposition of great arteries with constrictive ductus arteriosus revisited. Pediatr Cardiol. 2008;29:827-829.
23 Waldman J.D., Paul M.H., Newfeld E.A., Muster A.J., Idriss F.S. Transposition of the great arteries with intact ventricular septum and patent ductus arteriosus. Am J Cardiol. 1977;39:232-238.
24 Boesen I. Complete transposition of the great vessels: importance of septal defects and patent ductus arteriosus. Analysis of 132 patients dying before age 4. Circulation. 1963;28:885-887.
25 Chiou H.-L., Moon-Grady A., Rodriguez R., Konia T., Parrish M., Milstein J. A rare lethal combination of premature closure of the foramen ovale and d-transposition of the great arteries with intact ventricular septum. Int J Cardiol. 2008;130:e57-e59.
26 Milanesi O., Ho S.Y., Thiene G., Frescura C., Anderson R.H. The ventricular septal defect in complete transposition of the great arteries: pathologic anatomy in 57 cases with emphasis on subaortic, subpulmonary, and aortic arch obstruction. Hum Pathol. 1987;18:392-396.
27 Shrivastava S., Tadavarthy S.M., Fukuda T., Edwards J.E. Anatomic causes of pulmonary stenosis in complete transposition. Circulation. 1976;54:154-159.
28 Aziz K.U., Paul M.H., Idriss F.S., Wilson A.D., Muster A.J. Clinical manifestations of dynamic left ventricular outflow tract stenosis in infants with d-transposition of the great arteries with intact ventricular septum. Am J Cardiol. 1979;44:290-297.
29 Schneeweiss A., Motro M., Shem-Tov A., Neufeld H.N. Subaortic stenosis: an unrecognized problem in transposition of the great arteries. Am J Cardiol. 1981;48:336-339.
30 Waldman J.D., Schneeweiss A., Edwards W.D., Lamberti J.J., Shem-Tov A., Neufeld H.N. The obstructive subaortic conus. Circulation. 1984;70:339-344.
31 Messeloff C.R., Weaver J.C. A case of transposition of the large vessels in an adult who lived to the age of 38 years. Am Heart J. 1951;42:467-471.
32 Becker A.E., Becker M.J. Juxtaposition of atrial appendages associated with normally oriented ventricles and great arteries. Circulation. 1970;41:685-688.
33 Charuzi Y., Spanos P.K., Amplatz K., Edwards J.E. Juxtaposition of the atrial appendages. Circulation. 1973;47:620-627.
34 Deutsch V., Shem-Tov A., Yahini J.H., Neufeld H.N. Juxtaposition of atrial appendages: angiocardiographic observations. Am J Cardiol. 1974;34:240-244.
35 Hunter A.S., Henderson C.B., Urquhart W., Farmer M.B. Left-sided juxtaposition of the atrial appendages. Report of 4 cases diagnosed by cardiac catheterization and angiocardiography. Br Heart J. 1973;35:1184-1189.
36 Huhta J.C., Edwards W.D., Danielson G.K., Feldt R.H. Abnormalities of the tricuspid valve in complete transposition of the great arteries with ventricular septal defect. J Thorac Cardiovasc Surg. 1982;83:569-576.
37 Moene R.J., Oppenheimer-Dekker A. Congenital mitral valve anomalies in transposition of the great arteries. Am J Cardiol. 1982;49:1972-1978.
38 Rosenquist G.C., Stark J., Taylor J.F. Congenital mitral valve disease in transposition of the great arteries. Circulation. 1975;51:731-737.
39 Mair D.D., Ritter D.G. Factors influencing intercirculatory mixing in patients with complete transposition of the great arteries. Am J Cardiol. 1972;30:653-658.
40 Powell T.G., Dewey M., West C.R., Arnold R. Fate of infants with transposition of the great arteries in relation to balloon atrial septostomy. Br Heart J. 1984;51:371-376.
41 Rashkind W.J., Miller W.W. Creation of an atrial septal defect without thoracotomy. A palliative approach to complete transposition of the great arteries. JAMA. 1966;196:991-992.
42 Mair D.D., Ritter D.G. Factors influencing systemic arterial oxygen saturation in complete transposition of the great arteries. Am J Cardiol. 1973;31:742-748.
43 Satomi G., Nakazawa M., Takao A., et al. Blood flow pattern of the interatrial communication in patients with complete transposition of the great arteries: a pulsed Doppler echocardiographic study. Circulation. 1986;73:95-99.
44 Lang P., Freed M.D., Bierman F.Z., Norwood W.I.Jr, Nadas A.S. Use of prostaglandin E1 in infants with d-transposition of the great arteries and intact ventricular septum. Am J Cardiol. 1979;44:76-81.
45 Graham T.P.Jr, Atwood G.F., Boucek R.J.Jr, Boerth R.C., Nelson J.H. Right heart volume characteristics in transposition of the great arteries. Circulation. 1975;51:881-889.
46 Huhta J.C., Edwards W.D., Feldt R.H., Puga F.J. Left ventricular wall thickness in complete transposition of the great arteries. J Thorac Cardiovasc Surg. 1982;84:97-101.
47 Keane J.F., Ellison R.C., Rudd M., Nadas A.S. Pulmonary blood flow and left ventricular volumes in transposition of the great arteries and intact ventricular septum. Br Heart J. 1973;35:521-526.
48 Maroto E., Fouron J.C., Douste-Blazy M.Y., et al. Influence of age on wall thickness, cavity dimensions and myocardial contractility of the left ventricle in simple transposition of the great arteries. Circulation. 1983;67:1311-1317.
49 Smith A., Wilkinson J.L., Arnold R., Dickinson D.F., Anderson R.H. Growth and development of ventricular walls in complete transposition of the great arteries with intact septum (simple transposition). Am J Cardiol. 1982;49:362-368.
50 Van Doesburg N.H., Bierman F.Z., Williams R.G. Left ventricular geometry in infants with d-transposition of the great arteries and intact interventricular septum. Circulation. 1983;68:733-739.
51 Daliento L., Cuman G., Isabella G., et al. Ventricular development and function in complete transposition: angiocardiographic evaluation. Int J Cardiol. 1986;12:341-352.
52 Hurwitz R.A., Caldwell R.L., Girod D.A., Mahony L., Brown J., King H. Ventricular function in transposition of the great arteries: evaluation by radionuclide angiocardiography. Am Heart J. 1985;110:600-605.
53 Newfeld E.A., Paul M.M., Muster A.J., Idriss F.S. Pulmonary vascular disease in complete transposition of the great arteries: a study of 200 patients. Am J Cardiol. 1974;34:75-82.
54 Wagenvoort C.A., Nauta J., Van Der Schaar P.J., Weeda H.W., Wagenvoort N. The pulmonary vasculature in complete transpossition of the great vessels, judged from lung biopsies. Circulation. 1968;38:746-754.
55 Yamaki S., Tezuka F. Quantitative analysis of pulmonary vascular disease in complete transposition of the great arteries. Circulation. 1976;54:805-809.
56 Haworth S.G., Radley-Smith R., Yacoub M. Lung biopsy findings in transposition of the great arteries with ventricular septal defect: potentially reversible pulmonary vascular disease is not always synonymous with operability. J Am Coll Cardiol. 1987;9:327-333.
57 Dick M.2nd, Heidelberger K., Crowley D., Rosenthal A., Hees P. Quantitative morphometric analysis of the pulmonary arteries in two patients with D-transposition of the great arteries and persistence of the fetal circulation. Pediatr Res. 1981;15:1397-1401.
58 Rabinovitch M., Haworth S.G., Castaneda A.R., Nadas A.S., Reid L.M. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation. 1978;58:1107-1122.
59 Aziz K.U., Paul M.H., Rowe R.D. Bronchopulmonary circulation in d-transposition of the great arteries: possible role in genesis of accelerated pulmonary vascular disease. Am J Cardiol. 1977;39:432-438.
60 Viles P.H., Ongley P.A., Titus J.L. The spectrum of pulmonary vascular disease in transposition of the great arteries. Circulation. 1969;40:31-41.
61 Newfeld E.A., Paul M.H., Muster A.J., Idriss F.S. Pulmonary vascular disease in transposition of the great vessels and intact ventricular septum. Circulation. 1979;59:525-530.
62 Flyer D.C. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:375-461.
63 Digilio M.C., Casey B., Toscano A., et al. Complete transposition of the great arteries: patterns of congenital heart disease in familial precurrence. Circulation. 2001;104:2809-2814.
64 Shaher R.M., Kidd L. Acid-base balance in complete transposition of the great vessels. Br Heart J. 1967;29:207-211.
65 Maeno Y.V., Kamenir S.A., Sinclair B., Van Der Velde M.E., Smallhorn J.F., Hornberger L.K. Prenatal features of ductus arteriosus constriction and restrictive foramen ovale in d-transposition of the great arteries. Circulation. 1999;99:1209.
66 Schmaltz A.A., Knab I., Seybold-Epting W., Apitz J. Prognosis of children with transposition of the great arteries, treated in a regional heart centre between 1967 and 1979. Eur Heart J. 1982;3:570-576.
67 Sterns L.P., Baker R.M., Edwards J.E. Complete transposition of the great vessels. Unusual longevity in a case with subpulmonary stenosis. Circulation. 1964;29(suppl):610-613.
68 Nichol A.D., Segal A.J. Complete transposition of the main arterial stems; report of a case. J Am Med Assoc. 1951;147:645-648.
69 Shaher R.M. Prognosis of transposition of the great vessels with and without atrial septal defect. Br Heart J. 1963;25:211-218.
70 Kato K. Congenital transposition of cardiac vessels: a clinical and pathologic study. Arch Pediatr Adolesc Med. 1930;39:363.
71 Fischbein C.A., Rosenthal A., Fischer E.G., Nadas A.S., Welch K. Risk factors of brain abscess in patients with congenital heart disease. Am J Cardiol. 1974;34:97-102.
72 Rosenthal G.L., Wilson P.D., Permutt T., Boughman J.A., Ferencz C. Birth weight and cardiovascular malformations: a population-based study. The Baltimore-Washington Infant Study. Am J Epidemiol. 1991;133:1273-1281.
73 Cleland W.P., Goodwin J.F., Steiner R.E., Zoob M. Transposition of the aorta and pulmonary artery with pulmonary stenosis. Am Heart J. 1957;54:10-22.
74 Mathew R., Rosenthal A., Fellows K. The significance of right aortic arch in D-transposition of the great arteries. Am Heart J. 1974;87:314-317.
75 Wells B. The sounds and murmurs in transposition of the great vessels. Br Heart J. 1963;25:748-754.
76 Perloff J.K. Auscultatory and phonocardiographic manifestations of pulmonary hypertension. Prog Cardiovasc Dis. 1967;9:303-340.
77 Fouron J.C., Douste-Blazy M.Y., Ducharme G., Van Doesburg N., Davignon A. Hang-out time of pulmonary valve in d-transposition of great arteries. Br Heart J. 1982;47:277-280.
78 Zuberbuhler J.R., Bauersfeld S.R., Pontius R.G. Paradoxic splitting of the second sound with transposition of the great vessels. Am Heart J. 1967;74:816-819.
79 Calleja H.B., Hosier D.M., Grajo M.Z. The electrocardiogram in complete transposition of the great vessels. Am Heart J. 1965;69:31-40.
80 Shaher R.M., Deuchar D.C. The electrocardiogram in complete transposition of the great vessels. Br Heart J. 1966;28:265-275.
81 Elliott L.P., Anderson R.C., Tuna N., Adams P.Jr, Neufeld H.N. Complete transposition of the great vessels: II. An electrocardiographic analysis. Circulation. 1963;27:1118.
82 Khoury G.H., Shaher R.M., Fowler R.S., Keith J.D. Preoperative and postoperative electrocardiogram in complete transposition of the great vessels. Am Heart J. 1966;72:199-205.
83 Vidne B.A., Duszynski D., Subramanian S. Pulmonary blood flow distribution in transposition of the great arteries. Am J Cardiol. 1976;38:62-66.
84 Carey L.S., Elliott L.P. Complete transposition of the great vessels. Roentgenographic findings. Am J Roentgenol Radium Ther Nucl Med. 1964;91:529-543.
85 Jue K.L., Adams P.Jr, Pryor R., Blount S.G.Jr, Edwards J.E. Complete transposition of the great vessels in total situs inversus. Anatomic, electrocardiographic and radiologic observations. Am J Cardiol. 1966;17:389-394.
86 Schneeweiss A., Blieden L.C., Shem-Tov A., Fiegal A., Neufeld H.N. Wide vascular pedicle on thoracic roentgenogram in complete transposition of the great arteries. Clin Cardiol. 1982;5:75-77.
87 Bream P.R., Elliott L.P., Bargeron L.M.Jr. Plain film findings of anatomically corrected malposition: its association with juxtaposition of the atrial appendages and right aortic arch. Radiology. 1978;126:589-595.
88 Pasquini L., Sanders S.P., Parness I.A., et al. Conal anatomy in 119 patients with d-loop transposition of the great arteries and ventricular septal defect: an echocardiographic and pathologic study. J Am Coll Cardiol. 1993;21:1712-1721.
89 Moro E., Ten Cate F.J., Tirtaman C., Leonard J.J., Roelandt J. Doppler and two-dimensional echocardiographic observations of systolic anterior motion of the mitral valve in d-transposition of the great arteries: an explanation of the left ventricular outflow tract gradient. J Am Coll Cardiol. 1986;7:889-893.
90 Vitarelli A., D’Addio A.P., Gentile R., Burattini M. Echocardiographic evaluation of left ventricular outflow tract obstruction in complete transposition of the great arteries. Am Heart J. 1984;108:531-538.
91 Kim S.J., Kim W.H., Lim C., Oh S.S., Kim Y.M. Commissural malalignment of aortic-pulmonary sinus in complete transposition of great arteries. Ann Thorac Surg. 2003;76:1906-1910.
92 Riggs T.W., Muster A.J., Aziz K.U., Paul M.H., Ilbawi M., Idriss F.S. Two-dimensional echocardiographic and angiocardiographic diagnosis of subpulmonary stenosis due to tricuspid valve pouch in complete transposition of the great arteries. J Am Coll Cardiol. 1983;1:484-491.
93 Rice M.J., Seward J.B., Hagler D.J., Edwards W.D., Julsrud P.R., Tajik A.J. Left juxtaposed atrial appendages: diagnostic two-dimensional echocardiographic features. J Am Coll Cardiol. 1983;1:1330-1336.