CHAPTER 66 COMPARTMENT SYNDROMES
Compartment syndrome is a diagnosis that demands a decision. The diagnosis should be followed by immediate operation. Whether it occurs in a limb or the abdomen, compartment syndrome is a crisis that can be averted by surgical decompression. Fasciotomy treats compartment syndrome in limbs and laparotomy in the abdomen. Any alternative treatment should be compared with that standard.
Abdominal compartment syndrome (ACS) occurs in patients with massive intra-abdominal injuries and hemorrhagic shock. In the abdomen, elevated compartment pressure is manifested by oliguria and reduced cardiac output that do not improve with intravascular fluid replacement and increased airway pressures. Organ impairment and increased airway pressure can be detected at a pressure of 15 mm Hg. At 25–30 mm Hg, organ failure is evident and immediate laparotomy should be performed.1 Measuring intra-abdominal pressures will confirm the diagnosis.
INCIDENCE
Trauma, vascular, and orthopedic surgeons will likely diagnose and treat compartment syndrome because of its association with vascular injury and fractures. The incidence is relatively low, even though the presentation and treatment are dramatic and memorable. In our Level 1 trauma center, the overall incidence of compartment syndrome was 0.004 among trauma admissions over a 2-year period.2 This number includes compartment syndrome in the leg, arm, and abdomen.
Other published reports suggest that the incidence is much higher. A review of leg injuries reported an incidence of 30%–35% (up to 64%) of popliteal artery injuries with knee dislocations.3 A large series of brachial artery injuries reported 12.1% incidence of fasciotomy.4 Ivatury et al.5 reported 100% incidence of ACS among patients with damage control laparotomy and primary fascial closure. The incidence was reduced to 38% with open abdomen techniques.5 Improvements in open abdomen techniques and widespread appreciation of the syndrome have reduced the incidence of ACS even further. The low incidence of compartment syndrome among all trauma patients should not mitigate vigilance in patients with high-risk injuries.
MECHANISM OF INJURY
Arterial occlusion followed by reperfusion injury is a common presentation. Long bone fractures often precipitate compartment syndrome because of hematoma and tissue swelling at the site. Gulli and Templeton6 report that it occurs in 3%–17% of closed tibia fractures. Compartment syndrome associated with femur injuries is rare if the fracture occurs at the shaft and absent associated vascular injuries.7,8
Venous pathology also causes compartment syndrome. There are several reports of compartment syndrome occurring with phlegmasia cerulea dolens.9–11 The muscle compartments deserve attention in these patients.
In the upper arm and forearm, compartment syndrome can occur with supracondylar humerus fractures, IV drug abuse, electrical injuries, complications of IV sites, prolonged tourniquet use, and even weight lifting.12 Many of these patients will present in ambulatory settings, where the index of suspicion may be low. Deep pain and tense swelling of the limb should prompt further investigation.
Abdominal compartment syndrome often develops in trauma patients who have had laparotomies and resuscitation for hemorrhagic shock. The abdominal cavity will stretch anteriorly and superiorly (along the diaphragm) to accommodate visceral edema or accumulating blood until it reaches the limits of its compliance. At this point, the abdomen becomes a rigid compartment, and pressure rises sharply, impairing organ function. Increased vascular resistance and reduced venous return impair cardiac output. Reduced renal perfusion pressure causes oliguria. Encroachment into the chest and tension on the diaphragm increase ventilator and airway pressures. Loss of functional residual capacity (FRC) and ventilation-perfusion mismatch (V/Q) cause hypoxia.13
Intoxications and systemic disease can cause compartment syndrome. Rutgers et al.14 reported four cases of nontraumatic rhabdomyolysis and compartment syndrome in young male alcoholics receiving treatment with benzodiazepines. Ergotamine and cocaine intoxication have also been implicated in cases of compartment syndrome.15,16 Patients with type I diabetes mellitus can suffer spontaneous compartment syndrome.17–19
Systemic diseases or drugs that cause vasoconstriction can induce muscle ischemia and subsequent compartment syndrome. Local factors that increase mass within the inelastic fascial compartments can also raise intracompartment pressure sufficiently to cause the feared syndrome. These include hematoma, fluid injection, infection, and even metastatic melanoma.20 The careful clinician should remember the pathology, not only the common clinical settings, for compartment syndrome.
DIAGNOSIS
Physical Examination
The combination of inordinate muscle pain, pain on passive motion, muscle weakness or paralysis, hyperesthesia, and tense muscle compartments have been well described and repeated to generations of surgery residents.21–23 Recognition of the symptom constellation should prompt immediate measurement of compartment pressure. If accurate measurements cannot be performed, or if the results are conflicting, then clinical diagnosis should supersede. Once the diagnosis has been made, the patient should not suffer delay in fasciotomy.
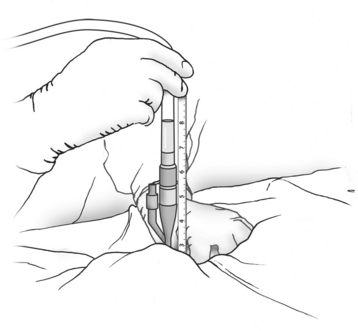
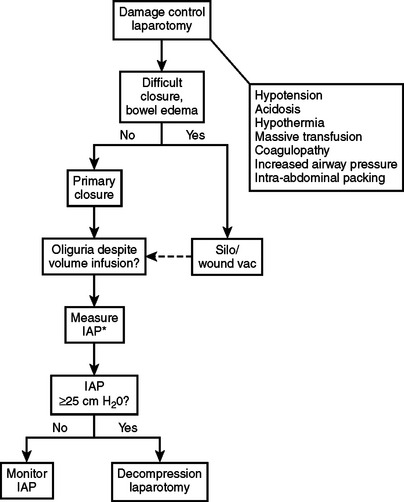
Abdominal compartment syndrome should be suspected in the patient with a tense, distended abdomen within a few hours after laparotomy for trauma or massive bleeding. Visceral swelling or continued bleeding push abdominal compliance beyond its limits. Oliguria that does not respond to fluid boluses is an early sign of intra-abdominal hypertension (IAH), and should prompt measurement of intra-abdominal pressure. This can be accomplished easily at the bedside by measuring the bladder pressure through a Foley catheter1,9 (Figure 1). Frequent ventilator alarms from high airway pressures as the ventilator pushes against abdominal pressures of 60 cm H2O or more herald the final stages of ACS. In this case, immediate abdominal decompression should be performed, even at the bedside, to permit ventilation and oxygenation.
Compartment Measurements
There are several techniques for measuring compartment pressures.24–26 There are two variations—wick and slit—of the catheter technique. The catheters are inserted into the muscle through large-bore needles and then connected to a pressure transducer or manometer via saline-filled tubing. Because insertion and connection of the catheters are cumbersome, measuring several compartment pressures is difficult. The new electronic transducer-tipped catheter is promising, but shares many of the shortcomings with the other catheter techniques, such as need for tubes, catheter kinking, and poor placement beneath the fascia.27 Other techniques for measuring compartments are readily available at the bedside and are easier to use.
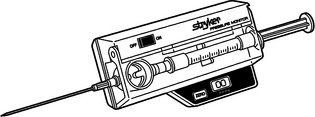
Manufactured pressure monitors such as the Stryker (Stryker Instruments, Kalamazoo, MI) (Figure 2) employ modifications of the needle technique. They measure the pressure directly through a needle inserted into the muscle compartment. They are self-contained units requiring no assembly, which makes multiple measurements easier at various sites or at different times. If a manufactured monitor is not available, a homemade version can be assembled using an 18-gauge needle, saline-filled pressure tubing, and a manometer or transducer.
Noninvasive Methods
There is a tenacious search for noninvasive diagnostics, and it extends to compartment syndrome. Several techniques that have clinical utility in other settings have been tried here. Near-infrared spectroscopy (NIRS) has been studied.28–30 It measures muscle perfusion, not pressure, and can reliably diagnose ischemic tissue. Oxyhemoglobin saturation of less than 60% correlates with muscle compromise of compartment syndrome. Champions for its use argue that it directly identifies ischemic tissue rather than compartment pressure, which is a proxy for tissue compromise. If clinicians monitor for tissue ischemia rather than a rise in pressure, unnecessary fasciotomies might be prevented. Conversely, skeptics argue that waiting until ischemia is manifest may delay surgery. Also, the probe’s range is limited to 2 cm or less below the skin surface. Therefore, it may miss deep muscle ischemia.
Digital pulse-oximetry is commonly available in the emergency room, operating room, and the intensive care unit. It is easy to use and inexpensive. It is not, however, sensitive in diagnosing compartment syndrome and muscle ischemia. It relies on pulsatile arterial flow to the distal digit to accurately measure the hemoglobin oxygen saturation. Because the arterial blood measured in the toe or finger bypasses the muscle compartments, measuring the former gives little useful information of the latter. A clinical series by Mars and Hadley31 confirms this theoretical limitation.
Scintigraphy using 99mTc-methoxyisobutyl isonitrile (99mTc-MIBI) has been used to diagnose chronic exertional compartment syndrome.32,33 The study requires a stable, ambulating patient, a trip to the nuclear medicine department, and a repeat study the next day with the patient at rest. With these limitations, nuclear medicine is no help with trauma patients.
Laboratory Studies
There are no laboratory tests to diagnose early compartment syndrome. Serum creatinine phosphokinase (CPK) is a marker for muscle cell injury, and is elevated in late or missed compartment syndrome.12,34 The surgeon should not wait for a rise in serum CPK before operating. Postoperative levels may be useful in monitoring the response to treatment.
SURGICAL MANAGEMENT
Surgery is the mainstay of treatment. Releasing the pressure through generous fascial incisions restores microvascular flow and rescues threatened tissue. For abdominal compartment syndrome, laparotomy accomplishes decompression. Medical therapies enjoy initial enthusiasm, but none so far have demonstrated adequate efficacy. Choices in operative treatment are choices of incision and wound closure. The necessity of fasciotomy for diagnosed compartment syndrome remains unassailable. Indications for prophylactic fasciotomies, however, have been questioned.23,36,37
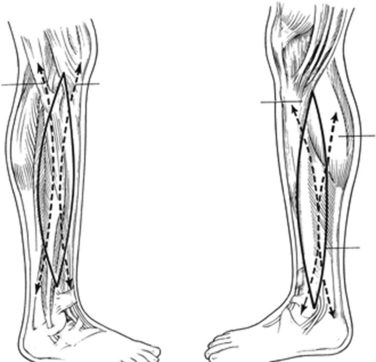
The four muscle compartments in the calf are the anterior, lateral, superficial posterior, and deep posterior. The anterior compartment is bounded by the tibia medially, the interosseous membrane posteriorly anterior crural intermuscular septum laterally, and the crural fascia anteriorly. It contains the tibialis anterior, the extensor digitorum longus, and the extensor hallucis longus muscles. It also contains the anterior tibial artery and vein, and the deep peroneal nerve. The lateral compartment contains the peroneus longus and brevis muscles and the superficial peroneal nerve. The superficial posterior compartment contains the bulky soleus muscle. The deep posterior compartment encloses the tibialis posterior, flexor digitorum longus, and flexor hallucis longus muscles. The posterior tibial vessels and the tibial nerve run within this compartment. Note that the saphenous vein courses in the subcutaneous tissue along the medial border of the superficial compartment. It can be damaged during fasciotomy if care is not taken to protect it. Also, the sural nerve runs along the posterior lateral border of the superficial posterior compartment. Fasciotomies of the calf are usually performed through a medial incision, to open the posterior and deep posterior compartments, and a lateral incision for the anterior and lateral compartments (Figure 3).
Prophylactic fasciotomies in the calves have been advocated for combined popliteal artery and vein injuries and for ischemia for more than 6 hours. Advocates argue that delays in diagnosing compartment syndrome may lead to severe dysfunction or amputation, and therefore, waiting until compartment pressures reach threshold cannot be justified.6 These authors recommend liberal fasciotomies, especially in the anesthetized or comatose patient.
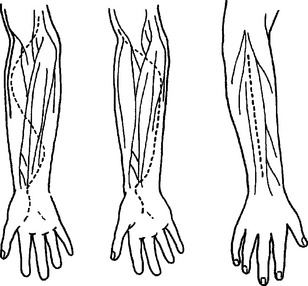
The forearm contains three compartments, the volar, dorsal, and the mobile wad. The volar compartment contains the flexor and pronator muscles, the radial and ulnar arteries, and the median and ulnar nerves. The dorsal compartment contains the extensor muscles. The mobile wad is closely associated with the dorsal compartment and contains the radial nerve. Fasciotomies of all compartments can be performed through volar and radial incisions. The volar incision can be curved or straight. The radial incision is straight along the forearm axis (Figure 4).
Hofmeister and Shin38 recommend prophylactic fasciotomy of all muscle compartments of the arm after replantation. They reason that because the replantation requires 5–10 hours and the compromised muscle relies on tenuous arterial and venous anastomoses, fasciotomy should be performed before compartment syndrome develops. Under these circumstances, fasciotomy is prudent.
Advocates of liberal fasciotomies tend to discount the morbidity of the scars. Conversely, other experts hold that the complications from fasciotomies, including prophylactic ones, can be significant.36,37,39 Wound complications include ulcers, skin tethering to the muscle, paresthesias, pruritus, muscle herniation, and disfigurement. Fitzgerald and colleagues39 report that 28% of their patients changed hobbies, and 12% changed their occupations because of the unsightly scars. The authors recommend primary closure of the wounds whenever possible, and hope for less invasive methods for fascial release.
The four compartments of the lower leg can be decompressed through a single lateral incision or through lateral and medial incisions. The two-incision technique is more common and the technique of choice because it is technically easier to reach the posterior compartments through the medial incision (see Figure 3). Fibulectomy has been described but has been abandoned because there are easier and less morbid operations that accomplish adequate decompression.6,40
Less invasive methods have been attempted. Ota et al.41 described endoscopic release of the anterior leg compartment using an arthroscope and a transparent outer tube for chronic compartment syndrome in an athlete. The patient enjoyed relief of her symptoms postoperatively, and the compartment pressures diminished. Other authors have been less enthusiastic about endoscopic fasciotomies. Havig and colleagues42 compared endoscopic and open forearm fasciotomies in cadavers. They found that the endoscopic procedure reduced compartment pressures, but not as dramatically as the open procedure. They caution against using the endoscopic forearm fasciotomy in the clinical setting.
Morbidity and Complication Management
In the abdomen, primary closure of the fascia is usually impossible. Sometimes, a skin-only closure can be accomplished. The most common forms of closure after laparotomy for abdominal compartment syndrome involve some form of temporary prosthesis such as a “Bogota bag” or vacuum pack.1,9 These maintain protection of the viscera while allowing loss of domain. They effectively increase the volume of the abdominal cavity. Removal of the prosthesis can be accomplished when the swelling recedes. If delayed primary closure cannot be performed, then skin grafting or component separation can cover the viscera.
Delayed primary closure of extremity wounds offers the benefit of a smaller scar, but is usually labor intensive. This method involves some daily manipulation of sutures, wires, or elastic bands. Steri-Strips® (3M Surgical Products, St. Paul, MN) have been used for gradual approximation of skin edges, closing the wound in 5–8 days.43 Chiverton and Redden44 used subcuticular Prolene suture to achieve skin closure. Harris45 described using rubber vessel loops stretched between skin staples in shoelace fashion. A new device, the WoundBullet™ (Boehringer Laboratories, Norristown, PA) is promising. It uses a small internal ratchet to add tension to sutures for gradual wound closure.
Because of the morbidity of fasciotomy, medical treatments have been advanced. The results are equivocal. Most are used to ameliorate the damage from oxygen-free radicals.38 They include deferoxamine to chelate iron, xanthine oxidase inhibitors, such as allopurinol, to block production of hypoxanthine, and superoxide dismutase, an enzyme to catalyze the superoxide radical to hydrogen peroxide. These antioxidants have been studied in many animal models, but not yet in human trials.
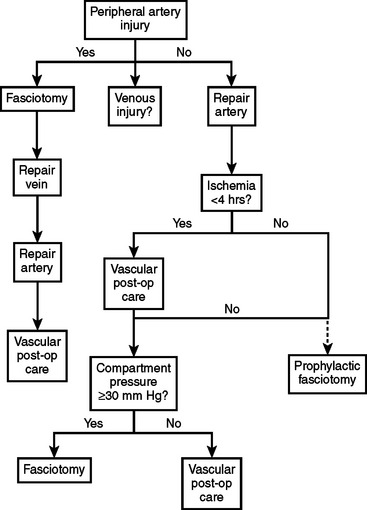
CONCLUSIONS AND ALGORITHMS
The following algorithms (Figures 5 and 6) show my approach to patients at risk for limb and abdominal compartment syndrome. They are not promoted as “the standard of care” nor to replace or supersede the judgment of a qualified surgeon. They have worked for me and my patients for many years, and I teach them to my residents.
1 Burch JAM, Moore EE, Moore FA, Françoise R. The abdominal compartment syndrome in complex and challenging problems in trauma surgery. Surge Clan North Am. 1996;76(4):833-843.
2 Garza R. Trauma Registry. Houston, TX: Ben Taub Trauma Center, October 2005.
3 Heyse MS, Richardson MW, Miller MD. The dislocated knee. Clan Sports Med. 2000;19(3):519-543.
4 Zellweger R, Hess F, Nicol A, et al. An analysis of 124 surgically managed brachial artery injuries. Am J Surge. 2004;188:240-245.
5 Ivatury RR, Diebel L, Porter JM, Simon RJ. Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am. 1997;77(4):783-800.
6 Gulli B, Templeton D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
7 Russel GV, Kregor PJ, Jarret CA, Zlowodowski M. Complicated femoral shaft fractures, in treatment of complex fractures. Orthop Clin North Am. 2002;33(1):1-17.
8 Schwartz JTJr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh: a spectrum of injury. J Bone Joint Surg [Am]. 1989;71:392-400.
9 Dennis C. Disaster following femoral vein ligation for thrombophlebitis, relief by fasciotomy: clinical case of renal impairment following crush injury. Surgery. 1945;17:264-269.
10 Cywes S, Louw JH. Phlegmasia cerulea dolens: successful treatment by relieving fasciotomy. Surgery. 1962;51:169-172.
11 Wood KE, Reedy JS, Pozniak MA, Coursin DB. Phlegmasia cerulean dolens with compartment syndrome: a complication of femoral vein catheterization. Crit Care Med. 2000;28(5):1626-1630.
12 Moore REIII, Friedman RJ. Current concepts in pathophysiology and diagnosis of compartment syndromes. J Emerg Med. 1989;7:657-662.
13 Ivatury RR, Sugerman HJ, Peiztman AB. Abdominal compartment syndrome: recognition and management. Adv Surg. 2001;35:251-269.
14 Rutgers PH, van der Harst E, Koumans RKJ. Surgical implications of drug induced rhabdomyolysis. Br J Surg. 1991;78:490-492.
15 Gilman AG, Goodman LS, Murad F. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 8th ed. New York: Macmillan, 1989.
16 Singhal P, Horowitz B, Quinones QC, et al. Acute renal failure following cocaine abuse. Nephron. 1989;52:76-78.
17 Lafforgue P, Janand-Delenne B, Lassman-Vague V, et al. Diabetes Metab. 1999;25(3):255-260.
18 Silberstein L, Britton KE, Marsh FP, et al. An unexpected cause of muscle pain in diabetes. Ann Rheum Dis. 2001;60(4):310-312.
19 Smith AL, Laing PW. Spontaneous tibial compartment syndrome in type I diabetes mellitus. Diabetes Med. 1999;16(2):168-169.
20 Simmons DJ. Compartment syndrome complicating metastatic malignant melanoma. Br J Plast Surg. 2000;53(3):255-257.
21 Matsen FA, Windquist RA, Krugmire RB. Diagnosis and management of compartmental syndromes. J Bone Joint Surg [Am]. 1980;62:286.
22 Perry MO. Compartment syndromes and reperfusion injury: vascular trauma. Surg Clin North Am. 1988;68(4):853-864.
23 Velmahos GC, Toutouzas KG. Vascular trauma and compartment syndromes. Surg Clin North Am. 2002;82(1):125-141.
24 Matsen FA, Mayo KA, Sheridan GW, Krugmire RB. Monitoring of intramuscular pressure. Surgery. 1976;79(6):702-709.
25 Perron AD, Brady WJ, Keats TE. Orthopedic pitfalls in the ED: acute compartment syndrome. Am J Emerg Med. 2001;19(5):413-416.
26 Hargens AR, Mubarak SJ, Owen CA, et al. Interstitial fluid pressure in muscle and compartment syndromes in man. Microvasc Res. 1977;14:1-10.
27 Willy C, Gerngross H, Sterk J. Measurement of intracompartmental pressure with use of a new electronic transducer-tipped catheter system. J Bone Joint Surg [Am]. 1999;81(2):158-168.
28 Garr JL, Gentilello LM, Cole PA, et al. Monitoring for compartmental syndrome using near-infrared spectroscopy: a noninvasive, continuous, transcutaneous monitoring technique. J Trauma. 1999;46(4):613-618.
29 Giannotti G, Cohn SM, Brown M, et al. Utility of near-infrared spectroscopy in the diagnosis of lower extremity compartment syndrome. J Trauma. 2000;48(3):396-401.
30 Gentilello LM, Sanzone A, Wang L, et al. Near-infrared spectroscopy versus compartment pressure for the diagnosis of lower extremity compartmental syndrome using electromyography-determined measurements of neuromuscular function. J Trauma. 2001;51(1):1-9.
31 Mars M, Hadley GP. Failure of pulse oximetry in the assessment of raised limb intracompartmental pressure. Injury. 1994;25(6):379-381.
32 Edwards PD, Miles KA, Owens SJ, et al. A new non-invasive test for detection of compartment syndromes. Nucl Med Commun. 1999;20(3):215-218.
33 Owens S, Edwards P, Miles K, et al. Chronic compartment syndrome affecting the lower limb: MIBI perfusion imaging as an alternative to pressure monitoring: two case reports. Br J Sports Med. 1999;33:49-51.
34 Robbs JV, Baker LW. Late revascularization of the lower limb following acute arterial occlusion. Br J Surg. 1979;78:490-493.
35 American Association for the Surgery of Trauma: October 26, 2005. http://www.aast.org/injury
36 Field CK, Senkowsky J, Hollier LH, et al. Fasciotomy in vascular trauma: is it too much, too often? Am Surg. 1994;60(6):409-411.
37 Velmahos GC, Theodorou D, Demetriades D, et al. Complications and nonclosure rates of fasciotomy for trauma and related risk factors. World J Surg. 1997;21:247-253.
38 Hofmeister EP, Shin AY. The role of prophylactic fasciotomy and medical treatment in limb ischemia and revascularization, in compartment syndrome and volkmann’s ischemic contracture. Hand Clin. 1998;14(3):457-465.
39 Fitzgerald AM, Gaston P, Quaba A, McQueen MM. Long-term sequelae of fasciotomy wounds. Br J Plast Surg. 2000;53:690-693.
40 Mubarak SJ, Owen CA. Double-incision fasciotomy of the leg for decompression in compartment syndromes. J Bone Joint Surg. 1977;59A:184-187.
41 Ota Y, Senda M, Hashizume H, Inoue H. Chronic compartment syndrome of the lower leg: a new diagnostic method using near-infrared spectroscopy and a new endoscopic fasciotomy. Arthroscopy. 1999;15(4):439-443.
42 Havig MT, Leversedge FJ, Seiler JG3rd. Forearm compartment pressure: an in vitro analysis of open and endoscopic assisted fasciotomy. J Hand Surg (Am). 1999;24(6):1289-1297.
43 Harrah J, Gates R, Carl J, Harrah JD. A simpler, less expensive technique for delayed primary closure of fasciotomies. Am J Surg. 2000;180(1):55-57.
44 Chiverton N, Redden JF. A new technique for delayed primary closure of fasciotomy wounds. Injury. 2000;31(1):21-24.
45 Harris I. Gradual closure of fasciotomy wounds using a vessel loop shoelace. Injury. 1993;24(8):565-567.