Chapter 24 Community-Acquired Pneumonia
Risk Factors
Certain risk factors are associated with higher frequency of infections with particular pathogens.
Patient Evaluation/Diagnostics
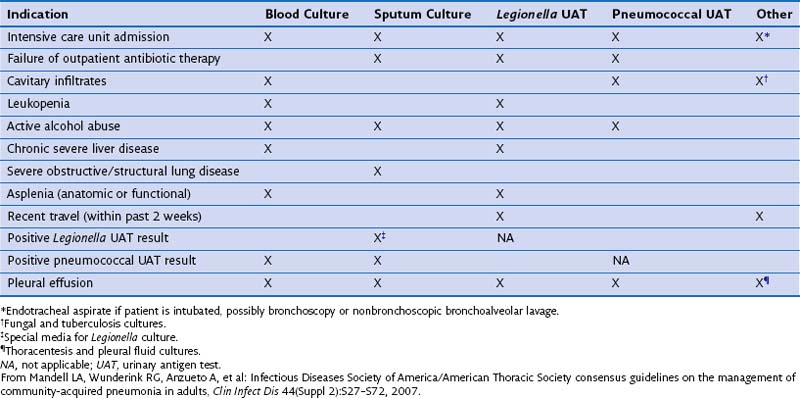
Clinical presentation of CAP can be rather nonspecific, especially in elderly and debilitated persons. Risk factors for specific causative agents, such as history of recent travel into endemic areas, is an important part of the clinical history in a patient with suspected CAP. Because of the inaccuracy of a purely clinical diagnosis, additional investigations are required to confirm the diagnosis, identify the causative pathogen, and initiate appropriate treatment. These are discussed in Chapter 23. Table 24-1 summarizes the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) recommendations of tests proven to be useful in different settings for investigating and treating CAP.
Specific Pathogens
Gram-Negative Bacilli
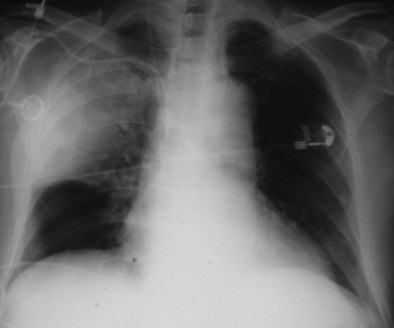
Friedländer’s pneumonia (caused by K. pneumoniae) typically occurs in men older than 40 years; alcoholism, diabetes mellitus, and chronic lung disease are predisposing factors. Historically, patients were thought to produce particularly large volumes of thick and bloody sputum; they were likely to present with prostration and hypotension and to have multiple patches of consolidation, particularly in the upper lobes, with bulging fissures (Figure 24-1) and multicavitation on chest radiographs (i.e., an expanding pneumonia).
Actinomyces israelii
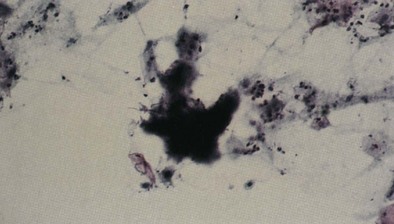
Both Actinomyces and Arachnia can cause actinomycosis, but Actinomyces israelii is the main responsible organism. These are anaerobic, gram-positive, filamentous, branching bacilli (Figure 24-2) that were incorrectly thought to be fungi for many years. They normally reside in the oropharynx and become invasive pathogens when there is a defect in the anatomic barrier or when they are inhaled, at which time the infection may extend directly from one place to an adjacent area. Bad dentition, bronchiectasis, and COPD are risk factors for pulmonary infection. Men are far more frequently affected than women. The clinical presentation suggests tuberculosis, carcinoma, or chronic fungal infection; asthenia, anorexia, weight loss, and low-grade fever may precede cough and chest pain by months. Cervicofacial and thoracic involvement coexists rarely. When infection progresses to the pleural space and chest wall, the opening of a sinus tract may disclose pus. Radiographic features are variable and include small cavitary nodules confined to one segment; cavitary infiltration; extension of infection to the interlobar fissure, chest wall, bone, or pleura; and empyema.
Treatment
Site of Care Decision
Several outcome tools have been developed to predict prognosis in CAP, and these are now being used to identify patients at low risk of mortality and appropriate for treatment at home rather than in hospital. Among the well-validated ones are the Pneumonia Severity Index (PSI) and the CURB-65 and CRB-65 indices. These are summarized in Chapter 23, Figures 23-5 and 23-6.
CURB-65 or CRB-65 (which is CURB-65 without the blood urea nitrogen level) indices are significantly more user-friendly scoring systems requiring consideration of four or five readily assessed variables (see Chapter 23, Figure 23-6).
Another sometimes challenging decision is whether and when to admit the patient to an ICU. Generally, patients with high scores on mortality prediction indices (such as class V PSI or group 3 CURB-65) are likely to benefit from early ICU admission. Emerging data suggest that early admission to the ICU in appropriate patients can improve outcomes. The challenge is identifying these patients among the larger group of patients at risk for higher mortality. Latest ATS/IDSA guidelines defined need for ICU admission as the presence of one of the major criteria (need for invasive mechanical ventilation or hemodynamic instability requiring vasopressor use) or three of nine minor criteria listed in Box 24-1. Several scoring systems (CURXO, SMART-COP, and CAP-PIRO) tested criteria similar to the ATS minor criteria set and found them to be similar in terms of predicting need for admission to intensive care. All of the aforementioned scoring systems, however, are not without limitations, especially when applied in younger populations. Development and validation of a dedicated scoring system to predict need for ICU treatment will be useful.
Box 24-1
American Thoracic Society Criteria for Admission of Patients with Community-Acquired Pneumonia to an Intensive Care Unit
Minor Criteria
Modified from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults, Clin Infect Dis 44(Suppl 2):S27–S72, 2007.
Antimicrobial Treatment
A major consideration determining empirical antibiotic choice in the current ATS/IDSA guidelines is the site of care, which serves as a surrogate marker for disease severity (outpatient, inpatient, and ICU settings) (Box 24-2 and Table 24-2). In the outpatient setting, choice of antibiotics is influenced primarily by presence of comorbid conditions and by recent antibiotic use. These two risk factors portend poor outcomes and infection by drug-resistant pathogens. Recent antibiotic use (within 3 months) should prompt choice of an agent from an antibiotic class other than the one to which the patient has already been exposed. Local resistance patterns also should be kept in mind in choosing agents for empirical antimicrobial therapy; for example, high-level macrolide resistance (minimum inhibitory concentration [MIC] of 16 µg/mL or higher) at a prevalence of 25% or more should discourage macrolide use as a single agent. Of the multitude of potential causative agents, five or six pathogens typically account for a majority of cases of CAP; nevertheless, the possibility of unusual pathogens should always be kept in mind. A thorough recent travel or exposure history and an awareness of specific pathogen association with certain epidemiologic settings are necessary to consider these unusual pathogens in CAP.
Box 24-2
Most Common Etiologic Agents of Community-Acquired Pneumonia*
Modified from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults, Clin Infect Dis 44(Suppl 2):S27–S72, 2007.
Table 24-2 ATS/IDSA Recommendations for Empirical Antibiotic Treatment of Community-Acquired Pneumonia
| In-Hospital Setting | Antibiotic Regimen | |
|---|---|---|
| Outpatient | Inpatient | |
| Non-ICU setting | ||
| Absence of risk factors | ||
ATS/IDSA, American Thoracic Society/Infectious Diseases Society of America; ICU, intensive care unit.
Data from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults, Clin Infect Dis 44(Suppl 2):S27–S72, 2007.
With CAP severe enough to warrant admission to an ICU, an expanded list of likely pathogens has been reported. Although S. pneumoniae remains the most common, Legionella, S. aureus, and gram-negative bacilli including P. aeruginosa are identified with increasing frequency and warrant consideration for empirical coverage (Table 24-3). Those patients with CAP admitted to the ICU are by definition severely ill, and available data point to improved outcomes with use of antibiotic combinations in these patients; thus, monotherapy with fluoroquinolones or beta-lactams is not indicated. All patients should receive antibiotic combinations active against the most likely offending organisms. Presence of certain comorbid conditions such as bronchiectasis and severe COPD and other factors such as frequent antibiotic and systemic corticosteroid use are associated with increased probability of infection with P. aeruginosa; in such instances, empirical treatment effective against this pathogen is warranted. If S. aureus infection is suspected, coverage for community-acquired MRSA with agents such as vancomycin or linezolid should be provided. If necrotizing pneumonia is evident, clindamycin should be added to the regimen.
Table 24-3 Empirical Coverage for Uncommon Pathogens Causing Community-Acquired Pneumonia
| Suspected Pathogen | Special Considerations |
|---|---|
| Pseudomonas | |
| Community-acquired methicillin-resistant Staphylococcus aureus |
Data from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults, Clin Infect Dis 44(Suppl 2):S27–S72, 2007.
Route and Duration of Therapy; Hospital Discharge
Most patients with CAP severe enough to warrant hospital admission are treated with intravenous antibiotics. Switching to oral therapy should be considered once the patient has achieved clinical stability (Box 24-3), is able to tolerate oral medications, and has a functioning gastrointestinal tract. In general, either the same agent or same class of antimicrobials should be used for the oral therapy regimen. Early initiation of oral therapy leads to earlier hospital discharge and overall decreased number of adverse events. In-hospital observation before discharge is unnecessary after the switch to oral medications if the patient meets at least four of the five stability criteria (see Box 24-3), is free from comorbid conditions of clinical significance, and has adequate social supports in place.
Box 24-3
Criteria for Clinical Stability in Management of Community-Acquired Pneumonia
 Systolic blood pressure ≥90 mm Hg
Systolic blood pressure ≥90 mm Hg
 Respiratory rate ≤24 breaths/minute
Respiratory rate ≤24 breaths/minute
 Oxyhemoglobin saturation ≥90% or PO2 ≥60 mm Hg on preadmission level of oxygen supplementation
Oxyhemoglobin saturation ≥90% or PO2 ≥60 mm Hg on preadmission level of oxygen supplementation
Modified from Mandell LA, Wunderink RG, Anzueto A, et al: Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults, Clin Infect Dis 44(Suppl 2):S27–S72, 2007.
Complications/Failure to Respond to Therapy
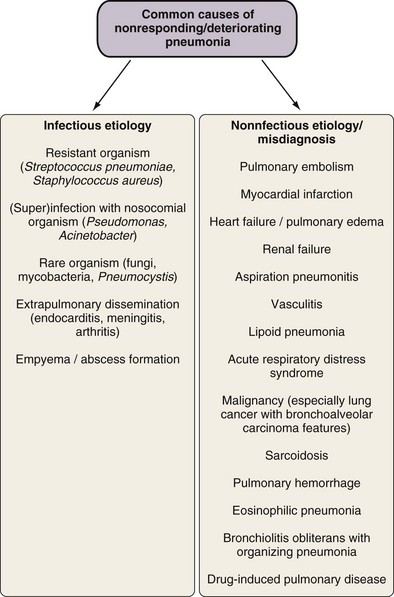
Several factors may potentially lead to lack of response to empirical therapy, including misdiagnosis of CAP, infection or superinfection with resistant organisms, infection dissemination with or without abscess formation, and others (Figure 24-3). However, no apparent cause for lack of response can be identified in up to 30% to 44% of such cases, with suggested reasons including comorbidity and possibly variations in inflammatory response.
Specific Complications
Aspiration Pneumonia
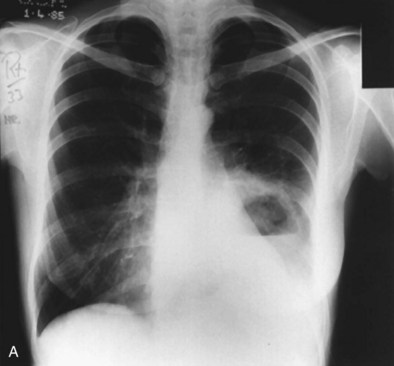
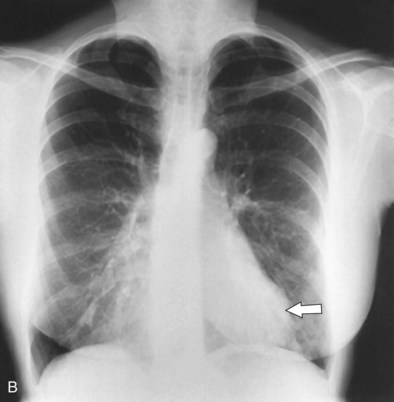
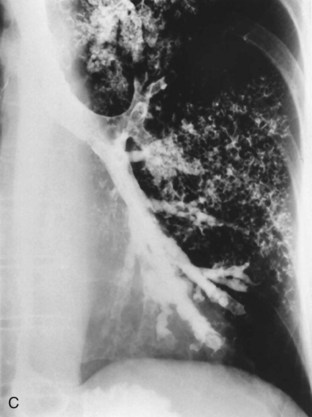
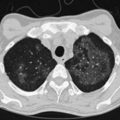
A number of clinical features help distinguish aspiration pneumonia from other CAPs. Aspiration pneumonia tends to have a more insidious course, such that the patient may have an empyema, lung abscess, or necrotizing pneumonia at the time medical care is first sought. The sputum may be putrid because of anaerobic bacteria, and weight loss is common. Chest imaging commonly shows necrotizing infiltrates or multiple abscesses, typically located in dependent regions of the lung (Figure 24-4).
Lung Abscess
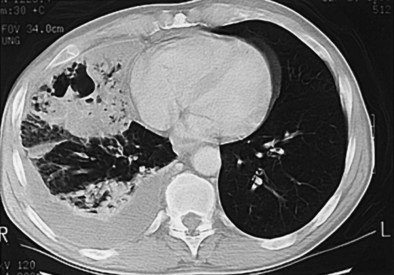
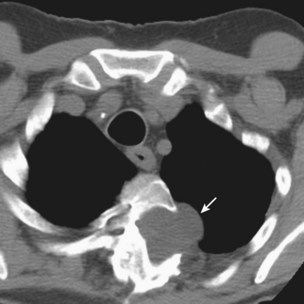
The incidence of pulmonary abscess has decreased over the past decade. Lung abscess is associated with several conditions, including poor dental status or periodontal disease, chronic alcoholism, intravenous drug use, and head and neck cancer. Lung abscess may complicate bronchiectasis (Figure 24-5) and the course of aspiration pneumonia in persons with impaired consciousness, dysphagia and gastroesophageal reflux, or acute or chronic neurologic diseases, but it also may occur with bronchial obstruction by a foreign body or bronchial carcinoma.
Parapneumonic Effusion and Empyema
All pleural fluid specimens should be submitted for Gram stain and culture, because the identification of significant bacterial cultures confirms the diagnosis of infection and aids in antibiotic choice. Unfortunately, approximately 40% of infected pleural effusions are culture negative, and in this situation, biochemical pleural fluid markers (pH, lactate dehydrogenase, white blood cell count, and glucose) are central to establishing a diagnosis. Fluid drainage by means of a chest tube and prolonged antibiotic therapy is necessary in all cases of complicated parapneumonic pleural effusion and empyema. (See Chapter 69 for a more detailed description of these two clinical entities.)
Organizing Pneumonia
Organizing pneumonia, sometimes known as cryptogenic organizing pneumonia (now the preferred designation for bronchiolitis obliterans organizing pneumonia [BOOP]), is a condition in which an organizing inflammatory exudate with fibroblast proliferation occurs after an episode of pneumonia. The consolidation often is patchy and may be fleeting. Organizing pneumonia subsequent to a bacterial infection is suggested when a residual consolidation (often fleeting) is observed despite adequate antibiotic treatment. Investigation includes examination of sputum or bronchial washings to exclude infection. CT may help to visualize the consolidation and exclude other causes. The definitive investigation is an open lung biopsy showing the typical histologic changes, which along with highly suggestive clinical findings constitute sufficient evidence for a confident diagnosis. A course of steroids usually leads to resolution. When the steroids are stopped, however, relapse may occur; in such cases, treatment for several months may be necessary (see Chapter 50).
Bronchiectasis
Permanent dilatation of the bronchus can be a sequela of severe pneumonia, causing localized bronchiectasis. CT scanning during or after an episode of acute pneumonia may show bronchial dilatation, so the diagnosis cannot be made with confidence until after the pneumonia has completely resolved. The presence of bronchiectasis is suggested by continual cough productive of sputum or recurrent infections in one part of the lung. Investigation consists of sputum examination, when organisms associated with bronchiectasis such as H. influenzae or P. aeruginosa may be isolated. The diagnostic test of choice is a thin-section, high-resolution CT scan. Management consists of postural drainage of the infected lobe and antibiotic treatment for any acute infection. In patients who have coexisting airflow obstruction, treatment with bronchodilators or inhaled steroids may be helpful. (See Chapter 45 for a full description of this clinical entity.)
Prevention
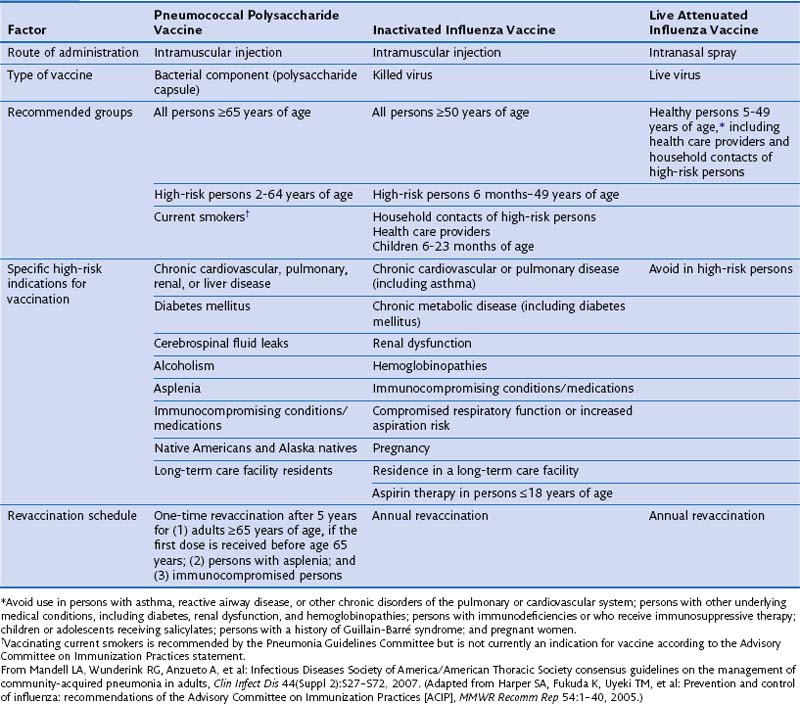
Administration of vaccines targeting S. pneumoniae and influenza viruses and risk factor modification such as smoking cessation, aspiration precautions, and cessation of alcohol abuse constitute the main preventive measures for CAP (Table 24-4). Vaccination status should be assessed at the beginning of the hospitalization, and vaccination may be performed either at discharge or during outpatient follow-up if needed. Vaccination at discharge is preferred in patients in whom compliance with outpatient follow-up care is likely to be unreliable.
Controversies and Pitfalls
Health care–associated pneumonia (HCAP) is a more recent designation for a form of pneumonia acquired in the community but warranting treatment more like that for nosocomial pneumonia, because of risk factors that predispose affected patients to infections by pathogens that usually are found in the local hospital setting (see Chapter 27). Current criteria used to distinguish HCAP from CAP are in question as being too broad in scope, potentially leading to excessive use of broad-spectrum antibiotics. It is likely that refinement of these criteria in the near future will redefine the boundaries between HCAP and CAP.
Acknowledgment
In preparation of this chapter, we retained some material from Chapter 27 on bacterial pneumonias, by Antoine Rabbat and Gérard J. Huchon, in the third edition of this book. The contribution of these authors is gratefully acknowledged.
Bochud PY, Moser F, Erard P, et al. Community-acquired pneumonia. A prospective outpatient study. Medicine (Baltimore). 2001;80:75–87.
Christ-Crain M, Opal SM. Clinical review: the role of biomarkers in the diagnosis and management of community-acquired pneumonia. Crit Care. 2010;14:203.
Feikin DR, Schuchat A, Kolczak M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am J Public Health. 2000;90:223–229.
File TMJr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–141.
Kee C, Palladino S, Kay I, et al. Feasibility of real-time polymerase chain reaction in whole blood to identify Streptococcus pneumoniae in patients with community-acquired pneumonia. Diagn Microbiol Infect Dis. 2008;61:72–75.
Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–iii55.
Mandell LA, Marrie TJ, Grossman RF, et al. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community-Acquired Pneumonia Working Group. Clin Infect Dis. 2000;31:383–421.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72.
Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066.
Waterer GW, Rello J, Wunderink RG. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med. 2011;183:157–164.

 10 billion in Europe. According to British Thoracic Society guidelines, age-standardized rate of hospital admissions due to CAP rose by 34% from 1997 to 2004. CAP also is the most common cause of severe sepsis. Admission to the intensive care unit (ICU) is required in up to 20% of cases of CAP. Because the incidence of pneumonia is substantially higher among elderly persons, it is anticipated that in view of the aging population, the incidence and health care burden of pneumonia will only increase. In the United States, approximately 4.2 million outpatient clinic visits for pneumonia were recorded for 2006, and this number has been increasing ever since. At the same time, despite introduction of novel antimicrobials, imaging modalities, and biomarker testing, mortality attributable to CAP has not changed significantly since the introduction of penicillin.
10 billion in Europe. According to British Thoracic Society guidelines, age-standardized rate of hospital admissions due to CAP rose by 34% from 1997 to 2004. CAP also is the most common cause of severe sepsis. Admission to the intensive care unit (ICU) is required in up to 20% of cases of CAP. Because the incidence of pneumonia is substantially higher among elderly persons, it is anticipated that in view of the aging population, the incidence and health care burden of pneumonia will only increase. In the United States, approximately 4.2 million outpatient clinic visits for pneumonia were recorded for 2006, and this number has been increasing ever since. At the same time, despite introduction of novel antimicrobials, imaging modalities, and biomarker testing, mortality attributable to CAP has not changed significantly since the introduction of penicillin.