CHAPTER 81 COMMON ERRORS IN TRAUMA CARE
Errors in management occur frequently in medicine. A recent Institute of Medicine report estimated that 44,000–98,000 deaths each year were caused by medical errors. This represents more deaths in the United States each year than are caused by breast cancer or AIDS. Most of these errors occur in low-intensity, nonemergent scenarios. Obviously, trauma care is a much more difficult setting to perform in an errorless fashion. Care of injured patients must occur in an emergent fashion. Decisions must be made rapidly, based on limited information. In many instances, interventions must be initiated before a complete evaluation is performed. Frequently, the history of the mechanism of injury is obscure, or injured patients involved with criminal activities may mislead the trauma team. Moreover, injured patients are frequently unresponsive, have a decreased level of consciousness, or are uncooperative due to intoxication. Seriously injured patients frequently present with multiple injuries that require the involvement of multiple providers. Routinely, numerous surgeons, surgical subspecialists, emergency medicine physicians, and residents must accurately communicate and coordinate care for an optimal outcome. The list of potential causes for errors in trauma care is infinite. Because of these many difficulties, the surgeon who cares for trauma must pay particular attention to the factors that cause errors in management and should make every effort to prevent these errors. In this chapter, a number of common errors in the management of injured patients are discussed. This discussion includes missed diaphragmatic injury, failure to recognize extremity compartment syndrome, failure to prevent or treat abdominal compartment syndrome, delayed damage-control laparotomy, missed hollow viscus injuries, failure to perform a tertiary survey, futile or emergency department thoracotomy, and the dogma of mandatory colostomy.
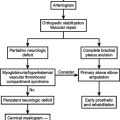
MISSED DIAPHRAGMATIC INJURY
A number of physical findings should increase the surgeon’s suspicion for diaphragmatic injury. These include penetrating thoracoabdominal injury or blunt trauma involving injuries to the abdomen or chest. Unfortunately, physical examination is unreliable in patients with diaphragmatic injury. In fact, 20%–40% of patients with isolated diaphragmatic injury have an initially normal physical examination. A number of noninvasive diagnostic adjuncts are routinely used in the evaluation of trauma patients. These include chest x-ray, focused assessment with sonography for trauma (FAST), and computed tomography (CT). Unfortunately, all of these diagnostic modalities used either alone or in combination are unreliable for the diagnosis of diaphragmatic injury. Additionally, diagnostic peritoneal lavage is nonspecific and fails to diagnose isolated diaphragmatic injury in a large percentage of cases. The only methods that evaluate the diaphragm with certainty are invasive operative procedures that directly visualize the diaphragm.
ABDOMINAL COMPARTMENT SYNDROME
The diagnosis of ACS is based on clinical parameters and the measurement of IAP. Findings of oliguria (<0.5 ml/kg/hr), hypoxia (oxygen delivery <600 ml/min/m2) with increasing airway pressures (peak >45 cm H2O), SVR greater than 1000, and a distended abdomen, are all suggestive of ACS. Two methods of IAP measurement are clinically useful: intragastric and intravesicular. The latter is the most widely employed. First described by Kron et al., the technique involves clamping the bladder catheter, followed by the injection of 50–100 ml of sterile saline into the bladder. The catheter is then connected to a pressure manometer.
THE MYTH OF MANDATORY COLOSTOMY
Interpersonal violence remains prevalent in North America. The colon is the second most commonly injured abdominal organ in penetrating trauma (Table 1). Multiple associated injuries and infectious complications are common. When these patients present, the trauma surgeon must decide to primarily repair the colon, resect and perform an anastomosis, or create a colostomy. There is continuing debate as to the optimal treatment for penetrating colon injuries. During World War I, many patients underwent primary repair. Colostomy was reserved for extensive injuries or left colon injuries. No matter what treatment was employed, there was a high mortality rate. These dismal survival statistics occurred in an era of delayed treatment, inadequate volume resuscitation, no blood transfusions or antibiotics, and the absence of critical care. The dogma of mandatory colostomy began after the U.S. Army Surgeon General W. H. Ogilvie published a letter in 1943 that required the performance of colostomy for colon injuries:
Table 1 American Association for the Surgery of Trauma (AAST) Colon Injury Scale
| Grade I | Hematoma or contusion without devascularization |
| Grade I | Laceration: partial thickness without perforation |
| Grade II | Laceration <50% of circumference |
| Grade III | Laceration >50% of circumference |
| Grade IV | Transection of colon |
| Grade V | Transection of colon with segmental tissue loss |
MISSED HOLLOW VISCUS INJURY
In 1999, Fang et al. examined patients with a delayed diagnosis of small bowel injury. Their retrospective review of 111 blunt trauma victims with small bowel injury showed no difference in mortality if the injury was treated within 24 hours. But patients with a delay in diagnosis of greater than 4 hours had an increased incidence of wound infection, abscess formation, enterocutaneous fistula, wound dehiscence, and sepsis. Those whose injury was repaired after 24 hours averaged a 20-day increase in hospital stay and a 6-day delay in initiation of oral intake when compared with those whose injury was repaired between 12 and 24 hours. Fakhry et al. found an increase in mortality and morbidity with diagnostic delays in small bowel injuries. Mortality was 2% when the injury was treated within 8 hours, 9.1% when treated between 8 and 16 hours, 16.7% when treated between 16 and 24 hours, and 30.8% when delayed more than 24 hours.
FUTILE RESUSCITATIVE THORACOTOMY
Some victims of penetrating trauma clearly may benefit from ED thoracotomy. Proper selection of potentially salvageable patients is key. Powell found that the duration of CPR was critical. All survivors of ED thoracotomy had CPR for 15 minutes or less. Any penetrating trauma patient with prolonged CPR (>15 minutes) should be pronounced dead upon arrival.
Biffl WL, Harrington DT, Cioffi WG. Implementation of a trauma survey decreases missed injuries. J Trauma. 2003;54(1):38-43.
Burch JM, Moore EE, Moore FA, Franciose R. The abdominal compartment syndrome. Surg Clin North Am. 1996;76:833-842.
Chappuis CW, Frey DJ, Dietzen CD, et al. Management of penetrating colon injuries: a randomized controlled trial. Ann Surg. 1991;213:492-498.
Eddy V, Nunn C, Morris JAJr. Abdominal compartment syndrome: the Nashville experience. Surg Clin North Am. 1997;77:801-812.
Enderson BL, Reath DB, Meadors J, et al. The tertiary survey: a prospective study of missed injury. J Trauma. 1990;30:666-669.
Grove L, et al. Emergency thoracotomy: appropriate use in the resuscitation of trauma patients. Am Surg. 2002;68:313-317.
Hirshberg A, Walden R. Damage control for abdominal trauma. Surg Clin North Am. 1997;77(4):813-820.
Houshian S, Larsen MS, Holm C. Missed injuries in a level I trauma center. J Trauma. 2002;52:715-719.
Janjua KJ, Sugrue M, Deane SA. Prospective evaluation of early missed injuries and the role of tertiary trauma survey. J Trauma. 1998;44:1000-1006.
Moore AFK, Hargest R, Martin M, Delicata RJ. Intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg. 2004;91:1102-1110.
Ogilvie WH. Abdominal wounds in the Western Desert. Surg Gynecol Obstet. 1944;78:225.
Powell DW, et al. Is emergency department resuscitative thoracotomy futile care for the critically injured patient requiring prehospital cardiopulmonary resuscitation? J Am Coll Surg. 2004;199(2):211-215.
Rhee P, et al. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg. 2000;190(3):288-298.
Rotondo M, Schwab C, McGonigal M, et al. Damage control: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375-382.
Saggi BH, Sugerman HJ, Ivatury RR, Bloomfield GL. Abdominal compartment syndrome. J Trauma. 1998;45(3):597-609.
Schreiber M. Damage control surgery. Crit Care Clin. 2004;20:101-118.
Shapiro MB, Jenkins DH, Schwab CW, et al. Damage control: collective review. J Trauma. 2000;49(5):969-978.
Stone H, Strom P, Mullins R. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532-535.
Sugrue M, D’Amours SK, Joshipura M. Damage control surgery and the abdomen. Injury. 2004;35:642-648.
Woodall JP, Oschner A. The management of perforating injuries of the colon and rectum in civilian practice. Surgery. 1951;29:305.
Working Group, Ad Hoc Subcommittee on Outcomes, American College of Surgeons, Committee on Trauma. Practice management guidelines for emergency department thoracotomy. J Am Coll Surg. 2001;193(3):303-309.