Chapter 48 Colon Cancer
Etiology and Epidemiology
In 2011 the American Cancer Society estimated that 141,210 patients were diagnosed with colorectal cancer and 49,380 deaths occurred.1 Although this malignancy may be linked to chemical carcinogens within the bowel lumen, it is not established whether these are ingested, the result of chemical activation of substances in the fecal stream, or a bacterial byproduct.2 Environmental and dietary factors have been established as contributing to colorectal cancer. Factors shown to increase the risk of developing this disease include increasing age, race, male sex, family history of colorectal cancer, increasing height, increasing body mass index, processed meat intake, excessive alcohol intake, and low folate consumption. Of these risk factors, only increasing age, male sex, and excessive alcohol use have been found to be associated with rectal cancer.3 The value of consumption of fruits and vegetables in the prevention of colon and rectal cancer remains controversial, although some studies have suggested that these associations have been overstated.4 Contemporary prospective and randomized data do not support a high fiber diet in the prevention of colorectal cancer.5 Other studies have suggested that nonsteroidal anti-inflammatory drugs may reduce the risk of colorectal cancer. The role of chemopreventive agents (carotenoids, polyamine inhibitors, aspirin and other nonsteroidal anti-inflammatory drugs) in colorectal cancer is under active investigation.
Prevention and Early Detection
Neoplastic polyps, including tubular adenomas, villous adenomas, and tubulovillous adenomas, are precursors of colon cancers. Most colorectal cancers arise from preexisting polyps.6 Patients with neoplastic polyps should be considered at high risk for large bowel cancer, and polypectomy may reduce this risk. With the availability of the flexible colonoscope and endoscopic polypectomy, polyps can be removed at a premalignant stage and patients observed closely.
Screening
Because the cumulative lifetime risk of developing colorectal cancer in the United States is about 6%,7 screening programs for the general population have been undertaken. The goal of screening is to detect preinvasive polyps or early invasive cancer. The presence of polyps increases the risk for cancer to approximately 15%. Data from programs in which proctoscopy is performed annually suggests that routinely scheduled polypectomy reduces the development of subsequent bowel cancer by 80% or more.2 The American Cancer Society has recommended that screening begin at age 50 in the average risk patient by (1) annual fecal occult blood test, fecal immunochemical test, and/or flexible sigmoidoscopy every 5 years; (2) double-contrast barium enema every 5 years; (3) colonoscopy every 10 years; or (4) CT colonography every 5 years. The interval for stool DNA tests has not been firmly established. Intensive surveillance is recommended for patients at high risk (patients with adenomatous polyps, history of colorectal cancer, a first-degree relative diagnosed with colorectal cancer or adenomas, inflammatory bowel disease, or high risk because of family history or genetic testing). CT colonography as well as genetic fecal testing continue to be studied as potential screening tools. Although screening methods can detect colorectal cancer at an early stage, less than 40% of patients are diagnosed with early disease, likely reflecting low rates of disease awareness as well as the infrequency of screening in eligible candidates.7,8
Biologic Characteristics/Molecular Biology
Microsatellites are mutated short-repeat DNA sequences usually consisting of one to five nucleotides. Microsatellite “instability” is found in a majority of patients with hereditary nonpolyposis colorectal cancer (HNPCC) as well as a minority of patients with sporadic colorectal cancers. It has been shown that this instability occurs in patients with mutations in genes encoding enzymes that repair DNA replication errors. These defects in mismatch repair lead to high-frequency microsatellite instability. Studies have suggested that patients with tumors possessing a high-frequency of microsatellite instability have a more favorable outcome and develop fewer metastases.9 Further elucidation of the genetic pathways in the development of colorectal cancer remains an active area of investigation and may ultimately impact therapy for this disease.
Clinical Manifestations, Patient Evaluation, and Staging
The clinical presentation of colon cancer is determined largely by site of the tumor. Cancers of the right colon are often exophytic and commonly associated with iron-deficiency anemia owing to occult blood loss. During the past 20 years the relative incidence of cancer of the right colon has increased and accounts for one third of cancers of the large bowel. Many of these are diagnosed late.2 Cancers of the left colon and sigmoid colon are often deeply invasive, annular, and accompanied by obstruction and rectal bleeding.
For patients undergoing resection, preoperative evaluation should include pathologic confirmation of adenocarcinoma, colonoscopy to evaluate the extent of tumor and rule out synchronous primary tumors (occurring in 3% to 5%), baseline blood cell counts with liver function tests, and carcinoembryonic antigen levels. Patients should undergo chest, abdominal, and pelvic CT to evaluate the extent of locoregional disease as well as the presence or absence of distant metastases. PET, MRI, and ultrasonography may be useful in evaluating patients with oligometastatic disease who may be appropriate candidates for resection of metastatic disease with curative intent. A diagnostic algorithm of the management of patients with potentially resectable colon cancer is shown in Figure 48-1.
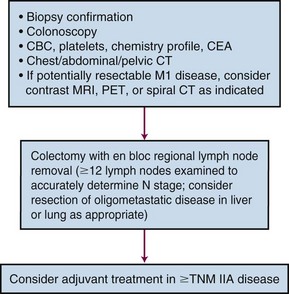
Figure 48-1 Diagnostic algorithm of the management of patients with potentially resectable colon cancer.
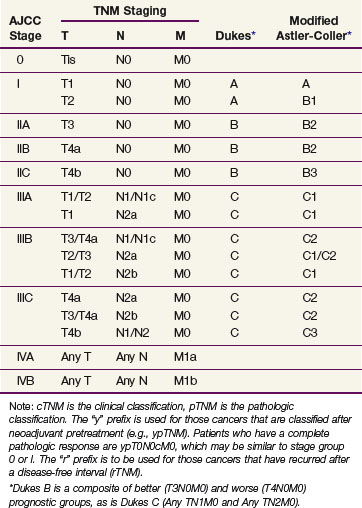
Prognostic factors influencing survival in colon cancer patients include depth of tumor invasion into and beyond the bowel wall, the number of involved regional lymph nodes, as well as the presence or absence of distant metastases. The TNM system of the American Joint Committee on Cancer (AJCC) can be used as a clinical (preoperative) or postoperative staging system (Tables 48-1 and 48-2). Additionally, site-specific prognostic factors have been identified and included in the AJCC staging (Table 48-3).
TABLE 48-1 American Joint Committee on Cancer TNM Staging for Colorectal Cancer (2010)
| Stage | Description |
|---|---|
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: intraepithelial or invasion of lamina propria |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades through the muscularis propria into pericolic tissues |
| T4a | Tumor penetrates to the surface of the visceral peritoneum* |
| T4b | Tumor is adherent to or directly invades other organs or structures*,† |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in one to three regional lymph nodes |
| N1a | Metastasis in one regional lymph node |
| N1b | Metastasis in two to three regional lymph nodes |
| N1c | Tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis |
| N2 | Metastasis in four or more regional lymph nodes |
| N2a | Metastasis in four to six regional lymph nodes |
| N2b | Metastasis in seven or more regional lymph nodes |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed. |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confined to one organ or site (e.g., liver, lung, ovary, nonregional node) |
| M1b | Metastases in more than one organ/site or the peritoneum |
Note: A satellite peritumoral nodule in the pericolorectal adipose tissue of a primary carcinoma without histologic evidence of residual lymph node in the nodule may represent discontinuous spread, venous invasion with extravascular spread (V1/2), or a totally replaced lymph node (N1/2). Replaced nodes should be counted separately as positive nodes in the N category, whereas discontinuous spread or venous invasion should be classified or counted in the site-specific factor category of Tumor Deposit (TD).
* Direct invasion in stage T4 includes invasion of other organs or segments of the colorectum by way of the serosa, as confirmed by microscopic examination (e.g., invasion of the sigmoid by a carcinoma of the cecum). For cancers in a retroperitoneal or subperitoneal location, direct invasion involves other organs or structures by virtue of extension beyond the muscularis propria (e.g., a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall or a mid or distal rectal cancer with invasion of the prostate with invasion of the prostate, seminal vesicles, cervix or vagina).
† Tumor grossly adherent to other organs or structures is classified as cT4b. However, if no tumor is present in the adhesion microscopically, the classification should be pT1 to pT4a depending on the anatomic depth of wall invasion. The V and L classifications should be used to identify the presence or absence of vascular or lymphatic invasion where the pN site-specific factor should be used for perineural invasion.
From Edge SB, Byrd DR, Compton CC, et al, editors: Colon and rectum. In American joint committee on cancer staging manual, ed 7, New York, 2010, Springer.
TABLE 48-3 Site-Specific Prognostic Factors for Colorectal Cancer
From Edge SB, Byrd DR, Compton CC, et al, editors: Colon and rectum. In American joint committee on cancer staging manual, ed 7, New York, 2010, Springer.
Primary Therapy
Surgery
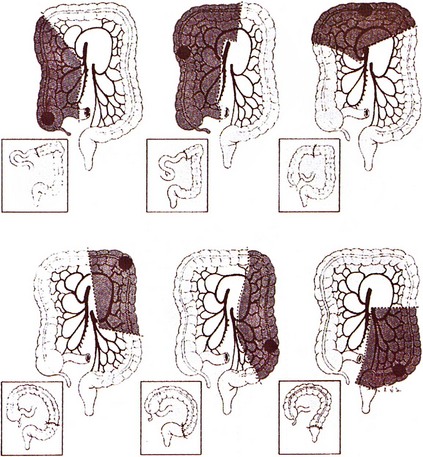
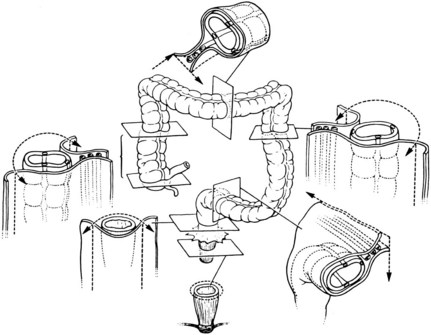
Surgery remains the primary treatment of colonic tumors. Resection with curative intent is possible in approximately 75% of patients.2 Surgical resection of primary colon cancer is based on the anatomy and mechanisms by which this disease spreads. Adenocarcinomas of the colon may grow by direct extension into the lymphatics of the submucosa and bowel wall. To avoid cutting across tumor in intramural lymphatics, sufficient lengths of bowel must be resected proximal and distal to the primary cancer. Colon cancer often extends through the serosa into mesenteric lymphatics that run along the blood vessels draining into the portal watershed at the root of the mesentery. Resection includes removal of the major lymphatic drainage system in the mesentery. Because anatomic resections designed to include named blood vessels also include the draining lymphatics, the boundaries for resecting large bowel cancer are relatively uniform (Fig. 48-2). Right hemicolectomy, transverse colectomy, left hemicolectomy, and sigmoid resection are performed by adherence to surgical oncologic principles without major sacrifice of large bowel function.
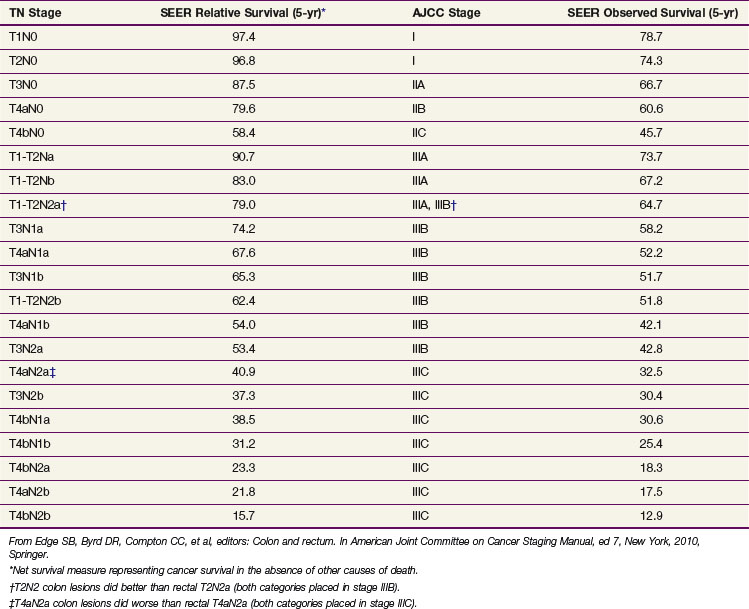
Resection results in excellent cure rates for lesions limited to the bowel wall with negative nodes (estimated 5-year survival, 97% for T1N0; 85% to 90% for T2N0 disease). With a single high-risk feature of extension beyond the colonic wall (T3 to T4, N0) or involved nodes (T0 to T2, N+), 5-year survival with surgery falls to 65% to 75% and adjuvant treatment is often indicated. When both high-risk features are present (T3 to T4, N+), 5-year survival with surgery alone drops to approximately 50% (T3N+) and 35% (T4N+) and adjuvant treatment is usually advised. Recent Surveillance, Epidemiology and End Results (SEER) analyses have allowed further refinement of survival outcomes based on the TNM stage (Table 48-4).
Adjuvant Chemotherapy
Initially, prospective randomized trials demonstrated that the addition of combined therapy with 5-FU and leucovorin improves survival for patients with resected stage III disease,10,11 leading to the incorporation of these agents as standard adjuvant therapy for these patients. However, more recently, newer agents have been investigated and have shown further benefit in the adjuvant setting. Capecitabine, an oral 5-FU prodrug, has demonstrated similar overall and disease-free survival rates to therapy with 5-FU/leucovorin in patients with resected stage III colon cancer in a recent randomized trial.12 More recently, final results of a large randomized trial of patients with stage II/III colon cancer undergoing resection with randomization to 5-FU/leucovorin, with or without oxaliplatin, demonstrated a significant improvement in 5-year DFS (67% vs. 73%, p = .003) as well as 6-year OS (76% vs. 78.5%, p = .046). When broken down by stage, this was significant for patients with stage III disease (73% vs. 69%, p = .023). The authors concluded that the addition of oxaliplatin improved 5-year DFS and 6-year OS and should be considered for patients with stage III disease.13 The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial also evaluated the addition of oxaliplatin to adjuvant 5-FU/leucovorin in stage II/III colon cancer patients. The addition of oxaliplatin resulted in a 20% reduction in the risk of recurrence. The authors concluded that the addition of oxaliplatin to 5-FU/leucovorin significantly improved disease-free survival in patients with stage II/III colon cancer.14
In contrast, patients in the PETACC-3 trial with stage II/III colon cancer were randomized to receive adjuvant 5-FU/leucovorin-based chemotherapy, with or without irinotecan. Study results demonstrated that irinotecan-based therapy did not confer a significant improvement in DFS or OS in patients with stage III disease compared with 5-FU/leucovorin alone.15 Similarly, a Cancer and Leukemia Group B (CALGB) study randomized patients with stage III colon cancer to receive bolus 5-FU/leucovorin with or without irinotecan. This study was closed prematurely secondary to an excessive number of treatment-related deaths in patients in the irinotecan study arm. No improvement in DFS or OS was observed.16
Ongoing randomized trials in the United States and Europe are evaluating the role of bevacizumab as well as cetuximab in the adjuvant colon cancer setting. Results of a randomized trial comparing 5-FU, leucovorin, and oxaliplatin with or without bevacizumab in patients with resected stage II/III colon cancer showed that the addition of bevacizumab did not result in a significant improvement in DFS.17
Adjuvant Irradiation With or Without Chemotherapy
Rationale: Patterns of Relapse, Surgery Alone
Because of the documented efficacy of adjuvant chemotherapy as well as the perception by many oncologists that colon (as opposed to rectal) cancer is much more likely to relapse distantly than locally, there has been little evaluation of the efficacy of postoperative irradiation with chemotherapy. The potential indications for adjuvant radiation therapy in colon cancer are based on analyses of patterns of failure after resection18,19 (Table 48-5). Advanced stage predicts for local failure in both colon and rectal cancer; however, local failure in colon cancer also depends on anatomic origin. The ascending and descending colon are considered “anatomically immobile,” and their close proximity to the retroperitoneal tissues often limits wide surgical resection (Fig. 48-3). Limitations in achieving satisfactory circumferential margins increase the risk of residual disease and consequently local failure. In contrast, the midsigmoid and midtransverse colon are relatively “mobile” with a wide mesentery, thus permitting the surgeon to obtain wide margins regardless of extent of disease invasion into the mesentery. Unless there is adjacent organ adherence/invasion by tumor, local failure at these sites is uncommon. Local failure rates for cecal, hepatic/splenic flexure, and proximal/distal sigmoid tumors are variable depending on the amount of mesentery present, tumor extension, and the adequacy of radial margins. When colon cancers adhere to or invade adjacent structures, local failure rates exceed 30% after surgery alone.
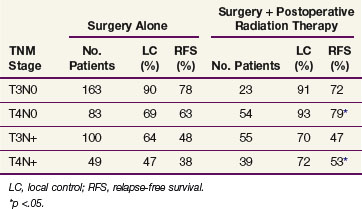
TABLE 48-5 Five-Year Actuarial Local Control and Relapse-Free Survival after Surgery plus Postoperative Radiotherapy versus Surgery Alone, according to Stage: Massachusetts General Hospital Series
Adjuvant Irradiation: Single-Institution Analyses
Until recently, data evaluating the use of adjuvant radiation therapy in high-risk colon cancer patients was limited to single-institution retrospective analyses.20–23 To summarize, these studies have suggested that operative bed failures in high-risk patients undergoing resection alone are at least 30% and that the risk of local failure is reduced by the administration of adjuvant radiation therapy.
Massachusetts General Hospital
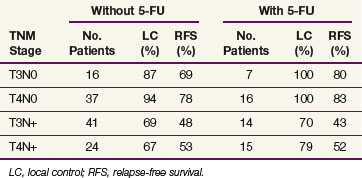
Table 48-5 shows 5-year actuarial local control and relapse-free survival (RFS) in the adjuvant group compared with patients undergoing surgery alone. Local control rates in patients with stage T4N0 and T4N+ disease treated with radiation therapy were 93% and 72%, respectively, versus 69% and 47%, respectively, in patients undergoing surgery alone. Similarly, RFS were 79% and 53%, respectively, in patients with stage T4N0/T4N+ disease undergoing adjuvant radiation versus 63% and 38%, respectively, undergoing surgery alone. No significant outcome differences were observed in patients with stage T3N0 and T3N+ lesions; however, there may be an element of selection bias in the radiation group given most patients were referred because of concerns of adequacy of local control after surgery alone. There was a trend toward improved local control in patients receiving 5-FU (Table 48-6). The rate of acute enteritis in patients receiving irradiation and 5-FU was 16% versus 4% in patients undergoing irradiation only. This rate of enteritis is similar to data from studies of concurrent 5-FU and radiation therapy in rectal cancer. Late bowel complication rates were not increased by concomitant 5-FU administration. The conclusion was that patients with stage T4 tumors or tumors with abscess/fistula formation or margin-positive resection may benefit from postoperative radiation therapy.20
TABLE 48-6 Five-Year Actuarial Local Control and Relapse-Free Survival of Adjuvantly Irradiated Patients Based on Administration of 5-Fluorouracil (5-FU): Massachusetts General Hospital Series
In an updated analysis from this institution, 152 patients with stage T4 tumors received adjuvant irradiation. On pathologic examination, 42 patients had tumors with positive margins. For patients with negative margins the 10-year actuarial local control in T4N0 and T4N+ disease was 78% and 48%, respectively. In patients with node-negative tumors, the 10-year actuarial local control and relapse-free survival rates were 87% and 58%, respectively, compared with 65% and 33%, respectively, in patients with node-positive tumors. For patients with one involved lymph node, local control and relapse-free survival rates were similar to those without nodal involvement; however, with increasing numbers of nodes involved, survival steadily decreased.23 The authors’ current policy is to consider adjuvant tumor bed irradiation in patients with tumors (1) invading adjoining structures, (2) when complicated by perforation or fistula, and (3) when incomplete excision is performed. Patients are generally given continuous 5-FU (225 mg/m2/24 h) or capecitabine (825 mg/m2 bid) 5 days per week throughout the course of radiation therapy.
Mayo Clinic
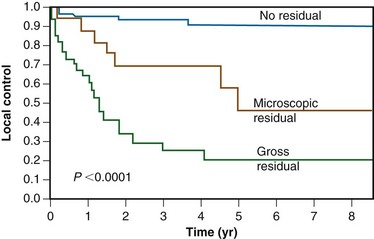
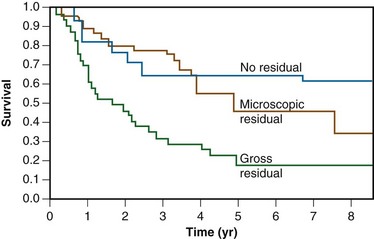
A report from the Mayo Clinic described a series of 103 patients receiving radiation therapy after surgery for locally advanced colon cancer. Microscopic and gross residual disease was present in 18 and 35 patients, respectively. Over 90% of patients had stage T4, N0 to N+ disease. A median dose of 50.4 Gy was delivered through multiple-field techniques and most patients received concurrent 5-FU–based chemotherapy. Eleven patients received an intraoperative “boost” of 10 to 20 Gy. The 5-year actuarial overall local control rate was 40%. Patients with margin negative tumors had a 5-year local control rate of 90%, compared with 46% for patients with microscopic residual tumor and 21% with gross residual tumor (Fig. 48-4). In patients with residual disease, local control rates in patients undergoing intraoperative boost irradiation were 89% compared with 18% in patients undergoing external irradiation alone (Fig. 48-5). Similarly, 5-year survival rates were improved in patients undergoing margin negative resection (66%) compared with those with microscopic residual (47%) or gross residual (23%) disease (Fig. 48-6). Additionally, patients undergoing intraoperative boost demonstrated improved survival (76% vs. 26%) (Fig. 48-7).
University of Florida Medical Center
A report from the University of Florida Medical Center of patients with locally advanced but completely resected colon cancers receiving adjuvant radiation therapy reported a local control rate of 88%, similar to the 90% reported at the Mayo Clinic in patients who had completely resected tumors.21 In addition, there appeared to be a dose-response relationship to local control. The 5-year rate of local control was 96% for patients receiving 50 to 55 Gy versus 76% for patients receiving less than 50 Gy (p = .0095).
Phase III Intergroup Trial
The initial trial accrual goal was 700 patients; however, the study was closed in 1996 owing to poor accrual (222 patients; 189 evaluable). Therefore total accrual was less than one third of initial goals and thus decreased statistical power to detect any differences between the groups. Nonetheless, no difference in OS or DFS was seen between the two groups. The 5-year OS rate of patients receiving chemotherapy only was 62% versus 58% for patients randomized to chemoirradiation (p >.50). Local recurrence rates were identical in both study arms (18 patients each). Grade 3/4 hematologic toxicity was higher in patients receiving radiation therapy. Interpretation of study results was handicapped by decreased statistical power, high ineligibility rates, and lack of surgical clips and/or preoperative imaging to assist the definition of appropriate EBRT fields in a high percentage of patients. No definitive conclusions can be made regarding the efficacy of routine postoperative irradiation with 5-FU and levamisole based on this study; however, this study provides no data supporting its routine use.24
Adjuvant Hepatic and Whole Abdomen Irradiation
In addition to local failure, patterns of failure analyses have shown that hepatic metastases are common in patients with locally advanced colon carcinoma. As a result, investigators have examined the efficacy of hepatic irradiation after resection in high-risk patients. The only randomized, prospective trial evaluating adjuvant hepatic irradiation was performed by the Gastrointestinal Tumor Study Group.25 Three hundred patients with resected, margin-negative Dukes B2/C colon cancer were randomized to observation only or adjuvant hepatic irradiation with concurrent 5-FU given in bolus fashion on week 1. Radiation was administered to the liver at 150 cGy per fraction to a total dose of 2100 cGy. After hepatic irradiation, patients received further 5-FU. The combination of 5-FU and hepatic irradiation did not improve OS or DFS.
Relapse within the peritoneal cavity is also commonly observed in patients undergoing resection of locally advanced colon cancer. Investigators have reported outcomes and toxicities after adjuvant whole abdominal irradiation in high-risk patients. The Southwest Oncology Group (SWOG) reported the results of a pilot study evaluating 41 patients with resected T3, N1 to N2, M0 colon cancer treated with continuous infusion of 5-FU (200 mg/m2/24 hr) with concomitant whole abdominal irradiation. Patients received 30 Gy given at 1 Gy per fraction followed by a 16-Gy boost to the tumor bed at 1.6 Gy per fraction. Further 5-FU therapy was administered after completion of radiation therapy. Five-year DFS and OS estimates were 58% and 67%, respectively. Patients with tumors exhibiting more than four lymph nodes involved experienced 5-year DFS and OS of 55% and 74%, respectively. Grade 3 and 4 toxicity was observed in 17% and 7% of patients, respectively. When compared with similarly staged patients from previous Intergroup trials treated with 5-FU/levamasole only, these survival results appeared favorable. The authors recommended that continuous infusion 5-FU and whole-abdominal irradiation with tumor bed boost should be studied in a randomized trial.26
Estes and associates27 reported a pattern of failure analysis in patients with T3N+ colon cancer treated with 5-FU/levamisole chemotherapy only (Intergroup), combined chemotherapy/whole-abdominal irradiation (SWOG), and patients undergoing surgery only (from the previously described study from the Massachusetts General Hospital). Their analysis showed 5-FU/levamisole reduced the rates of lung metastases but was less effective at preventing local and peritoneal recurrence. In contrast, patients receiving whole-abdominal irradiation with continuous 5-FU experienced a 12% tumor bed relapse rate, 22% hepatic relapse rate, and 15% peritoneal relapse rate, all of which were superior to the results of patients who did not receive irradiation. However, the use of whole-abdominal irradiation should be considered investigational.
Locally Advanced Disease and Palliation
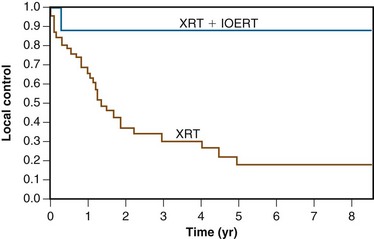
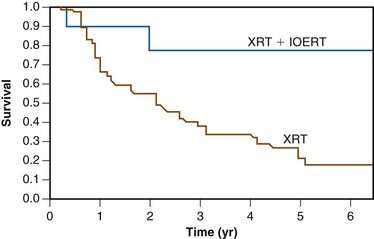
As previously described, the Mayo Clinic analyzed the results of 103 patients with advanced colonic carcinoma (microscopic or gross residual tumor [n = 53]; margin-negative tumors [n = 50]) treated by EBRT alone or in conjunction with intraoperative electron radiation therapy (IOERT).22 For patients with either microscopic or gross residual disease, local failure occurred in 11% of patients receiving IOERT plus EBRT versus 82% of patients receiving EBRT only (p = .02) (see Fig. 48-5). The 5-year actuarial survival rate was 66% for patients with no residual disease, 47% for patients with microscopic residual disease, and 23% for patients with gross residual disease (p = .0009) (see Fig. 48-6). The 5-year survival rate in patients with residual disease was 76% for patients receiving IOERT and 26% for patients receiving EBRT alone (p = .04) (see Fig. 48-7).
For patients with metastatic disease, 5-FU–based chemotherapy is usually administered. Prospective randomized trials have shown that use of multiple agents improves survival in patients with metastatic colorectal cancer. Saltz and colleagues28 reported the results of a three-arm randomized trial comparing (1) irinotecan, 5-FU, and leucovorin, (2) 5-FU/leucovorin, or (3) irinotecan alone. Patients receiving irinotecan, 5-FU, and leucovorin had an improved survival (median survival 14.8 vs. 12.6 months, p = .04) and response rate (39% vs. 21%, p <.001) compared with 5-FU and leucovorin alone. The incidence of grade 3 or higher diarrhea was significantly higher with the three-drug regimen. Hurwitz and associates29 reported a randomized trial comparing irinotecan, 5-FU, and leucovorin with or without bevacizumab, a monoclonal antibody directed against vascular endothelial growth factor. Median survival was significantly improved in the bevacizumab arm of the study (20.3 vs. 15.6 months, p <.001). Additionally, response rates were improved with bevacizumab (45% vs. 35%, p = .004). Cunningham and colleagues30 randomized 329 patients with metastatic colorectal cancer refractory to irinotecan-based chemotherapy regimens to receive cetuximab (a monoclonal antibody directed against the epidermal growth factor receptor [EGFR]) or cetuximab with irinotecan. Response rates in patients receiving combination therapy were significantly higher (23% vs. 11%, p = .007) as was median time to progression (4.1 vs. 1.5 months, p <.001). No difference in survival was observed.
A study by Goldberg and associates31 randomized 795 patients with previously untreated, metastatic colorectal cancer to receive (1) irinotecan, 5-FU, and leucovorin, (2) oxaliplatin, 5-FU, and leucovorin, or (3) irinotecan and oxaliplatin. Patients receiving oxaliplatin, 5-FU, and leucovorin had an improved median survival compared with those receiving irinotecan, 5-FU, and leucovorin or irinotecan and oxaliplatin (19.5 vs. 15 vs. 17.4 months; p <.05 for oxiliplatin-containing regimens vs. irinotecan-only regimen). Response rates in patients receiving 5-FU, leucovorin, and oxaliplatin were significantly higher than those receiving irinotecan, 5-FU, and leucovorin or irinotecan with oxaliplatin (45% vs. 31% vs. 35%, p <.05).
Another trial randomized patients with metastatic disease to receive irinotecan, 5-fluorouracil and leucovorin, with or without the EGFR inhibitor cetuximab.32 A significant improvement in progression-free (PFS) but not OS was seen in patients receiving cetuximab, with the benefit of cetuximab limited to patients with KRAS wild-type tumors. Results of a randomized trial comparing the combination of 5-FU, leucovorin, and oxaliplatin, with or without the anti-EGFR antibody panitumumab again showed an improvement in PFS (9.6 vs. 8 months, p = .02) in patients receiving panitumumab. KRAS wild-type tumors were again found to respond better to EGFR-based therapy.33 In contrast, a randomized study evaluating the combination of capecitabine, oxaliplatin, and bevacizumab, with or without cetuximab, demonstrated a significantly shorter PFS and inferior quality of life in patients receiving cetuximab, with KRAS mutational status again predicting response in patients receiving cetuximab.34 A similarly designed randomized trial evaluated the combination of chemotherapy plus bevacizumab, with or without panitumumab, in metastatic colorectal cancer patients; there was increased toxicity and decreased PFS in patients receiving panitumumab.35 These trial results suggest that dual antibody therapy may not be appropriate for the first-line treatment of metastatic colorectal cancer. Varying combinations of these drugs as well as other novel agents remain the focus of ongoing investigation in both metastatic and nonmetastatic settings.
Irradiation Techniques
Treatment field design in colon cancer is based on patterns of failure data. As is true in the treatment of rectal carcinoma, great care must be taken in the design of postoperative treatment of adenocarcinoma of the colon. Field arrangement will vary depending on the site of the primary disease as well as areas judged to be at high risk for local recurrence.36 Patient positioning (supine, prone, decubitus) should be considered in planning. Small bowel is often a dose-limiting structure in this therapy, and it is often advantageous to position patients in the right or left decubitus position for at least a portion of their treatment, allowing displacement of the small bowel away from the treatment field. Immobilization devices may improve reproducibility. A small-bowel series defines small bowel volume within the treatment field. It may be useful to compare films in both the decubitus and supine positions to determine the actual amount of small bowel displacement. CT-based planning facilitates definition of the tumor bed, determining beam orientation, as well as estimating the volume of small bowel included within the treatment fields. As in other abdominal malignancies, a portion of one kidney may be irradiated. Unilateral renal irradiation results in minimal long-term clinical sequelae, assuming baseline function in the contralateral kidney is normal.37
The total radiation dose used in the adjuvant treatment of colon carcinoma depends on the volume of suspected residual disease and tolerance constraints of surrounding normal tissue. Generally, an initial dose of 45 Gy in 25 fractions of 1.8 Gy per fraction is delivered through larger fields to the primary tumor and at-risk tissues. Reduced fields may be treated to 50 Gy if only a small portion of small bowel is included. For patients with stage T4 tumors, the general goal is to treat the tumor bed to a total dose of 54 to 60 Gy. Any treatment beyond 50 Gy generally mandates exclusion of all small bowel from the field. Spinal cord dose should be limited to 45 Gy. In addition, at least two thirds of one functional kidney should receive no more than 18 to 20 Gy and at least two thirds of the total liver volume should not receive more than 30 Gy. In a Mayo Clinic analysis, small bowel obstruction rates were lower when more than two treatment fields were used, and attempts should be made to implement multiple-field techniques, which is aided by CT-based planning.38 Three-dimensional treatment planning as well as the use of intensity-modulated radiation techniques may facilitate small bowel as well as other normal organ sparing.
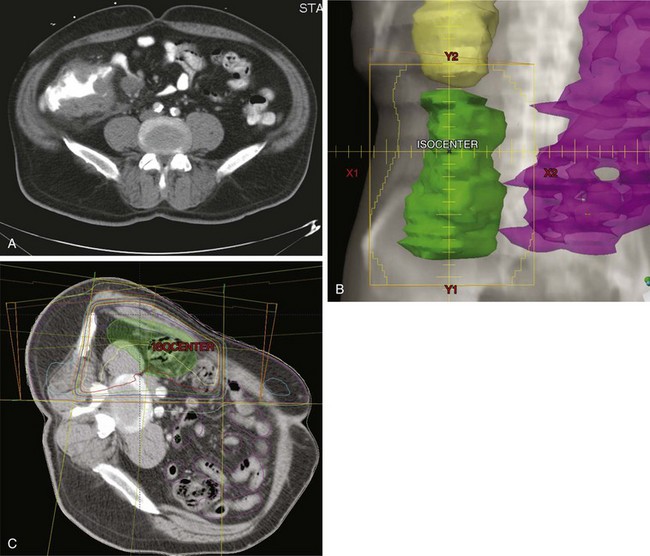
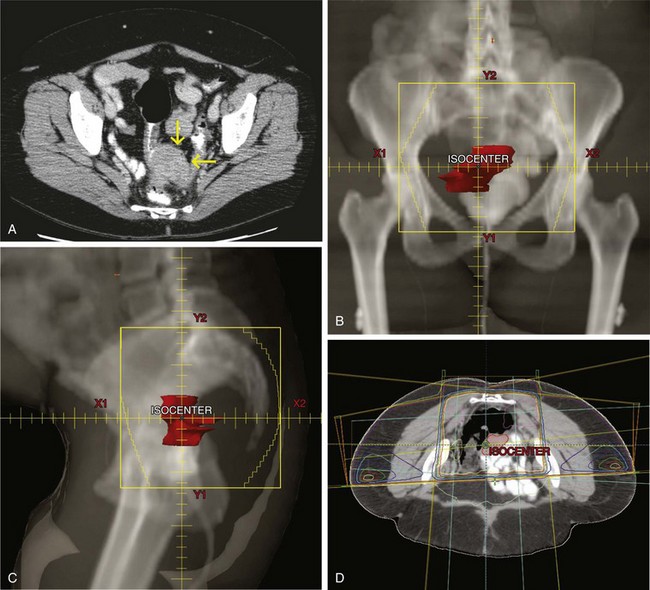
Generally, the primary tumor site should be covered with a 4- to 5-cm margin proximally and distally with a 3- to 4-cm margin medially and laterally to cover areas of potential residual disease. The nodal basins in the mesentery beyond surgical margins are usually not treated because satisfactory margin clearance is obtained in these sites. An exception to this may be tumors of the right colon where both small bowel and right colon are supplied by ileocolic vessels, limiting the extent of resection. In some instances, treatment of the para-aortic nodes may be indicated, particularly with extensive retroperitoneal involvement by tumor. Treatment of proximal mesenteric nodes may be appropriate if nodes adjacent to the surgical or resection margin are involved. Figures 48-8 and 48-9 show examples of locally advanced ascending and sigmoid carcinomas with their respective treatment fields, including isodose distributions. In many situations it may be appropriate to exclude treatment of para-aortic nodal basins, based on operative and pathologic findings.
Treatment Algorithm, Conclusions, and Future Possibilities
The value of adjuvant postoperative irradiation combined with systemic therapy for patients at high-risk for local relapse is unlikely ever to be further addressed in a definitive randomized trial. Treatment recommendations should be made on a case-by-case basis with existing data in the setting of an informed consent. A potential treatment algorithm for these patients is shown in Table 48-7 and reflects the personal preference of the authors.
TABLE 48-7 Treatment Algorithm: Approach to Therapy for Colon Cancer
The use of intraoperative irradiation as a supplement to EBRT in certain stage T4 tumors (i.e., those with uncertain margins) may also be appropriate. For patients with tumors adherent to or invading adjacent structures, the preferred treatment sequence would be preoperative EBRT plus 5-FU–based chemotherapy followed by resection with or without IOERT and postoperative systemic therapy, based on excellent results in preliminary IOERT reports from both U.S. and European institutions.22,38,39 A similar approach would be reasonable for patients with locally recurrent cancers or with regional nodal relapse.40–44
1 Siegel R, Ward E, et al. Cancer statistics, 2010. CA Cancer J Clin. 2011;61(4)::212-236.
2 Steele G, Mayer R, Podolsky DK, et al. Cancer of the colon, rectum, and anus. Cancer manual, ed 9. American Cancer Society, Framingham, MA, 1996..
3 Wei EK, Govannucci E, Wu K. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433-442.
4 Michaels KB, Govannucci E, Joshipura KJ, et al. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740-1752.
5 Gustin DM, Brenner DE. Chemoprevention of colon cancer. Current status and future prospects. Cancer Metastases Rev. 2002;21:323-348.
6 Winawer SJ, Zauber AG, Ho MH, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977-1981.
7 Smith R, Cokkinides V, Eyre H. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2004;54:41-52.
8 American Cancer Society, www.cancer.org. Accessed January 29, 2010
9 Gryfe R, Kim H, Hsieh E, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69-77.
10 Moertel CG, Fleming TR, MacDonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352-358.
11 O’Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of 5-FU and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246-250.
12 Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704.
13 André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116.
14 Kuebler J, Wieand H, O’Connell J, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer. Results from NSABP C-07. J Clin Oncol. 2007;25:2198-2204.
15 Van Cutsum E, LaBianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer. PETACC-3. J Clin Oncol. 2009;27:3117-3125.
16 Saltz S, Niedzwiecki D, Hollis D, et al. Irinotecan, fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer; results of CALGB 89803. J Clin Oncol. 2007;25:3456-3461.
17 Allegra CJ, Yothers G, O’Connell M, et al. A phase III trial assessing bevacizumab in stages II or III carcinoma of the colon. Results of NSABP protocol C-08. J Clin Oncol. 2011;29(1):11-16.
18 Willett CG, Tepper JE, Cohen AM, et al. Failure patterns following curative resection of colonic carcinoma. Ann Surg. 1984;200:685-690.
19 Gunderson LL, Sosin H, Levitt S. Extrapelvic colon—areas of failure in a reoperation series. Implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1985;11:731-741.
20 Willett C, Fung C, Kaufman D, et al. Postoperative radiation therapy for high-risk colon carcinoma. J Clin Oncol. 1993;11:1112-1117.
21 Amos EH, Mendenhall WM, McCarty PJ, et al. Postoperative radiotherapy for locally advanced colon cancer. Ann Surg Oncol. 1996;3:431-436.
22 Gunderson LL, Nelson H, Martenson J, et al. Locally advanced primary colorectal cancer. Intraoperative electron and external beam irradiation ± 5-FU. Int J Radiat Oncol Biol Phys. 1997;37:601-614.
23 Willett C, Goldberg S, Shellito P, et al. Does postoperative radiation play a role in the adjuvant therapy of stage T4 colon cancer? Cancer J Sci Am. 1999;5:242-247.
24 Martenson JA, Willett CG, Sargent DJ, et al. A phase III study of adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the surgical adjuvant treatment of colon cancer. Results of Intergroup Protocol 130. J Clin Oncol. 2004;22:3277-3283.
25 Gastrointestinal Tumor Study Group. Adjuvant therapy with hepatic irradiation plus 5-FU in colon carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:1151-1156.
26 Fabian C, Giri S, Estes N, et al. Adjuvant continuous infusion 5-FU, whole abdominal radiation and tumor bed boost in high-risk stage III colon carcinoma: A Southwest Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1995;32:457-464.
27 Estes N, Giri S, Fabian C. Patterns of recurrence for advanced colon cancer modified by whole abdominal radiation and chemotherapy. Am Surg. 1996;62:546-550.
28 Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905-914.
29 Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:235-242.
30 Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345.
31 Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30.
32 Van Cutsem E, Kohne C, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417.
33 Douillard J-Y, Siena S, Cassidy J, et al. Randomized phase III study of panitumumab with infusional fluorouracil, leucovorin, and oxalplatin (FOLFOX) versus FOLFOX 4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: PRIME trial. J Clin Oncol. 2010;28(31):4697-4705.
34 Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572.
35 Hecht J, Mitchell E, Chidiac T, et al. A randomized phase IIIb trial of chemotherapy, bevacizumab and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:655-658.
36 Gunderson LL, Martenson JA, Smalley SR, et al. Lower gastrointestinal cancer. Rationale, results and techniques of treatment. In The Lymphatic System and Cancer. Front Radiat Ther Oncol. 1994;28:140-154.
37 Willett CG, Tepper JE, Orlow E, et al. Renal complications secondary to radiation treatment of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 1986;12:1601-1604.
38 Schild SE, Gunderson LL, Haddock MW, et al. The treatment of locally advanced colon cancer. Int J Radiat Oncol Biol Phys. 1997;37:51-58.
39 Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for primary locally advanced rectal and rectosigmoid carcinoma. J Clin Oncol. 1991;9:843-849.
40 Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for recurrent locally advanced rectal and rectosigmoid carcinoma. Cancer. 1991;67:1504-1508.
41 Rutten H, Gosens M, Klaassenet R, et al: The treatment of locally recurrent rectal cancer with intraoperative electron beam radiotherapy (IOEBRT). Fourth International Conference of the International Society of Intraoperative Radiation Therapy. Hotel InterContinental. Miami, Florida, March 17-19, 2005.
42 Gunderson LL, Nelson H, Martenson J, et al. Intraoperative electron and external beam irradiation with or without 5-FU and maximal surgical resection for previously unirradiated locally recurrent colorectal cancer. Dis Colon Rectum. 1996;39:1380-1396.
43 Haddock MG, Gunderson LL, Nelson H, et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001;49:1267-1274.
44 Haddock MG, Nelson H, Donahue J, et al. IORT as a component of salvage therapy for colorectal cancer patients with advanced nodal metastases. Int J Radiat Oncol Biol Phys. 2003;56:966-973.