Colorectal Cancer
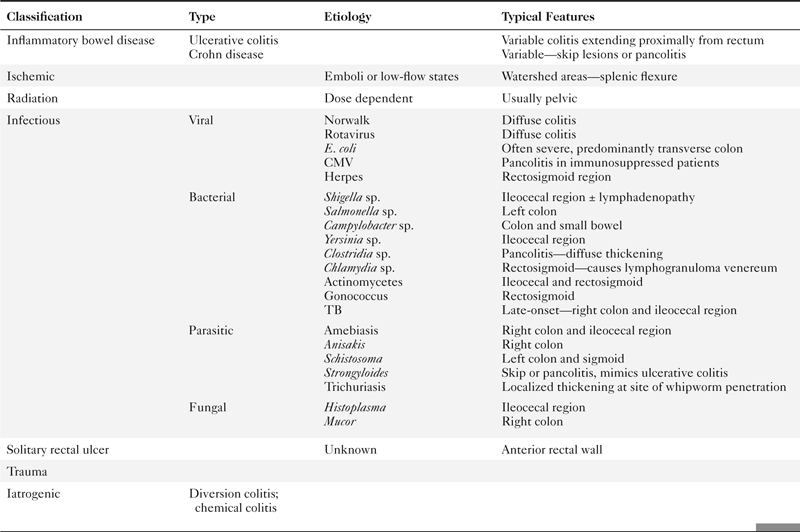
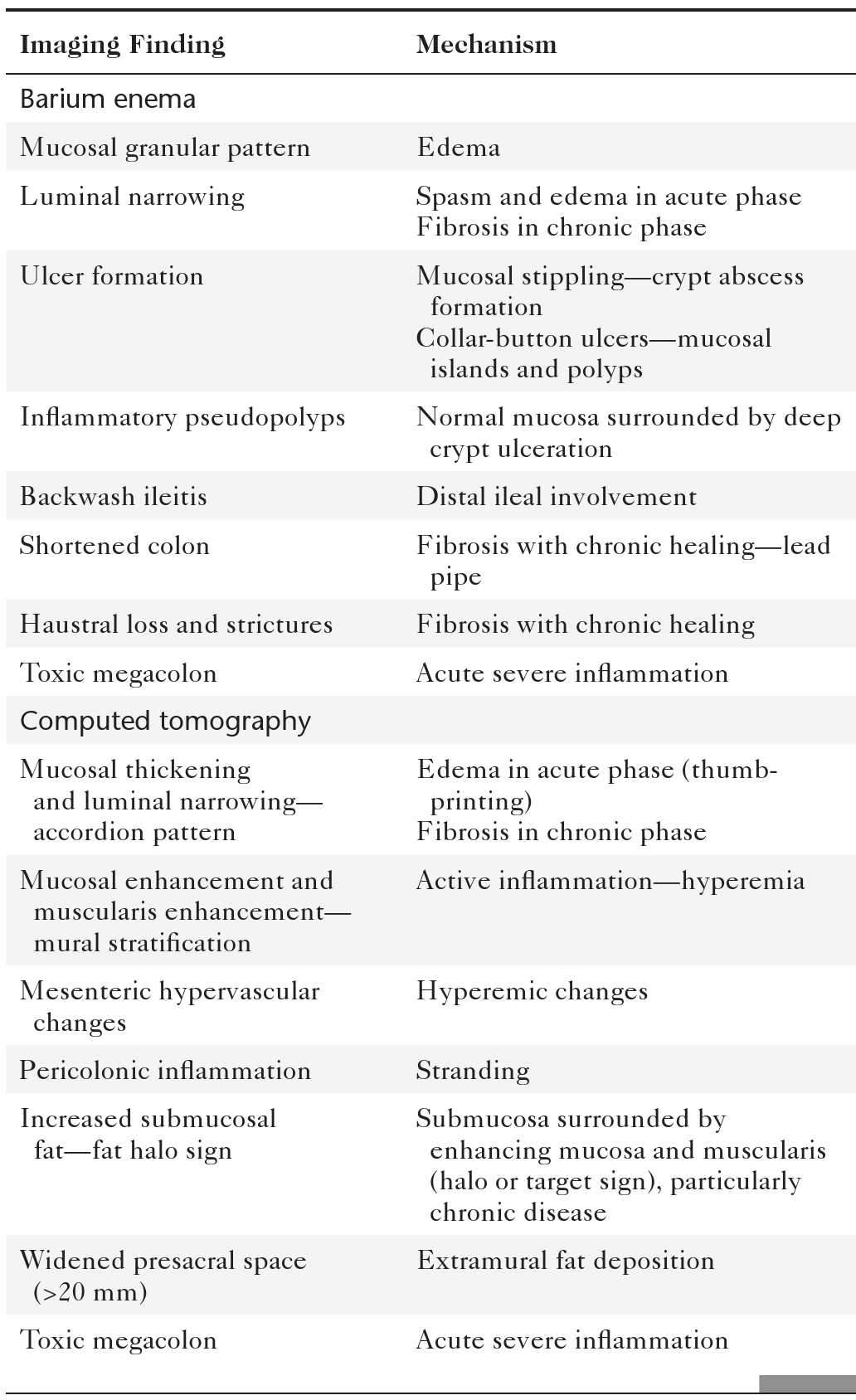
Colorectal cancer is responsible for up to 1.5 million cases diagnosed annually worldwide with as many as 600,000 deaths. It therefore remains a major killer even though the comprehensive colonic screening widely available in the West (where the disease is most common) could reduce this number to a small fraction. Survival is therefore related to early detection. Once colorectal cancer is detected, survival is predicted by TNM staging (
Table 5-7), as defined by the American Joint Committee on Cancer (AJCC) and now less commonly by the Dukes∗ classification system.
Table 5-7
Staging of Colon Cancer
| AJCC Stage |
TNM Stage |
Criteria |
| 0 |
Tis N0 M0 |
Confined to mucosa—cancer in situ |
| I |
T1 N0 M0 |
Submucosal invasion |
| I |
T2 N0 M0 |
Muscularis propria invasion |
| II-A |
T3 N0 M0 |
Serosal invasion |
| II-B |
T4 N0 M0 |
Invades adjacent organs |
| III-A |
T1-2 N1 M0 |
T1-2 + 1-3 regional nodes involved |
| III-B |
T3-4 N1 M0 |
T3-4 + 1-3 regional nodes involved |
| III-C |
T1-4 N2 M0 |
T1-4 + 4 regional nodes involved |
| IV |
T1-4 N1-2 M1 |
T1-4 N1-2 M1 |
AJCC, American Joint Committee on Cancer; T (in TNM), extent of tumor invasion of colorectal wall; N (in TNM), nearby lymph nodes that are involved; M (in TNM), distant metastases.
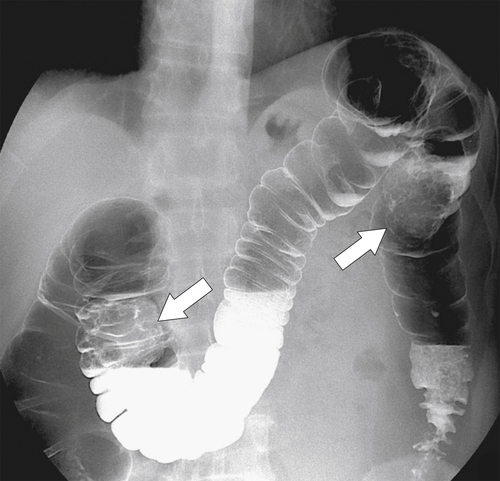
Most tumors (95%) are adenocarcinomas (approximately 5% of tumors are squamous cell), usually mucinous, and develop secondary to the adenoma-carcinoma sequence. All adenomas, whether acquired or congenital, are at risk for the development of adenocarcinoma. Other predisposing factors include IBD (both Crohn disease and UC). There is an increased risk in patients with hamartomatous syndromes (but not because of the hamartoma itself) and rarely in cystic fibrosis. Metachronous and synchronous adenocarcinomas are well recognized. The cumulative risk for metachronous disease (presentation of another tumor at a later date) is approximately 0.3% to 0.5% per year. Risk of synchronous disease (the presence of a second
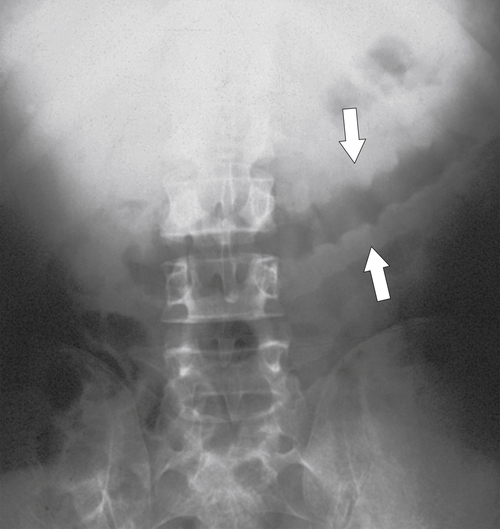
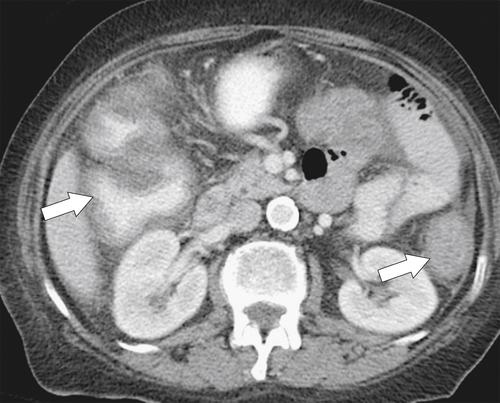
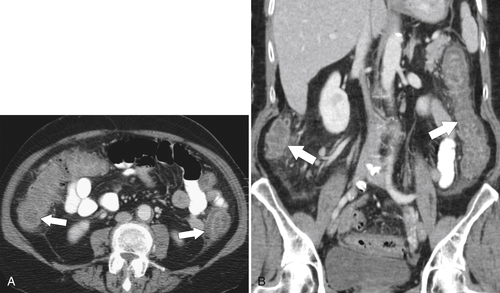
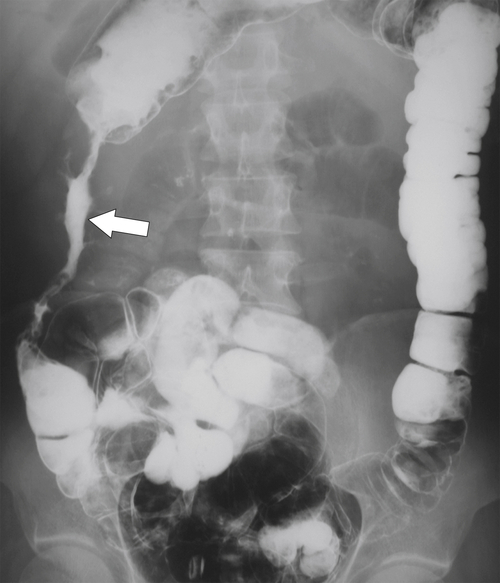
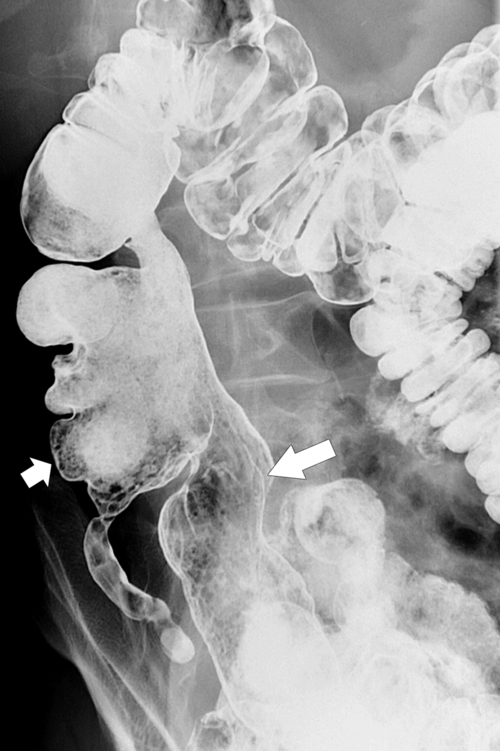
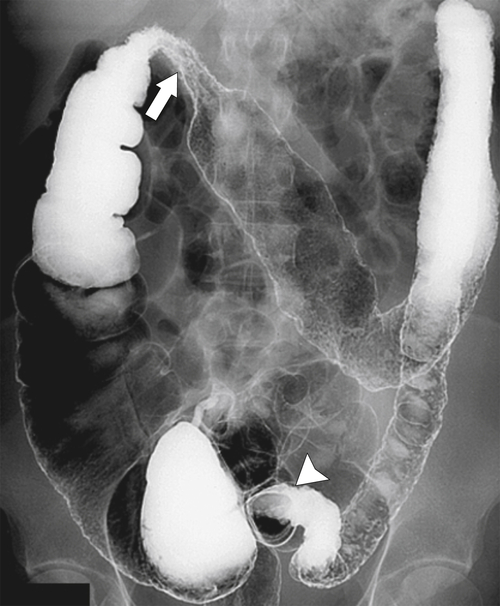
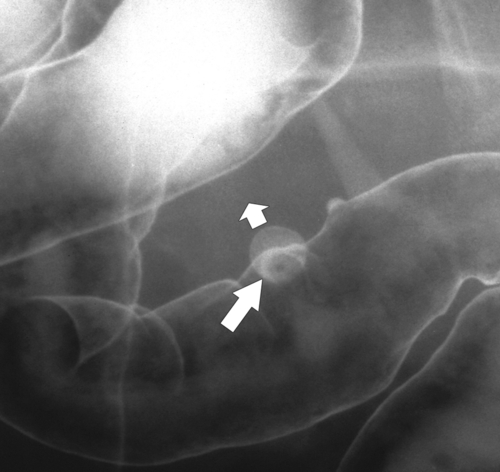
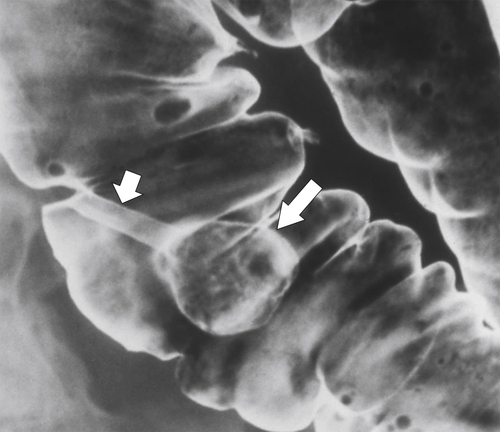
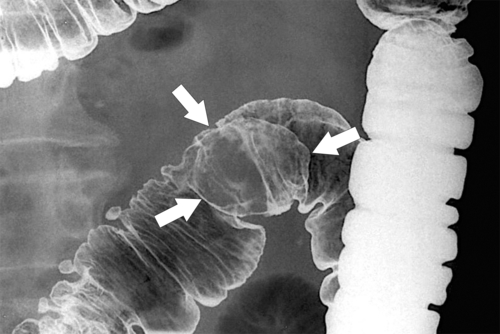
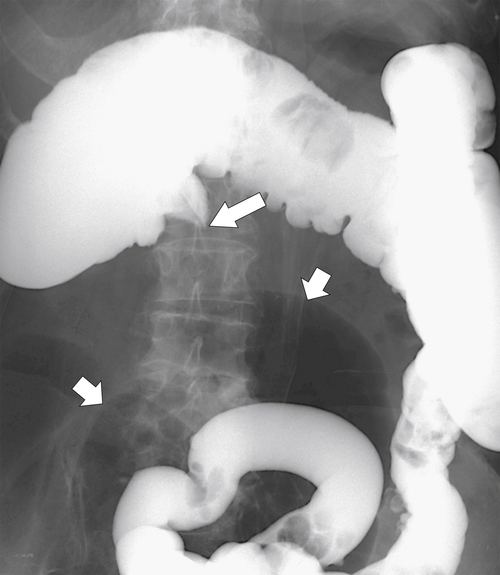
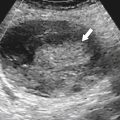
primary adenocarcinoma at the initial primary diagnosis) is approximately 5% (Fig. 5-102).
Figure 5-102 BE in a 71-year-old woman with synchronous carcinomas of the colon (arrows).
Squamous cell carcinoma, while representing the minority of colorectal tumors, are mostly found in the rectum. Predisposing factors include HIV and HPV infection and a rare form of rectal cancer arising from the remnant of the cloacal membrane at the anorectal junction. These tumors are usually aggressive and have metastasized at the time of presentation.
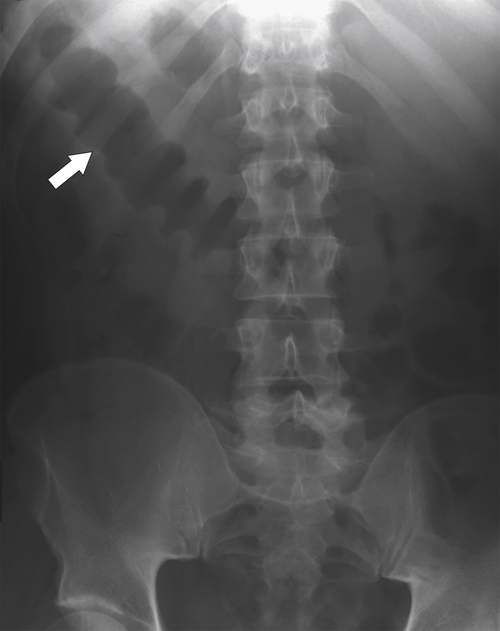
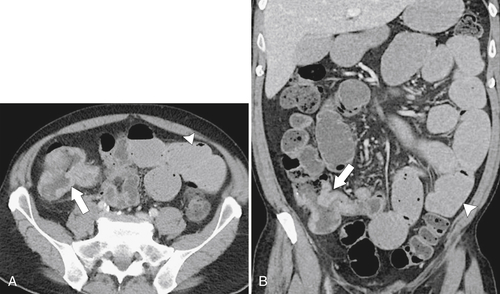
The location of the tumor usually dictates patient presentation. Tumors arising from the right side of the colon are more insidious, perhaps because the colon is wider and therefore tumors less often cause obstructive symptoms. This premise does not hold for cecal tumors that involve the ileocecal valve, which can cause early small bowel obstruction (
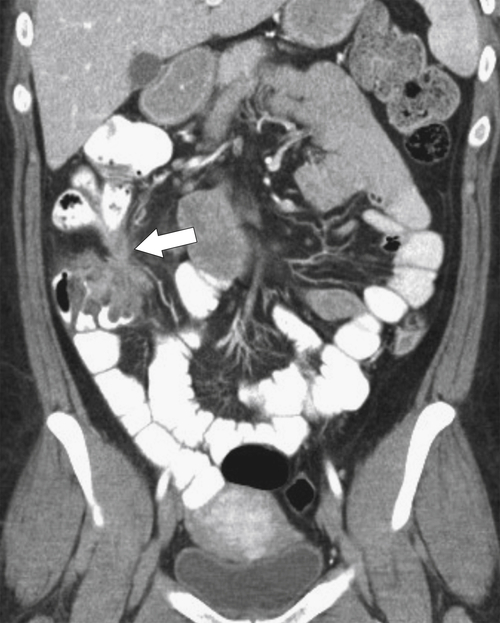
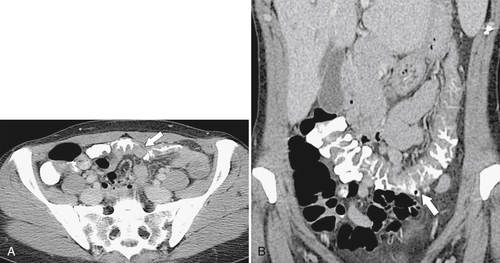
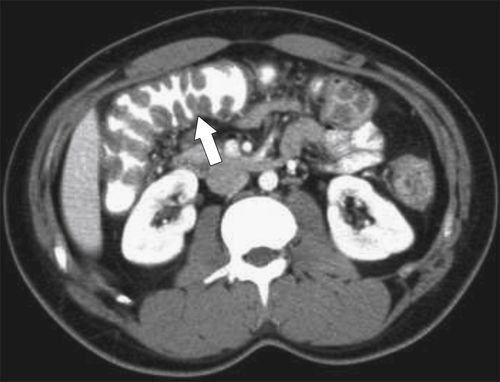
Figs. 5-63 and
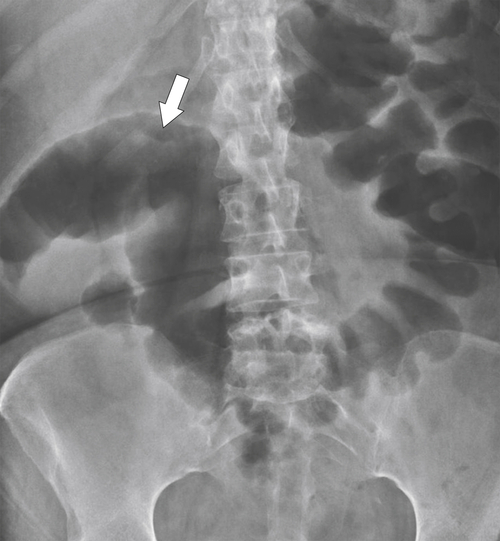
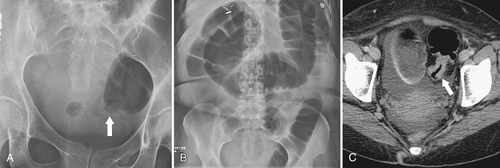
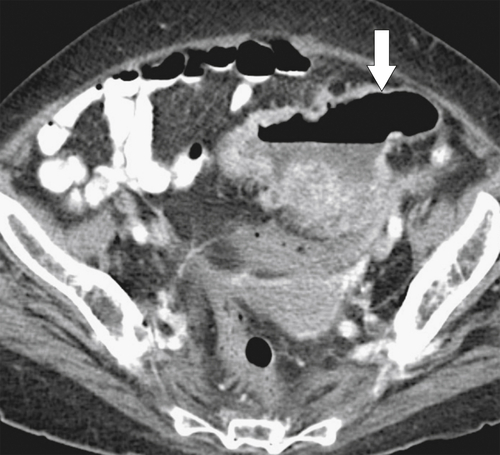
5-103). Patients may present with pain, but it is more likely that the tumor will bleed intermittently, often asymptomatically, and that the patients ultimately present with the symptoms of anemia rather than the direct local effects of the tumor. Left-sided colon tumors, in contrast, present more commonly with symptoms of obstruction because luminal narrowing is more prevalent (Fig. 5-104). Occasionally tumors are so indolent that the patient presents with a very large mass that has not yet declared itself with bleeding or obstruction.
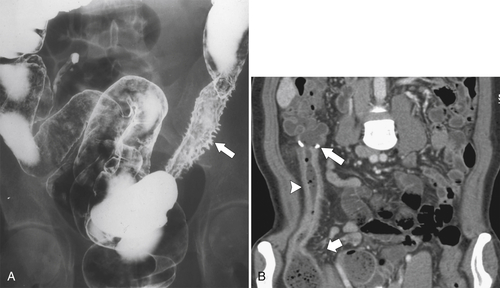
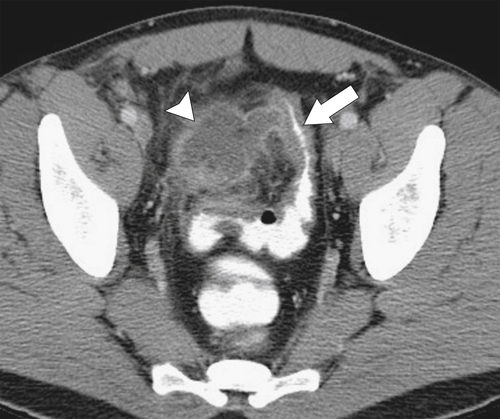
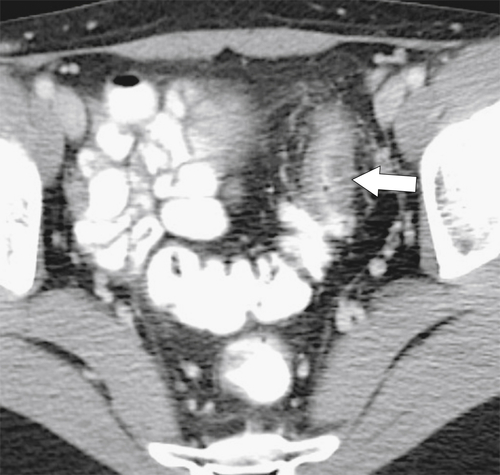
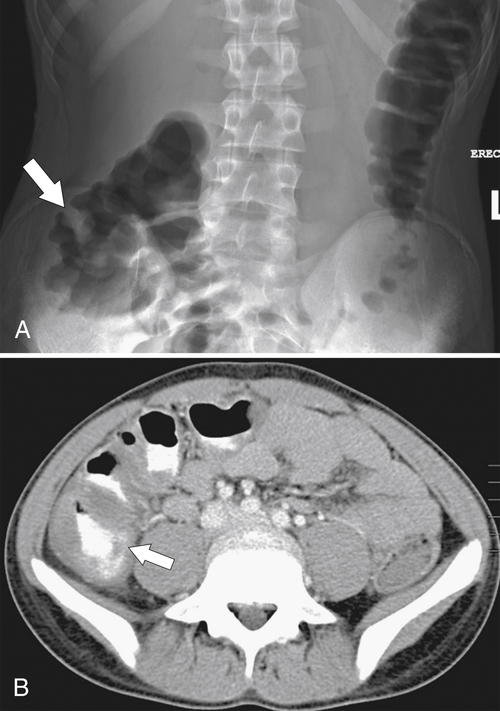
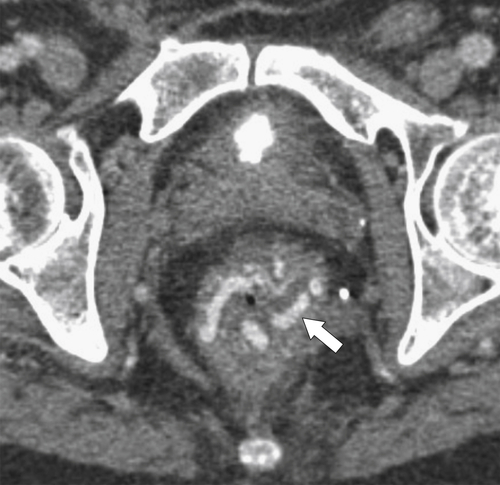
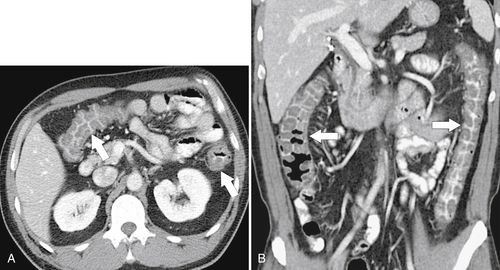
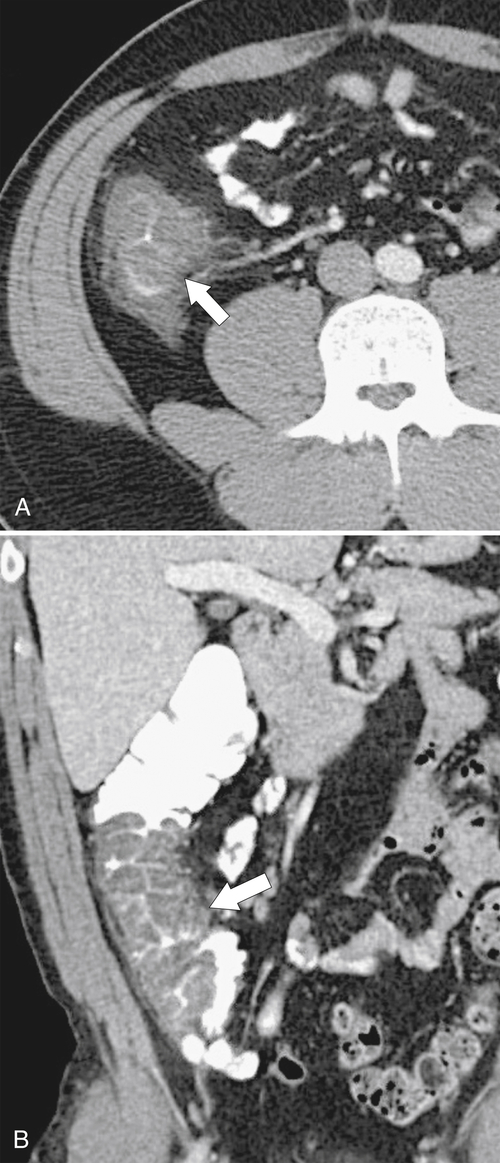
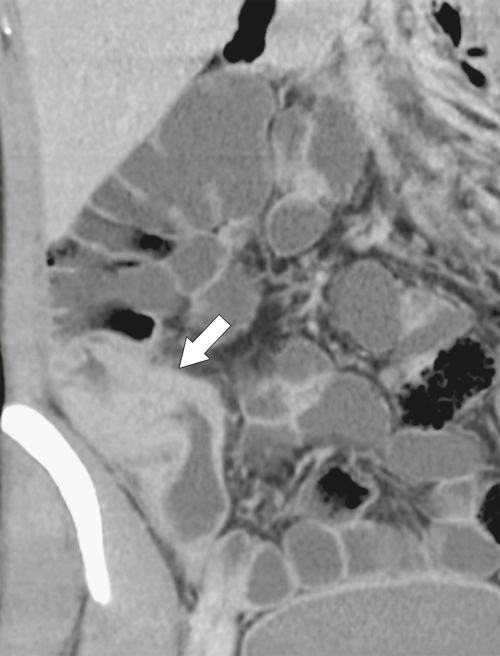
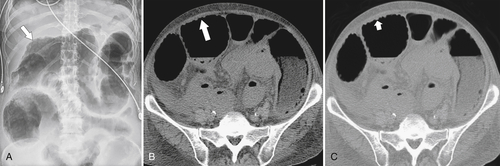
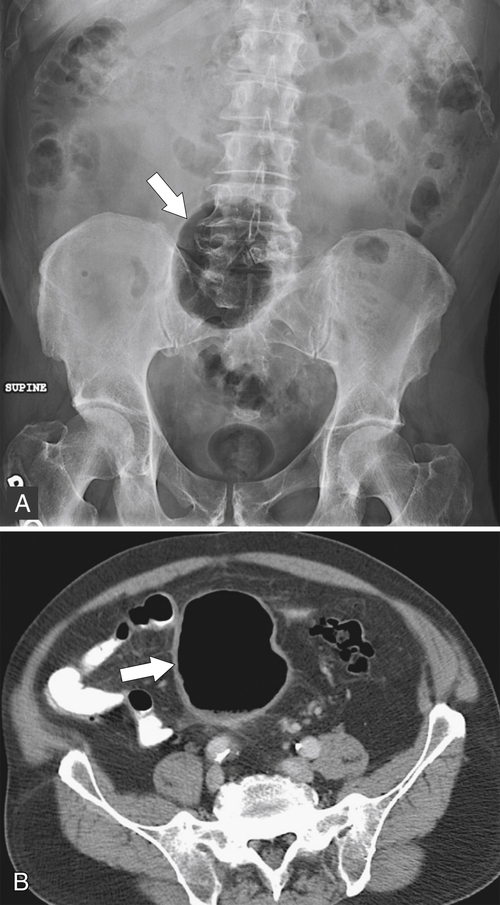
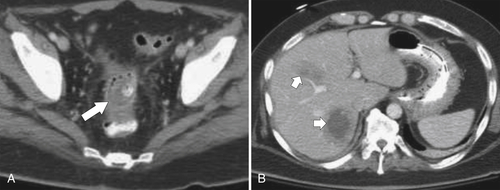
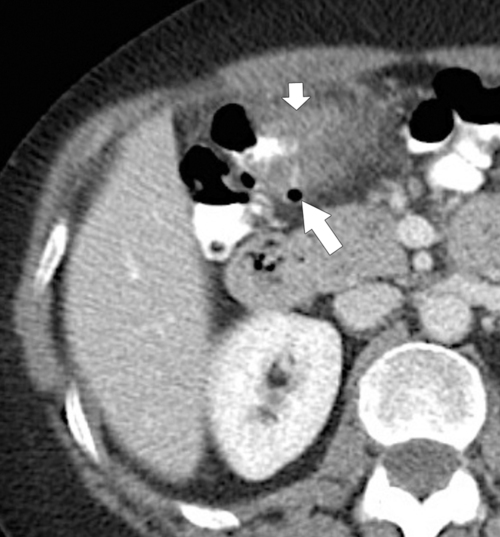
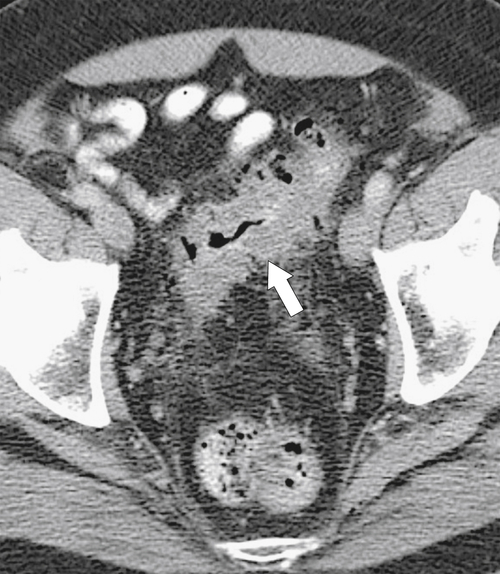
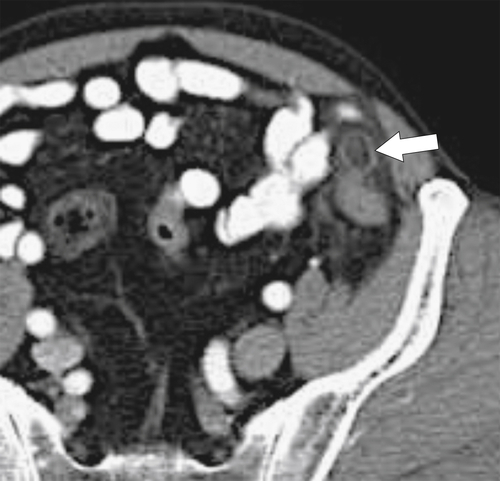
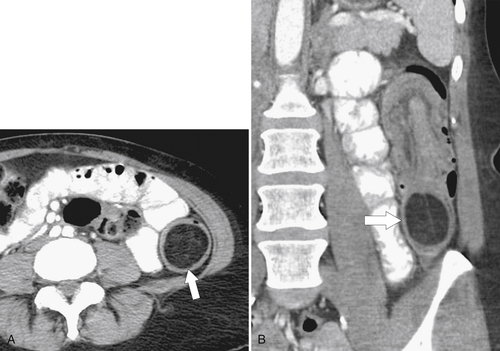
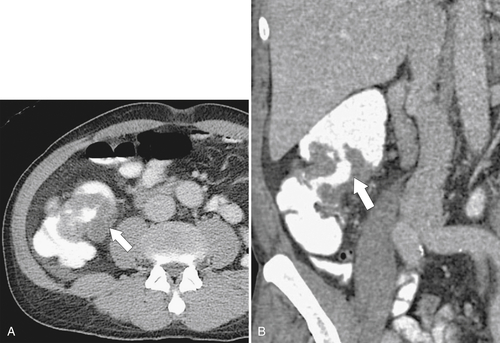
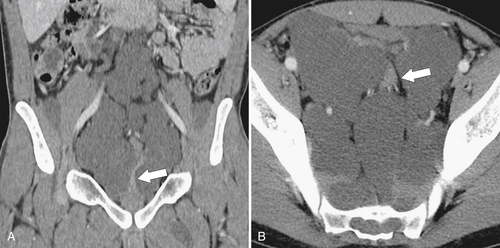
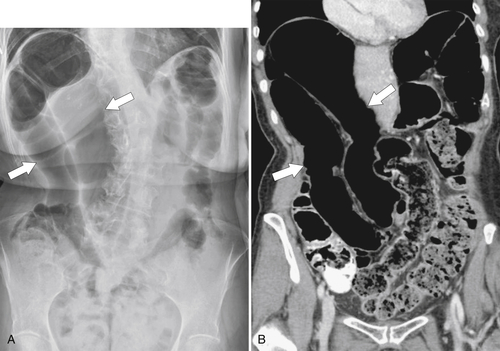
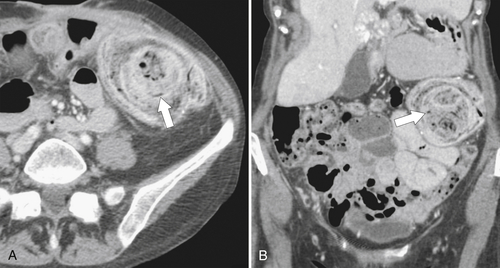
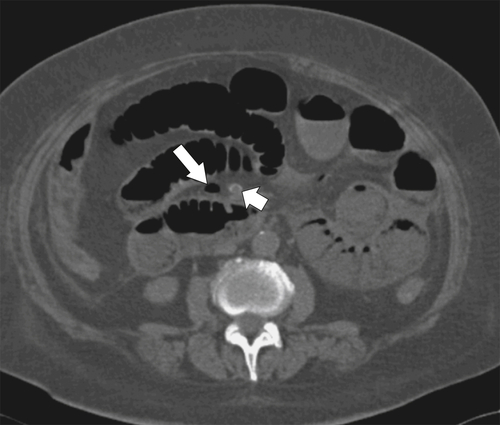
Figure 5-103 Axial (A) and coronal (B) contrast-enhanced CT in a 60-year-old man with small bowel obstruction (arrowheads) due to cecal adenocarcinoma (arrows).
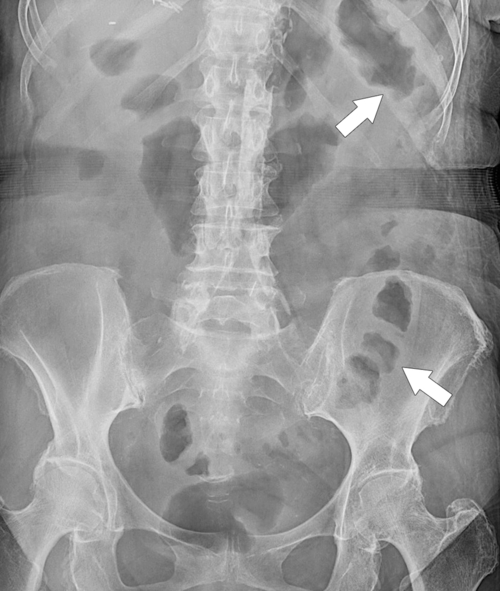
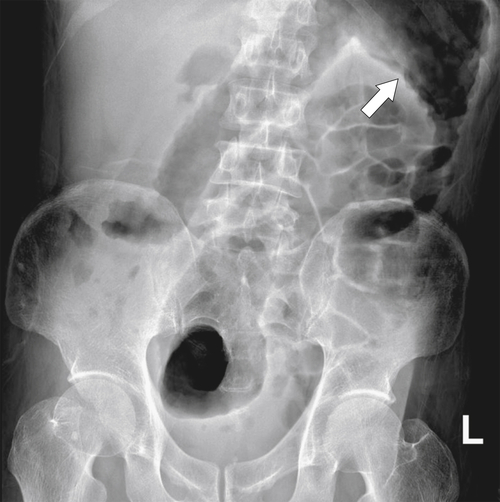
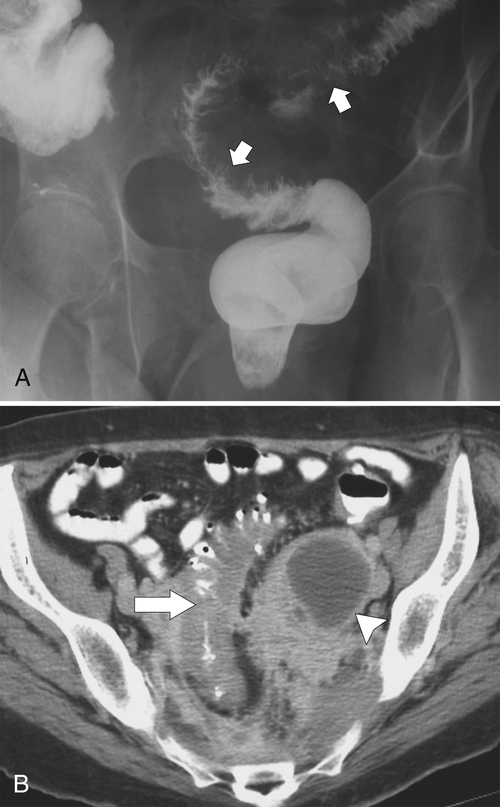
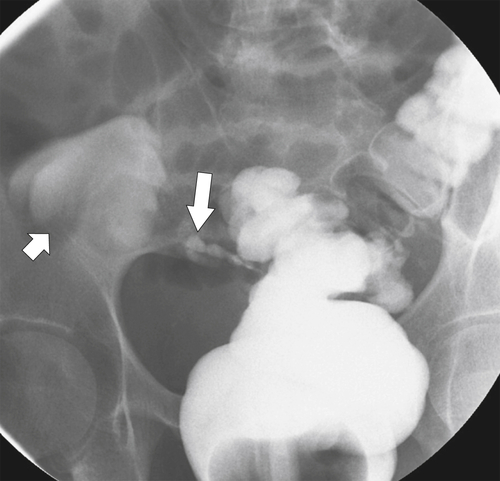
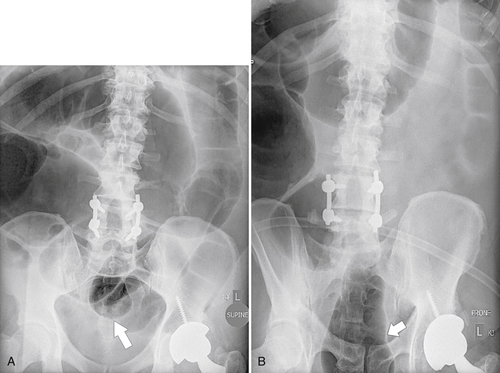
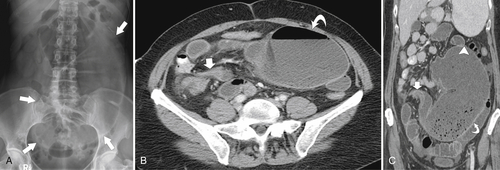
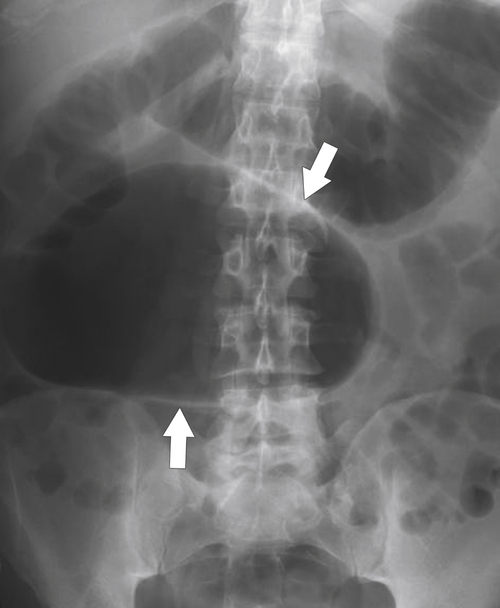
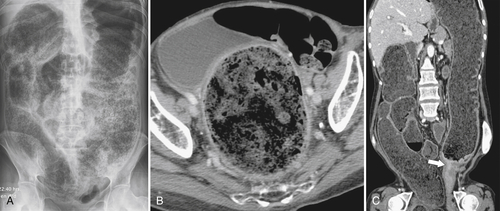
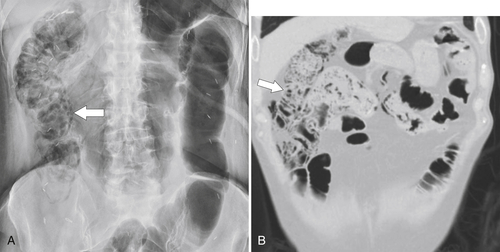
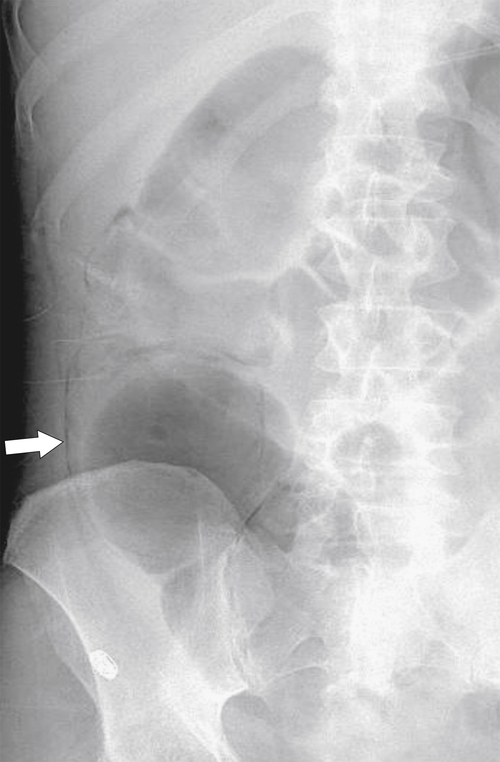
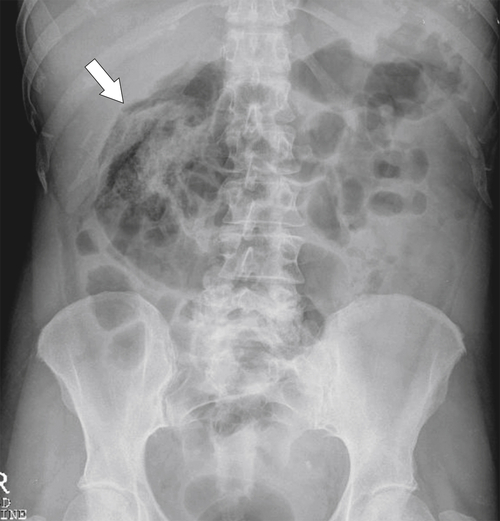
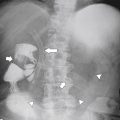
Figure 5-104 Plain radiograph of the abdomen (A and B) and axial contrast-enhanced CT (C) with large bowel dilatation and obstruction due to an obstructing sigmoid adenocarcinoma (arrows). There is also pelvic ascites.
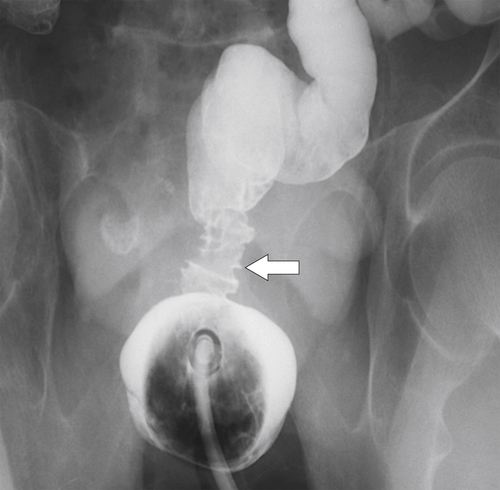
Lesions in the rectosigmoid region commonly present with rectal bleeding (often bright red) and a change in bowel habit. They therefore tend to present earlier and consequently are associated with better survival statistics. Rarely tumors from any location in the colon can present because of perforation and peritonitis or abscess formation (
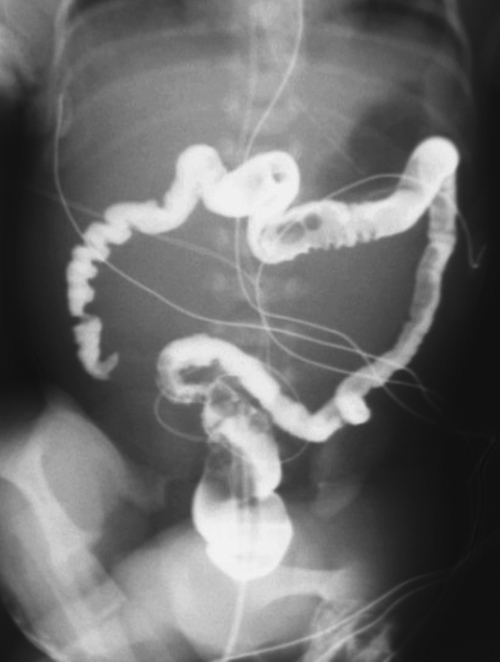
Fig. 5-105). A very occasional tumor can present with intussusception, with a predominantly intraluminal mass acting as the lead point (Fig. 5-106).
Figure 5-105 Axial contrast-enhanced CT in a 66-year-old man with a perforated sigmoid carcinoma and pelvic abscess (arrow).
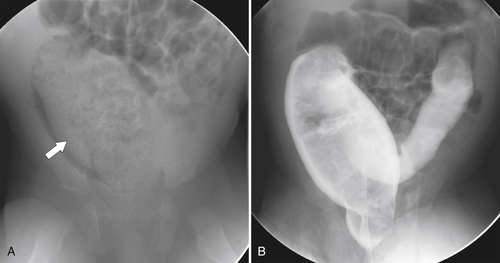
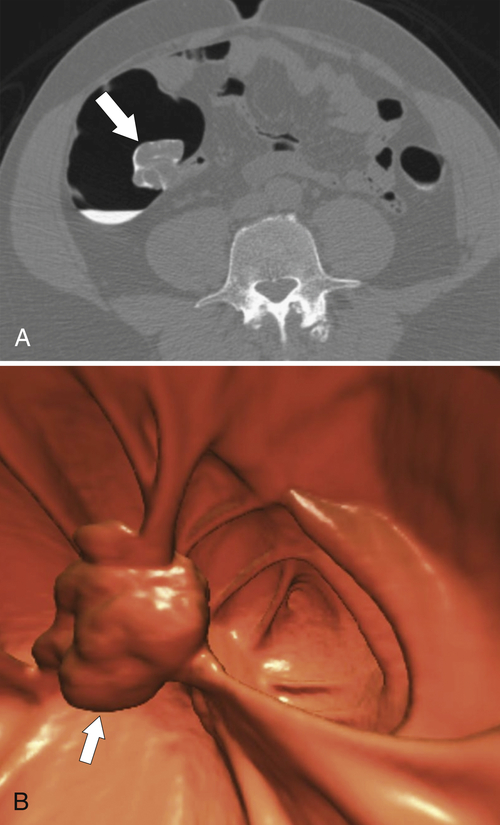
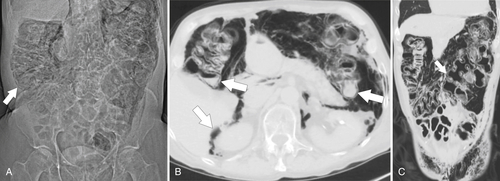
Figure 5-106 A through C, Axial and coronal non-contrast-enhanced CT in a 51-year-old man with a polypoid colonic carcinoma (arrows) that acts as a lead point for a colocolonic intussusception (small arrow).
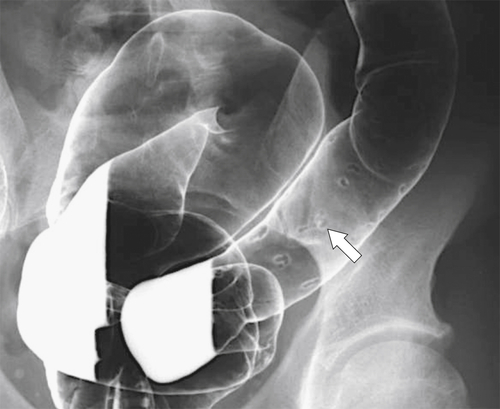
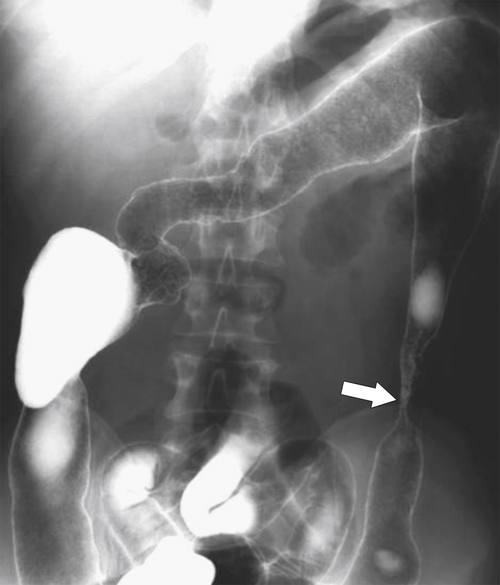
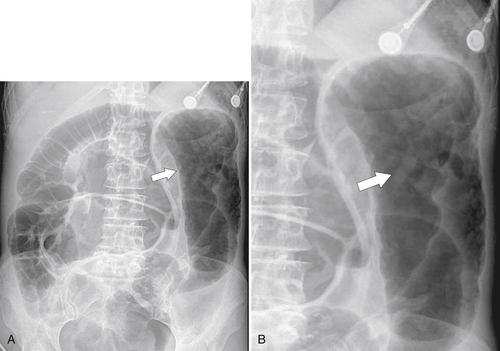
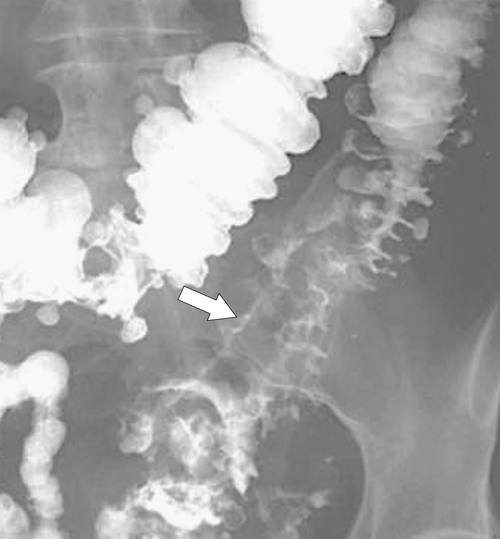
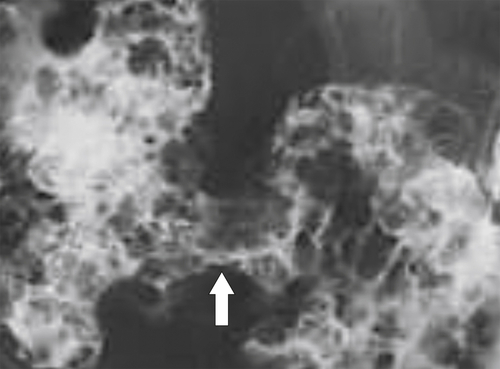
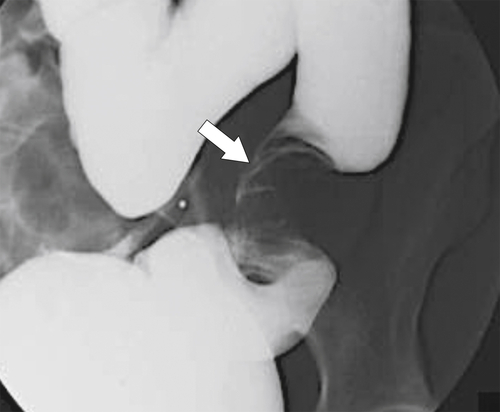
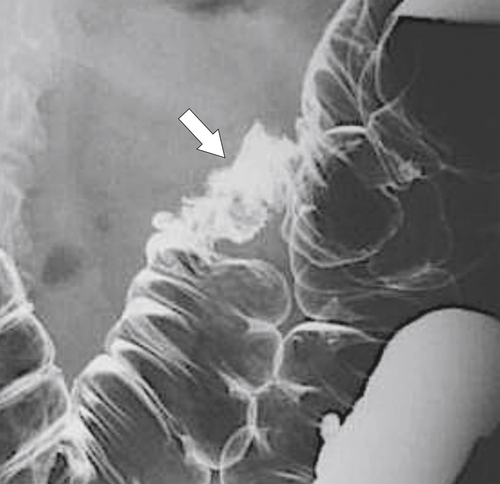
Characteristics of colorectal cancer lesions at DCBE range from subtle, slightly raised, plaque-like lesions (
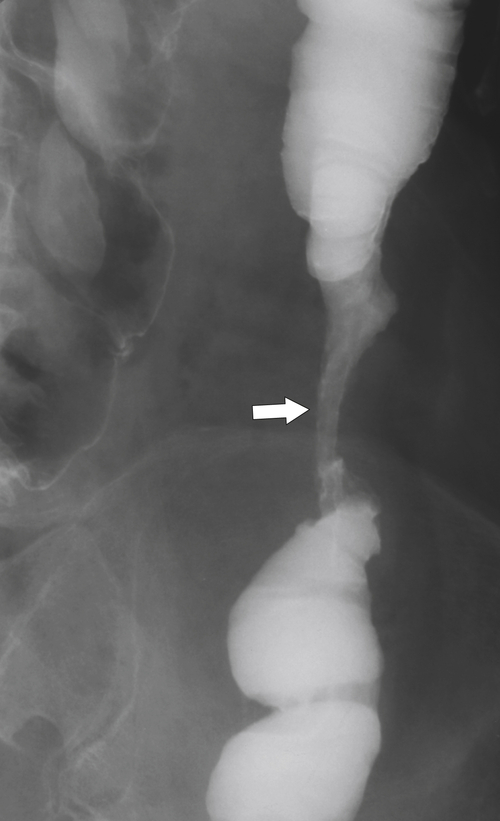
Fig. 5-107), to smooth or irregular polypoid intraluminal lesions (Fig. 5-108), to “carpet-like” flat nodular lesions (Fig. 5-109). The classic presentation is a circumferential annular constricting appearance, which has abrupt shelf-like margins with mucosal destruction in between (Fig. 5-110). The latter (the so-called apple-core lesion) is the cardinal sign of colorectal cancer and is almost diagnostic.
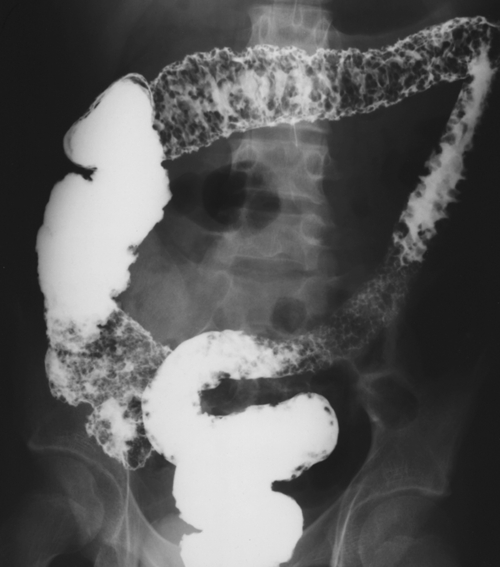
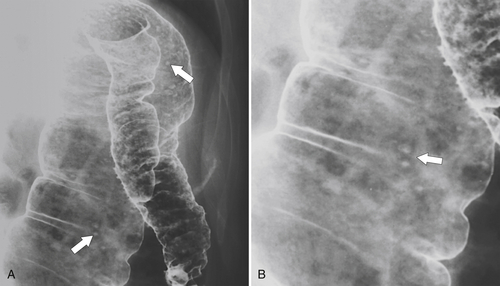
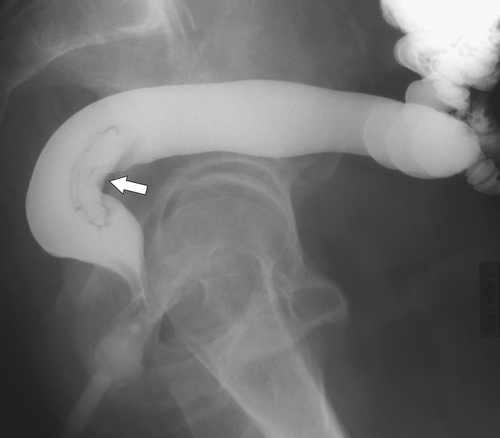
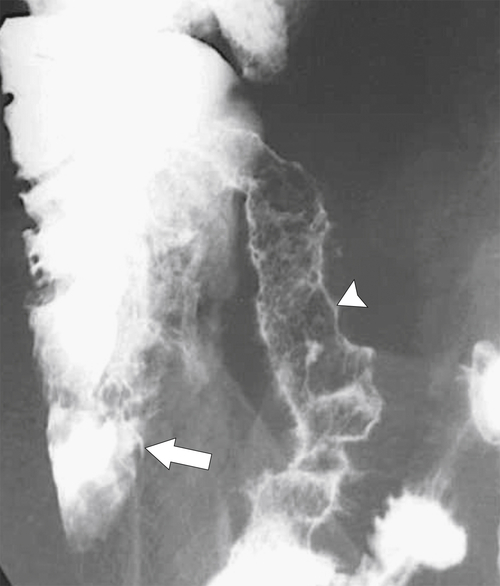
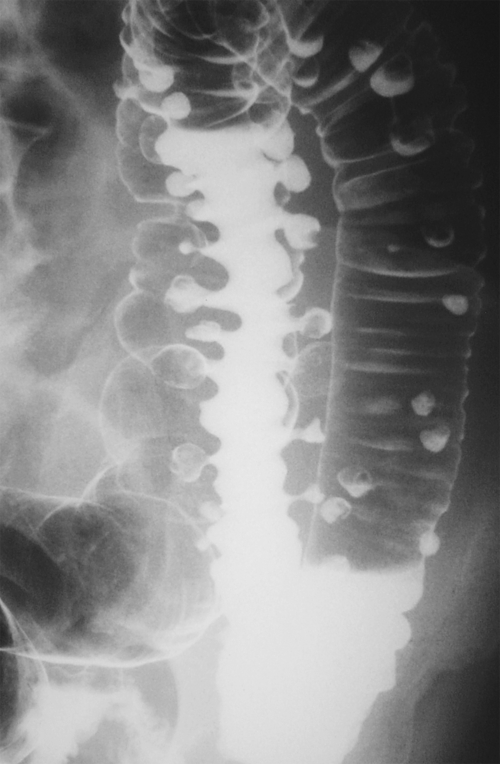
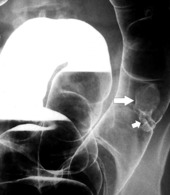
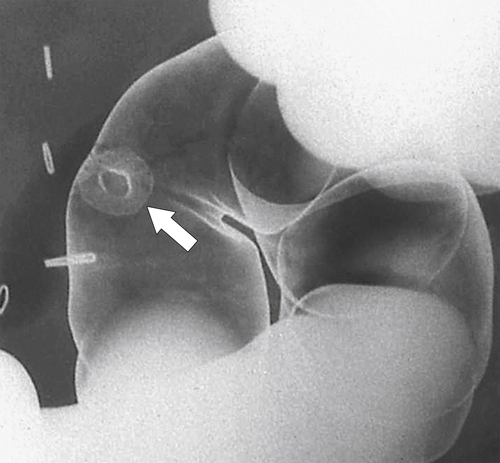
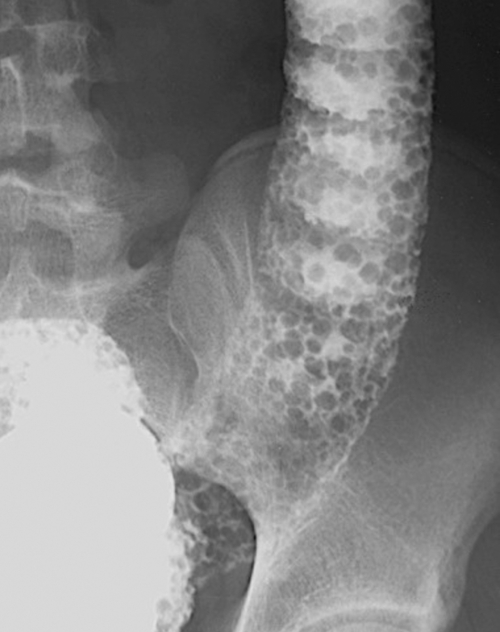
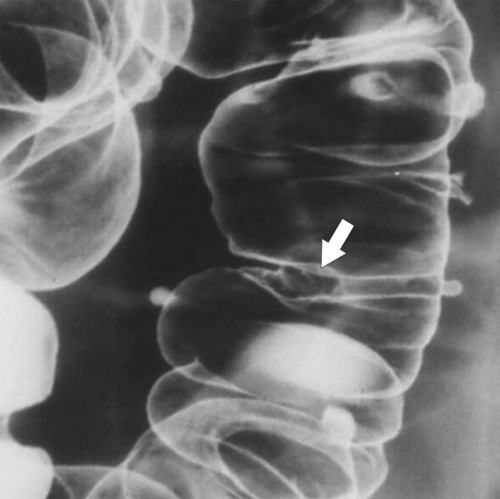
Figure 5-107 DCBE in a 73-year-old woman with a small plaque-like mucosal defect (arrow) in the left colon due to early colon adenocarcinoma.
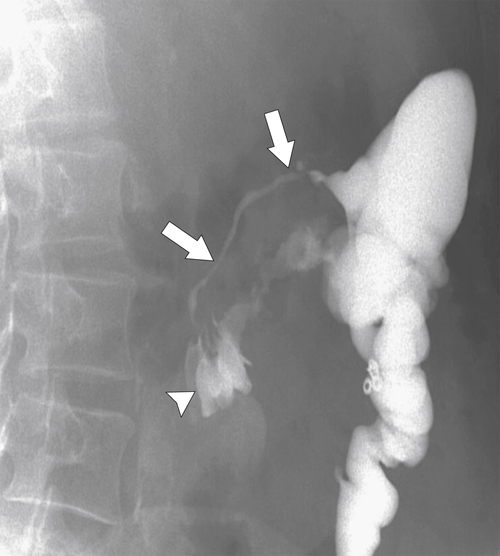
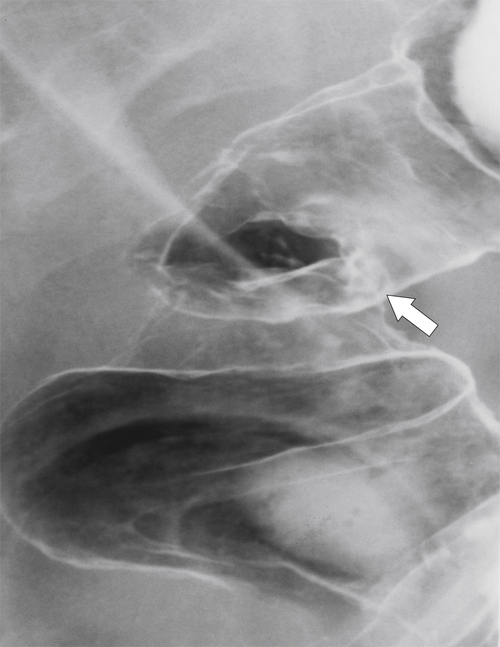
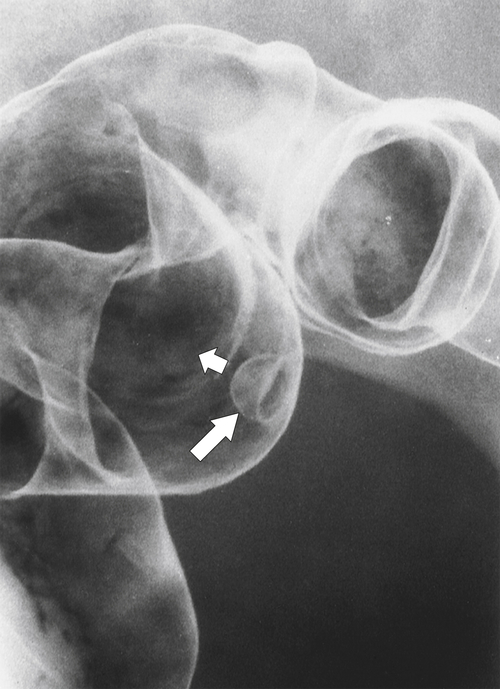
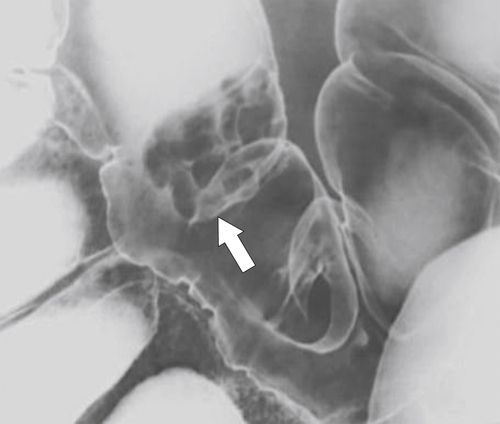
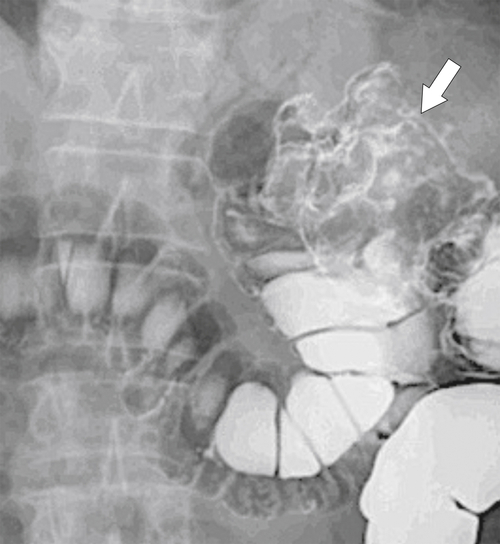
Figure 5-108 DCBE in a 67-year-old woman with a large polypoid mass (arrow) due to adenocarcinoma of the splenic flexure.
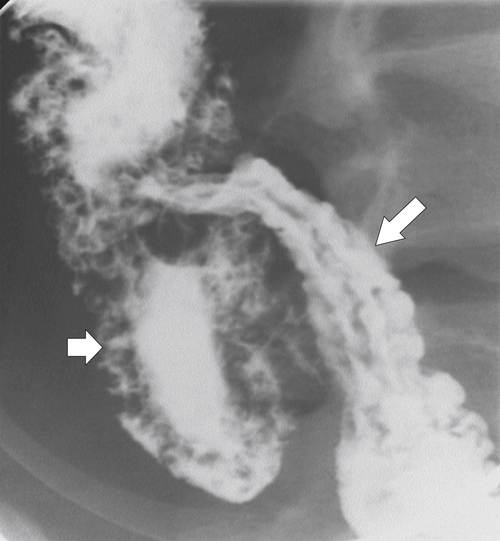
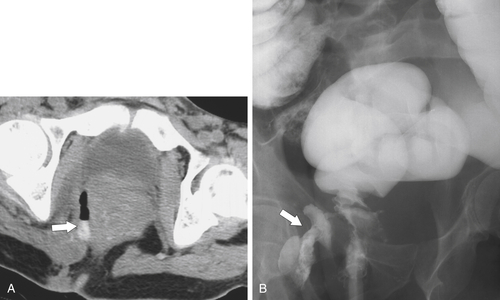
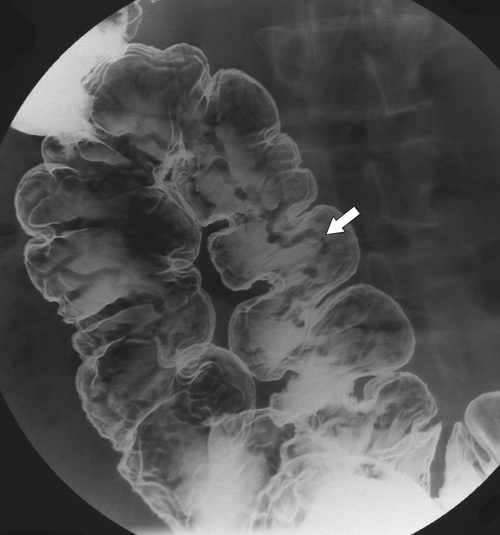
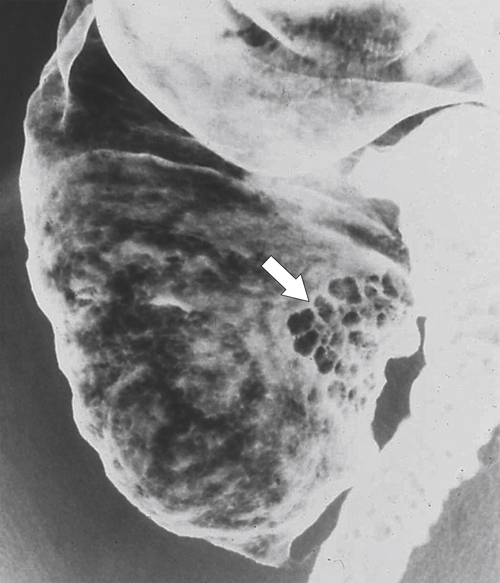
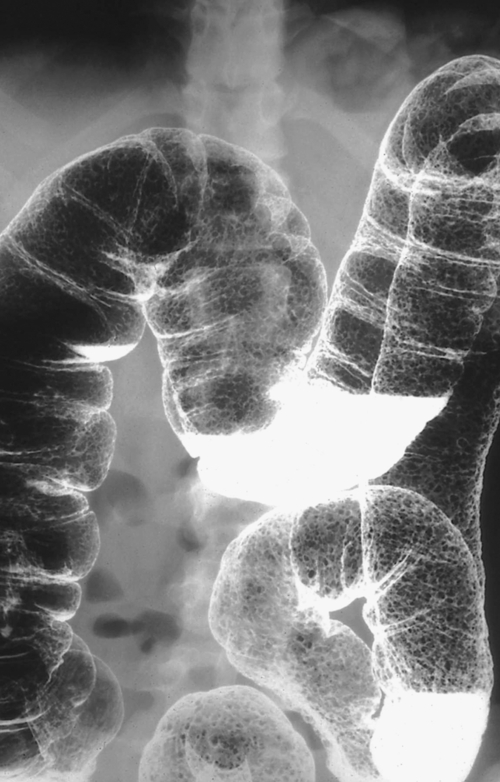
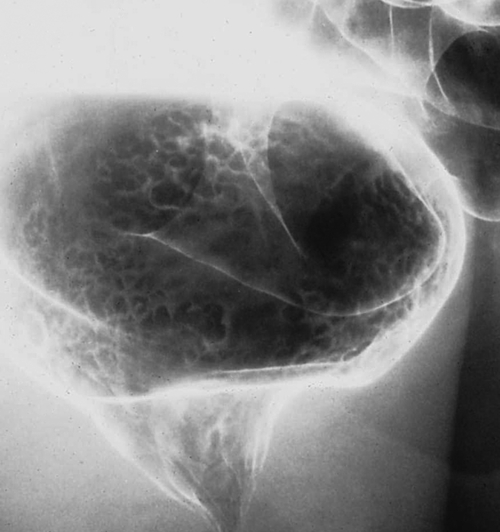
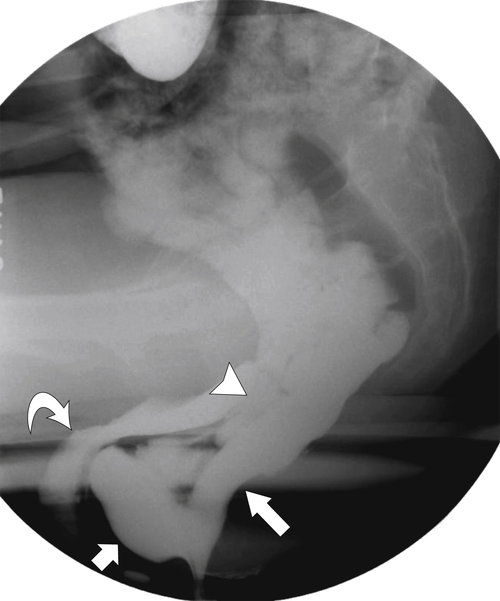
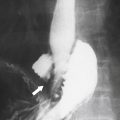
Figure 5-109 BE in a 77-year-old man with multiple polypoid rectal filling defects due to a superficial spreading “carpet lesion” from rectal adenocarcinoma.
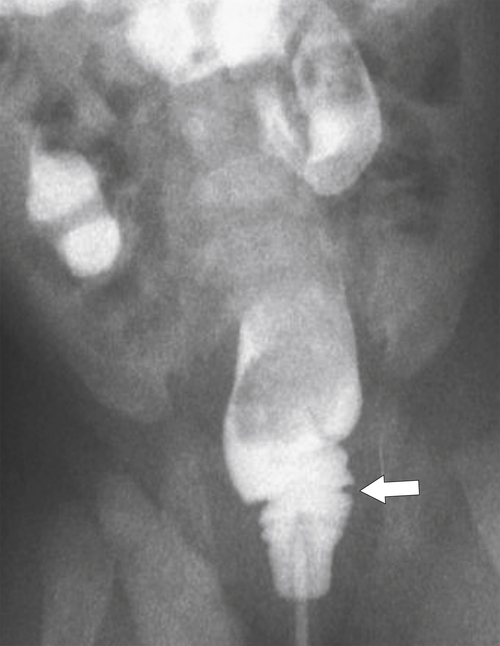
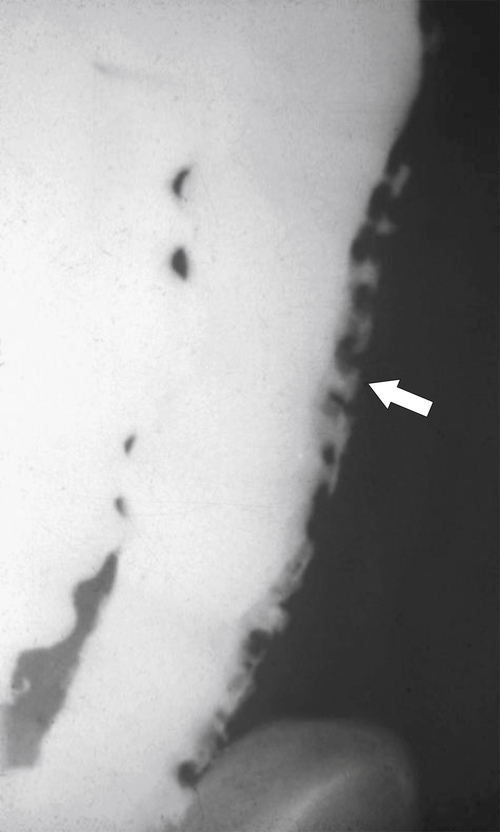
Figure 5-110 BE in a 61-year-old man with an “apple-core” rectal adenocarcinoma (arrow).
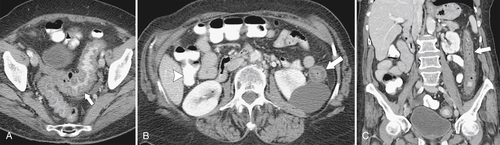
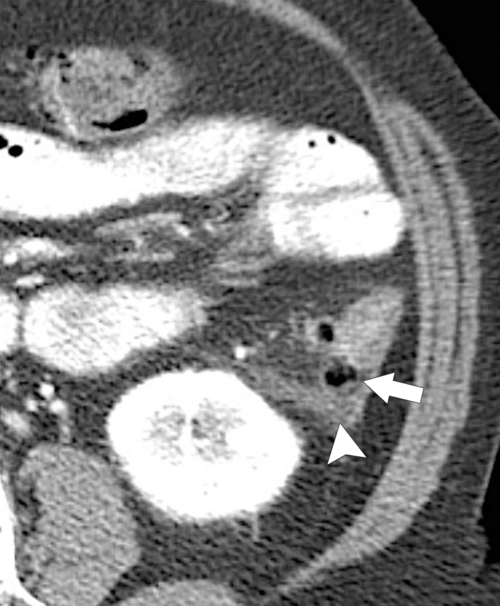
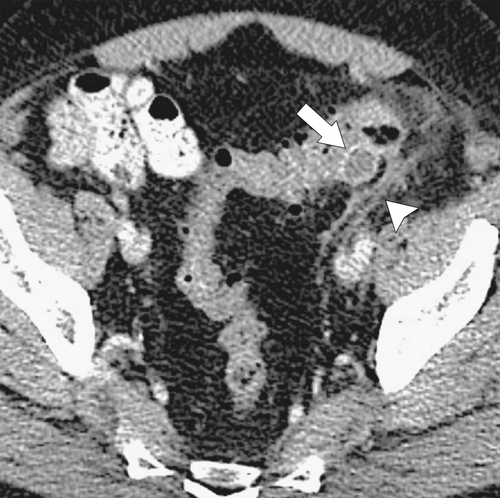
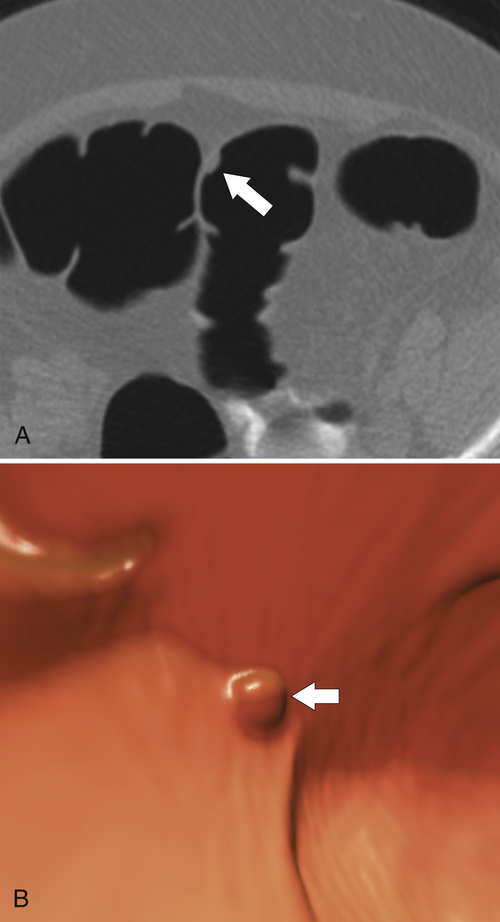
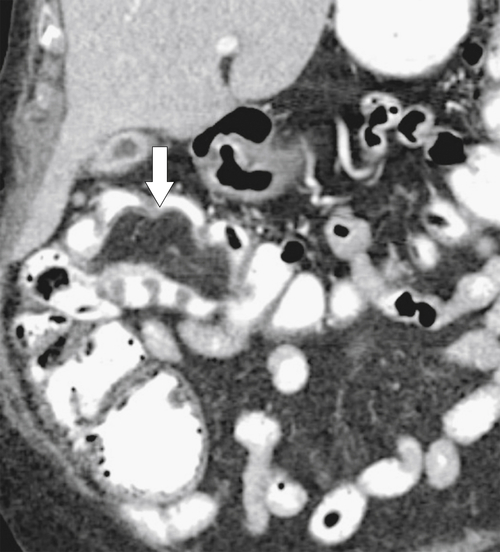
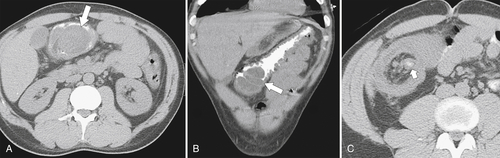
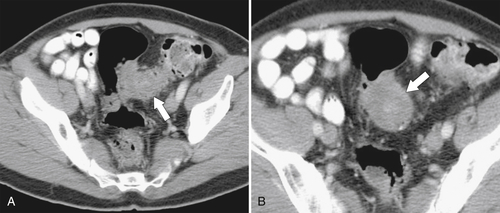
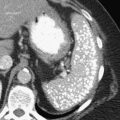
The majority of colorectal cancers are now detected by optical colonoscopy, and patients are then referred for a screening metastatic CT. Less commonly, cancer might first be detected by CT if it is performed for symptoms associated with the disease (abdominal pain, weight loss, anemia, change in bowel habit) or as an incidental finding when CT is performed for other reasons. Small tumors (<2 cm) are often missed on CT because they are easily mistaken for normal stool. Masses at the ileocecal valve may be missed because the valve often has a mass-like appearance, particularly if the cecum is decompressed. A clue that a malignant mass is present at the ileocecal valve is the degree of soft tissue thickening of the cecal wall (
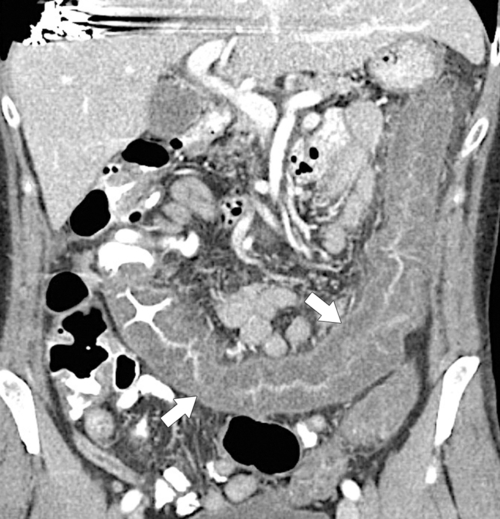
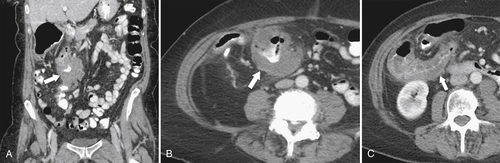
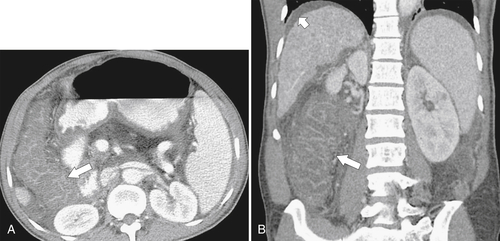
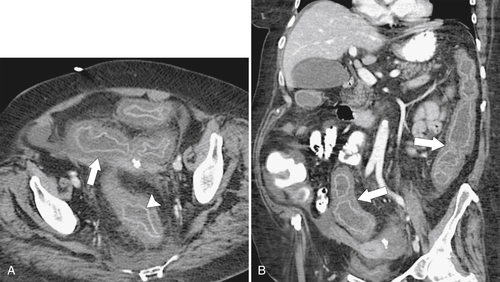
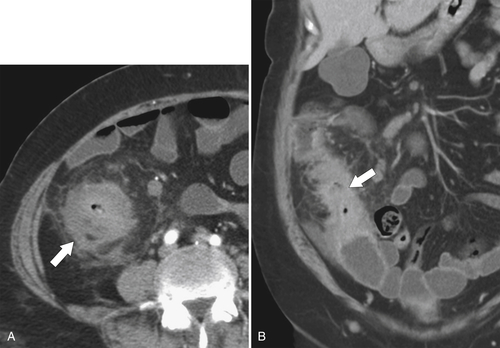
Fig. 5-111). Multiplanar imaging can be helpful in these circumstances. Other tumors will be detected with CT based on their size (Fig. 5-112). Sometimes a CT “apple-core” equivalent can be identified (Figs. 5-104 and 5-113). Tumors may present with a more inflammatory-type mass that can be difficult to differentiate from diverticulitis (Fig. 5-114). In the diagnosis of simple diverticulitis by CT imaging, it is critical to consider any underlying carcinoma as the cause. Signs that suggest an underlying malignancy include the degree of wall thickness and regional adenopathy (Fig. 5-71). More sinister features include extension of the tumor beyond the cecal wall, with edematous changes (fat-stranding) in the surrounding mesentery or retroperitoneum or local metastatic deposits and regional lymphadenopathy (Fig. 5-112). Sometimes the tumor may perforate, usually locally, with a walled-off abscess (Fig. 5-105) or fistulize with adjacent viscera (Fig. 5-115). Ideally, however, these tumors should be detected before they have metastasized either locally or distant. Given that colorectal cancer is relatively common and early detection is critical, radiologists should evaluate the colon and rectum carefully on every CT image to exclude subtle early tumors.
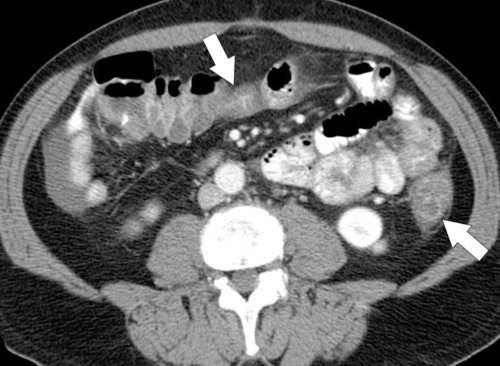
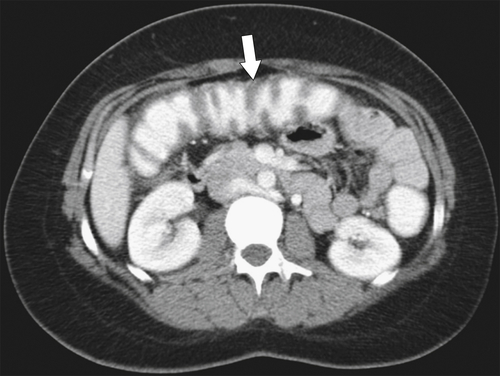
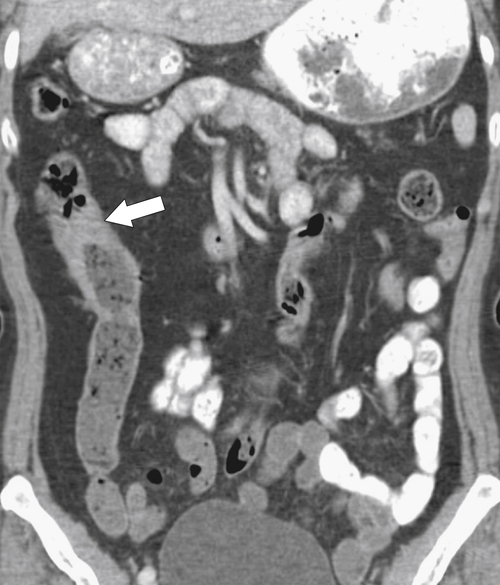
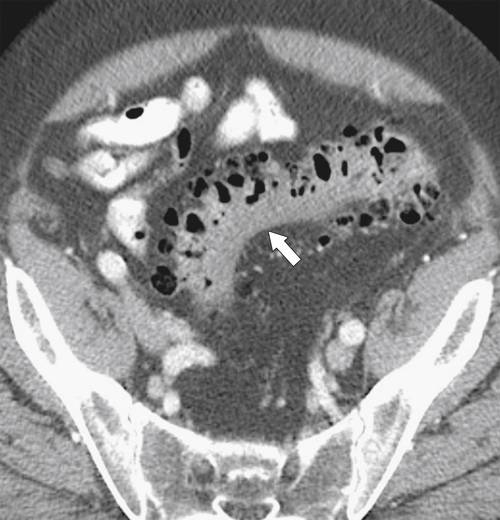
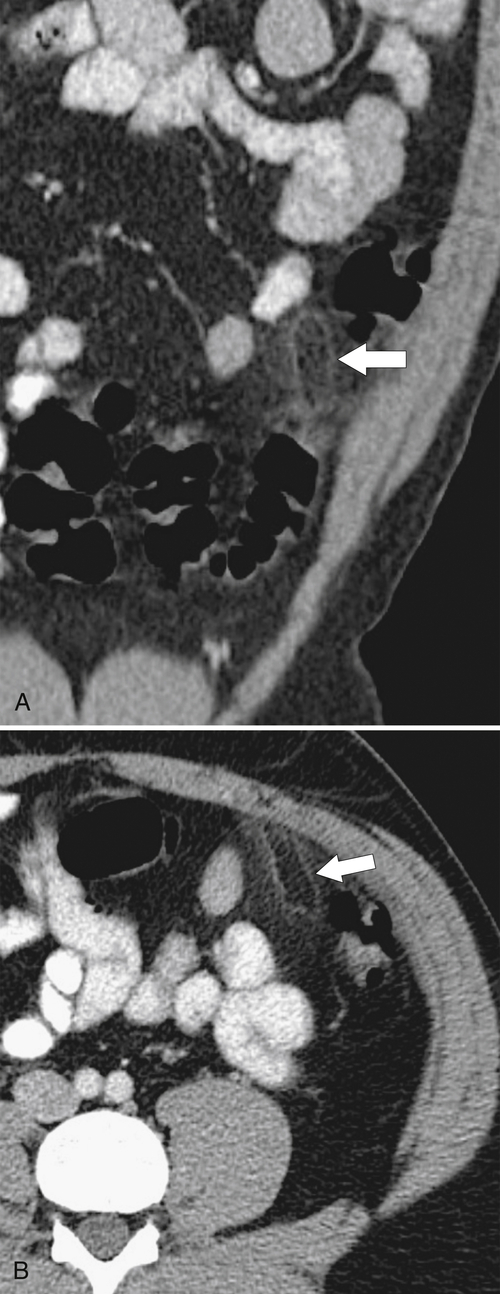
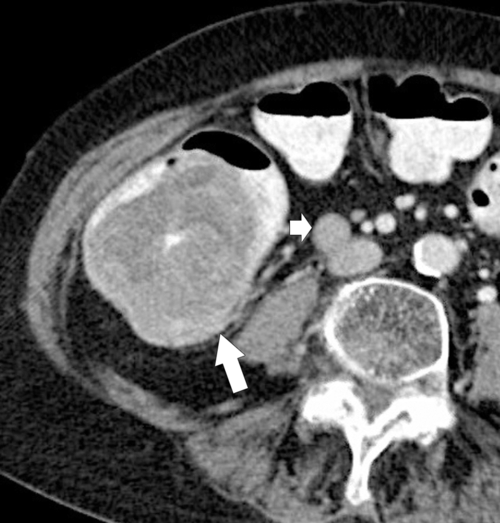
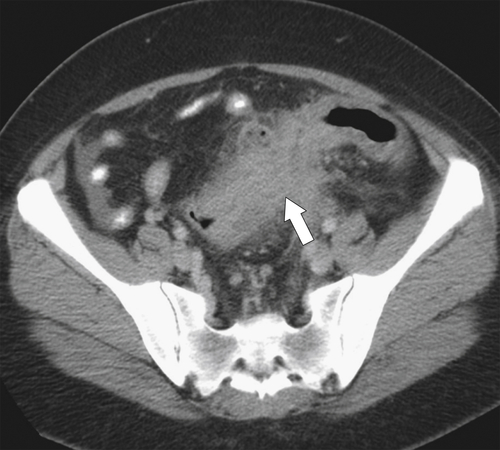
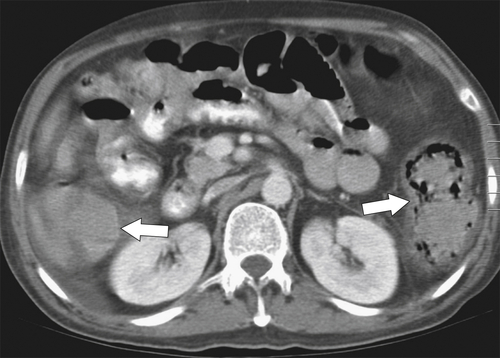
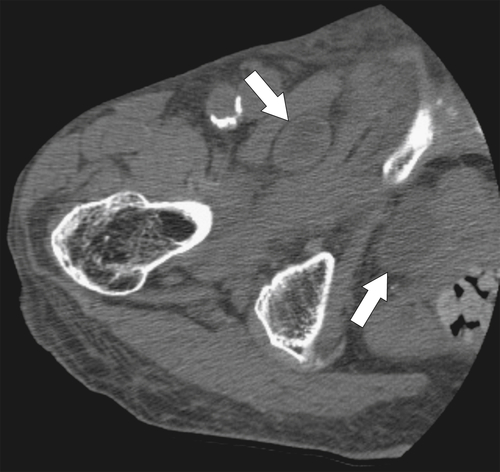
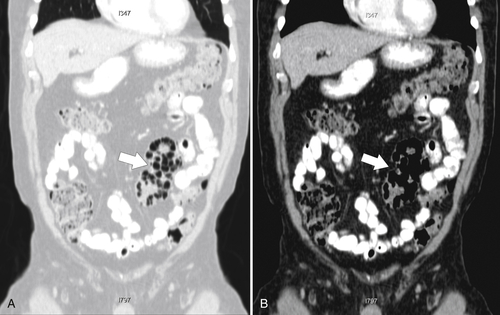
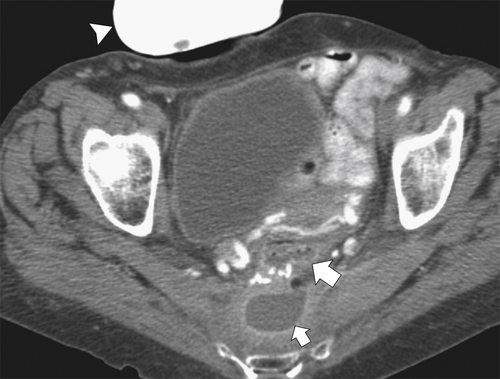
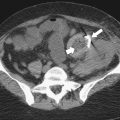
Figure 5-111 Axial (A) and coronal (B) contrast-enhanced CT in a 60-year-old man with a cecal mass (arrows) due to cecal adenocarcinoma.
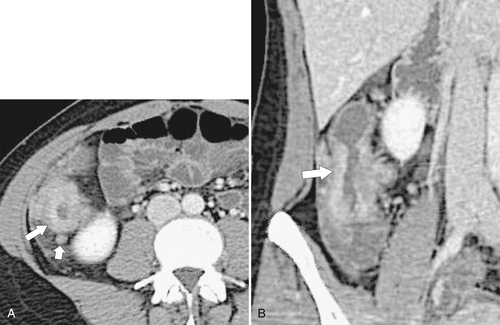
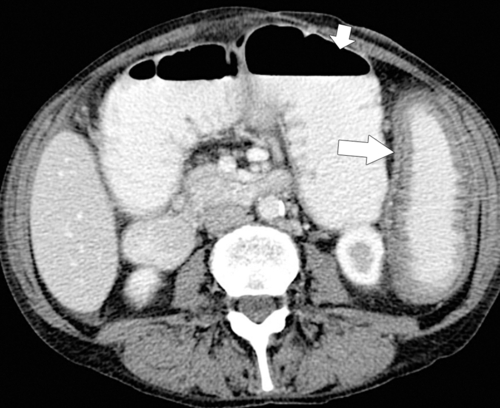
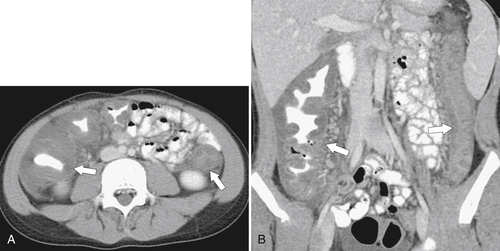
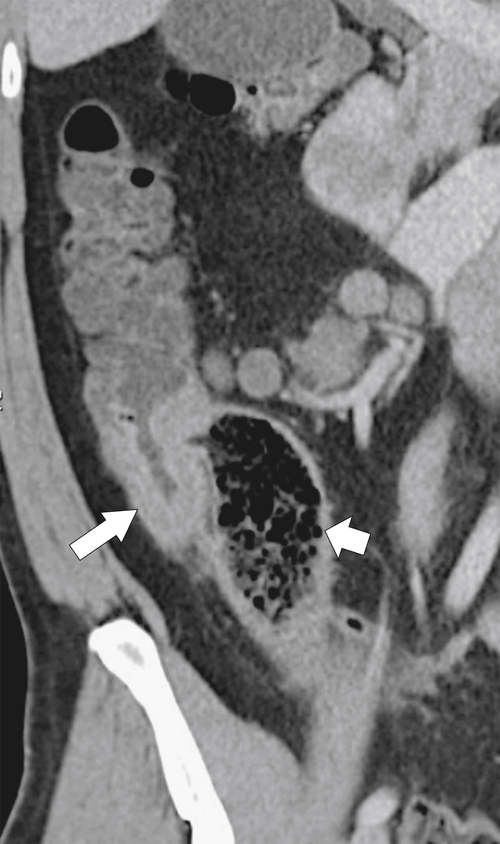
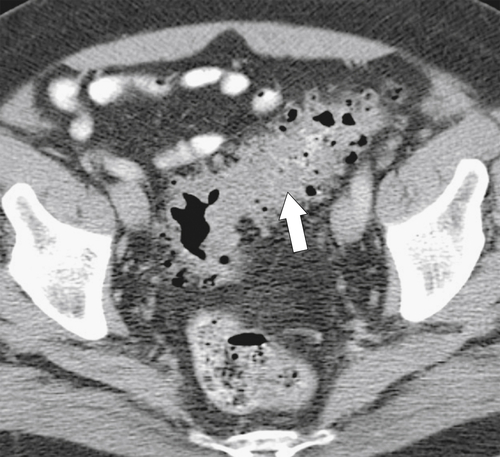
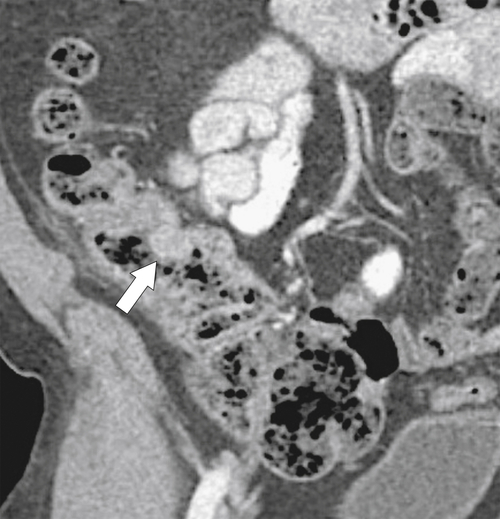
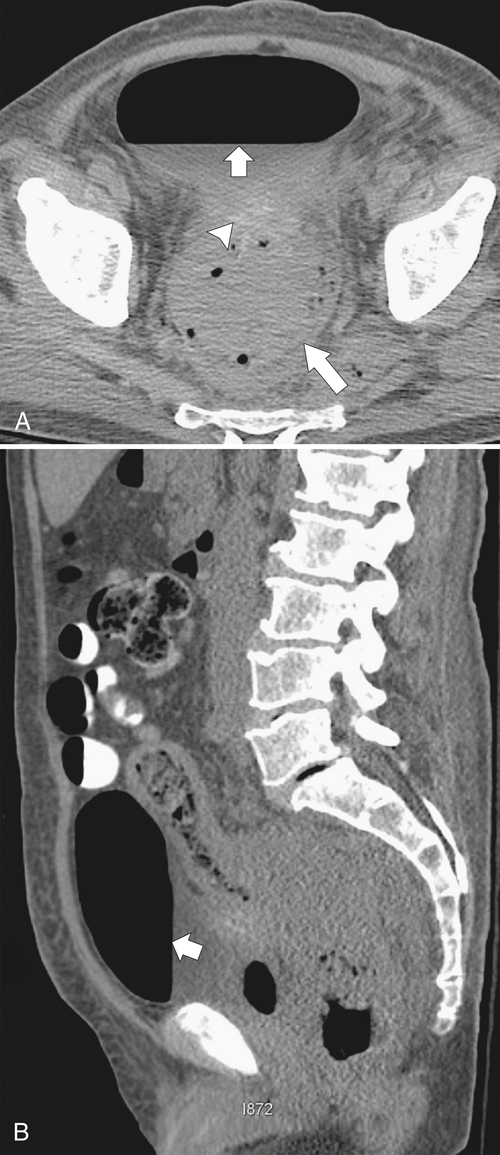
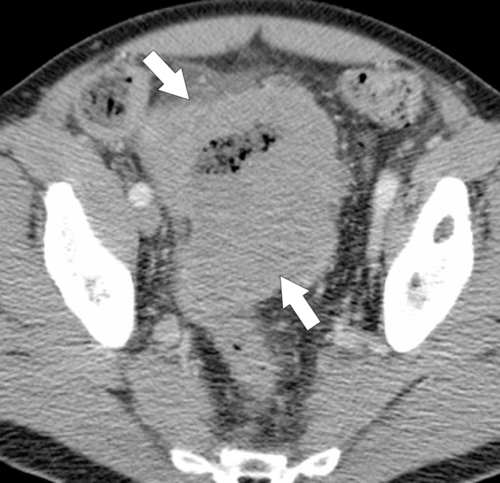
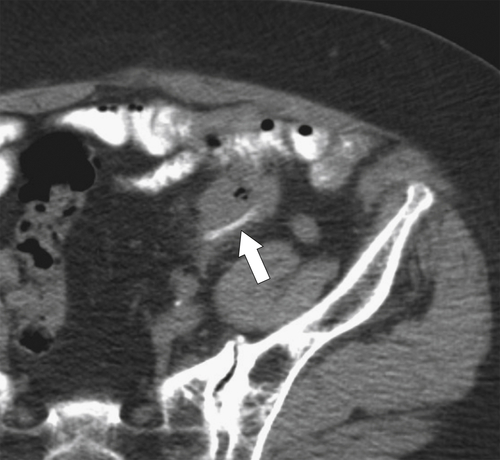
Figure 5-112 Axial contrast-enhanced CT in a 59-year-old woman with a large cecal mass (arrow) due to adenocarcinoma. There is regional metastatic adenopathy (small arrow).
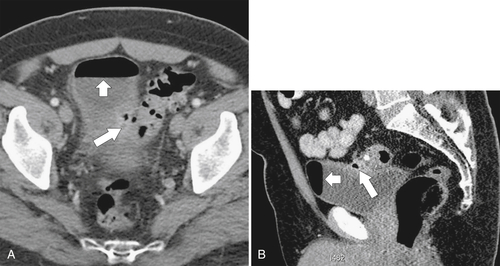
Figure 5-113 Axial (A) and coronal (B) contrast-enhanced CT in a 69-year-old man with a circumferential “apple-core” mass (arrows) of the rectosigmoid due to adenocarcinoma.
Figure 5-114 Axial contrast-enhanced CT in a 44-year-old woman with an inflammatory form of adenocarcinoma of the sigmoid (arrow).
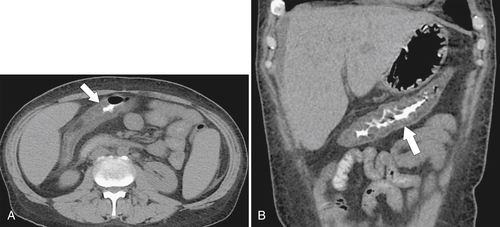
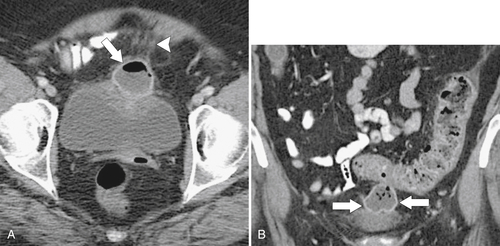
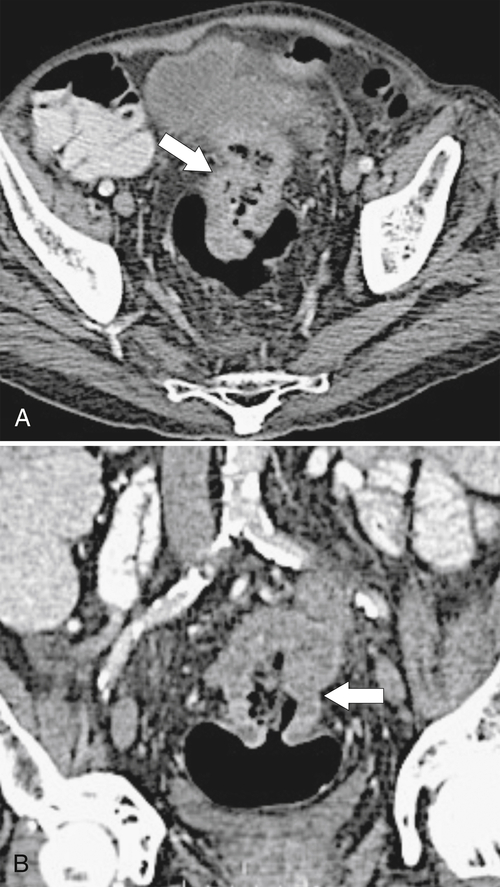
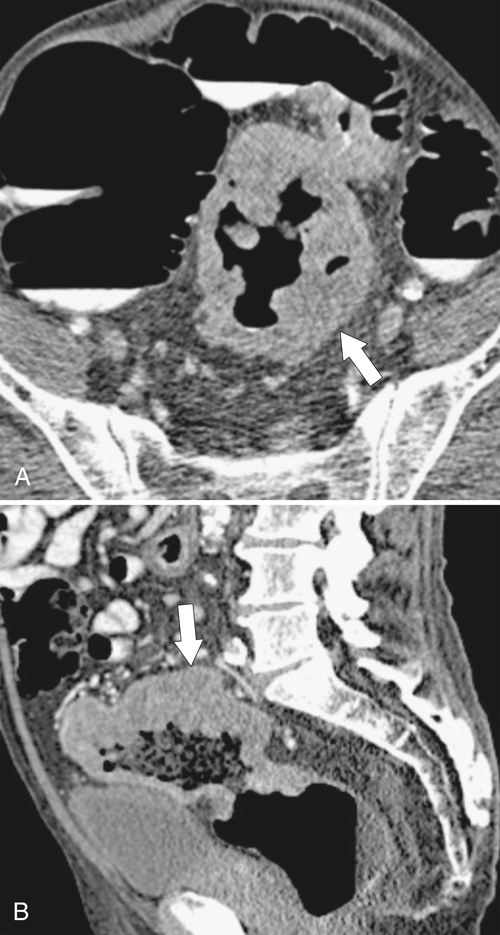
Figure 5-115 Axial (A) and sagittal (B) non-contrast-enhanced CT in a 76-year-old woman with a large rectal mass (arrow) due to rectal adenocarcinoma, which has directly invaded the posterior bladder wall (arrowhead ) and resulted in a rectovesical fistula and gas in the bladder (small arrows).
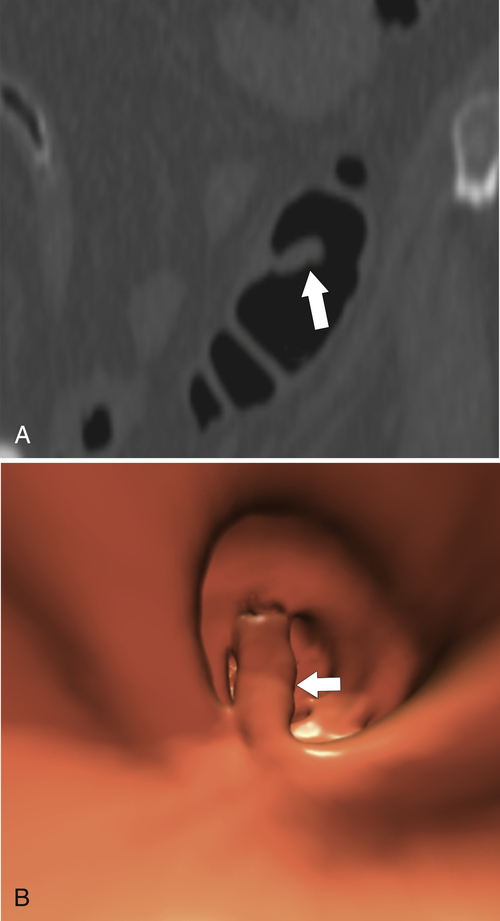
Experience with CTC is sufficiently widespread that all the features identified on conventional cross-sectional CT can now be observed (
Fig. 5-116). However, the main role of CTC is screening, ideally to detect adenomatous polyps before they degenerate into malignant lesions. Therefore large tumors are identified only uncommonly.
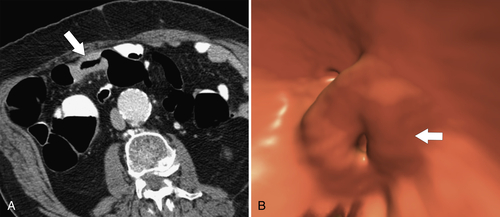
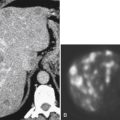
Figure 5-116 A and B, CTC in a 59-year-old man with an “apple-core” colon cancer in the transverse colon (arrows).
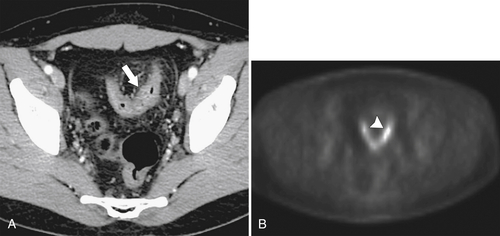
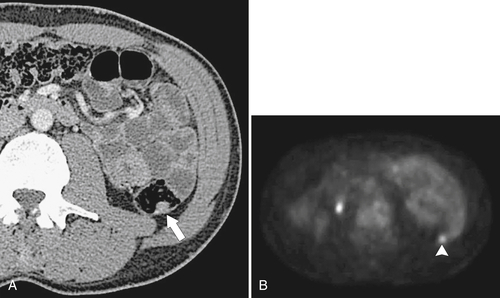
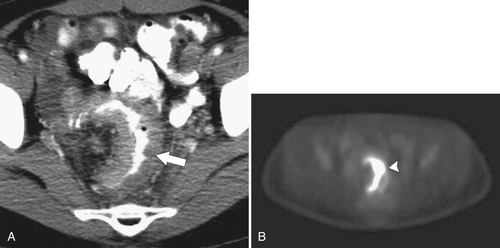
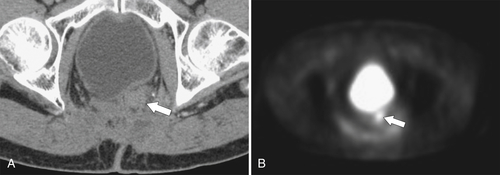
PET and PET/CT are used primarily for staging and monitoring of metastatic disease, but primary colorectal tumors, if large enough, should also be visualized (
Fig. 5-117). Small tumors may not be identified, either because FDG uptake is insufficient to be visible at PET or because the normal bowel can also have some FDG activity. Determination of what constitutes normal versus abnormal uptake is also aided by PET/CT fusion software, whereby the PET uptake findings can be correlated with the anatomical CT findings. Extracolonic spread of the tumor, including spread to regional nodes or distant metastatic disease, can also be identified with PET imaging. Some adenomatous polyps are also strongly FDG avid but cannot be identified with CT alone because they are small or obscured by feces (Fig. 5-93).
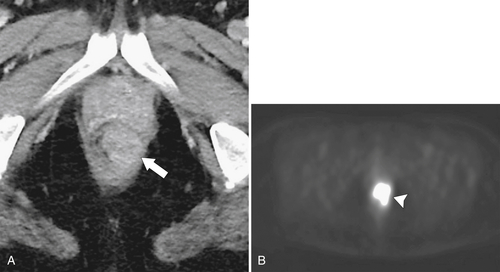
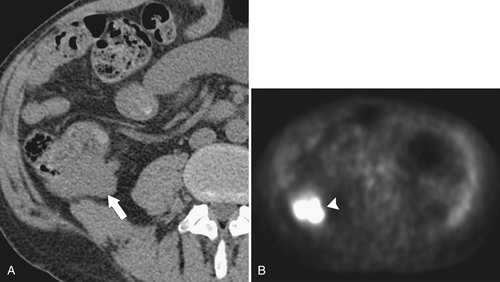
Figure 5-117 Axial contrast-enhanced CT (A) and PET (B) in a 35-year-old woman with rectal adenocarcinoma. There is a long segment of circumferential mural sigmoid thickening (arrow) that shows marked uptake on PET (arrowhead ).
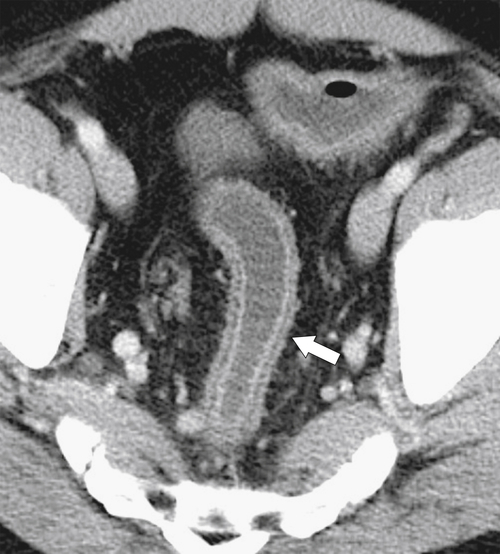
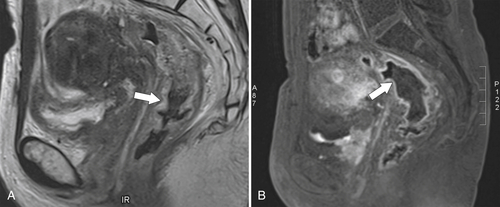
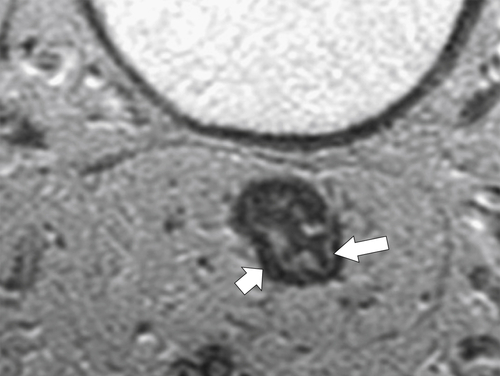
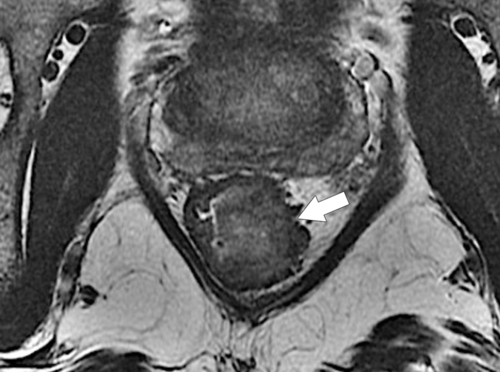
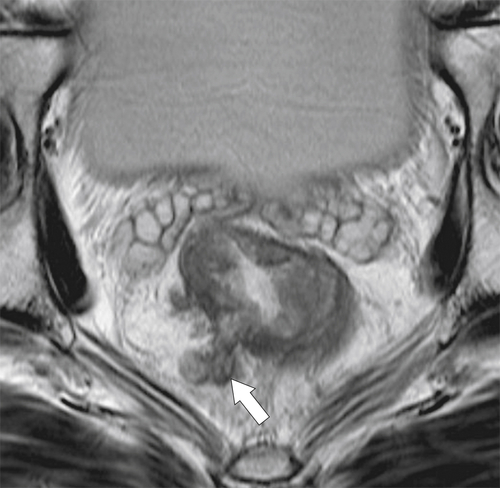
Preoperative staging is increasingly being performed using MRI, particularly using an endorectal coil, which has shown benefit in determining the TNM staging of rectal adenocarcinoma. It is important preoperatively to determine whether the disease extends into the mesorectal fascia because chemoradiation treatment may be required before the surgeon contemplates
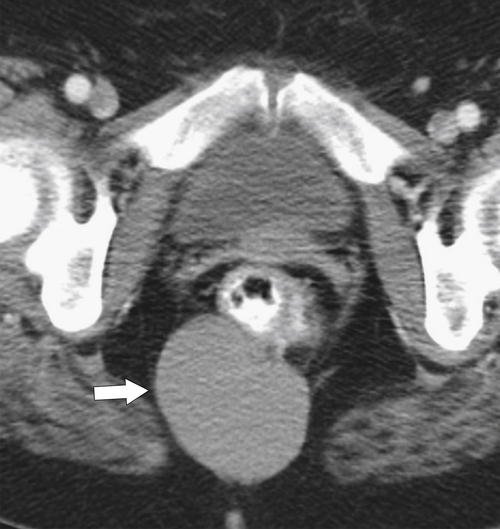
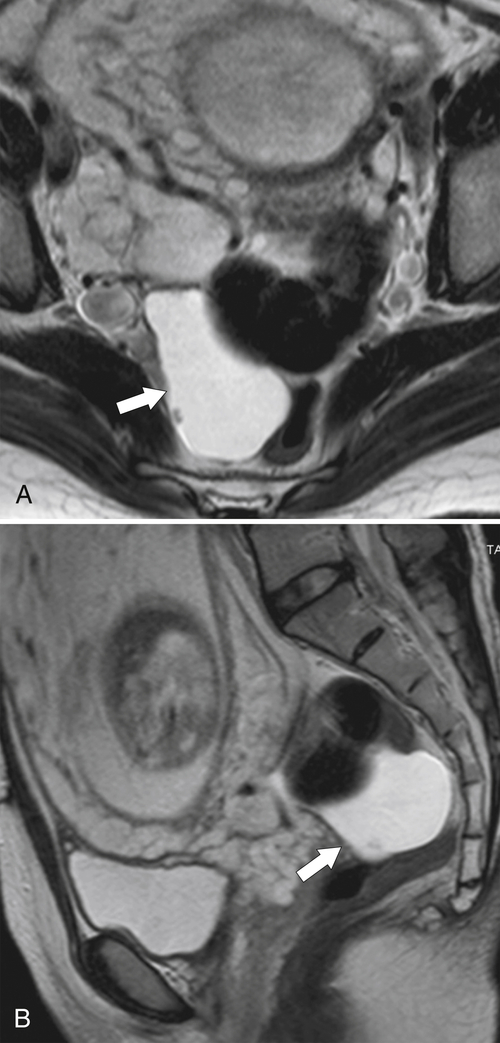
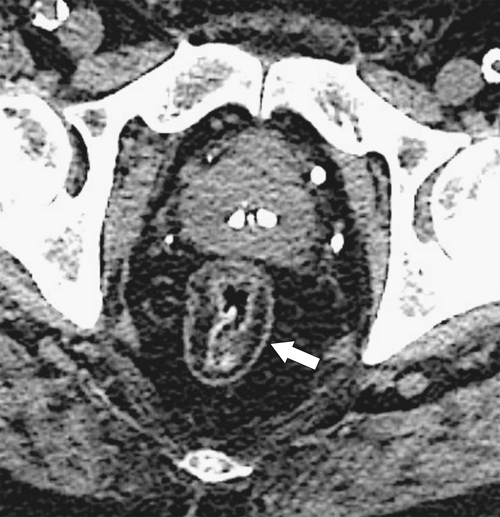
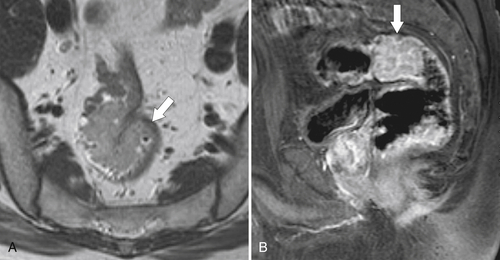
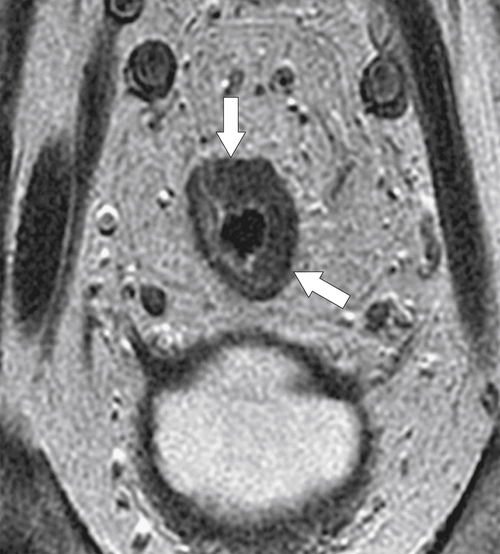
total mesorectal excision. The normal rectal submucosa has a T2 intermediate signal and hypointense serosa (Fig. 5-118). With T2 invasion, the brighter submucosa is replaced by tumor that is hypointense (Fig. 5-119). Further progression to T3 obliterates the distinction between the muscularis and serosa and extends into the perirectal fascia (Fig. 5-120). Differentiation between T2 and T3 disease can be difficult (Fig. 5-121).
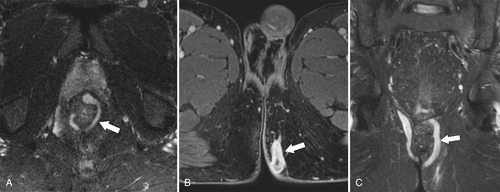
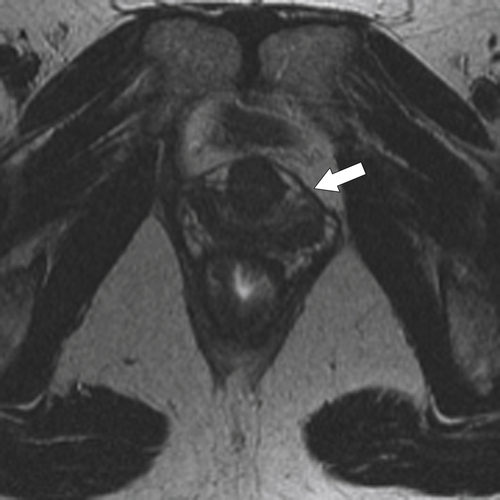
Figure 5-118 Axial T2-weighted MRI of normal rectal mucosa with T2 bright muscularis (arrow) and dark serosa (small arrow).
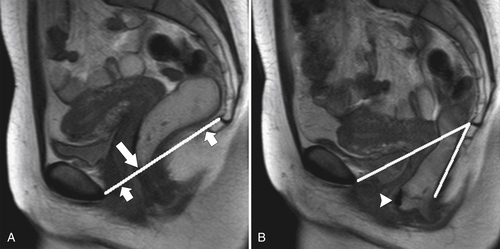
Figure 5-119 Axial T2-weighted MRI in a 56-year-old man with a T2 rectal adenocarcinoma. The hypointense (arrow) mass does not invade the serosa.
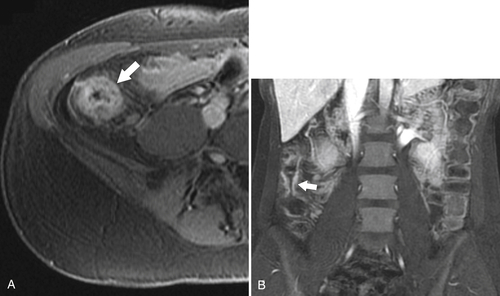
Figure 5-120 Axial T2-weighted MRI in a 66-year-old man with tumor extension through the serosa (arrow) consistent with a T3 tumor.
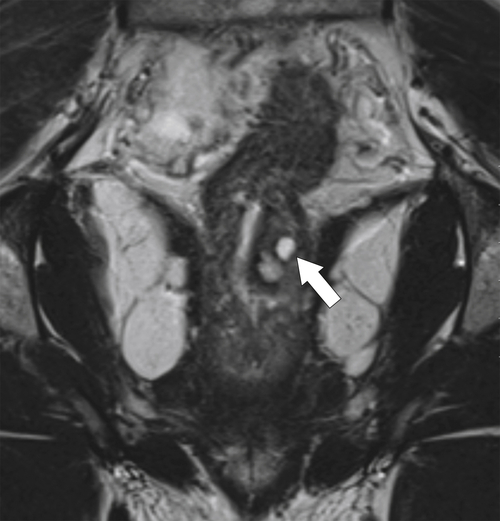
Figure 5-121 Coronal T2-weighted MRI in a 45-year-old man with a T2 rectal adenocarcinoma. The low signal tumor is between 11 o’clock and 5 o’clock (arrows), replacing the normal muscularis mucosa.
Colonic Lymphoma
Lymphoma has numerous subtypes and commonly affects the GI tract by direct extension from regional disease, by metastatic disease, or less commonly as a primary colonic non-Hodgkin lymphoma. Although specific imaging features can point to the diagnosis, colonic lymphoma can appear identical to adenocarcinoma anywhere in the GI tract (
Fig. 5-122). Although lymphoma is less common than adenocarcinoma, it should still be considered in the diagnosis, particularly if the lesion is focal, circumferential, or ulcerative (Fig. 5-122). Conversely, lesions can be diffuse and nodular, unlike adenocarcinoma. This rarer presentation is sometimes indistinguishable from polyposis syndromes, although the findings are usually confined to the cecum. Metastatic disease from either lymphomatous type is common, with local lymph node involvement.
Figure 5-122 Axial (A) and sagittal (B) contrast-enhanced CT in a 61-year-old man with a large irregular and ulcerating mass on the sigmoid colon due to colonic lymphoma (arrows).
When present in the large bowel, colonic lymphoma most commonly affects the cecum. The masses are often very large with diffuse circumferential bowel wall thickening (although eccentric polypoid masses also occur) and can be associated with bulky regional lymphadenopathy. A particular feature and a strong clue to the diagnosis is “aneurysmal” dilatation of the bowel lumen, as seen in the small bowel, because of destruction by lymphomatous cells of the myenteric plexus. It would be highly unusual, particularly if large, for an adenocarcinoma to show luminal dilatation. Rather, adenocarcinomas constrict the lumen and present with luminal narrowing and, if large enough, obstruction. Lymphoma typically does not present with bowel obstruction in either the small or large bowel. Increasingly, PET/CT is being used to evaluate the extent of disease and response to chemotherapy. Residual disease identified by CT may no longer represent active tumor by PET, termed a “metabolic response.”
Metastatic Disease
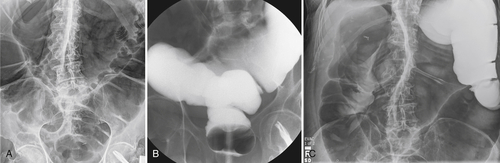
Metastases from noncolonic sites can invade the colon by one of three routes: intraperitoneal along the mesentery (e.g., stomach, pancreas), hematogenous (e.g., melanoma, breast, stomach, lung), or direct local extension (e.g., ovarian, bladder). The imaging appearances differ according to the form of metastatic spread. These range from small target-like (or bulls-eye) submucosal lesions, such as that seen in hematogenous spread from melanoma elsewhere in the GI tract, to a more substantial eccentric or circumferential mass from peritoneal seeding from stomach cancer (
Fig. 5-125). Direct spread from adnexal malignancies generally causes luminal distortion and stricture (Fig. 5-126). Small submucosal lesions are unlikely to be identified by CT, but larger lesions should be (Fig. 5-127). Metachronous or synchronous colorectal cancer should be considered in the diagnosis because of their frequency in patients with a known history of this disease (Fig. 5-102).
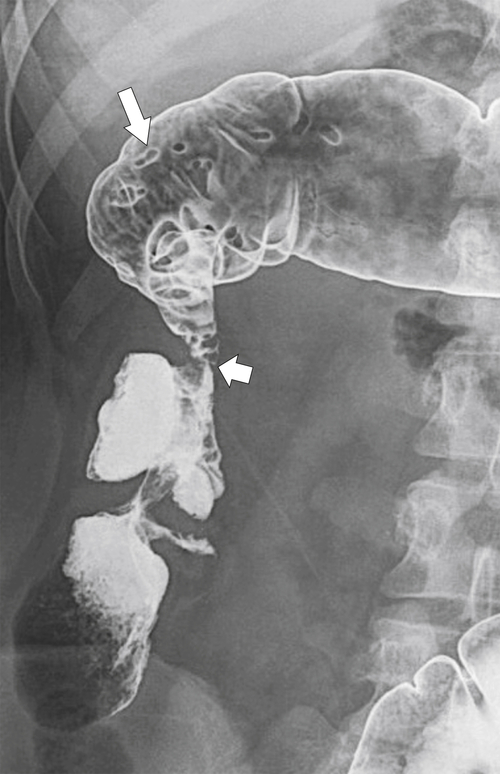
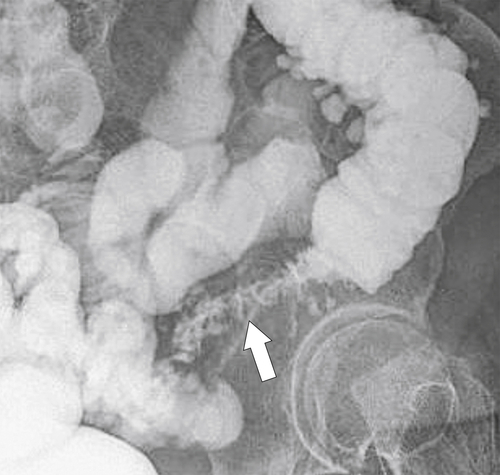
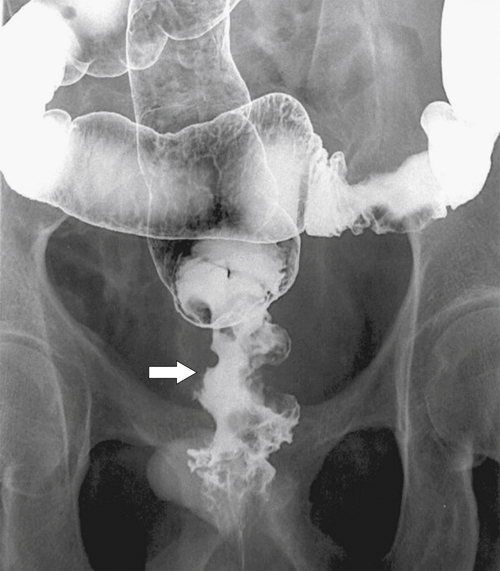
Figure 5-125 Single-contrast BE in a patient with metastatic gastric cancer and a circumferential upper rectal narrowing (arrow) due to serosal gastric metastases.
Figure 5-126 DCBE in a 56-year-old man with a stricture (arrow) of the distal transverse colon due to direct invasion from gastic cancer.
Figure 5-127 Axial contrast-enhanced CT (A and B) in a 39-year-old man with a 4-cm parasigmoid mass (arrows) due to a carcinoid metastasis.