CHAPTER 5
Colon
INTRODUCTION
The colon can be divided into six segments both anatomically and endoscopically. Unlike in the stomach, the histology of the colon is relatively uniform. These segments can be divided into the cecum, where the ileocecal valve and appendiceal orifice serve as important landmarks; ascending colon; transverse colon; descending colon; sigmoid colon; and rectum. Appreciation of the endoscopic differences between regions is important, particularly when dealing with colonic neoplasms, where accurate localization is essential.
In contrast with the upper gastrointestinal tract, in the colon, diagnosis and therapy of neoplasms assume a prominent role. Although adenomatous polyps are the most frequent neoplastic lesions, a variety of other polyps may masquerade endoscopically; subtle mucosal differences may aid in distinguishing these impostors. Inflammatory disorders such as Crohn’s disease and ulcerative colitis represent another important group of diseases. Other inflammatory disorders, including ischemia and infections (bacterial and viral), assume greater importance in the colon than in the upper gastrointestinal tract. Many of these inflammatory processes appear endoscopically similar; however, differentiation can usually be accomplished based on the characteristics of the patient, location of disease (pancolonic versus segmental), and characteristics of the disease in the involved segment (e.g., circumferential versus patchy; ulcer versus no ulcer).
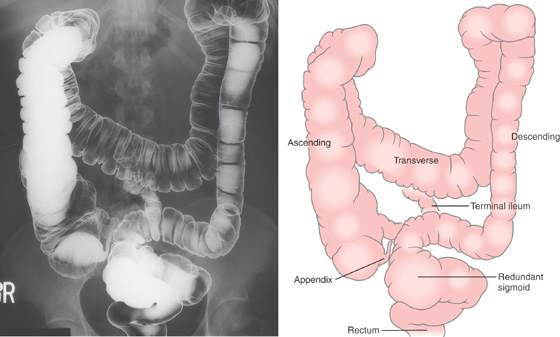
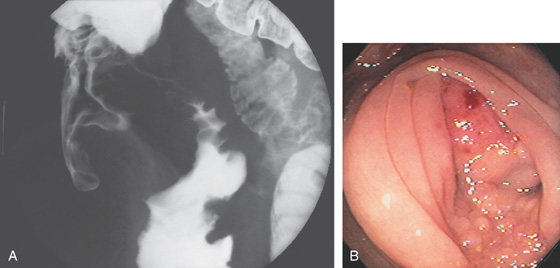
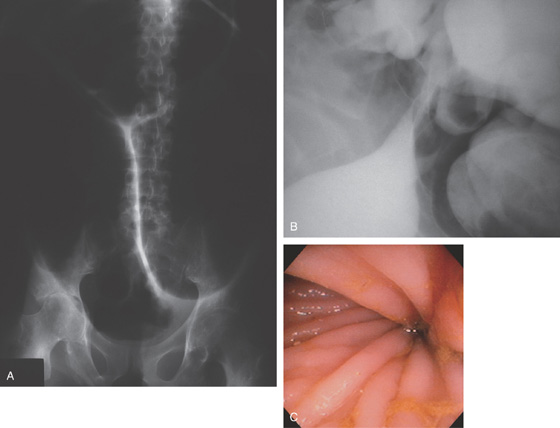
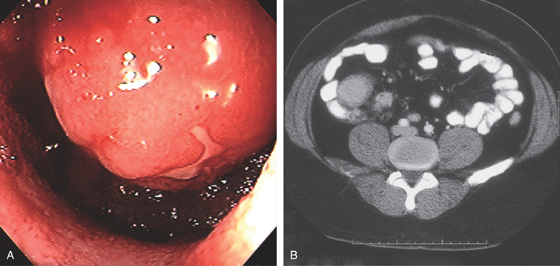
Figure 5.1 BARIUM ENEMA
An air-contrast barium enema demonstrates the normal anatomy of the colon. The sigmoid colon is redundant. The transverse colon dips inferiorly into the pelvis. The ascending and descending colon are retroperitoneal and fixed. The sigmoid and transverse colon have suspending mesentery and are thus mobile. The appendix is filled and seen in the pelvis. Reflux of barium demonstrates the distal terminal ileum.
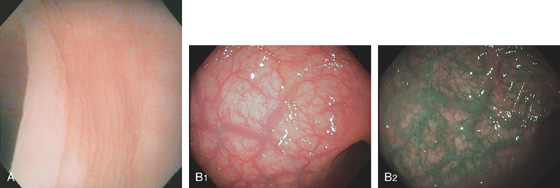
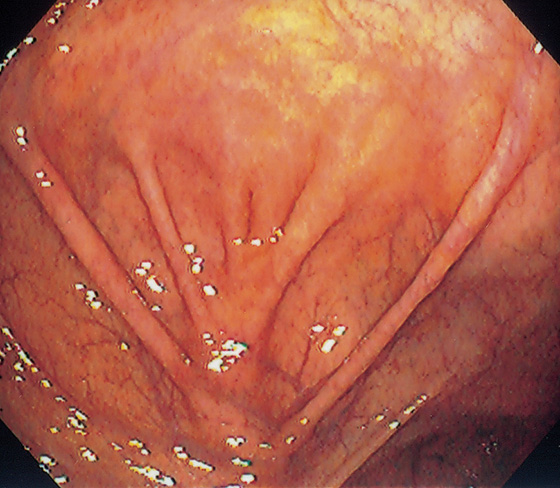
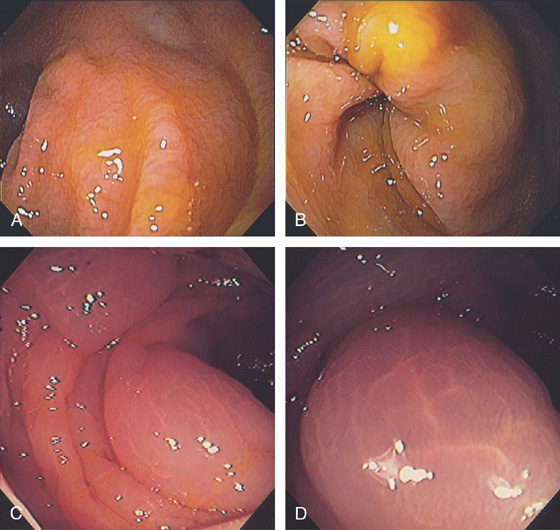
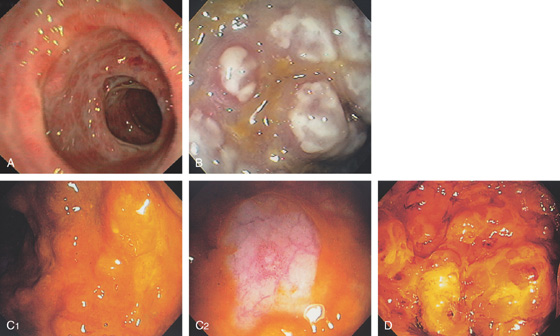
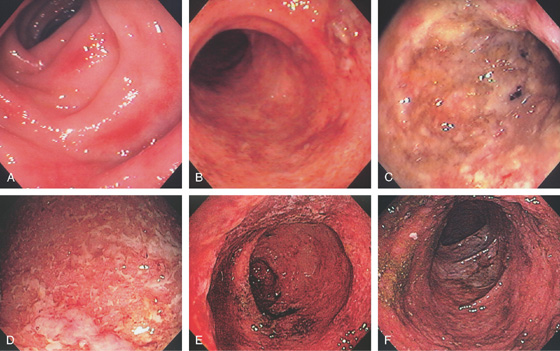
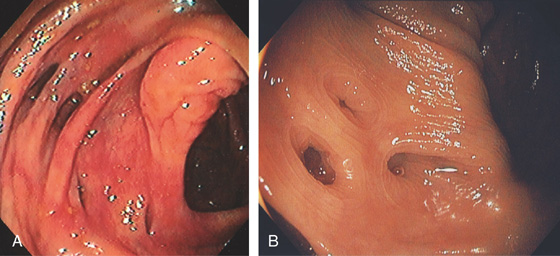
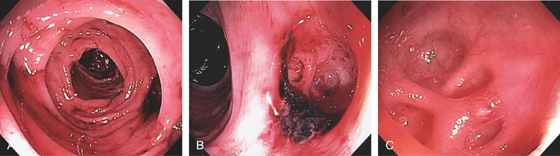
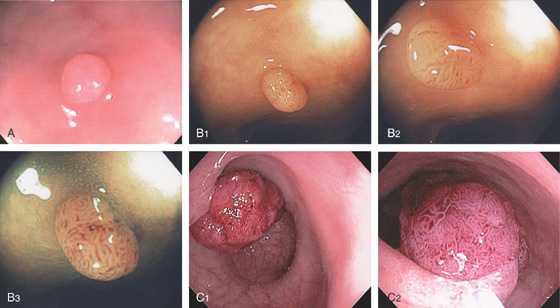
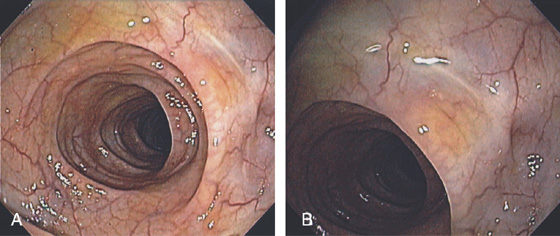
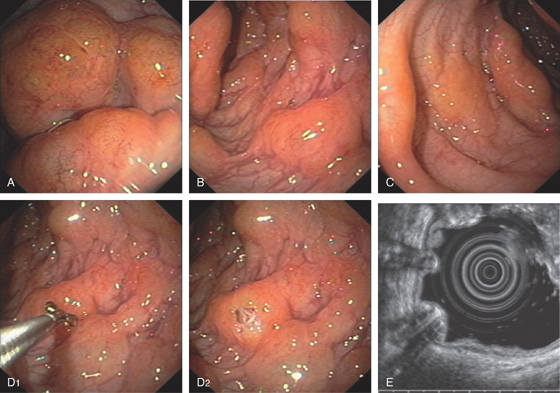
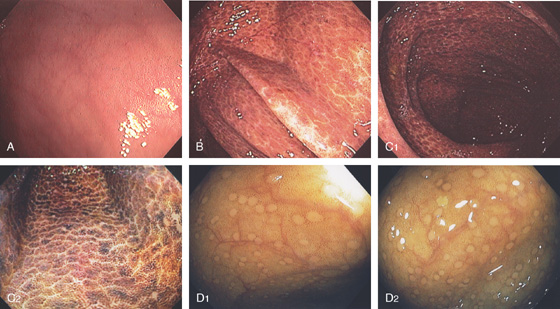
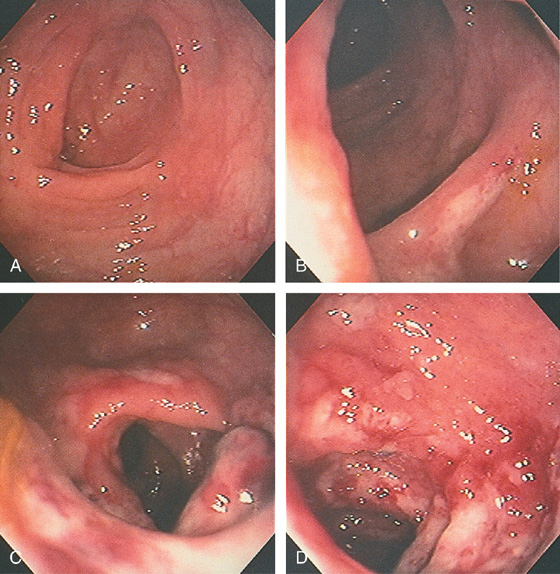
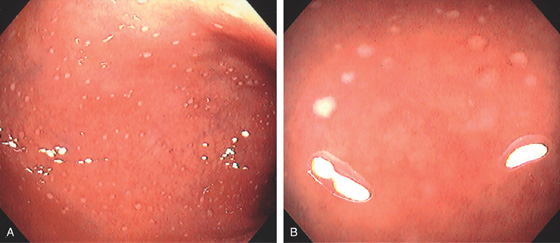
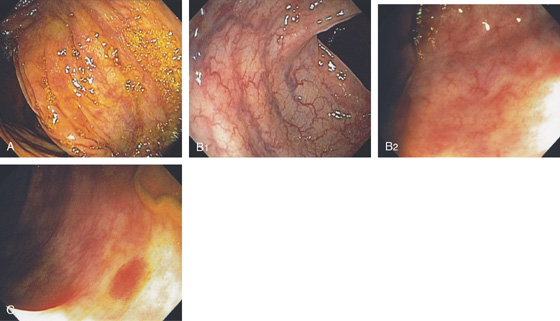
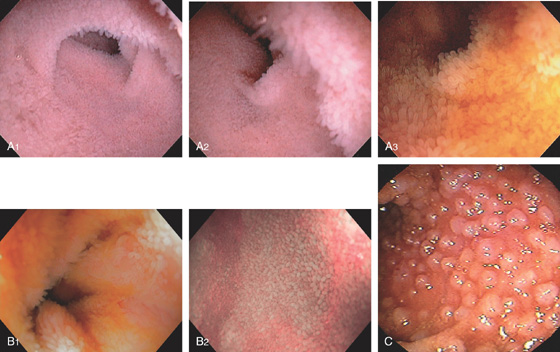
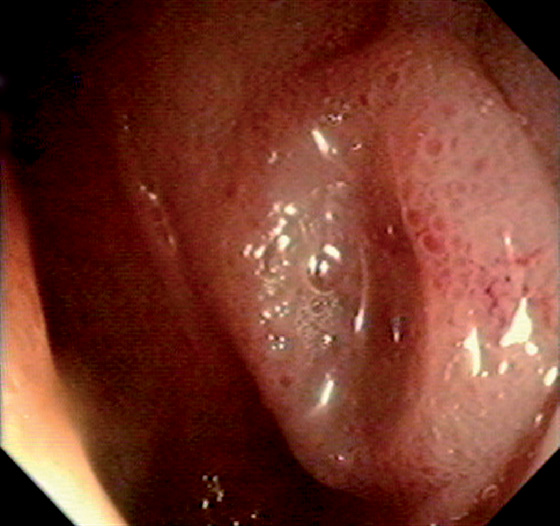
Figure 5.2 NORMAL MUCOSAL PATTERN
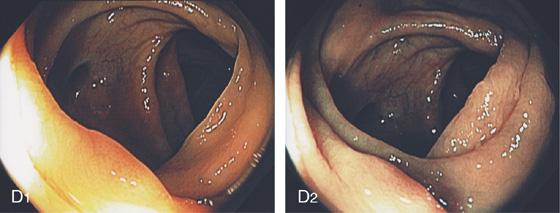
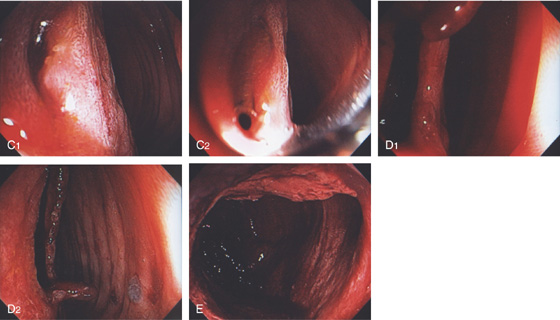
A, The normal descending colon visualized underwater demonstrates a linear appearance of the mucosa. B1, The normal colonic mucosal vascular pattern as seen on high-definition endoscopy. B2, The mucosal vascularity is now green when visualized by narrow band imaging.
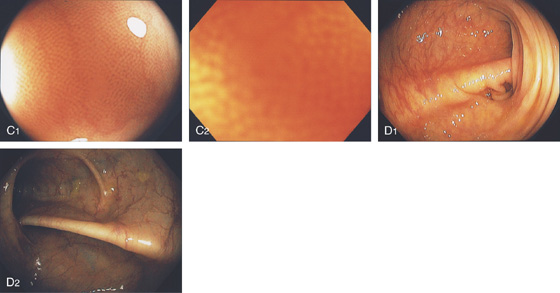
C1, C2, Close-up of the colonic mucosa demonstrates a honeycomb-type pattern. D1, At the flexures, one can oftentimes see the longitudinal colonic muscle, or teniae, which provides the direction of the lumen (D2).
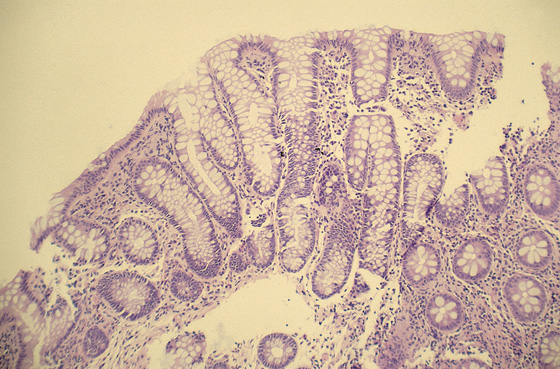
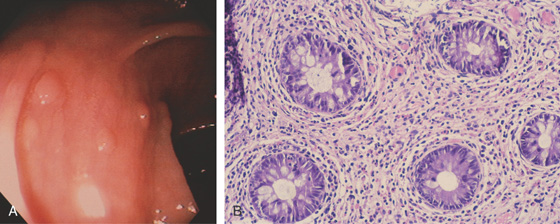
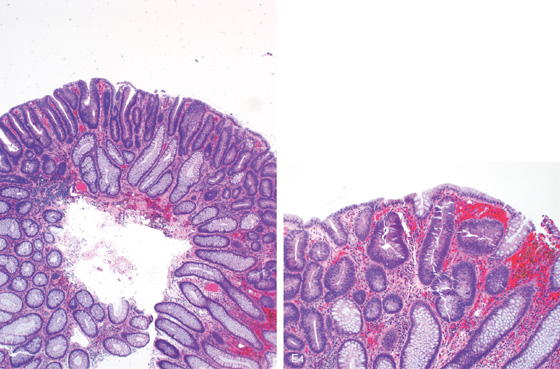
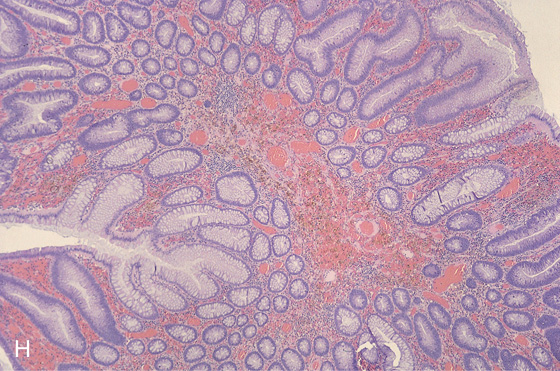
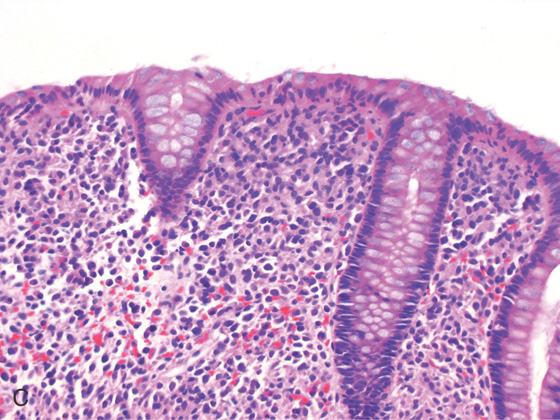
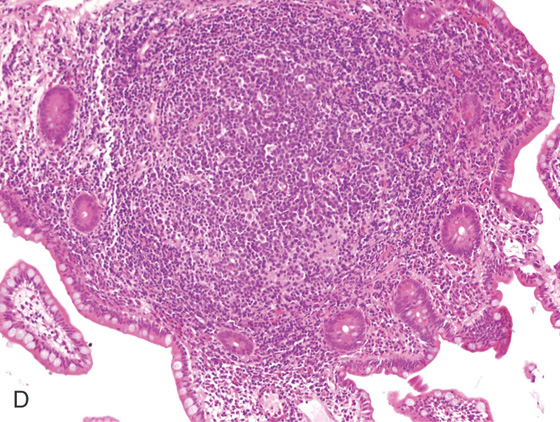
Figure 5.3 HISTOLOGY
Normal colonic architecture. The crypts form the multiple round structures seen in cross section.
Figure 5.4 SIGMOID COLON
A, Circular folds of modest thickness are identified. B, The sigmoid colon demonstrates thickened circular folds corresponding to hypertrophied musculature. Several diverticula are present.
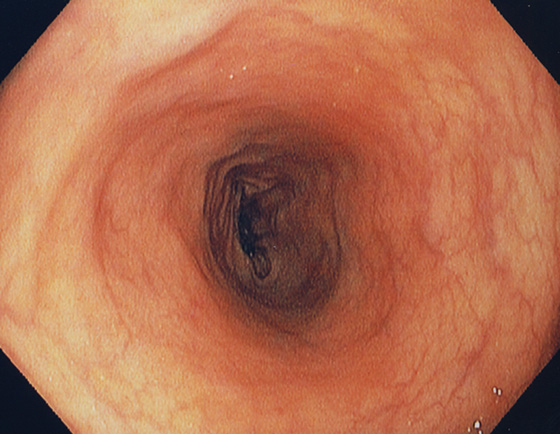
Figure 5.5 DESCENDING COLON
The descending colon forms a long tube and is relatively featureless.
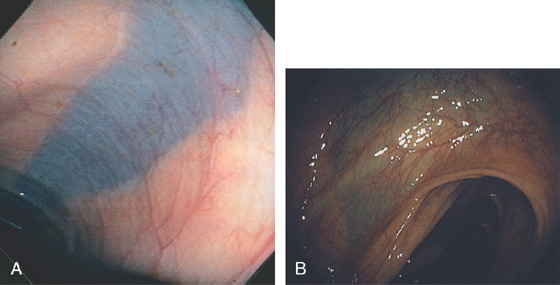
Figure 5.6 SPLENIC FLEXURE
A long, bluish indentation from the spleen. Normal colonic vasculature is seen. A, Long bluish indentation from the spleen, with normal overlying colonic vasculature. B, Dark area easily identified under a normal colonic vascular pattern.
Figure 5.7 TRANSVERSE COLON
A-C, The typical-appearing triangular folds of the transverse colon.
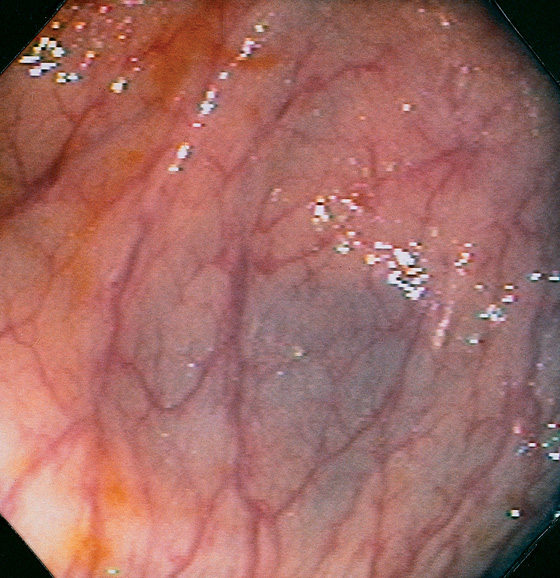
Figure 5.8 HEPATIC FLEXURE
The hepatic flexure is noted by the darkish hue from the liver. Normal colonic vasculature is seen overlying the bluish hue of the liver. Visualization of the adjacent liver will depend on the position of the patient.
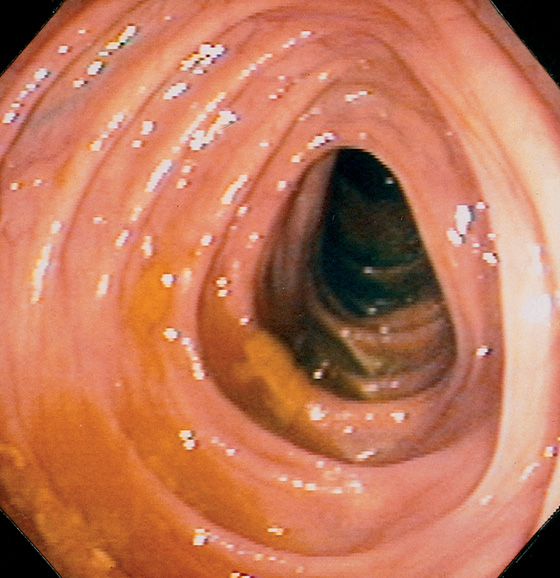
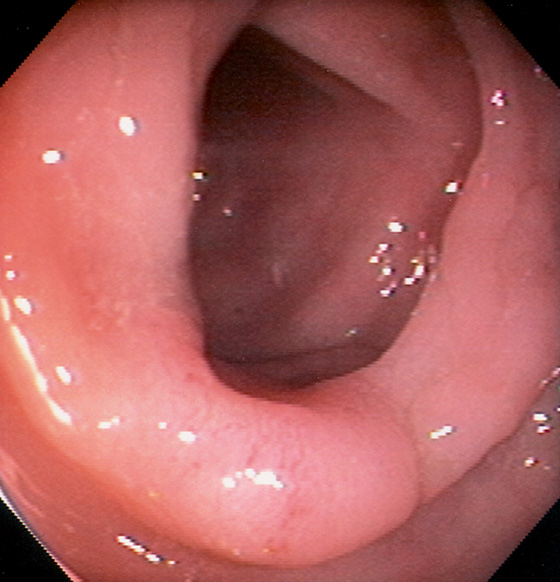
Figure 5.9 ASCENDING COLON
The ascending colon may also have triangular folds. The ileocecal valve is in the distance.
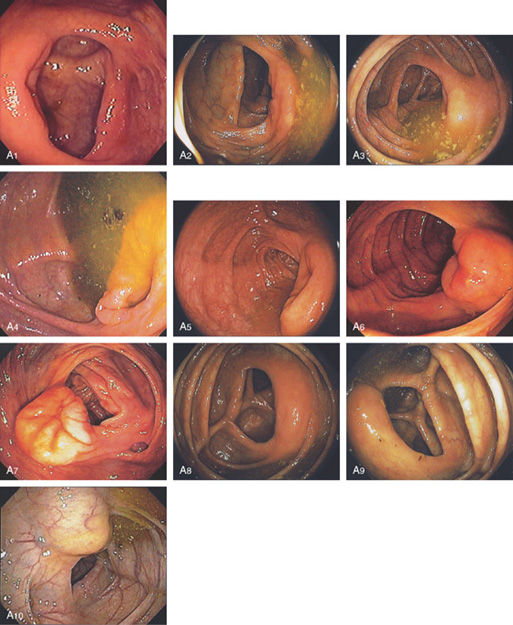
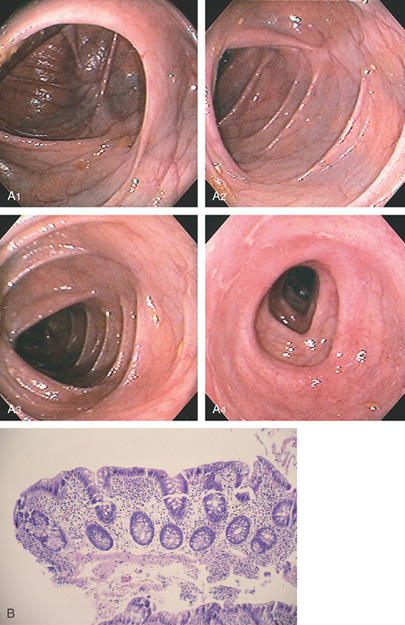
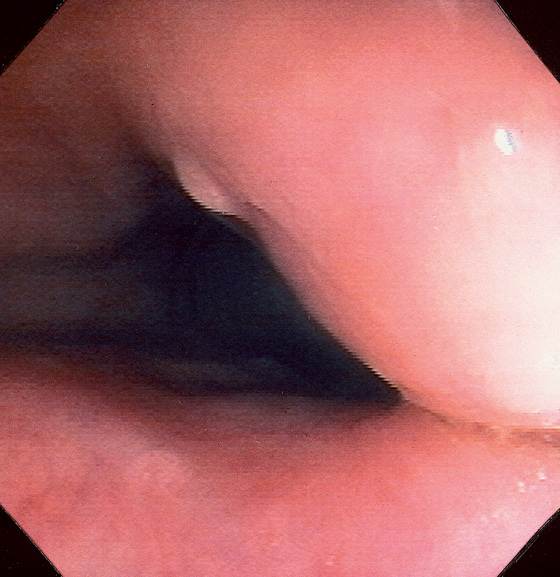
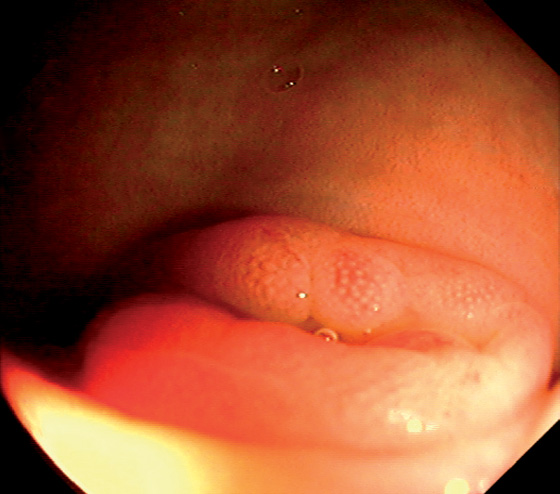
Figure 5.10 ILEOCECAL VALVE
A, Appearance of a normal ileocecal (IC) valve (A1-A10). Note that fluid in the right colon puddles by the IC valve when the patient is in the left lateral decubitus position regardless of the rotation of the endoscope and can be used as a landmark.
Figure 5.11 CECAL POLE
The slit of the appendiceal orifice resides at the base of the cecal pole. Folds radiate from the base of the cecum to the transverse cecal fold.
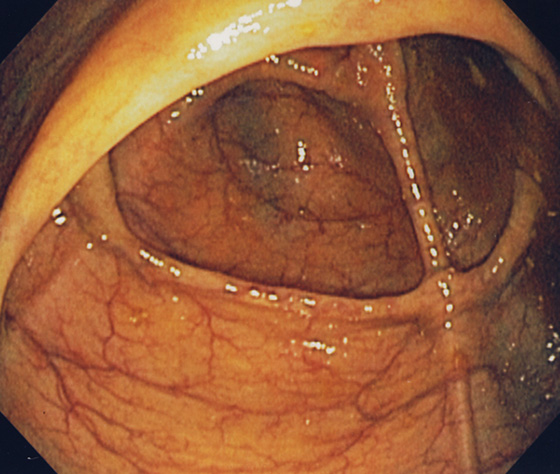
Figure 5.12 CECUM
The thickened yellow structure represents the ileocecal valve. On the contralateral wall, the thickened tinea coli converge with several others forming the transverse cecal fold, “crow’s foot” or cecal strap.
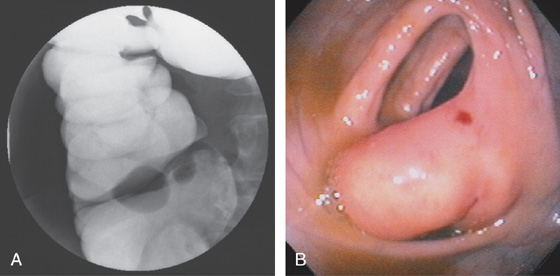
Figure 5.13 LIPOMATOUS ILEOCECAL VALVE
A, A smooth filling defect extending from the medial wall at the level of the ileocecal valve. B, The filling defect represents a bulbous (fatty) ileocecal valve. The red area on the fatty valve resulted from endoscopic trauma.
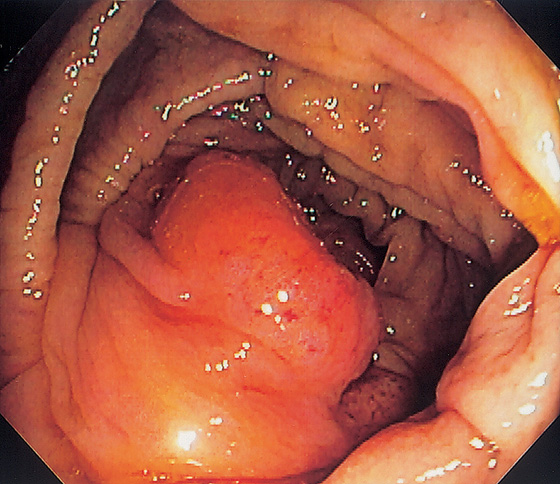
Figure 5.14 PROLAPSED ILEOCECAL VALVE
Masslike appearance from prolapse of the valve. Biopsy confirmed ileal epithelium.
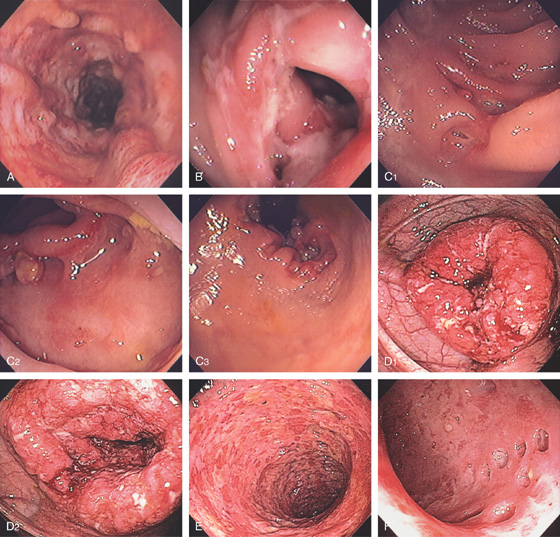
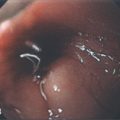
Figure 5.15 APPENDICEAL ORIFICE
A1, A2, Slitlike semilunar structure at the base of the cecum. B1, B2, Slitlike opening at the base of the cecum. The “leopard skin” pattern around the appendiceal orifice represents lymphoid follicles that are frequently identified in the cecal pole. B3, B4
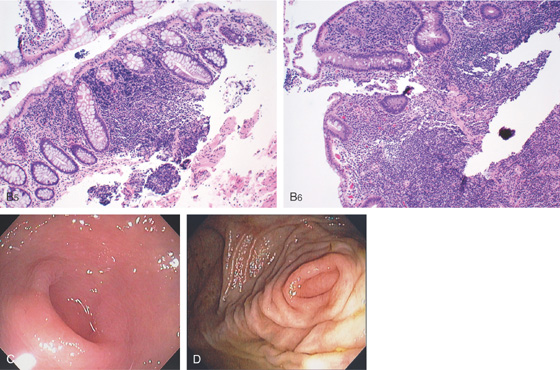
Narrow band imaging highlights the lymphoid follicles (B5, B6). C, Circular raised area at the base of the cecum. The appendiceal orifice is at the base of this structure. D, Multiple circular folds surround the appendiceal orifice.
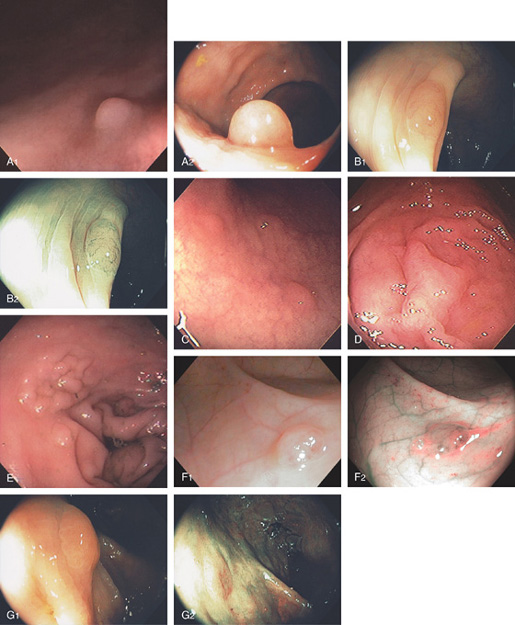
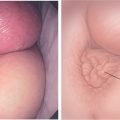
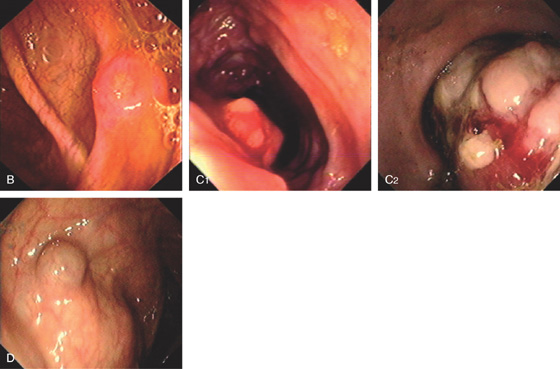
Figure 5.16 EVERTED APPENDIX
A1, Circular folds emanating from an indentation in the cecal pole. A2, With further observation, the appendix was seen to spontaneously evert, simulating a polyp. B, Fleshy tissue emanating from the base of the cecum.
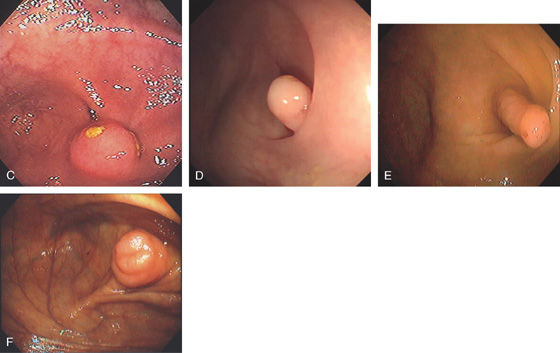
C, Round, submucosal-appearing lesion at the base of the cecum with overlying exudate. This patient had a prior appendectomy. D, Short finger-like projection from the base of the cecum resembling a pedunculated polyp. E, Long finger-like projection from the base of the cecum resembling a pedunculated polyp. F, Round polypoid lesion at the base of the cecum resembling a sessile polyp.
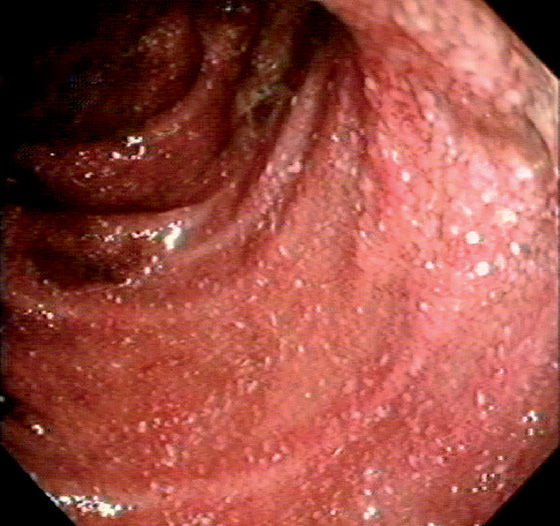
Figure 5.17 COLONIC EDEMA
A, B, Mild colonic edema highlights the normal mucosal pattern. C, Marked edema of the colon with loss of vascular pattern. D, Close-up shows the accentuated architecture of the mucosal pattern.
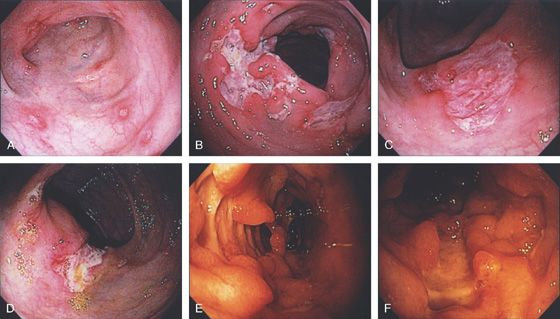
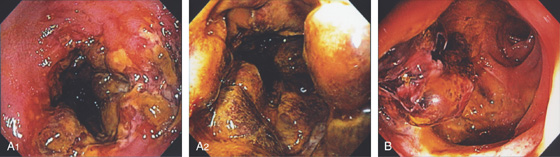
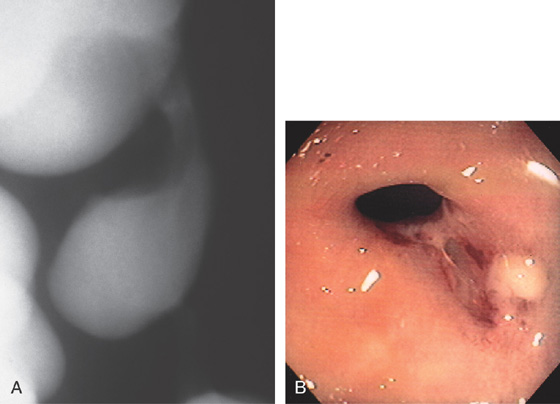
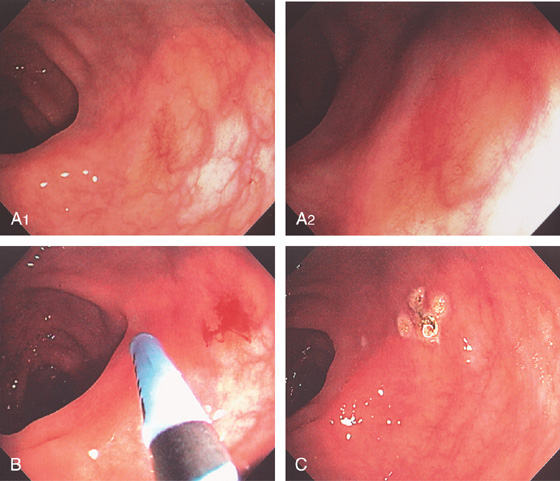
Figure 5.18 ACUTE CULTURE-NEGATIVE BACTERIAL COLITIS
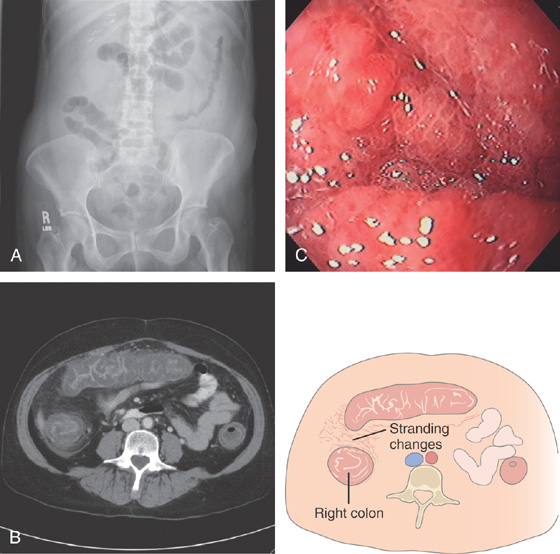
A1, Edema, loss of vascular pattern, subepithelial hemorrhage, and exudate in the descending colon. A2, Stool and pus are in the ascending colon. B1, Diffuse subepithelial hemorrhage with thick mucopus. B2, Striking subepithelial hemorrhage of the distal colon. Note the subepithelial hemorrhage spares the lymphoid follicles, creating a honeycomb pattern.
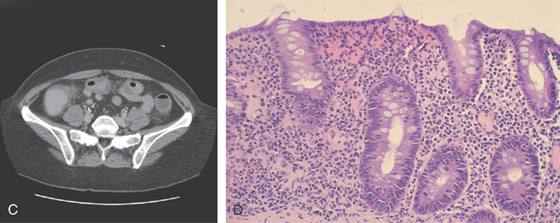
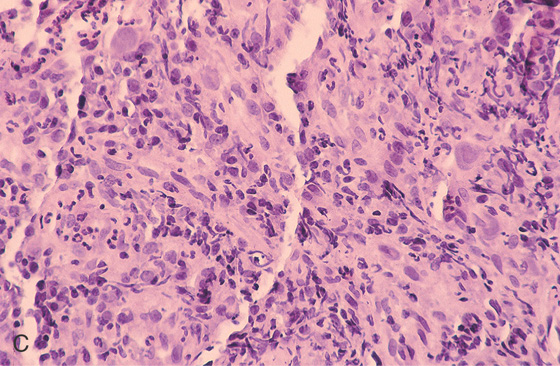
C, Pancolitis with circumferential wall thickening in each segment. The bowel wall thickening is accentuated by the fluid-filled colon. Note the inflammatory process (stranding) extends around the colon, most striking in the cecum and descending colon. D, The colonic mucosa has both an acute and a chronic inflammatory infiltrate and mild cryptitis. The crypt architecture is preserved, suggesting acute rather than chronic colitis.
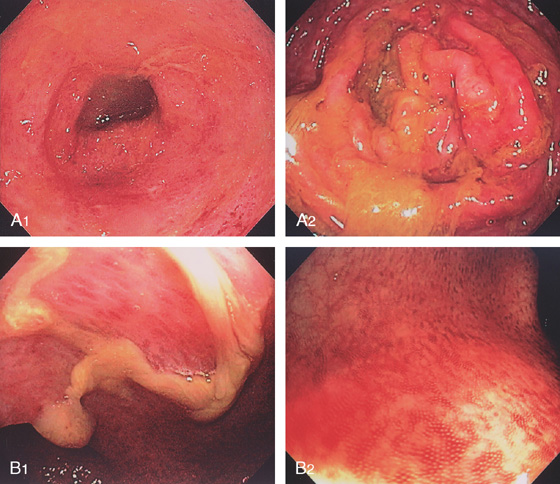
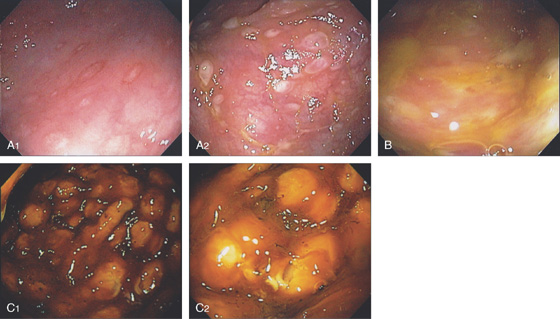
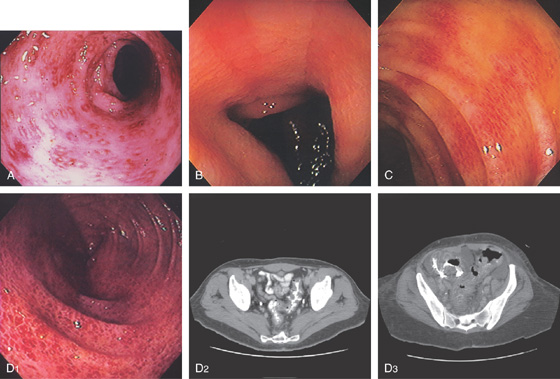
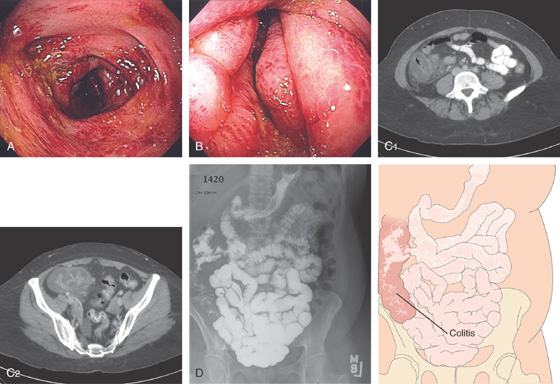
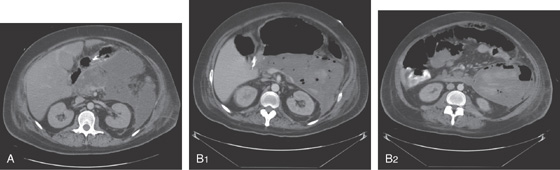
Figure 5.19 SALMONELLA COLITIS AND ILEITIS
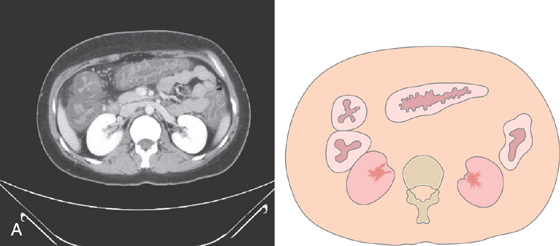
A, Edema, granularity, and mucopus are in the sigmoid colon. B, Similar findings are present in the ileum. C1, C2, The colon is mildly edematous. The colon wall enhances with intravenous contrast injection. There is mild thickening of the left colon (C3).
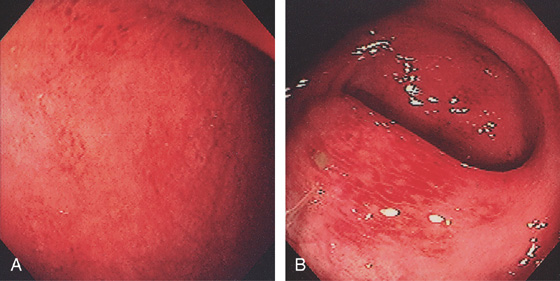
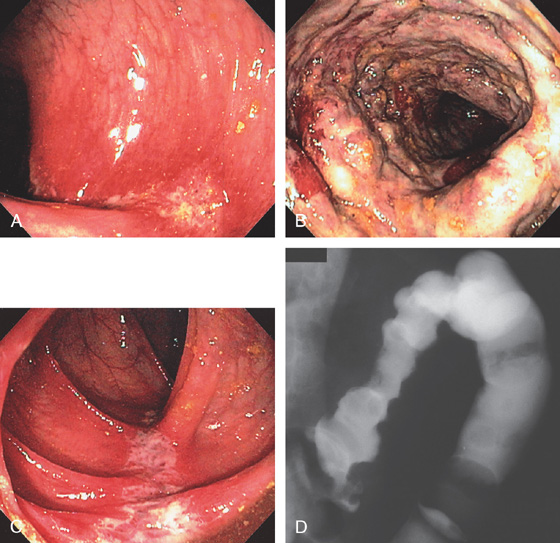
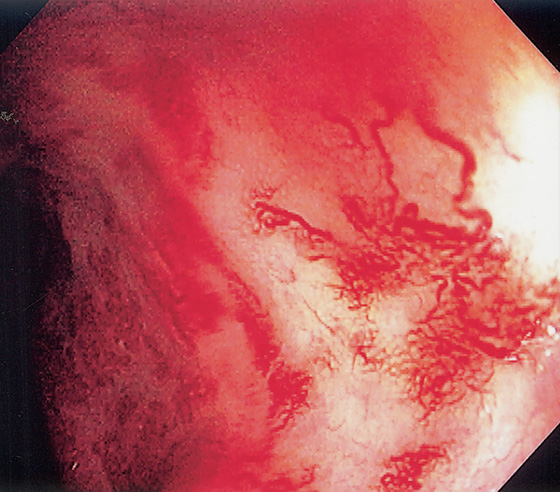
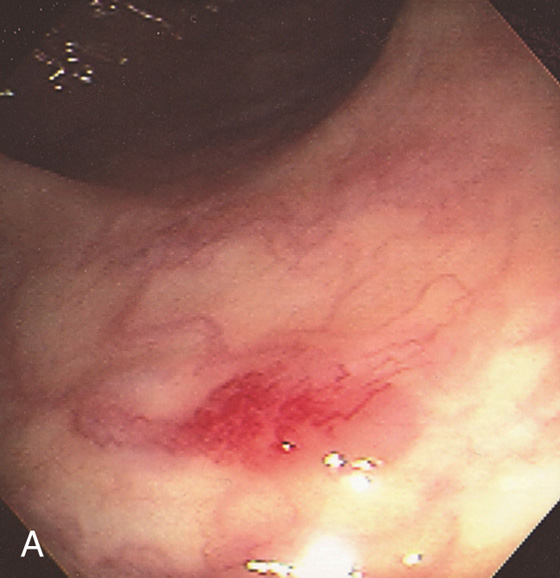
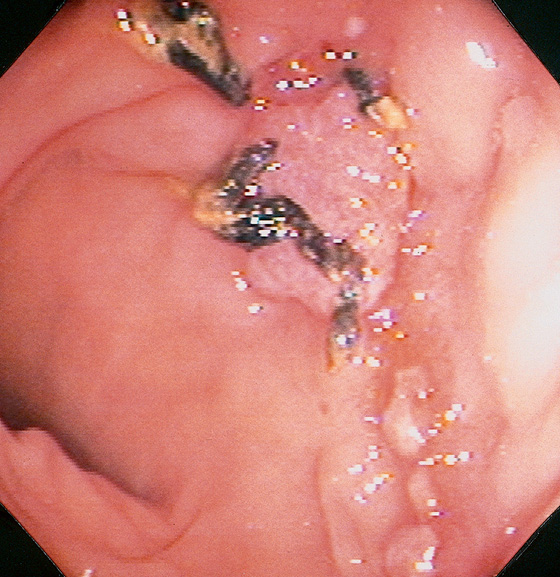
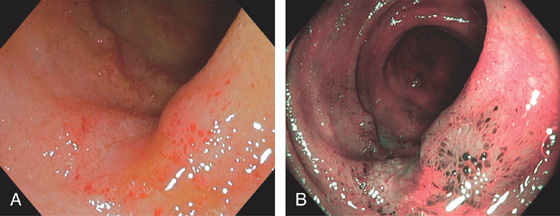
Figure 5.20 CAMPYLOBACTER COLITIS
A, Diffuse erythema with patchy subepithelial hemorrhage in the distal colon. Stool culture was positive for Campylobacter jejuni. B, A small ulcer surrounded by subepithelial hemorrhage is shown more proximally.
![]() Differential Diagnosis
Differential Diagnosis
Campylobacter Colitis (Figure 5.20)
Inflammatory bowel disease, ulcerative colitis
Ischemia
Other infections (bacterial, viral)
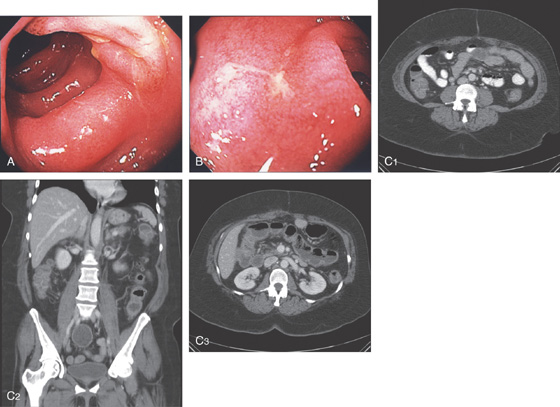
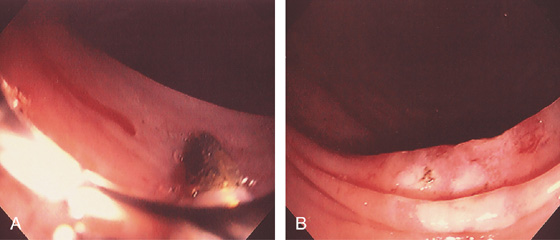
Figure 5.21 ESCHERICHIA COLI 0157 H7
A, Thumbprinting and luminal narrowing of the distal transverse colon. B, Pancolitis with mucosal enhancement. Note the stranding changes around the right colon. C, Diffuse edema and subepithelial hemorrhage of the distal colon. Stool culture was positive.
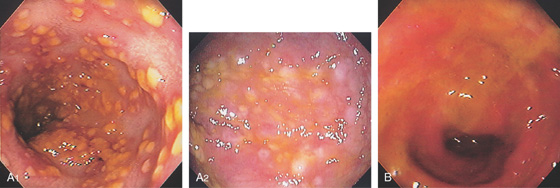
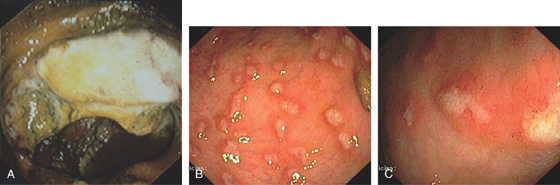
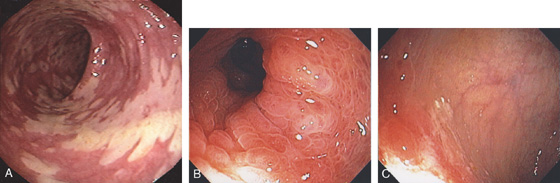
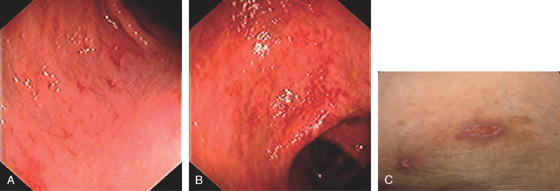
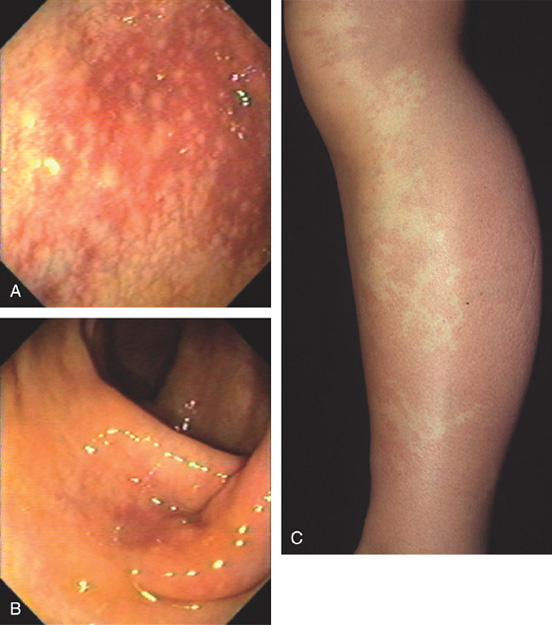
Figure 5.22 CLOSTRIDIUM DIFFICILE COLITIS
A1, A2, Characteristic multiple circular, plaquelike lesions of the distal colon. Note the halo of erythema and the loss of vascular pattern in the surrounding mucosa. B, Diffuse erythema and edema of the distal colon with overlying exudate.
Figure 5.23 CLOSTRIDIUM DIFFICILE COLITIS
A, Patchy circular subepithelial hemorrhage in the distal colon. There is colonic edema with loss of the normal mucosal vascular pattern. B, Multiple raised white plaquelike lesions. C1, Multiple nodules underneath a thin coating of stool. C2, Washing of the lesions demonstrates pinpoint erosions. D, Multiple bullae of the distal colon.
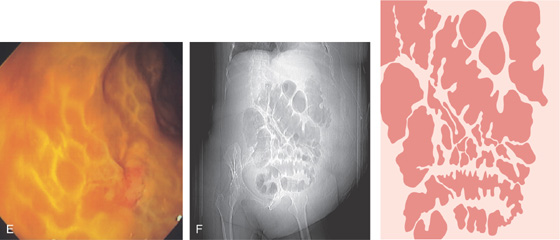
E, Severe edema with overlying exudate. The honeycomb appearance of the mucosa is apparent and is caused by the marked edema. F, Thumbprinting is apparent.
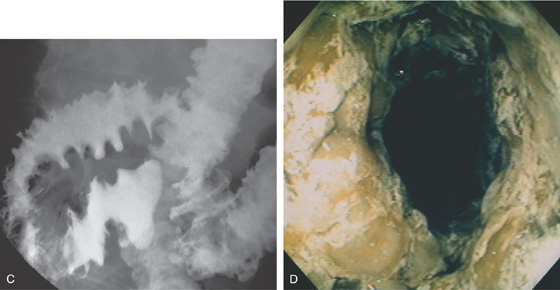
Figure 5.24 CLOSTRIDIUM DIFFICILE COLITIS
A1, Patchy, well-circumscribed areas of erosion with surrounding hyperemia. A2, More proximally, the colitis becomes more severe and the typical pseudomembranes are observed. B, Flat pseudomembranes with areas of mucopus. C1, Multiple pseudomembranes have a nodular appearance. C2, No normal mucosa can be appreciated.
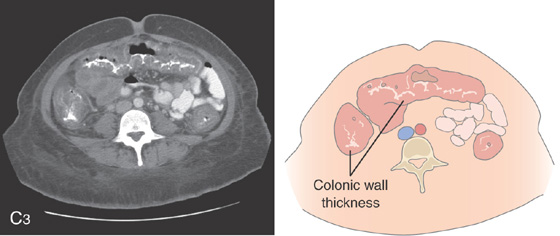
C3, Striking colonic wall thickness is present.
Note the nodular mucosa can also be seen on kidneys, ureter, and bladder termed thumbprinting (C4).
Figure 5.25 CLOSTRIDIUM DIFFICILE COLITIS
A, Diffuse thickening of the entire colon with contrast enhancement of the mucosa.
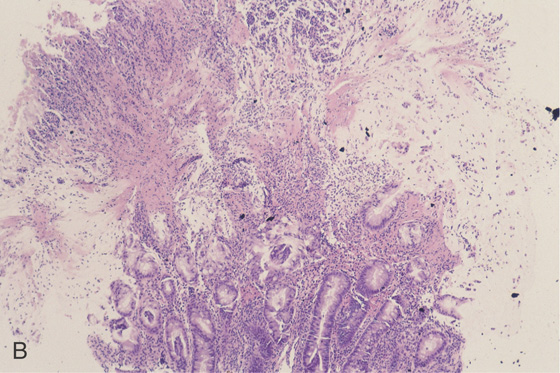
B, Severe colitis with acute and chronic inflammatory cells and edema. A mushroom-shaped pseudomembrane is shown. The colonic architecture is preserved.
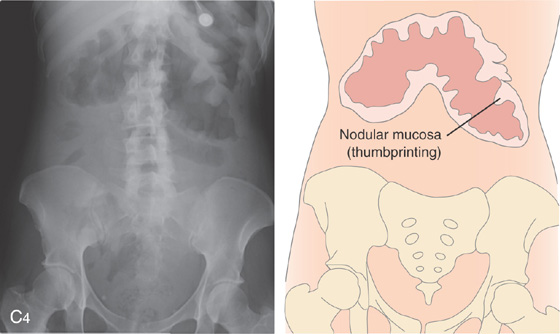
Figure 5.26 SEVERE CLOSTRIDIUM DIFFICILE COLITIS
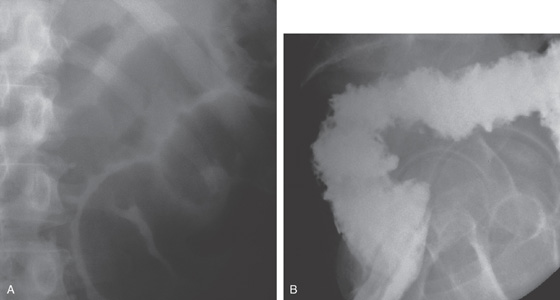
A, Abdominal radiograph shows subtle nodularity of the colon. B, Marked irregularity of the rectal wall.
C, The more proximal colon demonstrates marked nodularity (thumbprinting) of the wall. The mucosa in some areas is poorly coated by the barium. D, The colonic wall is covered by a thick, tenacious membrane. The rectum had multiple well-circumscribed yellow plaques characteristic of C. difficile colitis.
Figure 5.27 CYTOMEGALOVIRUS COLITIS
A, Multiple small, well-circumscribed, ringlike lesions with surrounding subepithelial hemorrhage. There is a diffuse colitis. B, Edema and erythema of the sigmoid colon. C, Prominent subepithelial hemorrhage of the descending colon. D1, Striking diffuse subepithelial hemorrhage. D2, Diffuse colonic wall thickening from rectum to right colon (D3).
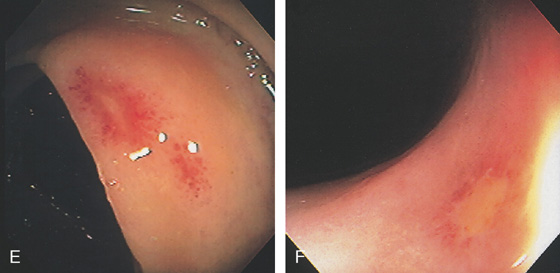
Figure 5.27 CYTOMEGALOVIRUS COLITIS
E, Well-circumscribed small ulcer with a halo of erythema and subepithelial hemorrhage. F, Flat ulcer with normal surrounding mucosa.
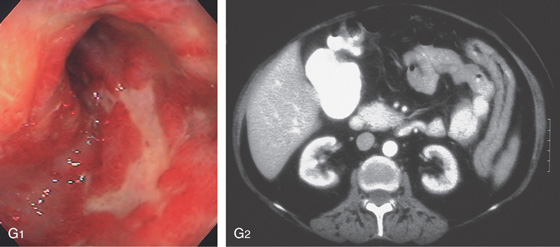
G1, Serpiginous ulcers in the rectum with an appearance suggestive of Crohn’s disease. G2, Marked thickening of the left colon.
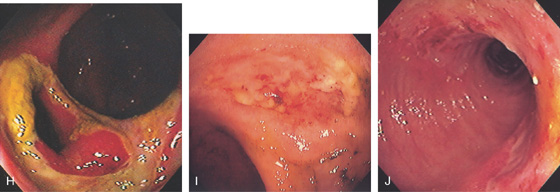
H, Large ulcer of the cecum involving the ileocecal valve. I, Large shallow ulcer with exudate. J, Hemicircumferential ulceration at an ileocolonic anastomosis.
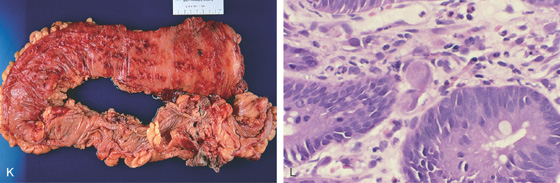
K, Diffuse petechial lesions throughout the left colon. L, Enlarged endothelial cell containing cytomegalovirus inclusions. A moderate amount of chronic inflammation is in the lamina propria.
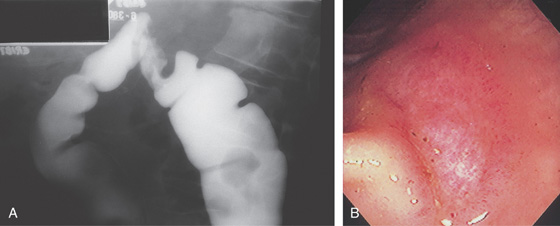
Figure 5.28 CYTOMEGALOVIRUS COLITIS
A, Barium enema shows a focal defect at the splenic flexure suggestive of neoplasm. B, Well-circumscribed ulceration with surrounding edema.
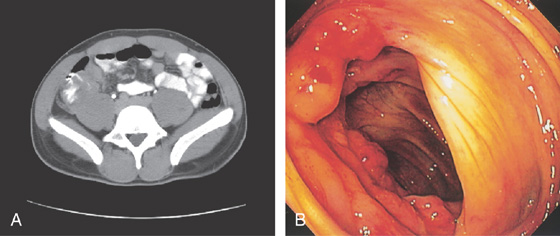
Figure 5.29 TUBERCULOUS COLITIS
A, Thickening of cecum. B, Marked nodularity and fresh hemorrhage around the ileocecal valve.
Figure 5.30 TUBERCULOUS COLITIS
A, Focal ulceration of the sigmoid colon. B, Serpiginous ulceration of the right colon. C, Circumferential ulceration with formation of a stricture. D, Marked stricturing of the cecum.
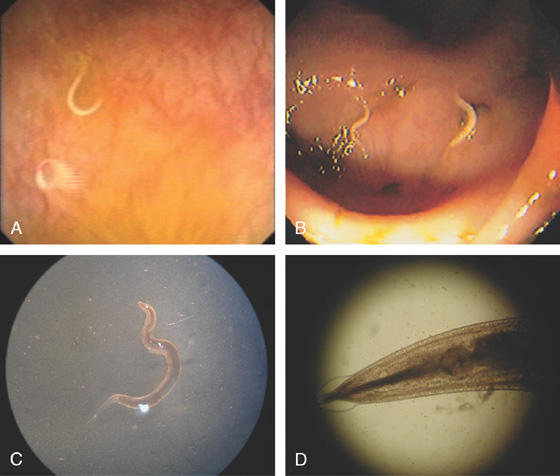
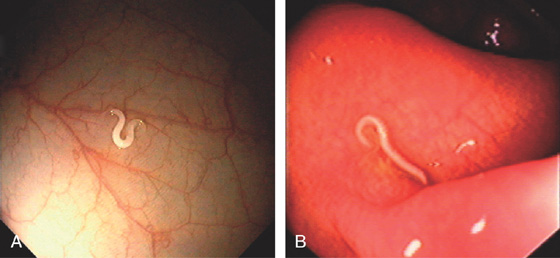
Figure 5.31 PINWORM INFECTION (ENTEROBIUS VERMICULARIS)
A, Solitary worm. B, Multiple small worms in the cecum characteristic of pinworms. C, D, Under the microscope, unique features of the worm are better appreciated.
Figure 5.32 WHIPWORM INFECTION (TRICHURIS TRICHIURA)
Solitary worms (A, B).
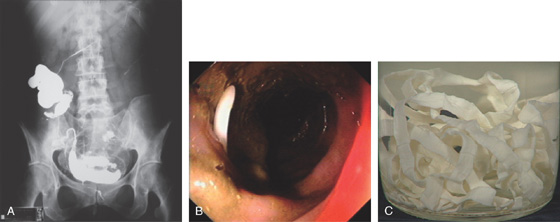
Figure 5.33 TAPEWORMS (TAENIA SPECIES)
A, Barium study shows worm in the cecum and ascending colon, as well as transverse colon. B, The worm appears as white object in the stool. C, The length of the worm is apparent after removal.
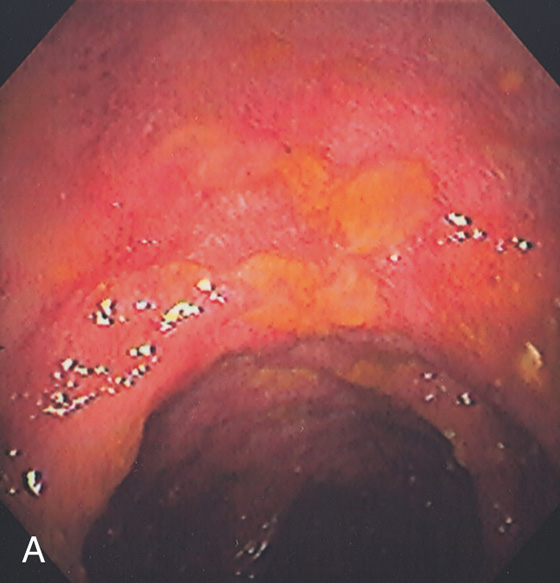
Figure 5.34 STRONGYLOIDES COLITIS
A, Marked colonic edema with patchy shallow ulceration.
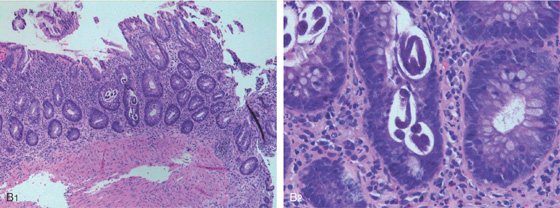
B1, Low-power view shows acute colitis with structures present in the crypts. B2, High-power view shows larvae in the crypts.
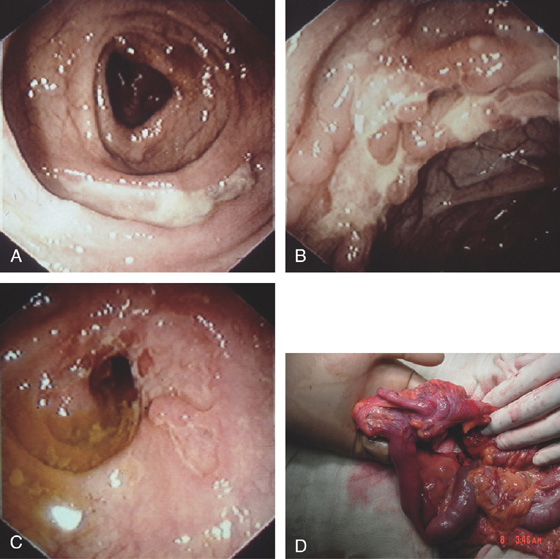
Figure 5.35 AMEBIC COLITIS
A, Large ulcer involving the ileocecal valve. Ulceration is also present in the cecum. B, Patchy well-circumscribed ulcers. C, Close-up shows the ulcers having a raised (volcano) appearance.
![]() Differential Diagnosis
Differential Diagnosis
Amebic Colitis (Figure 5.35)
Inflammatory bowel disease
Other infections (viral, bacterial)
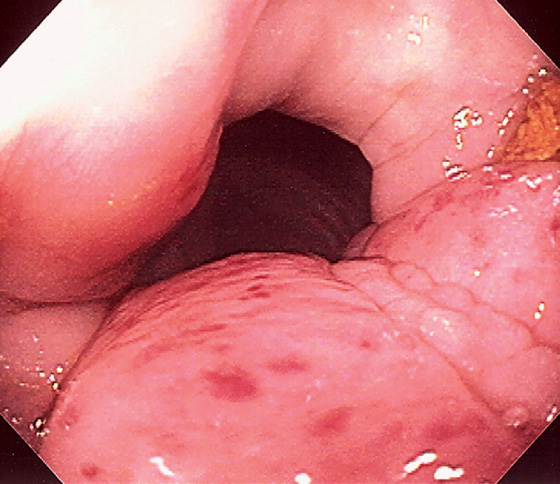
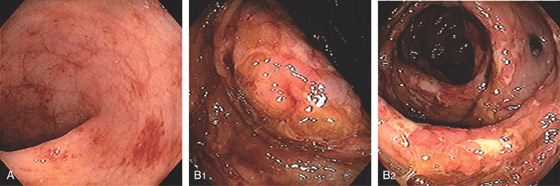
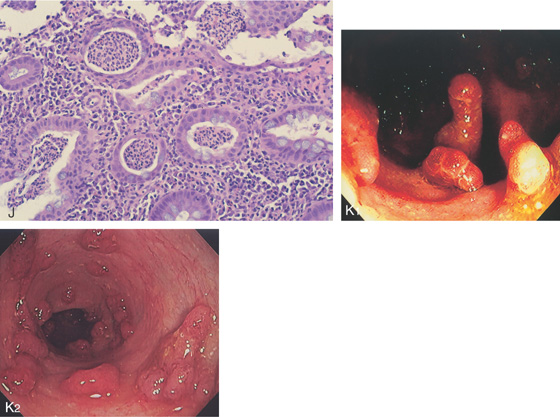
Figure 5.36 ULCERATIVE COLITIS
A, Mild ulcerative colitis demonstrated by edema, loss of the normal mucosal vascular pattern, and patchy subepithelial hemorrhage. B, Moderate colitis with loss of vascular pattern, subepithelial hemorrhage, and patchy mucopus. C, Diffuse mucopus coating the colon. D, Diffuse colitis with diffuse shallow ulceration. E, Diffuse colonic hemorrhage. F, Focal colitis with point of demarcation to normal mucosa.
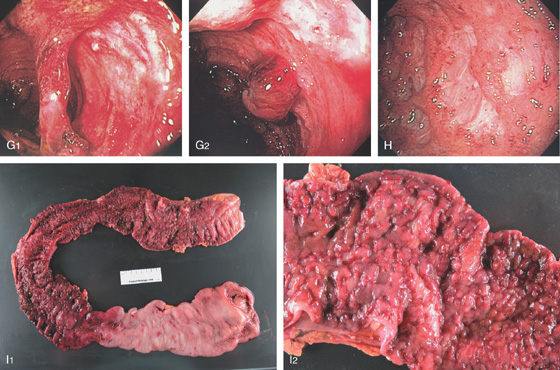
Figure 5.36 ULCERATIVE COLITIS
G1, Severe colitis with mucosal bridge. G2, Deep ulceration with a residual round area of preserved but hemorrhagic mucosa. H, Severe colitis with deep serpiginous ulcerations most suggestive of Crohn’s disease. I1, I2, Surgical specimen shows severe colitis with sparing of cecum.
J, Severe acute and chronic inflammatory process, with multiple crypt abscesses. K1, K2, Multiple filiform polyps in the setting of active colitis.
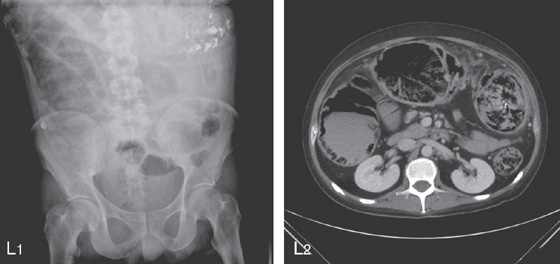
Figure 5.36 ULCERATIVE COLITIS
SEVERE ULCERATIVE COLITIS
L1, Kidneys, ureter, and bladder x-ray film shows haziness in the right colon compatible with pneumatosis. Also note the barium at the splenic flexure. L2, CT scan shows diffuse dilatation of the colon with air not only in the lumen but in the colonic wall.
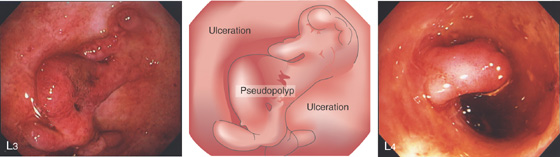
L3, Severe ulceration with mucosal loss and formation of pseudopolyps. L4, Pseudopolyp formation with surrounding ulceration.
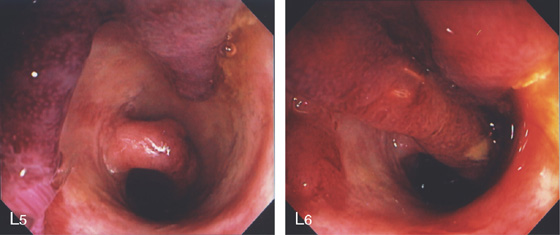
L5, The exudate is removed showing the underlying edema of the mucosa. L6, Formation of long pseudopolyps.
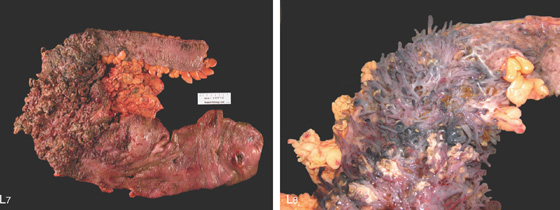
Figure 5.36 ULCERATIVE COLITIS
L7, Surgical specimen shows diffuse colitis, a cecal ulcer, and the marked pseudopolyposis (L8).
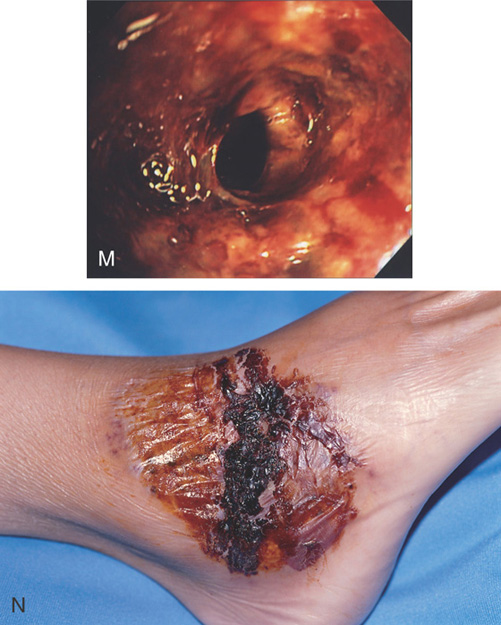
M, Severe ulceration with scarring and luminal narrowing. N, Lesion on the right ankle typical for pyoderma gangrenosum.
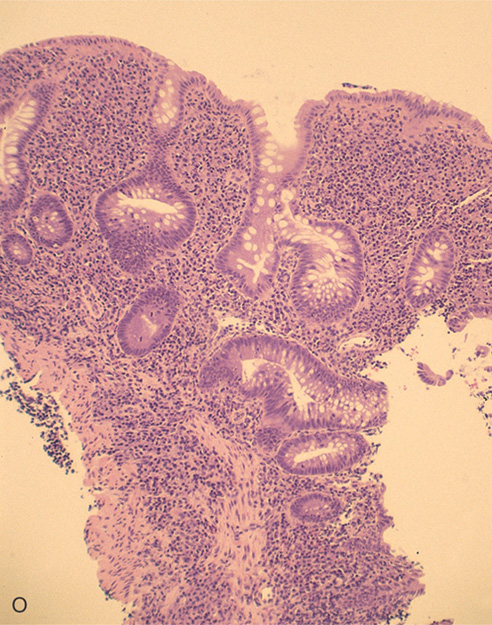
O, The colonic architecture is distorted, with a loss of crypts and abnormal branching of the crypts. The disordered architecture is useful in differentiating acute from chronic colitis.
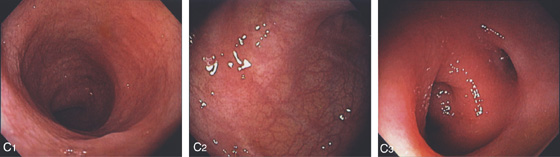
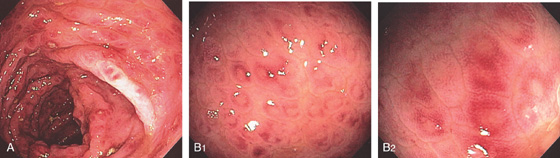
Figure 5.37 ULCERATIVE COLITIS
A, Surveillance colonoscopy in a patient with chronic ulcerative colitis. The ascending colon (A1), transverse colon (A2), and descending colon (A3) are normal, with active disease of the sigmoid colon (A4). B, Biopsy samples of the normal-appearing colon demonstrate abnormal architecture consisting of shortened crypts but no active colitis. C1, C2, Surveillance endoscopy shows mild granularity of the mucosa. The granularity and mild edema are more pronounced in the sigmoid colon (C3).
Figure 5.38 COLONIC CROHN’S DISEASE
A, Diffuse deep ulceration with a nodular appearance. B, Focal area of ulceration with distortion, luminal narrowing, and an early fistula. C1, Focal ulceration with an appearance of the ulcer burrowing underneath the mucosal fold. C2, More proximally a large deep ulcer was present, again with the appearance of the ulcer burrowing underneath the mucosa. C3, Marked involvement of the anorectum was present. D1, Focal area of severe colitis resembling a mass lesion in the right colon. D2, Proximally diffuse disease was evident. E, Circumferential ulceration of the distal colon resembling ulcerative colitis. F, Multiple punched-out ulcers with colitis.
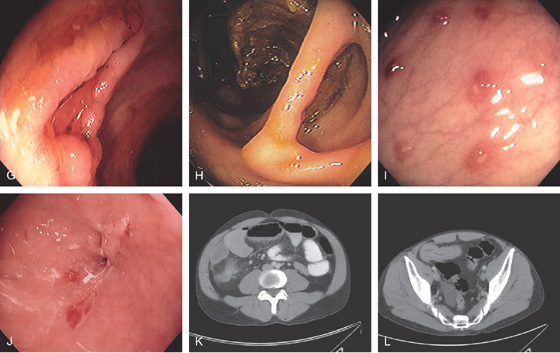
Figure 5.38 COLONIC CROHN’S DISEASE
G, Serpiginous ulcer. H, Mucosal bridge representing healing of a submucosal ulcer. I, Multiple aphthous ulcers. J, Pinpoint area in the distal colon representing a fistulous tract. K, Thickening with inflammatory changes (stranding) around the cecum. L, Thickened terminal ileum.
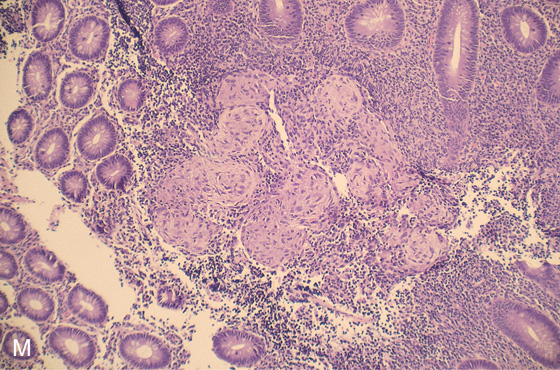
M, Multiple well-circumscribed, noncaseating granulomata. This finding supports the diagnosis of Crohn’s disease when an infectious cause is excluded.
Figure 5.39 COLONIC CROHN’S DISEASE
A, Multiple punctate ulcers in the descending colon. B, Large serpiginous ulceration of the descending colon. C, Solitary ulcer in transverse colon. D, Irregular ulceration involves the ileocecal valve. E, Deep ulceration with associated pseudopolyps. F, “Bear claw”-type ulceration.
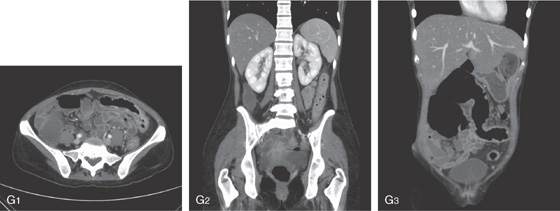
G1-G3, Routine and coronal CT images shows thickened colon. Coronal image shows colonic dilatation and colonic wall thickening.
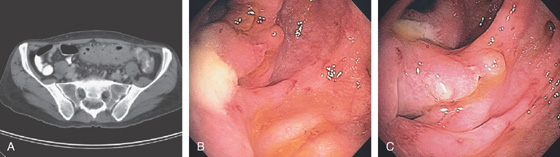
Figure 5.40 ILEOCECAL CROHN’S DISEASE
A, The ileocecal valve is patulous from involvement with Crohn’s disease. The valve is diffusely ulcerated and hemorrhagic. B, The terminal ileum is edematous and hemorrhagic, and has scattered ulcerations typical of Crohn’s ileitis. C, The terminal ileum appears nodular and narrowed (“string sign”). D, CT shows thickening of the right colon and pronounced thickening of a long segment of terminal ileum. E, There is also circumferential wall thickening at the splenic flexure.
Figure 5.41 COLONIC CROHN’S WITH PSEUDOPOLYPS
A, Diffuse edema, ulceration, and multiple pseudopolyps. B, The surgical specimen demonstrates a normal-appearing colon on the right. The involved area shows ulceration and diffuse polyposis representing inflammatory polyps. C, The surgical specimen demonstrates marked thickening of the colonic wall typical of Crohn’s disease. D, Recurrence at the anastomosis, with deep ulceration surrounding the sutures.
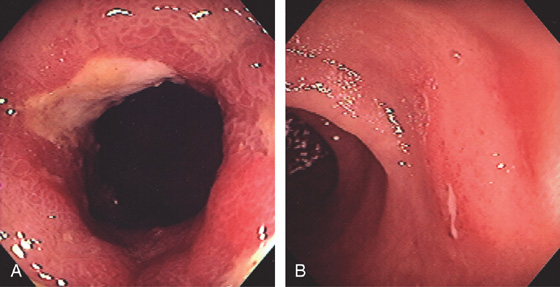
Figure 5.42 RECURRENT CROHN’S DISEASE WITH ANASTOMOTIC ULCER
A, Narrowing, edema, and ulceration at the site of a prior ileocolonic anastomosis. B, Typical serpiginous ulcer seen in the distal ileum just proximal to the anastomosis.
Figure 5.43 ANASTOMOTIC STRICTURE
A, Tight stricture at the site of a prior ileocolonic anastomosis. B, A balloon has been placed across the anastomosis and inflated. C, Improved luminal caliber after dilation.
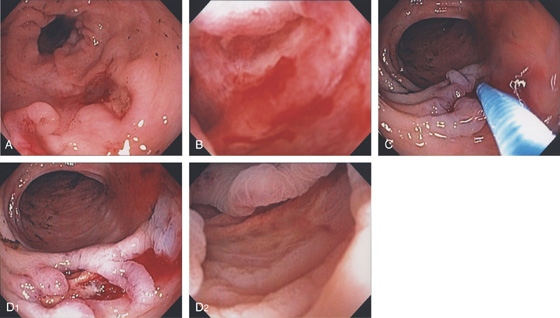
Figure 5.44 COLONIC CROHN’S WITH ULCER-RELATED BLEEDING
A, Deep serpiginous ulceration in the descending colon. B, The ulcer base is viewed underwater showing pinpoint mucosal oozing. C, Epinephrine is injected into the lesion, resulting in blanching of the mucosa and hemostasis (D1, D2).
Figure 5.45 ISCHEMIC COLITIS
A, Distinct demarcation manifested by focal subepithelial hemorrhage. B, Proximally, the hemorrhage becomes confluent with ulceration. C, Marked edema of the mucosa forming subepithelial blebs. D, More marked ulceration, edema, and luminal narrowing. E, Follow-up colonoscopy 2 months later shows scarring and distortion.
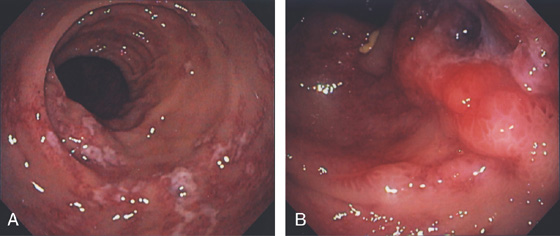
Figure 5.46 ISCHEMIC COLITIS
A, Patchy exudate overlying ulceration in the descending colon. B, More proximally, the ulceration becomes confluent and a deep ulceration with raised border is seen.
Figure 5.47 ISCHEMIC COLITIS WITH BLEEDING
A1, A2, The ulceration becomes confluent and nodular with marked luminal narrowing. Mucosal biopsy of such a lesion is generally firm with ischemic colitis. B, Large ulceration with fresh bleeding and an adherent blood clot.
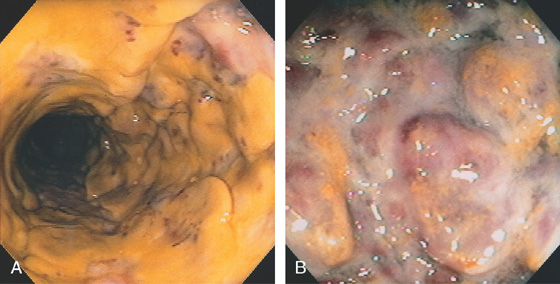
Figure 5.48 ISCHEMIC COLITIS
A, Circumferential ulceration with a thick membrane, resembling Clostridium difficile colitis. B, Marked nodularity and ulceration.
Figure 5.49 RIGHT-SIDED ISCHEMIC COLITIS
A, B, Marked edema and subepithelial hemorrhage of the right colon. The cecum is markedly edematous with luminal narrowing. C1, C2, CT shows edema of the right colon. D, Small-bowel follow-through shows a normal small bowel, but edema of the right colon.
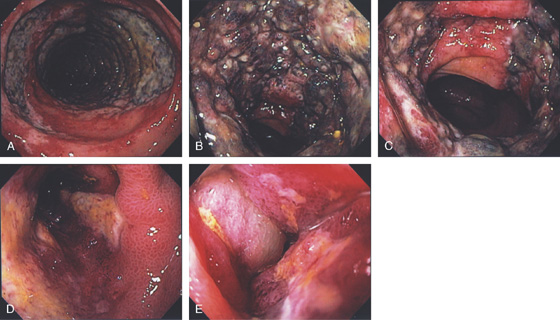
Figure 5.50 ISCHEMIC PROCTITIS
A, Edema of the proximal rectum with circumferential disease. B, Circumferential disease with nodularity and luminal narrowing. C, Note the ulceration stops relatively abruptly, typical for ischemia. D, E, Progression over time shows marked edema.
Figure 5.51 ISCHEMIC COLITIS
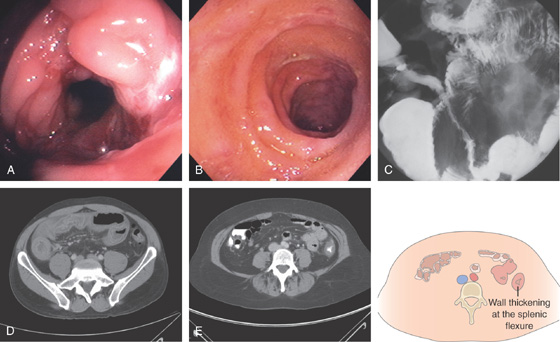
A, Focal ulceration with surrounding erythema and subepithelial hemorrhage just distal to the splenic flexure. B, More proximally, there is marked edema, ulceration, and subepithelial hemorrhage typical for ischemic colitis. The mucosa was firm on biopsy, also characteristic of ischemic colitis. C, More proximally in the distal transverse colon, the ulceration again follows the tinea with surrounding focal edema. D, Barium enema shows nodularity at the splenic flexure.
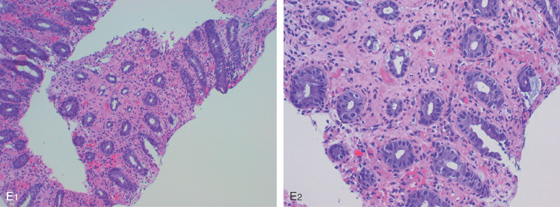
E1, E2, Dropout of glands with fibrosis of lamina propria.
Figure 5.52 ISCHEMIC COLITIS
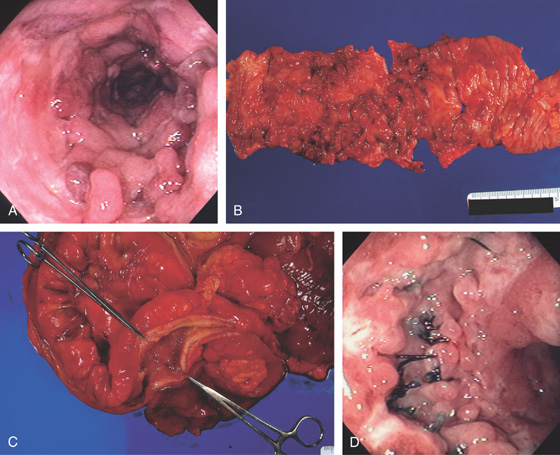
A, Patchy yellow exudate in the distal colon resembling Clostridium difficile colitis. B, Circumferential edema with a prominent mucosal pattern. C, Abrupt termination of ischemic colitis is characteristic.
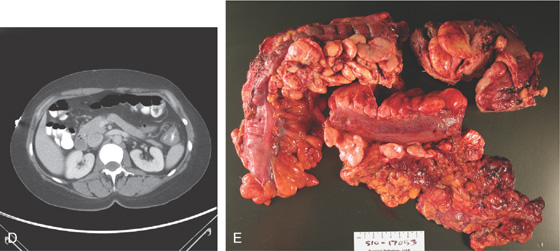
D, Colonic thickening at the splenic flexure. E, Resection specimen shows the marked thickening of the colonic wall.
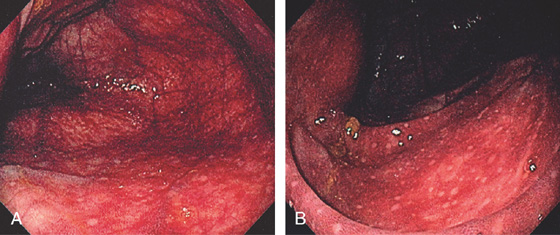
Figure 5.53 ISCHEMIC COLITIS
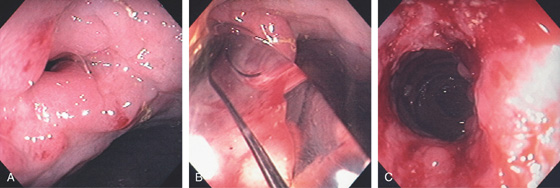
A, Marked diffuse subepithelial hemorrhage involving the cecum. B, Note the lymphoid aggregates are spared.
Figure 5.54 ISCHEMIC COLITIS STRICTURE
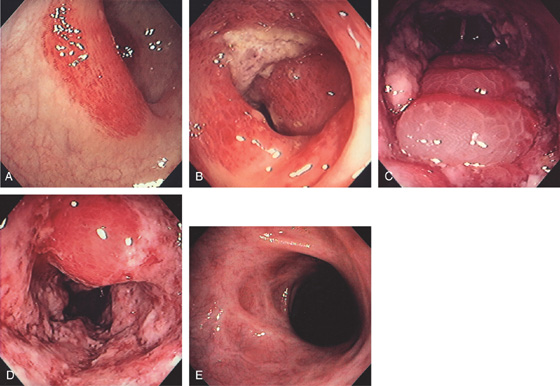
A, Focal smooth stricture at the splenic flexure. B, Narrowing of the colonic lumen with ulceration at the site of a prior episode of ischemia.
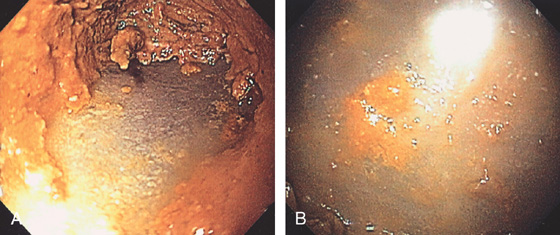
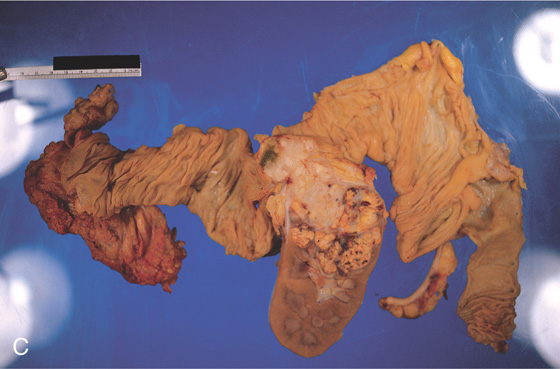
Figure 5.55 INFARCTED COLON
A, Stool coats a black colon. B, Close-up shows the dark discoloration with the absence of normal-appearing mucosa.
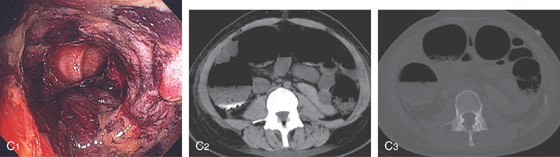
C1, Dusky appearance of the distal colon. C2, Air in the right colon wall best appreciated on bone windows (C3).
Figure 5.56 RADIATION PROCTOPATHY
Multiple ectatic blood vessels in the distal rectum. This patient previously underwent radiation therapy for prostate cancer.
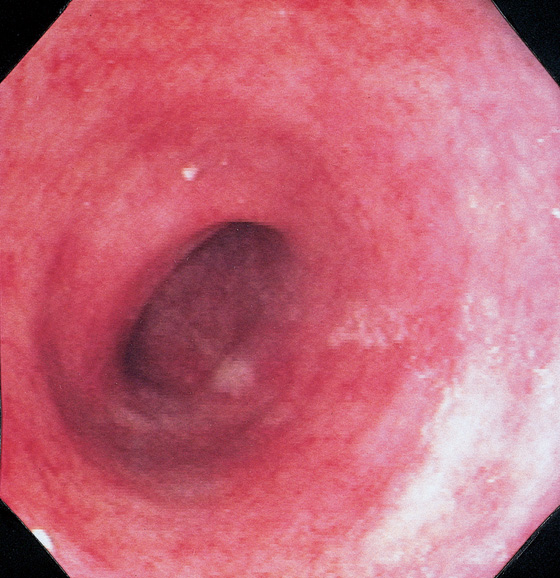
Figure 5.57 DIVERSION COLITIS
Hartmann’s pouch demonstrates loss of normal haustrations and vasculature, with diffuse subepithelial hemorrhage. Biopsy findings demonstrated normal architecture with edema, subepithelial hemorrhage, and mild chronic inflammatory infiltrate.
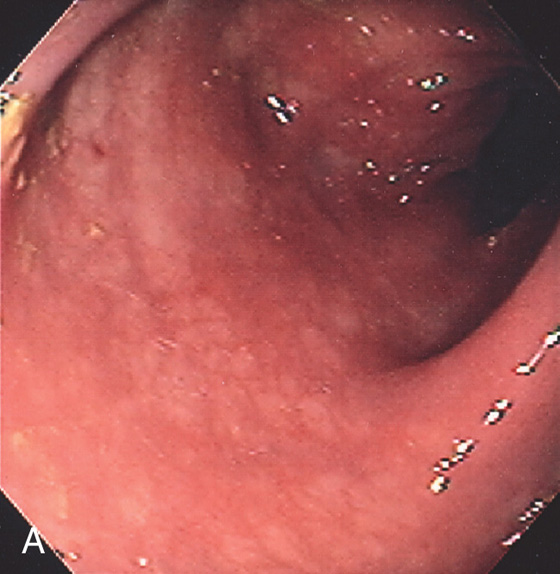
Figure 5.58 COLLAGENOUS COLITIS
A, Subtle loss of vascular pattern and erythema of the sigmoid colon. Typically, the colonic mucosa is normal.
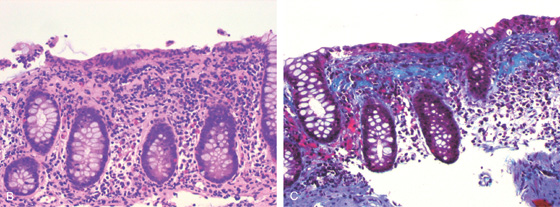
B, Hematoxylin and eosin stain suggests the diagnosis. C, Trichrome stain highlights the collagen layer in the subepithelium.
Figure 5.59 BEHÇET’S DISEASE
A, Edema with shallow ulcers in the distal colon. B, More extensive disease in the right colon with prominent subepithelial hemorrhage. C, Ulcer of the skin.
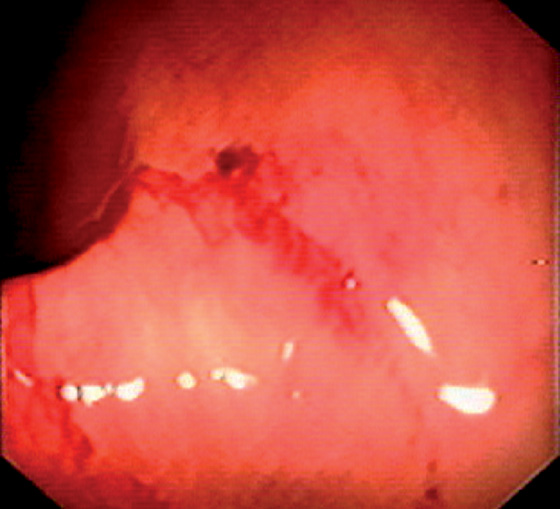
Figure 5.60 HENOCH-SCHÖNLEIN PURPURA
Subepithelial hemorrhage and recent bleeding.
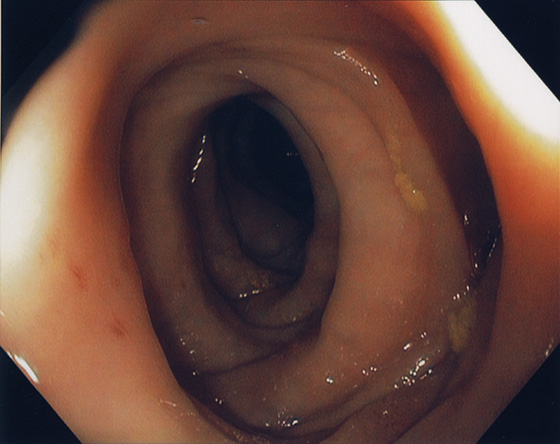
Figure 5.61 MUSCULATURE HYPERTROPHY ANTEDATING DIVERTICULOSIS
Circumferential thickening of musculature antedating diverticula formation.
Figure 5.62 DIVERTICULOSIS
A1, Large diverticula filled with stool. The haustra are thickened. A2, Barium enema demonstrated sigmoid diverticulosis. B, Multiple diverticula in the sigmoid colon with thickened haustra.
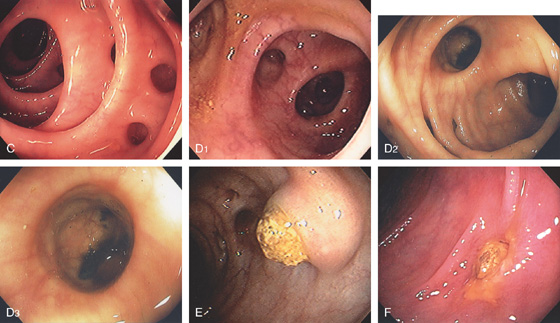
Figure 5.62 DIVERTICULOSIS
C, Multiple sigmoid diverticula between thickened haustra. D1, D2, Multiple large diverticula. Note the depth and large caliber of the diverticula (D3). E, Engorged diverticulum filled with stool. F, Isolated diverticula filled with stool with surrounding edema and exudate potentially portending subsequent diverticulitis.
G1, Large fecalith that spontaneously passed from a diverticulum. leaving the diverticulum with a wide-mouth appearance (G2). H1, H2, Multiple diverticula make identification of the colonic lumen difficult. Which way do I go?
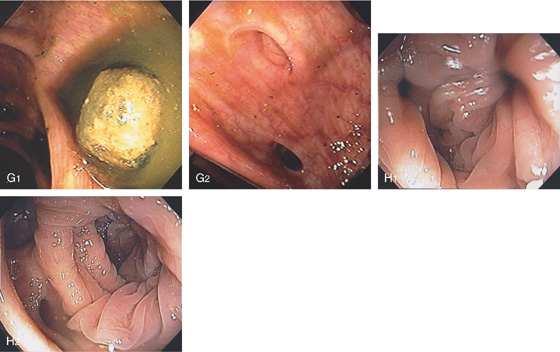
Figure 5.63 RIGHT-SIDED DIVERTICULOSIS
A, B, Multiple typical-appearing diverticula just proximal to the ileocecal valve.
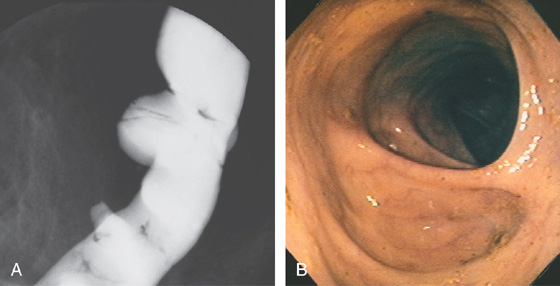
Figure 5.64 WIDE-MOUTHED COLONIC DIVERTICULA
A, A large diverticulum in the descending colon, with a small diverticulum distally. B, Large, hemicircumferential, wide-mouthed diverticulum. Wide-mouthed diverticula are most suggestive of scleroderma.
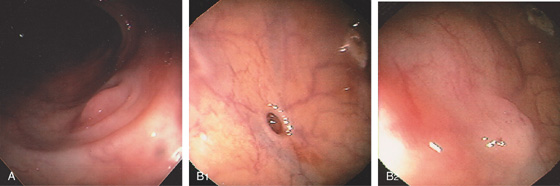
Figure 5.65 EVERTED DIVERTICULUM
A, Everted colonic diverticulum suggestive of a polyp. B1, Observation of this diverticulum showed intermittent eversion of colonic tissue (B2).
Figure 5.66 PERFORATED DIVERTICULUM FROM COLONOSCOPY
A, B, Yellow shiny surface is seen through the lateral colonic wall representing a perforation. The perforation presumably occurred at the site of a diverticulum.
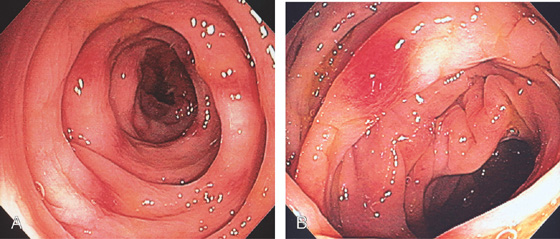
Figure 5.67 DIVERTICULAR COLITIS
A, B, Patchy subepithelial hemorrhage and edema of the sigmoid colon resembling mild idiopathic ulcerative colitis. Histologic changes of colitis were present.
![]() Differential Diagnosis
Differential Diagnosis
Diverticular Colitis (Figure 5.67)
Inflammatory bowel disease
Bacterial colitis
Ischemia/vasculitis
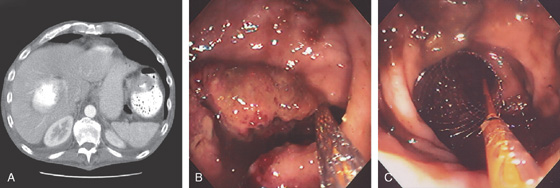
Figure 5.68 DIVERTICULITIS
A, Thickened sigmoid colon with several circumscribed areas of air-filled diverticula and possibly extraluminal gas (abscess). The surrounding fat has a “dirty” appearance, suggesting an inflammatory process. B, Edema, subepithelial hemorrhage, and mucopus are surrounding a diverticulum. The mucosal pattern is accentuated by the edema, as occurs with the areae gastricae of the stomach.
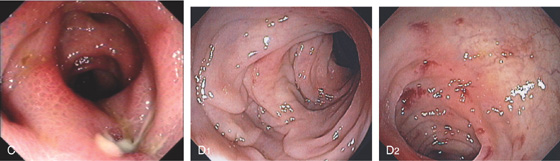
C, Pus exuding from a diverticulum. The surrounding mucosa is edematous. D1, D2, After resolution of the diverticulitis, areas of edema and subepithelial hemorrhage are identified.
Figure 5.69 CHRONIC DIVERTICULITIS
A, Marked edema, diverticula, luminal narrowing, and associated stranding suggestive of diverticulitis. B, Marked colonic edema with patchy subepithelial hemorrhage and presence of mucopus. C, Diverticula are present.
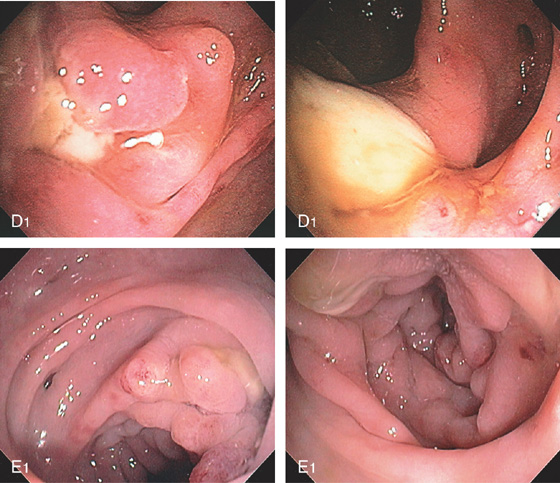
D1, Mucopus covers the diverticulum, and with further observation, a large amount of pus passed spontaneously (D2). E1, E2, After antibiotics, there is improvement in appearance, although there is still luminal narrowing and mucopus.
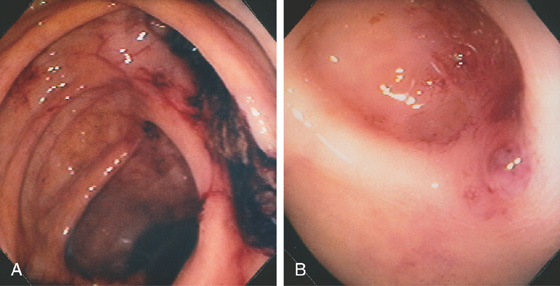
Figure 5.70 DIVERTICULAR HEMORRHAGE
A, A large blood clot is just proximal to the ileocecal valve. B, After the area is irrigated, a small amount of residual blood is in the diverticulum near a visible vessel at the entrance to the diverticulum.
Figure 5.71 DIVERTICULAR HEMORRHAGE
A, Fresh blood and clot in the sigmoid colon; multiple diverticula are present. B, One large diverticulum with several internal diverticula with adherent clot. C, With further washing, a small, nipple-like projection is apparent, suggesting a visible vessel.
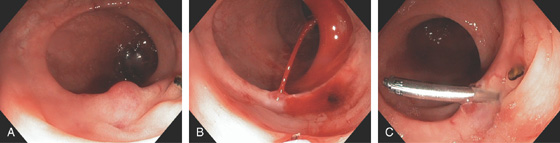
Figure 5.72 COLONIC BLEEDING AFTER BIOPSY
Mucosal biopsy of a small polypoid lesion (A) results in marked bleeding (B). C, A single clip is applied, resulting in hemostasis.
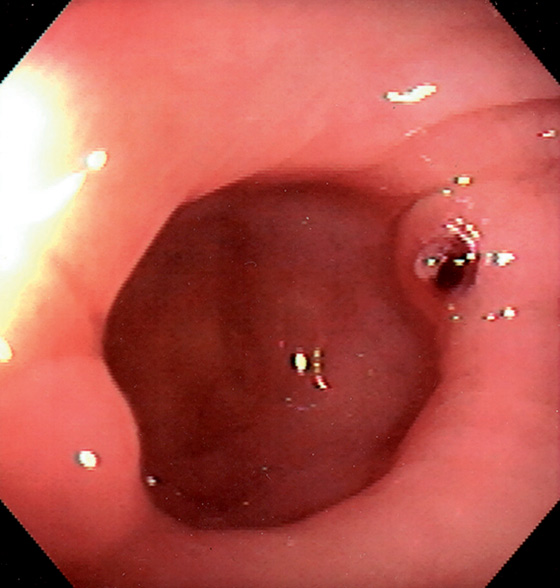
Figure 5.73 DIVERTICULAR HEMORRHAGE WITH VISIBLE VESSEL
Fleshy projection on the lip of a diverticulum in the right colon. This patient had significant lower gastrointestinal bleeding.
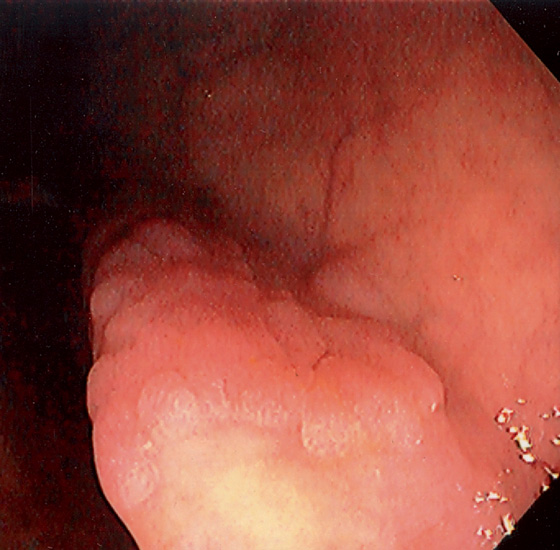
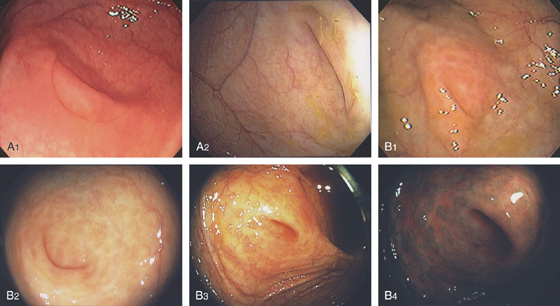
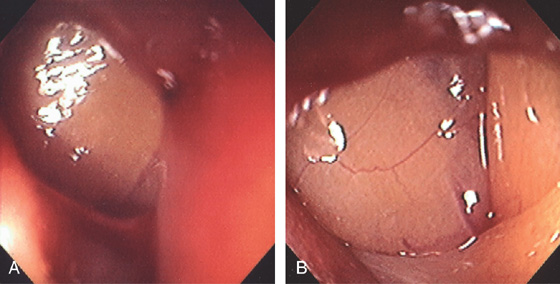
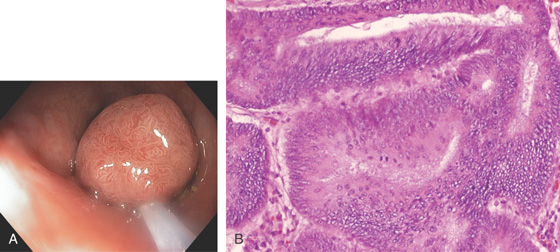
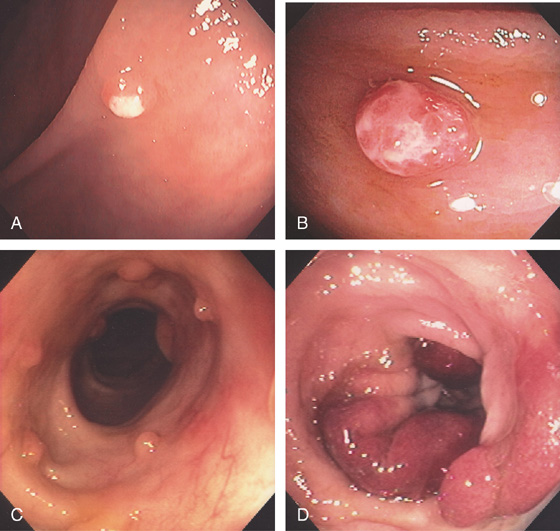
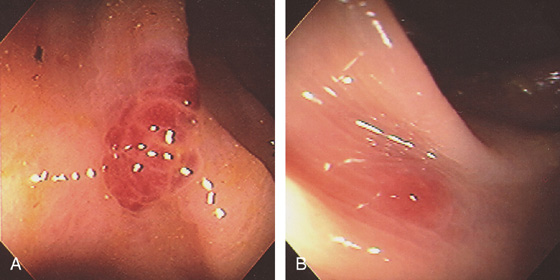
Figure 5.74 FLAT ADENOMA
Small, flat adenoma that blends into the surrounding mucosa.
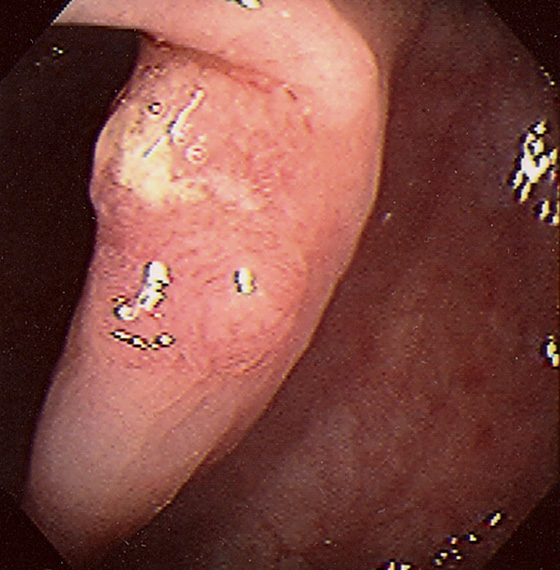
Figure 5.75 FLAT ADENOMA
A portion of the colonic fold is asymmetric and appears thickened and erythematous, with subepithelial hemorrhage. The texture of the mucosa appears different from that of the normal-appearing surrounding colon. Biopsy demonstrated adenomatous change with focal high-grade dysplasia. The high resolution of the endoscopic equipment allows such a flat adenomatous lesion to be discerned.
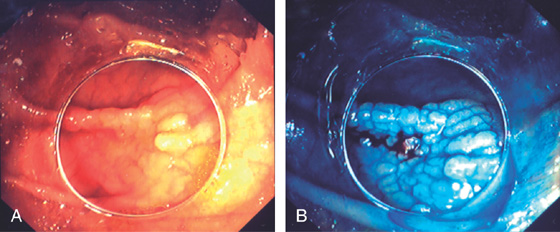
Figure 5.76 FLAT ADENOMA
A, Flat adenoma that is subtle. B, The adenoma is highlighted by the use of methylene blue and is now much easier to recognize.
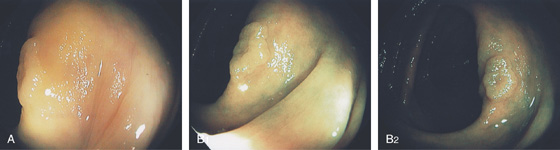
Figure 5.77 FLAT ADENOMA
A, Raised lesion on the ileocecal valve as seen on standard and narrow band imaging (B1, B2).
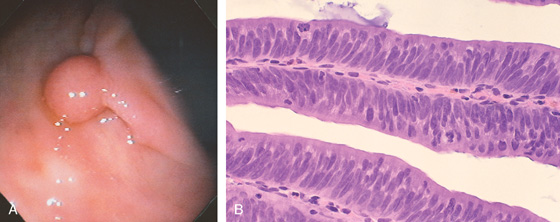
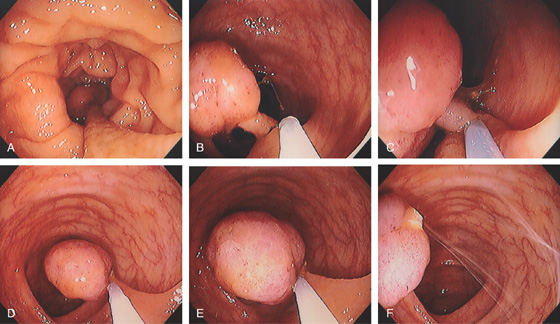
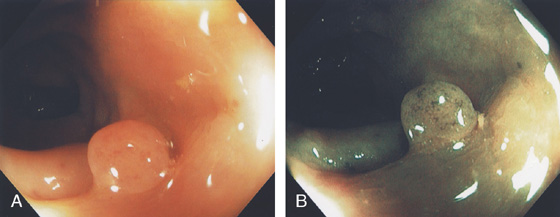
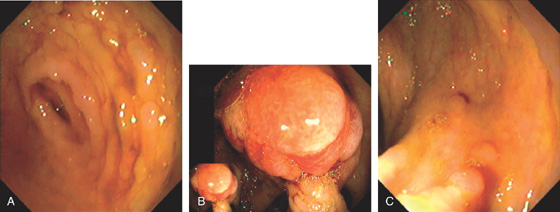
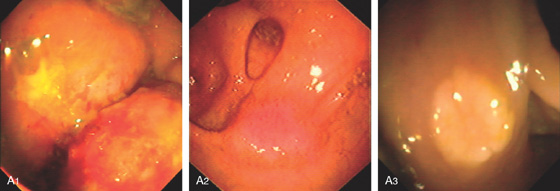
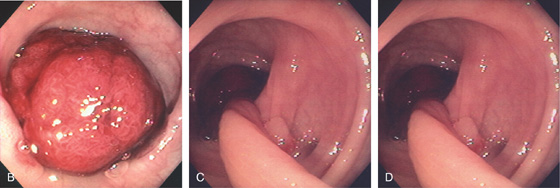
Figure 5.78 TUBULAR ADENOMA
A, A typical-appearing colonic adenoma. The head of the polyp has a textured appearance compared with the surrounding mucosa. A colonic fold is pulled to the side by the polyp. Just superior to the adenomatous polyp are two small hyperplastic polyps. B, Adenomatous colonic mucosa demonstrating atypical cells, with an increased nuclear/cytoplasmic ratio and a “picket-fence” appearance of the nuclei.
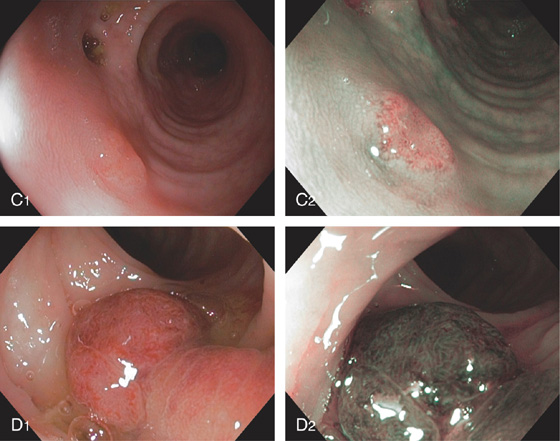
C, Tubular adenoma as seen by high-definition endoscopy (C1) and narrow band imaging (C2). D, Pedunculated adenoma as seen by high-definition endoscopy (D1) and narrow band imaging (D2).
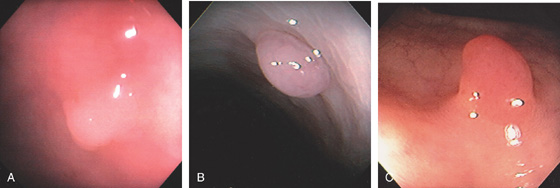
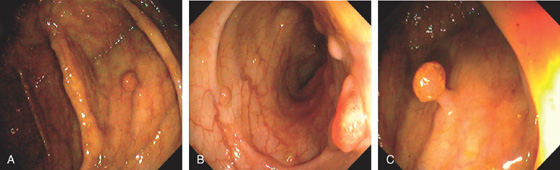
Figure 5.79 TUBULAR ADENOMA
A, Small white raised polyp with a subtle pattern distinct from normal surrounding mucosa. B, Small, well-circumscribed polyp. The mucosal surface is smooth. C, Small erythematous-appearing polyp. Note the overlying mucosa is different from the surrounding normal colonic mucosa.
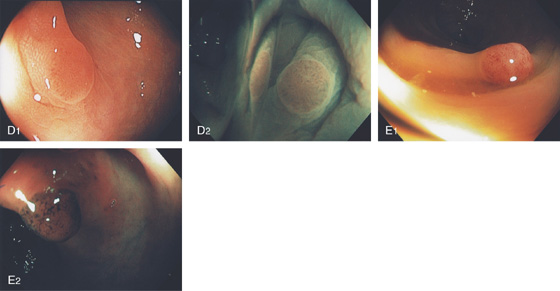
D1, Small polyp with abnormal mucosal pattern highlighted with narrow band imaging underwater for magnification (D2). E1, Small redheaded polyp. Mucosal pattern also can be seen on narrow band imaging (E2).
Histology shows features of a tubular adenoma on low and high power. Note the submucosal hemorrhage that results in the red appearance of the lesion (E3, E4).
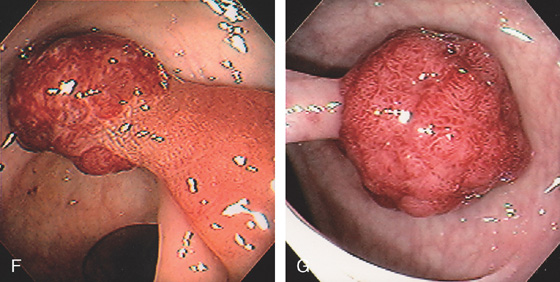
Figure 5.79 TUBULAR ADENOMA
F, Typical-appearing pedunculated adenoma. The polyp head is markedly hemorrhagic, and the stalk is a dark brown color. These color changes represent bleeding into the polyp with subsequent deposition of hemosiderin. G, Pedunculated polyp with a “red head.”
H, The red color of the polyp head results from diffuse subepithelial hemorrhage.
Figure 5.80 TUBULOVILLOUS ADENOMA
A, Small polyp with a whitish serpentine appearance. The polyp has a white granular mucosa distinct from the normal surrounding mucosa. This polyp could be mistaken for a hyperplastic polyp. B1, B2, Small sessile polyp as seen by white light and (B3) narrow band imaging with the mucosal pattern typical for villous adenoma better visualized. C1, Large pedunculated polyp with a typical-appearing reddish serpentine mucosa best seen on close-up (C2).
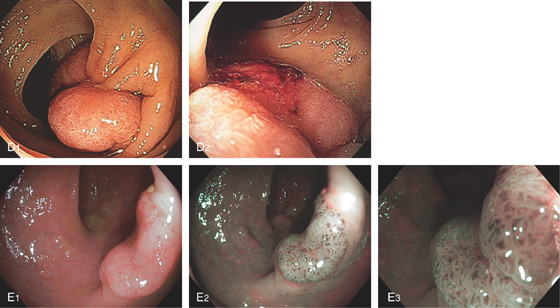
D1, Multilobed pedunculated polyp. D2, Area of fresh hemorrhage is visible on the mucosal surface. This patient, on chronic anticoagulation, experienced mild rectal bleeding. E1-E3, Polypoid lesion as seen by high-definition endoscopy (E1) and narrow band imaging (E2, E3).
Figure 5.81 TUBULOVILLOUS ADENOMA
A, Adenomatous polyp with tubulovillous features and areas of low- and high-grade dysplasia. The S and I represent areas where endomicroscopy was performed. B, Endomicroscopy of the lesion shows villous structure, irregular glands, and hyperchromatic nuclei with increase of the stroma.
Figure 5.82 TUBULOVILLOUS ADENOMA
A, Polyp in the descending colon with a long stalk.
B, The polyp head is hemorrhagic with spontaneous bleeding. The geographic appearance is typical for adenoma. C, The large size of the stalk is evident. D, Well-circumscribed sessile lesion. Note the same geographic appearance typical for adenoma.
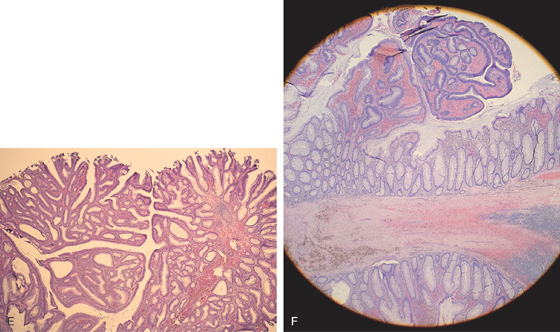
Figure 5.82 TUBULOVILLOUS ADENOMA
E, Typical appearance of a tubulovillous adenoma is noted. F, Transition zone from adenomatous to normal colonic mucosa is at the base of the polyp stalk.
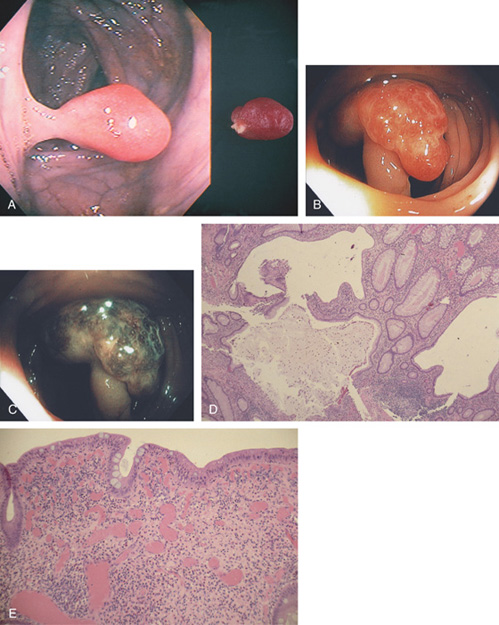
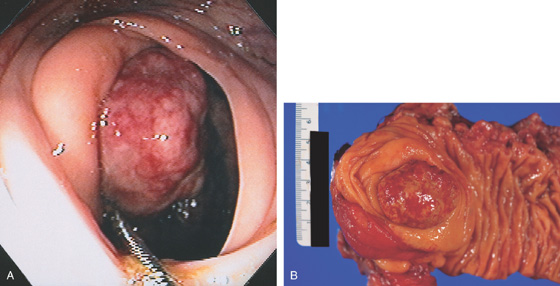
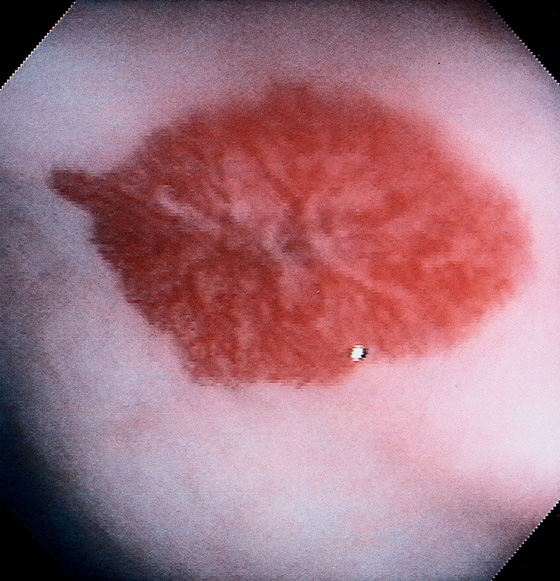
Figure 5.83 VILLOUS ADENOMA
A1, Sessile polyp just opposite the ileocecal valve. A2, Close-up shows the sharp demarcation of normal colon with the diffuse nodular appearance of the polyp. B, Both a snare and argon plasma coagulator were used to remove the polyp.
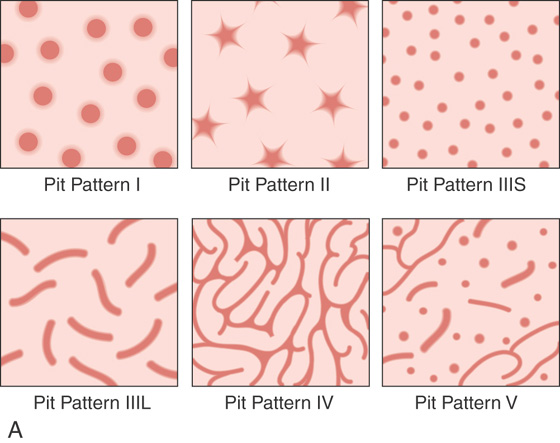
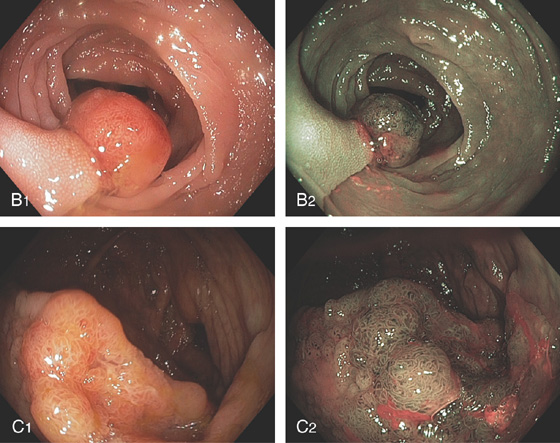
Figure 5.84 TUBULOVILLOUS ADENOMA
A, Cartoon of the different pit patterns. B1, B2, Pit pattern III as shown on standard and narrow band imaging. C1, C2, Pit pattern IV as shown on standard and narrow band imaging.
Figure 5.85 TUBULOVILLOUS ADENOMA
A, Pedunculated polyp. The mucosal pattern of the polyp would be classified as pit pattern IV. B, High-grade dysplasia on pathology. The crowding of the cells is more pronounced.
Figure 5.86 VILLOUS ADENOMA
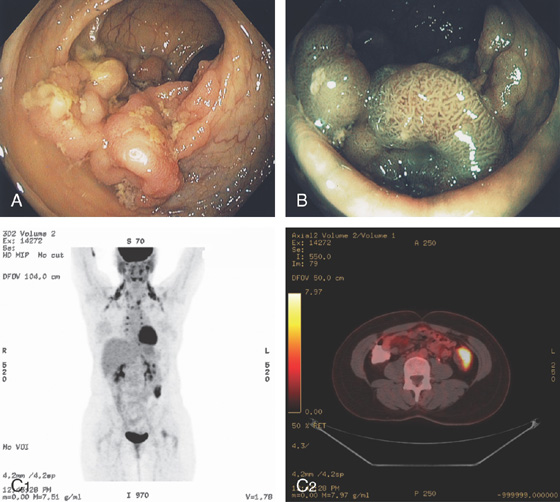
A, Large sessile lesion of the sigmoid colon as seen on standard and (B) narrow band imaging. The lesion was hypermetabolic on positron emission tomography computed tomography scanning (C1, C2).
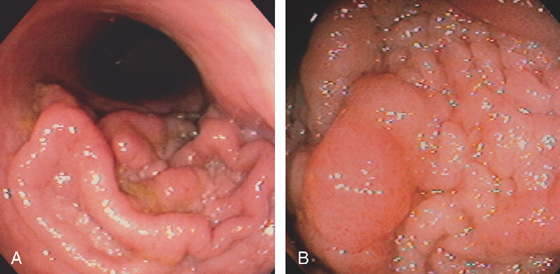
Figure 5.87 VILLOUS ADENOMA
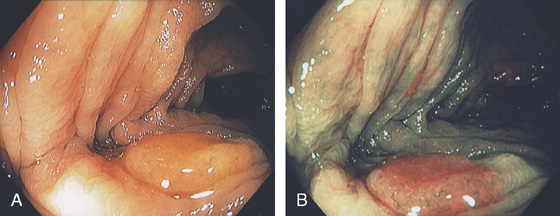
A, Flat-appearing villous adenoma with serpiginous borders in the sigmoid colon. B, The base of the villous adenoma has a whitish and serpentine appearance.
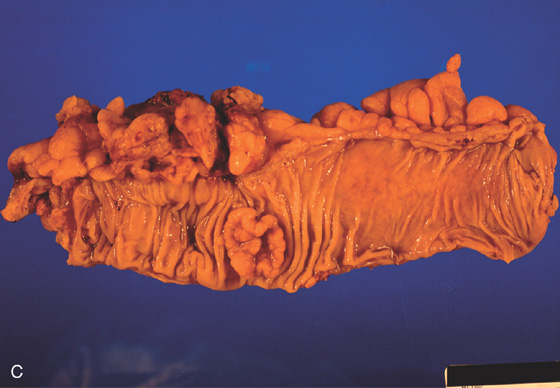
C, Well-circumscribed villous adenoma.
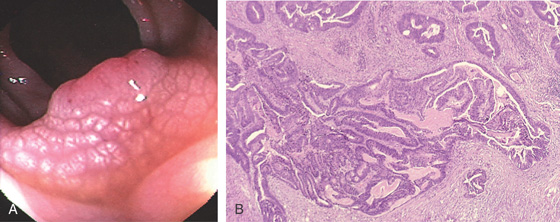
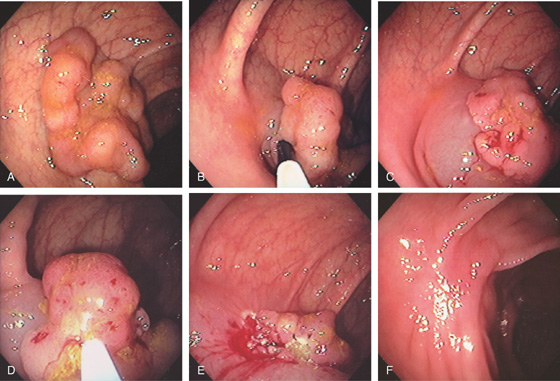
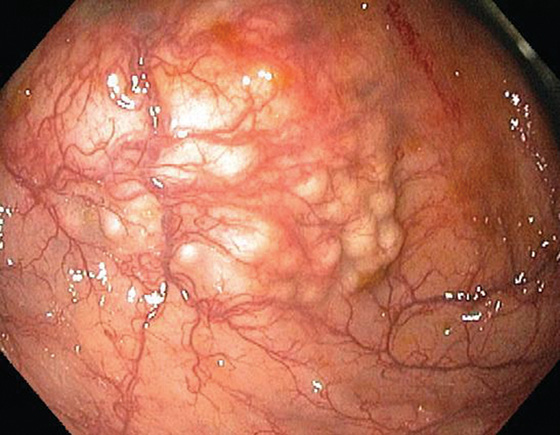
Figure 5.88 SESSILE SERRATED ADENOMA
A, Sessile polyp on standard and narrow band imaging (B).
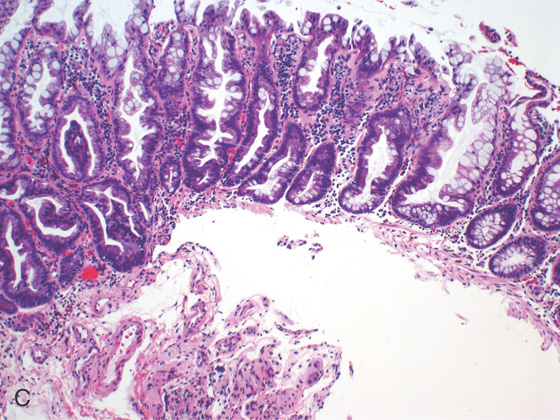
C, Typical features of a sessile serrated adenoma include flattening of the angulated crypt base, some of which have a T-shaped appearance, and serrations extending into the deep portion of the gland in focal areas showing an “invasive” growth pattern where the lesion dips into the muscularis mucosae.
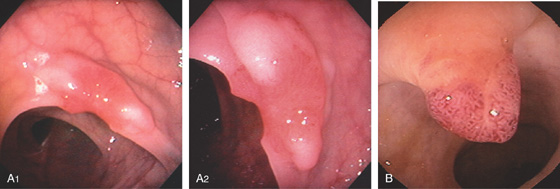
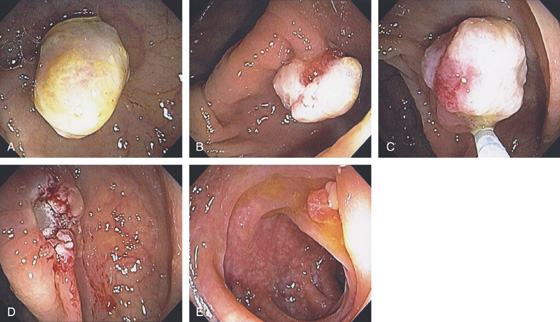
Figure 5.89 CARCINOMATOUS POLYP
A1, Sessile polyp with central area of retraction and hypervascularity. A2, Close-up shows the hypervascularity of the lesion. Biopsy showed tubulovillous adenoma with carcinoma in situ. B, Small, typical-appearing adenomatous polyp. Biopsy results showed carcinoma in situ.
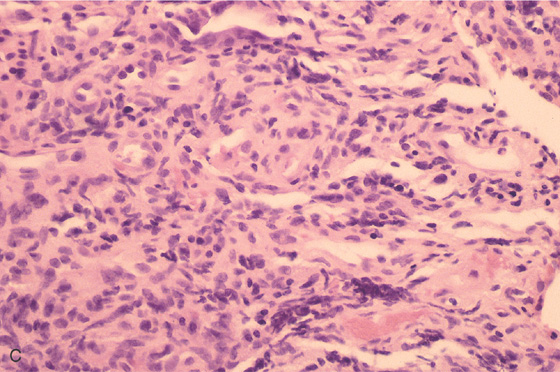
Figure 5.90 CARCINOMATOUS POLYP
A, A flat, sessile polyp in the descending colon. The polyp size and appearance suggest a benign adenoma. B, Malignant glands invade the muscularis mucosa at the edge of the polyp.
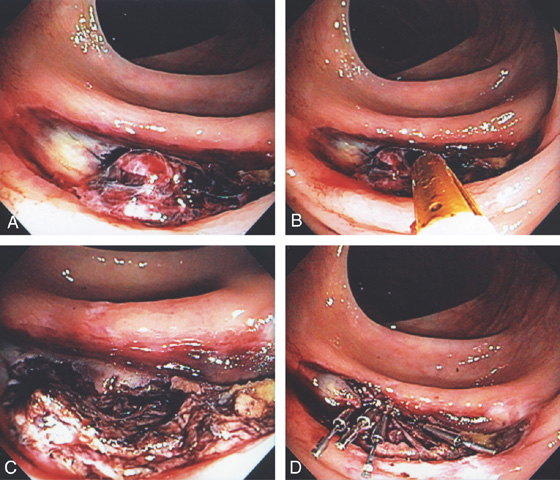
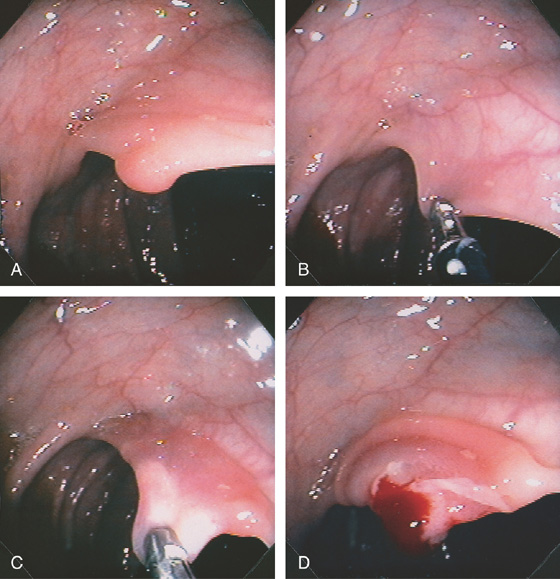
Figure 5.91 COLONIC POLYPECTOMY
A, Pedunculated polyp on a long stalk. B, A snare has been opened and positioned around the polyp to the midportion of the stalk. C, The snare is closed. D, The snare is advanced so that the polyp does not touch the contralateral wall. E, The snare has been closed for several seconds, ensuring ischemia to the polyp head. Note the now purplish discoloration. F, Coagulation current is used with the polyp completely incised, which falls away from the stalk. Note the “smoking gun.”
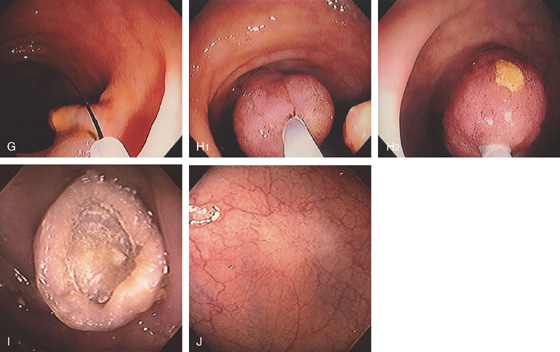
Figure 5.91 COLONIC POLYPECTOMY
G, The snare is now placed around the stalk, and if bleeding ensues, further coagulation may be performed. H1, The polyp is grasped and will be removed with the endoscope. The polyp stalk is free of bleeding. H2, An ulcer is present on the polyp. I, Note the appearance of the polyp on removal. There appears to be a central core with outer covering. J, Subsequent colonoscopy shows a scar at the site of the polypectomy.
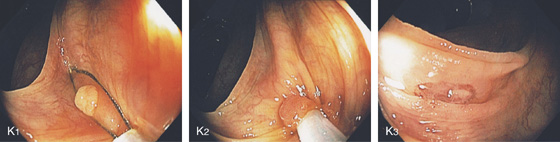
K1-K3, Cold snare polypectomy. A sessile polyp is identified (K1); a snare is placed around the polyp and snaring some normal colonic tissue (K2); mucosal defect seen after cold snare polypectomy (K3).
Figure 5.92 SALINE LIFT TECHNIQUE FOR POLYPECTOMY
A, Sessile adenomatous-appearing polyp. B, Sclerotherapy needle advanced to the base of the polyp with injection of dilute epinephrine and saline. C, The polyp has been elevated off the colon wall. D, The polyp is snared. E, Defect from polypectomy. The remaining polyp is removed. F, Follow-up colonoscopy shows scar at the site of polypectomy.
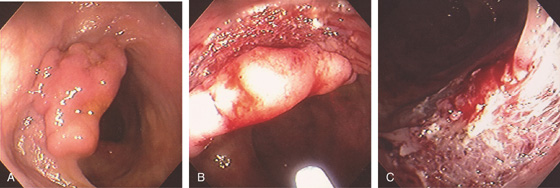
Figure 5.93 POLYPECTOMY
A, Sessile polyp. B, A snare was used without saline lift with removal in a piecemeal fashion. C, Note the large defect at the site of polypectomy with the submucosa visible.
Figure 5.94 ULCER SCAR
A, B, White linear area of fibrosis in the descending colon resulting from prior polypectomy.
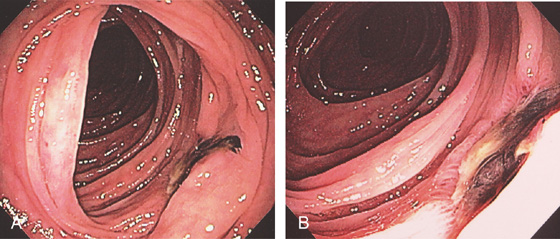
Figure 5.95 POSTPOLYPECTOMY ULCER WITH BLEEDING
A, Ulcer with adherent clot. B, Close-up shows the deep ulcer with clot.
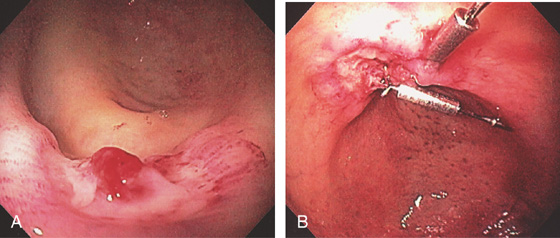
Figure 5.96 POSTPOLYPECTOMY BLEEDING
A, Deep ulcer with visible vessel at the site of recent polypectomy. B, Clips applied to the lesion. The patient experienced no further bleeding.
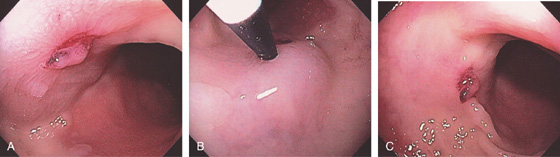
Figure 5.97 POSTPOLYPECTOMY BLEEDING
A, Small ulcer at the site of recent polypectomy. A small remnant of the polyp remains over the ulcer. B, Dilute epinephrine was injected into the base of the ulcer. C, The ulcer is somewhat raised off the mucosa because of the injection, and there is blanching of the surrounding mucosa.
Figure 5.98 POSTPOLYPECTOMY BLEEDING
A, Deep ulcer with adherent blood clot. B, Thermal therapy was applied to the lesion, resulting in an eschar (C). D, Given the apparent depth of the lesion, multiple clips were applied.
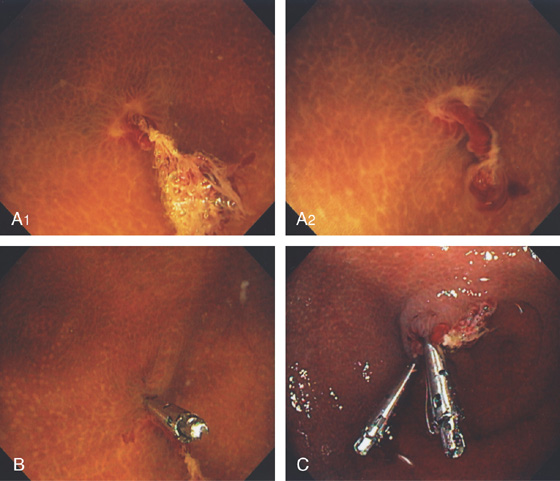
Figure 5.99 POSTPOLYPECTOMY BLEEDING
A1, A2, Area in the cecal pole with ulceration and long adherent blood clot/visible vessel. The lesion is well visualized underwater. B, The first clip is applied. C, Two clips applied to the lesion.
Figure 5.100 HOT BIOPSY TECHNIQUE
A, A small adenomatous-appearing polyp. B, The hot biopsy forceps is opened, and the head of the polyp is grasped and lifted away from the wall. C, With application of coagulation current, the base of the polyp assumes a white discoloration. Note the immediate edema present at the base of the polyp. D, A small amount of bleeding is present after biopsy, and edema is now more pronounced.
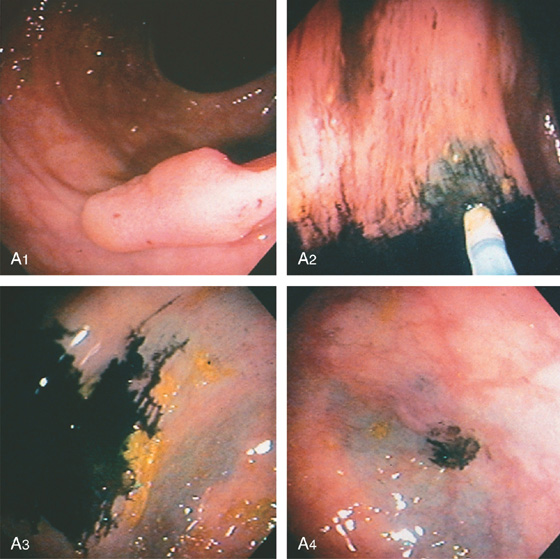
Figure 5.101 COLONIC TATTOOING
A, A sessile-appearing polyp in the descending colon (A1). A sclerotherapy needle filled with sterilized India ink is injected into the colonic wall surrounding the sessile lesion (A2-A4). After washing, a black discoloration is in the colonic wall at the point of injection (A3, A4).
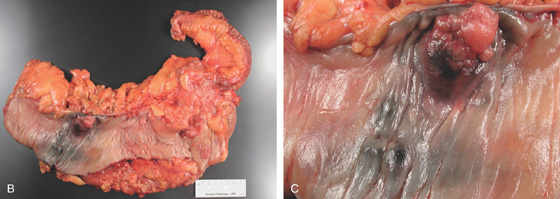
Figure 5.101 COLONIC TATTOOING
B, Postoperative specimen shows the polyp and submucosal India ink better seen on close-up (C).
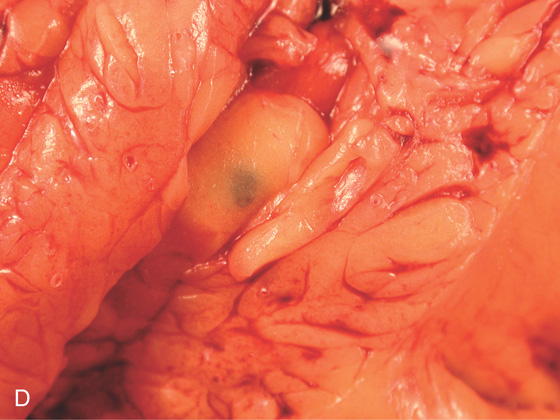
D, A small, dark area corresponding to the injection site is visible on the serosal surface.
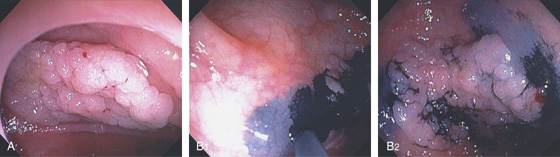
Figure 5.102 COLONIC TATTOOING
A, Large sessile polyp in the right colon. B1, B2, India ink is applied submucosally around the lesion.
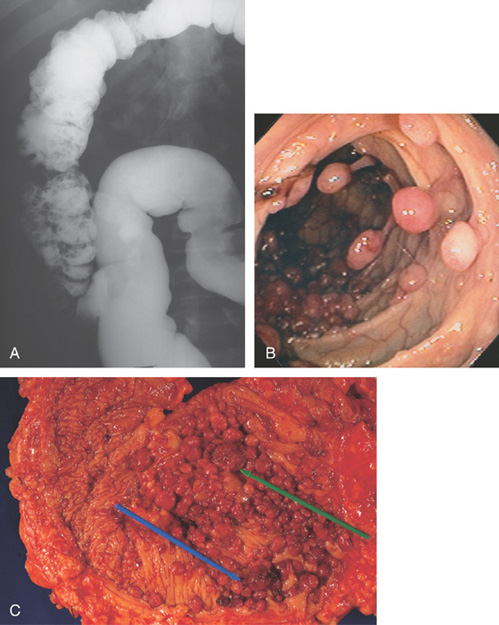
Figure 5.103 MULTIPLE COLONIC POLYPS
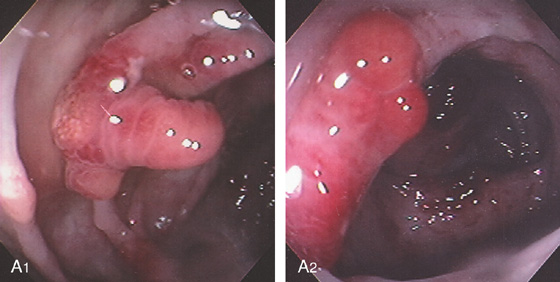
A, Multiple filling defects in the left colon and cecum, misinterpreted as a poor preparation with stool. B, Multiple colonic polyps, some on stalks and some sessile. Polyps were present throughout the colon. C, Resection specimens show the cluster of polyps. Arrows point to the larger polyps, which were villous adenomas.
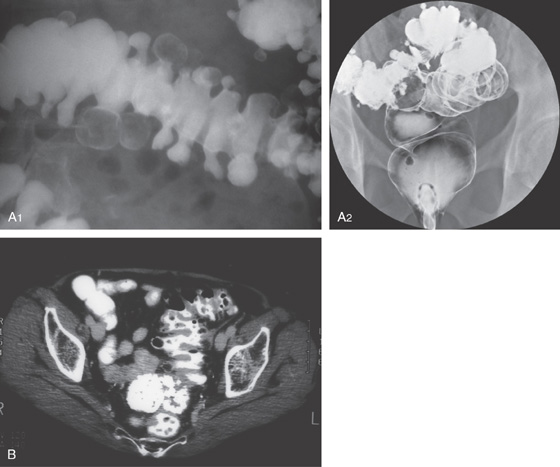
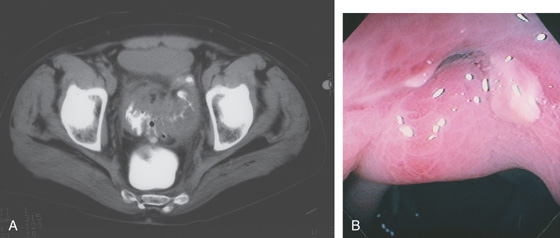
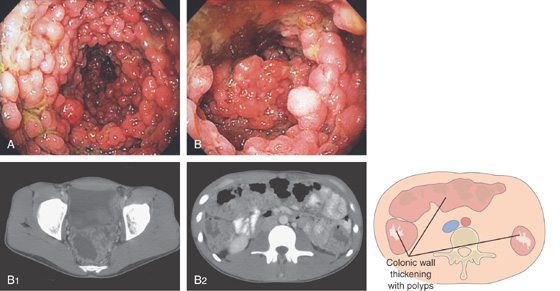
Figure 5.104 MULTIPLE COLONIC POLYPS IN FAMILIAL ADENOMATOUS POLYPOSIS
A, Multiple colonic polyps circumferentially coat the mucosa. B, Close-up of the polypoid lesions. B1, CT shows marked thickening of the rectum with multiple filling defects compatible with polyps. B2, A similar appearance of colonic wall thickening with polyps identified in the right and left colon.
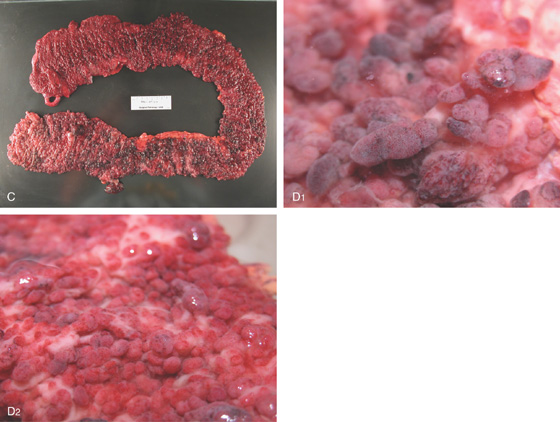
C, Surgical specimen demonstrates numerous polyps throughout the colon. D1, D2, Close-up of the multiple polyps.
Figure 5.105 HYPERPLASTIC POLYPS
A1, A2, Solitary small white polyp in the distal rectum. Note the overlying mucosa is smooth. B, Multiple hyperplastic polyps in the distal rectum. B1, B2, Solitary sessile polyp with smooth overlying mucosa in the descending colon on standard and narrow band imaging. C, Reddish polyp in the distal rectum. D, Flat polyp at the ileocecal valve. The mucosa blends in with the normal colonic tissue. E, Large sessile polyp resembling a villous adenoma. Note the nearby hyperplastic-appearing polyp. F, Hyperplastic polyp as shown on high-definition endoscopy (F1) and narrow band imaging (F2).
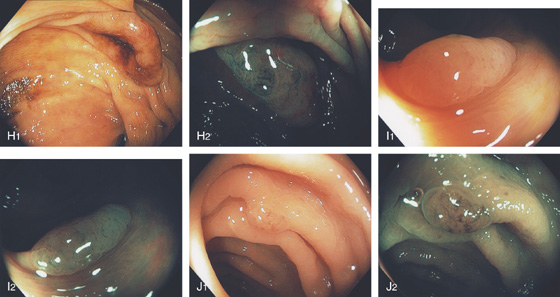
Figure 5.105 HYPERPLASTIC POLYPS
G-J, Polyps of varying sizes. Note the difference in the mucosal pattern on narrow band imaging in comparison with the adenomatous polyps.
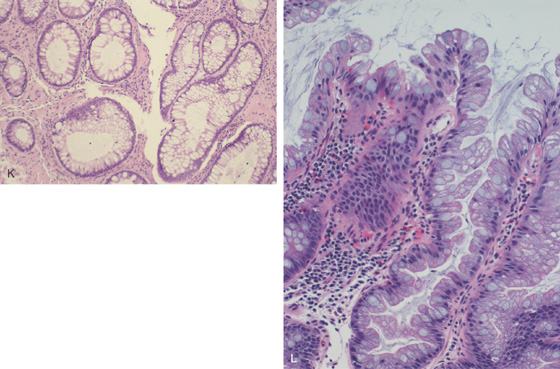
K, Typical mucosal changes of a hyperplastic polyp. The colonic crypts have an increased number of goblet cells, resulting in a “starfish” appearance. There is no inflammatory infiltrate or alteration in the nuclear/cytoplasmic ratio to suggest adenoma. L, Colonic mucosa with cells having increased cytoplasmic mucin content and arranged in a serrated configuration.
Figure 5.106 INFLAMMATORY POLYP
A, B, A polypoid lesion is completely coated with exudate. With some of the exudates removed, the polyp is edematous with overlying ulceration. C, The polyp is snared and removed, resulting in a mucosal defect (D). E, Small red polyp with overlying ulceration in the left colon.
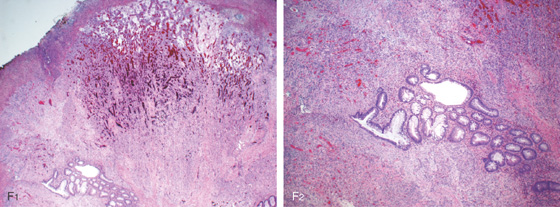
F1, F2, Polypoid mucosa with acute and chronic inflammation and ulceration with abundant granulation tissue.
Figure 5.107 INFLAMMATORY POLYP
A, Small polyp with ulcerated surface. B, Polyp with overlying exudate and ulcer. C, Multiple small polyps in the descending colon. This patient has a long-standing history of ulcerative colitis. D, Large inflammatory polyps with a reddish discoloration in a patient with long-standing ulcerative colitis.
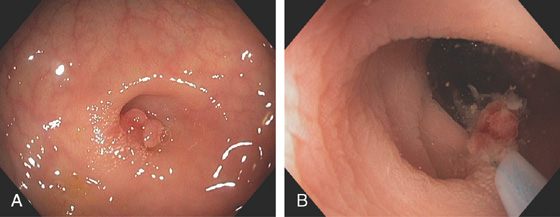
Figure 5.108 COLONIC INFLAMMATORY POLYP IN DIVERTICULUM
A, Small polyp in the base of a diverticulum. B, The polyp is removed using standard techniques.
Figure 5.109 FIBROVASCULAR POLYP
A, Solitary lesion with patchy vascular pattern as shown on standard and (B) narrow band imaging.
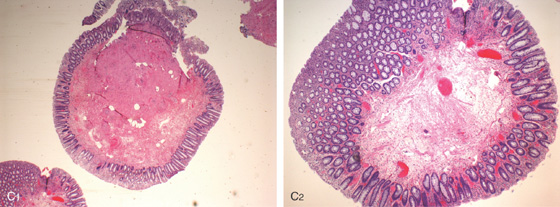
C1, C2, Polypoid colonic mucosa with focal surface erosion and prominent submucosa showing edema, fibrosis, focal adipose deposition, and vascular congestion/dilatation.
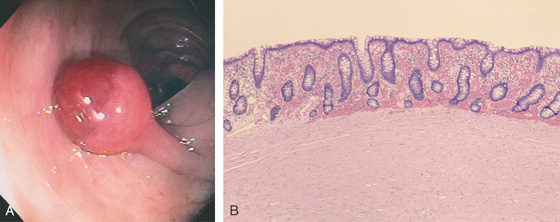
Figure 5.110 LEIOMYOMATOUS POLYP
A, The polyp has a short stalk and an unusual red demarcation of the head. The polyp head looks smooth and lacks the serpentine appearance of an adenomatous or a villous lesion. B, Normal colonic mucosa with subepithelial hemorrhage overlying a mass of smooth muscle bundles.
Figure 5.111 INFLAMMATORY FIBROID POLYPS
A, Multiple small polyps at the hepatic flexure. The polyps themselves are nondiagnostic in appearance. B, A moderate amount of inflammation is in the polyp. The striking finding in this polyp is the multiple eosinophils.
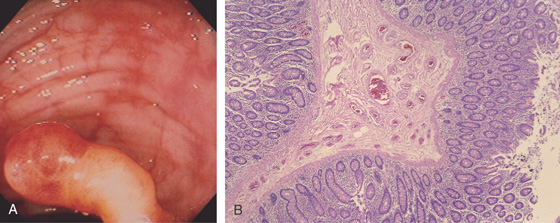
Figure 5.112 ARTERIOVENOUS MALFORMATION
A, A snakelike polyp with a yellowish stalk and red color of the head of the lesion. The polyp has been snared. B, The polypectomy specimen demonstrates an arterial malformation in which multiple, abnormally thick-walled arteries occupy the lamina propria.
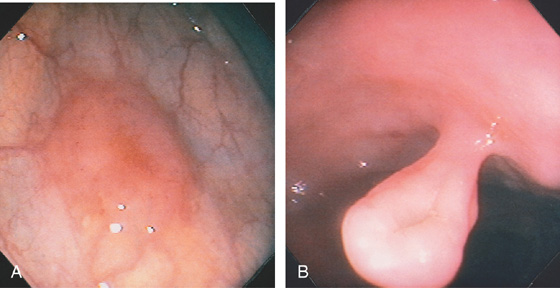
Figure 5.113 NORMAL COLON MIMICKING A POLYP
A, Well-demarcated sessile polyp in the descending colon. The surrounding mucosa has a normal vascular pattern. B, Pedunculated polyp. Note the absence of any adenomatous appearance of the polyp head.
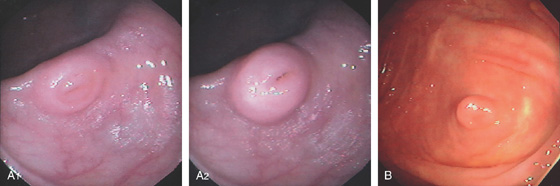
Figure 5.114 JUVENILE POLYP
A, This pedunculated polyp resembles an adenoma. The polyp was “rubbery” to palpation. B, C, Mushroom-shaped polyp as seen on standard and narrow band imaging. D, Typical features of a juvenile polyp include the large cystic structures. E, The red color of the polyp resulted from subepithelial hemorrhage and vascular engorgement.
![]() Differential Diagnosis
Differential Diagnosis
Juvenile Polyp (Figure 5.114)
Arterial venous malformation
Inflammatory polyp
Adenomatous polyp
Leiomyoma
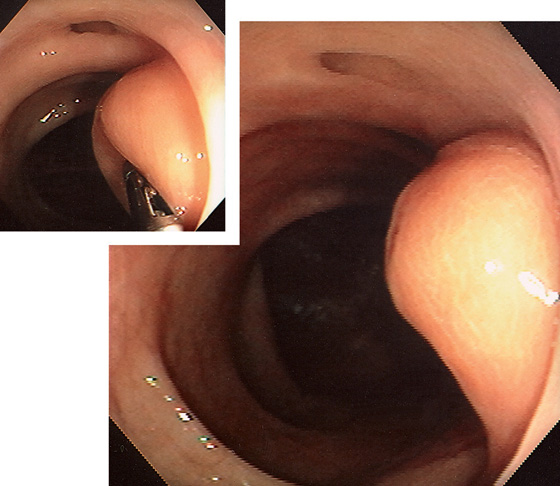
Figure 5.115 LIPOMA
Yellow sessile polyp in the right colon. Forceps demonstrate a pillow sign (inset).
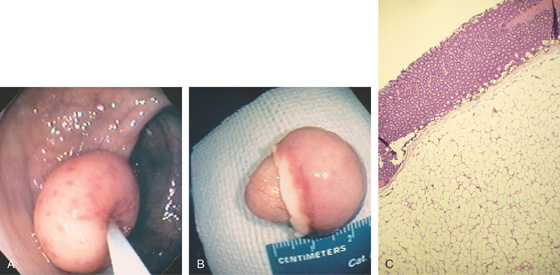
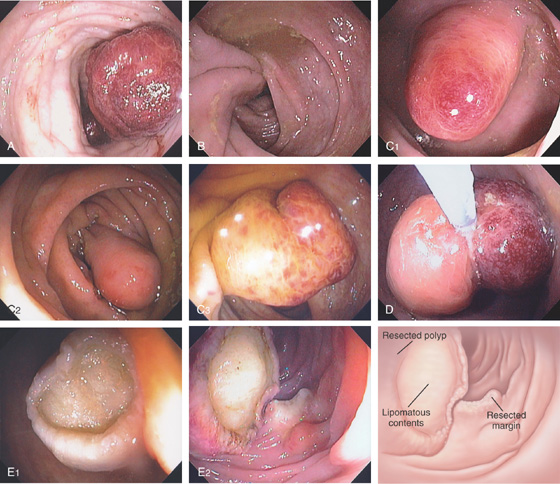
Figure 5.116 LIPOMA
A, Large polyp on a stalk; the lesion has areas of hypervascularity. A closed polyp snare is indenting the polyp (“pillow” sign). B, The lipoma measures about 2.5 cm. The lesion appears to be encapsulated. C, The lipoma is composed of mature adipocytes and compresses the overlying colonic mucosa.
Figure 5.117 LIPOMA
A, Large polyp with a hemorrhagic appearance. B, Note the large stalk. C1-C3, Hemorrhagic polyp. Note the overlying mucosa does not resemble an adenoma. D, Because of persistent bleeding, the larger polyps were removed using standard snare techniques. Coagulation current cuts very slowly through the lesion. E1, E2, After resection, note the mucosa surrounding the center of the polyp.
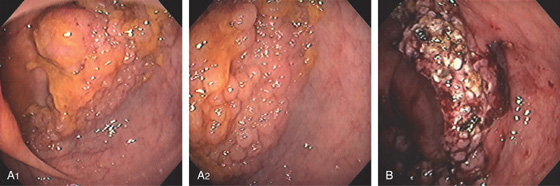
Figure 5.118 GIANT LIPOMA
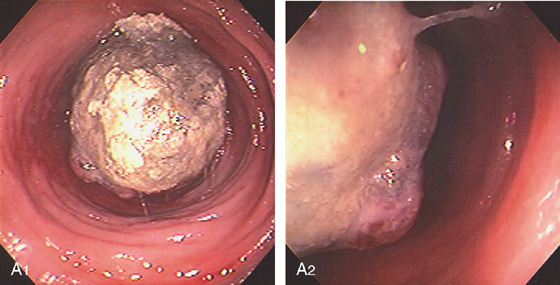
A1, Large mass lesion in the cecum. The surface appears ulcerated. A2, Close-up shows the exudate and nodularity of the lesion.
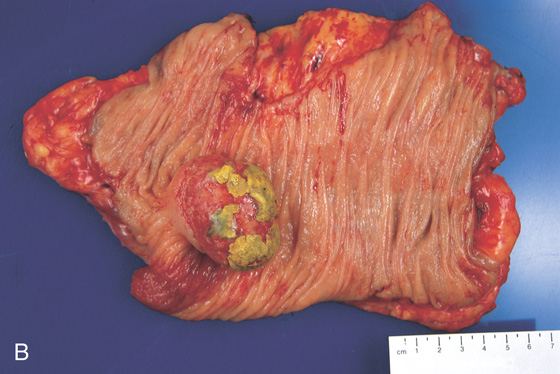
B, Colectomy specimen shows the well-circumscribed polyp with overlying ulcer.
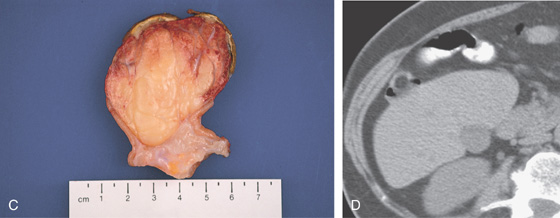
C, The fatty polyp after removal. D, Right-sided colonic polyp has a dark center, the density of fat.
Figure 5.119 NEUROFIBROMA AND GANGLIONEUROMA
A1, A2, Hemorrhagic-appearing polyps in the right colon. This patient has Von Recklinghausen syndrome.
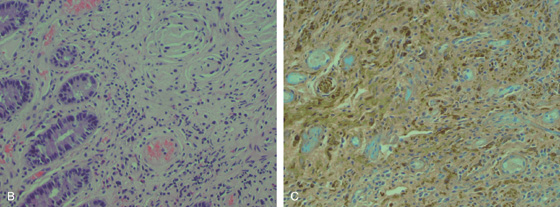
B, The lamina propria is hypercellular and composed of cytologically bland spindle-shaped cells. C, The cells are immunoreactive to the anti–S-100 protein antibody.
D1, D2, Small, nondescript polyp of the left colon.
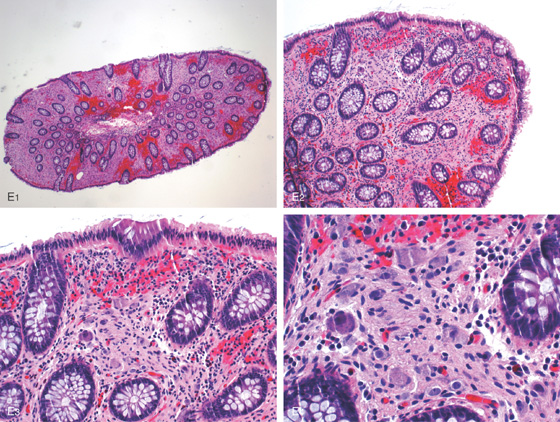
Figure 5.119 NEUROFIBROMA AND GANGLIONEUROMA
E1-E3, Colonic mucosa with lamina propria showing mild fibrosis, occasional wavy neural fibers, and numerous scattered ganglion cells consistent with ganglioneuroma seen on high power (E4). Usually, ganglion cells are located in the muscularis propria, not in the lamina propria.
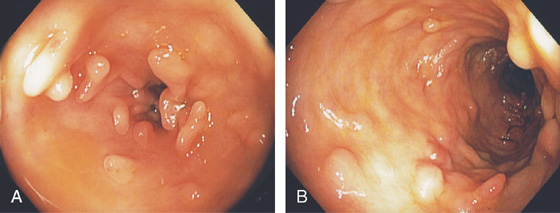
Figure 5.120 PSEUDOPOLYPS
A, B, Multiple filamentous polyps in the descending colon in a patient with prior active ulcerative colitis.
Figure 5.121 GARDNER’S SYNDROME
A, Multiple polyps in the cecum. B, Large pedunculated polyps. C, Multiple small flat polyps in the right colon.
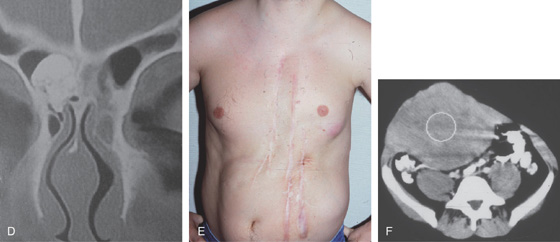
D, Osteoma in the ethmoid sinus. E, Multiple abdominal scars and deformity from desmoid tumors. F, Large desmoid tumor as shown on CT. (D courtesy J. L. Zubieta, MD, Pamplona, Spain.)
Figure 5.122 COWDEN SYNDROME
Multiple small colonic polyps (A-C). Histologically, these polyps are hamartomas.
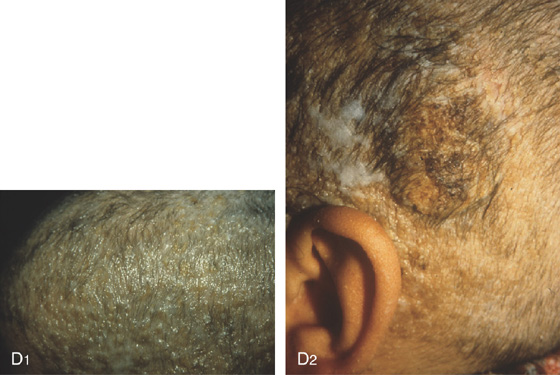
D1, D2, Typical skin lesions on the scalp (trichilemmomas) with hyperkeratotic papules and verrucous lesions. (Courtesy P. Redondo, MD, Pamplona, Spain.)
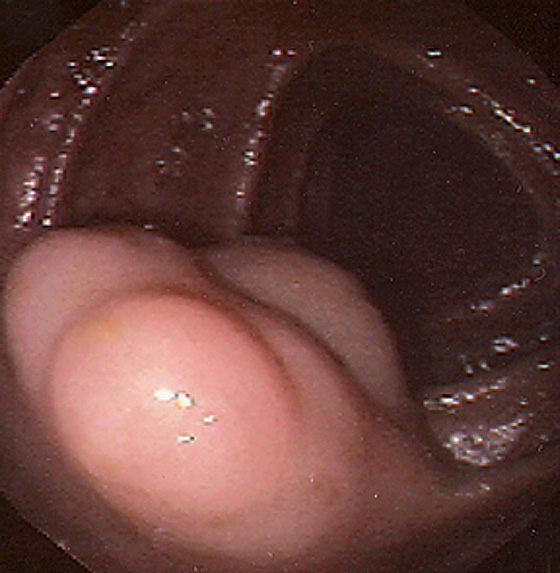
Figure 5.123 LYMPHATIC CYST
Multilobe cystic structure in the transverse colon.
Figure 5.124 PNEUMATOSIS COLI
A-C, Multiple submucosal lesions throughout the colon. D1, The lesion biopsy results show typical appearance of submucosal air (D2). E, Endoscopic ultrasonography demonstrates the submucosal lesions to be air represented by the hyperechoic areas with shadowing.
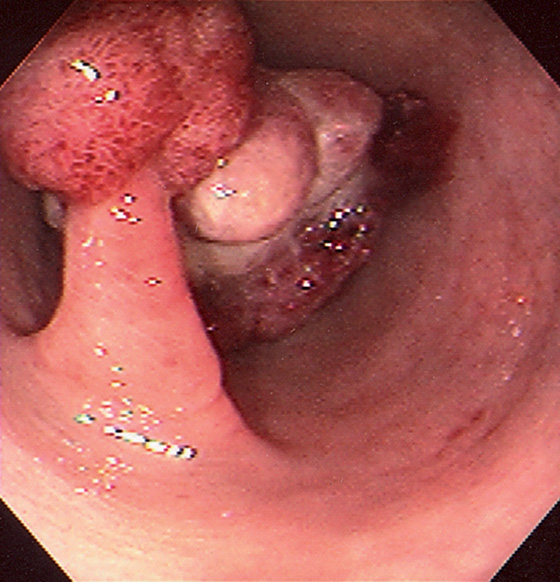
Figure 5.125 SENTINEL POLYP WITH CANCER
Adenomatous-appearing pedunculated polyp is just distal to a large mass lesion.
Figure 5.126 ADENOCARCINOMA
Small, flat polyp with overlying erosion.
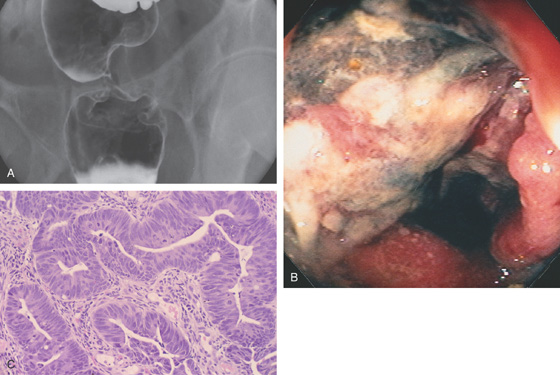
Figure 5.127 ADENOCARCINOMA OF THE RECTUM
A, A narrow stricture in proximal rectum. The margin of the stricture is nodular, and there is extrinsic compression proximal to the stricture. B, Circumferential ulcerated mass lesion. The black area with exudate represents tumor necrosis and ulceration. C, Well-differentiated adenocarcinoma with an increased nuclear/cytoplasmic ratio and atypical glands that appear “back to back.”
Figure 5.128 ADENOCARCINOMA OF SIGMOID
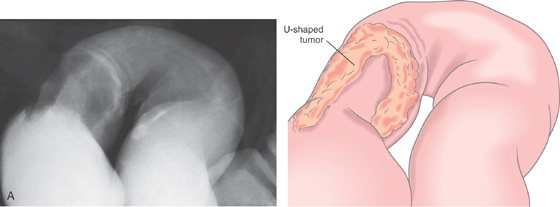
A, U-shaped mass lesion in the sigmoid colon.
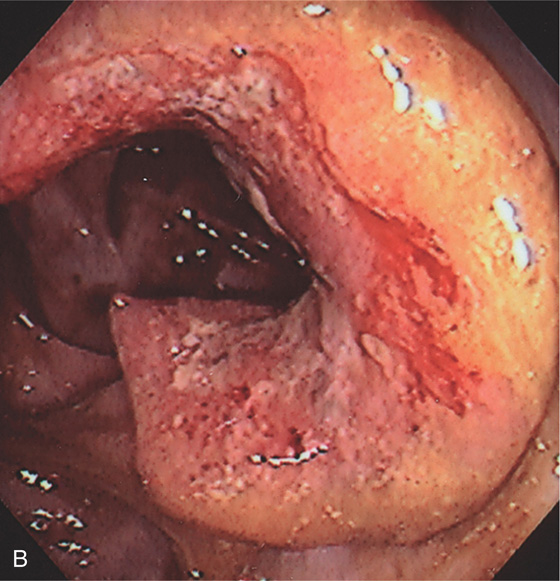
B, Hemicircumferential mass typical of carcinoma.
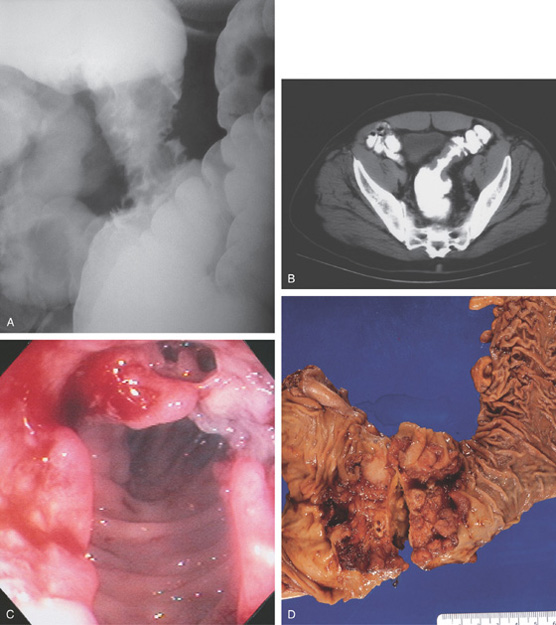
Figure 5.129 ADENOCARCINOMA OF SIGMOID
A, Annular lesion of the sigmoid colon is noted. B, “Apple-core” lesion demonstrated by computed tomography. C, Circumferential lesion shown with spontaneous bleeding. A cavity is in the superior portion of the polypoid tumor. D, Surgical resection demonstrates the circumferential polypoid nature of the sigmoid adenocarcinoma.
Figure 5.130 ADENOCARCINOMA OF TRANSVERSE COLON
A, Mass lesion covered with stool and exudate. B, C, With washing, a typical hemicircumferential tumor can be seen. D, The patient previously underwent distal resection. Note the anastomotic scar and neovascularity. D1, Mass lesion of transverse colon as shown on standard and coronal images (D2).
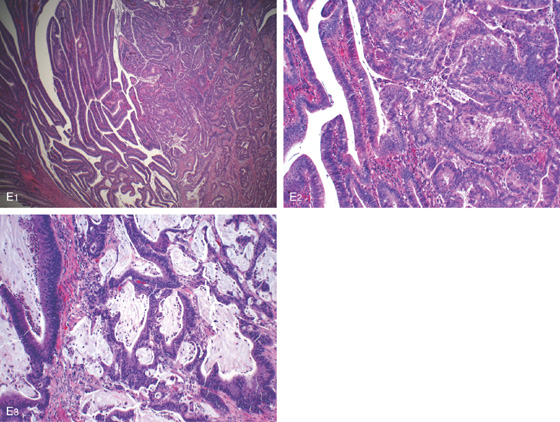
Figure 5.130 ADENOCARCINOMA OF TRANSVERSE COLON
E1, E2, Invasive well-differentiated to moderately differentiated adenocarcinoma arising in a tubulovillous adenoma. Mucin is present (E3), but the tumor has to consist of more than 50% mucin to be labeled a mucinous (colloid) carcinoma.
Figure 5.131 ADENOCARCINOMA OF ASCENDING COLON
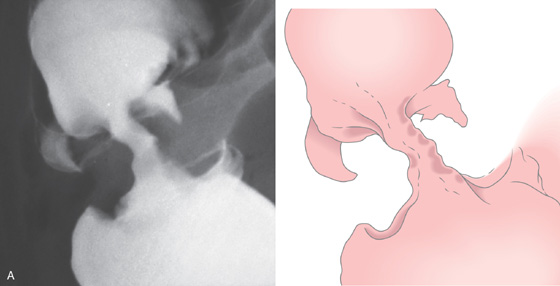
A, “Apple-core” lesion is shown.
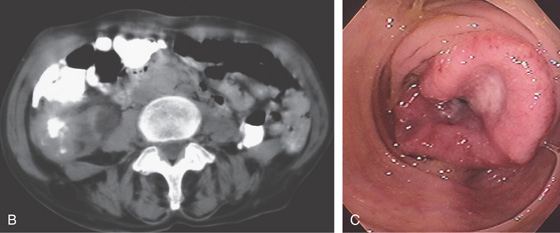
B, The mass lesion is easily identified. C, The distal portion of the neoplasm is demonstrated by the edematous circumferential fold. An ulcer is present in the center of the tumor.
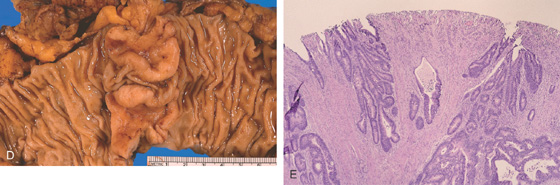
D, Surgical specimen confirms the circumferential neoplasm. E, Scirrhous nature of the colonic neoplasm is evident.
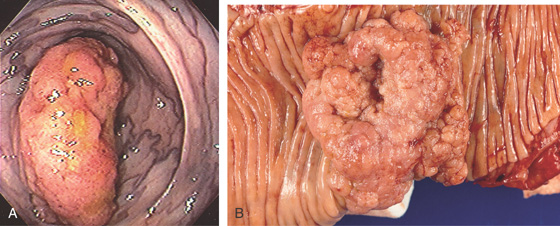
Figure 5.132 ADENOCARCINOMA ARISING IN VILLOUS ADENOMA
A, Large polypoid lesion in the right colon. Note the venous congestion. B, Large sessile polyp.
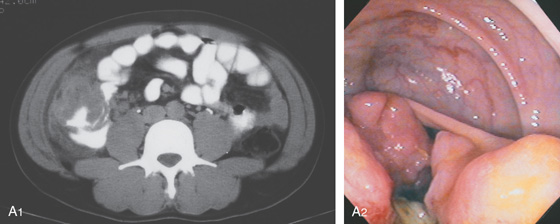
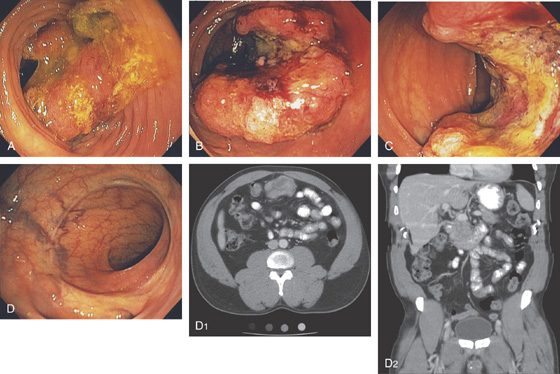
Figure 5.133 HEMICIRCUMFERENTIAL ADENOCARCINOMA OF THE CECUM
A1, Mass lesion appears hemicircumferential; the lesion appears to have an inflammatory component outside the colonic wall. A2, Hemicircumferential mass with central ulceration.
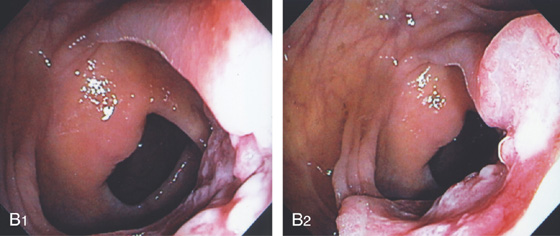
B1, B2, The tumor is opposite the ileocecal valve and demonstrates a raised ulcerated appearance.
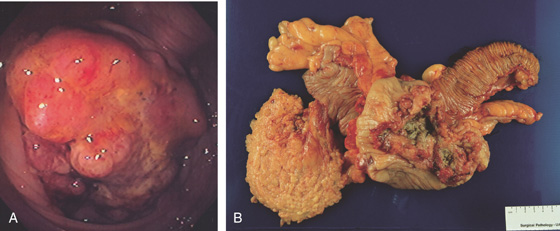
Figure 5.134 ULCERATED CECAL ADENOCARCINOMA
A, Large, ulcerated mass lesion occupying the cecum. B, Large cecal mass.
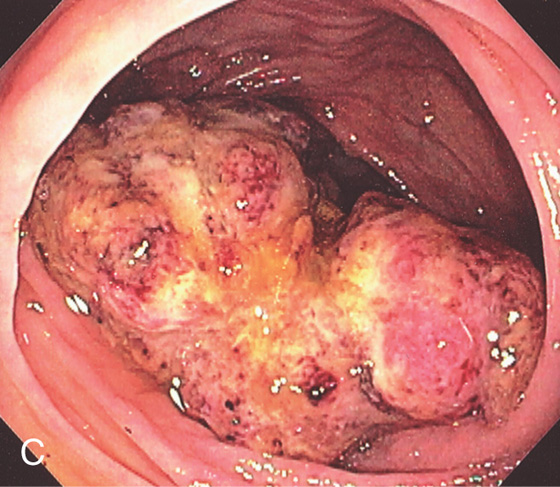
C, Ulcerated cecal mass with stigmata of recent bleeding.
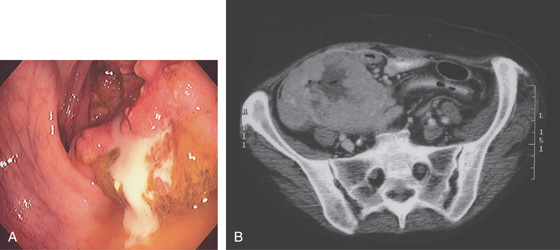
Figure 5.135 PERFORATED CECAL ADENOCARCINOMA
A, Mass lesion of the cecum exuding pus. B, Large mass lesion with a central hypodense area representing abscess.
Figure 5.136 ADENOCARCINOMA OF ILEOCECAL VALVE
A, Mass lesion in the cecal pole with extrinsic compression on the terminal ileum, associated with nodularity and narrowing of the distal ileum and proximal dilation. The cecum is contracted and thick-walled. B, The mass lesion occupies the ileocecal valve. The folds surrounding the valve are edematous. The center of the lesion has a nodular appearance.
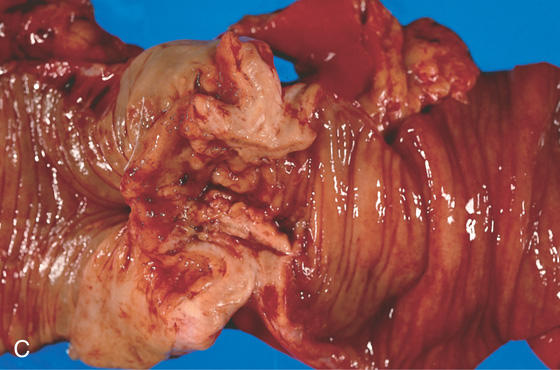
C, Carcinoma of the ileocecal valve with a nodular appearance. The terminal ileum is on the right and is dilated. Normal colonic mucosa is present on the left.
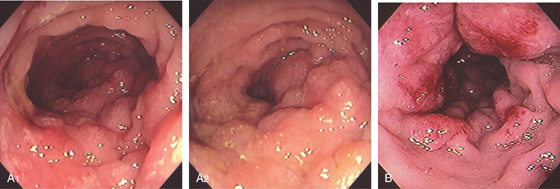
Figure 5.137 INFILTRATING COLON CANCER (LINITIS PLASTICA)
A1, A2, Diffuse nodularity of the right colon without mass lesion. This resembles a linitis plastica appearance. B, Hemorrhagic nodular lesions infiltrating the colonic wall.
Figure 5.138 ENTERAL STENTING OF OBSTRUCTING COLON CANCER
A, Obstructing mass lesion of proximal descending colon. B, The prosthesis is placed fluoroscopically through the lesion. C, The stent has been deployed.
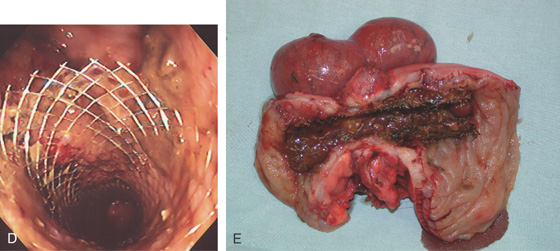
D, The stent is widely patent. E, Position of stent in tumor at resection.
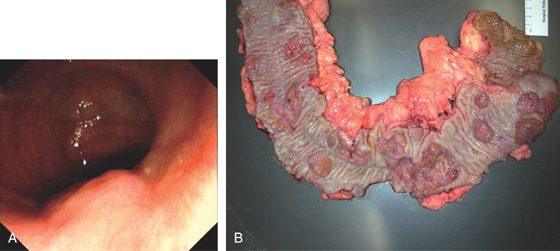
Figure 5.139 ADENOCARCINOMA IN ULCERATIVE COLITIS
A, Raised lesion in distal colon. The surrounding mucosa has a bland appearance. B, Multiple colonic polyps and cancers are present throughout colon.
Figure 5.140 NON-HODGKIN’S LYMPHOMA
Submucosal mass lesion with petechiae.
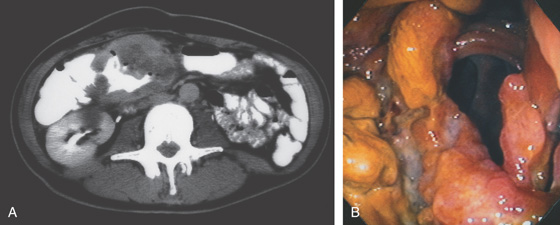
Figure 5.141 NON-HODGKIN’S LYMPHOMA
A, Large mass lesion in the proximal transverse colon. Hypodense areas in the tumor represent necrosis. The patient has acquired immunodeficiency syndrome with fever and abdominal pain. B, Circumferential ulcerated mass lesion is shown.
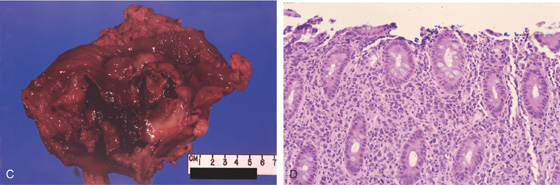
C, The circumferential necrotic nature of the mass is shown. D, The lamina propria is expanded by infiltration of lymphoma cells. For optimal pathologic diagnosis of lymphoma, a portion of the specimens should be placed in Bouin’s fixative or submitted on saline-dampened gauze immediately.
Non-Hodgkin’s Lymphoma (Figure 5.141)
Adenocarcinoma
Metastatic neoplasm
Gastrointestinal stromal tumor
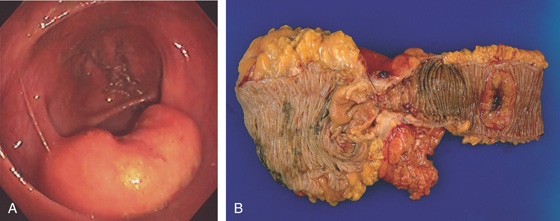
Figure 5.142 NON-HODGKIN’S LYMPHOMA OF THE ILEOCECAL VALVE
A, Bulbous ileocecal valve. The valve appears firm and fixed. B, Mass lesion involving the ileocecal valve. Note the ileum is dilated and a synchronous ileal lymphoma is present (right).
C, Close-up shows the ileocecal valve mass.
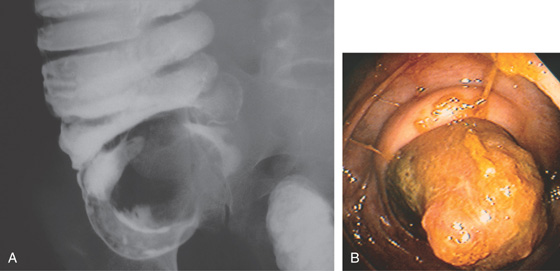
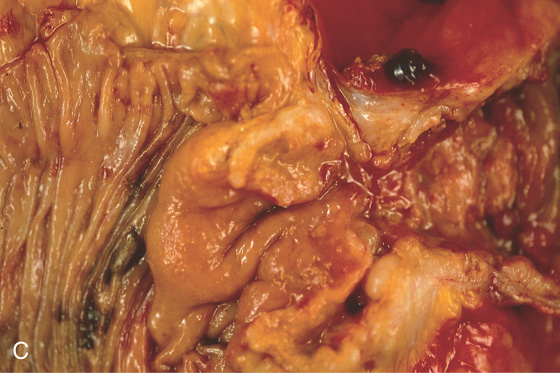
Figure 5.143 CECAL NON-HODGKIN’S LYMPHOMA IN ACQUIRED IMMUNODEFICIENCY SYNDROME
A, Mass lesion occupying the cecum. B, Mass lesion projecting from the base of the cecum.
Cecal Non-Hodgkin’s Lymphoma in Acquired Immunodeficiency Syndrome (Figure 5.143)
Carcinoid tumor
Adenocarcinoma
Gastrointestinal stromal tumor
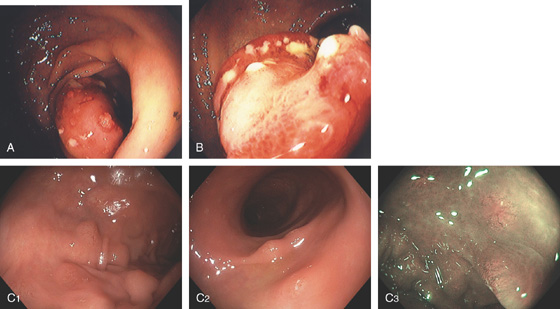
Figure 5.144 MANTEL CELL LYMPHOMA
A, B, Solitary polypoid lesion in the distal colon with overlying ulceration. C1, C2, Multiple sigmoid lesions shown on high-definition and narrow band imaging (C3).
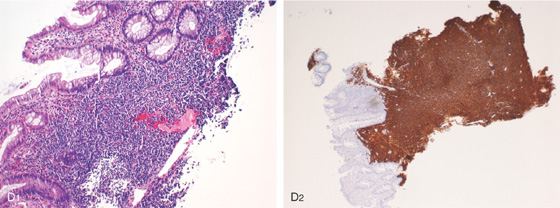
D1, Atypical lymphoid infiltrates are present. D2, CD20 immunostain highlights the malignant B lymphocytes.
Figure 5.145 LEUKEMIA
A, Circumscribed red lesions throughout the colon. B1, B2, Close-up shows the lesions to be well-circumscribed, round areas of subepithelial hemorrhage suggestive of lymphoid aggregates.
C, The mucosa is infiltrated by leukemic cells. (See also Figures 1.37, 2.74, and 5.207.)
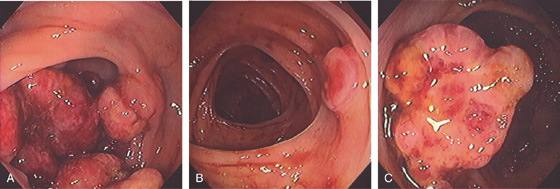
Figure 5.146 KAPOSI’S SARCOMA
A, Multiple confluent hemorrhagic mass lesions in the cecum. B, Solitary raised lesion in the transverse colon. C, Solitary mass lesion in the descending colon.
Figure 5.147 KAPOSI’S SARCOMA IN ACQUIRED IMMUNODEFICIENCY SYNDROME
A, B, Small submucosal hemorrhagic lesion.
C, Spindle cells in the submucosa.
Figure 5.148 METASTATIC LESIONS TO THE COLON
A, Breast cancer. A1, Large mass lesions occupying the colonic lumen. A2, Solitary raised lesion. A3, Raised white plaquelike lesion.
B, Cervical cancer. Raised lesion with central indentation. C, Metastatic neuroendocrine tumor of the pancreas. C1, Nodular lesion. C2, Large nodular mass lesion. D, Metastatic melanoma. Raised dark lesion.
Figure 5.149 CARCINOID TUMOR
A, Well-circumscribed mass is at the ileocecal valve. The surface of the lesion is smooth and was hard when probed with the biopsy forceps. These features are atypical for an adenoma. B, Surgical specimen shows well-demarcated lesion at ileocecal valve.
C, Typical pathologic appearance of carcinoid tumor.
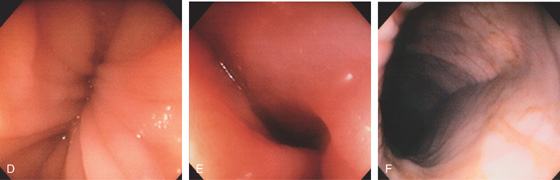
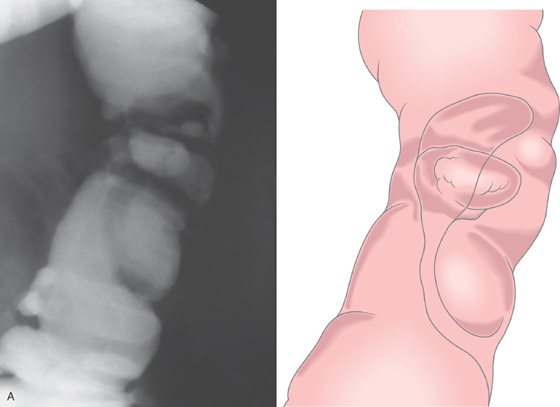
Figure 5.150 SIGMOID VOLVULUS
A, Typical kidney-bean shape of a sigmoid volvulus. B, The twist of the sigmoid colon is demonstrated. C, The colonic mucosa appears twisted around a central opening at barium enema examination. D, Midportion of the twist is shown. No associated colitis or mass lesion is present. E, Further advancement of the colonoscope demonstrates partial opening in the proximal end of the twist. F, Once past the twist, the colon is markedly dilated. The mucosa appears normal, with no evidence of ischemia.
Figure 5.151 MELANOSIS COLI
A, Mild disease with subtle pinpoint dark discoloration to the mucosa. B, More extensive dark discoloration. C1, C2, The colon is black. D1, Lymphoid tissue does not take up the pigment, and thus is easily identified, (D2) as shown on narrow band imaging.
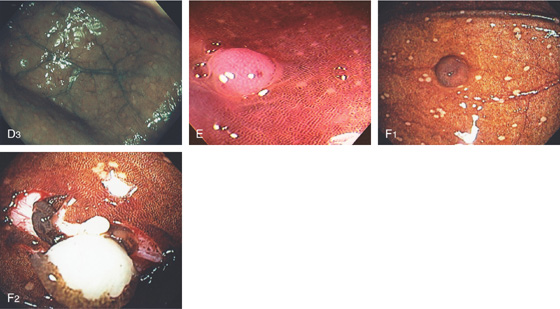
D3, Multiple lesions in the cecal pole. E, Likewise, polyps do not take up the pigment and are readily identified. F1, The mucosa overlying this polyp is stained. F2, The polyp is resected demonstrating a lipoma. Because the overlying mucosa was normal, the pigment was taken up.
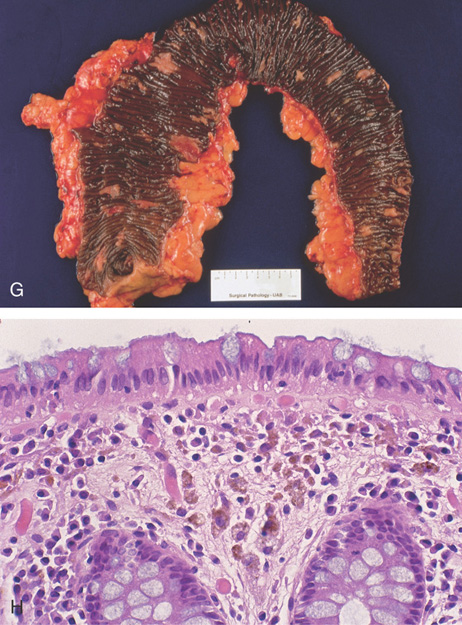
Figure 5.151 MELANOSIS COLI
G, Striking changes in this colectomy specimen. H, Normal-appearing colonic mucosa, with multiple pigment-containing macrophages.
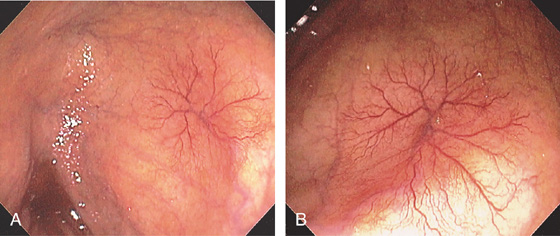
Figure 5.152 VASCULAR ECTASIAS
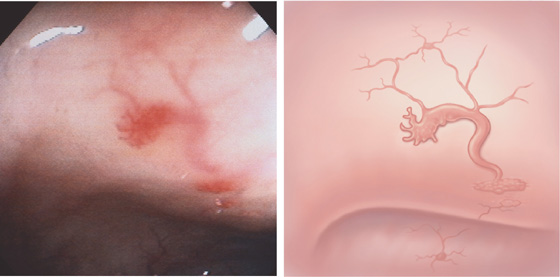
A tuft of blood vessels resembles spider angioma in the cecum. A large draining vein is emanating from the ectasia. Several other vascular ectasias are noted proximally.
Figure 5.153 VASCULAR ECTASIA
Large sessile ectasia in the cecal pole.
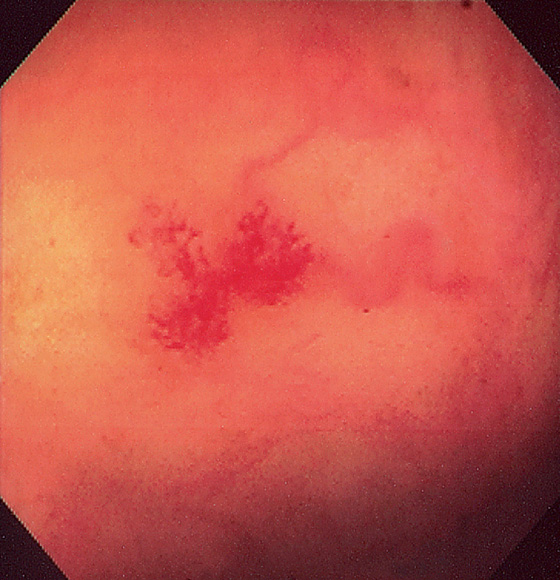
Figure 5.154 VASCULAR ECTASIA
Typical spider-web appearance of a vascular ectasia. Note the draining vein.
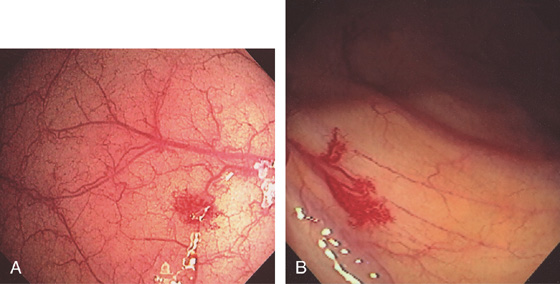
Figure 5.155 VASCULAR ECTASIA
Solitary ectasias in the right colon (A, B).
Figure 5.156 VASCULAR ECTASIA
A, Large ectasia in a patient with portal hypertension. B, Note the draining vein on close-up.
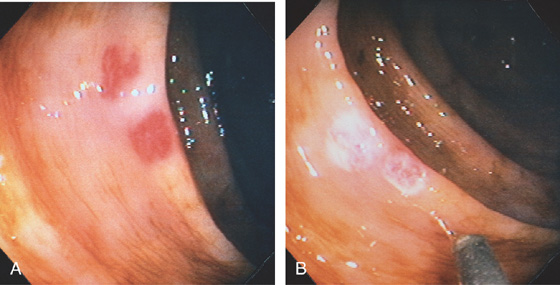
Figure 5.157 VASCULAR ECTASIAS
A, Two well-circumscribed vascular ectasias in a patient with chronic renal failure and hematochezia. The tuft of blood vessels in the ectasia does not have a spider angiomatous appearance. B, The 7-French heater probe is shown after coagulation of the lesions. The ectasias now appear white from the coagulation.
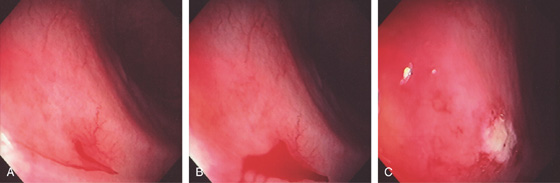
Figure 5.158 ACTIVE BLEEDING FROM VASCULAR ECTASIA
A, B, A stream of blood is emanating from an ectasia. C, Appearance of the lesion after thermal ablation.
Figure 5.159 LASER ABLATION OF VASCULAR ECTASIA
A1, A2, Ectasia in distal colon. Close-up shows the draining vein. B, The argon laser probe is visible. Spontaneous bleeding was present from the lesion with inadvertent trauma from the catheter. C, Lesion after ablation.
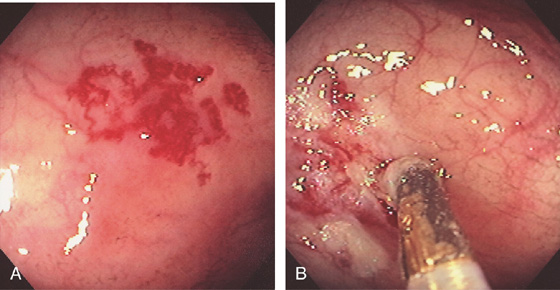
Figure 5.160 THERMAL TREATMENT OF VASCULAR ECTASIAS
A, Large tuft of ectasias. B, Thermal therapy being applied.
Figure 5.161 VASCULAR ECTASIA
A, Typical appearance of ectasia with draining veins.
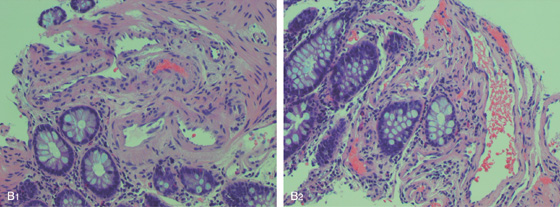
B1, Biopsy shows submucosal blood vessels. B2, Red blood cells in a vascular channel.
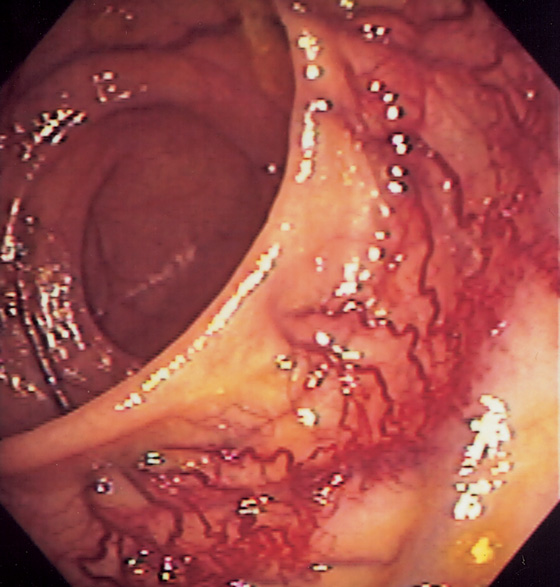
Figure 5.162 ANASTOMOTIC VARICES
Neovascularization is apparent at the site of prior surgical anastomosis.
Figure 5.163 DIEULAFOY LESION
A, Pinpoint area of active bleeding. The thermal probe is alongside the lesion. B, Hemostasis achieved after thermal ablation.
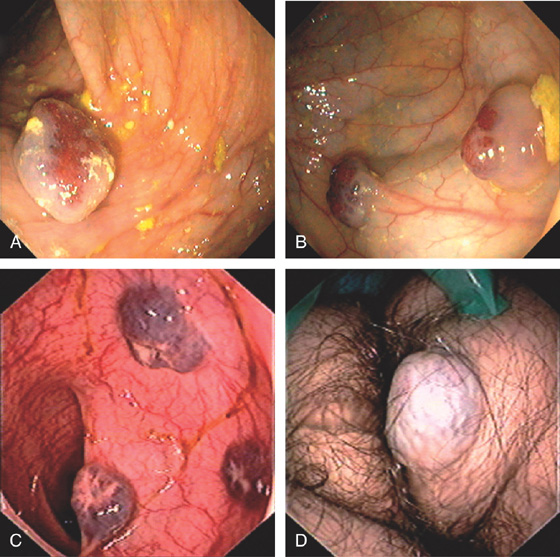
Figure 5.164 BLUE RUBBER BLEB NEVUS SYNDROME
A, Large solitary cavernous hemangioma. B, Two lesions. C, Three submucosal cavernous hemangiomas in the left colon. D, Vascular lesion on the buttocks.
Figure 5.165 KLIPPEL-TRENAUNAY-WEBER SYNDROME
A, Large, flat submucosal venous structure in the rectum sparing the lymphoid follicles. B, Subtle rectal hemangioma. C, Large flat hemangioma of the leg.
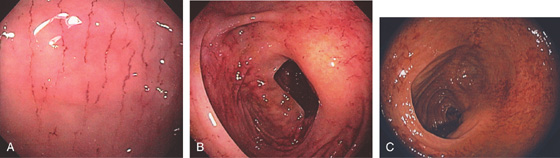
Figure 5.166 BAROTRAUMA
A-C, Multiple linear lesions in the right colon.
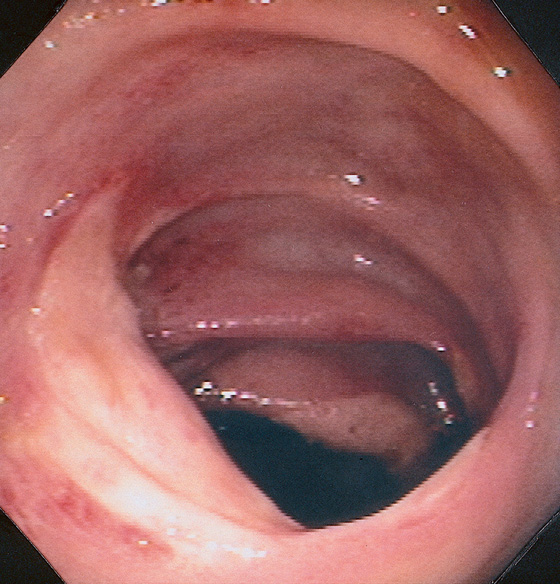
Figure 5.167 CECAL ULCERS
Large ulcerations in the cecum. The lesions were not secondary to ischemia but were idiopathic. Similar lesions may be observed in patients with Crohn’s disease or secondary to nonsteroidal antiinflammatory drug treatment.
Cecal Ulcer (Figure 5.167)
Infection (cytomegalovirus)
Inflammatory bowel disease (Crohn’s disease)
Vasculitis
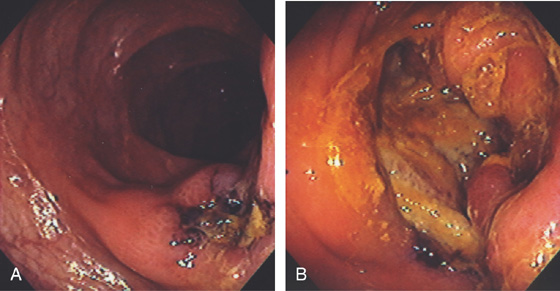
Figure 5.168 IDIOPATHIC COLONIC ULCER
Large ulceration in the right colon (A) and cecum (B) in a patient with acquired immunodeficiency syndrome.
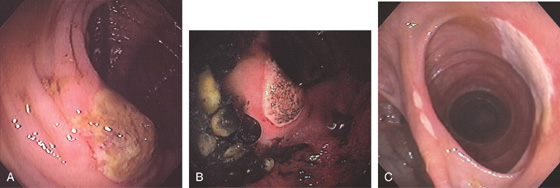
Figure 5.169 NONSTEROIDAL ANTIINFLAMMATORY DRUG-INDUCED ULCER
A, Solitary ulcer at the ileocecal valve. B, Large ulcer straddling the ileocecal valve. C, Ulceration of the ileocolonic anastomosis.
Figure 5.170 NONSTEROIDAL ANTIINFLAMMATORY DRUG-INDUCED COLITIS
Normal-appearing ascending colon, with the cecum in the distance (A). Withdrawal of the colonoscope demonstrates focal ulceration (B); further withdrawal demonstrates circumferential edema and ulceration, suggestive of ischemia (C, D). Segmental circumferential disease also occurs with ischemia, inflammatory bowel disease (Crohn’s disease), and some infections (cytomegalovirus colitis).
![]() Differential Diagnosis
Differential Diagnosis
Nonsteroidal Antiinflammatory Drug-Induced Colitis (Figure 5.170)
Crohn’s disease
Ischemia
Infection (cytomegalovirus)
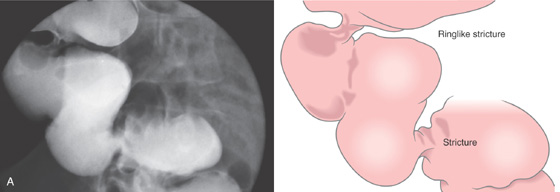
Figure 5.171 NONSTEROIDAL ANTIINFLAMMATORY DRUG (NSAID)-INDUCED COLONIC STRICTURES
A, Segmental strictures in the right colon, with normal-appearing mucosa.
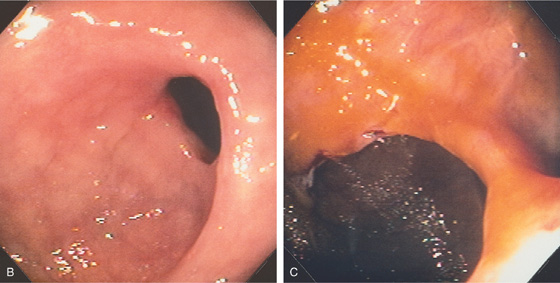
B, The distal stricture has a ringlike appearance. The mucosa appears normal. C, The proximal stricture is formed by a ring of fibrosis, and a small ulceration is present. The strictures and ulceration were secondary to chronic NSAID use.
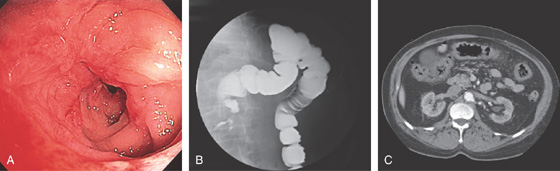
Figure 5.172 NONSTEROIDAL ANTIINFLAMMATORY DRUG-INDUCED COLONIC STRICTURE
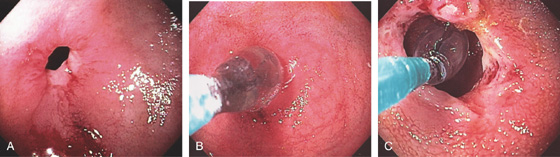
A, Tight stricture with cicatrization of the surrounding mucosa in the transverse colon. B, Barium enema shows the caliber of the transverse colon stricture. C, Thickening of the transverse colon in the area of the stricture. This patient also had gastric (see Figure 3.61B) and duodenal ulcers.
Figure 5.173 BENIGN COLONIC STRICTURE
A, Tight anastomotic stricture in the descending colon. B, A balloon is passed across the stricture and inflated. C, The appearance after dilatation.
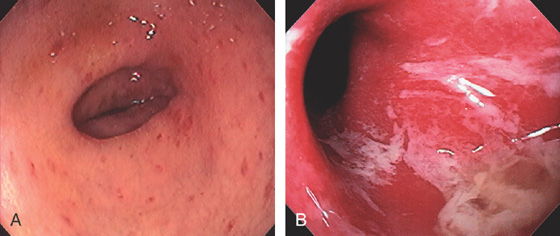
Figure 5.174 GRAFT-VERSUS-HOST DISEASE (GVHD)
A, Patchy subepithelial hemorrhage and edema of the descending colon. B, Severe disease with complete loss of the mucosa associated with mucopus.
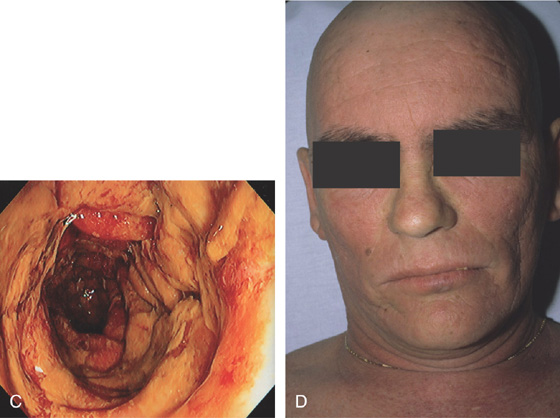
C, Diffuse edema with exudate covering the descending colon. D, Diffuse erythema is typical for GVHD of the skin.
Figure 5.175 AMYLOID COLOPATHY
A, Patchy subepithelial hemorrhage in the distal colon. B1, B2, Diffuse edema and ulceration of the right colon resembling an inflammatory bowel disease.
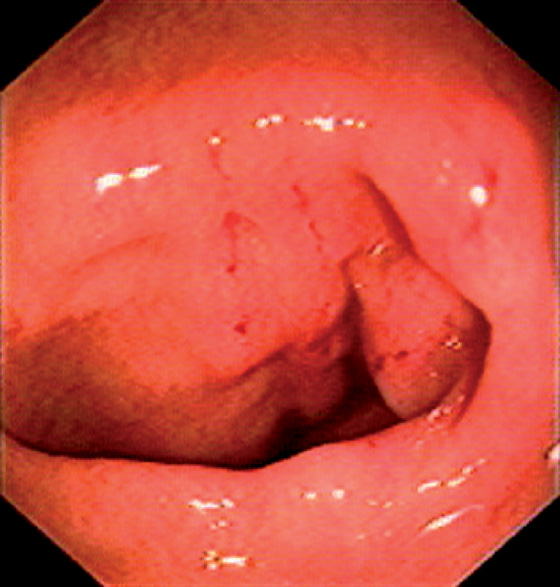
Figure 5.176 SYSTEMIC MASTOCYTOSIS
Two linear areas of edema with overlying subepithelial hemorrhage are related to touching the mucosa with biopsy forceps. Similar phenomenon have been described for the skin termed Darier sign (urticaria pigmentosa).
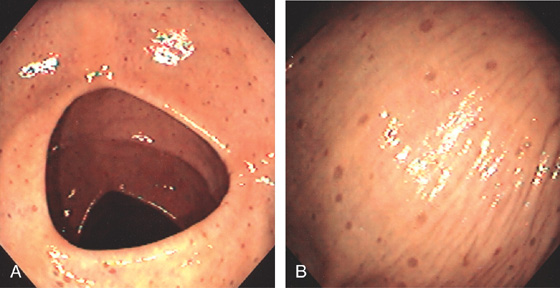
Figure 5.177 XANTHOMA
A, B, Multiple well-circumscribed, hyperpigmented lesions throughout the colon.
Figure 5.178 APHTHOUS ULCERS
A, B, Diffuse aphthous ulcers in the distal colon.
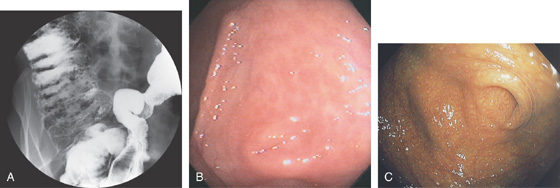
Figure 5.179 LYMPHOID HYPERPLASIA
A, Barium enema shows numerous filling defects in the right colon (new x-ray scan). B, Multiple small nodular lesions surrounding the appendiceal orifice are typical of lymphoid hyperplasia. Some of the lesions have a “red ring” appearance. The appendiceal orifice is in the center. C, Melanosis accentuates the finding.
Figure 5.180 END-TO-END ANASTOMOSIS
Sutures are seen at the anastomosis. The polypoid-appearing tissue at the suture site represents an inflammatory response rather than residual tumor or adenoma.
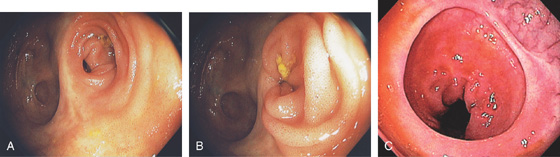
Figure 5.181 END-TO-END ANASTOMOSIS
A, The anastomosis is on the right. B, The small-bowel mucosa prolapses, confirming the endoscopic impression. C, Widely patent end-to-end distal colonic anastomosis.
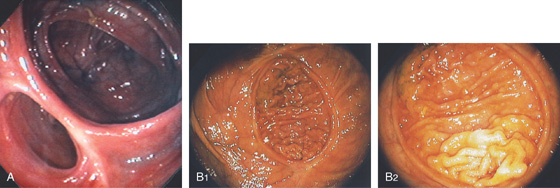
Figure 5.182 END-TO-SIDE ANASTOMOSIS
A, The blind colonic pouch is at the top right, with the ileum on the lower left. B1, B2, Large, circular fixed anastomosis with a small amount of prolapsing small-bowel mucosa.
Figure 5.183 EXTRINSIC LESION
Extrinsic compression in the proximal rectum, with normal-appearing overlying mucosa. The extrinsic compression resulted from a tumor mass in the pelvis.
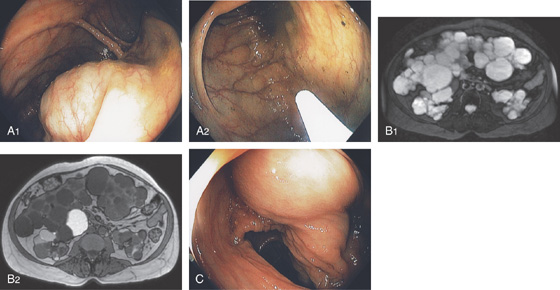
Figure 5.184 EXTRINSIC COMPRESSION
A1, A2, Multiple extrinsic lesions compressing the right colon in a patient with polycystic liver and kidney disease. B1, B2, Magnetic resonance imaging (MRI) shows the multiple lesions of the liver and kidneys. C, Corresponding compression in right colon.
Figure 5.185 EXTRINSIC COMPRESSION IN THE RIGHT COLON FROM CIRRHOSIS
Note the nodular appearance of the compressing liver.
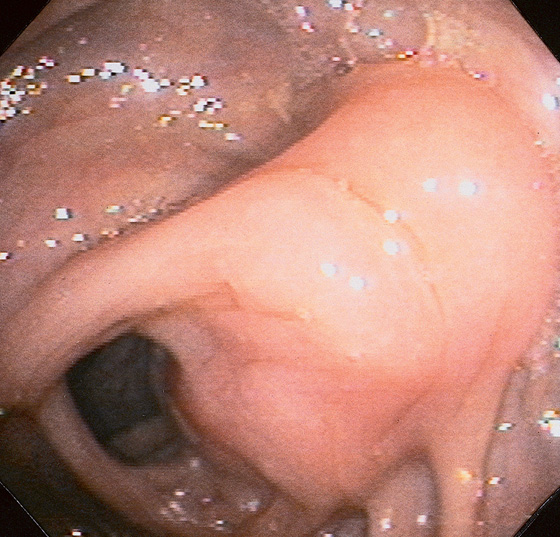
Figure 5.186 SEROSAL LESION
A well-circumscribed, edematous lesion that appears to be submucosal. A small ulceration is at the apex of the lesion. The surrounding colonic mucosa appears normal. This submucosal lesion represents a serosal metastasis from a distal colonic carcinoma.
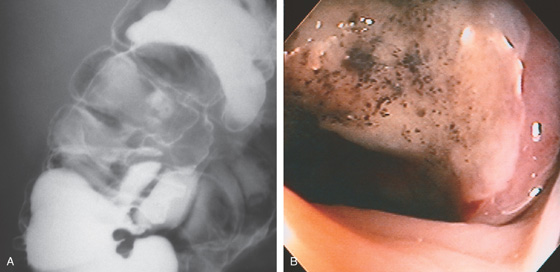
Figure 5.187 EXTRINSIC LESION
A, Round, masslike lesion at the hepatic flexure that appears to be submucosal. B, Ulcerated lesion with a well-circumscribed nodular border.
C, Renal cell carcinoma eroding into the colon.
Figure 5.188 EROSION OF PANCREATIC PSEUDOCYST INTO LEFT COLON
A, Fluid collection in the right and left colon. B1, B2, Note large amount of air fills the collection.
C1, C2, Mucosal defect is seen with fresh blood. D1, D2, The colonoscope is passed through the defect, demonstrating a large cavity representing the pseudocyst. E, Large pseudocyst cavity where the scope has entered.
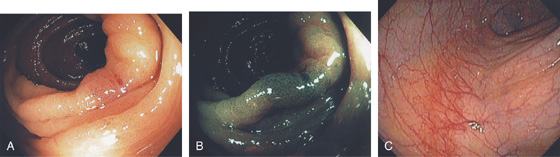
Figure 5.189 NONSPECIFIC ABNORMALITY
A, Focal area of edema and subepithelial hemorrhage of unclear significance. B, Narrow band imaging suggests the lesion is not adenomatous. Biopsy showed nonspecific findings. C, Linear area of hypervascularity related to the colonoscope. This linear lesion is commonly seen in the distal colon on colonoscopic withdrawal.
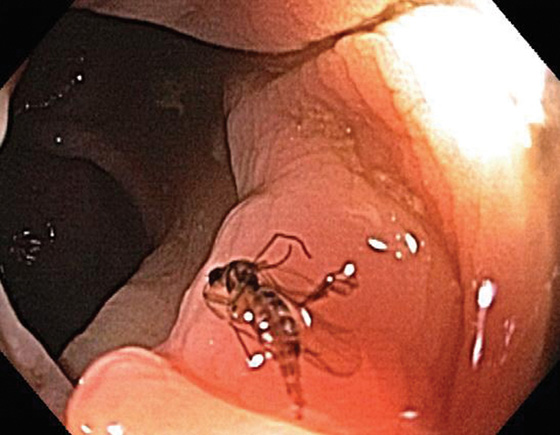
Figure 5.190 COLONIC FOREIGN BODIES
Fly. (Courtesy Leticia Luz, MD.)
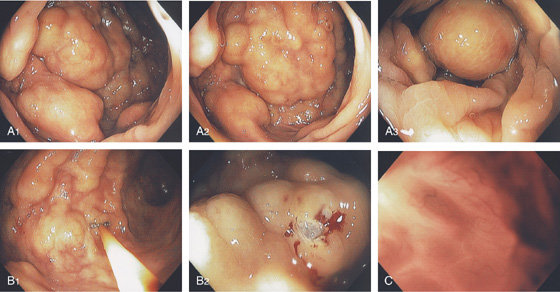
Figure 5.191 COLITIS CYSTIC PROFUNDA
A1-A3, Focal area of submucosal nodularity and cystic-appearing lesions. B1, B2, The lesion is firm, and biopsy results show the underlying mucosa has a cystic component. C, With the endoscope advanced to the cystic area, a honeycomb-type pattern represents the multiple cystic areas.
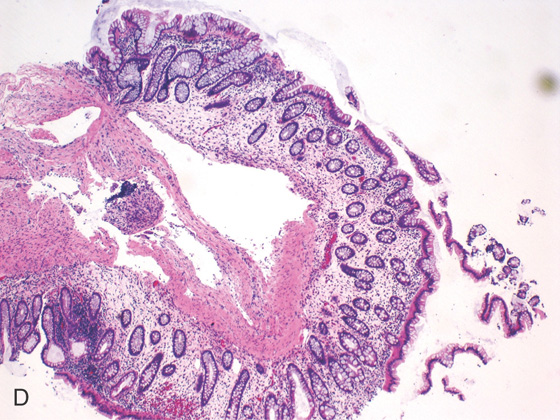
D, Pathology shows the submucosal cystically dilated spaces.
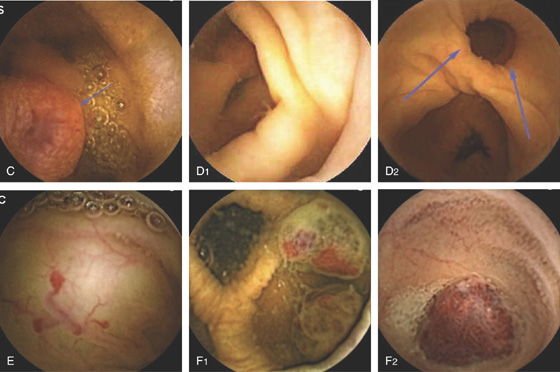
Figure 5.192 PORTAL HYPERTENSIVE COLOPATHY
A, Focal area of erythema in a circular pattern that is composed of blood vessels. B1, B2, Tortuous vascular pattern in the distal colon. C, Solitary vascular ectasia.
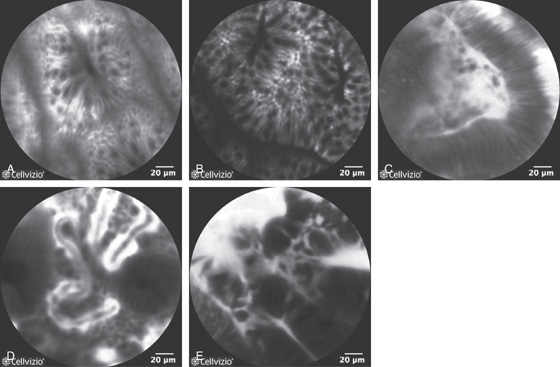
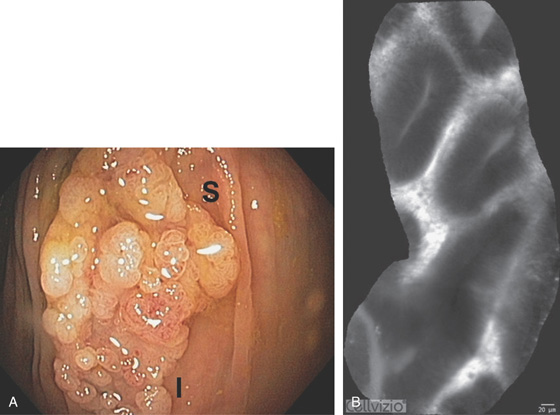
Figure 5.193 CONFOCAL ENDOMICROSCOPY
Endoscopic images of normal and abnormal colon. A, Normal colon. B, Hyperplasia. C, Adenoma. D, Ulcerative colitis. E, Adenocarcinoma. (A courtesy A. Meining, MD, Klinikum Rechts Der Isar, Munich, Germany; B courtesy E. Coron, MD, CHU Nantes, France; C-E courtesy Michael Wallace, MD, Mayo Clinic, Jacksonville, FL.)
Figure 5.194 PILLCAM COLON
A, Eosinophilic esophagitis. B1, Portal hypertensive gastropathy as shown on upper endoscopy and colon capsule (B2).
C, Ampulla of Vater. D1, D2, Colonic diverticula. E, Colonic ectasia. F1, F2, Blue rubber bleb nevus syndrome.
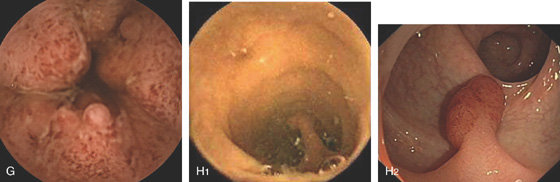
Figure 5.194 PILLCAM COLON
G, Ulcerative colitis.
COLONIC LESIONS ON STANDARD PILLCAM
H1, H2, Pedunculated colonic polyp.
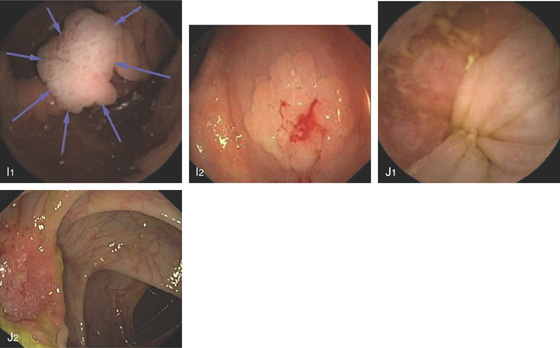
I1, I2, Sessile colonic polyp. J1, J2, Colonic carcinoma. (Courtesy I. Fernandez-Urièn, MD, Pamplona, Spain.)
Figure 5.195 NORMAL ILEUM
A1-A3, Normal-appearing ileum. Note the pronounced villi as viewed underwater, which is accentuated with bile. B1, B2, Normal ileum as shown with high-definition endoscopy (B1) and narrow band imaging (B2). C, Marked nodularity of the distal ileum representing normal lymphoid follicles.
D, Lymphoid follicle found on biopsy of the nodule. (Courtesy J. Pardo-Mindan, MD, Pamplona, Spain.)
Figure 5.196 APPENDICITIS
The appendiceal orifice appears masslike.
Figure 5.197 APPENDICEAL TUMOR
A, Prolapsing lesion emanating from the base of the cecum. B, Large appendix with extension into the base of the cecum caused by a neuroma.
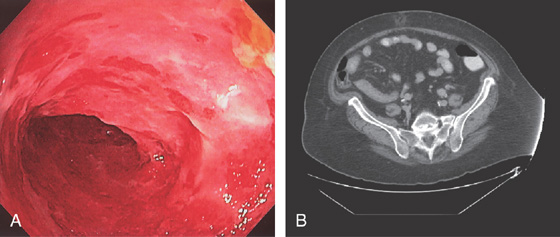
Figure 5.198 ACUTE ILEITIS
A, Diffuse edema and shallow ulceration of the distal ileum. B, Thickening of the distal ileum resulting in a tubular appearance on CT.
Figure 5.199 CROHN’S ILEITIS
Shallow ulcer with surrounding edema of the distal ileum as shown on high-definition endoscopy (A) and narrow band imaging (B).
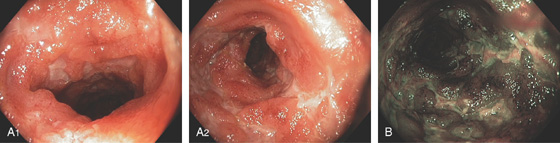
Figure 5.200 ILEAL CROHN’S DISEASE
Numerous ulcerations in the terminal ileum on high magnification (A1, A2) and narrow band imaging (B).
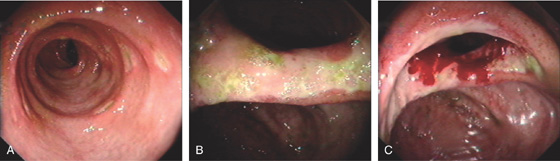
Figure 5.201 CYTOMEGALOVIRUS ILEITIS
A, Multiple well-circumscribed, punched-out ulcers of the ileum. B, Marked involvement of the ileocecal valve results in a stenotic appearance. C, The bleeding represents the appearance after biopsy.
Figure 5.202 WHIPPLE’S DISEASE
The mucosa is thickened with white plaques.
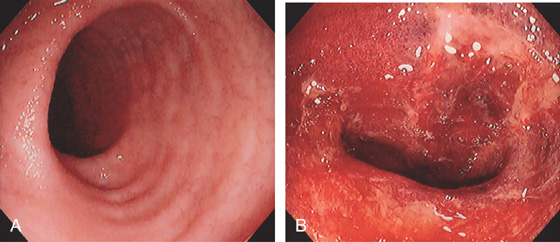
Figure 5.203 ILEAL GRAFT-VERSUS-HOST DISEASE
A, Minimal abnormalities in the terminal ileum. B, Complete loss of the ileal mucosa with hemorrhage and exudate, as seen with severe disease.
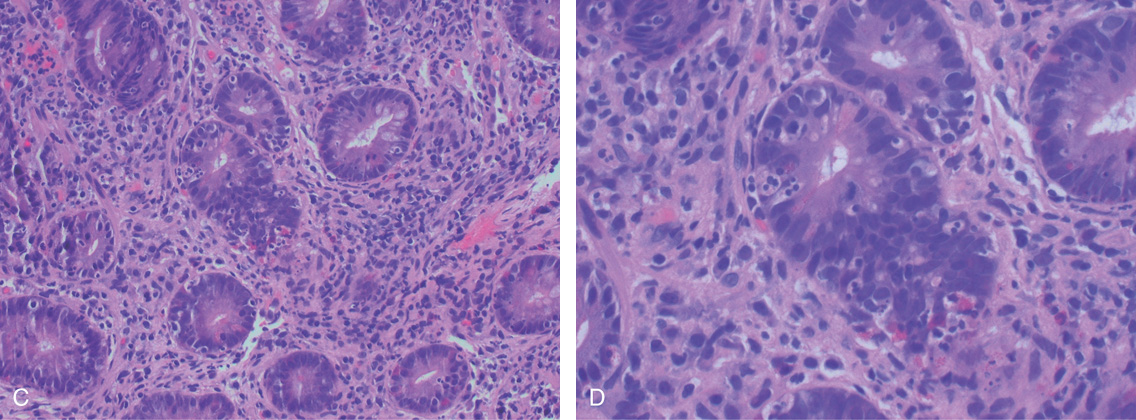
(C) Low- and (D) high-power views show abundant apoptosis involving the crypts.
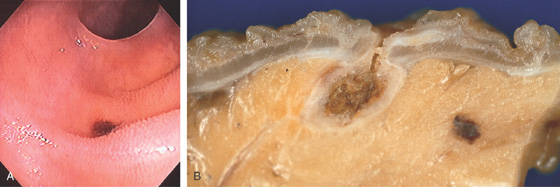
Figure 5.204 ILEAL DIVERTICULAR HEMORRHAGE
A, Small clot covered diverticulum in the distal ileum. B, Diverticulum apparent in the surgical specimen.
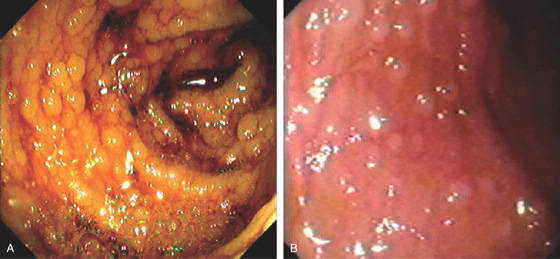
Figure 5.205 GARDNER SYNDROME
A, B, Multiple small polyps throughout the distal ileum.
Figure 5.206 CARCINOID TUMOR
A, Ulcerated mass lesion just inside the ileocecal valve. B, Round mass lesion in the terminal ileum.
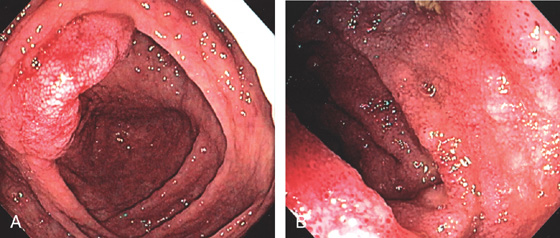
Figure 5.207 LEUKEMIA
A, Subepithelial hemorrhage and edema of the ileocecal valve. B, Edema, granularity, and subepithelial hemorrhage of the terminal ileum.
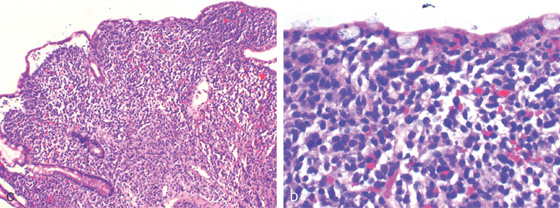
(C) Low- and (D) high-power views show infiltration of the ileal mucosa by leukemic cells. (See also Figures 1.37, 2.74, and 5.145.)
Figure 5.208 METASTATIC MELANOMA
Solitary donut-shaped lesion in the ileum.