Chapter 150 Collaborative Ocular Melanoma Study
Background
The choice of management of choroidal melanoma was controversial at the time the COMS was designed and initiated, and it remains controversial for tumors of small size. No data comparing enucleation or any other treatment with natural history were available; however, most ophthalmologists and oncologists were unwilling to undertake a randomized trial in which observation was one of the treatment arms. Large choroidal melanoma in the absence of metastasis traditionally has been treated with enucleation of the affected eye. Pre-enucleation irradiation of the eye had been proposed with the goal of minimizing the possibility of dissemination of viable tumor cells at time of enucleation.1–8 Other adjunctive treatments also had been proposed, including post-enucleation irradiation of the socket, cryotherapy before enucleation, and chemotherapy.
The length of survival after a diagnosis of choroidal melanoma is quite variable. A meta-analysis of data published from 1966 through 1988 regarding survival following enucleation, yielded 5-year survival rates of 50% for large choroidal melanoma, 70% for medium choroidal melanoma, and 85% for small choroidal melanoma.9 However, few studies reported survival or mortality rates according to tumor size. It is accepted that the most important predictor of survival is tumor cell type. However, because of concerns regarding complications and inadequate sampling during fine-needle aspiration biopsy, choroidal melanoma typically is not biopsied. Thus, cell type is not known until the eye is removed or the patient dies. Also, the cell type(s) might change over time.
Design of the collaborative ocular melanoma study (coms)
The COMS was designed as a set of clinical trials of treatment for choroidal melanoma to be conducted by a group of investigators in the USA and Canada. Initially, three separate studies were undertaken, two randomized clinical trials and one observational study.10 Initial funding was provided in 1985 by the National Eye Institute, National Institutes of Health, US Department of Health and Human Services; beginning in 1991, both the National Eye Institute and the National Cancer Institute provided funding to conduct the study.
Randomized trials of radiotherapy
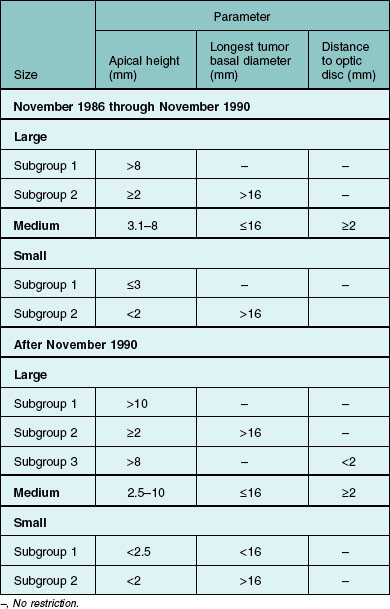
For COMS purposes, choroidal melanoma was categorized broadly by size. Size criteria are summarized in Table 150.1; other eligibility criteria are provided elsewhere11 and have been published.12,13
The COMS randomized clinical trial of primary interest was designed to compare enucleation alone with I-125 brachytherapy for treatment of “medium” choroidal melanoma, which was believed to comprise the majority of newly diagnosed cases. Brachytherapy was chosen as the most feasible method of radiation delivery to the melanoma with respect to standardization of dosimetry and ability to monitor adherence to the radiotherapy protocol. I-125 was selected as the isotope because of the ability to protect the surgeon and other tissues in the orbit from radiation damage by using a gold shield and because of the half-life of the isotope.14 Patients were assigned randomly with equal probability between enucleation and I-125 brachytherapy. All patients were to be followed for a minimum of 5 years or until death. The minimum sample size targeted a priori was 1250 patients for comparison of overall survival between treatment arms based on conventional Type I and Type II errors of 0.05 and 0.20, respectively. A desired sample size of 2400 patients also was established a priori to provide more precise estimates of survival overall and within patient subgroups and for evaluation of secondary outcomes.10,11 The desired sample size was based on Type I and Type II errors of 0.01 and 0.10, respectively.
The clinical trial for “large” (high-risk of metastasis and death) choroidal melanoma was designed to compare enucleation alone with pre-enucleation radiation treatment (PERT). Pre-enucleation radiation was chosen for comparison with enucleation alone because a similar approach had been shown to be effective in other types and sites of cancer in which surgery was employed. In addition, external radiation was widely available throughout the USA and Canada. Patients were assigned randomly, with equal probability, between the two treatment arms and were to be followed for a minimum of 5 years or until death. The sample size was estimated on the basis of overall survival. Taking account of possible losses to follow-up, treatment crossovers, and treatment refusals, a target sample size of 1000 patients was established a priori based on Type I and Type II errors of 0.01 and 0.10, respectively.10–12
A parallel study of quality of life of patients enrolled in the randomized trial of I-125 brachytherapy for medium choroidal melanoma was initiated in 1994.15 The purpose of the parallel study was to compare treatment arms over time with respect to general health, visual function, and anxiety and depression using scores from several standard interview instruments. The study had two components: (1) a prospective randomized component consisting of 209 patients who enrolled in the trial of I-125 brachytherapy and were interviewed prior to random treatment assignment, at 6 months following treatment, and on annual anniversaries of enrollment for up to 8 years; and (2) a cross-sectional component consisting of 645 additional patients who had enrolled in the randomized trial before the quality of life study was initiated and who were interviewed at least once during scheduled follow-up. These 854 patients represent 90% of patients who were eligible for the quality of life study and 65% of all patients who enrolled in the trial of I-125 brachytherapy.
Methods
The COMS design and many of the methods have been published; the COMS Manual of Procedures11 is available. Patients were evaluated for eligibility, enrolled, and treated at 43 different clinical centers, 41 in the USA and two in Canada. A standard schedule of clinical examinations was followed for data collection purposes. An unusual feature of the COMS design was that the participating ophthalmologists reported de-identified basic demographic information (age, gender, race, or ethnicity) and tumor dimensions for all cases of choroidal melanoma examined during the period of patient accrual, regardless of tumor size, eligibility for the COMS, or willingness of eligible patients to enroll in the COMS.
Chronology of the COMS
Accrual of patients to the randomized trial of pre-enucleation radiation (PERT) of large choroidal melanoma began in November 1986 and ended in December 1994, with 1003 patients enrolled. Scheduled clinical follow-up of all surviving patients for vital status, incidence of metastasis and second cancers, and complications continued until July 31, 2000. Interim mortality findings and related information that emphasized 5-year outcomes were published in 199812,16,17; mortality findings through 10 years and prognostic factors were published in 2004.18
Accrual of patients to the randomized trial of I-125 brachytherapy for medium choroidal melanoma began in January 1987 and ended in July 1998, with 1317 patients enrolled, at the recommendation of the Data and Safety Monitoring Committee. Scheduled clinical follow-up of all surviving patients, for clinical and vital status and for quality of life, continued until July 31, 2003, and October 31, 2003, respectively. Interim mortality findings were published in 200113; mortality findings through 12 years after enrollment and subgroup findings were published in 2006.19 Information about complications20–22 and related information23 also have been published.
Patient accrual to the nonrandomized study for small choroidal melanoma began in 1987 and ended in 1989, with 204 patients enrolled. Annual follow-up examinations were halted in 1991 as a result of funding constraints. Vital status and treatment status were reassessed in 1993 through 1994, and again in 1995 through 1996, for all patients who had not been lost to follow-up. Findings from this study have been published.24,25
The COMS database, copies of all publications from the COMS Group, and the “COMS Manual of Procedures” were deposited in the Alan Mason Chesney Medical Archives at the Johns Hopkins University in August 2008. An anonymized public use dataset, containing baseline characteristics and survival outcomes, the COMS Manual, and copies of all COMS publications are available by application to the Medical Archives (http://www.medicalarchives.jhmi.edu). Access to more extensive data, including images of original data forms received at the COMS Coordinating Center, is available to qualified researchers whose application to the COMS Archives Committee (via: schacha@ccf.org or bhawkins@jhmi.edu) is approved by the COMS Archives Committee and by the Medical Archives institutional review board.
Findings from the COMS trial of I-125 brachytherapy for medium choroidal melanoma
Participants
By July 1998, a total of 8712 patients with choroidal melanoma had been reported by COMS investigators; 5046 were classified to be of medium size by COMS criteria (Table 150.1). Among 2882 patients eligible for the randomized trial of I-125 brachytherapy versus standard enucleation, 1317 patients gave signed consent, enrolled, and were assigned randomly to treatment arm: 660 to standard enucleation and 657 to I-125 brachytherapy. Treatment arms were well balanced; adherence to the COMS protocol was excellent.13 All but 21 patients, seven in the brachytherapy arm and 14 in the enucleation arm, were treated promptly as assigned. Three patients assigned to brachytherapy crossed over to enucleation; seven enucleation patients crossed over to brachytherapy and two had proton beam radiation as the initial treatment. At the end of clinical follow-up in 2003, the vital status 5 years after enrollment was known for 1313 patients (99.7%), i.e., for all but four patients in the enucleation arm. Of 799 patients eligible for 10 years of follow-up, the vital status at 10 years was known for 791 (99.0%), i.e., for all but one patient in the brachytherapy arm and seven patients in the enucleation arm.
Survival estimates
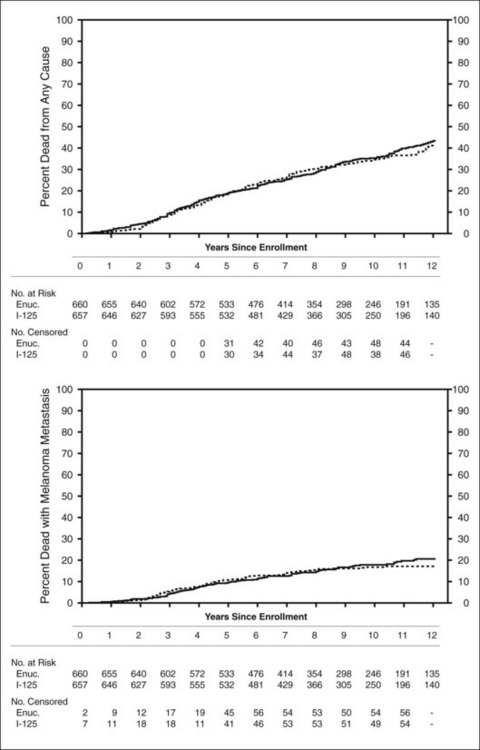
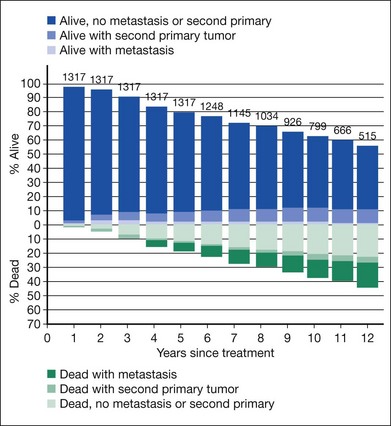
Clinical follow-up of patients in the trial of I-125 brachytherapy ended on July 31, 2003, after all patients had been followed for at least 5 years up to a maximum of 15 years. Five-year survival rates were similar to those published in 2001, i.e., 81% in each treatment arm, yielding a pooled 95% CI of 79–83%. Ten-year survival rates also were the same in the two arms: 65% (95% CI: 62–68%). Mortality rates by treatment arm for all causes and with melanoma metastasis are shown in Figure 150.1. Older age and maximum basal tumor diameter were the primary predictors of earlier death.19 Pooled data were used to summarize mortality, diagnosis of melanoma metastasis,26 and second primary cancers27 through 12 years19 (Fig. 150.2).
Complications
As reported in 2002,20 69 of the 650 patients whose eyes were treated with brachytherapy had the eye enucleated during the first 5 years after initial treatment, yielding a 5-year rate of 12% (95% CI: 10–16%); local treatment failure was reported for 57 eyes in the same time period, with a 5-year cumulative rate of 10% (95% CI: 8–13%). Local treatment failure accounted for 39 of the 69 enucleations. The 3-year cumulative rate of loss of six or more lines of visual acuity from baseline was 49% (95% CI: 44–53%) and the 3-year cumulative rate of loss of visual acuity to 20/200 or worse was 43% (95% CI: 38–48%).21 Five-year estimates of cataract among the 532 eyes treated with I-125 brachytherapy that were phakic at baseline and had no history of cataract in the eye, were 83% (95% CI: 79–87%).22
Quality of life
In the parallel study of quality of life, 206 of 209 patients who enrolled and were treated as assigned (103 each to enucleation or to I-125 brachytherapy) provided 5-year findings regarding quality of life.28 Patients treated with brachytherapy reported significantly better visual function than those treated with enucleation with respect to driving and peripheral vision for up to 2 years following treatment. Differences between treatment arms diminished thereafter, paralleling declining visual acuity in brachytherapy-treated eyes. Patients treated with brachytherapy reported more symptoms of anxiety through 5 years than those treated with enucleation.28
Findings from the COMS trial of pre-enucleation radiation for large choroidal melanoma
Participants
From November 1986 through December 1994, COMS investigators reported 6078 patients with choroidal melanoma. Of these, 1860 had tumors classified as large by COMS criteria (see Table 150.1 for definitions used). Of those classified as large, 1302 were judged eligible for enrollment; 1003 gave signed consent and were enrolled. The two treatment arms were well-balanced with respect to most of the many characteristics of patients, eyes, and tumors considered.12 Adherence to the COMS protocol was excellent, as was diagnostic accuracy.12,29 Only nine patients; three assigned to standard enucleation and six assigned to pre-enucleation radiation, were not treated as assigned at time of enrollment. When clinical follow-up ended in 2000, vital status 5 years after enrollment was known for 998 patients (99.5%), i.e., all but three patients in the enucleation alone arm and two patients in the pre-enucleation radiation arm.
Survival estimates
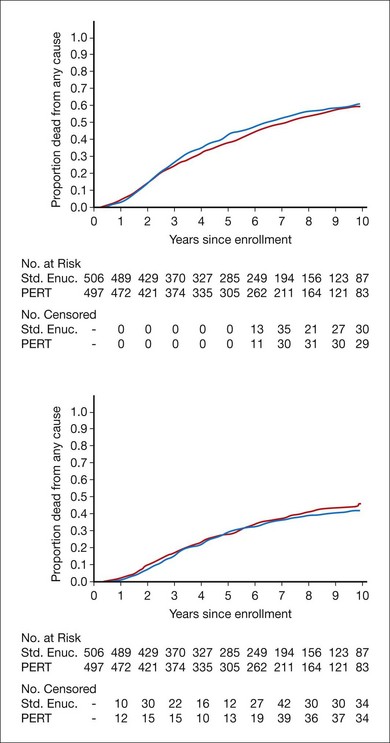
By July 31, 1997, the vital status at 3 years after enrollment was available for all but 26 patients who had enrolled during the last few months of patient accrual. The 5-year vital status was known for 801 (80%) of all patients enrolled; 238 patients assigned to enucleation alone (47%) and 219 patients assigned to pre-enucleation radiation (44%) were known to have died. The estimated 5-year cumulative survival rates and 95% confidence intervals were 57% (95% CI: 52–62%) for patients assigned to enucleation alone and 62% (95% CI: 57–66%) for patients assigned to pre-enucleation radiation. Neither 5-year survival rates nor survival rates over the first 8 years after enrollment differed between treatment arms, to either a statistically or clinically significant degree.12
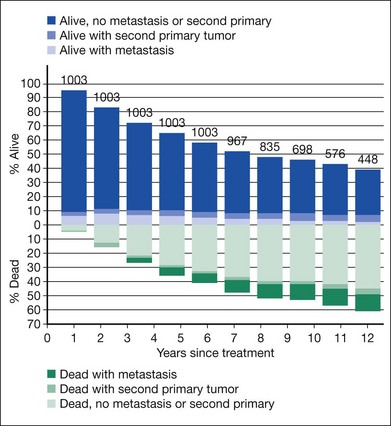
Mortality by treatment arm and by time since enrollment when clinical follow-up ended in July 2000 is summarized in Figure 150.3. Five-year survival rates were identical to those reported earlier; 10-year survival was similar in the two arms and yielded a pooled estimate of 39% (95% CI: 35–42%).18 Among baseline characteristics of the patients, eyes, and tumors evaluated for their potential as prognostic factors, only patient age at time of treatment and longest tumor basal diameter had statistically significant effects on the length of overall survival.18
Among patients assigned to enucleation alone, 130 (26%) died within 5 years of enrollment with histologically confirmed metastatic melanoma, compared with 139 (28%) patients assigned to pre-enucleation radiation, based on review of 435 of the 457 deaths by the Mortality Coding Committee.30,31 Time to death with histologically confirmed metastatic melanoma is displayed in Figure 150.3. The liver was the most common site of melanoma metastasis.31 Findings pooled by treatment arm for mortality, diagnosis of melanoma metastases, and second primary cancers are summarized through 10 years in Figure 150.4.
Complications
Only 17 patients treated with enucleation alone and 19 patients treated with pre-enucleation radiation had any surgical or anesthetic complication reported at the time of initial treatment.17 Orbital tumor recurrence was reported during the first 5 years of follow-up for six patients treated with enucleation alone and for one patient treated with pre-enucleation radiation; patients treated with enucleation alone had nearly twice the 5-year incidence of severe ptosis as reported for patients treated with pre-enucleation radiation.17
Findings from the coms nonrandomized prospective study of small choroidal melanoma
Of 300 patients with small choroidal melanoma (by COMS criteria) reported from December 1986 through August 1989, 220 were judged eligible for this COMS observational substudy; 204 gave signed consent and enrolled. The majority of the patients enrolled within 1 year of the initial diagnosis of choroidal melanoma.24 No attempt was made to establish uniform criteria for treatment timing or to recommend the type of treatment; the patients and their ophthalmologists made these decisions.
A total of 16 patients were treated shortly after enrollment; 20 additional patients were treated after the melanoma grew to medium or large size (COMS criteria) and the patients were enrolled in one of the COMS randomized trials. As of June 1996, an additional 47 patients had been treated during follow-up.24,25
By June 30, 1996, 27 patients had died. Survival findings are summarized in Figure 150.5. The estimated 5-year all-cause mortality rate was 6% (95% CI: 3–9%).25 Of 188 patients who were not treated at time of enrollment, 44 had tumors that had grown to medium or large size by February 1997, based on COMS criteria. The estimated 5-year proportion of initially small tumors that grew was 31% (95% CI: 23–39%).24
Histopathologic findings from enucleated eyes
The COMS Group has one of the highest rates of diagnostic accuracy ever documented.29,32 Of 1532 eyes enucleated in the COMS by June 1996, 994 from patients enrolled in the randomized trial for large choroidal melanoma and 536 from patients enrolled in the trial for medium choroidal melanoma, 1527 (99.7%) were confirmed histopathologically by the Pathology Review Committee to harbor choroidal melanoma.29,32 A detailed description of the characteristics of the 1527 confirmed cases has been published.32 Key findings include documentation of local tumor invasion: rupture of Bruch’s membrane in 88% of eyes; invasion of emissary canals in 55%; retinal invasion in 49%; tumor cells in the vitreous in 25%; invasion of tumor vessels in 14%; and vortex vein invasion in 22% of eyes with vortex veins. Scleral invasion was present in 56% of eyes and extrascleral extension in 8%. Histopathologic review confirmed that pre-enucleation radiation significantly reduced mitotic activity.12
In other published histopathologic investigations of enucleated eyes of COMS patients, silver-stained nucleolar organizer region scores have been evaluated as predictors of later metastasis, transillumination and histologic measurements of tumor dimensions have been compared,33 and a clear cell variant of choroidal melanoma has been identified.34
Other published findings
The multicenter organization of the COMS facilitated referral to a COMS clinical center of cases of choroidal melanoma from most of the USA and from a large part of eastern Canada. The large number of patients screened for the COMS and judged to have choroidal melanoma (totaling 8712 as of July 31, 1998, when accrual halted) provides the largest group of patients with this diagnosis for whom data have been collected systematically at time of presentation in accord with a common protocol. Trends in size of choroidal melanoma and treatments over time have been published.35 The COMS Group has reported the first published cases of choroidal melanoma in Native Americans.36 This large database permits comparison of tumor characteristics at time of screening and diagnosis among different racial subgroups. This information may provide clues for future investigation in epidemiologic and genetic studies of choroidal melanoma.
The COMS Group also has published information on surgical and postsurgical complications among the largest group of patients whose eyes have been enucleated because of choroidal melanoma and who have been examined in accord with a common follow-up protocol,17 documenting the low rates of serious complications following enucleation for this condition. The importance of liver function tests and other tests for metastasis has been evaluated; those findings have been published in the oncology literature.37 Also, 10-year changes in fellow eyes of patients enrolled in COMS randomized trials have been reported.38 Echographic characteristics of melanoma at baseline39 and post-brachytherapy changes observed on fluorescein angiograms and stereoscopic photographs40 also have been published. In addition to publications from the COMS Group that have important clinical information, several articles have been published that deal with research methodology.41–46
Conclusion
The COMS Group successfully enrolled sufficient numbers of eligible patients with choroidal melanoma so that valid comparisons between treatments could be made for mortality and other important clinical outcomes and, in the case of the trial for medium tumors, also on important patient-reported (“quality of life”) outcomes. Protocol adherence and data quality were exceptionally good in both randomized trials. Findings regarding primary and secondary outcomes have been published. The information in Figures 150.2 and 150.4 is useful for patient counseling.
1 Augsburger JJ, Eagle RC, Chiu M, et al. The effect of preenucleation radiotherapy on mitotic activity of choroidal and ciliary body melanomas. Ophthalmology. 1987;94:1627–1630.
2 Benediktsdóttir K, Edenholm M, Lindholm Å, et al. Preoperative irradiation of malignant melanoma: a multifactorial statistical analysis of survival. Acta Radiol Oncol. 1984;23:315–320.
3 Bornfeld N, Alberti W, Foerster MH, et al. External beam therapy of choroidal melanomata: preliminary report. Trans Ophthalmol Soc UK. 1983;103:68–71.
4 Burch FE, Camp WF. Results of irradiation of malignant melanomas of the uveal tract. Trans Am Acad Ophthalmol Otolaryngol. 1943;47:335–353.
5 Char DH, Phillips TL. The potential for adjuvant radiotherapy in choroidal melanoma. Arch Ophthalmol. 1982;100:247–248.
6 Char DH, Phillips TL. Preenucleation irradiation of uveal melanoma. Br J Ophthalmol. 1985;69:177–179.
7 Lommatzsch P, Dietrich B. The effect of orbital irradiation on the survival rate in patients with choroidal melanoma. Ophthalmologica. 1976;173:49–52.
8 Sanborn GE, Ngyuen P, Gamel J, et al. Reduction of enucleation-induced metastasis in intraocular melanoma by periorbital irradiation. Arch Ophthalmol. 1987;105:1260–1264.
9 Diener-West M, Hawkins BS, Markowitz JA, et al. A review of mortality from choroidal melanoma. II. A meta-analysis of mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol. 1992;110:245–250.
10 Collaborative Ocular Melanoma Study Group. Design and methods of a clinical trial for a rare condition: the Collaborative Ocular Melanoma Study. COMS report No. 3. Control Clin Trials. 1993;14:362–391.
11 Collaborative Ocular Melanoma Study Group. COMS Manual of Procedures. NTIS accession No. PB95–179693. Springfield: National Technical Information Service; 1995.
12 Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma. II. Initial mortality findings. COMS report No. 10. Am J Ophthalmol. 1998;125:779–796. 126:622
13 Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. III. Initial mortality findings. COMS report No. 18. Arch Ophthalmol. 2001;119:969–982.
14 Earle J, Kline RW, Robertson DM. Selection of iodine 125 for the Collaborative Ocular Melanoma Study. Arch Ophthalmol. 1987;105:763–764.
15 Moy CS, Melia BM. Quality of life assessment in the Collaborative Ocular Melanoma Study: design and methods. COMS–QOLS report no. 1. for the COMS Quality of Life Study Group. Ophthalmic Epidemiol. 1999;6:5–17.
16 Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) trial of pre-enucleation radiation of large choroidal melanoma. I. Characteristics of patients enrolled and not enrolled. COMS report No. 9. Am J Ophthalmol. 1998;125:767–778.
17 Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma. III. Local complications with and without pre-enucleation radiation. COMS report No. 11. Am J Ophthalmol. 1998;126:362–372.
18 Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report No. 24. Am J Ophthalmol. 2004;138:936–951.
19 Collaborative Ocular Melanoma Study (COMS) Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. V. Twelve-year mortality rates and prognostic factors. COMS report No. 28. Arch Ophthalmol. 2006;124:1684–1693.
20 Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report No. 19. Ophthalmology. 2002;109:2197–2206.
21 Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years. COMS report No. 16. Ophthalmology. 2001;108:348–366.
22 Collaborative Ocular Melanoma Study Group. Incidence of cataract and outcomes after cataract surgery in the first 5 years after iodine 125 brachytherapy in the Collaborative Ocular Melanoma Study. Ophthalmology. 2007;114:1363–1371.
23 Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. II. Characteristics of patients enrolled and not enrolled. COMS report No. 17. Arch Ophthalmol. 2001;119:951–965.
24 Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma. COMS report No. 5. Arch Ophthalmol. 1997;115:1537–1544.
25 Collaborative Ocular Melanoma Study Group. Mortality in patients with small choroidal melanoma. COMS report No. 4. Arch Ophthalmol. 1997;115:886–893.
26 Collaborative Ocular Melanoma Study Group. Development of metastatic disease after enrollment in the COMS trials of treatment of choroidal melanoma. Collaborative Ocular Melanoma Study Group report No. 26. Arch Ophthalmol. 2005;123:1639–1643.
27 Collaborative Ocular Melanoma Study Group. Second primary cancers after enrollment in the COMS trials for treatment of choroidal melanoma. COMS report No. 25. Arch Ophthalmol. 2005;123:601–604.
28 Collaborative Ocular Melanoma Study – Quality of Life Study Group. Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma. COMS QOLS report No. 3. Arch Ophthalmol. 2006;124:226–238.
29 Collaborative Ocular Melanoma Study Group. Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS report No. 1. Arch Ophthalmol. 1990;108:1268–1273.
30 Collaborative Ocular Melanoma Study Group. Cause-specific mortality coding: methods in the Collaborative Ocular Melanoma Study. COMS report No. 14. Controlled. Clin Trials. 2001;22:248–262.
31 Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study. COMS report No. 15. Arch Ophthalmol. 2001;119:670–676.
32 Collaborative Ocular Melanoma Study Group. Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol. 1998;125:745–766.
33 Collaborative Ocular Melanoma Study Group. Comparison of clinical, echographic, and histologic measurements from eyes with medium-sized choroidal melanoma in the Collaborative Ocular Melanoma Study. COMS report No. 21. Arch Ophthalmol. 2003;121:1163–1171.
34 Grossniklaus HE, Albert DM, Green WR, et al. for the Collaborative Ocular Melanoma Study Group. Clear cell differentiation in choroidal melanoma. COMS report No. 8. Arch Ophthalmol. 1997;115:894–898.
35 Collaborative Ocular Melanoma Study Group. Trends in size and treatment of recently diagnosed choroidal melanoma, 1987–1997. Findings from patients evaluated at Collaborative Ocular Melanoma Study centers. COMS report No. 20. Arch Ophthalmol. 2003;121:1156–1162.
36 Wells CG, Bradford RH, Fish GE, et al. for the COMS Group. Choroidal melanoma in American Indians. Arch Ophthalmol. 1996;114:1017–1018.
37 Diener-West M, Reynolds SM, Agugliaro DJ, et al. Screening for metastasis from choroidal melanoma: Experience of the Collaborative Ocular Melanoma Study. Collaborative Ocular Melanoma Study report No. 23. Am J Clin Oncol. 2004;22:2438–2444.
38 Collaborative Ocular Melanoma Study Group. Ten-year follow-up of fellow eyes of patients enrolled in the Collaborative Ocular Melanoma Study (COMS) randomized trials. COMS report No. 22. Ophthalmology. 2004;111:966–976.
39 Collaborative Ocular Melanoma Study Group. Baseline echographic characteristics of tumors in eyes of patients enrolled in the Collaborative Ocular Melanoma Study. COMS report No. 29. Ophthalmology. 2008;115:1390–1397.
40 Boldt HC, Melia BM, Liu JC, et al. for the Collaborative Ocular Melanoma Study Group. I-125 brachytherapy for choroidal melanoma. Photographic and angiographic abnormalities: The Collaborative Ocular Melanoma Study: COMS report No. 30. Ophthalmology. 2009;116:106–115.
41 Collaborative Ocular Melanoma Study Group. Sociodemographic and clinical predictors of participation in two randomized trials: Findings from the Collaborative Ocular Melanoma Study Group. COMS report No. 7. Controlled Clin Trials. 2001;22:526–537.
42 Collaborative Ocular Melanoma Study Group. Echography (ultrasound) procedures for the Collaborative Ocular Melanoma Study. COMS report No. 12. J Nurs Technol. 1999;18(4):143–149. (pt 1); 18(5):219–232 (pt 2)
43 Collaborative Ocular Melanoma Study Group. Consistency of observations from echograms made centrally in the Collaborative Ocular Melanoma Study. COMS report No. 13. Ophthalmic Epidemiol. 2002;9:11–27.
44 Diener-West M, Connor PB, Newhouse MM, et al. Feasibility of keying data from screen-displayed facsimile images in an ongoing trial: The Collaborative Ocular Melanoma Study. Controlled Clin Trials. 1998;19:39–49.
45 Goldsborough IL, Church RY, Newhouse MM, et al. How clinic coordinators spend their time in a multicenter clinical trial. Applied Clin Trials. 1998;7:33–40.
46 Mobley RY, Moy CS, Reynolds SM, et al. Time trends in personnel certification and turnover in the Collaborative Ocular Melanoma Study. Clinical Trials. 2004;1:377–386.