CHAPTER 36 Coincident cataract and glaucoma surgery
Introduction
The management of coincident cataract and glaucoma presents common and challenging decisions for the ophthalmic surgeon. Emerging data and technological advances continue to shape the approach to coexisting cataract and glaucoma1–3. Historically this generally involved sequential surgery with initial glaucoma surgery followed by cataract extraction months later when the intraocular pressure (IOP) was controlled and the bleb had matured. The rationale for the sequential approach was based on the poor success rate of combined extracapsular cataract extraction (10 mm scleral incision) and glaucoma filtration surgery. While the advantage of the sequential approach is the high success rate of primary filtration surgery, the disadvantages are that the patient undergoes at least two operations, prolonged poor vision, and the risk of bleb failure after the cataract operation. As techniques improved with clear cornea small incision phacoemulsification and guarded filtration surgery with anti-metabolites, glaucoma surgeons began to reconsider combined rather than sequential surgery4,5. As new procedures such as canaloplasty, ab interno trabeculectomy (Trabectome), endoscopic cyclophotocoagulation, and trabecular meshwork stents evolve, there may be a decreased need to perform traditional filtering procedures including glaucoma drainage devices. Modern techniques of clear cornea small incision cataract surgery alone lower IOP and may be the preferred initial step to manage most cases of coexisting glaucoma and cataract6–8. However, combined surgery remains an important option for select cases with advanced glaucoma or markedly elevated IOP. We describe techniques for the surgical treatment of coexisting cataracts and glaucoma.
Indications
Traditional indications for combined cataract and glaucoma surgery are uncontrolled glaucoma that requires surgery in a patient with visually significant cataract, or the need for cataract extraction in a patient with advanced or poorly controlled glaucoma. The mere coexistence of glaucoma and cataract is not in itself an indication for combined cataract and glaucoma surgery9. Mild to moderate glaucoma that is controlled well on medication is usually best managed by cataract extraction alone. In these instances, it is best to perform the cataract extraction with a conjunctival sparing approach such as a clear corneal incision. If the glaucoma later becomes uncontrolled, a glaucoma procedure can be performed readily. Small incision cataract extraction techniques have made the ‘cataract extraction first, glaucoma surgery later if necessary’ approach more feasible. (Table 36.1)7.
Table 36.1 Options for surgical management of coincident cataract and glaucoma*
| Trabeculectomy first; cataract extraction later | Combined cataract extraction–trabeculectomy/tube shunt† | Cataract extraction first; glaucoma surgery later if needed‡ |
|---|---|---|
| Consider if employing large incision 10–11 mm extracapsular cataract extraction | Mild to moderate glaucoma, well-controlled intraocular pressure with additional medical options available | |
| Far advanced glaucoma | Untreated or medically controlled ocular hypertension with additional medical options available | |
| Patients on MTMT or patients intolerant to medications Patients poorly compliant with medications Low target intraocular pressure |
MTMT = maximally tolerated medical therapy.
* Assumes visually significant cataract.
Anesthesia
The theory behind the use of external compression is that the pressure prevents bleeding into the retrobulbar space, facilitates diffusion of the anesthetic solution into the orbital tissues, and softens the eye, making the surgery safer9. As the risk of applying external compression to eyes with badly damaged optic nerves is not clear, it is advisable to limit the magnitude and duration of ocular compression to 20–30 mmHg and 5 to 10 minutes in such patients.
Preoperative considerations
Preoperative clinical assessment is vital. Gonioscopy should be performed. The conjunctiva should be examined carefully. Topical steroids should be administered if the conjunctiva is inflamed10. The surgeon should carefully examine the eye for the presence of exfoliative material, which can be associated with a more aggressive postoperative inflammatory response, weakened zonular fibers, and poor pharmacologic dilation11,12. It is important to note the pupillary response to pharmacologic dilatation. If dilatation is poor and pupil manipulation is anticipated, the patient should be informed, as such manipulation may result in anisocoria or pupillary distortion. This precaution is particularly important for younger patients with light irides in whom the cosmetic effect of anisocoria or irregular pupil will be more apparent. Depending on the surgical procedure, patients should be educated preoperatively about the specific features of the postoperative period with combined cataract and glaucoma surgery, which may require frequent doctor visits and prolonged visual recovery, beyond that expected for standard cataract surgery in non-glaucomatous eyes.
Surgical technique
Small incision cataract extraction techniques have vastly improved treatment of cataracts in patients with glaucoma. Standard cataract surgery is described in great detail in Chapter 8. Rarely, other techniques, such as extracapsular or intracapsular extraction may be necessary. Surgeons should either be competent in these other techniques or refer specific cases9. When possible, a clear cornea incision is preferable to preserve the conjunctiva and the trabecular meshwork for any future glaucoma procedures. Current techniques for combined glaucoma surgery using small incision phacoemulsification will be described here.
Cataract surgery alone as a glaucoma procedure
Studies following cataract extraction in the 1970s and 1980s demonstrated minimal reduction of IOP13–15. However, advances in surgical technique and intraocular lens technology means that these results probably don’t apply today. As extracapsular surgery became the standard, studies began to demonstrate an average lowering of IOP by 2–4 mmHg16–18. Studies that stratify patients based on preoperative IOP clearly demonstrated that patients with higher preoperative IOP enjoy the greatest reduction of IOP after cataract surgery and that IOP can be controlled in 20% of patients with cataract surgery alone1,19–21. Long-term studies have shown a drop in IOP of about 3 mmHg with 75–85% of patients maintaining an IOP reduction up to 10 years7,8,22–25.
The method of cataract extraction may influence the reduction in IOP. Phacoemulsification (particularly clear cornea phacoemulsification) seems to lower IOP more than manual extracapsular cataract extraction16,17,26,27. PXF patients may have an even greater long-term decrease in IOP than POAG patients, even though IOP often rises in the immediate postoperative period28–30. Factors that have been identified to be important in the perioperative control of IOP include pressurization of the eye at the time of surgery, immediate postoperative medications, and viscoelastic type31–34. The surgeon should remove all viscoelastic carefully and ensure that the eye is not over-pressurized at the time of surgery. Despite limitations, cataract surgery seems to be emerging as a safe way to lower IOP in patients with mild to moderate glaucoma while avoiding the morbidity of traditional glaucoma surgery.
Results of combined cataract extraction and trabeculectomy
Overall, combined phacotrabeculectomy and trabeculectomy alone have similar outcomes. A couple of studies have compared phacotrabeculectomy with trabeculectomy alone. In one retrospective study 85 eyes that underwent trabeculectomy were compared with 105 eyes that had had phacotrabeculectomy. The mean postoperative IOP was similar in both groups at all time points up to 2 years. However, the mean IOP decrease from baseline was higher in the trabeculectomy alone group35. Another study showed a similar success rate of 70% in patients having either combined phacotrabeculectomy or trabeculectomy alone36. In a retrospective study of 60 eyes that underwent phacotrabeculectomy, 95% achieved an IOP of 21 mmHg or less with or without medication, 50% of eyes had an IOP less than 15 mmHg, and 57% of eyes had a 30% reduction in IOP. Best corrected vision of 20/40 or better was maintained in 87% of eyes37.
A prospective study of phacotabeculectomy looked at 304 consecutive eyes over 1 year showing a mean IOP of 15.5 mmHg with nine eyes requiring a reoperation38. Two studies comparing one-site and two-site phacotrabeculectomy showed similarly safety and IOP control over a 3-year follow-up period in patients with POAG and pseudoexfoliative glaucoma (PXFG) with a mean postoperative IOP of 15 mmHg in all groups39,40.
Single-site combined filter and cataract extraction
When performing combined cataract extraction and filtering surgery, a one-site or two-site incision may be used. In a one-site approach the entire procedure can be performed through the conjunctival and scleral incisions used in standard trabeculectomy mimicking a guarded filtration procedure as described in Chapter 3541–43. Either a limbus-based or fornix c based conjunctival flap can be used and the merits of each technique are discussed in Chapter 219.
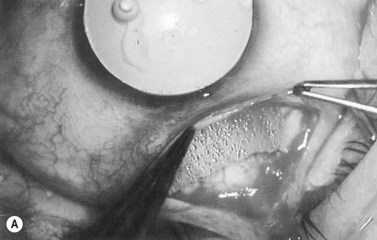
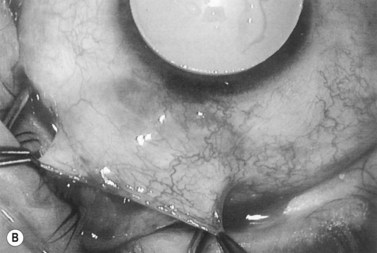
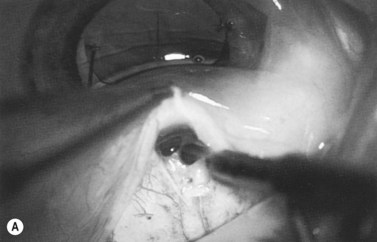
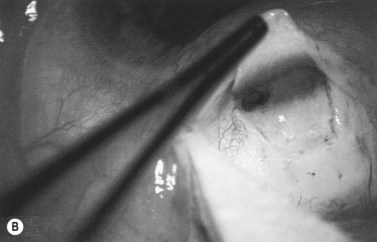
Anti-metabolites are typically used in combined cataract and filtration surgery since the goal of the procedure is a long-term functioning bleb. Mitomycin C (MMC) is applied to the proposed filtration site following dissection of the conjunctival flap (Fig. 36.1A) (see Chapter 25) with saturated pledget tips. The conjunctival incision should be protected from MMC exposure (Fig. 36.1B,C; Fig. 36.2). A complete discussion regarding the use of anti-metabolites in filtration surgery can be found elsewhere, but the role of MMC in combined cataract and glaucoma surgery is less clear5,44–49. Many surgeons believe the potential benefits of MMC in conjunction with meticulous surgical techniques outweigh the risks; others disagree. 5-fluorouracil can also be used safely for phacotrabeculectomy50.
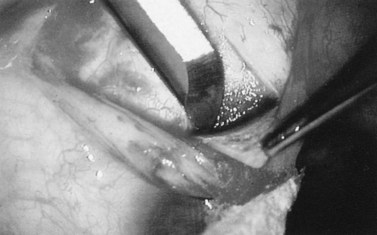
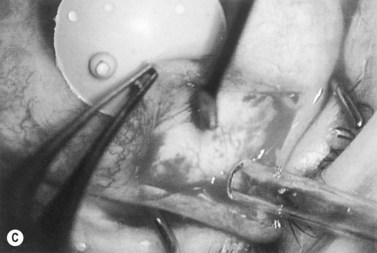
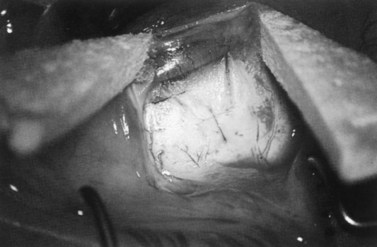
Fig. 36.2 A 3.5 mm scleral flap is dissected. The dissection is carried anteriorly well into clear cornea.
From Samuelson TW. Management of coincident glaucoma and cataract. Curr Opin Ophthalmol 1995;6:14–21. Copyright Rapid Science Publishers.
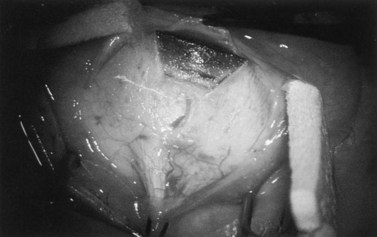
The scleral flap is identical to that used in standard guarded filtration procedure. Alternatively, some surgeons modify the scleral flap in combined glaucoma surgery to mimic the scleral tunnel approach of standard cataract surgery. The size and shape of the scleral flap are quite varied among surgeons yielding equally effective results (Fig. 36.3). A paracentesis tract is then performed using a no. 75 blade to facilitate two-handed phacoemulsification.
Next, the surgeon must excise the trabecular block. An incision has already been made with the keratome into the anterior chamber beneath the scleral flap. A Kelly Descemet’s punch completes the trabecular block excision (Fig. 36.4A). In general, the two most important factors ensuring patency of the filtration fistula are adequate bulk aqueous flow and retardation of the healing process. The trabecular block excision must be performed far enough anteriorly to avoid incising the vascularized structures of the angle, such as the scleral spur or even the ciliary processes.
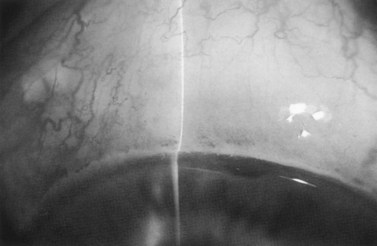
After completion of the sclerostomy, a peripheral iridectomy can be performed although, for most eyes undergoing combined surgery, an iridectomy may be omitted. After the trabecular block excision and iridectomy, the sclerostomy site should be examined carefully to ensure that it is entirely free of debris such as blood, cortex, iris, vitreous, or Descemet’s membrane (Fig. 36.4B). The scleral flap is then closed with interrupted sutures as in a standard trabeculectomy (Fig. 36.5). Filtration is assessed in three ways: First, the anterior chamber should maintain normal depth. A deep chamber alone is inadequate to gauge filtration: many eyes maintain a formed chamber intraoperatively despite profound hypotony. Therefore, the eye should be palpated digitally during re-formation. The eye should become firm, but not hard and then should soften slightly over the first several seconds after irrigation is discontinued. Finally, a surgical spear (sponge) should be placed in all regions around the scleral flap to observe for ‘passive flow’, that is spontaneous aqueous flow without external pressure on the globe. If anti-metabolites have been employed, it is desirable to have very little, if any, passive flow. However, it should be possible to produce flow readily by applying gentle pressure adjacent to the filtration site. This is the ‘active filtration test’. An important observation in filtration surgery is that it is much easier to increase filtration postoperatively than it is to slow it down. Therefore, filtration surgery should be performed by creating a leaky fistula and controlling the filtration in a reversible fashion. This is accomplished with releasable sutures or argon laser suture lysis. The ‘active flow test’ is intended to simulate the effect of eyelid activity on the filtration site. If there is considerable filtration on passive testing, then there is a substantial risk for overfiltration and hypotony. If the filtration proves to be inadequate postoperatively, suture lysis can be performed to augment flow (Fig. 36.6)51,52. Closure of limbus-based or fornix-based flaps is described in Chapter 35.
Two-site combined filter and cataract extraction
Two-site surgery (a separate clear cornea cataract incision and a scleral incision for the filter) has become the preferred approach for combined procedures with the increasing popularity of clear cornea cataract surgery. Such an approach typically provides better exposure for the phacoemulsification portion of the procedure as the surgeon can more easily manage in the setting of a prominent brow, blepharospasm, or deeply set eye. Since the clear corneal cataract incision does not interfere with the filter, either limbus-based or fornix-based surgery can performed per the surgeon’s preference as described in Chapter 35. The clear cornea incision should be made at or near the 0 or 180° meridian to ensure that the filter site is avoided. Since it is easier to complete the scleral flap on a firm eye, one technique is to perform as much of the filter portion of the surgery as possible before creating an intraocular wound. This includes creation of a peritomy, dissection of conjunctiva and Tenon’s over the filter site, cautery of the sclera bed, creation of a partial thickness sclera flap, and application of MMC. It is best to apply the MMC prior to creation of an intraocular incision to prevent intraocular toxicity53. After the steps above are completed a standard clear cornea cataract surgery can be performed. However, perhaps a more popular and practical approach is to complete the entire clear corneal cataract surgery prior to initiating the trabeculectomy portions of the procedure. This latter strategy has some distinct advantages.
Ergonomically, there is less need to change position of the operating microscope between the different stages of the procedure. Further, if there are unexpected events during cataract surgery, the surgeon could elect to delay or forego the trabeculectomy. The surgeon should ensure that the capsulectomy is not larger than 5 mm to prevent IOL dislocation secondary to postoperative IOP fluctuations. Viscoelastic is not removed immediately and a temporary 10-0 nylon suture is used to seal the clear corneal wound to maintain a firm eye for the rest of the procedure. Following completion of the cataract surgery, the trabeculectomy portion of the procedure may ensue or resume. Following the peritomy, scleral flap, and anti-metabolite application, a standard sclerectomy is performed. As an alternative to a standard sclerectomy, an ExPress mini shunt can be placed through a 25 g needle tract54. The scleral flap is secured with 10-0 nylon sutures. The clear cornea suture is loosened and the viscoelastic is aspirated from the eye. Then the cornea suture is retied to ensure an adequate seal. At this point the aqueous flow can be tested as described above and if flow is adequate the conjunctiva is reapproximated. A cholinergic agent such as acetylcholine (Miochol, Ciba-Vision) or carbachol (Miostat, Alcon) is injected intraocularly to protect the IOL and cornea in the event of postoperative hypotony.
Combined cataract extraction and glaucoma drainage devices
Glaucoma drainage devices (GDD) such as the Ahmed, Baerveldt, or Molteno have become increasingly popular in recent years. Data from the tube versus trabeculectomy controlled clinical trial have shown that GDDs have acceptable short-term safety and efficacy profile55. The principles of combined cataract and GDD surgery are similar to combined cataract and trabeculectomy. A two-site approach is preferred utilizing a temporal clear corneal incision for the cataract surgery. Prior to intraocular penetration, as much of the tube surgery should be performed as possible including the peritomy, cautery, posterior dissection of Tenon’s layer, identification of the extraocular muscles, and fixing the plate to the sclera. The basic approach to combined cataract extraction with GDD placement will be described below. Differences between the individual tube shunts and detailed surgical techniques are described in great detail in Chapter 39.
Combined cataract extraction and other glaucoma procedures
Cataract surgery can be performed simultaneously with other glaucoma procedures including canaloplasty (iScience)56, ab-internal trabecular meshwork excision (Trabectome, Neomedix)38, trabecular meshwork stents (iStent, Glaukos), and endoscopic cyclophotocoagulation (EndoOptiks)57. The specifics of these procedures are discussed in Chapters 43 and 45, but a brief description is below.
A standard cataract surgery is performed initially during combined cataract extraction and trabecular meshwork stent placement (Glaukos iStent)58. A cholinergic agent is used to constrict the pupil. With viscoelastic in the eye, a gonioprism is used to visualize the angle and one or more stents can be placed into the trabecular meshwork through the same clear cornea incisions used for the cataract surgery.
Postoperative care
Postoperative care for combined filter and cataract extraction
Postoperative care for combined filter and cataract extraction is delicate and requires diligent follow-up. Topical steroid administration may be continued for 3–4 months postoperatively following filtering procedures. Tapering of the steroid dose is based on the amount of inflammation in and around the bleb, not intraocular inflammation, which generally subsides long before the bleb becomes quiet. Inflammatory intraocular lens deposits, more common with earlier IOL biomaterials, have been shown to peak at approximately 3 months after cataract extraction in high risk eyes59,60. Therefore, the topical steroids are continued for at least 3 months unless the eye and the bleb are unusually quiet. Topical steroids may be tapered more rapidly in hypotonous eyes. Cycloplegia and mydriatic agents are used in the event of hypotony, shallow anterior chamber, and prominent inflammation or fibrin formation. However, these agents are not necessary on a routine basis.
The early postoperative period is critical: during this time procedures often fail and complications are most common. If the IOP is higher than the mid-teens during the first postoperative week after filtering procedures, focal pressure is applied adjacent to the filtration site with a cotton-tip applicator (CTM, Carlo Traverso Maneuver) to augment filtration61. This maneuver separates the flap edges, releasing aqueous from the anterior chamber through the filtration site. In most cases, the CTM pressure will instantaneously inflate the bleb, lowering the IOP. This technique is favored over releasing aqueous through the clear corneal paracentesis tract, because it encourages outflow through the sclerostomy site and can dislodge fibrin or other debris that may be limiting outflow. Not uncommonly, a single CTM procedure will bring the pressure into the favorable range. Finally, if the IOP is not lowered with CTM, gonioscopy should be performed. If the internal sclerostomy is patent, it may be assumed that the resistance to outflow is related to a tight scleral flap or conjunctival fibrosis. Argon laser suture lysis should then be considered. If suture lysis is planned on a given postoperative visit, CTM should not be performed because it is easier to lyse the suture when the eye is firm and the suture is under tension. The surgeon should resist the temptation to perform Argon laser suture lysis too early. It is not uncommon for retained viscoelastic material or perioperative inflammation to cause elevation of IOP during the early postoperative period. The IOP often falls as the viscoelastic material clears the eye. If anti-metabolites have not been used, the schedule for releasing sutures is accelerated (Table 36.2). The schedule of postoperative visits is individualized according to each patient’s progress. Typically, however, patients are seen at least two to four times during the first two postoperative weeks. During each visit the surgeon should record the visual acuity, IOP, bleb appearance, and chamber depth. The bleb appearance should reflect the IOP. For example, if the IOP is very low, one would expect to see an exuberant bleb. Any discrepancy between bleb appearance and IOP warrants search for a bleb leak. The entire bleb and wound should be painted with fluorescein and examined for Seidel positivity, using a fluorescein-impregnated ‘strip’ to paint the surface of the conjunctiva and then inspecting meticulously as the slit lamp into the blue filter in front of the brightest illuminating beam.
Table 36.2 Postoperative titration of intraocular pressure, timing of suture release, and suture lysis*

The importance of performing ‘controlled’ filtration surgery to avoid hypotony cannot be overemphasized. The risk to hypotonous eyes is substantial. Flat chambers, choroid effusions, suprachoroidal hemorrhage, and hypotonous maculopathy are risks associated with over-filtration. The risk of hypotonous maculopathy is greatest in young myopic patients62. Over-filtration in the early postoperative period may be exacerbated by decreased aqueous production by a ciliary body that is recovering from years of chronic aqueous suppression, as well as from perioperative inflammation. Postoperative outflow should be titrated based on the individual response to surgery. With controlled glaucoma filtration surgery the scleral flap is closed more tightly, but in a ‘reversible’ fashion. This approach limits filtration in the early postoperative period, decreasing the likelihood of hypotony. Once adequate aqueous production is established, manifest by a deep chamber and stable or rising intraocular pressure, the surgeon may augment filtration by performing suture lysis or release. The ability to titrate filtration and avoid early perioperative hypotony has greatly enhanced the safety of filtration surgery. Patients have faster visual rehabilitation when the IOP is in the physiological range. For example an IOP of 18 mmHg on postoperative day 1 is much more desirable than a pressure of 2 mmHg. With CTM and judicious suture lysis the target IOP can be achieved. Moreover, it is common for the IOP to fall spontaneously over the first postoperative week as residual viscoelastic material clears the eye.
Postoperative management of combined GDD and cataract extraction
Postoperative management of combined GDD and cataract extraction depends on the type of GDD used. The subtle differences between the GDDs and the postoperative care of each is described in Chapter 40. Generally, GDDs require less postoperative management and have fewer complications than filtering procedures55. During the immediate postoperative period steroid eye drops are used every 2 hours for the first week and then tapered for 3–4 months until they can be discontinued. Antibiotics are stopped at 1 week. One unique characteristic of GDDs is the so called ‘hypertensive phase’, which is a result of a breakdown in the blood–tissue barrier round the plate. This is typically addressed with ocular massage and/or temporary use of topical aqueous suppressants63,64. Hypotony can be seen in the early postoperative period and is addressed in a manner similar to filters. Problems with ocular motility are seen more commonly after tube shunts. Fortunately, persistent diplopia is less than 5% and can be corrected usually with strabismus surgery or glasses with prism.
Postoperative management of other glaucoma procedures and cataract extraction
The optimal postoperative management of canaloplasty, endoscopic cyclophotocoagulation, ab-interno trabecular meshwork excision, and trabecular bypass shunts is still being determined and is beyond the scope of this chapter (see Chapter 41). Generally, one of the main advantages to these procedures collectively is the relatively quick recovery and a less intensive postoperative medication and follow-up regimen. Steroids should probably be used more frequently than with cataract surgery alone in the initial postoperative period. However, a quick tapering can usually be employed and the patient can be completely off steroids 4–6 weeks after surgery. Immediately after surgery all glaucoma medications should be stopped and added back as needed. Prostaglandins are typically added back first, although this may increase the chances of postoperative cystoid macular edema. Aqueous suppressants also can be used.
Pupil management
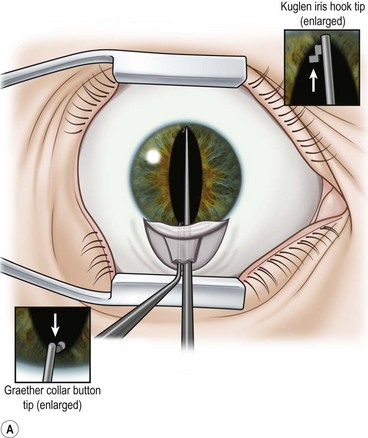
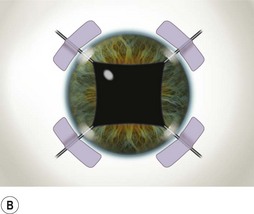
Perhaps the single most challenging aspect of combined cataract and glaucoma surgery is cataract extraction in the presence of poor pharmacologic pupillary dilatation. Chronic miotic therapy frequently reduces the pupillary response to mydriatic agents. Additionally, conditions such as PXF, floppy iris syndrome secondary to urinary outflow treatment with agents such as the alpha blocking agent tamsulosin65, or chronic inflammation may further limit dilatation of the pupil. A variety of techniques to enhance pupillary dilatation have been described. Traditional incisional iris surgery such as sector iridectomy or multiple small sphincterotomies has been replaced with less invasive methods. While incisional iris surgery adequately enhances visualization of the lens during phacoemulsification, the cut edges of the iris may be drawn into the phacoemulsification port during lens removal. This may result in significant iris trauma and postoperative inflammation. Most surgeons prefer non-incisional techniques to enlarge the pupil such as sphincter stretching, iris retraction hooks, or a Malyugin ring66. In eyes with poor dilatation, exposure can be improved with a simultaneous push–pull technique of sphincter stretching by means of two instruments such as Kuglen hooks or Graether ‘collar buttons’ (Fig. 36.7A). The sphincter is stretched first in the vertical direction, then in the horizontal meridian. The sphincter is stretched to the point of minute tears in the pupillary margin, which are typically seen as small, very focal hemorrhages in the iris sphincter. Additional viscoelastic material is then instilled into the anterior chamber to mechanically expand the pupil. This technique provides an additional 2 or 3 mm of pupillary dilatation. When stretching techniques are inadequate, flexible iris retraction hooks (Greishaber and Company, Langhorne, PA) or a Malyugin ring can be used. (Fig. 36.7B and Fig. 36.8A,B). The pupil stretch technique is simple, fast, effective, and avoids incisional iris surgery, while resulting in a round pupil postoperatively67–69. However, it is not as reliable as the use of hooks or a Malyugin ring. Moreover, stretching techniques are not recommended for floppy iris syndrome. In such cases, rings and hooks provide better stenting of the iris and are less likely to result in intraoperative miosis.
1 Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34(5):735-742.
2 Berdahl JP. Cataract surgery to lower intraocular pressure. Middle. East Afr J Ophthalmol. 2009;16(3):119-122.
3 Samuelson TW. Management of coincident glaucoma and cataract. Curr Opin Ophthalmol. 1995;6(1):14-21.
4 Stewart WC, Rogers GM, Crinkley CM, et al. Effect of cataract extraction on automated fields in chronic open-angle glaucoma. Arch Ophthalmol. 1995;113(7):875-879.
5 Munden PM, Alward WL. Combined phacoemulsification, posterior chamber intraocular lens implantation, and trabeculectomy with mitomycin C. Am J Ophthalmol. 1995;119(1):20-29.
6 Shingleton B, Laul A, Nagao K, et al. Effect of phacoemulsification on intraocular pressure in eyes with pseudoexfoliation Single-surgeon series. J Cataract Refract Surg. 2008;34(11):1834-1841.
7 Shingleton BJ, Gamell LS, O’Donoghue MW, et al. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25(7):885-890.
8 Shingleton BJ, Pasternack JJ, Hung JW, et al. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15(6):494-498.
9 Spaeth GL, editor. Ophthalmic Surgery, 3 edn, Philadelphia: WB Saunders, 1999.
10 Broadway DC, Grierson I, Sturmer J, et al. Reversal of topical antiglaucoma medication effects on the conjunctiva. Arch Ophthalmol. 1996;114(3):262-267.
11 Kuchle M, Vinores SA, Mahlow J, et al. Blood–aqueous barrier in pseudoexfoliation syndrome: evaluation by immunohistochemical staining of endogenous albumin. Graefes Arch Clin Exp Ophthalmol. 1996;234(1):12-18.
12 Kuchle M, Nguyen NX, Hannappel E, et al. The blood–aqueous barrier in eyes with pseudoexfoliation syndrome. Ophthalmic Res. 1995;27(Suppl 1):136-142.
13 Radius RL, Schultz K, Sobocinski K, et al. Pseudophakia and intraocular pressure. Am J Ophthalmol. 1984;97(6):738-742.
14 Bigger JF, Becker B. Cataracts and primary open-angle glaucoma: the effect of uncomplicated cataract extraction on glaucoma control. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(2):260-272.
15 Randolph ME, Maumenee AE, Iliff CE. Cataract extraction in glaucomatous eyes. Am J Ophthalmol. 1971;1(1 Part 2):328-330.
16 Tennen DG, Masket S. Short- and long-term effect of clear corneal incisions on intraocular pressure. J Cataract Refract Surg. 1996;22(5):568-570.
17 Hansen MH, Gyldenkerne GJ, Otland NW, et al. Intraocular pressure seven years after extracapsular cataract extraction and sulcus implantation of a posterior chamber intraocular lens. J Cataract Refract Surg. 1995;21(6):676-678.
18 Friedman DS, Jampel HD, Lubomski LH, et al. Surgical strategies for coexisting glaucoma and cataract: an evidence-based update. Ophthalmology. 2002;109(10):1902-1913.
19 Bowling B, Calladine D. Routine reduction of glaucoma medication following phacoemulsification. J Cataract Refract Surg. 2009;35(3):406-407. author reply 7
20 Leelachaikul Y, Euswas A. Long-term intraocular pressure change after clear corneal phacoemulsification in Thai glaucoma patients. J Med Assoc Thai. 2005;88(Suppl 9):S21-S25.
21 Hayashi K, Hayashi H, Nakao F, et al. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg. 2001;27(11):1779-1786.
22 Liu DT, Lee VY, Chiu TY, et al. Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. J Cataract Refract Surg. 2006;32(2):183. Author reply
23 Kim DD, Doyle JW, Smith MF. Intraocular pressure reduction following phacoemulsification cataract extraction with posterior chamber lens implantation in glaucoma patients. Ophthalmic Surg Lasers. 1999;30(1):37-40.
24 Pohjalainen T, Vesti E, Uusitalo RJ, et al. Phacoemulsification and intraocular lens implantation in eyes with open-angle glaucoma. Acta Ophthalmol Scand. 2001;79(3):313-316.
25 Poley BJ, Lindstrom RL, Samuelson TW, et al. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35(11):1946-1955.
26 Suzuki R, Tanaka K, Sagara T, et al. Reduction of intraocular pressure after phacoemulsification and aspiration with intraocular lens implantation. Ophthalmologica. 1994;208(5):254-258.
27 Sacca S, Marletta A, Pascotto A, et al. Daily tonometric curves after cataract surgery. Br J Ophthalmol. 2001;85(1):24-29.
28 Shingleton BJ, Heltzer J, O’Donoghue MW. Outcomes of phacoemulsification in patients with and without pseudoexfoliation syndrome. J Cataract Refract Surg. 2003;29(6):1080-1086.
29 Merkur A, Damji KF, Mintsioulis G, et al. Intraocular pressure decrease after phacoemulsification in patients with pseudoexfoliation syndrome. J Cataract Refract Surg. 2001;27(4):528-532.
30 Pohjalainen T, Vesti E, Uusitalo RJ, et al. Intraocular pressure after phacoemulsification and intraocular lens implantation in nonglaucomatous eyes with and without exfoliation. J Cataract Refract Surg. 2001;27(3):426-431.
31 Shingleton BJ, Rosenberg RB, Teixeira R, et al. Evaluation of intraocular pressure in the immediate postoperative period after phacoemulsification. J Cataract Refract Surg. 2007;33(11):1953-1957.
32 Jahn CE. Reduced intraocular pressure after phacoemulsification and posterior chamber intraocular lens implantation. J Cataract Refract Surg. 1997;23(8):1260-1264.
33 Shingleton BJ, Wadhwani RA, O’Donoghue MW, et al. Evaluation of intraocular pressure in the immediate period after phacoemulsification. J Cataract Refract Surg. 2001;27(4):524-527.
34 Arshinoff SA, Albiani DA, Taylor-Laporte J. Intraocular pressure after bilateral cataract surgery using Healon, Healon5, and Healon GV. J Cataract Refract Surg. 2002;28(4):617-625.
35 Murthy SK, Damji KF, Pan Y, et al. Trabeculectomy and phacotrabeculectomy, with mitomycin-C, show similar two-year target IOP outcomes. Can J Ophthalmol. 2006;41(1):51-59.
36 Kaplan-Messas A, Cohen Y, Blumenthal E, et al. Trabeculectomy and phaco-trabeculectomy with and without peripheral iridectomy. Eur J Ophthalmol. 2009;19(2):231-234.
37 Jin GJ, Crandall AS, Jones JJ. Phacotrabeculectomy: assessment of outcomes and surgical improvements. J Cataract Refract Surg. 2007;33(7):1201-1208.
38 Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008;34(7):1096-1103.
39 Bagli E, Gartzios C, Asproudis I, et al. Comparison of one-site versus two-site phacotrabeculectomy without the use of anti-metabolites intraoperatively in patients with pseudoexfoliation glaucoma and primary open-angle glaucoma. Clin Ophthalmol. 2009;3:297-305.
40 Shingleton BJ, Price RS, O’Donoghue MW, et al. Comparison of 1-site versus 2-site phacotrabeculectomy. J Cataract Refract Surg. 2006;32(5):799-802.
41 Samuelson TW. Management of coincident glaucoma and cataract. Curr Opin Ophthalmol. 1996;7(1):53-58.
42 Samuelson TW. Surgical management of coincident cataract and glaucoma. Curr Opin Ophthalmol. 1997;8(1):39-45.
43 Samuelson TW. Management of coincident glaucoma and cataract. Curr Opin Ophthalmol. 1999;10(1):66-72.
44 Joos KM, Bueche MJ, Palmberg PF, et al. One-year follow-up results of combined mitomycin C trabeculectomy and extracapsular cataract extraction. Ophthalmology. 1995;102(1):76-83.
45 Wong PC, Ruderman JM, Krupin T, et al. 5-Fluorouracil after primary combined filtration surgery. Am J Ophthalmol. 1994;117(2):149-154.
46 O’Grady JM, Juzych MS, Shin DH, et al. Trabeculectomy, phacoemulsification, and posterior chamber lens implantation with and without 5-fluorouracil. Am J Ophthalmol. 1993;116(5):594-599.
47 Shin DH, Hughes BA, Song MS, et al. Primary glaucoma triple procedure with or without adjunctive mitomycin. Prognostic factors for filtration failure. Ophthalmology. 1996;103(11):1925-1933.
48 Gandolfi SA, Vecchi M. 5-fluorouracil in combined trabeculectomy and clear-cornea phacoemulsification with posterior chamber intraocular lens implantation. A one-year randomized, controlled clinical trial. Ophthalmology. 1997;104(2):181-186.
49 Cotran PR, Roh S, McGwin G. Randomized comparison of 1-Site and 2-Site phacotrabeculectomy with 3-year follow-up. Ophthalmology. 2008;115(3):447-454. e1
50 Chang L, Thiagarajan M, Moseley M, et al. Intraocular pressure outcome in primary 5FU phacotrabeculectomies compared with 5FU trabeculectomies. J Glaucoma. 2006;15(6):475-481.
51 Hoskins HDJr, Migliazzo C. Management of failing filtering blebs with the Argon laser. Ophthalmic Surg. 1984;15(9):731-733.
52 Wilson RP, Steinmann WC. Use of trabeculectomy with postoperative 5-fluorouracil in patients requiring extremely low intraocular pressure levels to limit further glaucoma progression. Ophthalmology. 1991;98(7):1047-1052.
53 Nuyts RM, Pels E, Greve EL. The effects of 5-fluorouracil and mitomycin C on the corneal endothelium. Curr Eye Res. 1992;11(6):565-570.
54 Kanner EM, Netland PA, Sarkisian SRJr, et al. Ex-PRESS miniature glaucoma device implanted under a scleral flap alone or combined with phacoemulsification cataract surgery. J Glaucoma. 2009;18(6):488-491.
55 Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol. 2009;148(5):670-684.
56 Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg. 2009;35(5):814-824.
57 Lima FE, Magacho L, Carvalho DM, et al. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004;13(3):233-237.
58 Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19(3):393-399.
59 Carlson DW, Barad JP, Parsons MR. Reduced vision secondary to pigmented cellular membranes on silicone intraocular lenses. Am J Ophthalmol. 1995;120(4):462-470.
60 Shah SM, Spalton DJ. Natural history of cellular deposits on the anterior intraocular lens surface. J Cataract Refract Surg. 1995;21(4):466-471.
61 Traverso CE, Greenidge KC, Spaeth GL, et al. Focal pressure: a new method to encourage filtration after trabeculectomy. Ophthalmic Surg. 1984;15(1):62-65.
62 Stamper RL, McMenemy MG, Lieberman MF. Hypotonous maculopathy after trabeculectomy with subconjunctival 5-fluorouracil. Am J Ophthalmol. 1992;114(5):544-553.
63 Souza C, Tran DH, Loman J, et al. Long-term outcomes of Ahmed glaucoma valve implantation in refractory glaucomas. Am J Ophthalmol. 2007;144(6):893-900.
64 McIlraith I, Buys Y, Campbell RJ, et al. Ocular massage for intraocular pressure control after Ahmed valve insertion. Can J Ophthalmol. 2008;43(1):48-52.
65 Chang DF, Osher RH, Wang L, et al. Prospective multicenter evaluation of cataract surgery in patients taking tamsulosin (Flomax). Ophthalmology. 2007;114(5):957-964.
66 Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. J Cataract Refract Surg. 2008;34(5):835-841.
67 Dinsmore SC. Modified stretch technique for small pupil phacoemulsification with topical anesthesia. J Cataract Refract Surg. 1996;22(1):27-30.
68 Shepherd DM. The pupil stretch technique for miotic pupils in cataract surgery. Ophthalmic Surg. 1993;24(12):851-852.
69 Miller KM, Keener GTJr. Stretch pupilloplasty for small pupil phacoemulsification. Am J Ophthalmol. 1994;117(1):107-108.