Chapter 49
Cocaine and the Heart
1. How common is cocaine use in the United States?
2. What are the pharmacologic effects of cocaine?
Cocaine is a powerful sympathomimetic that acts by directly stimulating central sympathetic outflow and blocking presynaptic uptake of norepinephrine and dopamine (Fig. 49-1). This augmentation in postsynaptic catecholamines increases heart rate, mean arterial pressure, and left-ventricular contractility through stimulation of both α- and β-adrenergic receptors. Through enhanced α-adrenergic receptor activation, increased endothelin production, and diminished nitric oxide generation, cocaine initiates coronary artery vasoconstriction. Cocaine may also enhance platelet aggregation and thrombus formation through heightened production of adenosine diphosphate, thromboxane A2, and tissue plasminogen activator inhibitors, as well as reductions in protein C and antithrombin III. Cocaine causes toxic effects on cardiac muscle that arise primarily from Ca2+ overload during excessive β-adrenergic stimulation. Cocaine is well absorbed through all body mucous membranes and can be administered by nasal, sublingual, intramuscular, intravenous, and respiratory routes. The onset of action varies from 3 seconds to 5 minutes, depending on the administration route.
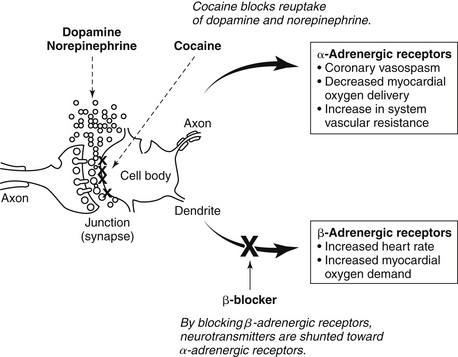
Figure 49-1 Proposed potential mechanism of the cardiovascular effects of cocaine when β-blockers are administered in cocaine-induced acute coronary syndrome.
3. What are the typical symptoms after cocaine ingestion?
4. What are the consequences of cocaine use?
5. How often does AMI occur after cocaine ingestion?
6. What else should be considered in the differential diagnosis after cocaine use?
Because patients who present to the emergency department after cocaine use are commonly hypertensive and tachycardic, aortic dissection needs to be considered in the differential diagnosis. Information concerning cocaine-induced aortic dissection is limited, but one study of 38 consecutive cases of aortic dissection demonstrated that a surprisingly high number (14, or 37%) were associated with cocaine use. However, among 921 patients in the International Registry of Aortic Dissection (IRAD), only 0.5% of aortic dissection cases were associated with cocaine use. In addition, an acute pulmonary syndrome, “crack lung,” has been described after inhalation of freebase cocaine. The syndrome presents with hypoxemia, hemoptysis, respiratory failure, and diffuse pulmonary infiltrates. Chronic cocaine use can lead to decreased left ventricular systolic function and congestive heart failure. This may relate to accelerated atherosclerosis or myocarditis, both of which are associated with cocaine use.
7. How does cocaine ingestion lead to AMI?
Cocaine can lead to AMI in a multifactorial fashion, including the following:
 Increasing myocardial oxygen demand by increasing heart rate, blood pressure, and contractility
Increasing myocardial oxygen demand by increasing heart rate, blood pressure, and contractility
 Decreasing oxygen supply as a result of vasoconstriction
Decreasing oxygen supply as a result of vasoconstriction
 Inducing a prothrombotic state by altering the balance between procoagulant and anticoagulant factors
Inducing a prothrombotic state by altering the balance between procoagulant and anticoagulant factors
8. Should younger patients with chest pain have a cocaine screening test?
9. Are there any specific electrocardiogram findings in patients that use cocaine?
10. Should all patients with cocaine-associated chest pain be admitted to the hospital?
11. Should all patients with cocaine-associated chest pain have a stress test?
12. How should patients with ST elevation myocardial infarction (STEMI) or non–ST elevation myocardial infarction (NSTEMI) be treated in the setting of cocaine use?
Rapid reperfusion by percutaneous coronary intervention (PCI) in a high-volume center by experienced operators is preferred over fibrinolytic therapy in the setting of STEMI, and this is even more desirable after cocaine use. Many young patients will have early repolarization, and only a small percentage of these patients will actually be experiencing an AMI. Furthermore, hypertensive patients after cocaine use are at higher risk for significant bleeding complications. There have been case reports of intracranial hemorrhage after fibrinolytic therapy in the setting of STEMI associated with cocaine use. Fibrinolytic therapy should only be considered for patients who are clearly having a STEMI but cannot receive timely PCI. Patients with NSTEMI should be treated in similar fashion as patients without cocaine, with a notable exception regarding beta-adrenergic blocking agents (β-blockers; see Question 14).
13. How should patients with cocaine-associated chest pain be treated?
Patients who ingest cocaine are commonly hypertensive, tachycardic, and anxious. In patients who use cocaine, the AHA recommends the early use of intravenous benzodiazepines. Benzodiazepines decrease the central stimulating characteristics of cocaine and lessen anxiety. Their use has been shown to relieve chest pain and to have beneficial hemodynamic effects. Many times the hypertension and tachycardia will not need to be directly treated after the use of benzodiazepines. Aspirin should also be given. In patients who remain hypertensive, nitroglycerin or nitroprusside can be administered. Phentolamine is also an alternative. Calcium channel blockers have not been well studied in this population, but can be considered in patients who do not respond to benzodiazepines and nitroglycerin. However, short-acting nifedipine should not be used, and verapamil and diltiazem should be avoided in the setting of heart failure or decreased left ventricular systolic function (Fig. 49-2).
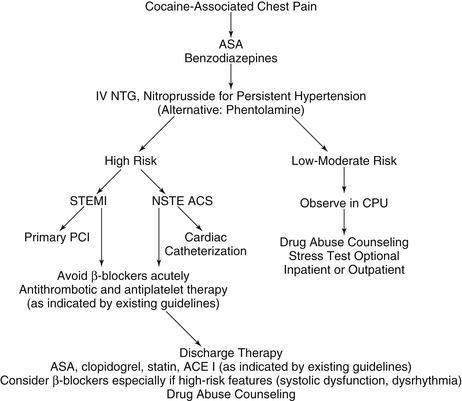
Figure 49-2 Therapeutic and diagnostic recommendations in cocaine-associated chest pain. ACE, Angiotensin-converting enzyme; ACS, acute coronary syndrome; ASA, aspirin; β-blockers, beta-adrenergic receptor blocking agents; CPU, chest pain unit; IV, intravenous; NSTE, non–ST segment elevation; NTG, nitroglycerin; PCI, percutaneous coronary intervention; STEMI, ST segment elevation myocardial infarction.
14. Should β-blockers be given to patients with cocaine-associated chest pain?
15. How should tachyarrhythmias be treated after cocaine use?
Bibliography, Suggested Readings, and Websites
1. Chang, A.M., Walsh, K.M., Shofer, F.S., et al. Relationship between cocaine use and coronary artery disease in patients with symptoms consistent with an acute coronary syndrome. Acad Emerg Med. 2010;18:1–9.
2. Feldman, J.A., Fish, S.S., Beshansky, J.R., et al. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med. 2000;36:469–476.
3. Hollander, J.E. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267–1272.
4. Hollander, J.E., Hoffman, R.S., Burstein, J.L., et al. Cocaine-associated myocardial infarction. Mortality and complications. Cocaine-Associated Myocardial Infarction Study Group. Arch Intern Med. 1995;155:1081–1086.
5. Hollander, J.E., Hoffman, R.S., Gennis, P., et al. Prospective multicenter evaluation of cocaine-associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med. 1994;1:330–339.
6. Maraj, S., Figueredo, V.M., Morris, L., et al. Cocaine and the Heart. Clin Cardiol. 2010;33:264–269.
7. McCord, J., Jneid, H., Hollander, J.E., et al. Management of cocaine-associated chest pain and myocardial infarction. Circulation. 2008;117:1897–1907.
8. Weber, J.E., Shofer, F.S., Larkin, G.L., et al. Validation of a brief observation period for patients with cocaine-associated chest pain. N Engl J Med. 2003;348:510–517.