CHAPTER 38 Coarctation of the Aorta
Definition
Coarctation refers to a stenosis of the proximal descending thoracic aorta that is almost always opposite the insertion of the ductus arteriosus—that is, at the junction of the distal aortic arch and the descending aorta just below the origin of the left subclavian artery (Fig. 38-1).1–4
Prevalence and Epidemiology
Coarctation of the aorta occurs in 3.2 of 10,000 births and accounts for 5% to 10% of all cases of congenital heart disease.1–4 Coarctation is more common in white males than in white females, with a male-to-female ratio of 1.3 to 2.0 : 1.3 Most cases are sporadic, but there may be a genetic inheritance.
Etiology and Pathophysiology
Coarctation is thought to be the result of a malformation of the aortic media, leading to a posterior infolding or shelf.1,5,6 Most often, the shelf is discrete and opposite the ductus arteriosus. However, the malformation may be a long segment and circumferentially surround the aorta. In the latter form, there is typically diffuse tubular hypoplasia of the transverse arch and isthmus.1,7,8
Histologic examination shows thickened intima and media that protrude posteriorly and laterally into the aortic lumen. The ductus arteriosus or ligamentum arteriosus inserts anteromedially at the same level.1
In milder forms of coarctation, the left ventricular myocardium hypertrophies to normalize myocardial wall stress and maintain normal systolic ventricular function. Left ventricular ejection fraction is often normal to increased. Collateral vessels also develop, which act as a source of lower body perfusion and help maintain normal flow to abdominal viscera.1
MANIFESTATIONS
Clinical Presentation
Critical or severe coarctation manifests in the first few days to first week of life. Symptoms include cyanosis (especially of the lower body), heart failure, shock, multiorgan failure and necrotizing enterocolitis.1–5
Milder forms of coarctation manifest later in life. Because left ventricular function is maintained and cardiac outflow is good, patients come to clinical attention because of hypertension or a heart murmur related to a bicuspid aortic valve.1–4 Blood pressure in the upper extremities is typically higher than that in the lower extremities. However, if there is an aberrant right subclavian artery, the pressure in the right upper arm may be equal to or lower than that in the left upper arm.
Imaging Studies
Techniques and Findings
Ultrasound
Two-dimensional echocardiography may show the site and extent of coarctation in young infants. Doppler imaging can help in determining the hemodynamic severity of the obstruction. In general, images in infants are of higher quality than those in older children and adults. In the latter populations, images of the distal arch may be suboptimal, requiring additional imaging with CT or MRI.1,2,4
Computed Tomography
CT angiography is performed with a pulmonary embolism protocol using thin collimation (<1 mm), pitch lower than 1.5,9,10 fast scan time, and a single breath-hold, when possible. In adolescents and adults, contrast medium is injected via a power injector at a high flow rate of 3 to 4 mL/sec using a contrast volume of 100 to 150 mL (280 to 320 mg I/mL). Scan delay time can be determined with an automatic bolus tracking system with the cursor over the aortic isthmus or empiric timing. Empiric timing, using a delay of 20 to 25 seconds after the start of the injection, is a default method if the bolus tracking fails to trigger. Electrocardiographic (ECG) gating is not needed for the evaluation of coarctation.
Special Considerations for Pediatric Patients
The contrast volume is 2 mL/kg (not to exceed 125 mL). Intravenous contrast medium can be administered with a power injector or manual (hand) injection. A power injector is used when a 22-gauge or larger cannula can be placed in an antecubital vein. For a 22-gauge catheter, flow rates are 1.5 to 2.5 mL/sec.10,11 Flow rates for larger gauge catheters are similar to those described above. With a manual injection, the contrast is pushed as quickly as possible. Determination of the scan initiation time can be made by an empiric or bolus tracking method. In pediatric patients weighing less than 10 kg, an empiric scan delay of 12 to 15 seconds after the start of the intravenous contrast injection suffices.10 In larger patients, the delay time is 15 to 25 seconds.
Magnetic Resonance Imaging
MRI, like CT, can accurately define the location, extent, and degree of aortic narrowing, collateral vessel formation, and valvular and ventricular morphology. In addition, MRI can provide an assessment of coarctation physiology, including information on blood flow and the pressure gradient across the stenosis.1,3 Because MRI yields functional information and does not rely on ionizing radiation, many centers prefer this modality over CT, especially for children.
Noncontrast-enhanced methods, both black blood and bright blood techniques, and three-dimensional contrast-enhanced MR angiography (CEMRA) are routinely acquired. CEMRA is performed by acquiring a three-dimensional gradient-echo sequence following a rapid bolus of gadolinium. There are several ways to time contrast delivery. The best guess method is the simplest, the automated detection method is the most operator-independent,12 and the timing run is the most reliable method.13
Velocity-encoded (VEC) phase contrast techniques are used to determine flow, peak flow rates, and the volume of flow per unit time (see Chapter 17, Fig. 17-8).14,15 The pressure gradient across the obstruction is calculated with a modified Bernoulli equation (product of the cube of the peak velocity as measured in meters per second).
Angiography
Angiography is no longer needed for the diagnosis of uncomplicated coarctation if conventional noninvasive imaging can clearly delineate the anatomy and physiology.1,4 If conventional studies are equivocal or indeterminate, or if there are possible associated intracardiac anomalies, angiography is performed.
Classic Signs
Chest Radiographs
In infants with critical coarctation, the chest radiograph demonstrates cardiomegaly and pulmonary edema (Fig. 38-2). In older children, the heart size is normal or slightly enlarged. There may be dilatation of the ascending aorta and a reverse 3 sign caused by aortic dilatation proximal and distal to the coarctation. Rib notching between the third and eighth ribs may be seen in older children, but rarely in children younger than 5 years (Fig. 38-3).4
Computed Tomography and Magnetic Resonance Imaging
The classic CT and MRI findings of coarctation are discrete short-segment aortic narrowing with an infolding or shelf-like appearance of the posterior wall (Fig. 38-4).9,16–18 The isthmus and possibly the transverse arch will also be narrow in the diffuse form of coarctation (Fig. 38-5). In older individuals, other CT and MRI findings include dilatation of the aorta proximal to the coarcted segment, collateral vessel formation, and rib notching (Fig. 38-6). The common collateral pathways are the intercostal arteries (usually third through eighth) and internal thoracic arteries (Fig. 38-5; see Fig. 38-4B).3 Other cardiac lesions, such as ventricular septal defect, bicuspid valve, and patent ductus arteriosus, may be seen.
Reconstructed images in the sagittal and parasagittal planes are best to show the location and extent of coarctation (see Fig. 38-4).19 Coronal and sagittal reconstructions may help delineate rib notching and/or collateral vessel formation (see Fig. 38-6).
DIFFERENTIAL DIAGNOSIS
From Clinical Presentation
The differential diagnosis of coarctation in the neonate includes other lesions that cause critical obstruction of the left ventricle outflow tract, including the following: (1) interrupted aortic arch; (2) hypoplastic left heart; and (3) critical aortic stenosis.5 Most neonates with critical left ventricular outflow obstruction present in the first few hours to first week of life with signs of shock and cardiac failure and radiographic findings of cardiomegaly and pulmonary edema.5
Interruption of the Aortic Arch
This is a rare anomaly, accounting for 1% to 1.5% of all critically ill infants with congenital heart disease.4,20 This is a marked form of coarctation in which a segment of the aortic arch is completely absent. There are three types of interrupted arch based on the site of interruption:
A dilated patent ductus arteriosus usually supplies the descending aorta beyond the interruption (Fig. 38-7).19,21 Associated cardiac defects include ventricular septal defect, bicuspid aortic valve, aortopulmonary window, truncus arteriosus, single ventricle subaortic stenosis.3,4 CT and/or MRI can have a role in diagnosis.
Hypoplastic Left Heart Syndrome
This is a spectrum of left heart obstructive lesions characterized by underdevelopment of the mitral valve, left ventricle, aortic valve, and aorta. The right heart and main pulmonary artery are enlarged. Atrial and ventricular septal defects and patent ductus arteriosus are common associated defects.22,23 Diagnosis is confirmed by echocardiography and cardiac angiography. CT and MRI usually have no role in preoperative assessment.
Critical Aortic Stenosis
This is a condition caused by fusion of the valve leaflets, resulting in a slit-like aortic orifice, severe outflow tract obstruction, left ventricular hypertrophy, and ultimately cardiac failure and pulmonary edema.5 Diagnosis is confirmed by echocardiography and cardiac catheterization. CT and MRI have no role in preoperative assessment.
From Imaging Findings
Pseudocoarctation is in the CT-MR differential diagnosis of coarctation in older patients. It is associated with an elongated, redundant aortic arch and appears as a buckle in the arch at the insertion of the site of the ligamentum arteriosum (Fig. 38-8). Luminal obstruction is absent. The blood pressures in the upper and lower extremities are almost always similar, although minor differences have been reported. There is no collateral circulation, which helps distinguish between pseudocoarctation and coarctation.
SYNOPSIS OF TREATMENT OPTIONS
Medical
In neonates with heart failure, prostaglandin E1 is administered to reopen the ductus arteriosus and establish perfusion to the distal aorta and abdominal viscera. Treatment of congestive heart failure with digoxin and diuretics is also instituted. As soon as the patient condition improves, primary surgical repair is performed.1,2,4
Surgical and Interventional
The surgical mortality rate from a primary repair is less than 5% in neonates and less than 1% in older patients.4 Late complications of surgical and interventional treatment include recoarctation or residual coarctation and aneurysm formation (Fig. 38-9).29 The incidence of aneurysm formation is higher after angioplasty and patch graft repair than after primary surgical repair.3
KEY POINTS
 Clinical presentation varies from congestive heart failure in the newborn to asymptomatic hypertension or a murmur in older patients
Clinical presentation varies from congestive heart failure in the newborn to asymptomatic hypertension or a murmur in older patients CT and MRI findings—focal or diffuse narrowing, collateral vessel formation, rib notching, bicuspid valve, thick left ventricular wall
CT and MRI findings—focal or diffuse narrowing, collateral vessel formation, rib notching, bicuspid valve, thick left ventricular wallGaca AM, Jaggers JJ, Dudley LT, Bisset GS. Repair of congenital heart disease: a primer—Part 2. Radiology. 2008;248:44-60.
Goo HW, Park I-S, Ko JK, et al. CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics. 2003;23:S147-S165.
Haramati LB, Glickstein FS, Issenberg HF, et al. MR imaging and CT of vascular anomalies and connections in patients with congenital heart disease. Radiographics. 2002;22:337-349.
Lee ED, Siegel MJ, Hildebolt CF, et al. Multidetector CT evaluation of pediatric thoracic aortic anomalies: comparison of axial, multiplanar, and three-dimensional images. AJR. 2004;182:777-784.
1 Beekman RH. Coarctation of the aorta. In: Alan HD, Gutgesell HP, Clark EB, Driscoll DJ, editors. Heart Disease in Infants, Children, and Adolescents. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:988-1010.
2 Joshi VM, Sekhavat S. Acyanotic congenital heart defects. In: Vetter VL, editor. Pediatric Cardiology: Requisites. St. Louis: Mosby; 2006:79-96.
3 Kaemmerer H. Aortic coarctation and interrupted aortic arch. In: Gatzoulis MA, Webb GD, Daubeney PEF, editors. Adult Congenital Heart Disease. Edinburgh: Churchill Livingstone; 2003:253-264.
4 Park MK. Obstructive lesions. In: Park MK, editor. Pediatric Cardiology for Practitioners. 5th ed. Philadelphia: Elsevier Mosby; 2008:205-214.
5 Zeltser H, Tabbutt S. Critical heart disease in the newborn. In: Vetter VL, editor. Pediatric Cardiology: Requisites. St. Louis: Mosby; 2006:31-50.
6 Edwards JE, Christensen NA, Clagett OT, et al. Pathologic considerations in coarctation of the aorta. Mayo Clin Proc. 1948;23:324-332.
7 Ho SY, Anderson RH. Coarctation, tubular hypoplasia, and the ductus arteriosus: histologic study of 35 specimens. Br Heart J. 1979;41:268-274.
8 Bharati S, Lev M. The surgical anatomy of the heart in tubular hypoplasia of the transverse aorta (preductal coarctation). J Thorac Cardiovasc Surg. 1986;91:79-85.
9 Becker C, Soppa C, Fink U, et al. Spiral CT angiography and three-dimensional reconstruction in patients with aortic coarctation. Eur Radiol. 1997;7:1473-1477.
10 Siegel MJ. Heart. In: Siegel MJ, editor. Pediatric Body CT. Philadelphia: Lippincott Williams & Wilkins; 2007:145-175.
11 Siegel MJ. CT angiography: optimizing contrast use in pediatric patients. Appl Radiol. 2003;32:S43-S49.
12 Foo TK, Saranathan M, Prince MR, Chenevert TL. Automated detection of bolus arrival and initiation of data acquisition in fast, three-dimensional, gadolinium-enhanced MR angiography. Radiology. 1997;203:275-280.
13 Earls JP, Rofsky NM, DeCorato DR, et al. Breath-hold single-dose gadolinium-enhanced three-dimensional MR aortography: usefulness of a timing examination and MR power injector. Radiology. 1996;201:705-710.
14 Rebergen SA, van der Wall EE, Doornbos J, de Roos A. Magnetic resonance measurement of velocity and flow: technique, validation, and cardiovascular applications. Am Heart J. 1993;126:1439-1456.
15 Varaprasathan GA, Araoz PA, Higgins CB, Reddy GP. Quantification of flow dynamics in congenital heart disease: applications of velocity-encoded cine MR imaging. Radiographics. 2002;22:895-905.
16 Gilkeson RC, Ciancibello L, Zahka K. Multidetector CT evaluation of congenital heart disease in pediatric and adult patients. AJR. 2003;180:973-980.
17 Goo HW, Park I-S, Ko JK, et al. CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics. 2003;23:S147-S165.
18 Haramati LB, Glickstein FS, Issenberg HF, et al. MR imaging and CT of vascular anomalies and connections in patients with congenital heart disease. Radiographics. 2002;22:337-349.
19 Ed Lee, Siegel MJ, Hildebolt CF, et al. Multidetector CT evaluation of pediatric thoracic aortic anomalies: comparison of axial, multiplanar, and three-dimensional Images. AJR. 2004;182:777-784.
20 Celoria CG, Patton RB. Congenital absence of the aortic arch. Am Heart J. 1959;58:407-413.
21 Cinar A, Haliloglu M, Karagoz T, et al. Interrupted aortic arch in a neonate: multidetector CT diagnosis. Pediatr Radiol. 2004;34:901-903.
22 Bardo DM, Frankel DG, Applegate KE, et al. Hypoplastic left heart syndrome. Radiographics. 2001;21:705-717.
23 Freedom RM, Black MD, Benson LN. Hypoplastic left heart syndrome. In: Alan HD, Gutgesell HP, Clark EB, Driscoll DJ, editors. Heart Disease in Infants, Children, and Adolescents. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1011-1026.
24 Van Son JA, van Asten WN, van Lier HJ, et al. Detrimental sequelae on the hemodynamics of the upper limb after subclavian flap angioplasty in infancy. Circulation. 1990;81:996-1004.
25 Hijazi ZM, Fahey JT, Kleinman CS, Hellenbrand We. Balloon angioplasty for recurrent coarctation of the aorta. Intermediate and long-term results. Circulation. 1991;84:1150-1156.
26 Siblini G, Rao PS, Nouri S, et al. Long-term follow up results of balloon angioplasty of postoperative aortic recoarctation. Am J Cardiol. 1998;81:61-67.
27 Fletcher SE, Nihill MR, Grifka RG, et al. Balloon angioplasty of native coarctation of the aorta: midterm follow-up and prognostic factors. J Am Coll Cardiol. 1995;25:73-734.
28 Mendelsohn AM, Lloyd TR, Crowley DC, et al. Late follow-up of balloon angioplasty in children with a native coarctation of the aorta. Am J Cardiol. 1994;25:696-700.
29 Shih M-CP, Tholpady A, Kramer CM, et al. Surgical and endovascular repair of aortic coarctation: normal findings and appearance of complications on CT angiography and MR angiography. AJR. 2006;187:W302-W312.

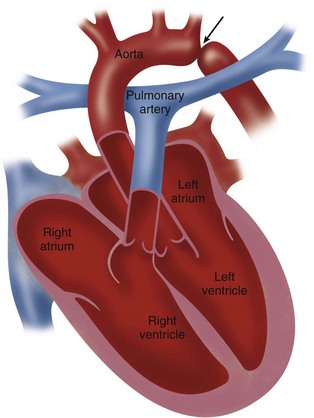
 FIGURE 38-1
FIGURE 38-1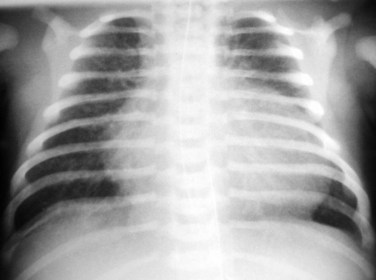
 FIGURE 38-2
FIGURE 38-2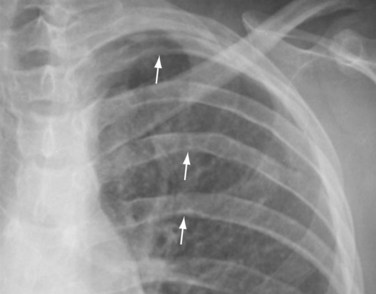
 FIGURE 38-3
FIGURE 38-3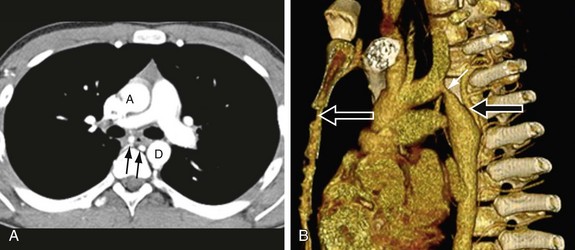
 FIGURE 38-4
FIGURE 38-4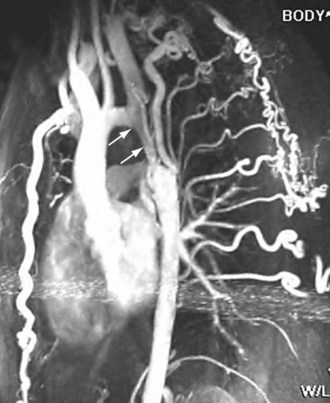
 FIGURE 38-5
FIGURE 38-5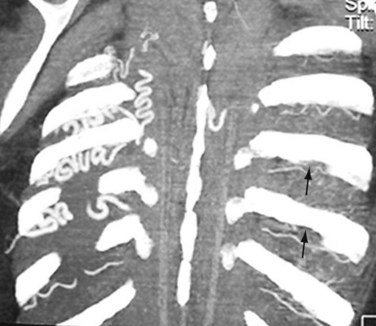
 FIGURE 38-6
FIGURE 38-6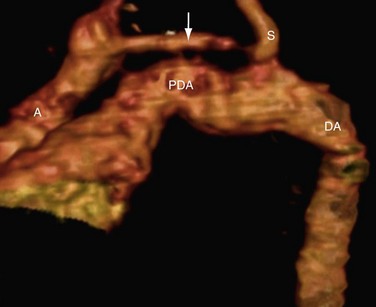
 FIGURE 38-7
FIGURE 38-7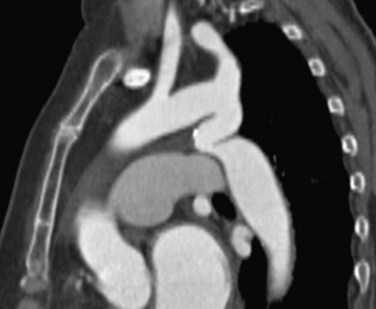
 FIGURE 38-8
FIGURE 38-8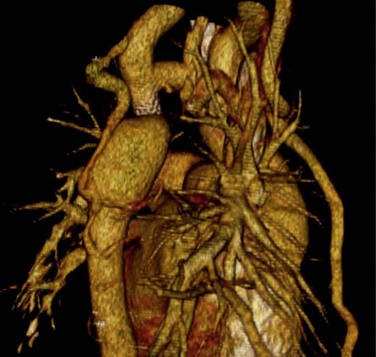
 FIGURE 38-9
FIGURE 38-9




