Chapter 8 Coarctation of the Aorta and Interrupted Aortic Arch
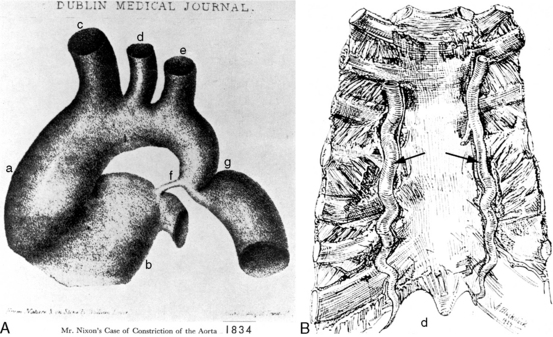
In 1760, the Prussian anatomist, Johann Friedreich Meckel, characterized coarctation of the aorta as an “extraordinary dilatation of the heart which came from the fact that the aortic conduit was too narrow.”1 Saul Jarcho’s1–5 historic papers underscored the accuracy of early accounts of this congenital malformation.
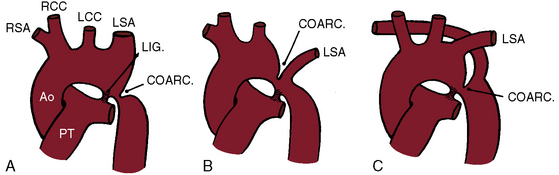
Coarctation of the aorta is typically located near the aortic attachment of the ligamentum arteriosum or patent ductus arteriosus (Figures 8-1A and 8-2). An obtuse indentation in the posterolateral wall of the aorta corresponds to the location of an internal ridge or shelf that eccentrically narrows the aortic lumen, hence the term coarctatus (Latin, contracted or tightened). The ridge that forms the coarctation consists of smooth muscle, fibrous tissue, and elastic tissue similar in composition to a muscular arterial ductus (see Chapter 20).6 Intimal proliferation distal to the ridge narrows the lumen.6 The junction between the ductus and the elastic aorta is clearly defined, with extension of ductal tissue into the aortic wall not exceeding 30% of the aortic circumference. In preductal coarctation, however, ductal tissue forms a circumferential sling that extends around the aorta.7 In juxtaductal coarctation, ductal tissue is not a significant part of the aortic wall.7 The coarctation ridge is thought to represent the original wall of the distal left sixth aortic arch. The neural crest is thought to play a role in the pathogenesis of some types of coarctation.7
The common form of aortic coarctation is represented by a localized constriction that contains the ridge or shelf as just described.6 Less commonly, a relatively long segment of constriction extends beyond the left subclavian artery. Rarely, congenital coarctation is in the mid thoracic aorta.8 Tubular hypoplasia refers to uniform narrowing within the aortic arch.6 Localized coarctation and tubular hypoplasia sometimes coexist.
The mechanisms that account for the typical location of coarctation take into account a number of variables: (1) the quantitative morphology and growth of the aortic arch in the normal fetus9–11; (2) the site of the aortic orifice of the ductus arteriosus; (3) the presence of ductal tissue in the coarctation6; (4) constriction of ductal tissue immediately after birth; and (5) coarctation in the presence of a widely patent ductus in the fetus12,13 and after birth.6 Current consensus favors an interplay between aortic growth and blood flow.9–11 High-resolution echocardiographic imaging in the healthy fetus has disclosed progressive tapering of the diameter of the aortic arch at all gestational ages, with the smallest diameter at the isthmus.9 Tapering is thought to reflect the relative proportion of fetal cardiac output traversing each aortic segment. The smallest proportion traverses the isthmus, which maintains its smaller dimension relative to the remainder of the aortic arch well into postnatal life, especially in premature infants.9 Neonatal coarctation is characterized by hypoplasia of the transverse aorta in the presence of a relatively large pulmonary trunk, a combination that is thought to reflect an in utero decrease in aortic arch flow together with an increase in flow through the main pulmonary artery and ductus.11
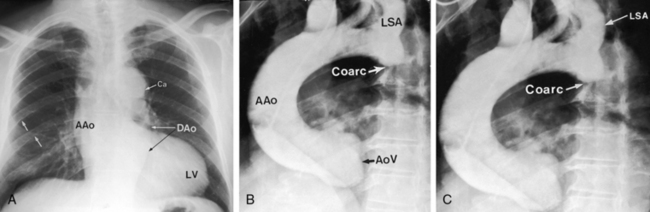
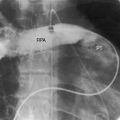
The coarctation ridge is located either immediately proximal to the aortic insertion of the ductus, opposite the aortic insertion (juxtaductal), or immediately distal to the aortic insertion.6,14,15 Infants with a juxtaductal coarctation show no signs of aortic obstruction as long as the ductus is widely patent, assuring unobstructed pulmonary trunk/ductus/descending aortic continuity. When the ductus closes, the coarctation becomes apparent immediately and dramatically and the femoral pulses disappear. The left subclavian artery is dilated because coarctation is usually located immediately distal to its origin (Figure 8-1A, 8-3D, and 8-4A). However, coarctation may lie at or proximal to the left subclavian orifice, compromising its lumen (Figure 8-1B). Exceptionally, the right subclavian artery arises distal to the coarctation (Figure 8-1C) or the coarctation is in a right aortic arch.16
Coarctation of the abdominal aorta can be part of a noncongenital systemic vascular disorder, such as Takayasu’s arteritis or von Recklinghausen’s disease, but can also be congenital and is therefore appropriate for inclusion here.17–20 Abdominal coarctation is rare, accounting for 0.5% to 2% of all varieties of aortic coarctation,18 and can be suprarenal, infrarenal, or interrenal.18 Systemic hypertension is believed to result from involvement of the renal artery.20 A congenital etiology is based on the combination of a localized hourglass deformity with an intraluminal membrane, occurrence at a young age, absence of a systemic vascular disorder, and slow progression with development of extensive collaterals.20
Pseudocoarctation refers to a rare anomaly characterized by buckling or kinking of the aorta in the vicinity of the ligamentum arteriosum that results in elongation, tortuosity, and dilation of the distal aortic arch and the proximal descending aorta.21–25 Occasionally, pseudocoarctation involves the abdominal aorta (Figure 8-5).26 Pseudo signifies absence of a gradient at the site of the localized external deformity, absence of collaterals, and absence of systemic hypertension, although refractory hypertension has been reported.27 Occasionally, transformation from pseudocoarctation to true coarctation occurs.28 The femoral pulses are sometimes reduced because of the damping effect of sharp angulation of the aortic arch (see Figure 8-5).22 Thin-walled saccular aneurysms may form in the distal segment,21 spontaneous rupture and dissection have been reported in the proximal descending thoracic aorta29 and in the ascending aorta,30 and bicuspid aortic valve may coexist.31
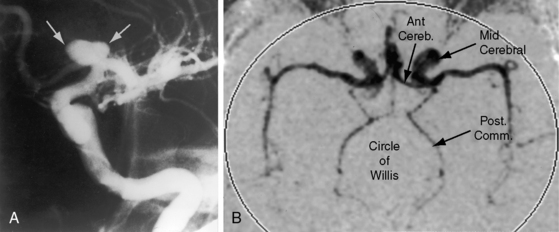
Coarctation of the aorta is usually and simplistically regarded as isolated obstruction of the aortic isthmus32 but in fact is a widespread disorder in which isthmic obstruction is only one of a cluster of abnormalities that include the proximal and distal paracoarctation aorta, the ascending and transverse aorta, the coronary arteries, conduit arteries (radial, brachial, carotid), the retinal vascular bed, dissecting aneurysm, cerebral aneurysms, vascular rings, and systemic hypertension. The strongest association is with a functionally normal, stenotic, or incompetent bicuspid aortic valve (Figure 8-6B; see Chapter 7)33,34 characterized by fusion of the right and left coronary cusps.34,35 A decrease in left ventricular interpapillary muscle distance is common36and culminates in the single papillary muscle of a parachute mitral valve (see Chapter 9).37,38 Coarctation in the fetus tends to be associated with a left superior vena cava.39 Endocardial fibroelastosis is patchy rather than confluent.40 Two shunts accompany coarctation (patent ductus arteriosus41 and ventricular septal defect41–44), which is usually characterized by leftward malalignment that curtails the amount of blood that reaches the aortic isthmus.42–44 Dissecting aneurysm with hemopericardium was reported in 1830.45 Aneurysmal dilation, which can be large, saccular, and calcified, occurs in the low-pressure high-velocity distal paracoarctation segment.46 The substrate for aneurysm formation is an inherent abnormality of medial smooth muscle and extracellular matrix that is a consistent feature of the proximal and distal paracoarctation aorta.47,48 Aneurysms of the circle of Willis set the stage for intracranial hemorrhage (Figure 8-7). Relatively benign aneurysms occasionally develop in intercostal arteries.49 A large aneurysm of the left subclavian artery was acutely dissected.50 The retinal arterioles are characteristically U shaped (see subsequent discussion).51
Arterial collaterals are important vascular sequelae of coarctation and depend on subclavian artery patency for their development (see Figures 8-3 and 8-6A). When coarctation obstructs the orifice of the left subclavian artery, ipsilateral collaterals fail to develop (see Figure 8-27). A subclavian steal may result from retrograde flow down the ipsilateral vertebral artery.52 Systemic-to-pulmonary collaterals normally present after birth gradually disappear.53
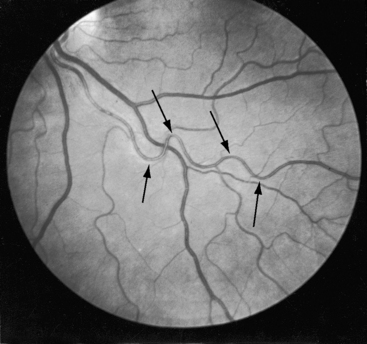
Retinal arterioles are the site of a distinctive vascular pattern.51,54 In 1948, at the annual meeting of the Swedish Ophthalmological Society, Professor K.O. Granstrom55 stated, “As in other cases of hypertension, these patients are sent as a matter of routine for examination to the eye department. As soon as I had seen a few such cases, it became evident that the retinal picture in coarctation of the aorta is often somewhat characteristic, the principal feature being pronounced tortuosity of the arteries.” The U-shaped tortuous retinal arterioles (Figure 8-8) may be accompanied by serpentine pulsations synchronous with the arterial pulse.54 Granstrom55 correctly concluded that the distinctive retinal abnormality in coarctation was benign and that hypertensive retinopathy was conspicuous by its absence.
Coarctation of the aorta can contribute to a vascular ring that consists of a double aortic arch or a right aortic arch, a left ligamentum arteriosum, and an aberrant subclavian artery.56–58 An anomalous retroesophageal right subclavian artery occasionally accompanies coarctation of the aorta (see Figure 8-1C) but does not cause tracheoesophageal compression.
The pathogenesis of systemic hypertension in coarctation of the aorta has been the subject of three theories. The mechanical theory focuses on resistance at the site of obstruction. This theory in itself cannot be sustained but is central to the neural theory that involves the sensitivity of carotid sinus baroreceptors and the distensibility characteristics of the precoarctation aorta.59 The proximal aortic segment of coarctation has an increase in collagen and a decrease in smooth muscle that are responsible for an increase in stiffness and a decrease in distensibility.60 The poorly compliant precoarctation aorta is responsible for the elevation of systolic blood pressure at rest, the disproportionate rise during isotonic exercise,61,62 and the resetting of carotid sinus baroreceptors to operate at higher pressures.59,61 The renal theory of pathogenesis takes into account a unique feature of coarctation hypertension, namely, that the elevated blood pressure is confined to the upper extremities. Accordingly, the renal arteries are exposed to the neurohumeral effects of hypertension but not the hydraulic effects. The coarctation gradient increases during isotonic exercise, which is accompanied by a disproportionate increase in systolic pressure,63 an exaggeration of the inherent disproportionate systolic hypertension related to rigidity of the precoarctation aortic wall. The response of the mean blood pressure to exercise is approximately the same as in control subjects.64 Experimental hypertension has been produced by aortic constriction that assigns the renal arteries to the distal low pressure zone.65 When the constriction is below the renal arteries, blood pressure remains normal.65,66 Toxemia of pregnancy is not a feature of coarctation hypertension. Eye grounds do not exhibit hypertensive retinopathy but instead exhibit distinctive serpentine retinal arterioles (see Figure 8-8).51,54
The increase in left ventricular mass induced by the afterload of coarctation is characterized as myocyte replication rather than myocyte hypertrophy,67 which is thought to account for reduced end-systolic wall stress and enhanced left ventricular ejection performance.68
History
The male:female ratio of coarctation is from 1.4:1 to 3:1.69 Familial recurrence has occurred in siblings, in monozygotic twins, and in parent and child70–75 and as autosomal dominant in four generations.76 True coarctation and pseudocoarctation have been reported in siblings.77 Turner’s syndrome (see section Physical Appearance) cannot be transmitted because of sterility, but seven women in three generations of one family had Turner’s syndrome attributed to loss of the short arm of one X chromosome that permitted transmission from phenotypically normal female carriers with a balanced X-1 translocation.78 A peak seasonal incidence of coarctation is found from September through November and from January through March.79 The reported rarity of coarctation in African Americans is open to question.80,81
Coarctation of the aorta usually produces significant symptoms in early infancy69,82,83 and after age 20 to 30 years.84 Neonates with severe coarctation become acutely symptomatic when the ductus closes. Most who survive the hazards of infancy reach adulthood, although more than a quarter die by age 20 years, half by age 30 years, and more than three quarters by age 50 years.33,84,85 Figures 8-24 and 8-26 are from patients aged 54 years, 62 years, and 70 years. Survival has been reported at age 74 years and 76 years.86 Isolated atresia of the aortic arch was surgically repaired at age 65 years.87 The longest recorded survival was Reynaud’s account (1828) of a 92-year-old man with coarctation.3 The anatomic illustrations in this report are noteworthy. However, examples of exceptional longevity should not obscure the inherent risk of coarctation that results in death at an average age of 33 years.
Mild coarctation is not always benign, and severe coarctation is not always asymptomatic. A case in point, albeit rare, is a 20-year-old woman with severe coarctation, borderline hypertension, and no symptoms.88 Except for symptomatic infants, patients tend to be clinically well when the diagnosis is first made. Initial suspicion requires little more than attention to upper and lower extremity arterial pulses and blood pressure (see section The Arterial Pulse). Nevertheless, delayed recognition is not uncommon,89,90 and diagnoses have been made by chance in the fifth decade.91 Minor symptoms include epistaxis and leg fatigue. When coarctation compromises the orifice of the left subclavian artery in left-handed patients, muscular fatigue involves the left arm. Leg fatigue occurs in about half of patients, but claudication is reserved for abdominal coarctation. Patients are sometimes subjectively aware of amplified arterial pulsations in the neck, especially after effort or excitement. Dysphagia occurs when a retroesophageal right subclavian artery originates distal to the coarctation and passes behind the esophagus (see Figure 8-1C)92 or when coarctation is a component of a vascular ring.
Major symptoms are features of four eventualities: (1) congestive heart failure; (2) rupture or dissection of the aorta; (3) infective endarteritis or endocarditis; and (4) cerebral hemorrhage.83,84,93–95 Hypertension is chiefly responsible for morbidity and mortality with advancing age.83 The incidence rate of cardiac failure is highest in infants69,96 and is high again after the fourth decade.97 In a review of 234 patients with coarctation of the aorta aged 1 day to 72 years, congestive heart failure occurred in two thirds of patients less than 1 year of age and more than age 40 years but in only 4% of patients between 1 year and 40 years of age.83 Many, if not most, neonates and infants with congestive heart failure have a coexisting ventricular septal defect or patent ductus arteriosus.98,99 In brief, more than 90% of infants and children with uncomplicated coarctation experience little or no difficulty.100
When coarctation is juxtaductal or proximal to the neonatal ductus, continuity between the pulmonary trunk and descending aorta is maintained, so the femoral arterial pulses remain palpable.6,96 When the ductus closes, the femoral pulses disappear, pulmonary blood flow is diverted into the lungs, the left ventricle is suddenly volume overloaded, and blood pressure and blood flow proximal to the coarctation suddenly increase. A high left atrial pressure opens the pliant valve of the foramen ovale, initiating a left-to-right shunt that imposes a volume load on the right ventricle that is already pressure overloaded because of pulmonary hypertension.101 Initial closure of the pulmonary arterial end of the ductus can leave the aortic end sufficiently patent to permit the proximal aorta to decompress.15 When the aortic end of the ductus closes, the isthmus is suddenly obstructed. Distal aortic pressure and flow fall, renal perfusion falls, and the renin-angiotensin system is activated.65,102
Rupture or dissection of the aorta is a dramatic complication with peak incidence in the third and fourth decades.83,85,94,97 The rupture originates either in a paracoarctation aneurysm47,48,94 or above a coexisting bicuspid aortic valve because of an inherent medial abnormality of the ascending aorta (see Chapter 7).48,94,97 Rupture of a postcoarctation aneurysm may be accompanied by bleeding into the esophagus that is announced by hematemesis and melena. In XO Turner’s syndrome (see section Physical Appearance), rupture or dissection of an ascending aortic aneurysm occurs because of an inherent medial abnormality,48 whether or not a coexisting coarctation or a bicuspid aortic valve exists (Figure 8-9).103–105 Aneurysms of intercostal arteries are almost always occult and relatively benign.49 Pseudocoarctation has been regarded as a disorder that incurs little or no risk, but the malformation is not necessarily benign and is not necessarily pseudo (see previous discussion). Pseudocoarctation has been reported with Turner’s syndrome.106 Cerebral arterial aneurysms are discussed in the section on cerebrovascular accidents (see subsequent discussion).
Infective endarteritis or endocarditis is a major complication of coarctation of the aorta, although the more susceptible site is a coexisting bicuspid aortic valve (see Chapter 7).83,97 Saccular septic aneurysms are occasional sequelae of infective endarteritis.94
Cerebrovascular accidents are the fourth major eventuality in coarctation of the aorta.83,93,107 Hypertension is not a necessary precondition because cerebral complications can occur with normotensive conditions long after successful repair. An aneurysm of the circle of Willis, first described in 1927,108 is the chief offender (see Figure 8-7) and sets the stage for rupture and cerebral hemorrhage.109 Less common, but not less important, are aneurysms in other cerebral arteries.93 Infective endocarditis on a bicuspid aortic valve can give rise to septic cerebral aneurysms that rupture. Rarely and oddly, an unruptured intracranial aneurysm triggers musical hallucinations.110
Coarctation hypertension is a risk factor for premature atherosclerotic coronary artery disease (see Figure 8-4B; see previous discussion).83,111,112 Evidence also exists of structural abnormalities of terminal intramural coronary arteries.111 Gonadal dysgenesis of Turner’s syndrome reportedly increases atherosclerotic cardiovascular risk.113
Physical Appearance
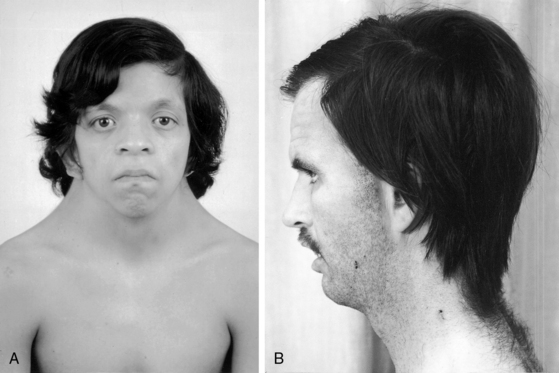
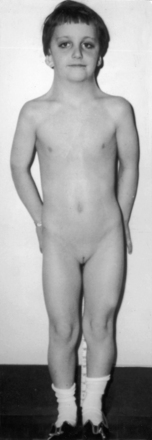
XO Turner’s syndrome with its distinctive physical appearance (Figure 8-10) arouses suspicion of coarctation of the aorta.114,115 Chromosomal patterns 45 XO and X-mosaicism are coupled with additional cardiac expressions.116–119 Aortic rupture or dissection in Turner’s syndrome was mentioned previously (see Figure 8-9).103,104,120 The typical XO Turner’s phenotypic female (Figure 8-11) is of short stature with webbing of the neck, absent or scanty auxiliary and pubic hair (ovarian dysgenesis), broad chest with widely spaced hypoplastic or inverted nipples, low anterior and posterior hairlines, small chin, prominent ears because of large auricles, cubitus valgus, short fourth metacarpals and metatarsals, distal palmar triaxial radii, narrow hyperconvex nails, and pigmented cutaneous nevi.115 Infants with Turner’s syndrome exhibit lymphedema of the neck with loose skin, puffiness of the dorsum of the hands and feet, and low hairlines.115,121 Congenital heart disease is much more frequent in Turner’s syndrome with webbing of the neck than in Turner’s syndrome without webbing,116,121 and coarctation of the aorta is eight times as frequent when Turner’s syndrome is accompanied by webbing of the neck.121 Noonan’s syndrome (Turner’s phenotype with normal genotype; see Figure 8-10) is only occasionally accompanied by coarctation of the aorta122 and rarely by ascending aortic aneurysm.95,115,123 Systemic hypertension in Turner’s syndrome occurs without coarctation. Another distinctive physical appearance associated with coarctation of the aorta is the PHACE syndrome124: P, posterior fossa brain malformations; H, hemangioma of the head or neck; A, arterial abnormalities of the head or neck; C, cardiac abnormalities, including coarctation of the aorta; E, eye abnormalities.
Arterial Pulse
Abnormal differences in upper and lower extremity arterial pulses and blood pressure are hallmarks of coarctation of the aorta. If proper attention were paid to these pulses, few or no diagnoses would be missed.90,125 Two methods have been advocated for comparison of upper and lower extremity arterial pulsations. One method compares femoral and radial pulses; the other method compares femoral and brachial pulses. Normally, radial and femoral pulses are sensed as synchronous, so that any femoral delay is considered abnormal. Positioning the patient’s wrist next to the groin is believed to facilitate comparison. In infants and newborns, the brachial artery is more readily palpated than the tiny radial artery, which is surrounded by subcutaneous fat pads (Figure 8-12B). The author’s preference is comparative palpation of brachial and femoral pulses, which is accomplished with placing a thumb on each (Figure 8-12A).125 The slight delay in perceived arrival time of the normal femoral pulse (15 msec in adults, less in infants and children) is easily sensed as a norm against which even slight deviations can be judged. With palpation of the femoral pulse in an infant, the patient must be allowed to relax the legs voluntarily because forceful restraint can decrease or abolish a normal femoral pulse.
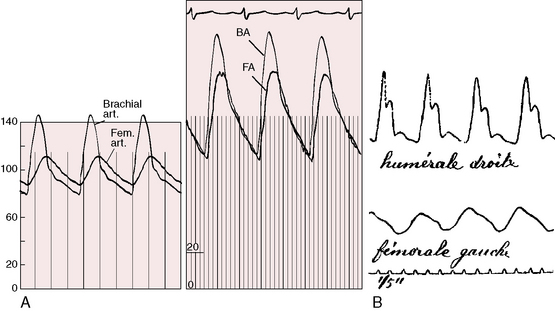
What is perceived as an abnormal delay of the femoral pulse in coarctation is not a delay in arrival time but instead a slow rate of rise to a delayed peak (Figure 8-13). The presence of a normal femoral pulse effectively eliminates all but mild coarctation, provided the aortic valve is functioning normally (see subsequent discussion). Palpation begins with applying to the brachial and femoral arterial pulses the amount of digital pressure necessary to elicit the maximal systolic impact.125 Stiffness of the precoarctation aortic wall results in disproportionate systolic hypertension and forceful carotid and suprasternal notch pulsations, which are sensed by the patient and become increasingly apparent with age and exercise (see previous discussion).59,61,62
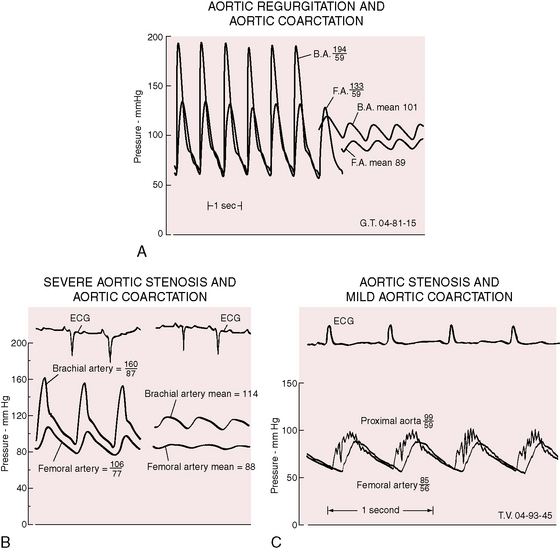
Bicuspid aortic regurgitation reinforces the femoral pulse, which can be misjudged unless properly compared with the brachial pulse (Figure 8-14A).126 Bicuspid aortic stenosis may not dampen the brachial arterial pulse because coarctation amplifies the ascending aortic and brachial arterial systolic pressures (Figure 8-14B).126 Conversely, if aortic stenosis dampens the ascending aortic systolic pressure, evidence of coarctation is obscured (Figure 8-14C).126
The right and left brachial arteries should be compared with palpation and with cuff blood pressure (see Figure 8-12B). A decreased or absent left brachial arterial pulse indicates that the coarctation has compromised the lumen of the left subclavian artery (see Figure 8-1B) or is proximal to its origin (see previous discussion). In comparison of the two brachial pulses, account must be taken of normal systolic pressure differences of 10 to 15 mm Hg lower in the left arm.125 Diminution or absence of the right brachial pulse implies that the right subclavian artery originates distal to the coarctation (see Figure 8-1C). Absence of both brachial arterial pulses signifies that coarctation is compromising the lumen of the left subclavian artery and that the right subclavian artery arises distal to the coarctation.127 At birth, femoral arterial pulses are temporarily palpable if the coarctation is juxtaductal, if the ductus is distal to the coarctation, or if the aortic end of the ductus is patent.6 The abdominal aorta is readily palpable proximal to abdominal coarctation but is not palpable when coarctation is in the aortic isthmus. Pseudocoarctation leaves the arterial pulses normal because there is no aortic obstruction, although angulation at the level of the ligamentum arteriosum occasionally dampens the femoral pulses (see Figure 8-5).22
Collateral arterial pulsations are specifically sought with patients who are old enough standing and bending forward with arms hanging at the sides while the examiner scrutinizes the patient’s back, especially around and between the scapulae.128 A tangential light in a darkened room highlights subcutaneous collaterals in shadowed relief. Collateral arteries occasionally appear around the shoulders, along the right and left sternal borders, and rarely over the upper abdominal wall.
Cuff blood pressure is an important supplement to palpation of the arterial pulses. Blood pressure should be taken in the right arm, left arm, and leg. Correct cuff size is essential because improper size results in inaccurate readings.125,129–133 An undersized cuff overestimates and an oversized cuff underestimates intraarterial blood pressure by as much as 10 to 30 mm Hg.133 The American Heart Association recommendations for cuff size130 are shown in Table 8-1. References to cuff size apply to the width and length of the inner inflatable bladder, not to the cloth covering. Cuff size is determined by limb circumference, not by patient age. The width of the inflatable bladder within the cuff should be 40% of the circumference of the midpoint of the limb. The length of the inflatable bladder must be sufficient to encircle the limb without overlapping.129,130
Table 8-1 Recommended Bladder Dimensions for Blood Pressure Cuff
| Arm Circumference at Midpoint* (cm) | Bladder Width (cm) | Bladder Length (cm) |
|---|---|---|
| 5-7.5 Newborn | 3 | 5 |
| 7.5-13 Infant | 5 | 8 |
| 13-20 Child | 8 | 13 |
| 17-26 Small adult | 11 | 17 |
| 24-32 Average adult | 13 | 24 |
| 32-42 Large adult | 17 | 32 |
| 42-50 Thigh | 20 | 42 |
* Midpoint is defined as half the distance from the acromion to the olecranon.
From Kirkendall WM, Feinleib M, Freis ED, Mark AL. AHA committee report: recommendations for human blood pressure determinations by sphygmomanometers. Circulation 1980;62:1146A. Reprinted with permission of the American Heart Association.
The examining room should be quiet, and the patient must be comfortable and reassured. Sufficient time should elapse for recovery from recent activity and tension. When an infant is quieted with a bottle or a pacifier, blood pressure can be obtained with the usual auscultatory method. An alternative is the flush technique, which requires two examiners.129 An uninflated cuff is applied to the infant’s forearm, which is elevated while the limb distal to the cuff is massaged to induce blanching. The cuff is inflated above anticipated systolic pressure while the arm remains elevated and blanched. The arm is then slowly lowered into a horizontal position while the cuff is slowly deflated. The point at which the blanched hand becomes flushed is an estimate of the mean arterial pressure. Doppler ultrasound or oscillometric techniques can be used by a single examiner and accurately determine systolic and diastolic blood pressure.90,129
Upper extremity blood pressure in infants and adults is usually recorded in the supine position, but a comfortable sitting position in a parent’s lap is acceptable for relaxation of infants and young children. Lower extremity blood pressure is best determined from the popliteal artery with the patient prone.125 While the popliteal artery is palpated, a thigh cuff is applied and must be inflated slowly to avoid discomfort. The cuff is inflated to a level just above the brachial arterial systolic pressure until the popliteal pulse vanishes. Systolic and diastolic pressures are then estimated with auscultation of popliteal Korotkoff’s sounds. Diagnostic differences in arm and leg blood pressures are based on systolic levels when only systolic Korotkoff’s sounds are heard. However, the diastolic pressures are important in confirmation of accuracy because they are not significantly different in the upper and lower extremities (see Figure 8-13). When intraarterial systolic, diastolic, and mean brachial and femoral pressures are compared in healthy persons, no significant difference is found, although the femoral auscultatory systolic pressure is about 10 mm Hg higher than the direct femoral arterial pressure.133 Differences between arm and leg systolic pressures in coarctation are exaggerated by exercise, which causes an increase in brachial arterial pressure, with no change or a reduction in femoral arterial pressure.134
Measurements of blood pressure should be a routine part of the physical examination after infancy and are desirable in infants at least once.90,135 Normal arterial pressure is based on age and gender.129,136,137 The average systolic blood pressure in females rises to a plateau at around age 14 years and remains almost constant during the early reproductive years.136 The average systolic blood pressure in males may not plateau until around age 20 years.129,136,137 Diastolic pressure differences are in the same direction but smaller in degree.
From birth to 6 months, normal upper extremity blood pressure averages 80/45 mm Hg. From 2 years to puberty, the average upper extremity blood pressure is 90/60 to 100/60 mm Hg,129,136 with the diastolic pressure more consistent than the systolic pressure. Coarctation of the aorta is accompanied by a progressive age-related increase in blood pressure, especially systolic, so the pulse pressure widens.61,73
In pregnant females with coarctation, blood pressure fluctuations are similar in direction to fluctuations in uncomplicated pregnancy but occur from a higher baseline.138 Coarctation is not a resistance vessel disease, so the incidence rate of toxemia is lower than in pregnant women with other forms of hypertension.138,139 Gestational hypertension is related to a significant coarctation gradient.140 Pregnancy increases the risk of aortic rupture and intracranial hemorrhage because gestational changes in connective tissue reinforce the medial abnormalities inherent in the arterial walls of coarctation.48,138 In abdominal coarctation, hypertension is likely to be caused by renal artery stenosis rather than by the coarctation.20
Precordial Movement and Palpation
The left ventricular impulse varies from normal to the sustained heaving impulse of pressure overload hypertrophy.125 In symptomatic infants with biventricular failure, the right ventricular impulse is dynamic because the pressure overload of pulmonary hypertension is coupled with volume overload of a right-to-left shunt through the foramen ovale. Suprasternal notch systolic thrills are frequent in uncomplicated coarctation, but precordial thrills are generated by coexisting bicuspid aortic stenosis.
Auscultation
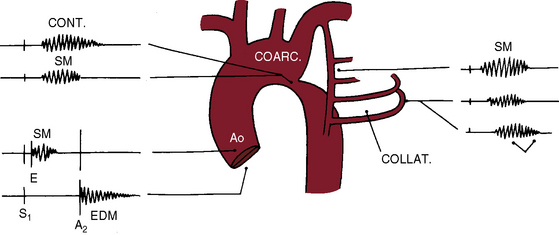
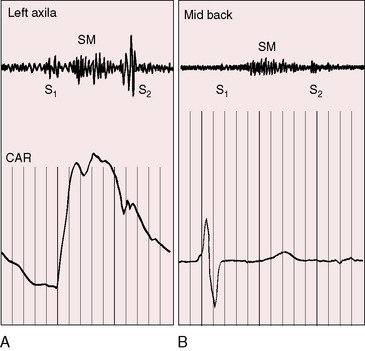
Coarctation of the aorta is associated with systolic, diastolic, and continuous murmurs (Figure 8-15). An ejection sound is an auscultatory sign of a coexisting bicuspid aortic valve (Figures 8-15 and 8-16). Systolic murmurs originate from three sources (see Figure 8-15): (1) arterial collaterals; (2) the coarctation itself; and (3) the brachiocephalic arteries. That murmurs can originate in collateral arteries is based on observations that localized superficial collateral murmurs are abolished by compression and that murmurs have been recorded from the surfaces of surgically exposed collateral arteries.141 A murmur cannot be generated across coarctation when obstruction is complete (see Figure 8-3B), but murmurs are prominent because arterial collaterals are well developed (see Figure 8-3A,C).141 The widespread anatomic distribution of the collateral arterial circulation accounts for the widespread thoracic distribution of accompanying murmurs.142 Collateral murmurs are crescendo-decrescendo and are delayed in onset and termination because they originate at a distance from the heart (Figures 8-15, 8-17, and 8-18).141 Widely distributed thoracic murmurs constitute presumptive evidence of collateral circulation, but in young children, collaterals may be well-developed without generating murmurs and in occasional adults with abundant collaterals, murmurs are inconspicuous.
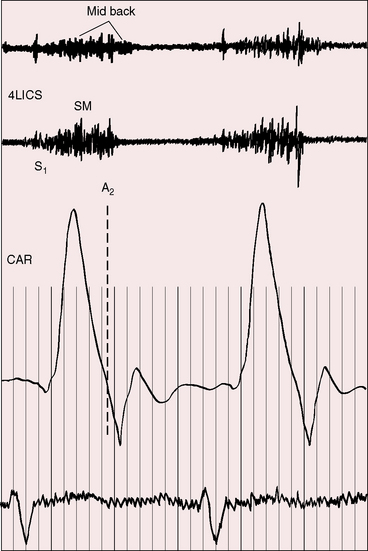
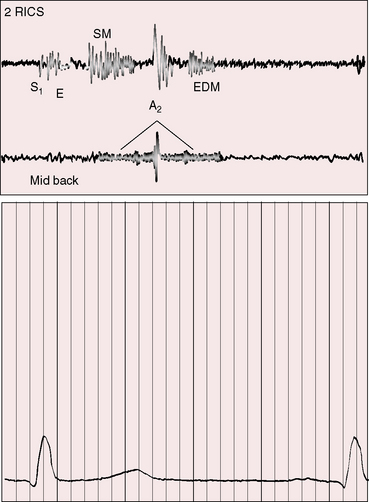
Figure 8-17 Tracings from a 22-year-old man with coarctation of the aorta. The phonocardiogram from the mid back over the site of coarctation shows a systolic murmur that is delayed in onset and continues into diastole (paired arrows). The systolic murmur in the fourth left intercostal space (4LICS) originated in a left internal mammary collateral (see Figure 8-3). (CAR = carotid.)
The coarctation itself is responsible for a systolic murmur141 that correlates with the size of the coarctation, and the localized posterior position over the thoracic spine correlates with the coarctation site (see Figures 8-15 and 8-18B). Typical isthmic coarctation generates a posterior murmur at the level of the fourth or fifth thoracic spinous process (see Figures 8-15 through 8-18).141 In infants, murmurs are typically absent, except in this posterior location. Heart failure causes the coarctation murmur to decrease or disappear.
Infants are best examined prone, and older patients are examined prone or in a relaxed sitting position that minimizes muscle tremor. Posterior auscultation begins in the midline at the upper thoracic spine and descends to the midthoracic and lumbar spine. The murmur overlying a coarctation is soft and high-frequency, so detection is best achieved with the stethoscopic diaphragm or firm pressure of the bell. As severity increases, the short posterior thoracic systolic murmur lenthens (see Figures 8-15 and 8-18B) and continues into diastole (see Figures 8-15 through 8-17). When the coarctation diameter decreases to 2.5 mm, the murmur occupies all of the cardiac cycle.141 The continuous murmur is soft and high-frequency, so assessment requires a quiet room and a cooperative patient. Rarely, the coarctation murmur is loud enough to radiate to the anterior chest.141 A loud brachiocephalic systolic murmur accompanied by a thrill is heard in the suprasternal notch, and prominent systolic murmurs are heard over the right and left subclavian arteries. A short low-frequency to medium-frequency diastolic murmur over the left ventricular impulse is caused by a decrease in interpapillary distance, which culminates in the single papillary muscle of a parachute mitral valve (see previous discussion; see Chapter 9).37 The murmur of abdominal coarctation is heard anteriorly in the epigastrium or just below and posteriorly over the lower thoracic or lumbar spine.20 Pseudocoarctation generates a systolic murmur posteriorly over the site of the aortic kink (see Figure 8-5).23,143
The second heart sound in coarctation of the aorta is either single or normally split with increased intensity of the aortic component. A loud single second sound in the second left interspace is the result of augmented aortic valve closure (see Figure 8-16). In symptomatic infants with pulmonary hypertension, the loud single second sound is the summation of aortic and pulmonary components.
Electrocardiogram
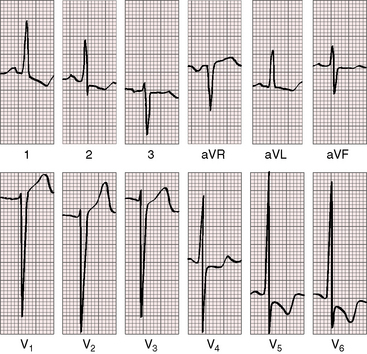
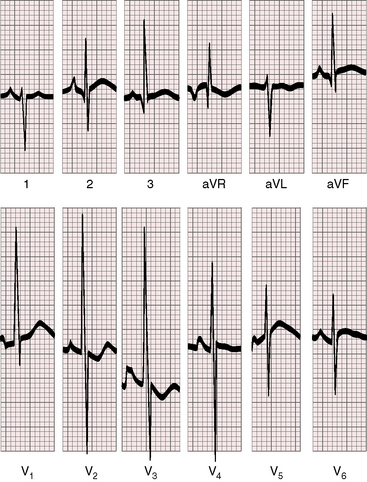
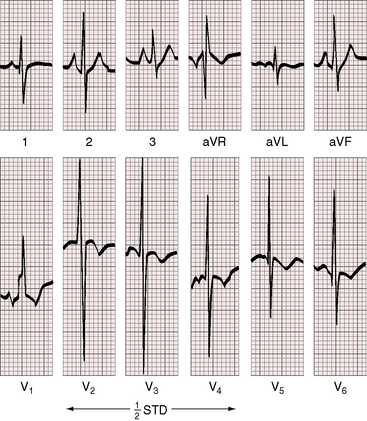
Electrocardiographic patterns are related to two age-groups: namely, symptomatic neonatal coarctation and coarctation after childhood. Left atrial P wave abnormalities occur in adults (Figure 18-19), and right atrial P wave abnormalities occur in symptomatic infants (Figure 8-20). The mean QRS axis in adults is normal, although axis is occasionally leftward in older patients (Figure 8-21). Right axis deviation with right ventricular hypertrophy is typical of the electrocardiogram in symptomatic infants with right ventricular pressure and volume overload (see previous discussion). Right ventricular hypertrophy that persists beyond infancy is rare in uncomplicated coarctation.
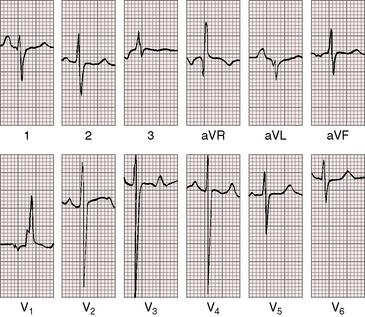
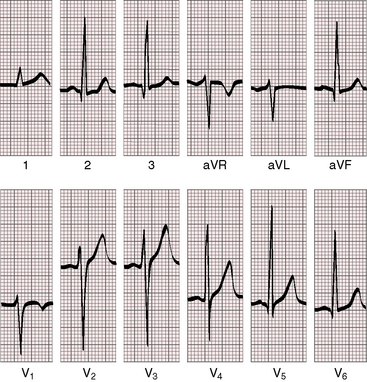
Figure 8-20 Electrocardiogram from a 6-year old-boy with coarctation of the aorta and congestive heart failure in infancy (see Figure 8-18). The QRS axis is rightward. There is a tall right atrial P wave in lead 1. Right ventricular hypertrophy is manifested by a tall monophasic R wave in led V1 and deep S waves in left precordial leads.
Left ventricular hypertrophy is characterized by tall R waves and low, flat, or inverted T waves in left precordial leads (see Figure 8-19). Prominent coved ST segment depressions with deeply inverted T waves are exceptional (see Figure 8-21) and imply coexisting bicuspid aortic stenosis. Prominent left precordial Q waves suggest the volume overload of bicuspid aortic regurgitation.
X-Ray
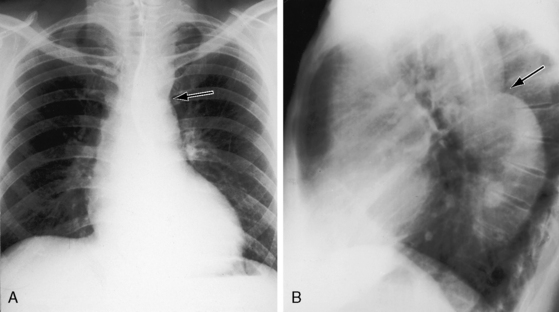
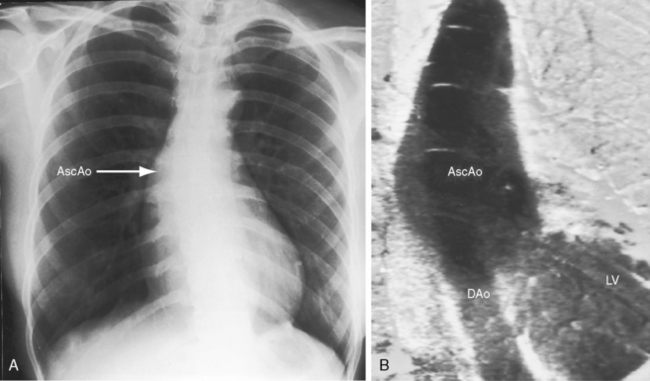
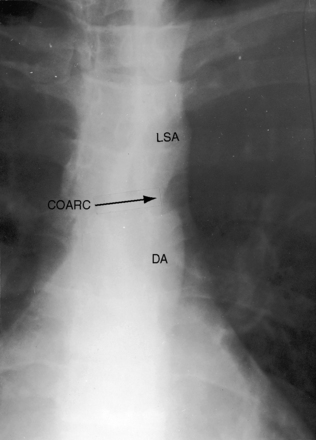
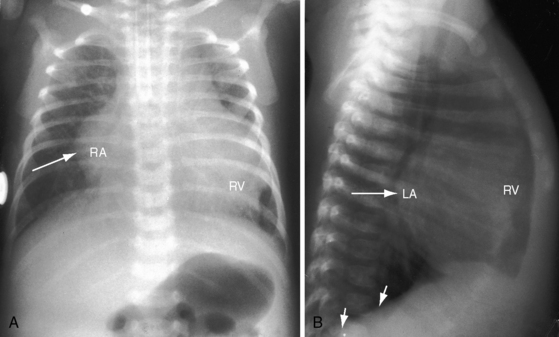
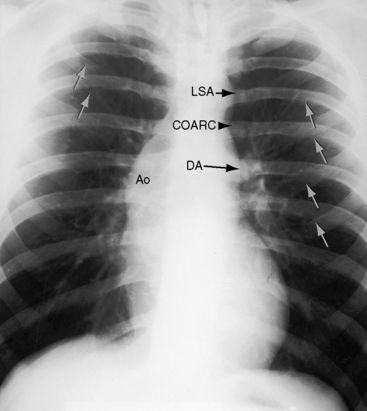
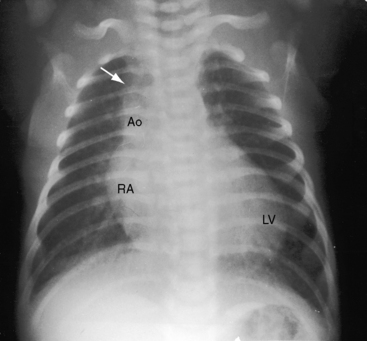
In asymptomatic infants and young children, the x-ray is normal. The descending thoracic aorta is a straight line that runs parallel to the left edge of the vertebral column, but in children and young adults, the postcoarctation descending thoracic aorta has a distinctive leftward convexity that is accompanied by dilation of the left subclavian artery (Figure 8-22).144 The x-ray in symptomatic infants discloses pulmonary venous congestion with dilation of the right ventricle and the right and left atria (Figure 8-23). Left ventricular size remains normal or nearly so.
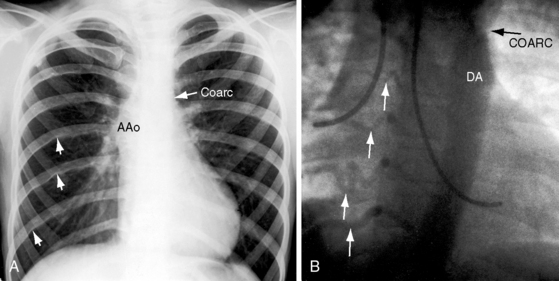
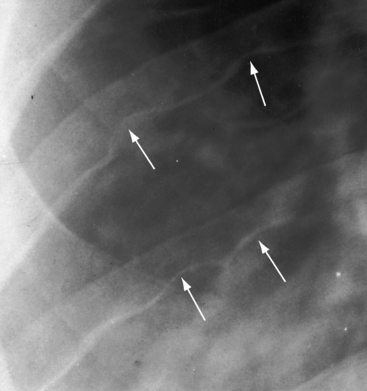
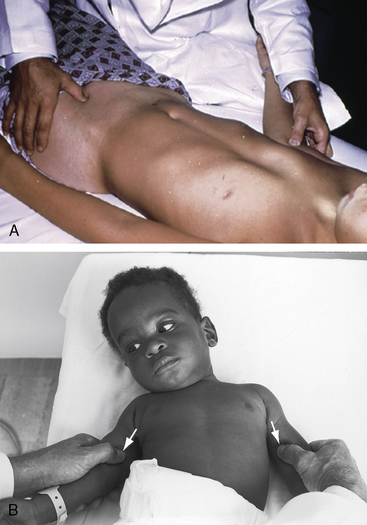
Notching of the ribs is a classic radiologic sign of coarctation caused by collateral flow through dilated, tortuous, pulsatile posterior intercostal arteries (Figures 8-3, 8-24, and 8-25). Notches vary from rib to rib and from patient to patient and may be single, multiple, shallow, deep, broad, or narrow.41,135 Notching originates in posterior intercostal arteries that run in intercostal grooves (see Figure 8-24), so the anterior ribs are spared because anterior intercostal arteries do not run in intercostal grooves.135,145 Rarely, the superior margin of a rib is notched because of contact with an overhanging intercostal artery.146 Rib notching seldom appears before age 6 years, although exceptional examples have been described as early as 2 years of age.41,145,147
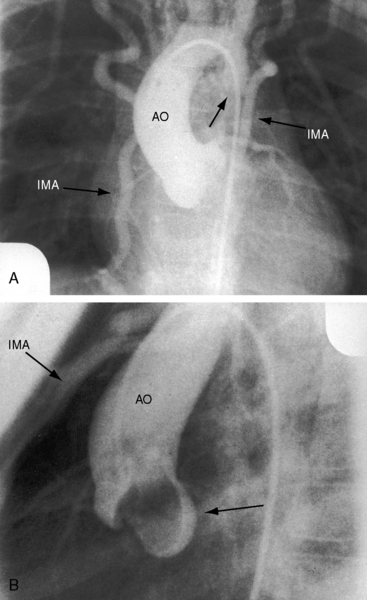
Typical coarctation distal to the left subclavian artery results in bilateral notching between the third and the eighth posterior ribs, but rarely above the third or below the ninth rib (Figures 8-25 and 8-26A).135 Anatomic variations of the coarctation site are accompanied by variations in the radiologic patterns. The development of arterial collaterals depends on patency of the ipsilateral subclavian artery; so when the left subclavian lumen is compromised, ipsilateral collaterals fail to develop, and unilateral rib notching is confined to the right hemithorax (Figure 8-27).135,145 Anomalous origin of the right subclavian artery distal to the coarctation (see Figure 8-1C) results in failure of collateral development in the right hemithorax, so unilateral notching is confined to the left hemithorax.92,145 Lateral x-rays show retrosternal notching or scalloping caused by dilated tortuous internal mammary arteries (see Figure 8-3A,B).41,147 When coarctation is in the abdominal aorta, notching, if present at all, is confined to the lower ribs.148
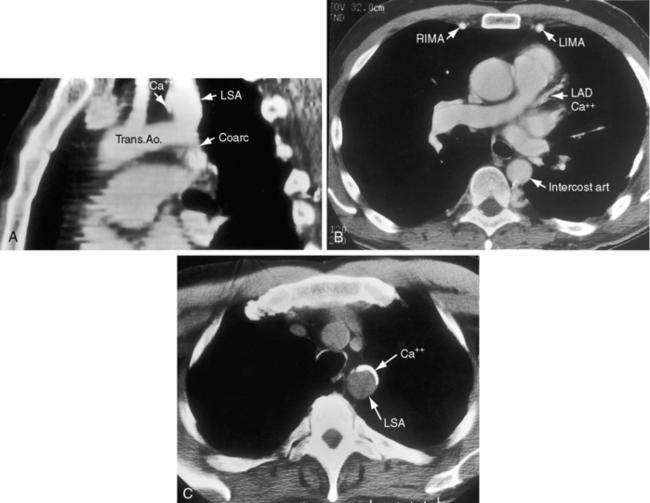
In older children and adults with coarctation, the ascending aorta is moderately to markedly dilated (see Figure 8-25).147 In XO Turner’s syndrome, the ascending aorta may be aneurysmal (see Figure 8-9). The proximal and distal paracoarctation aorta are typically dilated (see Figure 8-2).41,48,144 Postcoarctation aortic dilation is sometimes aneurysmal, and calcification is occasionally visible in the wall of the aneurysm. The combination of a dilated left subclavian artery proximal to the coarctation and a dilated aorta distal to the coarctation produces a figure 3 silhouette in the x-ray (see Figures 8-22 and 8-25).41,144 The mirror image of the figure three sign is seen when the left subclavian artery and descending aorta indent a barium esophagram (Figure 8-28).41,147 The left subclavian cannot dilate when its lumen is compromised by the coarctation, so dilation of the distal paracoarctation aorta exists alone (see Figure 8-27). A retroesophageal aberrant right subclavian artery is sometimes identified as a posterior indentation of the barium esophagram.135
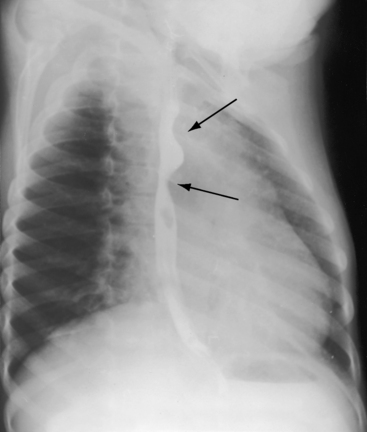
Figure 8-28 Right anterior oblique view from a 6-year-old boy with coarctation of the aorta. The barium esophagram exhibits the E sign (arrows) caused by compression from the dilated left subclavian artery above and the dilated descending aorta below. The E sign is the mirror image of the 3 sign (see Figure 8-25).
The kinked aorta of pseudocoarctation has a transverse arch and a descending aorta that form a large 3 sign above and below the kink (see Figure 8-5).24,77,143,149 Pseudocoarctation is conspicuous, but rib notching is conspicuous by its absence (see Figure 8-5).
Echocardiogram
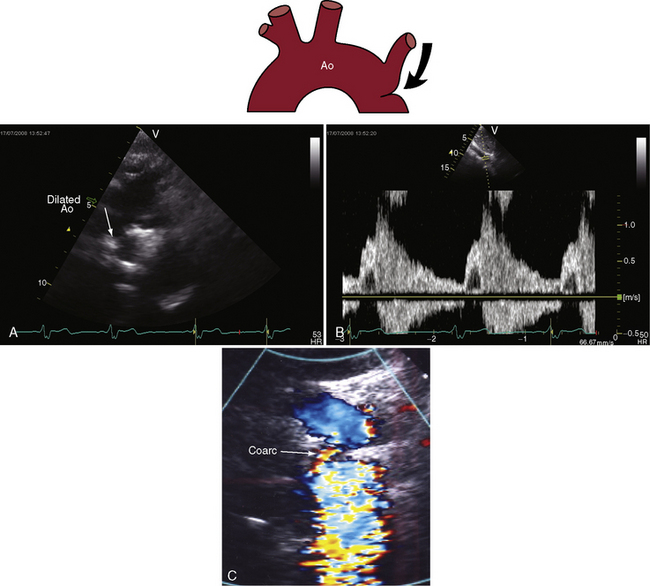
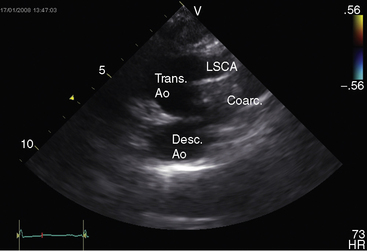
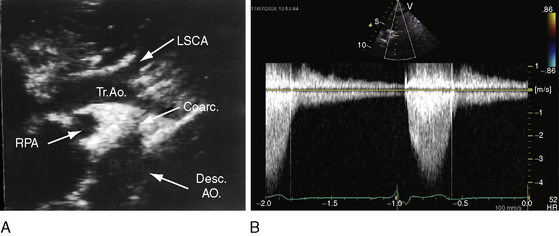
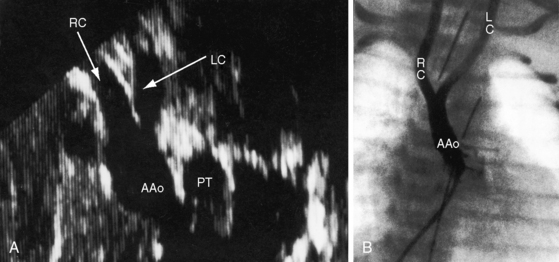
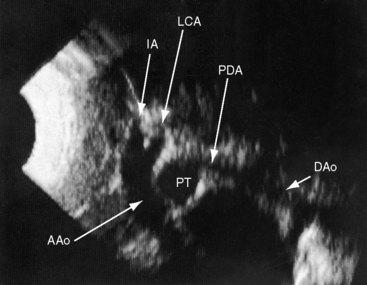
Transthoracic and transesophageal echocardiography with Doppler scan interrogation and color flow imaging (Figures 8-29 [and video 8-1], 8-30, and 8-31) permit segment-by-segment analysis of the ascending aorta, the aortic arch, the brachiocephalic arteries, the aortic isthmus, and the proximal descending aorta from birth to maturity. 150,151 A long segment of luminal narrowing can be identified. The suprasternal notch window with color flow imaging is identifies the coarctation by a zone of localized accelerated flow and by providing the target through which the continuous wave Doppler beam can be aligned to determine the gradient (see Figures 8-29A,B, 8-30, and 8-31).150,151 Peak systolic velocity reflects the maximal systolic gradient, and persistent high-velocity diastolic forward flow indicates severity (see Figures 8-29B and 8-31B).150–152 The Doppler pattern across the coarctation is affected by reduced proximal aortic compliance.153 Color flow identifies the level at which laminar flow becomes turbulent (see Figure 8-29C). An echocardiographic diagnosis of coarctation can be made in utero.12 In the fetus and neonate, normal tapering of the aortic isthmus9 can be mistaken for isthmic obstruction, and artifacts from the ductus insertion sometimes make interpretation difficult.151 The relationship between the site of coarctation and the aortic insertion of the ligamentum arteriosum establishes preductal, juxtaductal, or postductal location.151
Interruption of the aortic arch
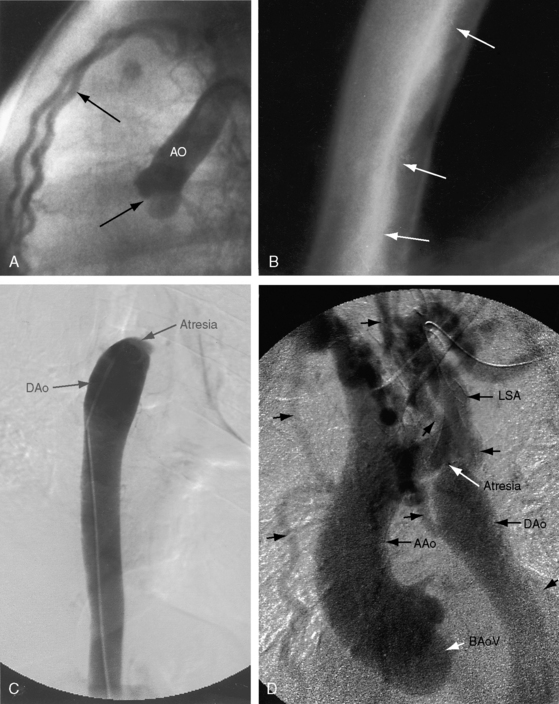
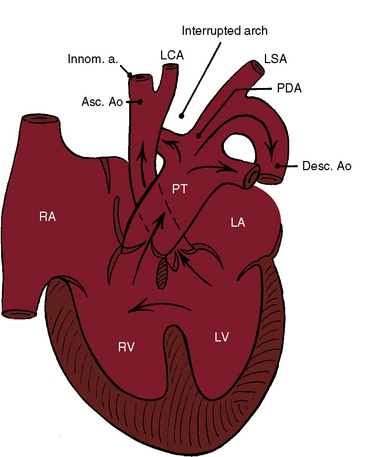
Interruption of the aortic arch was first described in the 18th century by Raphael Steidele154 of the University of Vienna. Steidele’s complex is occasionally applied as an eponym to this rare malformation,155,156 which is characterized by complete anatomic discontinuity or by an atretic fibrous strand between the aortic arch and the descending aorta (Figure 8-32).157–160 The classification of complete interruption by Celoria and Patton158 refers to one of three sites,155,156,160,161 two of which were described in 1927 by Maude Abbott.162 Interruption between the left common carotid and left subclavian arteries (type B; Figures 8-32 and 8-33) is more common than interruption distal to the left subclavian artery (type A). The least common site is between the innominate artery and the left carotid artery (type C; Figure 8-34).158,163,164 Subtypes are based on anomalous origin of the right subclavian artery.127,163,165 Rarely, interruption is in a right aortic arch.166
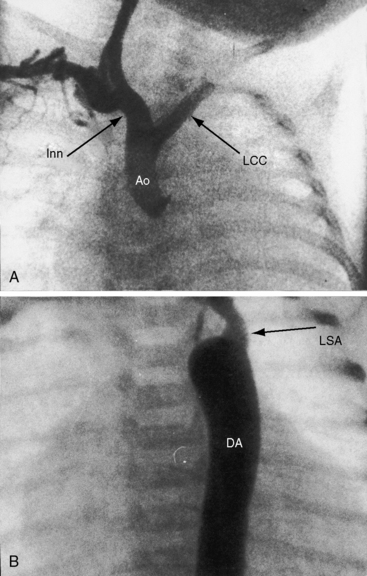
Figure 8-33 A, Aortogram from a female neonate with complete interruption of the aortic arch distal to the left common carotid artery (LCC). The innominate artery (Inn) gives rise to the right common carotid artery and to the right subclavian artery. B, Aortogram from a 3-day-old male with complete interruption of the aortic arch distal to the left common carotid artery. The left subclavian artery (LSA) originates from the descending aorta (DA). Compare with Figure 8-32. (Ao = ascending aorta.)
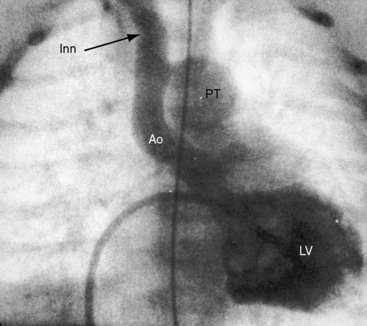
Figure 8-34 Left ventriculogram (LV) from a female neonate with complete interruption of the aortic arch distal to the innominate artery (Inn). The ascending aorta (Ao) ascends vertically and is small compared with the dilated pulmonary trunk (PT). The left common carotid artery and left subclavian artery originated from the descending aorta. Compare with Figure 8-32.
Interruption of the aortic arch seldom occurs as an isolated anomaly.167–170 Patent ductus arteriosus and ventricular septal defect are coexisting malformations on which tenuous neonatal survival depends. The clinical picture in infants is largely determined by patency of the ductus arteriosus, which provides nonrestrictive flow from the pulmonary trunk into the descending aorta (see Figure 8-32).155,159,160 In most patients with interrupted aortic arch, the ductus is histologically mature with prominent intimal cushions that prefigure constriction. In a minority of patients, an immature ductus is devoid of intimal cushions and has marked elastification that set the stage for persistent patency. A ventricular septal defect almost invariably coexists when interruption is between the left carotid and left subclavian arteries (type B) and also coexists in about 50% of interruptions distal to the left subclavian artery (type A).42,155,159–161,163,168 A posterior malaligned ventricular septal defect is the rule when the interrupted arch is between the left carotid and left subclavian arteries (type B).157,161,171 Defects in the perimembranous and muscular septum are uncommon.42 Posterior deviation of the infundibular septum encroaches on the left ventricular outflow tract and causes subaortic obstruction.42,161 When the ventricular septum is intact, a physiologically equivalent aortopulmonary window is almost always present,172 and the interrupted arch is distal to the left subclavian artery (type A).172
Theories on pathogenesis have focused on the role of reduced aortic flow during early morphogenesis, analogous to theories proposed for the pathogenesis of coarctation (see previous discussion).165,172 The malaligned ventricular septal defect in type B interruption encroaches on the left ventricular outflow tract, reducing aortic blood flow165 and favoring pulmonary blood flow.161,165 These anatomic arrangements are in keeping with the small caliber of the ascending aorta because of the paucity of aortic blood flow during fetal development.42 Unique vascular morphology of the fourth aortic arches has been implicated in the pathogenesis of type B interruption with anomalous right subclavian artery.173 An unresolved morphogenic point is the relatively rare occurrence of interruption between the innominate artery and the left common carotid artery (type C, 4% of cases), which implies that this segment of the arch is less vulnerable to decreased flow or depends on a separate developmental defect. Aortic arch interruption distal to the left subclavian artery (type A) and coarctation of the aorta are believed to be morphogenetically related.
The physiologic consequences of interrupted aortic arch with patent ductus arteriosus and ventricular septal defect consist of ascending aortic blood flow from the left ventricle and descending aortic blood from the pulmonary trunk through the ductus (see Figure 8-32).159,168 The left-to-right interventricular shunt is reinforced by resistance to left ventricular outflow caused by the malaligned ventricular septal defect. The magnitude of the right-to-left shunt through the ductus depends on the relative resistances in the pulmonary and systemic vascular beds. Constriction of the ductus is catastrophic, suddenly abolishing flow into the descending aorta and diverting virtually all pulmonary blood flow into the already overloaded lungs.174 Nevertheless, interruption or atresia of the aortic arch without a patent ductus is compatible with life,164,175 even adult survival,87,174,176,177 provided that both subclavian arteries arise from the descending aorta, an arrangement that potentially permits retrograde flow to the entire body except for the head.164,175,176 A variation on this theme is the congenital subclavian steal that occurs when both subclavian arteries arise distal to the interruption.52,178 Constriction of the ductus prompts a fall in pressure in the subclavian arteries and a steal into those arteries through collateral channels from ascending to descending aorta via the circle of Willis and the vertebral arteries.178
History
Complete interruption of the aortic arch is estimated to occur in 19 per million live births according to the New England Regional Infant Cardiac Program.179 Males and females are equally represented.155,156,160 Recurrence has been reported in siblings,180–183 and a 2.5% recurrence rate of congenital heart disease has been reported in siblings of patients with type B interruption.184
The typical clinical picture is represented by a neonate who becomes acutely ill as the ductus closes, prompting a sudden increase in pulmonary blood flow and a sudden decrease in circulation to the trunk and lower limbs.156,160,167 The result is shock, acidosis, renal failure, intracranial hemorrhage,174 and a mean survival time of 4 to 10 days.156,160,161 Three quarters of infants who do not undergo operation are dead within a month, with only 10% reaching their first birthday.155,160,185 Occasional survivals into childhood, adolescence, and young adulthood have been reported,168,175,176,184,186,187 with three patients surviving to their early or mid thirties.156,177,188 Interruption of the aortic arch or aortic arch atresia without patent ductus arteriosus or ventricular septal defect resembles severe coarctation.87,167,176 Rupture of an intracranial aneurysm was reported in a teenager189 and in an adult.190
Physical Appearance
Cyanosis is inconspicuous until the acute advent of congestive heart failure. Differential cyanosis caused by right-to-left ductal flow into the descending aorta is, with rare exception, canceled by the left-to-right shunt through the ventricular septal defect (see Figure 8-32),155,159,160,168 a mechanism that was recognized in 1852 by Greig,191 who wrote:
A 22q 11.2 deletion and the DiGeorge syndrome (see Chapter 28, Figure 28-6), which consists of hypoplastic mandible, defective ears, short philtrum, and aplasia or hypoplasia of the thymus and parathyroid glands, are associated with type A or type B interruption.108,170,192–195
Arterial Pulse
Femoral arterial pulses are maintained because of continuity between the pulmonary trunk, ductus, and descending aorta (see Figure 8-32).160,168,186 A reduction in femoral pulses signifies ductal constriction, which initially may be intermittent.168 When the left subclavian artery originates from the descending aorta (type B), ductal constriction reduces the left brachial pulse in addition to the femoral pulse. Anomalous origin of the right subclavian artery places the right brachial pulse in the distal low pressure zone.165 On the rare occasion of interruption of the aortic arch or arch atresia without patent ductus or ventricular septal defect, palpation of the systemic arterial pulses is analogous to palpation in coarctation of the aorta.127,167–169,175,176 When interruption occurs proximal to both subclavian arteries (anomalous origin of the right subclavian), the carotid pulses are bounding, but the brachial and femoral pulses are diminished or absent.127,169,176 If either or both subclavian arteries originate proximal to the interruption, the arms are hypertensive, the carotid pulses are bounding, and the femoral pulses are weak or absent.167,176
Precordial Movement and Palpation
An isolated right ventricular impulse is palpated in infants with interruption of the aortic arch because of obligatory pulmonary hypertension and is associated with a nonrestrictive patent ductus arteriosus and ventricular septal defect. A left ventricular impulse is absent despite the left-to-right shunt at ventricular level because blood entering the pulmonary trunk is diverted through the ductus into the descending aorta rather than through the pulmonary circulation into the left side of the heart (see Figure 8-32). Interruption of the aortic arch without patent ductus or ventricular septal defect results in a left ventricular impulse analogous to coarctation of the aorta.176
Auscultation
Murmurs are negligible or absent.160 The ductus is silent because the shunt is entirely right-to-left through a single nonrestrictive arterial conduit formed by continuity of the pulmonary trunk, ductus, and descending aorta. Murmurs originating through the ventricular septal defect are early systolic, decrescendo, and grade 3/6 or less.186,196 A continuous murmur has been attributed to collateral circulation that connects the ascending and descending aortas.186 Diastolic murmurs from pulmonary hypertensive pulmonary regurgitation160 are introduced by a loud pulmonary component of the second heart sound. Interruption of the aortic arch without a ductus or ventricular septal defect presents with auscultatory signs similar to those of imperforate coarctation, with widespread collateral murmurs that include the head, especially behind the ears.167,176,197
Electrocardiogram
Peaked right atrial P waves and right ventricular hypertrophy are electrocardiographic features in the neonate with interruption of the aortic arch, ventricular septal defect, and patent ductus arteriosus (Figure 8-35).196 Biventricular hypertrophy is in response to left ventricular volume overload and persistent right ventricular pressure overload (Figure 8-36).160 Interruption without patent ductus or ventricular septal defect results in right ventricular hypertrophy in infants127 but left ventricular hypertrophy in older patients.
X-Ray
The cardiac silhouette enlarges and pulmonary venous congestion develops rapidly when closure of the ductus arteriosus suddenly causes an increase in pulmonary blood flow and volume overload of the left ventricle. The aortic knuckle is absent because the ascending aorta is small and ascends vertically (Figures 8-37 and 8-38).163 The trachea is not deviated by an aortic arch and is therefore midline.163 The x-ray shows increased pulmonary venous and pulmonary arterial vascularity with enlargement of the left ventricle (Figures 8-37 and 8-39).186,196 Isolated interruption of the aortic arch without a ductus or a ventricular septal defect is radiologically similar to coarctation of the aorta, including rib notching.164,167,197 Whether the notching is bilateral or unilateral depends on the site of interruption, the origin of the subclavian arteries, and the length of survival.163,164
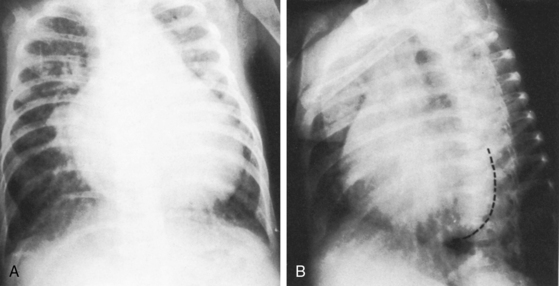
Figure 8-37 X-rays from the 6-month-old female whose electrocardiogram is shown in Figure 8-36. A, Prominent pulmonary soft tissue densities represent venous congestion in addition to increased pulmonary arterial vascularity. The pulmonary trunk is dilated in contrast to the inconspicuous ascending aorta, and the right atrium is markedly enlarged. B, In the left anterior oblique projection, the right and left ventricles are both enlarged.
Echocardiogram
Echocardiography with Doppler scan interrogation and color flow imaging is used to identify the major segments of the thoracic aorta, visualize the patent ductus and the ventricular septal defect, and distinguish interruption of the aortic arch from coarctation with tubular hypoplasia. In type C interruption, echocardiography is used to identify antegrade flow in the right carotid and basilar arteries and retrograde flow in the left carotid artery.198 Echocardiography is used to identify type B interruption of a right aortic arch.199
A normal aorta exhibits a continuous smooth curvature from its ascending to its descending portion, but when the arch is interrupted distal to the left carotid artery (type B), the ascending aorta rises vertically, exhibits little curvature, and bifurcates into the innominate and left carotid arteries,170 with the carotid appearing as an index finger pointing upward (see Figure 8-38). When the arch is interrupted distal to the left subclavian artery (type A), the ascending aorta exhibits a slight curvature and gives off three branches (the innominate, the left carotid, and the left subclavian arteries)170 because the right subclavian artery is prone to anomalous origin (Figure 8-40). A useful echocardiographic observation is the discrepancy between the small caliber of the ascending aorta and conspicuous dilation of the pulmonary trunk. The image created by continuity of pulmonary trunk, ductus, and descending aorta superficially resembles an uninterrupted aortic arch, but identification of the origins of the right and left pulmonary arterial branches, together with absence of brachiocephalic branches, prevents error (see Figure 8-40).170 Characterization of a ventricular septal defect is an important part of the echocardiographic examination. An intact ventricular septum with an interrupted aortic arch prompts search for an aortopulmonary window.170 A malaligned ventricular septal defect is associated with posterior and leftward deviation of the conal septum and varying degrees of subaortic narrowing.170,171,200
1 Jarcho S. Coarctation of the aorta (Meckel, 1750; Paris, 1791). Am J Cardiol. 1961;7:844-852.
2 Jarcho S. Coarctation of the aorta (Otto, 1824; Bertin, 1824). Am J Cardiol. 1961;8:843-845.
3 Jarcho S. Coarctation of the aorta (Reynaud, 1828). Am J Cardiol. 1962;9:591-597.
4 Jarcho S. Coarctation of the aorta (Legrand, 1833). Am J Cardiol. 1962;10:266-271.
5 Jarcho S. Aortic coarctation and aortic stenosis (Nixon 1834). Am J Cardiol. 1963;11:238-245.
6 Elzenga N.J., Gittenberger-De Groot A.C. Localised coarctation of the aorta. An age dependent spectrum. Br Heart J. 1983;49:317-323.
7 Kappetein A.P., Gittenberger-De Groot A.C., Zwinderman A.H., Rohmer J., Poelmann R.E., Huysmans H.A. The neural crest as a possible pathogenetic factor in coarctation of the aorta and bicuspid aortic valve. J Thorac Cardiovasc Surg. 1991;102:830-836.
8 Park H.K., Cho S.H., Park Y.-H. Atypical coarctation of the aorta: congenital stenosis of the mid-thoracic aorta. J Am Coll Cardiol. 2009;53:2098.
9 Hornberger L.K., Weintraub R.G., Pesonen E., et al. Echocardiographic study of the morphology and growth of the aortic arch in the human fetus. Observations related to the prenatal diagnosis of coarctation. Circulation. 1992;86:741-747.
10 Langille B.L., Brownlee R.D., Adamson S.L. Perinatal aortic growth in lambs: relation to blood flow changes at birth. Am J Physiol. 1990;259:H1247-H1253.
11 Morrow W.R., Huhta J.C., Murphy D.J.Jr, Mcnamara D.G. Quantitative morphology of the aortic arch in neonatal coarctation. J Am Coll Cardiol. 1986;8:616-620.
12 Allan L.D., Chita S.K., Anderson R.H., Fagg N., Crawford D.C., Tynan M.J. Coarctation of the aorta in prenatal life: an echocardiographic, anatomical, and functional study. Br Heart J. 1988;59:356-360.
13 Allan L.D., Crawford D.C., Tynan M. Evolution of coarctation of the aorta in intrauterine life. Br Heart J. 1984;52:471-473.
14 Rudolph A.M., Heymann M.A., Spitznas U. Hemodynamic considerations in the development of narrowing of the aorta. Am J Cardiol. 1972;30:514-525.
15 Talner N.S., Berman M.A. Postnatal development of obstruction in coarctation of the aorta: role of the ductus arteriosus. Pediatrics. 1975;56:562-569.
16 Mcmahon C.J., Vick G.W.3rd, Nihill M.R. Right aortic arch and coarctation: delineation by three dimensional magnetic resonance angiogram. Heart. 2001;85:492.
17 Bahabozorgui S., Nemir P.Jr. Coarctation of the abdominal aorta. Am J Surg. 1966;111:224-229.
18 Bergamini T.M., Bernard J.D., Mavroudis C., Backer C.L., Muster A.J., Richardson J.D. Coarctation of the abdominal aorta. Ann Vasc Surg. 1995;9:352-356.
19 Riemenschneider T.A., Emmanouilides G.C., Hirose F., Linde L.M. Coarctation of the abdominal aorta in children: report of three cases and review of the literature. Pediatrics. 1969;44:716-726.
20 Roques X., Bourdeaud’hui A., Choussat A., et al. Coarctation of the abdominal aorta. Ann Vasc Surg. 1988;2:138-144.
21 Bahabozorgui S., Bernstein R.G., Frater R.W. Pseudocoarctation of aorta associated with aneurysm formation. Chest. 1971;60:616-617.
22 Bilgic A., Ozer S., Atalay S. Pseudocoarctation of the aorta. Jpn Heart J. 1990;31:875-879.
23 Griffin J.F. Congenital kinking of the aorta (pseudocoarctation). N Engl J Med. 1964;271:726-728.
24 Smyth P.T., Edwards J.E. Pseudocoarctation, kinking or buckling of the aorta. Circulation. 1972;46:1027-1032.
25 Wang W.-B., Lin G.-M. Pseudocoarctation and coarctation. Int J Cardiol. 2009;133:e62-e64.
26 Schellhammer F., Von Den Driesch P., Gaitzsch A. Pseudocoarctation of the abdominal aorta. Vasa. 1997;26:308-310.
27 Joseph M., Leclerc Y., Hutchison S.J. Aortic pseudocoarctation causing refractory hypertension. N Engl J Med. 2002;346:784-785.
28 Yamada M., Horigome H., Ishii S. Pseudocoarctation of the aorta coexistent with coarctation. Eur J Pediatr. 1996;155:993.
29 Ikonomidis J.S., Robbins R.C. Cervical aortic arch with pseudocoarctation: presentation with spontaneous rupture. Ann Thorac Surg. 1999;67:248-250.
30 Safir J., Kerr A., Morehouse H., Frost A., Berman H. Magnetic resonance imaging of dissection in pseudocoarctation of the aorta. Cardiovasc Intervent Radiol. 1993;16:180-182.
31 Angelini G.D., Kulatilake E.N., Hayward M., Ruttley M.S. Pseudocoarctation of the aortic arch associated with bicuspid aortic valve lesion: is it a surgical entity? Thorac Cardiovasc Surg. 1985;33:36-37.
32 Mulder B.J.M., Van Der Wall E.E. Optimal imaging protocol for evaluation of aortic coarctation; time for a reappraisal. Int J Cardiovasc Imaging. 2006;22:695-697.
33 Abbott M.E. Coarctation of the aorta of adult type; statistical study and historical retrospect of 200 recorded cases with autopsy; of stenosis or obliteration of descending arch in subjects above age of two years. Am Heart J. 1928;3:574.
34 Folger G.M.Jr, Stein P.D. Bicuspid aortic valve morphology when associated with coarctation of the aorta. Cathet Cardiovasc Diagn. 1984;10:17-25.
35 Fernandes S.M., Sanders S.P., Khairy P., et al. Morphology of bicuspid aortic valve in children and adolescents. J Am Coll Cardiol. 2004;44:1648-1651.
36 Freed M.D., Keane J.F., Van Praagh R., Castaneda A.R., Bernhard W.F., Nadas A.S. Coarctation of the aorta with congenital mitral regurgitation. Circulation. 1974;49:1175-1184.
37 Celano V., Pieroni D.R., Morera J.A., Roland J.M., Gingell R.L. Two-dimensional echocardiographic examination of mitral valve abnormalities associated with coarctation of the aorta. Circulation. 1984;69:924-932.
38 Rosenquist G.C. Congenital mitral valve disease associated with coarctation of the aorta: a spectrum that includes parachute deformity of the mitral valve. Circulation. 1974;49:985-993.
39 Pasquini L., Fichera A., Tan T., Ho S.Y., Gardiner H. Left superior caval vein: a powerful indicator of fetal coarctation. Heart. 2005;91:539-540.
40 Oppenheimer E.H. The association of adult-type coarctation of the aorta with endocardial fibroelastosis in infancy. Bull Johns Hopkins Hosp. 1953;93:309-319.
41 Baron M.G. Radiologic notes in cardiology: obscuration of the aortic knob in coarctation of the aorta. Circulation. 1971;43:311-316.
42 Anderson R.H., Lenox C.C., Zuberbuhler J.R. Morphology of ventricular septal defect associated with coarctation of aorta. Br Heart J. 1983;50:176-181.
43 Moene R.J., Gittenberger-De Groot A.C., Oppenheimer-Dekker A., Bartelings M.M. Anatomic characteristics of ventricular septal defect associated with coarctation of the aorta. Am J Cardiol. 1987;59:952-955.
44 Smallhorn J.F., Anderson R.H., Macartney F.J. Morphological characterisation of ventricular septal defects associated with coarctation of aorta by cross-sectional echocardiography. Br Heart J. 1983;49:485-494.
45 Doyle L. Coarctation of the aorta with dissecting aneurysm and haemopericardium: an account by Joseph Jordan, Manchester, 1830. Thorax. 1991;46:268-269.
46 Celik T., Iyisoy A., Kursaklioglu H., et al. A large calcified saccular aneurysm in a patient with aortic coarctation. Int J Cardiovasc Imaging. 2006;22:93-95.
47 Isner J.M., Donaldson R.F., Fulton D., Bhan I., Payne D.D., Cleveland R.J. Cystic medial necrosis in coarctation of the aorta: a potential factor contributing to adverse consequences observed after percutaneous balloon angioplasty of coarctation sites. Circulation. 1987;75:689-695.
48 Niwa K., Perloff J.K., Bhuta S.M., et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393-400.
49 Wallace R.B., Nast E.P. Postcoarctation mycotic intercostal arterial pseudoaneurysm. Am J Cardiol. 1987;59:1014-1015.
50 Henderson R.A., Ward C., Campbell C. Dissecting left subclavian artery aneurysm: an unusual presentation of coarctation of the aorta. Int J Cardiol. 1993;40:69-70.
51 Shamsa K., Perloff J.K., Lee E., Wirthlin R.S., Tsui I., Schwartz S.D. Retinal vascular patterns after operative repair of aortic isthmic coarctation. Am J Cardiol. 2010;105:408-410.
52 Deeg K.H., Singer H. Dopplersonographic diagnosis of subclavian steal in infants with coarctation of the aorta and interrupted aortic arch. Pediatr Radiol. 1989;19:163-166.
53 Yu C.-H., Chen M.-R. Clinical investigation of systemic-pulmonary collateral arteries. Pediatr Cardiol. 2008;29:334-338.
54 Johns K.J., Johns J.A., Feman S.S. Retinal vascular abnormalities in patients with coarctation of the aorta. Arch Ophthal. 1991;109:1266-1268.
55 Granstrom K.O. Retinal changes in coarctation of the aorta. Br J Ophthalmol. 1951;35:143-148.
56 Chun K., Colombani P.M., Dudgeon D.L., Haller J.A.Jr. Diagnosis and management of congenital vascular rings: a 22-year experience. Ann Thorac Surg. 1992;53:597-602. discussion 602–593
57 Ettedgui J.A., Lorber A., Anderson D. Double aortic arch associated with coarctation. Int J Cardiol. 1986;12:258-260.
58 Brockmeier K., Demirakca S., Metzner R., Floemer F. Images in cardiovascular medicine. Double aortic arch. Circulation. 2000;102:E93-E94.
59 Sehested J., Baandrup U., Mikkelsen E. Different reactivity and structure of the prestenotic and poststenotic aorta in human coarctation. Implications for baroreceptor function. Circulation. 1982;65:1060-1065.
60 Brili S., Dernellis J., Aggeli C., et al. Aortic elastic properties in patients with repaired coarctation of aorta. Am J Cardiol. 1998;82:1140-1143. A1110
61 Igler F.O., Boerboom L.E., Werner P.H., et al. Coarctation of the aorta and baroreceptor resetting. A study of carotid baroreceptor stimulus-response characteristics before and after surgical repair in the dog. Circ Res. 1981;48:365-371.
62 O’rourke M.F., Cartmill T.B. Influence of aortic coarctation on pulsatle hemodynamics in the proximal aorta. Circulation. 1971;44:281-292.
63 Cumming G.R., Mir G.H. Exercise haemodynamics of coarctation of the aorta. Acute effects of propranolol. Br Heart J. 1970;32:365-369.
64 Earley A., Joseph M.C., Shinebourne E.A., De Swiet M. Blood pressure and effect of exercise in children before and after surgical correction of coarctation of aorta. Br Heart J. 1980;44:411-415.
65 Alpert B.S., Bain H.H., Balfe J.W., Kidd B.S., Olley P.M. Role of the renin-angiotensin-aldosterone system in hypertensive children with coarctation of the aorta. Am J Cardiol. 1979;43:828-834.
66 Warren D.J., Smith R.S., Naik R.B. Inappropriate renin secretion and abnormal cardiovascular reflexes in coarctation of the aorta. Br Heart J. 1981;45:733-736.
67 Perloff J.K. Normal myocardial growth and the development and regression of increased ventricular mass. In Perloff J.K., Child J.S., Aboulhosn J., editors: Congenital heart disease in adults, 3rd ed, Philadelphia: W.B. Saunders, 2009.
68 Borow K.M., Colan S.D., Neumann A. Altered left ventricular mechanics in patients with valvular aortic stenosis and coarction of the aorta: effects on systolic performance and late outcome. Circulation. 1985;72:515-522.
69 Gutgesell H.P., Barton D.M., Elgin K.M. Coarctation of the aorta in the neonate: associated conditions, management, and early outcome. Am J Cardiol. 2001;88:457-459.
70 Gough J.H. Coarctation of the aorta in father and son. Br J Radiol. 1961;34:670-672.
71 Moss A.J. Coarctation of the aorta in siblings. J Pediatr. 1955;46:707-709.
72 Rowen M.J. Coarctation of the aorta in father and son. Am J Cardiol. 1959;4:540-542.
73 Sehested J. Coarctation of the aorta in monozygotic twins. Br Heart J. 1982;47:619-620.
74 Simon A.B., Zloto A.E., Perry B.L., Sigmann J.M. Familial aspects of coarctation of the aorta. Chest. 1974;66:687-689.
75 Taylor R.R., Pollock B.E. Coarctation of the aorta in three members of a family. Am Heart J. 1953;45:470-475.
76 Beekman R.H., Robinow M. Coarctation of the aorta inherited as an autosomal dominant trait. Am J Cardiol. 1985;56:818-819.
77 Keller H.I., Cheitlin M.D. The occurrence of mild coarctation of the aorta (pseudocoarctation) and coarctation in one family. Am Heart J. 1965;70:115-118.
78 Leichtman D.A., Schmickel R.D., Gelehrter T.D., Judd W.J., Woodbury M.C., Meilinger K.L. Familial Turner syndrome. Ann Intern Med. 1978;89:473-476.
79 Miettinen O.S., Reiner M.L., Nadas A.S. Seasonal incidence of coarctation of the aorta. Br Heart J. 1970;32:103-107.
80 Hernandez F.A., Miller R.H., Schiebler G.L. Rarity of coarctation of the aorta in the American Negro. J Pediatr. 1969;74:623-625.
81 Van Der Horst R.L., Gotsman M.S. Racial incidence of coarctation of aorta. Br Heart J. 1972;34:289-294.
82 Becker A.E., Becker M.J., Edwards J.E. Anomalies associated with coarctation of aorta: particular reference to infancy. Circulation. 1970;41:1067-1075.
83 Liberthson R.R., Pennington D.G., Jacobs M.L., Daggett W.M. Coarctation of the aorta: review of 234 patients and clarification of management problems. Am J Cardiol. 1979;43:835-840.
84 Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970;32:633-640.
85 Reifenstein G.H., Levine S.A., Gross R.E. Coarctation of the aorta; a review of 104 autopsied cases of the adult type, 2 years of age or older. Am Heart J. 1947;33:146-168.
86 Miro O., Jimenez S., Gonzalez J., De Caralt T.M., Ordi J. Highly effective compensatory mechanisms in a 76-year-old man with a coarctation of the aorta. Cardiology. 1999;92:284-286.
87 Milo S., Massini C., Goor D.A. Isolated atresia of the aortic arch in a 65-year-old man. Surgical treatment and review of published reports. Br Heart J. 1982;47:294-297.
88 Barili F., Biglioli P., Polvani G. A case of asymptomatic high-grade aortic coarctation. Heart. 2008;94:82.
89 Strafford M.A., Griffiths S.P., Gersony W.M. Coarctation of the aorta: a study in delayed detection. Pediatrics. 1982;69:159-163.
90 Ward K.E., Pryor R.W., Matson J.R., Razook J.D., Thompson W.M., Elkins R.C. Delayed detection of coarctation in infancy: implications for timing of newborn follow-up. Pediatrics. 1990;86:972-976.
91 Braimbridge M.V., Yen A. Coarctation in the elderly. Circulation. 1965;31:209-218.
92 Silander T. Anomalous origin of the right subclavian artery and its relation to coarctation of the aorta. Acta Chir Scand. 1962;124:412-418.
93 Schievink W.I., Mokri B., Piepgras D.G., Gittenberger-De Groot A.C. Intracranial aneurysms and cervicocephalic arterial dissections associated with congenital heart disease. Neurosurgery. 1996;39:685-689. discussion 689–690
94 Edwards J.E. Aneurysms of the thoracic aorta complicating coarctation. Circulation. 1973;48:195-201.
95 Shachter N., Perloff J.K., Mulder D.G. Aortic dissection in Noonan’s syndrome (46 XY turner). Am J Cardiol. 1984;54:464-465.
96 Graham T.P.Jr, Atwood G.F., Boerth R.C., Boucek R.J.Jr, Smith C.W. Right and left heart size and function in infants with symptomatic coarctation. Circulation. 1977;56:641-647.
97 Baylis J.H., Campbell M. The course and prognosis of coarctation of the aorta. Br Heart J. 1956;18:475-495.
98 Graham T.P.Jr, Burger J., Boucek R.J.Jr, et al. Absence of left ventricular volume loading in infants with coarctation of the aorta and a large ventricular septal defect. J Am Coll Cardiol. 1989;14:1545-1552.
99 Shinebourne E.A., Tam A.S., Elseed A.M., Paneth M., Lennox S.C., Cleland W.P. Coarctation of the aorta in infancy and childhood. Br Heart J. 1976;38:375-380.
100 Hesslein P.S., Gutgesell H.P., Mcnamara D.G. Prognosis of symptomatic coarctation of the aorta in infancy. Am J Cardiol. 1983;51:299-303.
101 Jentsch E., Liersch R., Bourgeois M. Prolapsed valve of the foramen ovale in newborns and infants with coarctation of the aorta. Pediatr Cardiol. 1988;9:29-32.
102 Bailie M.D., Donoso V.S., Gonzalez N.C. Role of the renin-angiotensin system in hypertension after coarctation of the aorta. J Lab Clin Med. 1984;104:553-562.
103 Anabtawi I.N., Ellison R.G., Yeh T.J., Hall D.P. Dissecting aneurysm of aorta associated with Turner’s syndrome. J Thorac Cardiovasc Surg. 1964;47:750-754.
104 Lie J.T. Aortic dissection in Turner’s syndrome. Am Heart J. 1982;103:1077-1080.
105 Lin A.E., Lippe B.M., Geffner M.E., et al. Aortic dilation, dissection, and rupture in patients with Turner syndrome. J Pediatr. 1986;109:820-826.
106 Klein L.W., Levin J.L., Weintraub W.S., Agarwal J.B., Helfant R.H. Pseudocoarctation of the aortic arch in a patient with Turner’s syndrome. Clin Cardiol. 1984;7:621-623.
107 Shearer W.T., Rutman J.Y., Weinberg W.A., Goldring D. Coarctation of the aorta and cerebrovascular accident: a proposal for early corrective surgery. J Pediatr. 1970;77:1004-1009.
108 Woltman H.W., Shelden W.D. Neurologic complications associated with congenital stenosis of the isthmus of the aorta: a case of cerebral aneurysm with rupture and a case of intermittent lameness presumably related to stenosis of the isthmus. Arch Neurol Psychiatry. 1927;17:303.
109 Hodes H.L., Steinfeld L., Blumenthal S. Congenital cerebral aneurysms and coarctation of the aorta. Arch Pediatr. 1959;76:28-43.
110 Roberts D.L., Tatini U., Zimmerman R.S., Bortz J.J., Sirven J.I. Musical hallucinations associated with seizures originating from an intracranial aneurysm. Mayo Clin Proc. 2001;76:423-426.
111 Schneeweiss A., Sherf L., Lehrer E., Lieberman Y., Neufeld H.N. Segmental study of the terminal coronary vessels in coarctation of the aorta: a natural model for study of the effect of coronary hypertension on human coronary circulation. Am J Cardiol. 1982;49:1996-2002.
112 Vlodaver Z., Neufeld H.N. The coronary arteries in coarctation of the aorta. Circulation. 1968;37:449-454.
113 Cracowski J.L., Vanzetto G., Douchin S., Atger O., Bost M., Machecourt J. Myocardial infarction and Turner’s syndrome. Clin Cardiol. 1999;22:245-247.
114 Goldberg M.B., Scully A.L., Solomon I.L., Steinbach H.L. Gonadal dysgenesis in phenotypic female subjects. A review of eighty-seven cases, with cytogenetic studies in fifty-three. Am J Med. 1968;45:529-543.
115 Nora J.J., Torres F.G., Sinha A.K., Mcnamara D.G. Characteristic cardiovascular anomalies of XO Turner syndrome, XX and XY phenotype and XO-XX Turner mosaic. Am J Cardiol. 1970;25:639-641.
116 Mazzanti L., Cacciari E. Congenital heart disease in patients with Turner’s syndrome. Italian Study Group for Turner Syndrome (ISGTS). J Pediatr. 1998;133:688-692.
117 Prandstraller D., Mazzanti L., Picchio F.M., et al. Turner’s syndrome: cardiologic profile according to the different chromosomal patterns and long-term clinical follow-up of 136 nonpreselected patients. Pediatr Cardiol. 1999;20:108-112.
118 Mccrindle B.W. Coarctation of the aorta. Curr Opin Cardiol. 1999;14:448-452.
119 Miller M.J., Geffner M.E., Lippe B.M., et al. Echocardiography reveals a high incidence of bicuspid aortic valve in Turner syndrome. J Pediatr. 1983;102:47-50.
120 Edwards W.D., Leaf D.S., Edwards J.E. Dissecting aortic aneurysm associated with congenital bicuspid aortic valve. Circulation. 1978;57:1022-1025.
121 Lin A.E., Garver K.L. Monozygotic Turner syndrome twins—correlation of phenotype severity and heart defect. Am J Med Genet. 1988;29:529-531.
122 Digilio M.C., Marino B., Picchio F., et al. Noonan syndrome and aortic coarctation. Am J Med Genet. 1998;80:160-162.
123 Siggers D.C., Polani P.E. Congenital heart disease in male and female subjects with somatic features of Turner’s syndrome and normal sex chromosomes (Ullrich’s and related syndromes). Br Heart J. 1972;34:41-46.
124 Bijulal S., Sivasankaran S., Krishnamoorthy K.M., Titus T., Tharakan J.A., Krishnamanohar S.R. Unusual coarctation-the PHACE syndrome: report of three cases. Congenital Heart Disease. 2008;3:205-208.
125 Perloff J.K. Physical examination of the heart and circulation, 3rd ed. Philadelphia: W.B. Saunders; 1998.
126 Glancy D.L., Morrow A.G., Simon A.L., Roberts W.C. Juxtaductal aortic coarctation. Analysis of 84 patients studied hemodynamically, angiographically, and morphologically after age 1 year. Am J Cardiol. 1983;51:537-551.
127 Subramanian A.R. Coarctation or interruption of aorta proximal to origin of both subclavian arteries. Report of three cases presenting in infancy. Br Heart J. 1972;34:1225-1226.
128 Campbell M., Suzman S. Coarctation of the aorta. Br Heart J. 1947;9:185-212.
129 Blumenthal S., Epps R.P., Heavenrich R., et al. Report of the task force on blood pressure control in children. Pediatrics. 1977;59:I-II. 797–820
130 Kirkendall W.M., Feinleib M., Freis E.D., Mark A.L. Recommendations for human blood pressure determination by sphygmomanometers. Subcommittee of the AHA Postgraduate Education Committee. Circulation. 1980;62:1146A-1155A.
131 Manning D.M., Kuchirka C., Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation. 1983;68:763-766.
132 (Chair)Falkner B. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98:649-658.
133 Park M.K., Guntheroth W.G. Direct blood pressure measurements in brachial and femoral arteries in children. Circulation. 1970;41:231-237.
134 Dahlbaeck O., Dahn I., Westling H. Hemodynamic observations in coarctation of the aorta, with special reference to the blood pressure above and below the stenosis at rest and during exercise. Scand J Clin Lab Invest. 1964;16:339-346.
135 Boone M.L., Swenson B.E., Felson B. Rib notching: its many causes. Am J Roentgenol Radium Ther Nucl Med. 1964;91:1075-1088.
136 De Man S.A., Andre J.L., Bachmann H., et al. Blood pressure in childhood: pooled findings of six European studies. J Hypertens. 1991;9:109-114.
137 Miller R.A., Shekelle R.B. Blood pressure in tenth-grade students: results from the Chicago Heart Association Pediatric Heart Screening Project. Circulation. 1976;54:993-1000.
138 Perloff J.K. Pregnancy in congenital heart disease: the mother and the fetus. In: Perloff J.K., Child J.S., editors. Congenital heart disease in adults. 3rd ed. Philadelphia: W.B. Saunders; 2009:144.
139 Vriend J.W., Drenthen W., Pieper P.G., et al. Outcome of pregnancy in patients after repair of aortic coarctation. Eur Heart J. 2005;26:2173-2178.
140 Beauchesne L.M., Connolly H.M., Ammash N.M., Warnes C.A. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol. 2001;38:1728-1733.
141 Spencer M.P., Johnston F.R., Meredith J.H. The origin and interpretation of murmurs in coarctation of the aorta. Am Heart J. 1958;56:722-736.
142 Kirks D.R., Currarino G., Chen J.T. Mediastinal collateral arteries: important vessels in coarctation of the aorta. AJR Am J Roentgenol. 1986;146:757-762.
143 Nasser W.K., Helmen C. Kinking of the aortic arch (pseudocoarctation). Clinical, radiographic, hemodynamic, and angiographic findings in eight cases. Ann Intern Med. 1966;64:971-978.
144 Garman J.E., Hinson R.E., Eyler W.R. Coarctation of the aorta in infancy: detection on chest radiographs. Radiology. 1965;85:418-422.
145 Drexler C.J., Stewart J.R., Kincaid O.W. Diagnostic implications of rib notching. Am J Roentgenol Radium Ther Nucl Med. 1964;91:1064-1074.
146 Sloan R.D., Cooley R.N. Coarctation of the aorta; the roentgenologic aspects of one hundred and twenty-five surgically confirmed cases. Radiology. 1953;61:701-721.
147 Bjork L., Friedman R. Routine roentgenographic diagnosis of coarctation of the aorta in the child. Am J Roentgenol Radium Ther Nucl Med. 1965;95:636-641.
148 Ben-Shoshan M., Rossi N.P., Korns M.E. Coarctation of the abdominal aorta. Arch Pathol. 1973;95:221-225.
149 Kavanagh-Gray D., Chiu P. Kinking of the aorta (pseudocoarctation): report of six cases. Can Med Assoc J. 1970;103:717-720.
150 Child J.S. Transthoracic and transesophageal echocardiographic imaging: anatomic and hemodynamic assessment. In: Perloff J.K., Child J.S., editors. Congenital heart disease in adults. Philadelphia: W.B. Saunders, 1998.
151 Simpson I.A., Sahn D.J., Valdes-Cruz L.M., Chung K.J., Sherman F.S., Swensson R.E. Color Doppler flow mapping in patients with coarctation of the aorta: new observations and improved evaluation with color flow diameter and proximal acceleration as predictors of severity. Circulation. 1988;77:736-744.
152 Carvalho J.S., Redington A.N., Shinebourne E.A., Rigby M.L., Gibson D. Continuous wave Doppler echocardiography and coarctation of the aorta: gradients and flow patterns in the assessment of severity. Br Heart J. 1990;64:133-137.
153 Tacy T.A., Baba K., Cape E.G. Effect of aortic compliance on Doppler diastolic flow pattern in coarctation of the aorta. J Am Soc Echocardiogr. 1999;12:636-642.
154 Steidele R.J. Sammlung Verschiedener in der chirurgisch Praktik: Lehrschule Germachten Beobb. 1777;2:114
155 Lie J.T. The malformation complex of the absence of the arch of the aorta—Steidele’s complex. Am Heart J. 1967;73:615-625.
156 Takashina T., Ishikura Y., Yamane K., Yorifuji S., Iwasaki T. The congenital cardiovascular anomalies of the interruption of the aorta—Steidele’s complex. Am Heart J. 1972;83:93-99.
157 Blake H.A., Manion W.C., Spencer F.C. Atresia or absence of the aortic isthmus. J Thorac Cardiovasc Surg. 1962;43:607-614.
158 Celoria G.C., Patton R.B. Congenital absence of the aortic arch. Am Heart J. 1959;58:407-413.
159 Moller J.H., Edwards J.E. Interruption of aortic arch; anatomic patterns and associated cardiac malformations. Am J Roentgenol Radium Ther Nucl Med. 1965;95:557-572.
160 Roberts W.C., Morrow A.G., Braunwald E. Complete interruption of the aortic arch. Circulation. 1962;26:39-59.
161 Freedom R.M., Bain H.H., Esplugas E., Dische R., Rowe R.D. Ventricular septal defect in interruption of aortic arch. Am J Cardiol. 1977;39:572-582.
162 Abbott M.E. Congenital cardiac disease. In: Osler’s modern medicine. Philadelphia: Lea & Febiger; 1927.
163 Jaffe R.B. Complete interruption of the aortic arch. 1. Characteristic radiographic findings in 21 patients. Circulation. 1975;52:714-721.
164 Jaffe R.B. Complete interruption of the aortic arch. 2. Characteristic angiographic features with emphasis on collateral circulation to the descending aorta. Circulation. 1976;53:161-168.
165 Kutsche L.M., Van Mierop L.H. Cervical origin of the right subclavian artery in aortic arch interruption: pathogenesis and significance. Am J Cardiol. 1984;53:892-895.
166 Mishaly D., Birk E., Katz J., Vidne B.A. Interruption of right sided aortic arch. Case report and review of the literature. J Cardiovasc Surg. 1995;36:277-279.
167 Dische M.R., Tsai M., Baltaxe H.A. Solitary interruption of the arch of the aorta. Clinicopathologic review of eight cases. Am J Cardiol. 1975;35:271-277.
168 Higgins C.B., French J.W., Silverman J.F., Wexler L. Interruption of the aortic arch: preoperative and postoperative clinical, hemodynamic and angiographic features. Am J Cardiol. 1977;39:563-571.
169 Judez V.M., Maitre M.J., De Artaza M., De Miguel J.M., Valles F., Marquez J. Interruption of aortic arch without associated cardiac abnormalities. Br Heart J. 1974;36:313-317.
170 Riggs T.W., Berry T.E., Aziz K.U., Paul M.H. Two-dimensional echocardiographic features of interruption of the aortic arch. Am J Cardiol. 1982;50:1385-1390.
171 Al-Marsafawy H.M., Ho S.Y., Redington A.N., Anderson R.H. The relationship of the outlet septum to the aortic outflow tract in hearts with interruption of the aortic arch. J Thorac Cardiovasc Surg. 1995;109:1225-1236.
172 Braunlin E., Peoples W.M., Freedom R.M., Fyler D.C., Goldblatt A., Edwards J.E. Interruption of the aortic arch with aorticopulmonary septal defect. An anatomic review. Pediatr Cardiol. 1982;3:329-335.
173 Bergwerff M., Deruiter M.C., Hall S., Poelmann R.E., Gittenberger-De Groot A.C. Unique vascular morphology of the fourth aortic arches: possible implications for pathogenesis of type-B aortic arch interruption and anomalous right subclavian artery. Cardiovasc Res. 1999;44:185-196.
174 Schumacher G., Schreiber R., Meisner H., Lorenz H.P., Sebening F., Buhlmeyer K. Interrupted aortic arch: natural history and operative results. Pediatr Cardiol. 1986;7:89-93.
175 Morgan J.R., Forker A.D., Fosburg R.G., Neugebauer M.K., Rogers A.K., Bemiller C.R. Interruption of the aortic arch without a patent ductus arteriosus. Circulation. 1970;42:961-965.
176 Sharratt G.P., Carson P., Sanderson J.M. Complete interruption of aortic arch, without persistent ductus arteriosus, in an adult. Br Heart J. 1975;37:221-224.
177 Wong C.K., Cheng C.H., Lau C.P., Leung W.H., Chan F.L. Interrupted aortic arch in an asymptomatic adult. Chest. 1989;96:678-679.
178 Garcia O.L., Hernandez F.A., Tamer D., Poole C., Gelband H., Castellanos A.W. Congenital bilateral subclavian steal: ductus-dependent symptoms in interrupted aortic arch associated with ventricular septal defect. Am J Cardiol. 1979;44:101-104.
179 Flyer D.C., Buckley L.P., Hellenbrand W.E., Cohn H.E. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:375-461.
180 Buch J., Wennevold A., Efsen F., Andersen G.E. Interrupted aortic arch in two siblings. Acta Paediatr Scand. 1980;69:783-785.
181 Pankau R., Funda J., Wessel A. Interrupted aortic arch type B1 in a brother and sister: suggestion of a recessive gene. Am J Med Genet. 1990;36:175-177.
182 Gobel J.W., Pierpont M.E., Moller J.H., Singh A., Edwards J.E. Familial interruption of the aortic arch. Pediatr Cardiol. 1993;14:110-115.
183 Nakada T., Yonesaka S. Interruption of aortic arch type A in two siblings. Acta Paediatr Jpn. 1996;38:63-65.
184 Pierpont M.E., Gobel J.W., Moller J.H., Edwards J.E. Cardiac malformations in relatives of children with truncus arteriosus or interruption of the aortic arch. Am J Cardiol. 1988;61:423-427.
185 Gokcebay T.M., Batillas J., Pinck R.L. Complete interruption of the aorta at the arch. Am J Roentgenol Radium Ther Nucl Med. 1972;114:362-370.
186 Merrill D.L., Webster C.A., Samson P.C. Congenital absence of the aortic isthmus; report of a case with successful surgical repair. J Thorac Surg. 1957;33:311-320.
187 Rangel A., Chavez E., Espinosa I. Interruption of the aortic arch in adults. Arch Inst Cardiol Mex. 1999;69:144-148.
188 Kerkar P., Dalvi B., Kale P. Interruption of the aortic arch with associated cardiac anomalies. Survival to adulthood. Chest. 1993;103:279-280.
189 Baysal T., Kutlu R., Sarac K., Karaman I. Ruptured intracranial aneurysm associated with isolated aortic arch interruption. Neuroradiology. 2000;42:842-844.
190 Hu W.Y., Sevick R.J., Tranmer B.I., Maitland A., Gray R.R. Aortic arch interruption associated with ruptured cerebral aneurysm. Can Assoc Radiol J. 1996;47:20-23.
191 Greig D. Case of malformation of the heart and blood vessels of the foetus; pulmonary artery giving off the descending aorta and left subclavian. Monthly Journal of Medical Science. 1852;15:28-30.
192 Jonas R.A. Interrupted aortic arch. Curr Opin Cardiol. 1988;3(September/October):776-780.
193 Van Mierop L.H., Kutsche L.M. Cardiovascular anomalies in DiGeorge syndrome and importance of neural crest as a possible pathogenetic factor. Am J Cardiol. 1986;58:133-137.
194 Moerman P., Dumoulin M., Lauweryns J., Van Der Hauwaert L.G. Interrupted right aortic arch in DiGeorge syndrome. Br Heart J. 1987;58:274-278.
195 Takahashi K., Kuwahara T., Nagatsu M. Interruption of the aortic arch at the isthmus with DiGeorge syndrome and 22q11.2 deletion. Cardiol Young. 1999;9:516-518.
196 Tabakin B.S., Hanson J.S. Congenital absence of the aortic arch associated with patent ductus arteriosus and ventricular septal defect. Am J Cardiol. 1960;6:689-696.
197 Starreveld J.S., Van Rossum A.C., Hruda J. Rapid formation of collateral arteries in a neonate with interruption of the aortic arch. Cardiol Young. 2001;11:464-467.
198 Thomson P.S., Teele R.L. Reversal of left carotid arterial flow as a sign of type C interruption of the aortic arch. Pediatr Radiol. 1994;24:300-301.
199 Geva T., Gajarski R.J. Echocardiographic diagnosis of type B interruption of a right aortic arch. Am Heart J. 1995;129:1042-1045.
200 Kreutzer J., Van Praagh R. Comparison of left ventricular outflow tract obstruction in interruption of the aortic arch and in coarctation of the aorta, with diagnostic, developmental, and surgical implications. Am J Cardiol. 2000;86:856-862.