CHAPTER 15 Clinical Techniques of Cardiac Magnetic Resonance Imaging
Function
The assessment of cardiac function provides valuable diagnostic and prognostic information when managing patients with cardiovascular disease. Advancements in magnetic resonance imaging (MRI) technology have allowed for routine noninvasive assessment of left and right ventricular regional and global systolic and diastolic function.1 With the image acquisition methods described in Chapter 13, MRI is well suited to measure these crucial parameters, as evidenced by results produced from studies of phantoms, cadavers, animals, and human clinical trials.
Technical Requirements
Technical requirements for evaluating resting cardiac function require an MRI scanner with capabilities and protocols described in Chapter 13. Evaluating for dynamic measures of ventricular function, such as with dobutamine or exercise, necessitates further equipment (see later).
DETERMINING GLOBAL MEASURES OF CARDIAC FUNCTION
Indications and Clinical Utility
An accurate assessment of LV function is important for determining prognosis and appropriate therapy. MRI determinations of LV volumes using Simpson’s rule have been rigorously validated with cadaver studies. An early study showed excellent correlation (r = 0.997) between MRI-derived volume measurements of latex casts of excised human ventricles and the actual volume measurements from the casts themselves.2 Data derived from multiple autopsy series have shown a similarly high degree of correlation,3 as have animal studies using canine, porcine, rat, and mouse models.4,5,6
Several studies have demonstrated high reproducibility of MRI in the assessment of ejection fraction (EF) via Simpson’s rule methods. In one such investigation, intraobserver, interobserver, and interexamination variability were analyzed rigorously and measurements of LV volumes using MRI were more consistent compared with those obtained by other imaging modalities in prior studies.7 Another study found a 5% interobserver and interstudy variability in EF with cardiac MRI,8 superior to the 10% that has been reported for echocardiography.9 Notably, such consistency has been demonstrated in normal and morphologically abnormal (dilated and hypertrophied) left ventricles,10 for which other methods are vulnerable to inaccuracy.
Direct comparisons between MRI and other imaging techniques in the evaluation of EF have further bolstered the value of MRI in clinical practice. One of the first such studies compared MRI with ventricular angiography in calculating EF using the area-length method and found excellent correlation between the two (r = 0.88).11 More recently, MRI assessment of EF using Simpson’s rule has proved more reproducible (less interstudy, intraobserver, and interobserver variability) than two-dimensional echocardiography, currently the most widely available imaging modality to assess cardiac function.12,13 Also of note, direct comparisons among MRI, echocardiography, and radionuclide ventriculography revealed considerable differences in calculated EF, suggesting that MRI is the more accurate and preferred method to assess cardiac function, especially in dilated ventricles.14 Because of its consistent performance in clinical evaluations, MRI assessment of LV volumes using Simpson’s rule is now widely accepted as the most accurate and precise noninvasive estimation of EF.15
These benefits of MRI have research and clinical implications. Better reproducibility has resulted in the need for smaller sample sizes (sometimes by a factor of 80% to 90%) in studies using MRI measures of ventricular volumes than in those using other imaging modalities.3 Furthermore, MRI is now routinely used as the gold standard when evaluating other imaging techniques, such as three-dimensional echocardiography.16,17 MRI may be particularly important in evaluating patients for prophylactic implantable cardioverter-defibrillator placement,18 in which an EF of 35% is commonly used to guide the implantation of a device. MRI may also be useful in serial evaluations to detect changes in EF that are smaller than the limits of sensitivity of echocardiography. This can be especially relevant in identifying the subclinical cardiotoxic effects of chemotherapy.19
Contraindications
Because no stimulant or contrast agent is required to assess resting EF, the contraindications for this technique coincide with those pertaining to any MRI scan, such as the presence of certain metal implants or devices (see Chapter 19) or severe claustrophobia.
Technique Description
Determination of resting EF by MRI requires no intravenous contrast agent or stimulant. The protocols that are most commonly used include steady-state free precession (SSFP) and gradient echo sequences, which are discussed in detail in Chapter 13.
RESTING REGIONAL WALL MOTION ABNORMALITIES
Indications and Clinical Utility
Although ventricular EF provides a global assessment of cardiac function, it is still a relatively insensitive index of myocardial pathology. A patient with an EF of 50% may have regional wall motion abnormalities indicative of underlying coronary disease. Because of its excellent spatial resolution, cine MR images of the left ventricle allow a qualitative assessment of regional cardiac function without the acoustic limitations inherent in echocardiography.20 In addition, because the quality and resolution of white blood MR images is not closely related to body habitus or cardiac orientation, images can be acquired in almost any tomographic plane, allowing for a more comprehensive evaluation of regional function.3
Quantitation of regional myocardial function can be accomplished with MRI using a variety of techniques (see Chapter 14). Local wall thickening can be assessed and has been shown to correlate well with the size and extent of myocardial infarction, as measured by enzymatic release.21 Myocardial tagging can also be used to calculate regional strain, an appealing concept because the dynamics of ventricular systole are complex and consist not only of thickening but also of contraction, expansion, twisting, and through-plane motion.20 Strain calculation can potentially detect more subtle wall motion abnormalities than visual qualitative assessment and may provide valuable functional information in patients who have infarctions, hypertension, and nonischemic cardiomyopathies.22–24 It is again worth noting that many of these patients may have a preserved global EF, despite having abnormal segmental myocardial mechanics. Although myocardial tagging and quantification of regional wall function is currently not a routine part of the cardiac MRI examination at most centers, considerable data suggest that it may have significant clinical utility in the future.
Description
Also as for the assessment of global EF, evaluation of regional wall motion abnormalities usually involves SSFP or gradient echo sequences. Although qualitative visual assessment of wall motion is most often used and is often feasible, given the formidable spatial resolution of MRI, quantitation of wall thickening using myocardial tagging may have a more significant clinical role in the future (see earlier, “Indications and Clinical Utility”). Myocardial tagging techniques are discussed in detail in Chapter 13.
RIGHT VENTRICULAR FUNCTION
Indications and Clinical Utility
Just as for the left ventricle, RV function has important clinical and therapeutic implications. A depressed RV function predisposes patients to hypotension and confers a poor prognosis for patients with acute myocardial infarctions and chronic LV dysfunction.25 In addition, RV function is a focus of attention in those with congenital heart disease. Because of its geometry and location beneath the sternum, the right ventricle is particularly difficult to assess with echocardiography and radionuclide ventriculography (Fig. 15-1). MRI is not bound by these constraints and thus allows for an accurate assessment of this cardiac chamber.
MRI-derived volumes and mass of the right ventricle have shown robust correlations with in vitro casts and with clinical standards, such as invasive dilution techniques and radionuclide ventriculography.26,27 Studies have also shown high reproducibility, with low intraobserver, interobserver, and interstudy variability in normal volunteers28 and low interstudy variability in a diverse patient population.29 The accuracy and precision of MRI allow for rigorous clinical evaluation of the right ventricle while obviating the risk of invasive procedures and radiation exposure.
DIASTOLIC FUNCTION
Image Interpretation
In addition to evaluating the motion and strain of the myocardial tissue, which is currently not widely used clinically for diastole, MRI can also determine LV flow velocities and propagation during diastole. Using phase contrast imaging (see Chapter 17), the transmitral flow pattern can be determined much like it is with echocardiography. It is important to note, however, that some aspects of the transmitral E signal, such as acceleration and deceleration time, have not been validated with phase contrast MRI. Further characterization of diastolic LV flow can be achieved using a combination of phase contrast imaging and color vector mapping (Fig. 15-2). This is an experimental technique at present and is not yet widely used in clinical practice, but it does represent the potential of phase contrast imaging for assessing flow propagation in LV diastole. There is currently no standardized method of reporting diastolic function assessed by MRI.
DYNAMIC LEFT VENTRICULAR FUNCTION USING DOBUTAMINE
Indications and Clinical Utility
Like resting cardiac function, LV response to chronotropic and inotropic stimulation provides considerable clinical information, both physiologic and prognostic. Advances in software and hardware, specifically the development of phased-array surface coils, faster scanning, and advanced gating have increased the practicality and clinical utility of cardiac MRI wall motion stress testing.3 The focus of this subject will be the use of dobutamine and exercise stress testing in clinical practice. See Chapter 53 for a discussion of myocardial contrast perfusion imaging.
Physiology and Safety Profile of Dobutamine
Dobutamine is a synthetic catecholamine with mild β1 receptor selectivity.30,31 It is a positive inotrope and, because of its peripheral vasodilatory effects, it induces a reflex tachycardia.32 At doses of up to 10 µg/kg/min, dobutamine augments myocardial contractility and promotes coronary vasodilation; doses of 20 to 40 µg/kg/min increase heart rate and myocardial oxygen demand33 and, in the setting of significant epicardial coronary artery stenoses, can induce wall motion abnormalities by way of a supply-demand mismatch (Fig. 15-3).34,35 Several large- scale investigations have evaluated the safety of dobutamine as a stress agent and have found a low incidence (often less than 1%) of major complications.36,37
Dobutamine Magnetic Resonance Imaging and Myocardial Ischemia
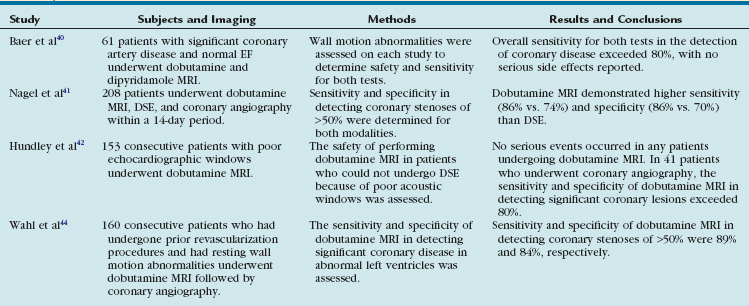
Perhaps the most common use of dobutamine stress imaging is to identify those patients who have physiologically significant coronary stenoses. The first clinical trials investigating the use of MRI in dobutamine studies were relatively small but showed excellent agreement between wall motion abnormalities seen on MRI at peak dobutamine levels and areas of abnormal myocardial perfusion as assessed by dobutamine thallium perfusion.38,39 The sensitivity of dobutamine MRI compared favorably with that of dipyridamole MRI in detecting coronary stenoses in another study consisting exclusively of patients with high-grade (70% or more) lesions of at least one major coronary vessel.40 In this series, the sensitivity of dobutamine MRI in detecting this degree of coronary stenosis was higher than 80% and increased with multivessel involvement. These early studies implied the safety and effectiveness of dobutamine as a pharmacologic stress agent and the utility of cardiac MRI in assessing for wall motion abnormalities.
A larger, more recent study compared dobutamine MRI with dobutamine stress echocardiography (DSE),41 a routinely used method of detecting inducible ischemia. In this study, 208 consecutive patients underwent DSE, dobutamine MRI, and biplane coronary angiography within a 14-day period. Dobutamine MRI demonstrated better sensitivity (86% vs. 74%) and specificity (86% vs. 70%) than DSE in detecting coronary artery disease (defined as more than 50% luminal narrowing) seen on angiography. Examinations were feasible in approximately 90% of patients with both DSE and dobutamine MRI, although the reasons for exclusion differed for the two techniques. Insufficient image quality was the major reason for exclusion from DSE and claustrophobia for dobutamine MRI. On the basis of these results, it was concluded that dobutamine MRI can detect wall motion abnormalities with significantly higher diagnostic accuracy than DSE because of improved image quality.
In keeping with this hypothesis, another large-scale, single-center trial evaluated 163 patients referred for DSE who did not have adequate imaging windows, despite the use of second harmonic imaging and contrast agents.42 These patients underwent dobutamine MRI instead and the vast majority (more than 80%) were able to complete their study without complications. Of patients who underwent coronary angiography, dobutamine MRI had a sensitivity and specificity higher than 80% in the detection of coronary arterial luminal narrowing of at least 50%, similar to the results of the study detailed earlier. Of note, this study included patients who had resting wall motion abnormalities, whereas the study by Nagel and colleagues41 specifically excluded patients with previous myocardial infarction and reduced resting EF. The authors concluded that dobutamine MRI could be performed safely using fast imaging techniques and that dobutamine MRI had potentially greater clinical utility than DSE, without compromising sensitivity or specificity.
Further studies have increasingly substantiated the clinical utility of dobutamine MRI. In a small series consisting exclusively of patients with known epicardial coronary disease, low-dose dobutamine MRI was effective in differentiating normal, ischemic, hibernating, and nonviable myocardium.43 There was also robust correlation seen with stress perfusion in these patients. In another study, which looked exclusively at patients with known coronary disease, Wahl and associates44 performed high-dose dobutamine MRI prior to coronary angiography on 160 consecutive patients who had undergone prior coronary revascularization and had resting wall motion abnormalities. The sensitivity and specificity of dobutamine MRI in detecting coronary luminal narrowing of at least 50% was 89% and 84%, respectively. This patient population (with underlying wall motion abnormalities and low EF) is especially difficult to evaluate with DSE because of frequently poor acoustic windows. The fact that the diagnostic accuracy of dobutamine MRI is maintained in this group further broadens its clinical utility (Table 15-1).
TABLE 15-1 Summary of Studies Validating the Clinical Utility of Dobutamine Magnetic Resonance Imaging in Detecting Significant Coronary Disease
In these studies and in current routine clinical practice, wall motion abnormalities are identified and graded in a qualitative manner. The heightened image clarity of MRI, however, is well suited for quantitative analysis; this may attenuate the subjectivity involved in dobutamine stress assessment. Dobutamine MRI was performed in 39 consecutive patients referred for coronary angiography and in 10 normal volunteers; wall thickening was quantified using the modified center line technique.45 This technique yielded a sensitivity of 91% and specificity of 80% in the detection of significant coronary artery disease. Myocardial tagging has also been applied to dobutamine MRI studies and yields significantly different strain and strain rates in ischemic and infarcted myocardium than in remote normal segments.46 Comparing tagged and nontagged images in dobutamine MRI studies, Kuijpers and coworkers47 found that tagged images detected inducible wall motion abnormalities in 10 patients who were judged to have normal nontagged studies. All these patients were found to have coronary disease on subsequent coronary angiography. Of note, this trial did not use quantitative methods to determine strain via the tagged images. Further studies are required to determine whether myocardial tagging and quantitative techniques are truly superior to visual assessment of wall motion and whether they should become a standard part of clinical practice (Fig. 15-4).
Dobutamine Magnetic Resonance Imaging and Myocardial Viability
Following an ischemic event, differentiating between viable and nonviable myocardial tissue is an important clinical distinction that can help determine the utility of revascularization. Early studies evaluating the use of cardiac MRI for this found that wall thinning and shortened T2 relaxation time at rest could identify regions of chronic infarction (i.e., nonviable myocardium).48 Corroborating the importance of wall thickness in assessing for myocardial viability was a study by Baer and colleagues,49 which correlated a diastolic wall thickness more than 2.5 standard deviations below the normal mean value and systolic akinesis to markedly reduced technetium uptake.
Another study by this group sought to determine which markers could predict contractile recovery of dysfunctional myocardium following revascularization, a functional definition of myocardial viability.50 In this series, 43 patients who suffered a remote myocardial infarction underwent resting and low-dose dobutamine cardiac MRI as well as a follow-up resting MRI after a revascularization procedure (either bypass grafting or angioplasty). A resting diastolic wall thickness of 5.5 mm or more was 92% sensitive but only 56% specific in predicting contractile recovery on postrevascularization MRI. Dobutamine-induced systolic wall thickening of 2 mm or more was highly sensitive (89%) but also very specific (94%) in predicting functional recovery. The authors concluded that low-dose dobutamine cardiac MRI provided more information on myocardial viability than resting MRI alone. Other studies evaluating the use of low-dose dobutamine MRI in assessing for contractile recovery in patients with a recent myocardial infarction have also yielded favorable results, with diagnostic accuracy exceeding 80%.51,52
Just as for the detection of coronary stenoses, DSE is also widely used to identify myocardial viability. An early study comparing dobutamine MRI with DSE found a high degree of concordance in the diagnosis of viability.53 Baer and associates54 compared dobutamine transesophageal echocardiography (TEE) with dobutamine MRI using positron emission tomography (PET) scanning as the reference standard for viability. Both modalities yielded similar results but MRI was slightly more sensitive and specific than echocardiography. In a follow-up study, a similar head-to-head comparison was performed, this time using contractile recovery following revascularization as the end point. The investigators determined that there was no significant difference between dobutamine TEE and dobutamine MRI in the assessment of viability.55 These studies indicated that dobutamine MRI compared favorably with an already accepted technique in the assessment of myocardial viability.
Dobutamine MRI has also been compared with contrast MRI imaging for the assessment of viability (Fig. 15-5). Delayed hyperenhancement techniques using gadolinium contrast are widely used in determining the extent of viable and scarred myocardium, discussed in detail in Chapter 57. Partial wall thickness enhancement (less than 75%) represents an area of uncertainty in predicting contractile recovery, which may be clarified with low-dose dobutamine testing. Kaandorp and colleagues sought to investigate the relevance of intermediate degrees of enhancement56 and found that regions with only subendocardial enhancement (encompassing 1% to 50% of total ventricular wall thickness) often exhibited contractile reserve with low-dose dobutamine, whereas areas with transmural involvement (enhancement extending to 76% to 100% of wall thickness) did not improve with dobutamine. Segments with 51% to 75% enhancement varied in their response, with 61% of these segments improving with dobutamine and 39% demonstrating no contractile reserve. The authors concluded that in cases of intermediate hyperenhancement, assessment of contractile reserve with dobutamine may allow optimal identification of segments that are likely to improve with revascularization.
In keeping with this hypothesis, two studies have compared dobutamine MRI with delayed contrast-enhanced MRI in predicting functional improvement after a revascularization procedure in patients with coronary artery disease.57,58 Dobutamine MRI exhibited a greater diagnostic accuracy than contrast enhancement; this advantage was largest in segments with intermediate degrees of enhancement (51% to 75%). Low-dose dobutamine MRI compares favorably with echocardiography and delayed hyperenhancement, which is a reflection of its clinical accuracy and utility in evaluating for myocardial viability.
Dobutamine Magnetic Resonance Imaging and Patient Prognosis
Aside from providing physiologic information, the results of a clinically relevant cardiac study should have prognostic implications and risk stratification value. To this end, Hundley and colleagues59 followed 279 patients over an average of 20 months after the completion of full-dose dobutamine MRI. Evidence of inducible ischemia or a resting EF lower than 40% was associated with myocardial infarction and cardiac death independently of the presence of coronary risk factors (Fig. 15-6). Of note, these patients had previous transthoracic echocardiography that was not adequate for wall motion analysis and so the authors concluded that in patients with poor echocardiograms, the results of dobutamine MRI stress tests can be used to forecast myocardial infarction or cardiac death.
The results of another investigation by Kuijpers and associates60 further suggested the clinical utility of dobutamine MRI in patient risk stratification. Consecutive patients (299) suspected of myocardial ischemia but with indeterminate resting and bicycle exercise electrocardiograms underwent resting and full-dose dobutamine MRI. Patients who had resting wall motion abnormalities were at higher risk for major adverse cardiac events at 2-year follow-up than patients with normal resting wall motion (18% vs. 0.56%; P < .001). Furthermore, the results of dobutamine MRI could classify patients with unknown or intermediate risk (e.g., patients in this study with no prior history of coronary disease, indeterminate resting electrocardiograms, and no resting wall motion abnormalities) more definitively into a high or low coronary risk group. It was concluded that dobutamine MRI may be a valuable tool in risk-stratifying patients, especially those with unclear cardiac risk.
Dobutamine MRI has also been used in the assessment of preoperative cardiovascular risk in patients undergoing noncardiac surgery.61 In 102 consecutive patients unable to undergo stress echocardiography because of inadequate acoustic windows, 6 patients suffered a perioperative cardiac event and 5 of them had inducible ischemia on their dobutamine MRI. Just as in the earlier study, the investigators though that dobutamine MRI was a significant predictor of cardiac events and could be especially useful for clarifying risk in patients who have intermediate clinical predictors of perioperative cardiac events.
DYNAMIC LEFT VENTRICULAR FUNCTION USING EXERCISE
Indications and Clinical Utility
In patients who are capable of physical exertion, it may be possible to perform exercise rather than pharmacologic stress MRI. Both bicycle and treadmill exercise have been evaluated as stress agents (Fig. 15-7) and have shown favorable preliminary results. In one study, 16 healthy patients successfully completed supine bicycle stress tests and underwent MR imaging using an ultrafast sequence.62 Both the right and left ventricles demonstrated physiologic changes with exercise, such as increased stroke volume and EF and decreased end-systolic volumes, all of which were consistent with previous exercise physiology data. Because of the difficulty in imaging the right ventricle with conventional modalities, this is one of the few reports detailing the response of the right ventricle to exercise. In another trial, which used treadmill exercise, 27 patients with exertional chest pain completed an exercise protocol and subsequent MRI imaging.63 The sequence was 79% sensitive and 85% specific for detecting more than 70% coronary artery stenoses on coronary angiography. These values are similar to those obtained by stress echocardiography and nuclear studies. The authors noted that one of the major limitations of performing treadmill MRI tests is the inability to acquire images within 60 to 90 seconds of cessation of exercise. This report suggests that treadmill MRI testing is at least feasible and further studies are needed to determine the clinical utility of exercise stress MRI in the evaluation of ischemic heart disease.
SUMMARY AND CONCLUSION
1 Ganz W, Donoso R, Marcus HS, et al. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol. 1971;27:392-396.
2 Rehr RB, Malloy CR, Filipchuk NG, et al. Left ventricular volumes measured by MR imaging. Radiology. 1985;156:717-719.
3 Walsh TF, Hundley G. Assessment of ventricular function with cardiovascular magnetic resonance. Cardiol Clin. 2007;25:15-33.
4 Nahrendorf M, Hiller KH, Hu K, et al. Cardiac magnetic resonance imaging in small animal models of human heart failure. Med Image Anal. 2003;7:369-375.
5 Koch JA, Poll LW, Godehardt E, et al. Right and left ventricular volume measurements in an animal heart model in vitro: first experiences with cardiac MRI at 1.0 T. Eur Radiol. 2000;10:455-458.
6 Caputo GR, Tscholakoff DT, Sechtem U, Higgins CB. Measurement of canine left ventricular mass by using MR imaging. Am J Roentgenol. 1987;148:33-38.
7 Pattynama PMT, Lamb HJ, van der Velde EA, et al. Left ventricular measurements with cine and spin-echo MR imaging: a study of reproducibility with variance component analysis. Radiology. 1993;187:261-268.
8 Semelka RC, Tomei E, Wagner S, et al. Normal left ventricular dimensions and function: interstudy reproducibility of measurements with cine MR imaging. Radiology. 1990;174:763-768.
9 Gordon EP, Schnittger I, Fitzgerald PJ, et al. Reproducibility of left ventricular volumes by two-dimensional echocardiography. J Am Coll Cardiol. 1983;2:506-513.
10 Semelka RC, Tomei E, Wagner S, et al. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990;119:1367-1373.
11 Stratemeier EJ, Thompson R, Brady TJ, et al. Ejection fraction determination by MR imaging: comparison with left ventricular angiography. Radiology. 1986;158:775-777.
12 Grothues F, Smith GC, Moon JCC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29-34.
13 Bellenger NG, Marcus NJ, Davies C, et al. Left ventricular function and mass after orthotopic heart transplantation: a comparison of cardiovascular magnetic resonance with echocardiography. J Heart Lung Transplant. 2000;19:444-452.
14 Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J. 2000;21:1387-1396.
15 Rumberger JA, Behrenbeck T, Bell MR, et al. Determination of ventricular ejection fraction: a comparison of available imaging methods. Mayo Clin Proc. 1997;72:360-370.
16 Ioannidis JPA, Trikalinos TA, Danias PG. Electrocardiogram-gated single-photon emission computed tomography versus cardiac magnetic resonance imaging for the assessment of left ventricular volumes and ejection fraction: a meta-analysis. J Am Coll Cardiol. 2002;39:2059-2068.
17 Chuang MI, Hibberd MG, Salton CJ, et al. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol. 2000;35:477-484.
18 Zimetbaum PJ. A 59-year-old man considering implantation of a cardiac defibrillator. JAMA. 2007;297:1909-1916.
19 Oberholzer K, Kunz RP, Dittrich M, Thelen M. Anthracycline-induced cardiotoxicity: cardiac MRI after treatment for childhood cancer. Fortschr Roentgenstr. 2004;176:1245-1250.
20 Bellenger NG, Grothues F, Smith GC, Pennell DJ. Quantification of right and left ventricular function by cardiovascular magnetic resonance. Herz. 2000;25:392-399.
21 Holman E, van Jonbergen HPW, van Dijkman PRM, et al. Comparison of magnetic resonance imaging studies with enzymatic indexes of myocardial necrosis for quantification of myocardial infarct size. Am J Cardiol. 1993;71:1036-1040.
22 Kramer CM, Rogers WJ, Theobald TM, et al. Remote noninfarcted region dysfunction soon after first anterior myocardial infarction. A magnetic resonance tagging study. Circulation. 1996;94:660-666.
23 Palmon LC, Reichek N, Yeon SB, et al. Intramural myocardial shortening in hypertensive left ventricular hypertrophy with normal pump function. Circulation. 1994;89:122-131.
24 MacGowan GA, Shapiro EP, Azhari H, et al. Noninvasive measurement of shortening in the fiber and cross-fiber directions in the normal human left ventricle and in idiopathic dilated cardiomyopathy. Circulation. 1997;96:535-541.
25 Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med. 1993;328:981-988.
26 Longmore DB, Underwood SR, Hounsfield GN, et al. Dimensional accuracy of magnetic resonance in studies of the heart. Lancet. 1985;1:1360-1362.
27 Culham JA, Vince DJ. Cardiac output by MR imaging: an experimental study comparing right ventricle and left ventricle with thermodilution. Can Assoc Radiol J. 1988;39:247-249.
28 Lorenz CH, Walker ES, Morgan VL, et al. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7-21.
29 Grothues F, Moon JC, Bellenger NG, et al. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218-223.
30 Vallet B, Dupuis B, Chopin C. Dobutamine: mechanisms of action and use in acute cardiovascular pathology. Ann Cardiol Angiol (Paris). 1991;40:397-402.
31 Ruffolo RRJr. The pharmacology of dobutamine. Am J Med Sci. 1987;294:244-248.
32 Baig MW. Pharmacologic perfusion imaging. Who needs it and why? Postgrad Med J. 1992;91:185-187.
33 Iskandrian AS, Verani MS, Heo J. Pharmacologic stress testing: mechanism of action, hemodynamic responses, and results in detection of coronary artery disease. J Nucl Cardiol. 1994;1:94-111.
34 Kugiyama K, Inobe Y, Ohgushi M, et al. Comparison of coronary hemodynamics during infusions of dobutamine and adenosine in patients with angina pectoris. Jpn Circ J. 1998;62:1-6.
35 Warltier DC, Zyvoloski M, Gross GJ, et al. Redistribution of myocardial blood flow distal to a dynamic coronary arterial stenosis by sympathomimetic amines: comparison of dopamine, dobutamine and isoproterenol. Am J Cardiol. 1981;48:269-279.
36 Kuijpers D, Janssen CH, van Dijkman PR, et al. Dobutamine stress MRI. Part I: safety and feasibility of dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol. 2004;14:1823-1828.
37 Hamilton CA, Link KM, Salido TB, et al. Is imaging at intermediate doses necessary during dobutamine stress magnetic resonance imaging? J Cardiovasc Magn Reson. 2001;3:297-302.
38 Pennell DJ, Underwood SR. The cardiovascular effects of dobutamine assessed by magnetic resonance imaging. Postgrad Med J. 1991;67(Suppl 1):S1-S8.
39 Pennell DJ, Underwood SR, Manzara CC, et al. Magnetic resonance imaging during dobutamine stress in coronary artery disease. Am J Cardiol. 1992;70:34-40.
40 Baer FM, Theissen P, Smolarz K, et al. Dobutamine versus dipyridamole magnetic resonance tomography: safety and sensitivity in the detection of coronary stenoses. Z Kardiol.. 1993;82:494-503.
41 Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763-770.
42 Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697-1702.
43 Sensky PR, Jivan A, Hudson NM, et al. Coronary artery disease: combined stress MR imaging protocol—one-stop evaluation of myocardial perfusion and function. Radiology. 2000;215:608-614.
44 Wahl A, Paetsch I, Roethemeyer S, et al. High-dose dobutamine-atropine stress cardiovascular MR imaging after coronary revascularization in patients with wall motion abnormalities at rest. Radiology. 2004;233:210-216.
45 Van Rugge FP, van der Wall EEE, Spanjersberg SJ, et al. Magnetic resonance imaging during dobutamine stress for detection and localization of coronary artery disease. Circulation. 1994;90:127-138.
46 Edvardsen T, Gerber BL, Garot J, et al. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106:50-56.
47 Kuijpers D, Ho KY, van Dijkman PR, et al. Dobutamine cardiovascular magnetic resonance for the detection of myocardial ischemia with the use of myocardial tagging. Circulation. 2003;107:1592-1597.
48 McNamara MT, Higgins CB. Magnetic resonance imaging of chronic myocardial infarcts in man. AJR Am J Roentgenol. 1986;146:315-320.
49 Baer FM, Smolarz K, Theissen P, et al. Regional 99mTc-methoxyisobutyl-isonitrile-uptake at rest in patients with myocardial infarcts: comparison with morphological and functional parameters obtained from gradient-echo magnetic resonance imaging. Eur Heart J. 1994;15:97-107.
50 Baer FM, Theissen P, Schneider CA, et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040-1048.
51 Dendale P, Franken PR, Holman E, et al. Validation of low-dose dobutamine magnetic resonance imaging for assessment of myocardial viability after infarction by serial imaging. Am J Cardiol. 1998;82:375-377.
52 Sandstede JJW, Bertsch G, Beer M, et al. Detection of myocardial viability by low-dose dobutamine cine MR imaging. Magn Reson Imaging. 1999;17:1437-1443.
53 Dendale PA, Franken PR, Waldman G-J, et al. Low-dosage dobutamine magnetic resonance imaging as an alternative to echocardiography in the detection of viable myocardium after acute infarction. Am Heart J. 1995;130:134-140.
54 Baer FM, Voth E, LaRosee K, et al. Comparison of dobutamine transesophageal echocardiography and dobutamine magnetic resonance imaging for detection of residual myocardial viability. Am J Cardiol. 1996;78:415-419.
55 Baer FM, Theissen P, Crnac J, et al. Head to head comparison of dobutamine–transoesophageal echocardiography and dobutamine–magnetic resonance imaging for the prediction of left ventricular functional recovery in patients with chronic coronary artery disease. Eur Heart J. 2000;21:981-991.
56 Kaandorp TAM, Bax JJ, Schuijf JD, et al. Head-to-head comparison between contrast-enhanced magnetic resonance imaging and dobutamine magnetic resonance imaging in men with ischemic cardiomyopathy. Am J Cardiol. 2004;93:1461-1464.
57 Motoyasu M, Sakuma H, Ichikawa Y, et al. Prediction of regional functional recovery after acute myocardial infarction with low dose dobutamine stress cine MR imaging and contrast enhanced MR imaging. Cardiovasc Magn Reson. 2003;5:563-564.
58 Wellnhofer E, Olariu A, Klein C, et al. Magnetic resonance low-dose dobutamine test is superior to scar quantification for the prediction of functional recovery. Circulation. 2004;109:2172-2174.
59 Hundley WG, Morgan TM, Neagle CM, et al. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328-2333.
60 Kuijpers D, van Dijkman PRM, Janssen CHC, et al. Dobutamine stress MRI. Part II. Risk stratification with dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol. 2004;14:2046-2052.
61 Rerkpattanapipat P, Morgan TM, Neagle CM, et al. Assessment of preoperative cardiac risk with magnetic resonance imaging. Am J Cardiol. 2002;90:416-419.
62 Roest AAW, Kunz P, Lamb HJ, et al. Biventricular response to supine physical exercise in young adults assessed with ultrafast magnetic resonance imaging. Am J Cardiol. 2001;87:601-605.
63 Rerkpattanapipat P, Gandhi SK, Darty SN, et al. Feasibility to detect severe coronary artery stenoses with upright treadmill exercise magnetic resonance imaging. Am J Cardiol. 2003;92:603-606.

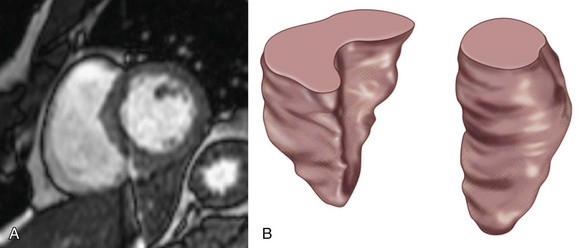
 FIGURE 15-1
FIGURE 15-1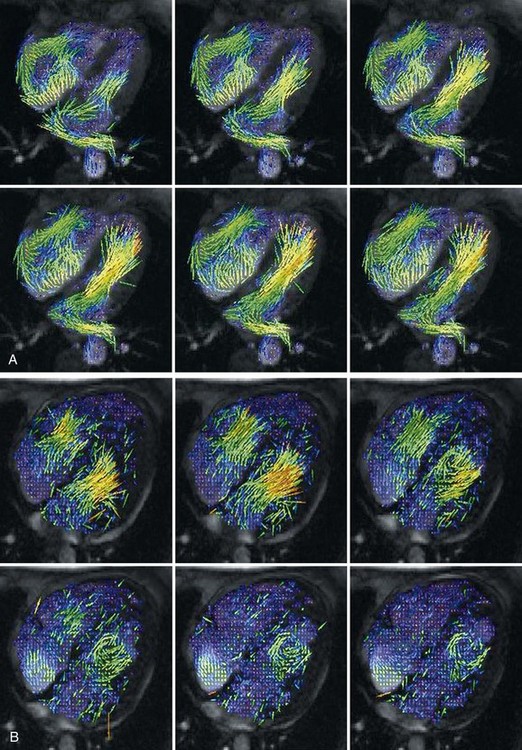
 FIGURE 15-2
FIGURE 15-2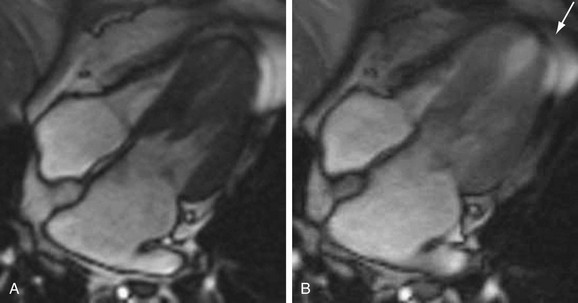
 FIGURE 15-3
FIGURE 15-3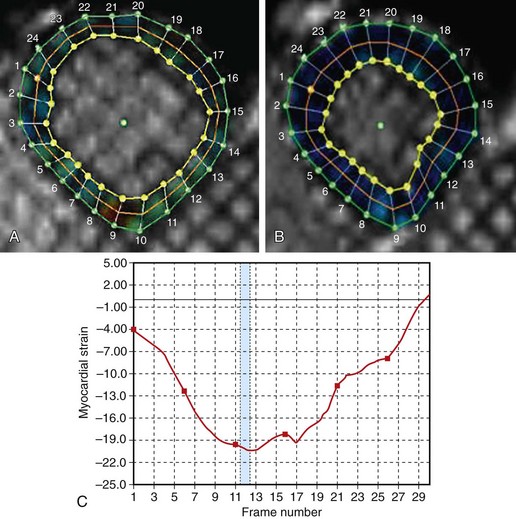
 FIGURE 15-4
FIGURE 15-4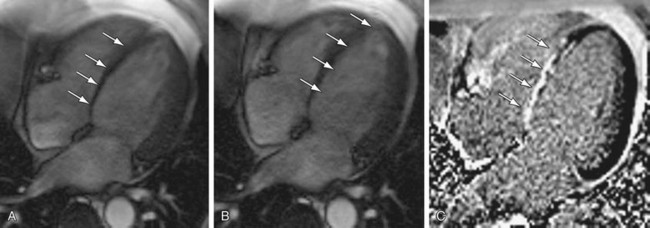
 FIGURE 15-5
FIGURE 15-5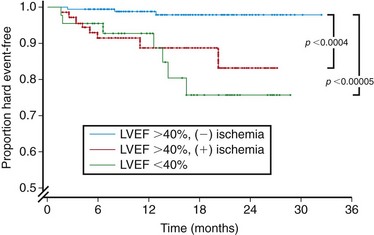
 FIGURE 15-6
FIGURE 15-6
 FIGURE 15-7
FIGURE 15-7



