CHAPTER 21 Clinical Single Photon Emission Computed Tomography Cardiac Protocols
Nuclear medicine has become a remarkable diagnostic tool to identify and to stratify patients with suspected coronary artery disease (CAD) with pivotal prognostic implications. Myocardial perfusion imaging (MPI) provides an accurate measurement of coronary narrowing leading to inducible perfusion abnormalities with prognostic implications (Fig. 21-1). The relationship between the degree of coronary stenosis and the maximal hyperemic response was first reported more than 30 years ago.1 Myocardial regions with relatively decreased post-stress radiotracer uptake and resting normalization indicate a misbalance between oxygen supply and myocardial demands, characteristic of ischemia. Nonreversible myocardial perfusion defects normally relate to necrosis or infarction. Current imaging protocols allow the accurate assessment of relative regional perfusion and myocardial function at rest and stress based on regional blood flow heterogeneity. Gated single photon emission computed tomography (SPECT) represents nearly 95% of all procedures performed today in cardiac nuclear medicine. This chapter reviews the different imaging SPECT acquisition protocols that are currently in use as well as the principal indications.
TECHNICAL REQUIREMENTS
201Tl Imaging Protocols
During the last two decades, thallium 201 (201Tl) has been used as a myocardial perfusion tracer. After an intravenous injection, the initial myocyte uptake is mainly determined by regional myocardial perfusion, whereas the integrity of the cell membrane is predominantly important for delayed imaging of tracer retention (potassium ion total distribution). Regional thallium activity on delayed images acquired early (3 to 4 hours) or late (8 to 72 hours) after stress has been used to demonstrate the presence and extent of viable myocardium based on the phenomenon termed thallium redistribution.2 Intracellular thallium uptake through the sarcolemmal membrane is maintained during long periods if regional myocardial blood flow is sufficiently preserved to be able to deliver the isotope to the myocytes. Redistribution is thought to represent areas of ischemic but viable myocardium, whereas fixed, non-redistributing defects are thought to represent nonviable, fibrotic scar.
When 201Tl alone is used, a variety of different acquisition protocols of stress imaging have been employed, including redistribution and reinjection imaging. Although different 201Tl imaging protocols have been employed, stress-redistribution-reinjection and rest-redistribution SPECT imaging protocols are the most currently used to assess myocardial viability.3 Reinjection of 1 mCi of 201Tl after 3 to 4 hours of redistribution imaging detects viable tissue in 35% to 49% of segments with fixed irreversible 201Tl defects with conventional stress-redistribution protocols.4 An inverse relationship has been reported between regional thallium activity in irreversible defects and the amount of myocardial fibrosis.5 Perrone-Filardi and colleagues6 demonstrated the nearly linear relation between thallium activity and the likelihood of recovery of regional function after revascularization. Overall sensitivity of several stress-redistribution-reinjection studies averaged 85% with a lower specificity (averaging 47%), suggesting that this protocol tends to overestimate the potential for contractile function recovery.2
Rest-redistribution 201Tl imaging is a valid alternative to discriminate viable from nonviable myocardium when the clinical question is to identify viability and not inducible ischemia.7,8 Studies have reported that 24-hour imaging after rest injection detects additional areas of viable myocardium compared with 4-hour imaging alone, possibly related to higher resting blood flow levels.9
Tc 99m Labeled Imaging Protocols
One-Day Protocol
Regarding viability assessment, earlier studies using primarily planar scintigraphy and visual interpretation suggested that Tc 99m sestamibi underestimates myocardial viability in chronic CAD and left ventricular dysfunction. Dilsizian and colleagues10 described the utility of quantitative Tc 99m sestamibi imaging when the severity of decrease in Tc 99m sestamibi uptake within irreversible defects was considered or when an additional redistribution image was acquired after the rest injection for detection of dysfunctional but viable myocardium. A significant inverse linear relationship has been described between Tc 99m sestamibi uptake and myocardial fibrosis in biopsy specimens.11 Several approaches have been introduced to increase the diagnostic performance of Tc 99m sestamibi for the identification of viable myocardium, such as functional evaluation of the left ventricle by first-pass radionuclide ventriculography, gated SPECT acquisition perfusion images,12 and intravenous administration of nitrates to reduce resting hypoperfusion and to increase the correspondence of the resting images with myocardial viability.13
Fatty acid analogues marked with iodine isotopes (123I, 131I) have been employed to study myocardial metabolism on the basis of the principle that in a viable but ischemic myocardium, fatty acids are mainly esterified and incorporated into the triglyceride and phospholipid blood pool and are slowly metabolized, with subsequent clearance rate reduction.14 Early data suggested that this method may provide data comparable to 201Tl protocols in patients with left ventricular dysfunction or after an acute myocardial infarction. These tracers may prove to be of more value in the near future, considering the key role that oxidative metabolism plays in preservation of myocardial function.
Dual-Isotope Protocols
Dual-isotope imaging protocols using Tc 99m labeled compounds and 201Tl are based on the ability of the Anger camera to collect data from the two different energy windows representing each radiotracer. Simultaneous and separate dual-isotope imaging protocols have been described.15,16 Two of the major advantages of these protocols are the possibility of shortening the duration of a complete stress or rest redistribution protocol and the superior capability of 201Tl to assess myocardial viability. Separate acquisition times can reduce the necessity of downscatter correction that can diminish 201Tl contrast images, leading to an overestimation of defect reversibility; this can be achieved by acquiring 201Tl data sets before the administration of Tc 99m because of the very limited (2.2%) contribution of 201Tl into the Tc 99m energy window.
Gated SPECT
Most of the current imaging protocols perform a simultaneous ECG gating during at least the stress data set acquisition.17 Gated SPECT can be performed with 201Tl or Tc 99m compounds. One of the major advantages is the possibility of measuring contractile function and the left ventricular ejection fraction.
STRESS PROTOCOLS
Exercise Testing
This is the most common form of stress during MPI. Exercise testing is performed on the treadmill according to the Bruce protocols and allows the assessment of different hemodynamic variables, such as exercise capacity, blood pressure, and heart rate responses. It is imperative that the intravenous injection of the radiotracer be performed at maximal stress and that exercise continue for at least an additional 60 seconds to ensure optimal myocardial concentration. The traditional goal of the test as an acceptable level of cardiac workload has been the achievement of at least 85% of the maximum predicted heart rate (220 − age). Prognostic information from the exercise test should be integrated with MPI because the patients with mild to moderate perfusion defects who achieved a heart rate higher than 80% had a very low risk for major cardiac events and cardiac death.18 At the same time, patients with a reported normal MPI study but abnormal heart rate reserve were at the same risk for suffering a major cardiac event as those with normal heart rate reserve achieved and abnormal MPI studies (Tables 21-1 and 21-2). A maximal stress test may satisfy diagnostic purposes if it goes beyond the hemodynamic threshold of triggering the ischemic symptoms. However, it may not reveal the full amount of jeopardized myocardium and may be inadequate for the evaluation of cardiac risk in a patient scheduled to have major noncardiac surgery. A submaximal exercise test (not achieving 85% of the targeted heart rate) may still be a valid alternative for evaluation of ischemic risks after cardiac events. To achieve the most adequate level of cardiac stress and to avoid suboptimal stress testing, patients should discontinue antianginal medications (β blockers and calcium blockers for 36 to 48 hours and long-acting nitrates for 12 hours).19 Furthermore, all patients should be studied in the fasting state, having been in a nothing-by-mouth status for at least 4 hours before the study, regardless of the stress method to be employed. All caffeine, including beverages and chocolate, especially before pharmacologic stress testing, should be avoided for at least 24 to 48 hours to avoid block of the endothelial receptors and their dilatory effect.
| Bicycle or treadmill |
| Maximal exercise and at least 85% of age-predicted maximum heart rate |
| Monitor ECG continuously (9 or 12 leads) |
| Blood pressure recorded every 2 to 3 minutes |
| Tracer injection at peak exercise; keep exercising for at least 1 additional minute |
| Assess electrical changes or symptoms |
TABLE 21-2 Exercise Stress Contraindications
| Acute aortic dissection |
| Symptomatic aortic stenosis |
| Uncontrolled cardiac arrhythmias |
| Severe pulmonary hypertension |
| Acute myocardial infarction within the last 5 days |
| Uncontrolled, severe hypertension (blood pressure > 200/110 mm Hg) |
| Decompensated congestive heart failure |
| Uncontrolled unstable angina |
Pharmacologic Stress Protocols
Vasodilators
Dipyridamole and adenosine are the most commonly used coronary vasodilators (Figs. 21-2 and 21-3). Briefly, dipyridamole is a pyrimidopyrimidine that has been widely used since 1987. Its blocks the cellular reuptake of adenosine, increasing its extracellular concentration, which produces vasodilation. Dipyridamole denies the extracellular access to the activity of red cell membrane–bound adenosine deaminase. The coronary dilating effect is related to the A2 receptor binding and activation mediated by G proteins, which ultimately result in vascular smooth muscle relaxation and vasodilation. The stimulation of the A1 receptor in the sinus and atrioventricular nodes reduces the sinus rate and the atrioventricular conduction that may cause heart block during stress testing.
Individuals with normal coronary arteries increase their blood flow up to four times of the resting levels. In patients with CAD, there is heterogeneity of perfusion without ischemia that may be observed in cases of severe CAD, often in association with a coronary steal.20 It has been reported that diagnostic accuracy for the detection of CAD with pharmacologic stress MPI is similar to that with exercise MPI, despite the fact that coronary flow may be increased to a greater degree than during exercise. Symptomatic myocardial ischemia is less commonly produced with vasodilators, possibly owing to the lower oxygen demands as opposed to exercise.21 Unlike with exercise, patients are instructed to withhold the use of methylxanthines, such as theophylline and caffeine, for at least 24 hours before the study to minimize interference with vasodilators due to the competition for the adenosine receptors. As with exercise, it appears preferable to withhold antianginal medications and calcium blockers for at least 24 hours before imaging; some studies have suggested that they may diminish the extent of myocardial perfusion defects.22 On the other hand, patients with known CAD should be imaged without withholding of medications, similar to exercise testing, to evaluate the effect of medical therapy.
A mild increase in the incidence of ischemia has been described by the addition of low-level exercise to the pharmacologic stress, which may add more diagnostic sensitivity to the test. The low-level exercise reduces the splanchnic blood flow and therefore the liver uptake of the radiopharmaceuticals.23
Dipyridamole is infused intravenously at rest, in the supine position, at a rate of 0.142 mg/kg/min for 4 minutes for a total dose of 0.57 mg/kg. The maximal vasodilator effect is achieved 3 minutes after completion of the infusion, the time of injection of the radiopharmaceutical. It persists at this level for 30 to 60 minutes. Injection of the radiotracer can be done earlier than 7 to 8 minutes if severe hemodynamic effects develop or clear symptoms are noted or there are acute ischemic ECG changes that can jeopardize the safety of the test. A wide range of side effects occur in up to 30% to 40% of all patients. They are mostly mild and nonspecific and should be accepted as an indicator of the drug effect. They include symptoms such as chest discomfort, dizziness, shortness of breath, and headache.24 Side effects are commonly reversed with a single intravenous dose of aminophylline (75 to 125 mg), although the dose may have to be repeated on some occasions, or rarely nitroglycerin is used. Aminophylline occupies endothelial adenosine binding sites and ends dipyridamole-induced vasodilation. Aminophylline is administered 3 to 4 minutes after administration of the radionuclide to allow adequate time for radiotracer extraction, and then the dipyridamole test is ended. It should be given slowly, however, to avoid some of its own side effects, including nausea, tachycardia, and hypotension. Unlike with adenosine, whose hemodynamic effects occur during the drug infusion, a fall in blood pressure and an increase in heart rate occur when the dipyridamole infusion is completed. There is generally no significant change in the double product and myocardial oxygen demand.
Inotropic Agents
Dobutamine, an adrenergic agonist, is generally used in patients with severe chronic obstructive pulmonary disease or asthma (Fig. 21-4). Dobutamine stimulates α1 receptors and β1 receptors, increasing myocardial contractility, and β2 receptors, which may cause hypotension due to peripheral vasodilation. As a consequence, there is an increase in heart rate, systolic blood pressure, cardiac output, and stroke volume. The cardiac effect varies, depending on the infused dose. At doses up to 10 µg/kg/min, dobutamine preferentially stimulates α1 and β1 receptors to increase contractility, making it a good tool for assessment of myocardial viability. Increased chronotropic effects occur at higher doses, with a subsequent increase in contractility and thickening that may be useful for identification of hibernating myocardium. When it is performed with echocardiography, the infusion is stopped when wall motion abnormalities develop. Dobutamine infusion should be maintained while the radiotracer is distributed. In recent years, different protocols have been recommended.25
Dobutamine is infused in a stepwise increased titration at 3-minute intervals, from low (5 to 10 µg/kg/min) to high (40 µg/kg/min) doses, with increments that are based on the patient’s symptoms to produce ischemia. The endpoint of the dobutamine test is a true ischemic response. The main side effect is nonsustained tachycardia; however, because of its ischemic mechanism, it has increased risks for development of serious events in patients with known severe proximal CAD or dynamic coronary syndromes. Because of its peripheral vasodilatory effect, dobutamine has been described to produce hypotension at higher doses in 20% of the patients.26 β Blockers are the antidote to dobutamine side effects. Esmolol drip should also be available in serious situations because of the short half-life of dobutamine (2.4 minutes). Dobutamine has been reported to have a diagnostic sensitivity of 91% and specificity of 86% in patients with suspected CAD.27 Dobutamine stress SPECT is more sensitive than dobutamine echocardiography, and it is generally used as an alternative to dipyridamole or adenosine in the presence of their contraindications. Dobutamine is the only pharmacologic stress for use with echocardiography.
BASIC IMAGE INTERPRETATION
As a general rule, use of a computer monitor and a linear color scale is recommended to interpret the results, even though the gray scale has been largely used as well. It is critical to follow a systematic approach to the images and to properly align stress and rest images, displayed on the three standard image sets—short axis, vertical long axis, and horizontal long axis (Figs. 21-5 to 21-16). Review of the projection (raw) data is crucial to provide evidence for motion of the patient that would create artifacts and reduce diagnostic accuracy. Projection data also provide information on the cardiac size and other potential areas of thoracic radiotracer uptake, such as lung uptake. Lung uptake in the presence of reversible perfusion defects is another sign of more severe disease. Although it is sometimes difficult to fully appreciate lung uptake in SPECT images, additional projection images should help accurately identify this finding.28 Extracardiac radiopharmaceutical activity may reflect a variety of neoplastic lesions, either primary or metastatic, that may involve the lung, breast, thyroid, parathyroid glands, mediastinum, or liver. In addition, intense subdiaphragmatic activity from the gastrointestinal tract or from the liver may confound the interpretation of the inferior wall by creating virtual perfusion defects, or it may obscure the presence of a true perfusion abnormality. When substantial activity is noted in the gastrointestinal tract, the image acquisition should be repeated. Soft tissue attenuation, such as breast activity, may reduce the specificity for the detection of CAD. Rotating SPECT images will clarify this point and help in the correct image interpretation.
1 Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87-94.
2 Pohost GM, Zir LM, McKusick KA, et al. Differentiation of transiently ischemic from infarcted myocardium by serial imaging after a single dose of thallium-201. Circulation. 1977;55:294-302.
3 Dilsizian V, Bonow R. Current diagnostic techniques of assessing myocardial viability in patients with hibernating and stunned myocardium. Circulation. 1993;87:1-20.
4 Dilsizian V, Rocco TP, Freedman NMT, et al. Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress-redistribution imaging. N Engl J Med. 1990;323:141-146.
5 Zimmerman R, Mall G, Rauch B, et al. 201Tl activity in irreversible defects as a marker of myocardial viability: clinicopathological study. Circulation. 1995;91:1016-1021.
6 Perrone-Filardi P, Pace L, Prastaro M, et al. Assessment of myocardial viability in patients with chronic coronary artery disease: rest–4-hour–24-hour 201Tl tomography versus dobutamine echocardiography. Circulation. 1996;94:2712-2719.
7 Galassi AR, Centamore G, Fiscella A, et al. Comparison of rest-redistribution thallium-201 imaging and reinjection after stress-redistribution for the assessment of myocardial viability in patients with left ventricular dysfunction secondary to coronary artery disease. Am J Cardiol. 1995;75:436-442.
8 Qureshi U, Nagueh SF, Afridi I, et al. Dobutamine echocardiography and quantitative rest-redistribution 201Tl tomography in myocardial hibernation: relation of contractile reserve to 201Tl uptake and comparative prediction of recovery of function. Circulation. 1997;95:626-635.
9 Wagdy HM, Christian TF, Miller TD, Gibbons RJ. The value of 24-hour images after rest thallium injection. Nucl Med Commun. 2002;23:629-637.
10 Dilsizian V, Arrighi JA, Diodati JG, et al. Myocardial viability in patients with chronic coronary artery disease. Comparison of 99mTc-sestamibi with thallium reinjection and 18F-fluorodeoxyglucose. Circulation. 1994;89:578-587.
11 Maes AF, Borgers M, Flameng W, et al. Assessment of myocardial viability in chronic coronary artery disease using technetium-99m sestamibi SPECT. Correlation with histologic and positron emission tomographic studies and functional follow-up. J Am Coll Cardiol. 1997;29:62-68.
12 Levine MG, McGill CC, Ahlberg AW, et al. Functional assessment with electrocardiographic gated single-photon emission computed tomography improves the ability of technetium-99m sestamibi myocardial perfusion imaging to predict myocardial viability in patients undergoing revascularization. Am J Cardiol. 1999;83:1-5.
13 Rambaldi R, Poldermans D, Bax JJ, et al. Dobutamine stress echocardiography and technetium-99m-tetrofosmin/fluorine 18-fluorodeoxyglucose single-photon emission computed tomography and influence of resting ejection fraction to assess myocardial viability in patients with severe left ventricular dysfunction and healed myocardial infarction. Am J Cardiol. 1999;84:130-134.
14 Vanzetto G, Janier M, Fagret D, et al. Metabolic myocardial viability assessment with iodine 123–16-iodo-3-methylhexadecanoic acid in recent myocardial infarction: comparison with thallium-201 and fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1997;24:170-178.
15 Berman DS, Kiat HS, Van Train KF, et al. Myocardial perfusion imaging with technetium-99m-sestamibi: comparative analysis of available imaging protocols. J Nucl Med. 1994;35:681-688.
16 Kiat H, Germano G, Friedman J, et al. Comparative feasibility of separate or simultaneous rest thallium-201/stress technetium-99m-sestamibi dual-isotope myocardial perfusion SPECT. J Nucl Med. 1994;35:542-548.
17 Bateman TM, Berman DS, Heller GV, et al. American Society of Nuclear Cardiology position statement on electrocardiographic gating of myocardial perfusion SPECT scintigrams. J Nucl Cardiol. 1999:470-471.
18 Azarbal B, Hayes SW, Lewin HC, et al. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol. 2004;44:423-430.
19 Klocke FJ, Bairb MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary. J Am Coll Cardiol. 2003;42:1318-1333.
20 Takeishi Y, Chiba J, Abe S, et al. Adenosine-induced heterogeneous perfusion accompanies myocardial ischemia in the presence of advanced coronary artery disease. Am Heart J. 1994;127:1262-1268.
21 Iskandrian A. State of the art for pharmacologic stress imaging. In: Zaret BL, Beller G, editors. Nuclear Cardiology: State of the Art and Future Directions. 2nd ed. St. Louis: Mosby; 1998:312-330.
22 Sharir T, Rabinowitz B, Livschitz S, et al. Underestimation of extent and severity of coronary artery disease by dipyridamole stress thallium-201 single-photon emission computed tomographic myocardial perfusion imaging in patients taking antianginal drugs. J Am Coll Cardiol. 1998;31:1540-1546.
23 Pennell DJ, Mavrogeni SI, Forbat SM, et al. Adenosine combined with dynamic exercise for myocardial perfusion imaging. J Am Coll Cardiol. 1995;25:1300-1309.
24 Lette J, Tatum JL, Fraser S, et al. Safety of dipyridamole testing in 73,806 patients: the Multicenter Dipyridamole Safety Study. J Nucl Cardiol. 1995;2:3-17.
25 Secknus MA, Marwick TH. Evolution of dobutamine echocardiography protocols and indications: safety and side effects in 3011 studies over 5 years. J Am Coll Cardiol. 1997;29:1234-1240.
26 Marcovitz PA, Bach DS, Mathias W, et al. Paradoxic hypotension during dobutamine stress echocardiography: clinical and diagnostic implications. J Am Coll Cardiol. 1993;21:1080-1086.
27 Bonow RO. Diagnosis and risk stratification in coronary artery disease: nuclear cardiology versus stress echocardiography. J Nucl Cardiol. 1997;4:S172-S178.
28 Veilleux M, Lette J, Mansur A, et al. Prognostic implications of transient left ventricular cavity dilation during exercise and dipyridamole-thallium imaging. Can J Cardiol. 1994;10:259-266.

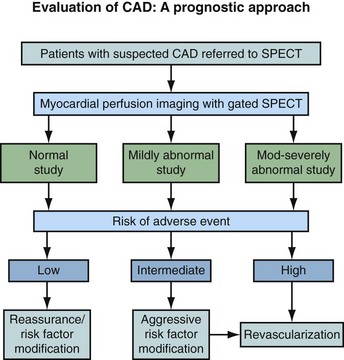
 FIGURE 21-1
FIGURE 21-1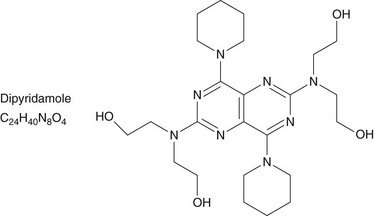
 FIGURE 21-2
FIGURE 21-2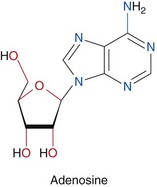
 FIGURE 21-3
FIGURE 21-3
 FIGURE 21-4
FIGURE 21-4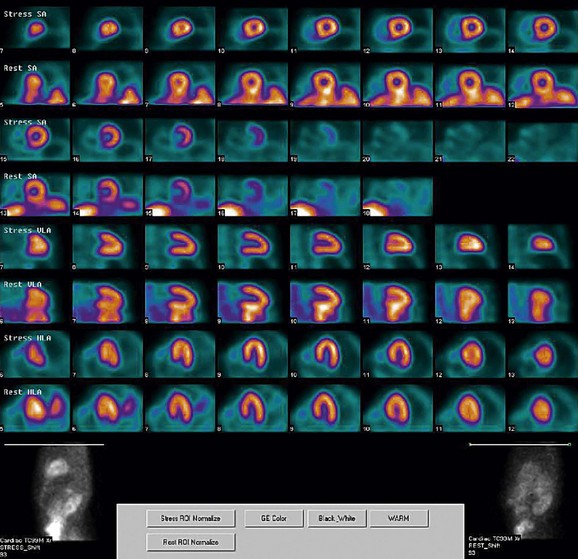
 FIGURE 21-5
FIGURE 21-5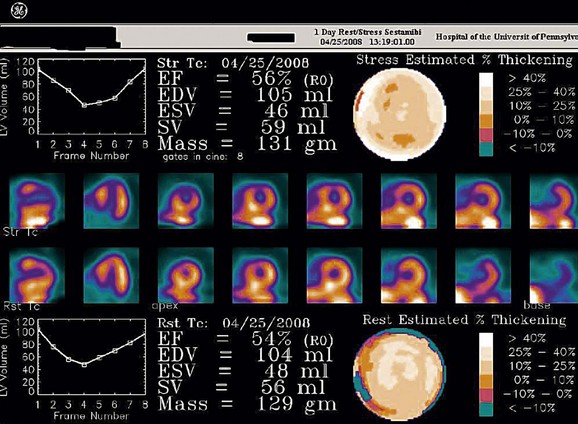
 FIGURE 21-6
FIGURE 21-6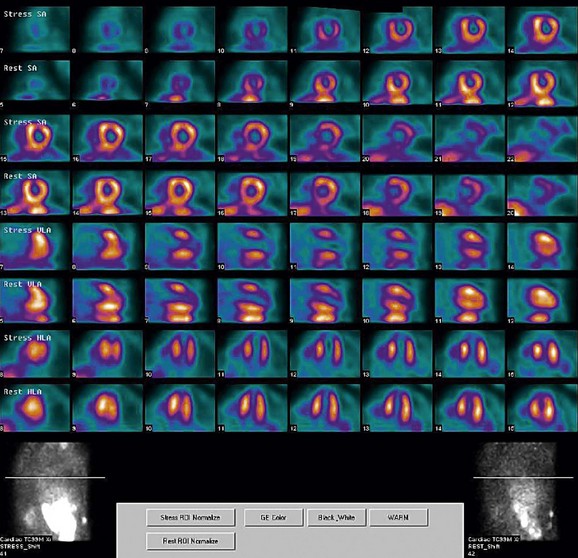
 FIGURE 21-7
FIGURE 21-7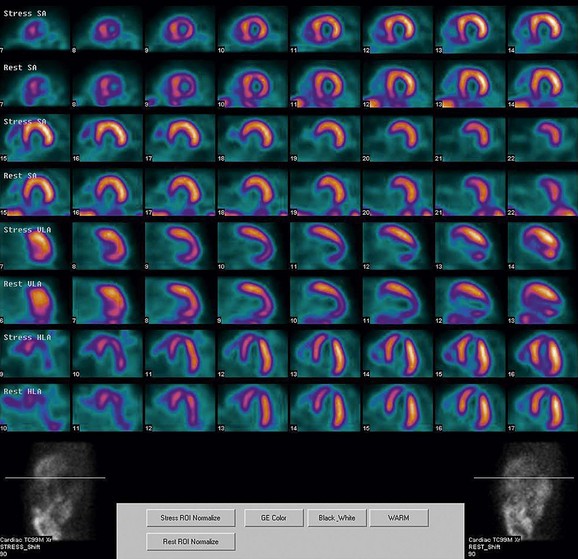
 FIGURE 21-8
FIGURE 21-8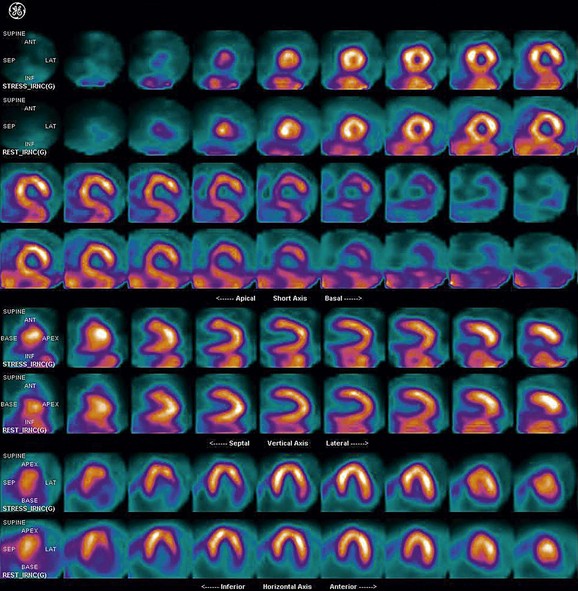
 FIGURE 21-9
FIGURE 21-9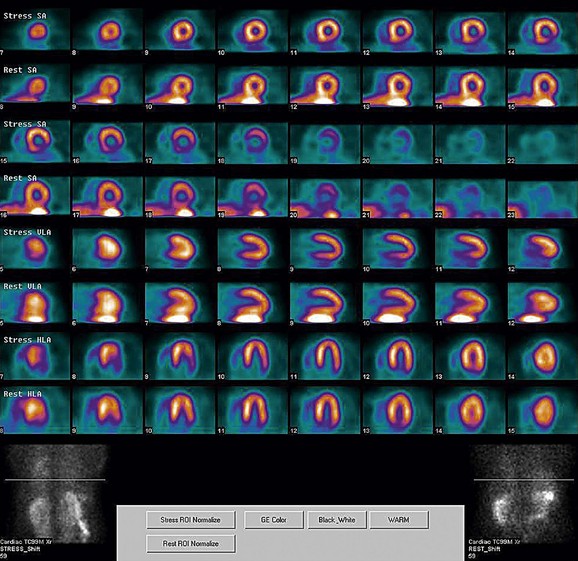
 FIGURE 21-10
FIGURE 21-10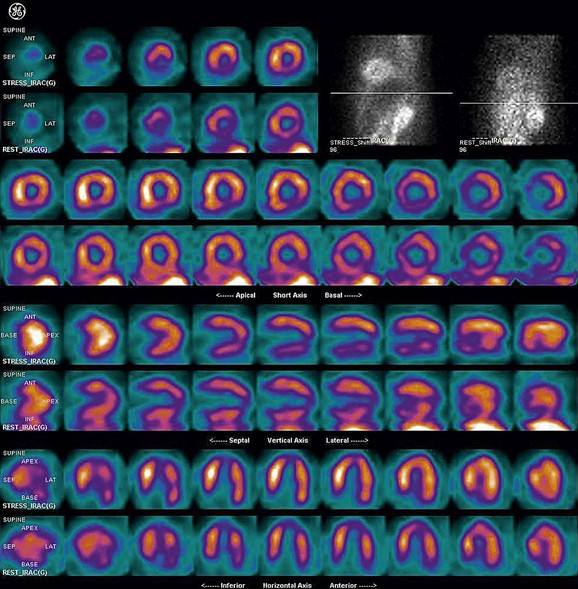
 FIGURE 21-11
FIGURE 21-11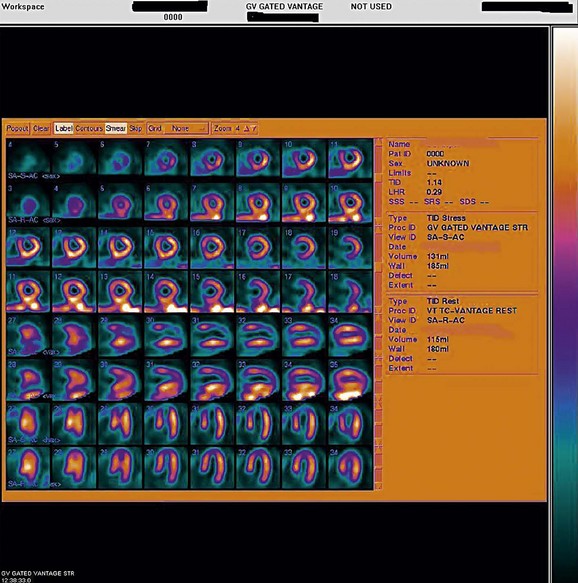
 FIGURE 21-12
FIGURE 21-12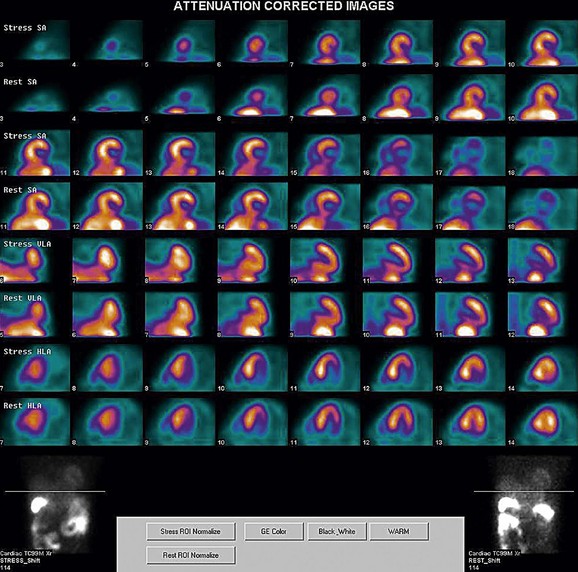
 FIGURE 21-13
FIGURE 21-13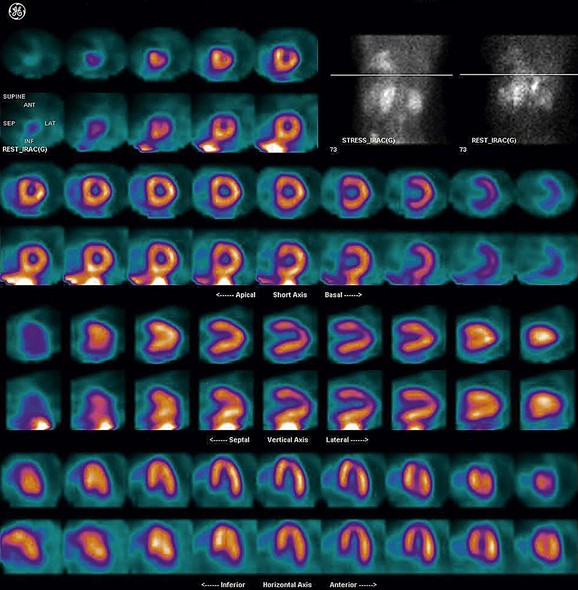
 FIGURE 21-14
FIGURE 21-14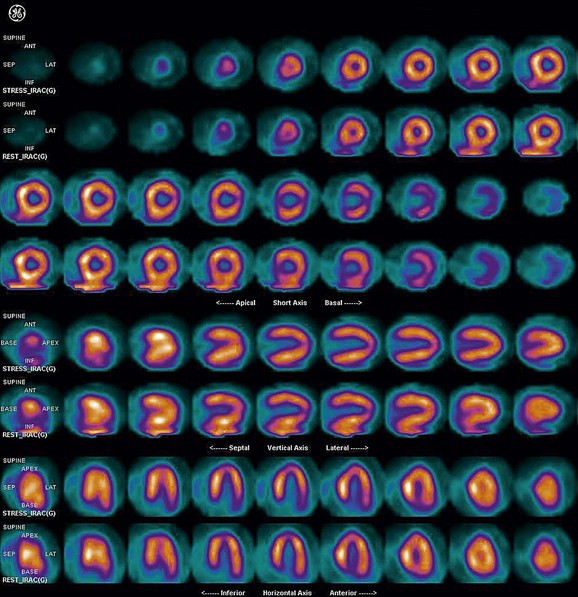
 FIGURE 21-15
FIGURE 21-15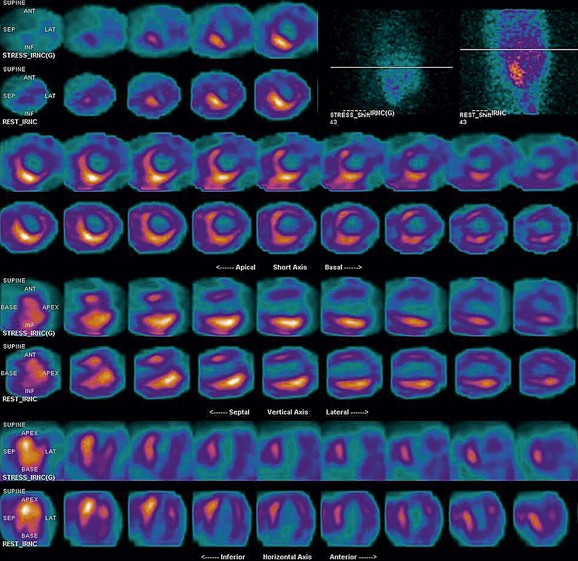
 FIGURE 21-16
FIGURE 21-16


