Chapter 30 Clinical Presentation and Diagnosis of Cerebrovascular Disease
Stroke is a common and serious disorder. Each year stroke affects almost 800,000 people in the United States and about 16 million people throughout the world.1 Associated high morbidity and mortality provide impetus for improving diagnosis, acute management, and prevention of strokes. A full understanding of how patients with stroke and cerebrovascular disease come to medical attention, along with a logical approach for defining the mechanism of stroke, are needed for safe and effective implementation of acute therapies and prevention strategies. This chapter will focus on clinical manifestations of all types of cerebrovascular disease and how clinicians can approach diagnostic evaluation.
Overview of Clinical Stroke
Stroke and cerebrovascular disease are caused by some disturbance of the cerebral vessels in almost all cases. In simple terms, we can divide stroke into two major types: ischemic and hemorrhagic. Ischemic stroke is the most common variety and is responsible for 80% to 85% of all strokes; hemorrhagic stroke accounts for the remainder.2 On occasion, an ischemic stroke can undergo secondary hemorrhagic transformation; likewise, a cerebral hemorrhage (particularly a subarachnoid hemorrhage [SAH]) can cause a secondary ischemic stroke via vasospasm.
Clinical Manifestations of Stroke and Cerebrovascular Disease
Stroke is similar to real estate in that much of its presentation and prognosis depend on size and location. The area of brain involved dictates presenting symptoms. Blood vessels that supply different parts of the brain are affected by different types of cerebrovascular disease and have different mechanisms (pathophysiology) for the stroke. This concept greatly influences and defines the approach a vascular neurologist or neurosurgeon uses when assessing patients with a stroke or cerebrovascular disease.3,4
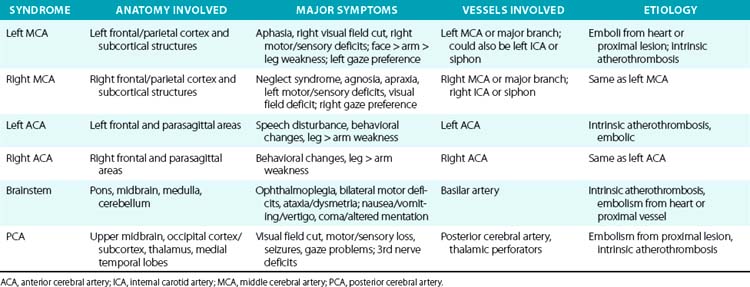
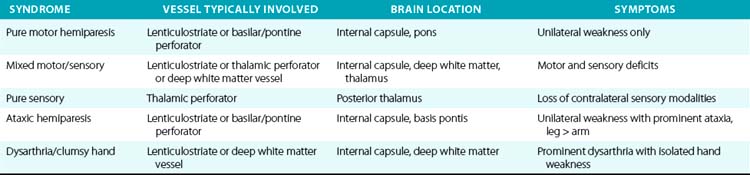
For example, a patient with evidence of involvement of the left hemispheric cortex (e.g., aphasia, visual field defect, weakness of contralateral face and arm) is likely to have a process involving the left middle cerebral artery (MCA). If head computed tomography (CT) does not show evidence of a hemorrhage, likely etiologies would include an embolic event from the heart (e.g., atrial fibrillation) or an artery-to-artery embolism (as might be seen with a high-grade lesion at the carotid bifurcation in the neck). Another patient with a pure motor hemiparesis but no other deficits is likely to have a lesion affecting the motor pathways in the internal capsule, often due to occlusion of a small penetrating artery (lenticulostriate vessel) deep in the brain. Most ischemic strokes will respect the vascular territory of one or more arteries.5 Indeed, lesions that do not respect typical arterial territories lead to concern for a nonvascular process (e.g., tumor, infection). Common ischemic stroke syndromes can be found in Tables 30-1 and 30-2.
Similar reasoning holds true for most cases of hemorrhagic stroke. Intracerebral hemorrhage often presents with abrupt onset of symptoms, but close questioning may reveal that symptoms actually progressed over 15 to 30 minutes as the hematoma grew and expanded.6 Subarachnoid hemorrhage is often characterized by sudden onset of the worst headache of one’s life, with significant nausea, vomiting, and stiff neck in many cases. The phrase “worst headache of my life” is so characteristic of SAH that a patient who presents to the physician or emergency department with that symptom complex is assumed to have SAH until proven otherwise.7
Transient Ischemic Attack
A transient ischemic attack (TIA) is often a prodrome to an ischemic stroke. Symptoms of a TIA are identical to those of a stroke, but with resolution within 24 hours (according to the old definition of a TIA). In reality, most TIA syndromes last just a few minutes, not many hours. In fact, modern brain imaging using magnetic resonance imaging (MRI) with diffusion-weighted sequences has now shown that 25% to 30% of patients with a TIA lasting 30 min to 2 hours will have a new diffusion-weighted imaging (DWI) lesion on MRI indicating a stroke based on a tissue definition.8 Transient ischemic attack symptoms lasting 6 hours or longer have a 50% likelihood of having a new stroke on MRI with DWI techniques. Therefore the perceived distinction between a TIA and a stroke should be viewed more as a continuum from minor transient neuronal dysfunction to permanent brain infarction.
Although it was once thought that the risk of stroke after a TIA was low, new imaging studies as well as epidemiological studies have proven this is not the case. Based on purely clinical criteria (not MRI results), several recent studies have shown that after a TIA, 10% of patients will have a stroke within 3 months, and half those strokes (5%) will occur within 48 hours of the initial TIA. About 25% of patients with a TIA will have a stroke, myocardial infarction (MI), death, recurrent TIA, or be hospitalized within the next 3 months.9 Based on these poor outcomes, recently published guidelines recommend hospital admission for patients with a recent TIA.8
Further studies have attempted to better define those patients with a TIA who are at higher risk of having a stroke within the next 2 to 7 days. Several scoring systems have been developed (Table 30-3) that may be useful for assessing such risks. Of course, any such assessment tool must be tempered by good clinical judgment and consideration of all clinical factors.
| ABCD | Age, blood pressure, clinical symptoms, duration |
| ABCD2 | Age, blood pressure, clinical symptoms, duration, diabetes |
| ABCD2I | Age, blood pressure, clinical symptoms, duration, infarction |
| Age: 60 years or greater = 1 point Blood pressure: systolic 140 mmHg or greater = 1 point or diastolic 90 mmHg or greater = 1 point Clinical symptoms: unilateral weakness = 2 points; speech disturbance without weakness = 1 point Duration: 60 minutes or more = 2 points; 10-59 minutes = 1 point Diabetes: 1 point (on antidiabetic medications) Infarction: evidence for acute ischemic stroke on CT or MRI |
|
CT, computed tomography; MRI, magnetic resonance imaging.
From Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al: Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 369:283–292, 2007.50
Several types of TIAs deserve special mention because of their unique presentations. One is sudden blindness in one eye, which typically occurs as a “shade coming down” over the eye. Some patients report a graying out of vision in the eye, like looking through a gray haze or cloud. This type of TIA is often referred to as amaurosis fugax. This symptom complex typically resolves in a few minutes, although it can last for several hours. There is sometimes pain in or around the eye, but patients usually do not have any other focal neurological complaints. Some cases of amaurosis are due to emboli to the retinal circulation from an ulcerated plaque in or near the carotid bifurcation in the neck. Other cases can be due to local disease in the ophthalmic artery or in the posterior ciliary artery that supplies the optic nerve.10
The other unique type of TIA is the limb-shaking TIA. This typically involves the arm or leg on one side of the body. Patients report uncontrollable shaking of a limb that can be precipitated by movement. These spells can last seconds to minutes. They are not epileptic in origin; electroencephalogram (EEG) is unremarkable. These TIAs are associated with severe stenosis of the contralateral internal or common carotid artery.11 Once the carotid artery is opened (usually with an endarterectomy), the spells cease.
Lastly is the topic of crescendo TIAs. This refers to a pattern where TIAs are recurrent, last longer, or are more severe in nature. This is a very worrisome type of TIA and is associated with a risk of stroke as high as 25% to 50% over the next few weeks.12
Some hemorrhagic strokes may also have a TIA equivalent, namely the sentinel headache before a SAH. The sentinel headache present as an acute headache that is unusual in terms of its nature, severity, and onset. It typically lasts more than an hour but does not have other impressive focal neurological findings and resolves prior to the definitive SAH presentation. Sentinel headaches occur in 25% to 50% of patients with a subsequent aneurysmal SAH and typically antedate the SAH by days to weeks (average 2 weeks).13,14 It is thought that most of these headaches are due to either minor leakage from a fragile aneurysm or enlargement of the aneurysm, resulting in pressure on a nearby structure that produces pain.
Ischemic Stroke Syndromes
There are numerous manifestations of ischemic stroke, and they can be classified based on brain location involved, artery affected, or symptoms produced. Although advanced diagnostic techniques have altered some of the clinical rules of stroke symptoms and etiology, there are still some useful concepts that can guide us in terms of stroke location and mechanism. Tables 30-1 and 30-2 list some classic ischemic stroke syndromes with their major clinical manifestations, vascular territory, and underlying pathophysiology.5
Broadly speaking, ischemic strokes typically involve one or more vessels or vascular territories and produce a clinical picture of focal neurological deficit. Typically, clinicians look for unilateral weakness or sensory deficits, unilateral visual field abnormalities, speech disturbance (aphasia or dysarthria), neglect syndromes, unilateral ataxia, ophthalmoplegias, or gaze abnormalities as clues of a stroke. Symptoms such as vague diffuse weakness alone, headaches alone, memory loss, abnormal behavior, or isolated dizziness are rarely caused by an ischemic stroke. The appearance of a lesion in a typical vascular territory (based on brain imaging) is a key feature of almost all stroke syndromes.4
Presence of cortical deficits (aphasia, visual field cuts, neglect syndromes) often indicates involvement of a major cerebral vessel in the cerebral hemispheres. Presence of ataxia, bilateral motor or sensory deficits, Horner’s syndrome, ophthalmoplegias, and crossed sensory findings (one side of the face and the other side of the body) often indicates a stroke in the posterior (vertebral-basilar) territory. There are specific syndromes that indicate small-vessel involvement deep in the brain. These so-called lacunar strokes are due to occlusion of small penetrating arteries that arise directly from larger parent vessels. Favored locations include the deep basal ganglia structures, thalamus, and brainstem (pons). A listing of large-vessel and lacunar syndromes appears in Tables 30-1 and 30-2.
Atherothrombosis accounts for the majority of ischemic strokes. These lesions can occur anywhere in the cerebral vasculature, but they tend to have a preference for specific locations such as the bifurcation of the carotid artery in the neck, intracranial carotid siphons, proximal portion of the middle cerebral artery, mid-portion of the basilar artery, and aortic arch. An atherosclerotic plaque forms over many years, then ruptures causing formation of a superimposed thrombus.15 This atherothrombotic lesion can totally occlude the vessel, produce severe narrowing (leading to watershed ischemia), or be a source of embolic material that embolizes to more distal parts of the cerebral vasculature (artery-to-artery emboli).
Cardiac embolism accounts for 15% to 20% of all ischemic strokes. A variety of conditions such as atrial fibrillation, endocarditis, prior myocardial infarction, valvular disease, and cardiomyopathy often lead to formation of intracardiac thrombi that subsequently embolize to the brain (and other organs).4,16 Most lacunar strokes are due to either lipohyalinosis or microatheromata occluding a small penetrating artery.
Special Cases
Ischemic Stroke in Young Adults
All clinicians see young adults (often defined as ≤ 45 years of age) with ischemic strokes. Such cases often entail a special evaluation because of the unique processes and conditions that can produce strokes in this age group. Many case series have examined the diseases leading to ischemic strokes in the young, and in general they fall into a few major categories: (1) premature atherosclerosis, (2) unusual vascular pathologies, (3) cardiac etiology, (4) coagulopathy, and (5) other diseases.17
Premature atherosclerosis typically occurs in patients with risk factors for atherosclerosis; in some cases these have not been diagnosed or not properly treated. Examples include hypertension, hyperlipidemia, diabetes, smoking, and obesity. The types of uncommon vascular pathologies often seen in young adults with a stroke include dissection of a vessel (often not related to any obvious trauma), fibromuscular dysplasia, moyamoya disease, or a vasculitis related to an inflammatory condition or drug abuse.17 Numerous cardiac processes can lead to strokes in the young, such as congenital heart disease, a patent foramen ovale (particularly with evidence of venous thrombi), valvular disease (infectious or inflammatory), cardiomyopathy, myxoma, papillary fibroelastoma, and many others. Myriad clotting disorders have been associated with strokes in young adults, the most common being lupus anticoagulants, anticardiolipin antibodies, and protein C and protein S deficiency.18 In general, these coagulopathies are more likely to cause venous thrombosis than arterial thrombosis. Clotting disorders related to hematological malignancies can cause both ischemic and hemorrhagic strokes.19 Various systemic diseases are also associated with hypercoagulable states such as inflammatory bowel disease, hemoglobinopathies, elevated homocysteine, and cancer.
The “other” category covers a host of conditions, some rare and some common, that cause strokes in young adults. Migraine headaches and pregnancy are the most common of these. Patients with complex or complicated migraines, prolonged auras, or taking contraceptives have a higher risk of stroke.20 Pregnancy, particularly in the third trimester and up to 3 months postpartum is associated with increased stroke risk, particularly venous thrombosis and cerebral hemorrhage.21,22 Drug abuse is another common cause of ischemic and hemorrhagic strokes in young adults.23 Other rare conditions include CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke), isolated central nervous system (CNS) vasculitis, Sneddon syndrome (combination of a livedo reticular rash, antiphospholipid antibodies, and ischemic stroke), Marfan’s syndrome, and a host of others (especially connective tissue disorders) have been known to cause strokes in this population.
Strokes Related to Systemic Disease
There are a number of unique systemic disorders that cause strokes in patients of any age. Autoimmune diseases, such as lupus, can produce strokes through a variety of mechanisms that include advanced or premature atherosclerosis, vasculitis, hypercoagulable states, and cardioembolic events.24 Sickle cell disease (SCD) also leads to ischemic strokes and hemorrhagic strokes due to myriad processes including a large-vessel arteriopathy, small-vessel occlusion, rupture of moyamoya vessels (producing ICH and/or SAH), and accelerated atherosclerosis due to hypertension and renal failure.25,26
Drug abuse, particularly cocaine, can produce ischemic strokes via a number of processes including vasospasm, cardiac emboli (due to cardiomyopathy), hypertension, and endocarditis.27 Drug abuse can produce an ICH or SAH due to extreme hypertension and necrotizing vasculitis. It is a fallacy to assume that drug abuse only occurs in young patients or those from certain demographic groups. All patients admitted with a stroke should be tested for drug abuse with urine toxicology screens, not excluding those older than 50 years and white-collar professionals.
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is now recognized as increasing the risk of stroke. This is partially because patients with HIV/AIDS are living longer, and some are having strokes as a result of accelerated development of typical stroke risk factors. It is also clear that modern drug therapy for AIDS can increase the risk of stroke (particularly ischemic stroke).28,29
Systemic cancer is a commonly overlooked cause of strokes. Sometimes the stroke diagnosis precedes diagnosis of the underlying cancer. Mechanisms for strokes related to cancer include a hypercoagulable state and nonbacterial thrombotic endocarditis. Oftentimes these strokes are multiple, variable in size, and in different vascular territories.30,31 Such patients may also have deep venous thrombosis (DVT). Liver failure appears to increase risk of ischemic and hemorrhagic stroke.
Intracerebral Hemorrhage
Chronic or acute hypertension is the most common etiology for nontraumatic ICH, and this type of bleed typically occurs in specific brain locations (Table 30-4). As with ischemic stroke, location of the ICH is highly correlated with the type of symptoms produced. Recent studies using serial brain scans have shown that 30% to 40% of ICHs will expand over the first 24 hours after admission; such expansion is almost always associated with clinical worsening.6,32 High blood pressure may be a risk factor for ICH expansion, although this association has not been mechanistically proven.
Table 30-4 Location and Symptoms for Common Types of Intracerebral Hemorrhage
| ICH Location | Likely Etiology | Common Symptoms |
|---|---|---|
| Basal ganglia | Hypertension | Contralateral hemiparesis, speech changes, gaze deviation, altered mentation if large |
| Lobar | Hypertension, CAA | Cortical syndromes, weakness, visual field lesions, altered mentation if large |
| Thalamus | Hypertension | Altered mentation, sensory changes, gaze abnormalities |
| Pons | Hypertension | Coma, gaze and pupil abnormalities, quadriparesis |
| Cerebellum | Hypertension, AVM | Ipsilateral ataxia, dizziness, vertigo, nausea/vomiting |
| Hemispheric cortex | AVM, extreme hypertension, mycotic aneurysm | Headaches, seizures, cortical syndromes |
AVM, arteriovenous malformation; CAA, cerebral amyloid angiopathy; ICH, intracerebral hemorrhage.
Another increasingly common etiology for ICH is cerebral amyloid angiopathy (CAA), which typically affects patients older than 70 years of age. Cerebral amyloid angiopathy is caused by deposition of one or more amyloid proteins within the wall of cerebral small arterioles. A typical CAA bleed occurs in a lobar region (junction of gray matter and white matter), most commonly in the parietal, temporal, and occipital lobes. Intracerebral hemorrhages due to CAA can be multiple and recurrent.33–35 There is a clear association between CAA, ICH, and Alzhemier’s disease. Sometimes an ischemic stroke can undergo hemorrhagic transformation and become an ICH. This occurs in up to 15% of cases of ischemic stroke and is associated with large strokes, cardioembolic strokes, and the use of anticoagulants and thrombolytic agents.
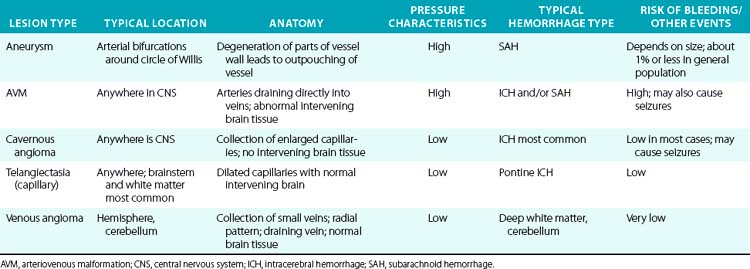
A variety of vascular malformations can cause an ICH, particularly arteriovenous malformations (AVMs) and cavernous malformations (less commonly, capillary telangiectasias and developmental venous anomalies). Arteriovenous malformations are the most common and serious type of vascular malformation that cause an ICH, and recurrent ICHs, as well as producing seizures and local neurological deficits.36 The characteristics and hemorrhagic risk of each of these lesions is shown in Table 30-5.
Intracerebral hemorrhage can occur as a consequence of anticoagulation use, administration of thrombolytic therapy (either for a stroke or another systemic condition), other coagulopathies, hematological disorders, endocarditis, infections (fungal, bacterial, viral), drug abuse (cocaine, heroin, amphetamines), brain tumors (typically metastases), and venous thrombosis.6 Iatrogenic causes of ICH deserve special mention, since there is now extensive use of powerful antiplatelet agents and anticoagulants for a variety of conditions. The use of tissue plasminogen activator (tPA) as well as endovascular therapies (thrombectomy, stenting) as therapies for acute ischemic stroke can lead to ICH (and less commonly SAH).37
Subarachnoid Hemorrhage
As noted earlier, most cases of nontraumatic SAH are due to rupture of a saccular aneurysm that typically occurs at the bifurcation of blood vessels around the circle of Willis at the base of the brain. However, using modern imaging techniques, we can now image aneurysms that occur more distally in the arterial tree. Such lesions are often due to an underlying infection (most commonly endocarditis), although they can be seen as a complication of vasculitis or inherited conditions (polycystic kidney disease, Marfan’s syndrome).38
Subarachnoid hemorrhage typically produces a severe and sudden headache along with nausea/vomiting, nuchal rigidity, and elevated blood pressure. Depending on the location of the ruptured aneurysm, some patients may have additional focal neurological findings. For example, an aneurysm involving the posterior communicating artery can produce an ipsilateral third nerve palsy that involves the pupil. Rupture of an aneurysm of the anterior communicating artery can produce speech and behavioral changes. Aneurysmal rupture that leads to extensive bleeding around the brain and into the ventricles can lead to altered mental status, coma, and sometimes early or sudden death due to dramatic increases in intracranial pressure. A listing of common aneurysm locations and symptoms can be found in Table 30-6.
Table 30-6 Common Locations for Saccular Aneurysms and Related Symptoms in Subarachnoid Hemorrhage
| Location | Clinical Symptoms |
|---|---|
| Anterior communicating artery | Leg weakness, speech disturbance, personality changes, seizures, memory loss |
| Posterior communicating artery/internal carotid artery (ICA) junction | Ipsilateral 3rd nerve palsy |
| Bifurcation of middle cerebral artery (MCA) | Contralateral weakness, sensory changes, speech changes |
| Basilar artery tip | Altered mentation, pupil and gaze abnormalities |
Stroke Mimics
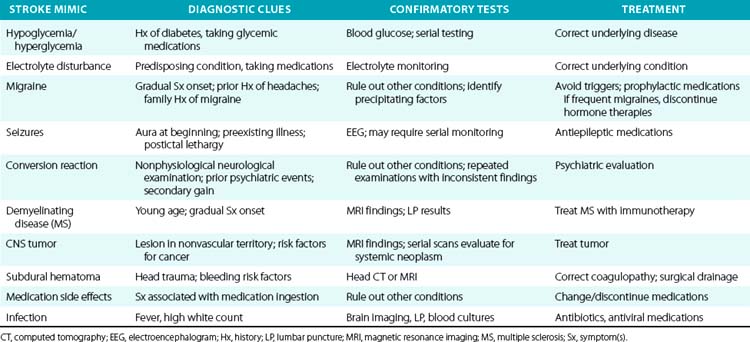
It is incumbent upon the clinician to ensure that a patient with a presumed stroke is having a real cerebrovascular event. Many medical conditions can present with stroke-like symptoms and even physical findings, but with a different etiology. This has obvious implications in terms of acute therapy, ongoing care, and secondary prevention. Table 30-7 lists some common stroke mimics and diagnostic tests that may be helpful for making the diagnosis.
Clinical Assessment Tools
History and Physical
Another key aspect is time of onset of stroke symptoms, since this will determine whether the patient is a candidate for acute intervention (this is of particular importance for ischemic stroke). Time of stroke onset is often (and incorrectly) assumed to be when the patient is found with evidence of a stroke. The correct definition of time of onset is when stroke symptoms first began. If a patient has been under constant observation, the time of onset will be when the patient was first noticed to have stroke symptoms. But if a patient has been home alone and discovered with stroke symptoms by a family member, the time of onset has to be when the patient was last known to be normal (assuming the patient cannot determine the time of onset). Therefore, in the case of a patient who awakens in the morning unable to speak at 7 AM, time of onset is assumed to be when the patient went to bed normal the night before, unless there is clear documentation otherwise. This strict definition essentially rules out many patients with so-called wakeup strokes from receiving acute therapies such as tPA.39
Physical examination will provide valuable information about the likely location of the stroke and suggest the vascular territory and blood vessel or vessels most likely to be involved. As already noted, this is a key step in determining stroke mechanism and etiology. Besides vital signs and a thorough neurological examination, there are particular aspects of the general medical examination that provide important diagnostic information to the clinician. These include an assessment for cervical bruits, a complete cardiac examination, checking blood pressure and pulses in both arms, a skin examination, and evidence of trauma to the head and neck. Table 30-8 offers an outline of a neurological assessment for patients with known or suspected cerebrovascular disease.
Table 30-8 Typical Components of a Neurological Examination Pertinent to Stroke
| Testing Domain | Specific Functions Tested |
|---|---|
| Mentation and cognition | Level of alertness, orientation, speech, naming/repetition, memory, personality, apraxia, agnosia, neglect syndromes |
| Cranial nerves | Testing of nerves II-XII typically performed, including visual acuity and funduscopic examination |
| Motor function | Tone, bulk, abnormal movements; strength, fine movements |
| Cerebellar function | Coordination, rapid movements, balance |
| Gait | Ability to walk, balance, tandem gait |
| Sensory | Pain/pin prick, light touch, vibration/proprioception |
| Reflexes | Deep tendon reflexes, plantar response (Babinski); cutaneous reflexes, primitive reflexes (snout, suck, grasp, palmomental) |
| Vascular system | Auscultation of neck and heart; blood pressure measurements in both arms; check pulses in hands and feet; consider ankle-brachial index (ABI) |
Brain Imaging
Our ability to rapidly and accurately image the brain and cerebral vasculature has been an important step and driver in our capability to determine the type of stroke, its locations, and likely mechanism.40 Almost every hospital in the United States is able to perform a head CT scan on patients in the emergency department. On-site personnel or remote radiology reading technologies and services can provide a reading within 30 to 60 minutes. The ability to rapidly perform and interpret brain imaging is a key component of a primary stroke center.41
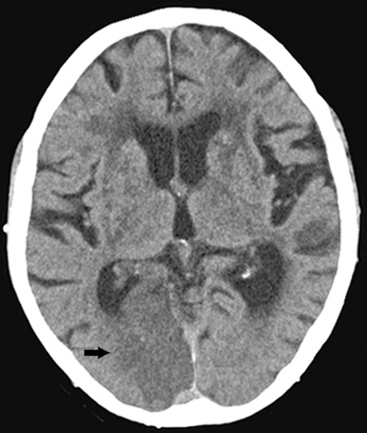
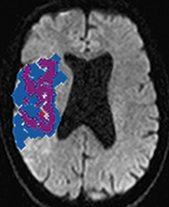
A head CT scan can easily, rapidly, and safely be used to diagnose an acute stroke, especially if it is a hemorrhagic stroke. In some cases when a patient with an ischemic stroke is imaged very soon after symptom onset, the head CT may be negative or show only subtle changes. In such cases, a repeat head CT in 12 to 24 hours will almost certainly show changes indicative of a large or medium-sized ischemic stroke (Fig. 30-1). However, a head CT can miss small and acute strokes, particularly if they are in the brainstem or posterior fossa.
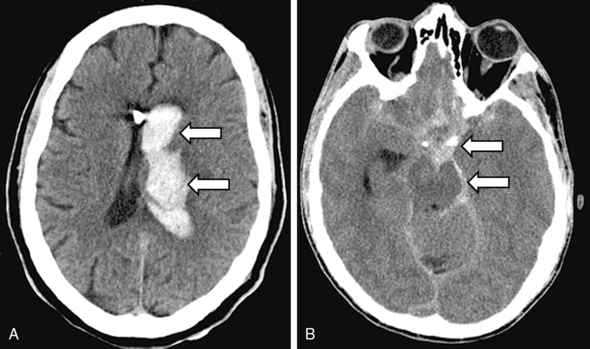
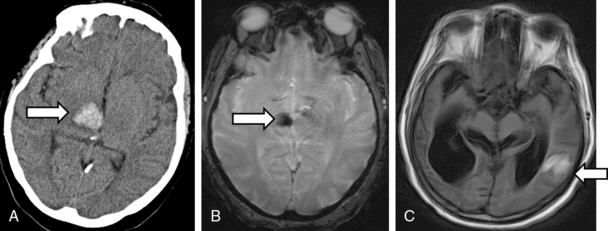
Head CT is very sensitive for imaging hemorrhagic strokes, particularly ICH. Intracerebral hemorrhages appear as white lesions in the brain parenchyma that represent the actual hematoma (Fig. 30-2A). Often there is early evidence of edema around the ICH, which can worsen over several hours and days. In 30% to 40% of ICH cases, the actual hematoma will expand and lead to clinical worsening. In patients with a large SAH, the head CT will show bright signal (blood) at the base of the brain and within some cortical sulci (Fig. 30-2B). However, a small SAH or sentinel bleed may be missed by CT and even MRI. Hence a lumbar puncture is needed to definitively rule out a small SAH.
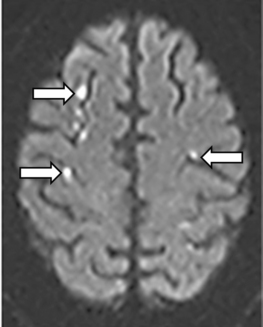
Numerous studies as well as recent guidelines have supported use of brain MRI for assessment of patients with known or suspected strokes.42 Magnetic resonance imaging using DWI techniques is extremely sensitive and accurate for diagnosing essentially all types of ischemic strokes (and some types of hemorrhagic stroke). Magnetic resonance imaging is particularly useful for imaging small strokes, acute strokes, and those in the posterior fossa (Fig. 30-3). In addition to DWI sequences, use of gradient echo sequences allows detection of small amounts of blood. Using this technique, studies have shown that up to 40% of ischemic strokes may have microhemorrhages within the area of ischemia.43
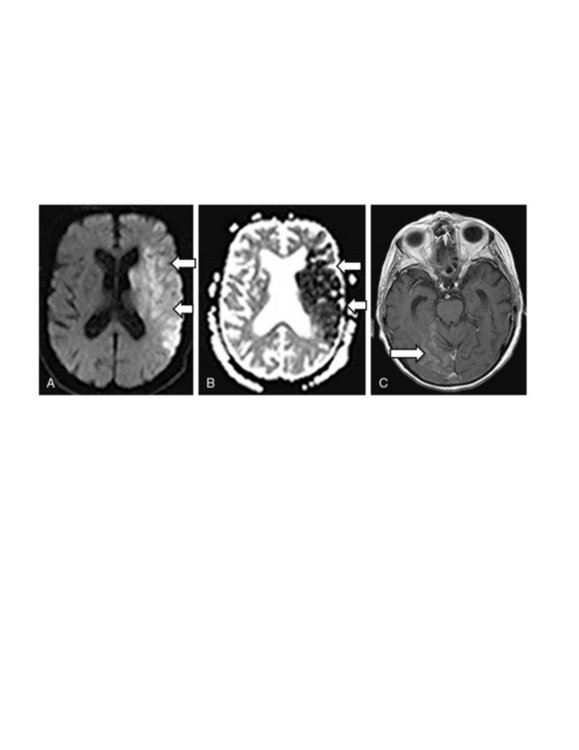
Another advantage of MRI is that it can accurately distinguish acute strokes from subacute strokes using lesion characteristics. An acute ischemic stroke will be bright on DWI, dark on apparent diffusion coefficients (ADC) and not show enhancement with gadolinium (Fig. 30-4A-B). A stroke that is 7 to 10 days old will be less bright on DWI, less dark on ADC, and show enhancement with gadolinium (Fig. 30-4C). A chronic stroke may be bright on DWI (due to T2 shine through), bright on ADC, and show no enhancement.
Advanced MRI techniques are now available that can identify potentially salvageable brain from infarcted brain based on comparing DWI lesions with magnetic resonance (MR) perfusion lesions (Fig. 30-5). Patients with an acute stroke who have a large area of perfusion abnormality on an MR perfusion study but a smaller area of ischemia (DWI lesion) may benefit from reperfusion therapy using lytic or endovascular therapies even 6 to 8 hours after stroke onset. Use of these advanced MRI techniques to select patients with an apparent “ischemic penumbra” is an area of active research.42,44
Magnetic resonance imaging of an ICH is more complex, owing to signal changes caused by metabolism of various blood constituents. About 48 to 72 hours after an ICH, the hemoglobin (Hb) in the hematoma is metabolized into intracellular methemoglobin, which appears bright on the MRI using T1 sequences and dark on T2 sequences. After a week or more, methemoglobin becomes extracellular and becomes bright on T1 and T2 sequences. Hemosiderin is then formed in the hematoma and produces a dark signal in gradient echo sequences45 (Fig. 30-6).
Imaging Cerebral Vasculature
Of equal importance to imaging brain parenchyma is detailed imaging of the cerebral vasculature, both extracranial (aorta, carotid and vertebral arteries) and intracranial vessels.40 Although in most cases we are focusing on the arterial vasculature, in certain cases it is also important to image the cerebral veins (so-called sinuses) to rule out a cerebral venous thrombosis.
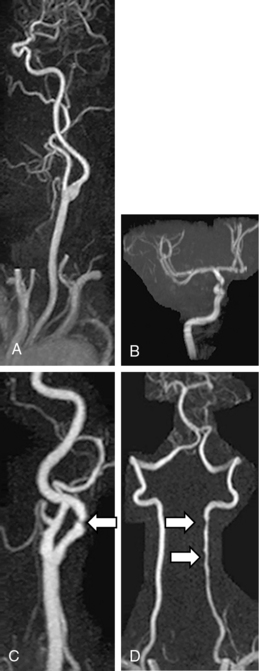
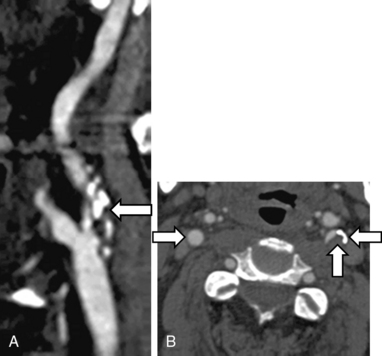
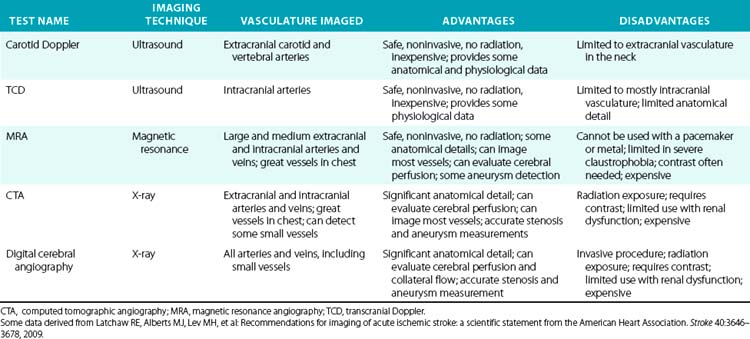
There are several noninvasive modalities available for imaging the cerebral vessels, including magnetic resonance angiography (MRA) (see Chapter 13), computed tomographic angiography (CTA) (see Chapter 14), duplex Doppler ultrasound (see Chapter 12), and transcranial Doppler (TCD) (Figs. 30-7 and 30-8). Each method has certain advantages as well as some limitations (Table 30-9). We typically use either MRA or CTA because they are capable of imaging the entire cerebral vasculature (from the great vessels in the chest to medium-sized intracranial vessels) at one time in only 5 to 10 minutes for CTA, and 25 to 30 minutes for MRA. Computed tomographic angiography requires intravenous contrast agents, whereas Magnetic resonance angiography can be done either with intravenous gadolinium or without (using a time-of-flight [TOF] protocol). Magnetic resonance angiography with contrast (vs. no contrast) provides better images, permitting visualization of small lesions such as dissections or a vasculitis. Computed tomographic angiography provides modestly more precise anatomical detail in terms of its ability to detect small aneurysms and small dissection flaps, and accurately determine the degree of arterial stenoses. However, CTA does expose patients to ionizing radiation.
Laboratory Tests
All patients with a stroke (ischemic or hemorrhagic) require standard testing that should include a complete blood cell count (CBC), chemistry panel, coagulation studies, chest x-ray, electrocardiogram (ECG), urinalysis, and the brain imaging already detailed.46 Again, all patients should undergo toxicology screening for drug use, since this is a common condition, and patients are often not forthcoming about drug abuse. The Centers for Disease Control and Prevention (CDC) recommends HIV testing for most adults who are hospitalized, and this would include patients with an acute stroke.47Table 30-10 lists routine laboratory testing for patients with ischemic stroke.
Table 30-10 Suggested Laboratory Testing for Patients with Stroke
| Routine Tests | Tests for Special Cases* | Comments (for Special Tests) |
|---|---|---|
| CBC with differential | Hb electrophoresis | Useful if SCD or thalassemia suspected |
| Comprehensive chemistry profile | Vasculitis screen (ANA, rheumatoid factor, etc.) | Useful if arteries show beading |
| APTT, PT, INR | Hypercoagulable screen (lupus anticoagulant, anticardiolipin antibodies, protein C and S activity; factor V Leiden and prothrombin gene mutations; cryoglobulin screen; antithrombin III level | Useful for ischemic strokes in young adults; postpartum; cryptogenic strokes |
| Sedimentation rate | Fibrinogen, DIC screen | Detects ongoing thrombosis |
| Fasting lipid profile | CADASIL gene mutation | Characteristic MRI and positive family history |
| Hb A1C | Apolipoprotein E genotype | CAA with recurrent ICH, dementia, MRI changes |
| Homocysteine | Fabry’s disease test | Positive family history; skin changes; renal disease |
| Vitamins B12 and folate | Blood cultures | Suspected endocarditis; multiple strokes |
| Thyroid panel | Assay of clotting factors | Recurrent cerebral hemorrhages |
| HIV | CT of chest/abdomen/pelvis | Look for cancer in patient with recurrent stroke |
| Urine toxicology screen (drug abuse) | ||
| Platelet function tests† |
ANA, antinuclear antibody; APTT, activated partial thromboplastin time; CAA, cerebral amyloid angiopathy; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; (CBC), complete blood cell count; DIC, disseminated intravascular coagulopathy; Hb, hemoglobin; HIV, human immunodeficiency virus; ICH, intracerebral hemorrhage; INR, International Normalized Ratio; MRI, magnetic resonance imaging; PT, prothrombin time; SCD, sickle cell disease.
* Special cases refer to unusual or atypical presentations or stroke syndromes, including cryptogenic etiologies.
† Benefits of platelet function testing for improving efficacy and safety of stroke therapy remain experimental; however, such testing may be important for detecting platelet dysfunction in patients with hemorrhagic stroke.
Special blood tests are warranted if a hypercoagulable state is suspected based on the patient’s age, lack of other risk factors, or if another condition is suspected.48 An elevated D-dimer may indicate ongoing thrombosis. Blood cultures may be obtained in patients with multiple embolic strokes to rule out endocarditis. In patients with suspected vasculitis or a possible autoimmune disorder, tests for inflammatory conditions, such as a sedimentation rate, antinuclear antibody (ANA) titers, and other serologies, may be performed. Hemoglobin electrophoresis can rule out SCD or trait, as well as thalassemia.
Since the heart can be the cause of up to 25% of all strokes, a thorough cardiac assessment is needed in most cases. Beyond the cardiac clinical examination and an electrocardiogram, all patients with an ischemic stroke should receive at least 48 hours of cardiac monitoring using computerized telemetry to detect rhythm changes that could cause a stroke (e.g., atrial fibrillation) as well as dysrhythmia that could indicate underlying coronary artery disease (CAD) or cardiomyopathy (e.g., ventricular tachycardia).46 In some cases, more prolonged monitoring using the Holter device is warranted to further assess for paroxysmal atrial fibrillation. Cardiac imaging including a transthoracic echocardiogram (TTE) or a transesophageal echocardiogram (TEE) is often performed to evaluate cardiac function and assess for presence of a cardiac clot or other structural lesion. Typically we begin with a TTE, and if negative then proceed to the TEE.49
1 Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954.
2 Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421.
3 Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–722.
4 Caplan LR. Diagnosis and treatment of ischemic stroke. JAMA. 1991;266:2413–2418.
5 Kumar S, Caplan LR. Why identification of stroke syndromes is still important. Curr Opin Neurol. 2007;20:78–82.
6 Fewel ME, Thompson BGJr, Hoff JT. Spontaneous intracerebral hemorrhage: a review. Neurosurg Focus. 2003;15:E1.
7 Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396.
8 Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293.
9 Johnston SC, Gress DR, Browner WS, et al. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906.
10 Adams HPJr, Putman SF, Corbett JJ, et al. Amaurosis fugax: the results of arteriography in 59 patients. Stroke. 1983;14:742–744.
11 Baquis GD, Pessin MS, Scott RM. Limb shaking–a carotid TIA. Stroke. 1985;16:444–448.
12 Donnan GA, O’Malley HM, Quang L, et al. The capsular warning syndrome: pathogenesis and clinical features. Neurology. 1993;43:957–962.
13 Gorelick PB, Hier DB, Caplan LR, et al. Headache in acute cerebrovascular disease. Neurology. 1986;36:1445–1450.
14 de Falco F. Sentinel headache. Neurol Sci. 2004:S215–S217.
15 Kannel WB, Wolf PA. Peripheral and cerebral atherothrombosis and cardiovascular events in different vascular territories: insights from the Framingham Study. Curr Atheroscler Rep. 2006;8:317–323.
16 White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331.
17 Adams HPJr, Kappelle LJ, Biller J, et al. Ischemic stroke in young adults. Experience in 329 patients enrolled in the Iowa Registry of stroke in young adults. Arch Neurol. 1995;52:491–495.
18 Urbanus RT, Siegerink B, Roest M, et al. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol. 2009;8:998–1005.
19 Hart RG, Kanter MC. Hematologic disorders and ischemic stroke. A selective review. Stroke. 1990;21:1111–1121.
20 Chen M. Stroke as a complication of medical disease. Semin Neurol. 2009;29:154–162.
21 Feske SK. Stroke in pregnancy. Semin Neurol. 2007;27:442–452.
22 Skidmore FM, Williams LS, Fradkin KD, et al. Presentation, etiology, and outcome of stroke in pregnancy and puerperium. J Stroke Cerebrovasc Dis. 2001;10:1–10.
23 Sloan MA. Illicit drug use/abuse and stroke. Handb Clin Neurol. 2009;93:823–840.
24 Kitagawa Y, Gotoh F, Koto A, et al. Stroke in systemic lupus erythematosus. Stroke. 1990;21:1533–1539.
25 Earley CJ, Kittner SJ, Feeser BR, et al. Stroke in children and sickle-cell disease: Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;51:169–176.
26 Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–2691.
27 Caplan LR, Hier DB, Banks G. Current concepts of cerebrovascular disease–stroke: stroke and drug abuse. Stroke. 1982;13:869–872.
28 Dobbs MR, Berger JR. Stroke in HIV infection and AIDS. Expert Rev Cardiovasc Ther. 2009;7:1263–1271.
29 Ortiz G, Koch S, Romano JG, et al. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257–1261.
30 Cestari DM, Weine DM, Panageas KS, et al. Stroke in patients with cancer: incidence and etiology. Neurology. 2004;62:2025–2030.
31 Kwon HM, Kang BS, Yoon BW. Stroke as the first manifestation of concealed cancer. J Neurol Sci. 2007;258:80–83.
32 Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–2023.
33 Towfighi A, Greenberg SM, Rosand J. Treatment and prevention of primary intracerebral hemorrhage. Semin Neurol. 2005;25:445–452.
34 Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612.
35 Greenberg SM, Eng JA, Ning M, et al. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420.
36 Wilkins RH. Natural history of intracranial vascular malformations: a review. Neurosurgery. 1985;16:421–430.
37 Flaherty ML, Woo D, Kissela B, et al. Combined IV and intra-arterial thrombolysis for acute ischemic stroke. Neurology. 2005;64:386–388.
38 Schuknecht B. High-concentration contrast media (HCCM) in CT angiography of the carotid system: impact on therapeutic decision making. Neuroradiology. 2007;49(Suppl 1):S15–S26.
39 Hills NK, Johnston SC. Why are eligible thrombolysis candidates left untreated? Am J Prev Med. 2006;31:S210–S216.
40 Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646–3678.
41 Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA. 2000;283:3102–3109.
42 Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177–185.
43 Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171.
44 Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: a scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke. 2003;34:1084–1104.
45 Anzalone N, Scotti R, Riva R. Neuroradiologic differential diagnosis of cerebral intraparenchymal hemorrhage. Neurol Sci. 2004;25(Suppl 1):S3–S5.
46 Adams HPJr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–e534.
47 Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11-CE14.
48 Bushnell CD, Goldstein LB. Diagnostic testing for coagulopathies in patients with ischemic stroke. Stroke. 2000;31:3067–3078.
49 de Bruijn SF, Agema WR, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–2534.
50 Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292.