1
Clinical pelvic anatomy
Introduction
A thorough understanding of pelvic anatomy is essential for clinical practice. Not only does it facilitate an understanding of the process of labour, it also allows an appreciation of the mechanisms of sexual function and reproduction, and establishes a background to the understanding of gynaecological pathology. Congenital abnormalities are discussed in Chapter 5.
Obstetric anatomy
The bony pelvis
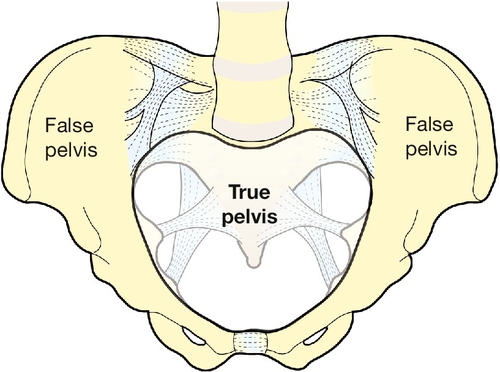
The girdle of bones formed by the sacrum and the two innominate bones has several important functions (Fig. 1.1). It supports the weight of the upper body, and transmits the stresses of weight bearing to the lower limbs via the acetabulae. It provides firm attachments for the supporting tissues of the pelvic floor, including the sphincters of the lower bowel and bladder, and it forms the bony margins of the birth canal, accommodating the passage of the fetus during labour.
The birth canal is bounded by the true pelvis, i.e. that part of the bony girdle which lies below the pelvic brim – the lower parts of the two innominate bones and the sacrum. These bones are bound together at the sacroiliac joints, and at the symphysis pubis anteriorly. The brim is outlined by the promontory of the sacrum, the sacral alae, the iliopectineal lines and the symphysis. The pelvic outlet is bounded by bone and ligament including the tip of the sacrum, the sacrotuberous ligaments, the ischial tuberosities, and the subpubic arch (of rounded ‘Norman’ shape) formed by the fused rami of the ischial and pubic bones. In the erect posture the pelvic brim is inclined at an angle of 65–70° to the horizontal. Because of the curvature of the sacrum, the axis of the pelvis (the pathway of descent of the fetal head in labour) is a J-shaped curve (Fig. 40.4).
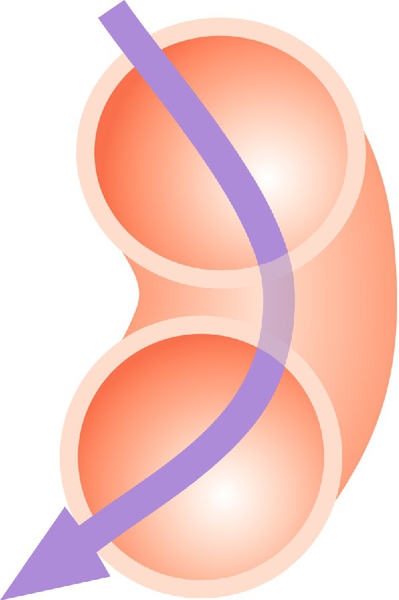
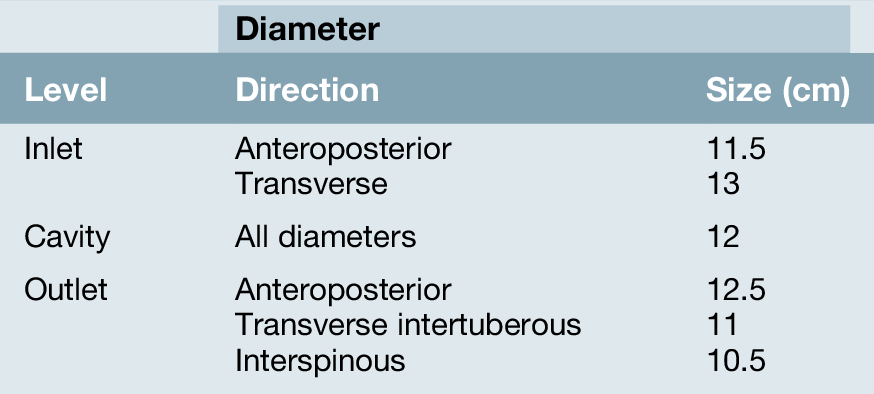
The change in the cross-sectional shape of the birth canal at different levels is fundamentally important in understanding the mechanics of labour. The canal can be envisaged initially as a sector of a curved cylinder of about 12 cm diameter (Fig. 1.2). The stresses of weight bearing at the brim level in the average woman tend to flatten the inlet a little, reducing the anteroposterior diameter, but increasing the transverse diameter. In the lower pelvis, the counterpressure through the necks of the femora tends to compress the pelvis from the sides, reducing the transverse diameters of this part of the pelvis (Figs 40.2 and 40.3). At an intermediate level, opposite the third segment of the sacrum, the canal retains a circular cross-section. With this picture in mind, the ‘average’ diameters of the pelvis at brim, cavity, and outlet levels can be readily understood (Table 1.1).
The distortions from a circular cross-section, however, are very modest. If, in circumstances of malnutrition or metabolic bone disease, the consolidation of bone is impaired, more gross distortion of the pelvic shape is liable to occur, and labour is likely to involve mechanical difficulty. This is termed cephalopelvic disproportion. The changing cross-sectional shape of the true pelvis at different levels – transverse oval at the brim and anteroposterior oval at the outlet – usually determines a fundamental feature of labour, i.e. that the ovoid fetal head enters the brim with its longer (anteroposterior) diameter in a transverse or oblique position, but rotates during descent to bring the longer head diameter into the longer anteroposterior diameter of the outlet before the time of birth. This rotation is necessary because of the relatively large size of the human fetal head at term, which reflects the unique size and development of the fetal brain (Fig. 45.1).
In most affluent countries, marked pelvic deformation is rare. Pelvimetry using X-rays, CT or MRI scans can be used to measure the pelvic diameters but is of limited clinical value in predicting the likelihood of a successful vaginal delivery. Mechanical difficulty in labour is assessed by close observation of the progress of dilatation of the cervix, and of descent, assessed by both abdominal and vaginal examination.
The pelvic organs during pregnancy
The uterus
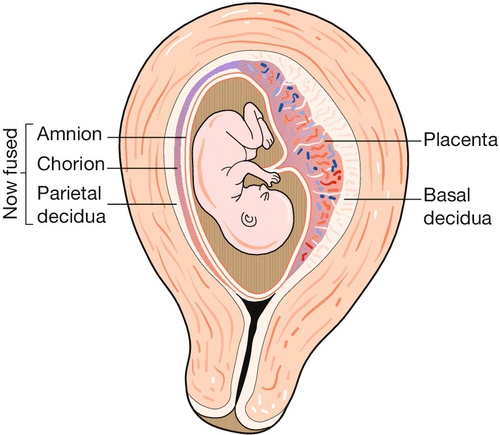
The uterus is a remarkable organ, composed largely of smooth muscle, the myometrium, which increases in weight during pregnancy from about 40 g to around 1000 g as the myometrial muscle fibres undergo both hyperplasia and hypertrophy (Fig. 1.3). It provides a ‘protected’ implantation site for the genetically ‘foreign’ fertilized ovum, accommodates the developing fetus as it grows, and finally expels it into the outside world during labour.
Whereas the body of the uterus is formed from a thick layer of plain muscle, the cervix, which communicates with the upper vagina, is largely composed of denser collagenous tissue. This forms a rigid collar, retaining the fetus in utero as the myometrium hypertrophies and stretches. The junctional area between the body and cervix is known as the isthmus, which, in late pregnancy and labour, undergoes dilatation and thinning, forming the lower segment of the uterus. It is through this thinned area that the uterine wall is incised during caesarean section.
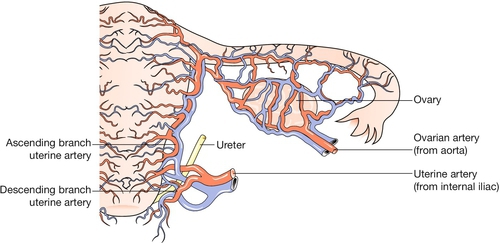
The uterine arteries, branches of the anterior division of the internal iliac arteries, become tortuous and coiled within the uterine wall (Fig. 1.4). Innervation of the uterus is derived from both sympathetic and parasympathetic systems, and the functional significance of the motor pathways is incompletely understood. Drugs which stimulate alpha-adrenergic receptors activate the myometrium, whereas beta-adrenergic drugs have an inhibitory effect, and both beta-agonists and alpha-antagonists have been used in attempts to inhibit premature labour. Afferent fibres from the cervix enter the cord via the pelvic splanchnic (parasympathetic) nerves (S2,3,4). Pain stimuli during labour from the fundus and body of the uterus travel via the hypogastric (sympathetic) plexus, and enter the cord at the level of the lower thoracic segments.
The cervix
This becomes more vascular and softens in early pregnancy. The mucous secretion from the endocervical glands becomes thick and tenacious, forming a mechanical barrier to ascending infection. In late pregnancy the cervix ‘ripens’ – the dense mesh of collagen fibres loosens, as fluid is taken up by the hydrophilic mucopolysaccharides which occupy the interstices between the collagen bundles. This allows the cervix to become shorter as its upper part expands.
Additional changes
The ligaments of the sacroiliac and symphyseal joints become more extensible under the influence of pregnancy hormones. As a result, the pelvic girdle has more ‘give’ during labour. The increased mobility of the joints may result in backache or symphyseal pain.
The urinary tract in pregnancy
Frequency of micturition is often noticed in early pregnancy. As pregnancy advances, the ureters become dilated, probably due to the relaxing effect of progesterone on the smooth muscle wall, but also in part due to the mechanical effects of the gravid uterus. The urinary tract is therefore more vulnerable to ascending infection (acute pyelonephritis) in comparison to non-pregnancy.
The perineum
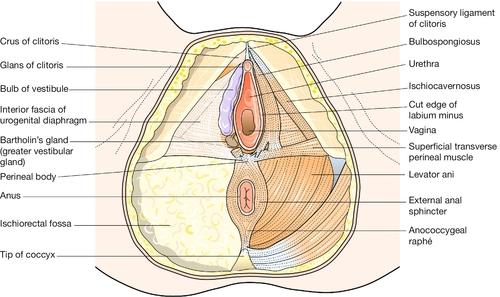
This term usually refers to the area of skin between the vaginal orifice and the anus. The underlying musculature at the outlet of the pelvis, surrounding the lower vagina and the anal canal, is important in the maintenance of bowel and urinary continence, and in sexual response. The muscles intermesh to form a firm pyramidal support, the perineal body, between the lower third of the posterior vaginal wall and the anal canal (Fig. 1.5). The tissues of the perineal body are often markedly stretched during the expulsive second stage of labour and may be torn as the head is delivered. Injury to the anal sphincters may lead to impaired anal continence of faeces and/or flatus. Poor healing of an episiotomy or tear is liable to result in scarring, which may cause dyspareunia (pain during intercourse).
Anatomical points for obstetric analgesia
Pudendal nerve block
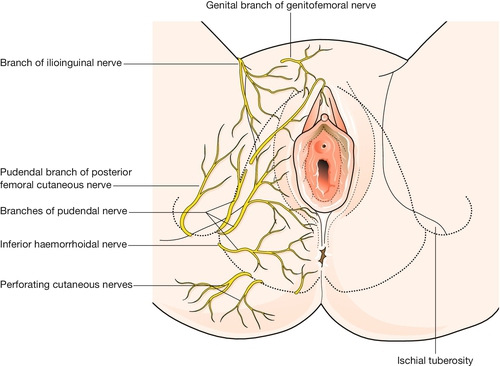
Knowledge of the pudendal nerves is important in obstetrics because they may be blocked to minimize pain during instrumental delivery, and because their integrity is vital for visceral muscular support and for sphincter function. These nerves, which innervate the vulva and perineum, are derived from the second, third and fourth sacral roots (Fig. 1.2). On each side the nerve passes behind the sacrospinous ligament close to the tip of the ischial spine, and re-enters the pelvis along with the pudendal blood vessels in the pudendal canal. After giving off an inferior rectal branch, they divide into the perineal nerves and the dorsal nerves of the clitoris. Motor fibres of the pudendal nerve supply the levator ani, the superficial and deep perineal muscles, and the voluntary urethral sphincter. Sensory fibres innervate the central areas of the vulva and perineum. The peripheral skin areas are supplied by branches of the ilioinguinal nerve, the genitofemoral nerve, and the posterior femoral cutaneous nerve (Fig. 1.6). The pudendal nerve can be blocked by an injection of local anaesthetic just below the tip of the ischial spine, as described on page 346.
Spinal block
The spinal cord ends at the level of L1–2. A spinal injection at the level of the L3–4 space will produce excellent analgesia up to around the level of the T10 nerve root or above, depending on the position of the patient and the volume of local anaesthetic used.
Epidural block
The epidural space, between the dura and the periosteum and ligaments of the spinal canal, is about 4 mm deep. Epidural injection of local anaesthetic blocks the spinal nerve roots as they traverse the space.
Gynaecological anatomy
The uterus
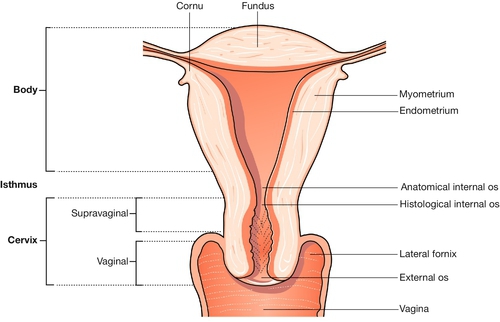
The uterus has the shape of a slightly flattened pear, and measures 7.5 × 5.0 × 2.5 cm. Its principal named parts are the fundus, the cornua, the body and the cervix (Fig. 1.7).
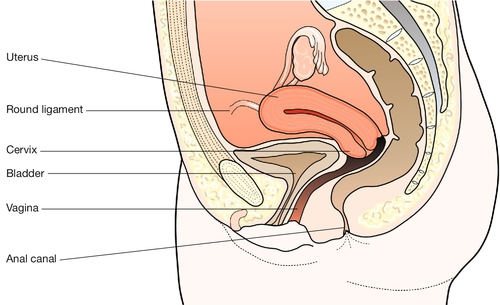
It forms part of the genital tract, lying in close proximity to the urinary tract anteriorly, and the lower bowel behind. All three tracts traverse the pelvic floor in the hiatus between the two bellies of the levator ani muscle. Clinically this means that a problem in one tract can readily affect another (Fig. 1.8).
The uterine cavity is around 6 or 7 cm in length, and forms a flattened slit, with the anterior and posterior walls in virtual contact. The wall has three layers: the endometrium (innermost), the myometrium and the peritoneum (outermost).
Endometrium
The endometrium is the epithelial lining of the cavity. The surface consists of a single layer of columnar ciliated cells, with invaginations forming uterine mucus-secreting glands within a cellular stroma. It undergoes cyclical changes in both the glands and stroma, leading to shedding and renewal about every 28 days.
There are two layers: a superficial functional layer which is shed monthly, and a basal layer which is not shed, and from which the new functional layer is regenerated. The epithelium of the functional layer shows active proliferative changes after a menstrual period until ovulation occurs, when the endometrial glands undergo secretory changes. Permanent destruction of the basal layer will result in amenorrhoea. This fact forms the basis for ablative techniques for the treatment of menorrhagia.
The normal changes in endometrial histology during the menstrual cycle, are determined by changing secretion of ovarian steroid hormones. If the endometrium is exposed to sustained oestrogenic stimulation, whether endogenous or exogenous, it may become hyperplastic. Benign hyperplasia may precede malignant change.
Myometrium
The smooth muscle fibres of the uterine wall do not form distinct layers. While the outermost fibres are predominantly longitudinal, continuous with the musculature of the uterine tubes above and the vaginal wall below, the main thickness of the uterine wall is formed from a mesh of criss-crossing spiral strands. The individual muscle cells contain filaments of actin and myosin, which interact to generate contractions. During labour the propagation of contractile excitation throughout the uterine wall is facilitated by the formation of ‘gap junctions’ between adjacent muscle cells. As a result, the spread of excitation resembles that in a syncytium.
Peritoneum
The posterior surface of the uterus is completely covered by peritoneum, which passes down over the posterior fornix of the vagina into the pouch of Douglas. Anteriorly, the peritoneum is reflected off the uterus at a much higher level onto the superior surface of the bladder.
The cervix
The cervix connects the uterus and vagina, and projects into the upper vagina. The ‘gutter’ surrounding this projection comprises the vaginal fornices – lateral, anterior and posterior. The cervix is about 2.5 cm long; the shorter part of it, which lies above the fornices, is termed the ‘supravaginal part’. The endocervical canal is fusiform in shape between the external and internal os. After childbirth, the external os loses its circular shape and resembles a transverse slit. The epithelial lining of the canal is a columnar mucous membrane with an anterior and posterior longitudinal ridge, from which shallow palmate folds extend; hence the name ‘arbor vitae’.
There are numerous glands secreting mucus which becomes more abundant and less viscous at the time of ovulation in mid-cycle. The vaginal surface of the cervix is covered with stratified squamous epithelium, similar to that lining the vagina. The squamocolumnar junction (histological external os) commonly does not correspond to the anatomical os, but may lie either above or external to the anatomical os. This ‘tidal zone’, within which the epithelial junction migrates at different stages of life, is termed the transformation zone. The ebb and flow of the squamocolumnar junction is influenced by oestrogenic stimulation. In the newborn female, and in pregnancy particularly, outgrowth of the columnar epithelium is very common, forming a bright pink ‘rosette’ around the external os. This appearance has been misnamed an ‘erosion’, but the epithelial covering, though delicate, is intact. In cases where the cervix has undergone deep bilateral laceration during childbirth, the resulting anterior and posterior lips tend to evert, exposing the glandular epithelium of the canal widely. This appearance is termed ‘ectropion’.
Clinical aspects
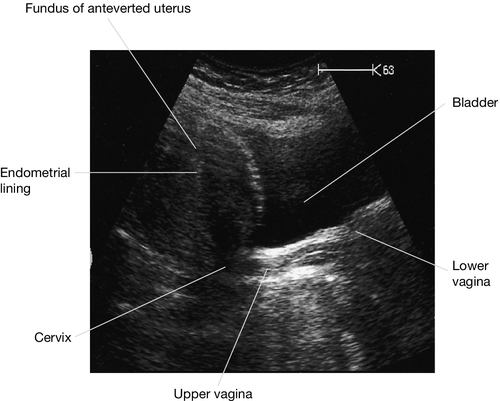
The transformation zone is typically the area where precancerous change occurs. This can be detected by microscopic assessment of a cervical cytological smear. If the duct of a cervical gland becomes occluded, the gland distends with mucus to form a retention cyst (or Nabothian follicle). Multiple follicles are not uncommon, giving the cervix an irregular nodular feel and appearance. The body of the uterus is usually angled forward in relation to the cervix (anteflexion), while the uterus and cervix as a whole lean forward from the upper vagina (anteversion). In about 15% of women the uterus leans backwards towards the sacrum, and is described as retroverted. The cervical os then faces down the long axis of the vagina, rather than at right angles to it. In most instances retroversion is an asymptomatic variant of normality.
It is especially important to distinguish retroversion from anteversion before introducing a sound or similar instrument into the uterine cavity, to avoid perforation of the uterine wall. After the menopause the uterus and cervix gradually become atrophic, and cervical mucus is scanty. The amount of cervix projecting into the vagina also diminishes.
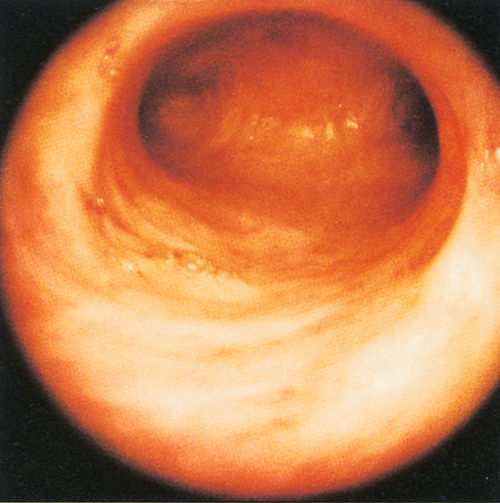
Because the uterus lies immediately behind the bladder, and between the lower parts of the ureters, particular care must be taken not to damage these structures during hysterectomy (Fig. 1.9). The endometrium and uterine cavity can be examined by hysteroscopy. The tubal ostiae can be seen (Fig. 1.10). Because the anterior and posterior walls are normally in contact, the cavity must be inflated with gas or fluid to obtain an adequate view of the surfaces.
The uterine attachments and supports
Structures attached to the uterus include (Fig. 1.11):
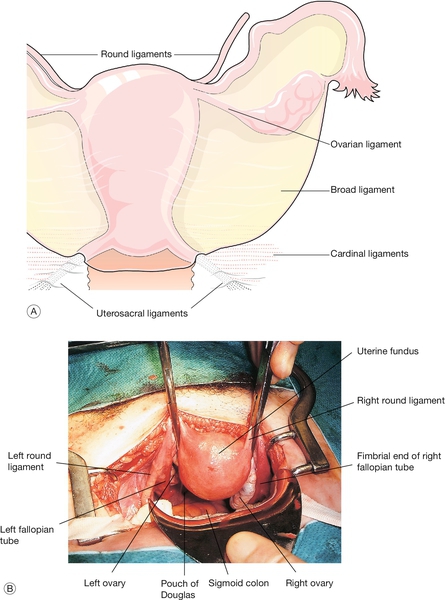
Fig. 1.11The uterus and appendages.
(A) Schematic view of the uterine ligaments seen from behind. (B) View of the uterus, fallopian tubes and ovaries at abdominal hysterectomy.
![]() round ligament
round ligament
![]() ovarian ligament
ovarian ligament
![]() uterosacral ligament or fold
uterosacral ligament or fold
![]() cardinal ligament/transverse cervical ligament (of Mackenrodt).
cardinal ligament/transverse cervical ligament (of Mackenrodt).
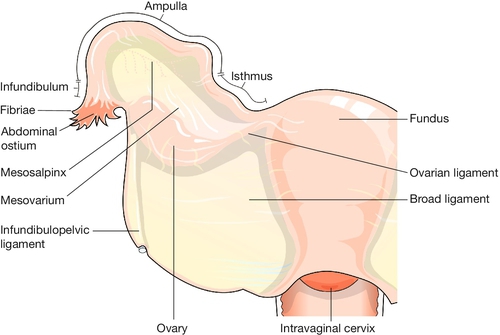
The broad ligament is merely a double fold of peritoneum extending laterally from the uterus towards the pelvic sidewall. The hilum of the ovary arises from its posterior surface. The portion of the fold lateral to the ovary and tube is termed the infundibulopelvic ligament. Between the leaves of this fold, the uterine and ovarian blood vessels form an anastomotic loop. The ovarian ligament forms a ridge on the posterior leaf of the broad ligament, from the cornu of the uterus to the medial pole of the ovary. Developmentally, it is part of the gubernaculum of the ovary, in continuity with the round ligament, which curves round anteriorly from the cornu towards the inguinal canal, through which it passes. The uterosacral ligaments pass upwards and backwards from the posterior aspect of the cervix towards the lateral part of the second piece of the sacrum. In their lower part, they contain plain muscle along with fibrous tissue and autonomic nerve fibres. In their upper part, they dwindle to shallow peritoneal folds. The ligaments divide the pouch of Douglas from the pararectal fossa on each side.
The main ligaments providing support to the internal genital organs are the cardinal ligaments. The traditional name ‘transverse cervical ligaments’ is a misnomer. The cardinal ligaments are essentially dense condensations of connective tissue around the venous and nerve plexuses and arterial vessels, which extend from the pelvic sidewall towards the genital tract. Medially, they are firmly fused with the fascia surrounding the cervix and upper part of the vagina. They pass upwards and backwards towards the root of the internal iliac vessels. These condensations of fibrous and elastic tissue, together with plain muscle fibres, are sometimes referred to as the ‘parametrium’. They support the upper vagina and cervix, helping to maintain the angle between the axis of the vagina and that of the anteverted uterus. Inferiorly they are continuous with the fascia on the upper surface of the levator muscles.
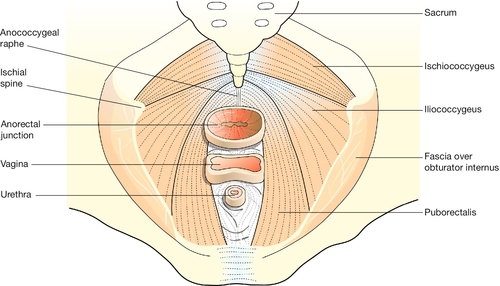
The pelvic diaphragm
Below the level of the cardinal ligaments, the pelvic organs are supported by a sloping shelf of muscle on each side, formed by the levator ani muscle (Fig. 1.12). The disposition of the muscle bundles is comparable with that of the abdominal musculature. Near to the midline there is a longitudinal muscle bundle, the puborectalis (cf. the rectus abdominis). Laterally, the muscle sheets (iliococcygeus and ischiococcygeus) are oblique/transverse. The most medial fibres of puborectalis are inserted into the upper part of the perineal body. The succeeding fibres turn medially behind the anorectal flexure, and are inserted into the anococcygeal raphe and the tip of the coccyx, along with the fibres of ilio- and ischiococcygeus. Thus all three visceral tubes reach the body surface via a hiatus between the medial margins of puborectalis, and all are supported from behind by the sling action of the muscle when it contracts. Innervation is from the pudendal nerve (S2,3,4). The fascia on the upper surface of the pelvic diaphragm blends with the lower part of the cardinal ligaments. The fascia on the inferior surface of levator ani forms the roof of the ischiorectal fossa.
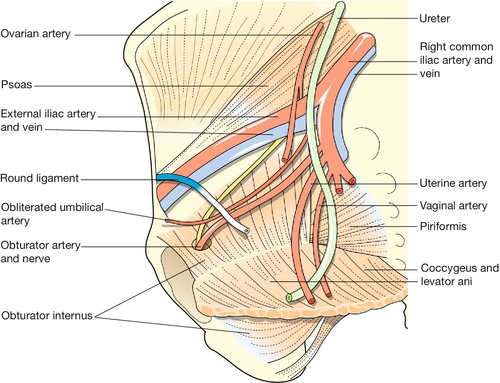
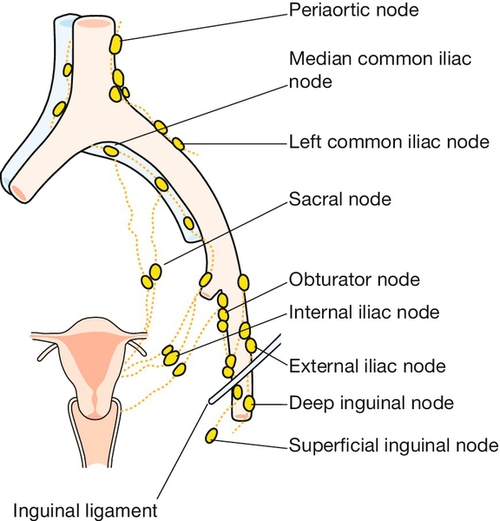
The main blood supply of the uterus is from the uterine arteries, which are branches of the internal iliac vessels (Fig. 1.13). Each passes medially in the base of the broad ligament above the ureter, and ascends along the lateral aspect of the uterus, forming an anastomotic loop in the broad ligament with the ovarian artery (see Fig. 1.4). The uterine veins form a plexus in the parametrium below the uterine arteries, draining into the internal iliac veins. The principal lymph drainage is to iliac and obturator glands on the pelvic sidewall. From the fundus and cornua, lymph drains via the ovarian pathway to aortic nodes, while a few lymphatics in the round ligaments drain into the inguinal nodes (Fig. 1.14). The uterus is supplied by sympathetic and parasympathetic nerves, the exact functional significance of which is uncertain.
Congenital abnormalities of the uterus
Most of the female genital tract develops from the two paramesonephric (Müllerian) ducts, the caudal portions of which approximate in the midline and fuse to form the uterus, cervix and upper part of the vagina. The upper divergent portions of the ducts form the uterine tubes.
Congenital abnormality can result from:
![]() failure of or incomplete fusion
failure of or incomplete fusion
![]() failure of canalization
failure of canalization
![]() asymmetrical maldevelopment.
asymmetrical maldevelopment.
The diagrams in Figure 5.5 illustrate some of abnormalities that may be encountered. Failures of canalization are likely to present at puberty, as menstrual blood has no way to escape. Incomplete fusion is associated with late miscarriage, pre-term labour and malpresentation. Because of the intimate association during development, congenital abnormality of the female genital tract is commonly associated with abnormality of the urinary tract.
The vulva
The term vulva generally encompasses all the external female genitalia, i.e. the mons pubis, the labia majora and minora, the clitoris, and the structures within the vestibule – the external urinary meatus and the hymen. The mons pubis is a thickened pad of fat, cushioning the pubic bones anteriorly. The labia majora contain fatty tissue overlying the vascular bulbs of the vestibule and the bulbospongiosus muscles. The skin of the labia majora bears secondary sexual hair on the lateral surfaces only. There are abundant sebaceous, sweat, and apocrine glands. The folds of the labia minora vary considerably in size, and may be concealed by the labia majora or may project between them. They contain no fat, but are vascular and erectile during sexual arousal; the skin contains many sebaceous glands. Anteriorly, the folds bifurcate before uniting to form a hood above the clitoris and a frenulum along its dorsal surface. Posteriorly, the labia minora are linked by a fine ridge of skin, ‘the fourchette’.
The labia minora and the fourchette form the boundaries of the vestibule. Between the fourchette and the posterior part of the hymen there is a crescentic furrow termed the ‘navicular fossa’. The urethral meatus lies within the vestibule, close to the anterior margin of the vaginal orifice. There are pairs of small mucus-secreting paraurethral glands in the lower part of the posterior wall of the urethra. These rudimentary tubules are homologous with the glands in the male prostate. If they become infected and blocked, they may form a paraurethral abscess, cyst or urethral diverticulum. Two mucus-secreting glands, known as Bartholin’s glands (or greater vestibular glands), lie posterolateral to the vaginal orifice on each side, embedded in the posterior pole of the vascular vestibular bulb (see Fig. 1.5). Their ducts open near the lateral limits of the navicular fossa. The glands only become palpable, and the duct orifices become visible, if infection is present.
Blood supply
The main sources of the vascularity of the vulva are branches of the internal pudendal arteries. There are also branches from the superficial and deep external pudendal arteries.
Nerve supply
The main sensory supply to the vulva is via the pudendal nerves. Peripheral parts of the vulvar skin are supplied by filaments from the iliohypogastric and ilioinguinal nerves, and from the perineal branches of the posterior cutaneous nerves of the thigh (see Fig. 1.6). The pudendal nerve provides motor fibres to all the muscles of the perineum, including the voluntary urinary and bowel sphincters, as well as the levator ani.
Lymph drainage
The main pathway of drainage is to the superficial inguinal glands, and on through the deep inguinal to the external iliac glands. Some lymphatics from the deeper structures of the vulva pass with vaginal lymphatics to the internal iliac nodes.
The fallopian tubes
The tube extends on each side from the cornu of the uterus within the upper border of the broad ligament for about 10 cm. The tubes and ovaries together are commonly described as the uterine appendages, or adnexa (Fig. 1.15).
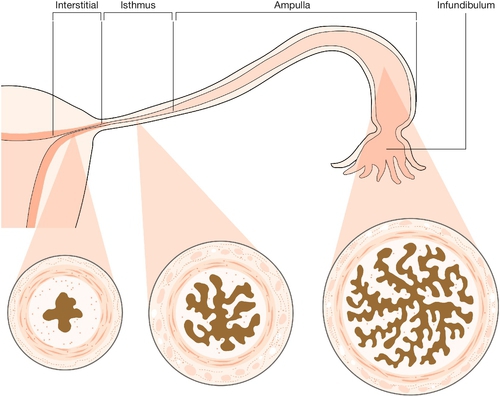
The tube can be divided into four parts (Fig. 1.16). The interstitial (intramural) part forms a narrow passage through the thickness of the myometrium. The isthmus, extending out from the cornu for about 3 cm, is also narrow. The ampulla is thin-walled, ‘baggy’, and tortuous; its lateral portion is free from the broad ligament, and droops down behind it towards the ovary. Near its lateral limit the abdominal ostium is constricted, but opens out again to form the infundibulum. This trumpet-shaped expansion is fringed by a ring of delicate fronds (or fimbriae), one of which is attached to the surface of the ovary.
The walls of the tubes include outer longitudinal and inner circular layers of smooth muscle. The delicate lining (endosalpinx), containing columnar ciliated and secretory cells, has longitudinal folds in the isthmic segment, which change into a highly intricate branching pattern in the ampulla.
Tubal function
At the time of ovulation, the fimbriae clasp the ovary in the area where the stigma (or point of follicular rupture) is forming. Usually, therefore, the ovum is discharged into the infundibulum (funnel) and is carried by tubal peristalsis into the ampulla of the tube, which is where fertilization occurs. Transit of the zygote to the site of implantation in the uterus takes several days.
Sterilization is effected by occluding both tubes, preferably in the narrow isthmic portion, using clips, sutures, rings or diathermy.
Patency of the tubes can be tested by injecting a watery dye (methylthioninium chloride – methylene blue) through the cervix, and observing spill from the abdominal ostia by laparoscopy. The contours of the uterine cavity and tubal lumen may also be demonstrated with radio-opaque fluid during a hysterosalpingogram.
The vagina
The vagina, which links the external and internal parts of the female genital tract, has a dual function: it forms the coital canal, affording access for spermatozoa to reach the cervix, and, with the cervix, it forms the soft-tissue birth canal. It lies in close proximity to the urethra and bladder anteriorly, and to the anal canal and rectum posteriorly. All three canals traverse the pelvic floor, passing between the medial (puborectalis) portions of the levator ani muscles. The insertion of these muscle fibres into the anococcygeal raphe creates a sling behind the bowel so that, at the junction of the lower rectum and the anal canal, a sharp angle is created, which is opened when the muscle relaxes. Other muscle fibres are inserted into the perineal body near its apex, creating a similar sling which angulates the axis of the vagina at that level. In turn, the anterior vaginal wall in the area of the bladder neck receives support.
There are differences in the anatomy of the vagina above and below this level. The lower third of the vagina is closely invested by the superficial and deep muscles of the perineum. It:
![]() incorporates the urethra in its anterior wall
incorporates the urethra in its anterior wall
![]() is separated from the bowel by the perineal body
is separated from the bowel by the perineal body
![]() has a rich arterial blood supply from branches of the vaginal arteries, and from both external and internal pudendal vessels.
has a rich arterial blood supply from branches of the vaginal arteries, and from both external and internal pudendal vessels.
The upper two-thirds of the vagina, above the levator shelf:
![]() is not invested by muscles, but is wide and capacious
is not invested by muscles, but is wide and capacious
![]() is in apposition with the bladder base anteriorly, and with the rectum (and, above that, the pouch of Douglas) posteriorly
is in apposition with the bladder base anteriorly, and with the rectum (and, above that, the pouch of Douglas) posteriorly
![]() is supported laterally and at the vault by the parametrium (cardinal and uterosacral ligaments). During sexual arousal the smooth muscle fibres within the parametrium elevate the vaginal vault and cervix, thereby elongating the vagina, and straightening its long axis.
is supported laterally and at the vault by the parametrium (cardinal and uterosacral ligaments). During sexual arousal the smooth muscle fibres within the parametrium elevate the vaginal vault and cervix, thereby elongating the vagina, and straightening its long axis.
Vaginal structure
The vaginal walls form an elastic fibromuscular tube with a multilayered structure. The lining of stratified squamous epithelium is corrugated into transverse folds (or rugae), which facilitates stretching during childbirth. The epithelium contains no glands, but during the reproductive years, the more superficial cells contain abundant glycogen. This polysaccharide is broken down by lactobacilli which form the normal flora of the vagina, producing lactic acid. This accounts for the low pH in the vaginal lumen (average pH 4.5).
Between the epithelium and the muscle, there is a layer of areolar tissue containing an extensive venous plexus. Vascular engorgement during sexual arousal, analogous to erection in the male, is most marked in the lower part of the vagina, encroaching on the vaginal lumen as the rugae distend. The vasocongestive response also results in increased transudation into the vaginal lumen.
The smooth muscle layers (outer longitudinal, inner circular) are not distinct, and an interlacing pattern is usual. Deep to the muscle, there is another extensive plexus of veins, within the outer vaginal fascia.
The ovary
The ovaries are attached on each side to the posterior surface of the broad ligaments through a narrowed base, termed the ‘hilum’. The ovaries are also attached to the cornua of the uterus by the ovarian ligaments. Developmentally, these are the upper portions of the gubernacula ovarii, and they are responsible for drawing the ovaries down into the pelvis from the posterior wall of the abdominal cavity. Typically, each ovary lies in an ovarian fossa, a shallow peritoneal depression lateral to the ureter, near the pelvic sidewall. The position may vary, however, and when the uterus is retroverted, one or both ovaries may lie in the pouch of Douglas.
The ovaries are ovoid in shape, with an irregular surface and a firm, largely solid, stroma, which can be divided indistinctly into an outer cortex and an inner medulla. The surface epithelium of cuboidal coelomic cells forms an incomplete layer, beneath which is a fibrous investment – the ‘tunica albuginea’. The germ cells from which the ova are derived are embedded in the substance of the ovaries.
The ovarian blood vessels and nerves enter through the hilum from the broad ligament. The ovarian arteries are direct branches of the aorta. Within the broad ligaments, they form an anastomotic loop with branches of the uterine arteries.
Anatomy of the lower urinary tract
The descending ureters are narrow thick-walled muscular tubes which cross into the pelvis close to the bifurcation of the common iliac arteries. They lie immediately under the peritoneum of the pelvic sidewall, behind the lateral attachment of the broad ligaments. Curving medially and forwards, they pass through the base of the broad ligaments below the uterine arteries, about 2 cm lateral to the supravaginal part of the cervix, a short distance above the lateral fornices of the vagina.
Approaching the bladder, the ureters pass medially in front of the upper vagina, and enter the bladder base obliquely at the upper angles of the trigone.
The wall of the ureter is composed of three elements: an external fibrous sheath, layers of smooth muscle and a lining of transitional epithelium. There may be partial or complete duplication of one or both ureters. An ectopic ureter is one that opens anywhere but the trigone of the bladder, and this may even be into the vagina or the vestibule. Urinary incontinence inevitably results.
The bladder
The urinary reservoir, lined with transitional epithelium, has the shape of a tetrahedron when empty, but the mesh of smooth muscle in the bladder wall can readily distend to contain a volume of half a litre or more. This muscle coat (the detrusor muscle) is thus normally relaxed and capable of considerable stretching without a contractile response. If urinary outflow during micturition is chronically impeded, however, the detrusor muscle becomes irritable and ultimately hypertrophic, producing prominent trabecular bands visible at cystoscopy.
The bladder is covered with peritoneum on its superior surface only. The peritoneum is reflected onto the anterior abdominal wall at a varying level, dependent on the degree of bladder filling. The oblique passage of the terminal part of each ureter through the bladder wall creates a one-way valve, which normally prevents urinary backflow from the bladder. This protects the kidneys from ascending infection. The triangular area within the bladder base defined by the two ureteric orifices and the internal urethral orifice is termed the ‘trigone’. Over this area, the epithelium remains smooth, even when the bladder is empty.
The urethra
The female urethra is about 4 cm long. Below the bladder neck it is embedded in the anterior vaginal wall, and the smooth muscle layers of the two structures intermingle. The urethral tissues also reflect the vascularity and turgidity of the vagina itself. Many of the urethral muscle fibres near the bladder neck are longitudinal and continuous with those of the bladder above, forming a funnel which opens out when these fibres contract, flattening the angle between the bladder base and the upper urethra. There are also abundant elastic fibres at this level, whose action helps to restore urethral closure after micturition. Around the lower part of the urethra, there is a fusiform collar of voluntary muscle – ‘the external urethral sphincter’. This segment of the urethra passes through the perineal membrane, which keeps it in a stable position. The upper urethra, on the other hand, shares the mobility of the bladder neck. The urethra is lined by transitional epithelium in its upper part, and by squamous epithelium below.
Nerve supply
Apart from the external sphincter, the efferent nerve supply controlling bladder function is from the pelvic parasympathetic system (S2,3,4), which provides the main motor fibres to the detrusor muscle. Afferent fibres conveying the normal sensations of bladder filling also return through the parasympathetic pathway, though some sympathetic sensory fibres convey the feelings of bladder overdistension via the hypogastric plexus. At the level of the second, third and fourth sacral segments of the spinal cord, the sensory and motor parasympathetic nerves form spinal reflex arcs, which are moderated by interaction with higher centres in the brain. Urinary continence depends upon a variety of factors. These include the elastic fibres surrounding the bladder neck which normally maintain urethral closure; and the tone or reflex contraction of the levator ani muscles which, through their insertion into the perineal body, elevate the urethrovesical junction, creating an angulation at the junction of the mobile (upper) and fixed (lower) portions of the urethra. The turgidity of spongy tissue underlying the urethral epithelium also assists in occluding the urethra, as does the action of the voluntary sphincter.