Chapter 32C Clinical Neurophysiology
Transcranial Magnetic Stimulation
At the beginning of the 1980s, Merton and Morton developed the first method of noninvasive brain stimulation, transcranial electrical stimulation (TES), and this had obvious clinical application. They used a single, brief, high-voltage electric shock and produced a relatively synchronous muscle response, the motor evoked potential (MEP). The latency of MEPs was compatible with activation of the fast-propagating corticospinal tract. It was immediately clear that this method would be useful for many purposes, but a problem with TES is that it is painful owing to simultaneous stimulation of pain fibers in the scalp. Five years later, Barker and colleagues demonstrated that it was possible to stimulate the brain (and nerves as well) using external magnetic stimulation (transcranial magnetic stimulation; TMS), with little or no pain. TMS is now commonly used in clinical neurology to study central motor conduction time. Depending on the stimulation techniques and parameters, TMS can excite or inhibit the brain activity, allowing functional mapping of cortical regions and creation of transient functional lesions. It is now widely used as a research tool to study aspects of human brain physiology including motor function and the pathophysiology of various brain disorders. Because TMS can influence the brain, there have been attempts to use it as therapy, particularly when used repetitively (repetitive transcranial magnetic stimulation, rTMS). Therapeutic investigations have included stroke rehabilitation, amelioration of movement disorders, and alleviation of pain and tinnitus, but effects demonstrated so far have generally been mild. There is currently only one therapeutic indication for rTMS: medically intractable depression (Padberg and George, 2009). In this situation it can be used as an alternative to electroconvulsive therapy (ECT) to induce a remission which would then be pharmacologically maintained.
Methods and Their Neurophysiological Background
In experimental animals, a single electrical stimulus applied at threshold intensity to the motor cortex produces descending volleys in the pyramidal tract with the same velocity at intervals of about 1.5 ms. The first volley is termed the D-wave (D for direct wave), which is thought to originate from the direct activation of the pyramidal tract. The subsequent volleys are termed I-waves (I for indirect wave), presumed to be elicited by transsynaptic activation of the pyramidal tract via intrinsic corticocortical circuitry. TMS also produces both D- and I-waves in descending pyramidal neurons. In contrast to electrical stimulation that preferentially evokes D-wave first, TMS at threshold intensity often produces a corticospinal volley with I-waves but no early D-wave (Ziemann and Rothwell, 2000). This finding suggests that TMS activates pyramidal neurons indirectly through synaptic inputs but does not activate them directly, presumably because of the direction of its current flow. Standards for the use of TMS and review of side effects have been published (Rossi et al., 2009).
Motor Excitability Measurements
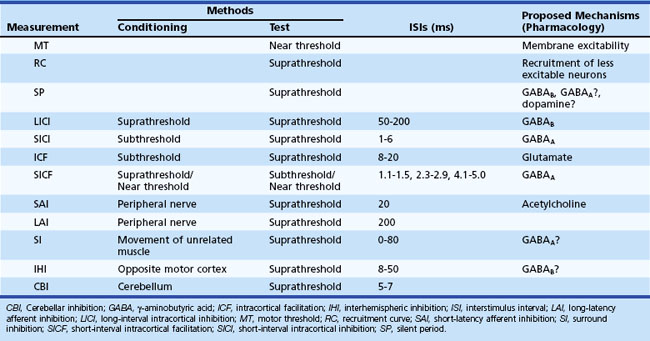
Silent Period and Long-Interval Intracortical Inhibition
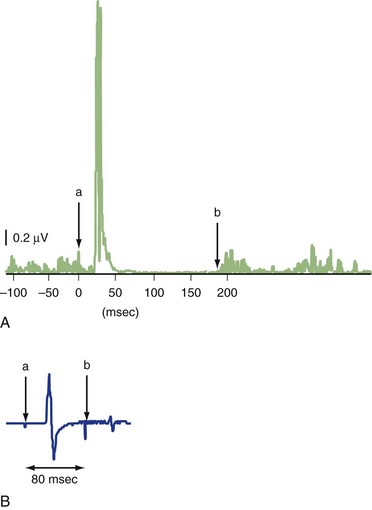
The silent period (SP) is a pause in ongoing voluntary electromyography (EMG) activity produced by TMS (Fig. 32C.1, A). The SP is usually measured with a suprathreshold stimulus in moderately active (usually 5%−10% of maximal voluntary contraction) muscle. SP duration is usually defined as the interval between the magnetic stimulus and the first recurrence of rectified voluntary EMG activity (Chen et al., 1999; Curra et al., 2002). While the first part of the SP is due in part to spinal cord refractoriness, the latter part is entirely due to cortical inhibition (cortical silent period, CSP). If a second suprathreshold test stimulation (TS) is given during the SP following suprathreshold conditioning stimulus (CS) (usually 50−200 ms after the first stimulus), its MEP is significantly suppressed (long intracortical inhibition; LICI) (see Fig. 32C.1, B) (Chen et al., 1999; Curra et al., 2002). SP and LICI appear to assess GABAB function, although other drugs affecting membrane excitability or dopaminergic transmission also influence SP. Although LICI and SP share similar mechanisms, they may not be identical, because they are affected differently in various diseases (Berardelli et al., 1996).
Short-Interval Intracortical Inhibition and Intracortical Facilitation
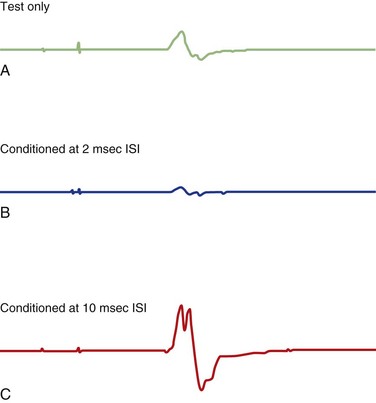
Various inhibitory and excitatory connections in the motor cortex can be evaluated by TMS using a paired-pulse technique. A subthreshold CS preferentially excites interneurons, by which MEPs from a following TS are suppressed at interstimulus intervals (ISIs) of 1 to 5 ms (short intracortical inhibition; SICI for such inhibition at short intervals) or facilitated at ISIs of 8-20 ms (intracortical facilitation; ICF) (Fig. 32C.2) (Ziemann, 1999; Ilic et al., 2002). SICI and ICF reflect interneuronal activity in the cortex. SICI is likely largely a GABAergic effect, especially related to GABAA receptors, whereas ICF is largely a glutamatergic effect. SICI can be divided into two phases, with maximum inhibition at ISI of 1 ms and 2.5 ms. SICI at 1 ms ISI is presumably caused either by neuronal refractoriness resulting in desynchronization of the corticospinal volley or by different inhibitory circuits; SICI at ISI of 2 ms or longer is most likely a synaptic inhibition.
Short-Interval Intracortical Facilitation
Short-interval intracortical facilitation (SICF) is also known as facilitatory I-wave interactions and is measured in a paired-pulse TMS protocol. In contrast to SICI and ICF, however, SICF is elicited by a suprathreshold first stimulus and a subthreshold second stimulus, or two near-threshold stimuli. SICF is usually observed at discrete ISIs of 1.1 to 1.5 ms, 2.3 to 2.9 ms, and 4.1 to 4.4 ms. ISIs producing facilitatory response in SICF are about 1.5 ms apart, similar to the intervals of different I-waves, which suggests that SICF originates in those neural structures responsible for the generation of I-waves (Ziemann and Rothwell, 2000). The second pulse is thought to excite the initial axon segments of excitatory interneurons, which are depolarized by excitatory postsynaptic potentials from the first pulse without firing an action potential. GABAA agonists reduce SICF.
Short-Latency and Long-Latency Afferent Inhibition
Afferent inhibition can be measured by applying a conditioning sensory stimulus such as median nerve stimulation followed by a test stimulus over the contralateral motor cortex. MEP inhibition usually occurs at ISIs of approximately 20 ms (short-latency afferent inhibition, SAI) and 200 ms (long-latency afferent inhibition, LAI) (Chen, 2004). SAI is thought to be of cortical origin because the recordings of corticospinal volleys demonstrate a strong suppression of later I-waves, with unaffected earlier descending waves. SAI is reduced by the acetylcholine antagonist, scopolamine, suggesting that SAI can be used to test the integrity of cholinergic neural circuits. Accordingly, SAI is reduced in patients with Alzheimer disease and is improved with a single dose of rivastigmine, an acetylcholinesterase inhibitor. The mechanism mediating LAI is still unclear but is thought to be different from that of SAI.
Surround Inhibition
Surround inhibition (SI), suppression of excitability in an area surrounding an activated neural network, has been proposed to be an essential mechanism in the motor system, where it could aid the selective execution of desired movements. Using a self-triggered TMS technique in which TMS is set to be triggered by EMG activity from the activated muscle (agonist), MEPs of the surrounding muscles (i.e., muscles near the agonist but unrelated to its movement) are suppressed during the movement (up to 80 ms after EMG onset) despite enhanced spinal excitability (Sohn and Hallett, 2004c). SI is reduced in patients with focal hand dystonia and may be altered in other disorders of human motor control such as Parkinson disease (PD).
Other Inhibitory Phenomena of the Motor Cortex
Interhemispheric inhibition (IHI) can also be assessed by TMS by applying a conditioning stimulus to the motor cortex, which suppresses MEPs produced by a test stimulus over the contralateral motor cortex at ISIs of between 6 and 50 ms (Di Lazzaro et al., 1999). IHI is thought to occur at the cortical level, although subcortical structures may also be involved. Long-latency IHI at ISIs between 20 and 50 ms is likely mediated by GABAB receptors.
Magnetic stimulation of the cerebellum, which can be performed using a double-cone coil, inhibits the MEPs produced by stimulation of the contralateral motor cortex 5 to 7 ms later (cerebellar inhibition, CBI) (Iwata and Ugawa, 2005). Cerebellar stimulation is thought to activate Purkinje cells in the cerebellar cortex, leading to inhibition of the deep cerebellar nuclei, such as dentate nucleus, which have a disynaptic excitatory pathway to the motor cortex via the ventral thalamus. CBI is reduced or absent in patients with cerebellar degeneration or lesions in the cerebellothalamocortical pathway.
Table 32C.1 summarizes the characteristics of different motor excitability measures using TMS.
Clinical Applications
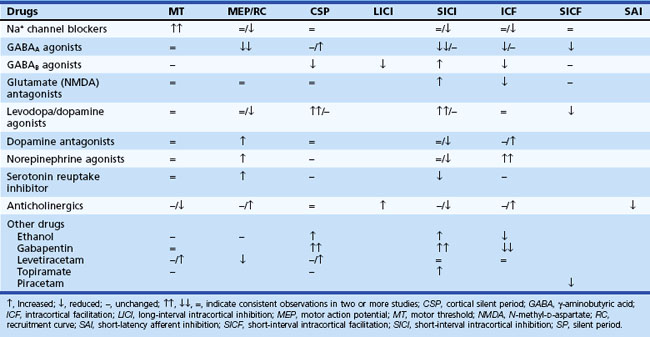
Since its introduction, TMS has been increasingly used to evaluate the underlying neurophysiological mechanisms in various neurological disorders (Curra et al., 2002; Chen, 2004; Sohn and Hallett, 2004b) (Table 32C.2). In addition, many studies have been performed to investigate the effect of various neurologically acting drugs on TMS measurements (Ziemann, 2004) (Table 32C.3), and these provide useful information about the mechanisms mediating various TMS techniques, as well as better understanding of the mechanism of these drugs. A report regarding the clinical diagnostic utility of TMS has been published by a committee of the IFCN (International Federation of Clinical Neurophysiology) (Chen et al., 2008).
Table 32C.2 Changes in Transcranial Magnetic Stimulation Measurements in Various Neurological Disorders
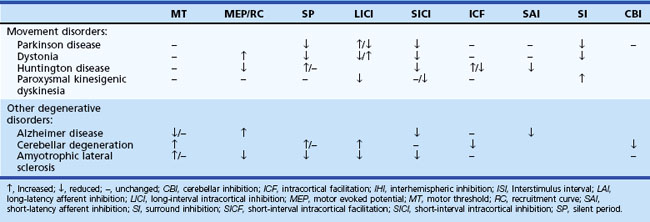
Movement Disorders
Parkinson Disease and Parkinson-Plus Syndromes
CCT is normal in Parkinson disease (PD) but can be prolonged in patients with multisystem atrophy (MSA) and progressive supranuclear palsy (PSP), which suggest a possible role of CCT measurements in patients with Parkinson-plus syndromes involving the pyramidal tracts. A reduction in SICI has been observed in various movement disorders regardless of the nature of the disturbances (Berardelli, 1999). In PD, reduced SICI is only observed at high CS intensities, suggesting increased facilitation rather than reduced inhibition (MacKinnon et al., 2005). In patients with corticobasal degeneration, SICI is often markedly reduced or turned into facilitation along with reduced IHI (Pal et al., 2008). SP is shortened in PD. Increased LICI has been noted in patients with PD and dystonia, but a recent study demonstrated a reduced LICI and reduced presynaptic inhibition in the motor cortex in PD (Chu et al., 2009). SAI is normal in patients with PD but is reduced in patients with MSA. LAI is reduced in PD, which is not affected by dopaminergic medications. SI is reduced or absent on the asymptomatic side of patients with unilateral PD (Shin et al., 2007). Dopaminergic drugs enhance SICI and prolong SP, whereas dopamine-blocking agents reduce SICI and increase ICF. These observations suggest that motor cortex excitability depends on the balance between different inhibitory mechanisms, some of which are under basal ganglia control. Several studies measured changes in TMS parameters after subthalamic nucleus deep brain stimulation (DBS). The SP is lengthened and ICF is enhanced after DBS, but no SP change was observed in another study. Reduced SAI, presumably associated with dopaminergic medications, and reduced LAI were restored by DBS.
Dystonia
In patients with focal dystonia, reduced SICI is not site specific and was also observed on unaffected sides in patients with upper-limb dystonia and in hand muscles of patients with cervical and facial dystonia. In patients with dystonia, shortening of SP is observed but only in dystonic muscles. In writer’s cramp, decrease in LICI was observed in the symptomatic hand during muscle activation. Both SICI and LICI were found to be abnormal in psychogenic dystonia, which limits the value of these measures in differentiating organic from psychogenic disorders (Espay et al., 2006; Quartarone et al., 2009). SICI is normal in dopa-responsive dystonia (DYT5) (Hanajima et al., 2007). Recruitment curve, SP, SICI, LICI, ICF, and SICF were all normal in patients with myoclonus-dystonia (DYT15) (Li et al., 2008; van der Salm et al., 2009). In patients with focal dystonia, LAI is diminished or absent, but SAI is normal. SI is reduced or absent in patients with focal hand dystonia (Sohn and Hallett, 2004a) but may be extended in patients with paroxysmal kinesigenic dyskinesia (Shin et al., 2010).
Huntington Disease
Patients with Huntington disease (HD) have shown prolonged SP that correlates with the severity of chorea. However, preclinical and early-stage patients with HD showed normal SP, normal or increased MT, normal SICI with slightly higher SICI threshold, enhanced ICF, and reduced SAI (Nardone et al., 2007; Schippling et al., 2009).
Other Neurodegenerative Disorders
In patients with various types of cerebellar degeneration, MT, CSP and LICI are often increased. ICF is reduced without change in SICI, and CBI is usually reduced or absent. However, these changes are different among the various types of cerebellar degeneration (Schwenkreis et al., 2002). In patients with inherited cerebellar ataxia, reduced ICF can be more specific for spinocerebellar ataxia (SCA) 2 and 3, whereas prolonged CCT was found in patients with Friedreich ataxia and SCA 1, 2, and 6. The alterations in TMS measurement in patients with amyotrophic lateral sclerosis (ALS) are different according to the main lesions involved in the disease, but are usually increased MT and decreased MEP, CSP, LICI, and SICI. In some patients whose clinical manifestations are atypical, these TMS findings may help diagnose the disease.
Epilepsy and Antiepileptic Drugs
There are several different mechanisms for the genesis of epileptic seizures and for the modes of action of antiepileptic drugs (AEDs). TMS can be used to give information about these mechanisms by measuring cortical excitability. For example, motor threshold is decreased in untreated patients with idiopathic generalized epilepsy. On the other hand, in progressive myoclonic epilepsy, threshold is normal, but there is loss of cortical inhibition demonstrated with paired pulses at 100 to 150 ms and an increase in facilitation at 50 ms. Prolonged SP was found in idiopathic generalized epilepsy and also in partial motor seizures. Within 48 hours after a generalized tonic-clonic seizure, ICF is reduced but SICI is normal, presumably representing a protective mechanism against spreading or recurrence of seizures. Increased motor cortical excitability including reduced MT, increased ICF, and reduced SICI and LICI was observed in the 24 hours before a seizure; opposite changes in motor cortical excitability measures were seen in the 24 hours after a seizure (Badawy et al., 2009). SICI is reduced but ICF is normal in progressive or juvenile myoclonic epilepsy. LICI is also reduced in progressive myoclonic epilepsy and idiopathic generalized epilepsy. Weaker SICI and ICF in the hemisphere ipsilateral to seizure onset were found to have a predictive value for seizure attacks in the subsequent 48 hours in temporal lobe epilepsy patients with acute drug withdrawal (Wright et al., 2006). A long-term follow-up study in drug-naïve patients with generalized or partial epilepsy demonstrated that a decrease in cortical excitability such as increased MT and increased SICI and LICI after medication predicted a high probability of being seizure free after 1 year of treatment (Badawy et al., 2010).
Specific effects can be seen with various AEDs in normal subjects. AEDs that enhance the action of GABA (e.g., vigabatrin, gabapentin, lorazepam) increase SICI but have no effect on MT. In contrast, the AEDs blocking voltage-gated sodium or calcium channels (phenytoin, carbamazepine, lamotrigine) increase MT without significant effects on SICI (Ziemann et al., 1996). In addition to elucidating these mechanisms, TMS can potentially be used to quantify physiological effects in individual patients, and this may be more valuable in some circumstances than monitoring blood levels of AEDs.
Stroke
Several studies have attempted to correlate clinical recovery from stroke to the characteristics of MEPs. MEPs are often absent in severely affected stroke patients. In mildly affected patients, MEPs are usually of longer latency, smaller amplitude, and higher motor threshold. The presence of MEPs in the early stage of stroke is associated with a good functional recovery (Hendricks et al., 2002). Conversely, absence of MEPs in a paretic limb, with concomitantly increased MEP amplitudes in the unaffected limb, predicts poor recovery. In addition, the presence of ipsilateral MEPs in the paretic limb in response to the stimulation of the unaffected hemisphere is also associated with poor recovery. The recovery of MEP latency is highly correlated with return of hand function. Higher MT than normal is often associated with the signs of spasticity. Noninvasive mapping of the motor cortex can be carried out with TMS using the figure-of-eight-shaped coil. This technique has been used to evaluate cortical reorganization in various conditions. In stroke patients, it has been demonstrated that cortical reorganization of the motor output still occurs up to several months after insult. There is progressive enlargement of the motor maps of the recovering affected part. SICI is reduced in the affected hemisphere in the acute phase of a motor cortical stroke and remains reduced regardless of functional recovery. SICI also tends to be reduced in the unaffected hemisphere but subsequently returns to normal or can be greater than that in the affected hemisphere in patients showing good recovery. Enhanced SICI may lead to reduced activity in the unaffected hemisphere, which enhances activity of the affected hemisphere and promotes recovery.
Multiple Sclerosis
CCT measurement has been applied in the evaluation of patients with multiple sclerosis (MS), where there is frequent involvement of the corticospinal tract. Typically there is either a unilateral or bilateral prolongation of CCT consistent with demyelinating lesions in the corticospinal tract (Schmierer et al., 2002). Prolonged CCT is more pronounced in progressive MS than in relapsing-remitting MS. Similar to other evoked potential studies, MEPs vary considerably in latency, amplitude, and shape in patients with MS when they are measured consecutively. An increased MT is also frequently observed in patients with MS.
Cervical Myelopathy and Other Spinal Cord Lesions
Along with measurements of somatosensory evoked potentials, TMS is a useful tool for detecting cervical myelopathy. In patients with this disorder, MEPs as well as the MEP : CMAP ratio (compound muscle action potential), are usually reduced. CCT is prolonged, and their interside difference is increased. Simultaneous recordings from muscles innervated by different cord segments can help define the spinal level of a lesion. Prolonged CCT often correlates with the clinical severity and degree of cord compression observed with magnetic resonance imaging (MRI). In a study recruiting large numbers of patients with cervical myelopathy (Lo et al., 2004), the sensitivity of TMS in differentiating the presence and absence of MRI cord abnormality was 100%, and the specificity was around 85%. CCT measures of the muscles innervated by cranial nerves (e.g., trapezius, tongue) may help differentiate ALS from cervical myelopathy. Abnormal CCT to these muscles indicates high probability of ALS.
Badawy R., Macdonell R., Jackson G., et al. The peri-ictal state: cortical excitability changes within 24 h of a seizure. Brain. 2009;132:1013-1021.
Badawy R.A., Macdonell R.A., Berkovic S.F., et al. Predicting seizure control: cortical excitability and antiepileptic medication. Ann Neurol. 2010;67:64-73.
Berardelli A. Transcranial magnetic stimulation in movement disorders. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:276-280.
Berardelli A., Rona S., Inghilleri M., et al. Cortical inhibition in Parkinson’s disease. A study with paired magnetic stimulation. Brain. 1996;119(Pt 1):71-77.
Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1-10.
Chen R., Cros D., Curra A., et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504-532.
Chen R., Lozano A.M., Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539-542.
Chu J., Wagle-Shukla A., Gunraj C., et al. Impaired presynaptic inhibition in the motor cortex in Parkinson disease. Neurology. 2009;72:842-849.
Curra A., Modugno N., Inghilleri M., et al. Transcranial magnetic stimulation techniques in clinical investigation. Neurology. 2002;59:1851-1859.
Di Lazzaro V., Oliviero A., Profice P., et al. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520-524.
Espay A.J., Morgante F., Purzner J., et al. Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol. 2006;59:825-834.
Hanajima R., Nomura Y., Segawa M., et al. Intracortical inhibition of the motor cortex in Segawa disease (DYT5). Neurology. 2007;68:1039-1044.
Hendricks H.T., van Limbeek J., Geurts A.C., et al. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629-1637.
Ilic T.V., Meintzschel F., Cleff U., et al. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153-167.
Iwata N.K., Ugawa Y. The effects of cerebellar stimulation on the motor cortical excitability in neurological disorders: a review. Cerebellum. 2005;4:218-223.
Li J.Y., Cunic D.I., Paradiso G., et al. Electrophysiological features of myoclonus-dystonia. Mov Disord. 2008;23:2055-2061.
Lo Y.L., Chan L.L., Lim W., et al. Systematic correlation of transcranial magnetic stimulation and magnetic resonance imaging in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2004;29:1137-1145.
MacKinnon C.D., Gilley E.A., Weis-McNulty A., et al. Pathways mediating abnormal intracortical inhibition in Parkinson’s disease. Ann Neurol. 2005;58:516-524.
Nardone R., Lochner P., Marth R., et al. Abnormal intracortical facilitation in early-stage Huntington’s disease. Clin Neurophysiol. 2007;118:1149-1154.
Padberg F., George M.S. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2-13.
Pal P.K., Gunraj C.A., Li J.Y., et al. Reduced intracortical and interhemispheric inhibitions in corticobasal syndrome. J Clin Neurophysiol. 2008;25:304-312.
Quartarone A., Rizzo V., Terranova C., et al. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009;132:2871-2877.
Rossi S., Hallett M., Rossini P.M., et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008-2039.
Schippling S., Schneider S.A., Bhatia K.P., et al. Abnormal motor cortex excitability in preclinical and very early Huntington’s disease. Biol Psychiatry. 2009;65:959-965.
Schmierer K., Irlbacher K., Grosse P., et al. Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology. 2002;59:1218-1224.
Schwenkreis P., Tegenthoff M., Witscher K., et al. Motor cortex activation by transcranial magnetic stimulation in ataxia patients depends on the genetic defect. Brain. 2002;125:301-309.
Shin H.W., Kang S.Y., Hallett M., et al. Extended surround inhibition in idiopathic paroxysmal kinesigenic dyskinesia. Clin Neurophysiol. 2010;121:1138-1141.
Shin H.W., Kang S.Y., Sohn Y.H. Disturbed surround inhibition in preclinical parkinsonism. Clin Neurophysiol. 2007;118:2176-2179.
Sohn Y.H., Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004;56:595-599.
Sohn Y.H., Hallett M. Motor evoked potentials. Phys Med Rehabil Clin N Am. 2004;15:117-131. vii
Sohn Y.H., Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004;158:397-404.
van der Salm S.M., van Rootselaar A.F., Foncke E.M., et al. Normal cortical excitability in myoclonus-dystonia—a TMS study. Exp Neurol. 2009;216:300-305.
Wright M.A., Orth M., Patsalos P.N., et al. Cortical excitability predicts seizures in acutely drug-reduced temporal lobe epilepsy patients. Neurology. 2006;67:1646-1651.
Ziemann U. Intracortical inhibition and facilitation in the conventional paired TMS paradigm. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:127-136.
Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717-1729.
Ziemann U., Lonnecker S., Steinhoff B.J., et al. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study [see comments]. Ann Neurol. 1996;40:367-378.
Ziemann U., Rothwell J.C. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397-405.