Chapter 32D Clinical Neurophysiology
Neuromodulation
Neuroscientists have defined neuromodulation as a dynamic process wherein a substance, usually a neurotransmitter, affects neuronal function by its action on the synapses and by modulating the response of the cell to other inputs, with potentially important behavioral implications. Neuromodulation has come to be more broadly defined as a process whereby the central, peripheral, or autonomic nervous system is electrically excited, inhibited, stimulated, modified, regulated, or therapeutically altered to achieve the desired effect (Krames et al., 2009). An ongoing dynamic process that affects targeted neural networks and utilizes electrical or neuropharmacological stimulation, neuromodulation may offer clinical outcomes that are potentially modifiable by simply adjusting stimulation parameters (Holsheimer, 2003). Other relevant terms that have been used to describe neuromodulation and its related techniques have included neurostimulation, neuroaugmentation, neuroprosthetics, functional electrical stimulation (FES), assisted technologies, and neural engineering. The International Neuromodulation Society has further defined neuromodulation “as a field of science, medicine and bio-engineering that encompasses both implantable and non-implantable technologies that may be electrical or chemical, and serve the purpose of improving quality of life and the overall functioning of humankind” (Sakas et al., 2007a).
Multiple specialties use neuromodulatory techniques for a variety of clinically relevant issues including augmentation of vision and hearing, cardiac pacing, and treating movement disorders, pain, epilepsy, stroke, depression, addiction, and gastrointestinal (GI) and urinary conditions. For more information on the history and classifications of neuromodulation, please go to www.expertconsult.com.
History of Neuromodulation
The first medical use of electricity was described by Scribbonius Largus, a Roman who used the electric torpedo fish to treat headache and address gout (Stillings, 1973). Modulation of brain activity by electrical stimulation of the dog motor cortex in an effort to induce limb movements was reported in 1870. Subsequently, stimulation of the human brain was tested by Sir Victor Horsely, who applied his technique to the occipital lobes (Vilensky and Gilman, 2002). Later, electrical stimulation was introduced as a more routine procedure. Brain lesions and biopsies were guided by what was referred to as “test stimulation,” and this technique was later refined by Spiegel and Wycis when they introduced the stereotactic head frame (Spiegel et al., 1947), which added precision to the targeting of very small brain regions. Movement disorder neurostimulation and psychosurgery continued to be performed in the 1960s and 70s; these surgeries used test or macrostimulation and then followed with alcohol-induced lesions and/or radiofrequency lesions once the target was carefully identified (Feldman and Goodrich, 2001; Guridi and Andres, 1997).
The potential benefits of chronic stimulation of brain targets have been refined by laboratory studies as well as intraoperative human stimulation. Following Melzack and Wall’s “gate theory” for pain (Melzack and Wall, 1965), implantable spinal cord neurostimulation and later brain stimulation were introduced as rational ways to “neuromodulate” relevant circuitry. Similarly in 1987, Professor Alim Benabid ameliorated a tremor in a chronic pain patient. Benabid left a chronic stimulating lead in place, and this has been considered the birth of a new application for neuromodulatory technology (Benabid et al., 1987). The current field of neuromodulation has evolved to include deep brain stimulators, pacemakers for breathing, urinary control systems, sacral nerve stimulation, cortical control systems, cochlear implants, visual prostheses, and neurorehabilitaton devices. All of these neuromodulatory devices have evolved in a relatively short period of time, and many have enhanced quality of life for various patient populations.
Classification
Although few attempts have been made, rapid proliferation and evolution of this field created a need for a more formal classification scheme for neuromodulation and related technologies. Classification has not been officially standardized, but many publications have recommended the following strategies: classified broadly (electrical versus pharmacological), classified by purpose (therapeutic, rehabilitative, or assistive), classified by body region placed (external, noninvasive versus internal, implanted), classified by directionality/functionality (open loop versus closed loop), and classified by nervous system (central versus peripheral) (Hatzis et al., 2007).
Neuromodulatory devices are thought to primarily work by modifying nervous signals in an effort to produce some sort of biological response (Sakas et al., 2007a). Some devices have been developed for neuropharmacological purposes and may thus be used to apply drugs in small doses to maximize benefits and limit side effects. Other devices may act in parallel with damaged parts of the nervous system to facilitate functional restoration. Facilitation can be provided by prosthetics and by neuro-orthotics specifically designed to take over a particular nervous function (Stieglitz, 2007).
It is important to distinguish between therapeutic devices and rehabilitative devices. Therapeutic neuromodulatory devices are generally used to maintain vital bodily functions. Rehabilitative devices, on the other hand, usually focus on assisting an individual to regain a bodily function. Therapeutic devices may be lifelong, whereas rehabilitatives may or may not be long-term adjuncts. Traditionally, many devices have used open-loop stimulation (constant neurostimulation without any feedback); however, smarter closed-loop devices (still in their infancy) may eventually have the potential to maximize battery life and minimize side effects (Halpern et al., 2008).
Applications of Neuromodulatory Devices
The human nervous system is composed of many complex and distinct neural networks. These networks may include chemical and electrical synapses, and they may communicate both within and between network structures. The structure and subsequently the behavior of these neural networks may be “shaped by the extrinsic as well as intrinsic neuromodulatory effects that include those of hormones and neurotransmitters” (Destexhe et al., 2004; Katz and Frost, 1996). The classifications and applications of neuromodulation devices are summarized in Table 32D.1.
| Stimulation | Electrical (e.g., DBS, MCS, SCS, PNS, TENS) |
| Pharmacological (e.g., infusion pumps) | |
| Application | Neuroprosthesis (auditory, retinal implants, phrenic/sacral nerve stimulators) |
| Neuro-orthosis | |
| Purpose | Therapeutic (neurostimulators for breathing, bladder and bowel functions) |
| Rehabilitation (neurostimulators for extremity functions) | |
| Placement | Implanted and invasive (DBS) |
| External and noninvasive (neurostimulators for extremity functions) | |
| Control and directionality | Open-loop (VNS, DBS) |
| Closed-loop (NeuroPace) | |
| Effect on nervous system | Central nervous system (DBS) |
| Peripheral nervous system (PNS) |
DBS, Deep brain stimulation; MCS, motor cortex stimulation; PNS, peripheral nerve stimulation; SCS, spinal cord stimulation; TENS, transcutaneous electrical nerve stimulation; VNS, vagus nerve stimulation.
Neurostimulation devices may involve either the application of deep brain/spinal cord leads or surface leads, or alternatively they may use direct stimulation of the spinal cord and/or peripheral nerves. Precisely placed leads may be connected via an extension cable to a pulse generator and ultimately to some sort of power source (battery). This process has the potential to generate the necessary electrical stimulation and to (hopefully) provide clinically relevant benefits (Panescu, 2008). Signals can be excitatory, inhibitory, or both (e.g., excite fibers and inhibit cells) (Vitek, 2002).
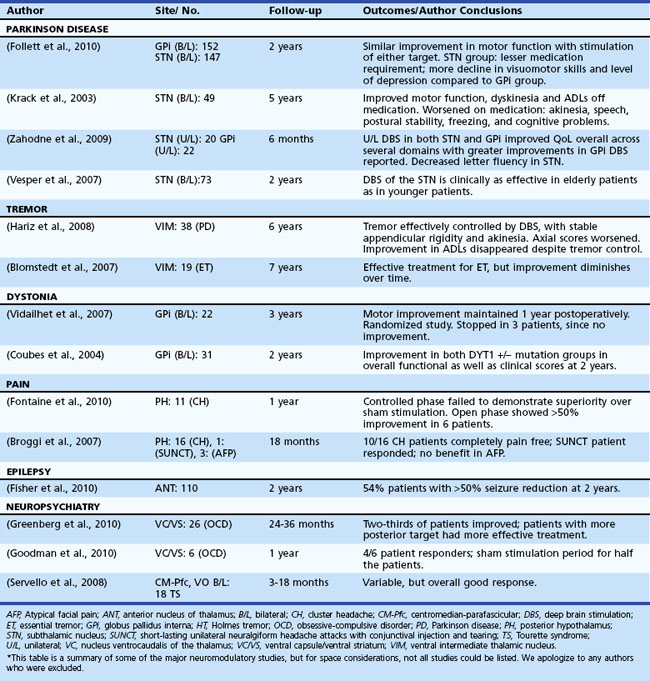
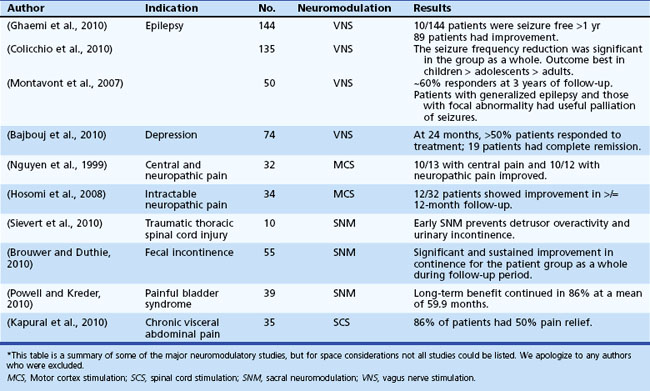
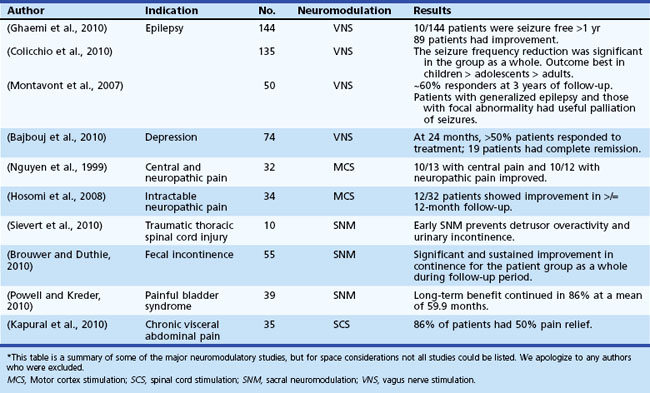
Table 32D.2 offers a selected summary of the literature on deep brain stimulation (DBS) neuromodulation for movement and neuropsychiatric disorders, and Table 32D.3 is a selected summary of the literature on non-DBS neuromodulation.
Extended versions of these tables are available online at www.ExpertConsult.com.
Table 32D.2 DBS Neuromodulation: Selected Summary of the Literature* (See Extended Online-Only Version)
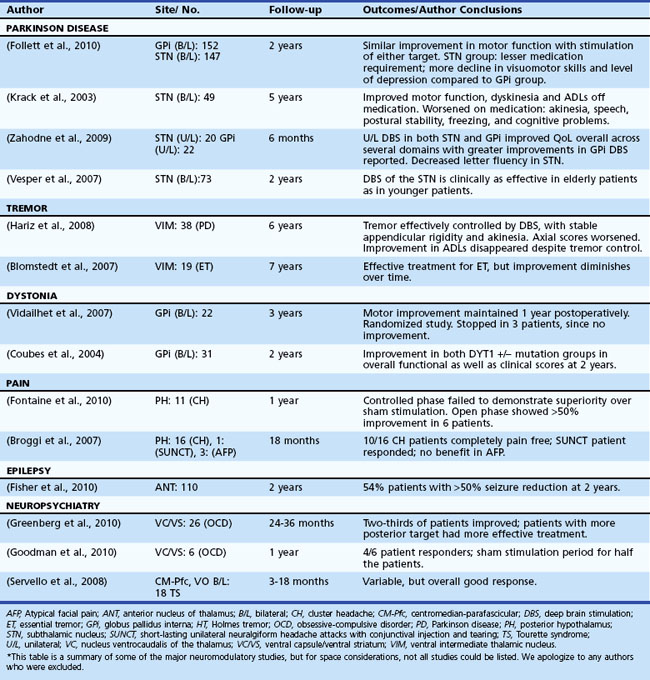
Table 32D.3 Non-DBS Neuromodulation: Selected Summary of the Literature* (See Extended Online-Only Version)
Motor Cortex Stimulation
Motor cortex stimulation (MCS) was utilized by Tsubokawa and colleagues, who originally presented this option as a possible safer alternative to DBS surgery in Dejerine syndrome (central thalamic pain syndrome) (Tsubokawa et al., 1991). It has been hypothesized that MCS induces antidromic activation of the sensory cortex, and this results in inhibition of the abnormal burst activity in a damaged and possibly deafferented sensory thalamus. Imaging studies “on” and “off” MCS have shown interesting blood flow changes within the ventrolateral (VL) tier of the thalamus, the medial thalamic regions, and the anterior cingulated cortex, orbitofrontal cortex, anterior insular cortex, and upper brainstem. Perhaps the most interesting aspect of post-MCS imaging has been the finding that blood flow does not seem to increase in the somatosensory cortex. Interpretations of this data have been variable, but the latest suggestions are that descending projections from the cortex to the thalamus can be activated and potentially lead to changes in basal ganglia pathophysiology within a loop responsible for both pain and emotional aspects of pain (Garcia-Larrea et al., 1999). It is thought that MCS may primarily stimulate γ-aminobutyric acid (GABA)-ergic interneurons that are found to be in parallel orientation to other layers of the cerebral cortex (Manola et al., 2007). Researchers have noticed that pain relief (≥40%\xE2\x80’50% improvement on the visual analog scale) from MCS seems to be greatest in cases where the corticospinal tract is found to be relatively intact (Katayama et al., 1998). Interestingly, when MCS is applied, patients with paretic extremities and concomitant central pain problems may have noticeable benefits in motoric weakness, rigidity, and spasticity. These improvements can, in select cases, aid in improving rehabilitation potential (Katayama et al., 1998).
MCS has been applied in cases of pharmacoresistant trigeminal neuralgia, painful peripheral neuropathy, and certain spinal cord pathologies. A greater than 50% pain relief has been reported in various studies of patients with central and neuropathic pain (Katayama et al., 1998; Nguyen et al., 1999). Data from randomized controlled studies for any application of MCS are lacking, however. There appears to be a large placebo effect with the therapy, and it has been noted that the effects may wane over time (Fontaine et al., 2009; Lima et al., 2008). Motor cortex stimulation has been attempted for therapeutic treatment of Parkinson disease (PD) and multiple system atrophy (MSA), but to date, results have been disappointing (Gutierrez et al., 2009; Kleiner-Fisman et al., 2003).
Transcranial Magnetic Stimulation
Information on transcranial magnetic stimulation (TMS) can be found in Chapter 32C as well as in the online version of this chapter at www.ExpertConsult.com.
Transcranial magnetic stimulation (TMS) is a noninvasive device consisting of an electromagnetic coil which when placed on the scalp, converts brief, high-current electrical activity into a rapidly cycling magnetic field. The magnetic field courses unimpeded through the scalp to induce localized intracranial electric current and thereby depolarizes the neurons, resulting in excitation or inhibition of the underlying neuronal circuit (Beric, 1993). Specific stimulation parameters such as current amplitude, duration, and direction can be used to calculate delivery of energy to a particular area in the brain. Repetitive TMS (rTMS) up to 60 Hz can be used to produce incrementally greater clinical effects (Andrews, 2003). The effects in general seem to be excitatory at fast frequencies (>1 Hz) and inhibitory at slow frequencies (≤1 Hz), and this may be similar to the published effects of long-term potentiation and long-term depression (Chen et al., 1997; Pascual-Leone et al., 1994). The state of brain activity, the integrity of the neural networks, and the interactions of the TMS with the targeted brain tissue can all be important in producing positive effects. Specific TMS stimulation parameters may also have positive and negative effects. The biological mechanism of action of TMS is not well understood, although changes at genetic levels and changes in concentrations of various neurotransmitters including monoamines, GABA, and glutamate have been suggested and may lead to corticospinal excitability and/or intracortical inhibition (Albert et al., 2009).
TMS has found applications in mapping the motor cortex and in treating post-stroke patients and several neurological disorders (movement disorders, neuropsychiatric disorders, epilepsy, tinnitus, and migraine), with varying degrees of success and failure. Some TMS targets include various regions of motor cortex in Parkinson disease (PD), dorsolateral prefrontal cortex (DLPFC) in depression, and left temporoparietal cortex in schizophrenia (Rossini and Rossi, 2007).
Different stimulation strategies for treatment of drug-resistant major depression have been attempted by randomized trials, suggesting the possibility of short-lived, clinically significant antidepressant effects (stimulation of the left DLPFC). Favorable responses to TMS may be predicted by previous medication treatment failures, lack of comorbid anxiety, and shorter duration of the current depressive episode (Avery et al., 2007; George, Lisanby et al., 2010; Lisanby et al., 2009; O’Reardon et al., 2007), although more data are needed to better understand positive clinical responses to TMS. It is unclear, however, whether the benefits of such treatment strategies will be sustained or whether longer-duration treatments may result in higher response and remission rates (Lam et al., 2008). A recent meta-analysis of rTMS for various neuropsychiatric disorders has supported its role in treating depressive symptoms and auditory hallucinations and addressing the negative symptoms of schizophrenia (Slotema et al., 2010). High-frequency rTMS has been shown to improve short-term motor scores in PD, potentially by enhancing motor cortex excitability (Elahi et al., 2009; Hemond and Fregni, 2007). In post-stroke patients, stimulation of the lesioned hemisphere (or alternatively, inhibition of the contralateral hemisphere) may aid in rehabilitation efforts. Repetitive TMS is also thought to have a potential role in treating neuropathic or visceral pain (Hemond and Fregni, 2007).
Seizure is the major adverse effect raising treating clinicians’ concern when applying TMS. Careful selection of patients may help avoid this complication. In general, patients with increased intracranial pressure, a metallic object(s) embedded in their head, or heart disease should avoid TMS. Other notable and not uncommon side effects from the stimulation include headaches, tinnitus, and facial muscle twitches (Andrews, 2003).
Deep Brain Stimulation
Over the last 2 decades, chronic DBS has become routine for several diagnoses in neurological practice (e.g., PD, dystonia, essential tremor) and has been used experimentally for selected neuropsychiatric indications (e.g., obsessive compulsive disorder [OCD], depression, Tourette syndrome [TS]). Interestingly, as early as the 1950s, temporary DBS electrodes were implanted into the septal region for pain control, and they were reported to have beneficial effects (Hamani et al., 2006). Over the ensuing years, there were various attempts at DBS, with most documented experiences revealing its usefulness in test stimulation prior to ablating brain lesions (Blomstedt and Hariz, 2010). In 1987 when Professor Benabid was operating on a chronic pain patient, he noticed that the patient’s tremor improved during test stimulation, and he decided to chronically stimulate this patient. Over the ensuing 2 decades, multiple DBS placements into multiple brain regions for a variety of clinical indications have occurred (Awan et al., 2009).
High-frequency stimulation (HFS) has been thought to effect a basal ganglia network–wide change, acting as a sort of informational lesion (Birdno and Grill, 2008; McIntyre et al., 2004a; McIntyre et al., 2004b). Thus, PD computer model systems have been developed to attempt to elucidate the mechanism of action of DBS (Birdno and Grill, 2008; McIntyre et al., 2004a; McIntyre et al., 2004b). HFS has been hypothesized to result in a decoupling of the cellular and axonal output within a thalamocortical relay circuit. The firing rates and patterns of the cell body may be suppressed, while the fibers and fibers of passage may be excited. DBS may ultimately effect a corticostriatopallido-thalamocortical (CSPTC) network and result in upstream as well as downstream changes within this complex basal ganglia network (McIntyre and Hahn, 2010). The specific effects of an electrical field are thought to reflect changes relative to the position and orientation of the axon to the actual DBS lead. Also, the electrical field is thought to exert trans-synaptic influences (McIntyre et al., 2004a; McIntyre et al., 2004b). The clinical benefits of DBS have been hypothesized to be due to more than just local neurotransmitter release (Stefani et al., 2005); however, several authors have argued that there is a collective effect and that transmitter release may be very important to the mechanism of action (Dostrovsky and Lozano, 2002; Lee KH et al., 2004; Vitek, 2002). Animal models of DBS have revealed increased extracellular concentrations of glutamate and GABA (Windels et al., 2003) and, most recently, adenosine and dopamine (Chang et al., 2009; Shon et al., 2010). Depolarization blockade, synaptic inhibition, and synaptic depression (McIntyre et al., 2004a; McIntyre et al., 2004b) have also been proposed to play a role in the potential mechanisms of action of DBS.
Selective placement of the DBS leads within different anatomical regions and different somatotopies may affect the neuronal network and, in the best possible cases, lead to improvement in clinical symptoms. For example, the placement of DBS electrodes in the ventralis intermedius nucleus of the thalamus for essential tremor may have an effect on an abnormal thalamocortical oscillatory loop and ultimately suppress tremor (Birdno et al., 2007). Similarly in PD, placement of a DBS lead in the ventralis intermedius may improve tremor; however, to affect bradykinesia and rigidity, one must either implant the ventralis oralis thalamic nucleus or, alternatively, either the subthalamic nucleus or globus pallidus internus (Mann et al., 2009; Oh et al., 2002).
DBS is a relatively simple technology. In its current form, it involves placement of a quadripolar (four contacts) lead into a specific and predetermined brain target. The lead is usually connected to a neurostimulator placed subcutaneously under the clavicle, although the battery can be placed in a multitude of regions. The neurostimulator can then be programmed or adjusted to tailor a setting to an individual patient. There are thousands of different combinations that may be chosen, and the voltage, frequency, and pulse width may be liberally changed. The optimal settings are patient and symptom specific, and they usually require that patients be reprogrammed frequently for the first 4 to 6 months and medications as well as stimulation monitored (Ondo et al., 2005; Rodriguez et al., 2007).
Each disorder or symptom considered for treatment with DBS should be carefully evaluated. Only a small fraction of any neurological or neuropsychiatric disorder may be eligible for this type of therapy. Most patients receiving DBS should be medication resistant, and they should undergo a complete multi-/interdisciplinary screening with a neurologist, psychiatrist, neuropsychologist, and neurosurgeon. Following screening, there should be a detailed interdisciplinary discussion about the goals of therapy (symptoms targeted, symptoms that will likely respond, symptoms not likely to respond). In cases of PD, patients should undergo an “off/on” levodopa medication challenge to determine which symptoms respond best to medication—these usually are the ones that respond best to stimulation (with the exceptions of tremor and dyskinesia). Risks and benefits of a potential DBS surgery, as well as the potential brain target(s), and unilateral versus bilateral DBS should all be carefully addressed in preoperative conversations with patients and families (Alberts et al., 2008; Kluger et al., 2009; Okun et al., 2004; Okun et al., 2007; Okun et al., 2009; Okun and Foote, 2004; Okun and Foote, 2009; Rodriguez et al., 2007; Skidmore et al., 2006; Ward et al., 2010). There are many potential adverse events that may occur as a result of DBS, some of which may constitute emergencies (Morishita et al., 2010).
Depending on the region of the world and the preference of individual surgical teams, leads and batteries may be placed in a single setting or may be staged (separate operating room procedures). One lead, two leads, or in exceptional circumstances, more than two leads may be implanted in a single session. One recent review of DBS hardware-related complications cited lead migration, lead fracture, lead erosion/infection, and lead malfunction as common occurrences (Lyons et al., 2004; Oh et al., 2002). DBS surgically related and stimulation-related complications can occur and may include (but are not limited to) hemorrhage, infections, strokes, seizures, paresthesias, dysarthria, hypophonia, dystonia, mood worsening, suicide, and worsening of comorbidities. Difficulty with verbal fluency and anger seem to be common sequelae in PD patients (Blomstedt and Hariz, 2005; Blomstedt and Hariz, 2006; Hariz et al., 2008a; Okun et al., 2008a; Saint-Cyr and Albanese, 2006). DBS teams must differentiate between lesion effects, stimulation-induced effects, and transient versus permanent neurological dysfunction. More DBS and device-related studies are needed to prospectively document all adverse effects; this constitutes a critical weakness of many published series (Hariz et al., 2008b).
Parkinson Disease
Parkinson Disease is a complex disorder thought to be the result of extensive loss of neurons and their projections within motor and nonmotor basal ganglia circuitry (Alexander et al., 1986). A rationale for neuromodulatory therapy has been developed as a result of models of basal ganglia physiology. Perhaps the most famous model reveals loss of dopaminergic neurons in the substantia nigra pars compacta, with a resultant abnormal neuronal activity in both the direct and indirect basal ganglia circuitry. These changes are thought to result in the genesis of many of the motor symptoms of PD. Initial treatment of PD is usually with dopaminergic therapy, though over time, disease progression may lead to limitations in medical therapy including such symptoms as wearing off between doses, on/off fluctuations, and hyperkinetic dyskinesia. Subthalamic nucleus (STN) or globus pallidus interna (GPi) DBS have been used to neuromodulate basal ganglia pathways and restore important functions in selected patients (Pahwa et al., 2005; Weaver et al., 2009). Studies are underway to define the selection criteria and help tailor the procedure for individual patients. To date, STN and GPi DBS have shown similar motor outcomes, but STN DBS may allow for larger dopaminergic medication reductions, and GPi DBS may provide better dyskinesia suppression and a relatively safer risk/benefit profile (Anderson et al., 2005; Follett et al., 2010; Mikos et al., 2010; Okun and Foote, 2009; Zahodne et al., 2009). These conclusions, however, will need to be bolstered by randomized clinical studies.
Long-term efficacy of DBS in PD has been good overall, but in all cases, the disease progresses. An important guideline is that symptoms that respond to levodopa continue to respond to DBS, with the exceptions of tremor and dyskinesia, which may have persistent benefits (Krack et al., 2003; Schüpbach et al., 2005; Wider et al., 2008).
Dystonia
Dystonia results from co-contraction of agonist and antagonist muscles, and patients may experience involuntary repetitive movements that result in twisted and sometimes painful postures. Dystonia may be focal, segmental, or generalized based on the body region affected. Other classification systems use age of onset or etiology. The most studied dystonia syndrome has been DYT-1 genetic dystonia; when the DYT-1 gene is expressed prior to age 26, the disease manifests. In those who live asymptomatic past age 26, the mutation (which occurs in the torsin A gene and is due to deletion of the GAG code) remains clinically silent (a carrier state) (Bressman, 2003). This is a phenomenon referred to as incomplete penetrance.
Lesion surgery (pallidotomy and thalamotomy) have both been successfully employed for various primary and secondary dystonias (Lozano et al., 1995; Yoshor et al., 2001), though most centers prefer DBS because bilateral lesions may result in speech or cognitive issues (Hua et al., 2003; Ondo et al., 1998). DBS therapy is mainly performed in the pallidal (GPi) target, because stimulation in this region has provided a reasonable alternative to lesion therapy. Most DBS cases have responded best if the dystonia has been of primary origin, although selected secondary dystonias as well as tardive dystonia have had meaningful improvements in small series (Coubes et al., 1999; Kumar R et al., 1999; Kupsch et al., 2003; Tronnier and Fogel, 2000; Vercueil et al., 2001). There have been two large randomized trials to date that addressed primary generalized dystonia, and both short- and long-term outcomes have been promising (Coubes et al., 2004; Vidailhet et al., 2005; Vidailhet et al., 2007). Additionally, the number of indications has been expanding within dystonia (e.g., cerebral palsy), and the number of brain targets also continues to expand (e.g., STN).
One interesting and unique aspect of DBS for dystonia has been the phenomenon that in many cases, the effects seem to be delayed and appear gradually after stimulation initiation (weeks to months). It has been hypothesized that this phenomenon may be the result of neuroplasticity, but its true mechanism remains a mystery. The other evolving story in dystonia DBS has been the use of lower stimulation frequencies for selected cases (Alterman et al., 2007). Which cases may respond to lower frequencies remains an area of investigation.
Tremor
Tremor has been broadly defined as an involuntary and rhythmic oscillation of a body part and has been classified according to its etiology and/or characteristics (e.g., phenomenology, physiology, etc.). It has been hypothesized that physiological disturbances in the cerebellothalamic and pallidothalamic pathways may be the genesis of some but not all tremor subtypes. According to Deuschl et al. (2001), four pathophysiological mechanisms are proposed to result in tremor: mechanical oscillation of the extremity, reflex activation of the oscillation network, central oscillation, and a disturbance in the regulatory feed-forward or feedback loops of an oscillation network. The ventralis intermedius (VIM) nucleus of the thalamus, which takes its input from the cerebellum, forms a vital piece of this regulatory network and has been frequently targeted for high-frequency (≥100 Hz) DBS to address various medication refractory tremors, with the most common being essential tremor (Benabid et al., 1996). DBS therapy has been reported to have similar efficacy as thalamotomy (Schuurman et al., 2000), and it may have fewer short-term side effects but more long-term (device related) side effects when compared to lesion therapy. Although VIM DBS is preferred for pure essential tremor and certain cases of PD tremor, other cerebellothalamic and pallidothalamic pathways may be altered with DBS. For example, outflow (cerebellar/midbrain) tremor, posttraumatic tremor, and multiple sclerosis (MS) tremor have all been treated in small case series by either single or multiple leads in VIM, ventralis oralis posterior, or zona incerta (Foote and Okun, 2005; Foote et al., 2006; Papavassiliou et al., 2008). The exact target(s) for these more complex tremor disorders remain to be investigated.
Typically, unilateral VIM DBS has been employed to control medication-refractory tremor in a contralateral extremity. Unilateral DBS commonly results in side effects of ataxia and speech problems, and these issues may be more commonly encountered when bilateral DBS is used (Pahwa et al., 2006). Midline tremor, head tremor, and voice tremor seem to respond less consistently to DBS (Ondo et al., 2001). Longitudinal follow-up studies have revealed good long-term benefits, although there has been an emerging concern in the field about tolerance and disease progression (Blomstedt et al., 2007; Pahwa et al., 2006; Sydow et al., 2003; Zhang et al., 2010). Other indications such as cerebellar tremor, Holmes tremor, and MS tremor have had worse efficacy when compared to essential tremor, but these outcomes may change with the emergence of better targets, better technology, and multiple lead approaches.
Neuropsychiatric Disorders
Tourette Syndrome
Tourette syndrome (TS) is a complex neuropsychiatric disorder with a usual onset in childhood (mean age 7 years). The disorder is characterized most prominently by changing motor and vocal tics that must be present for at least 1 year and be marked by fluctuations in number, frequency, and complexity (Robertson, 2000). Patients frequently have associated behavioral abnormalities including anxiety, attention deficit hyperactivity disorder, self-injurious behavior, and obsessive-compulsive behavior, which may persist in their adult life even when motor and phonic tics decline or disappear (Jankovic, 2001; Leckman et al., 1998). Only a small minority of patients diagnosed with TS progress to disabling refractory tic disorder or to malignant TS that is unresponsive to medical and behavioral therapy (Cheung et al., 2007). Only a very select group of TS patients may be candidates for DBS.
Although the mechanisms that cause TS are unknown, abnormalities within the limbic and motor loops of the cortical-basal ganglia/thalamocortical circuitry that involve both dopaminergic and serotonergic neurotransmission likely contribute to the motor and behavioral manifestations in mild and severe TS cases (Albin and Mink, 2006; Wichmann et al., 2006). The centromedian-parafascicular (CM-PF) complex of the thalamus (Houeto et al., 2005), the GPi (both motor and non-motor territories), and the anterior limb of the internal capsule have all been targets for DBS therapy. To date, the GPi and the CM-PF seem to respond better than the anterior limb, but more careful studies, including characterization of individual targets, will be needed (Burdick et al., 2010; Flaherty et al., 2005; Maciunas et al., 2007; Porta et al., 2009; Servello et al., 2008; Shields et al., 2008; Visser-Vandewalle et al., 2003). The heterogeneity of patient populations and the small size of these studies have limited interpretation of reported successes and failures. Because of the special risks in this population, the Tourette Syndrome Association has published guidelines for selection of DBS candidates and for the preferred standardized outcome measures that should be employed if attempting these surgeries (Mink et al., 2006).
Depression
Severe refractory depression is much more common than any other potential patient group for DBS therapy. The loss of quality of life, the impact on lost work hours, and the suicide rate make neuromodulatory therapy an attractive alternative for a select group of these patients (Ward et al., 2010). DBS for medication-refractory depression remains investigational and should only be considered when medication, psychotherapy, and electroconvulsive therapy are not helpful, and when an institutional review board experimental protocol has been obtained. Experts have hypothesized that there is an abnormality in the corticostriatal-thalamic-cortical (CSTC) network in severely depressed humans, and that by lesioning or neuromodulating at specific nodes, clinical symptoms may be reduced (e.g., anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, limbic leucotomy). It has been reported that up to two-thirds of well-selected patients may benefit, but these data are highly preliminary and not inclusive of the entire population of depression patients (Greenberg et al., 2003). Neuromodulatory targets and outcomes have been rapidly emerging and may include subgenual cingulate gyrus/outflow tract, ventral capsule/ventral striatum, nucleus accumbens, and the inferior thalamic peduncle (Greenberg et al., 2010; Mayberg et al., 2005; Ward et al., 2010). To date, the most encouraging results have been achieved with area 25 (cingulate) and anterior limb internal capsule stimulation.
Obsessive-Compulsive Disorder
Another DBS indication that has recently emerged and received U.S. Food and Drug Administration (FDA) approval under a humanitarian device exemption has been OCD. OCD has been characterized by recurrent intrusive thoughts or obsessions that may produce overwhelming anxiety, relieved in some cases by indulgence in ritualistic compulsive behaviors. Functional neuroimaging has revealed hyperactivity within the ventral striatum (VS), medial thalamic region, and orbitofrontal cortex as a potentially abnormal network in this disorder. Patients who are refractory to medical treatment or behavioral approaches could be candidates for a neurosurgical intervention (Tye et al., 2009). Neurosurgical interventions have in the recent past involved lesioning of the anterior limb of the internal capsule (ALIC), cingulotomies, leucotomies, and other approaches (Ward et al., 2010). The idea underpinning early therapies was to create a disconnection between frontal lobe and basal ganglia circuitry to attempt to disrupt the abnormally firing neural network. Apathy and other irreversible complications resulted from early lesion approaches, and most were abandoned in favor of selective lesioning of the VS or DBS. It has been reported that high-frequency DBS of the bilateral anterior limb of the internal capsule/nucleus accumbens region may achieve remission in more than 50% of well-selected patients (Goodman et al., 2010; Greenberg et al., 2010; Nuttin et al., 2008). Other brain areas that have been successfully targeted include the STN and the inferior thalamic peduncle (Mallet et al., 2008; Ward et al., 2010). It should be stressed that strong interdisciplinary teams including psychiatrists and psychologists should be employed to carefully screen and follow patients who undergo DBS for OCD, depression, and other neuropsychiatric disorders (Okun et al., 2005; Okun et al., 2008a).
Drug Addiction
Addiction is the behavior characterized by relentlessly seeking drugs despite knowledge of possible adverse consequences (Kreek, 2008). Addicts often relapse even after prolonged abstinence (Grant et al., 2004; Kalivas and Volkow, 2005; Substance Abuse and Mental Health Services Administration (SAMHSA), 2008). Imaging studies in humans have revealed that addiction seems to involve sudden surges in extracellular dopamine in limbic areas including the nucleus accumbens (NA) (shell and core) and dorsal striatum. The drug ingestion itself results in pleasurable effects (binge/intoxication stage). When abstaining, there seem to be changes in molecular targets of the specific drugs during an acute phase, and this leads to a proposed hypofunction of the dopamine pathways and may result in disrupted activity of frontal regions including dorsolateral prefrontal regions, cingulate gyrus, and orbitofrontal cortex (OFC) (withdrawal/negative affect stage). Prolonged drug abuse may result in reorganization of the reward and memory circuits and lead to increased sensitivity to various signals which may trigger relapse (craving stage) (Koob and Volkow, 2010). As an alternative to medical therapy, stereotactic neurosurgery-leucotomy (Knight, 1969), hypothalamotomy (Dieckmann et al., 1978), cingulotomy (Kanaka and Balasubramaniam, 1978), and ablation of the nucleus accumbens (Gao et al., 2003) have all been attempted in the past and shown some variable effectiveness. The irreversibility of lesions, the behavioral implications, and the trial designs of ablative procedures have led to uncertainty about their place in clinical practice (Stelten et al., 2008).
Subthalamic nucleus DBS in PD seemed to help with dopamine dysregulation syndrome (Witjas et al., 2005), and DBS in the accumbens seemed to help a single patient with OCD who was suffering from alcohol dependency (Kuhn et al., 2007). Preclinical studies involving DBS of the NA shell in mice (with cocaine-seeking behavior) seemed to reduce the craving for cocaine without affecting other functions. This data suggested that afferent and efferent neuronal activity in the NA may be inhibited or neuromodulated with DBS (Vassoler et al., 2008). Encouraging results from STN DBS and insular cortex DBS in animal models offer hope for this as a potential therapy (Forget et al., 2010; Rouaud et al., 2010).
Pain
Neuromodulatory approaches have been employed for persistent pain syndromes for more than half a century. Many initial studies focused on the hypothalamus as the treatment target, but in later studies, many regions emerged as potential targets for therapy, including various areas of the thalamus as well as periventricular (PV) and periaqueductal (PA) gray regions. PV/PA gray matter regions have been hypothesized to respond better to nociceptive pain, whereas targeting the sensory thalamus has been thought to be better for deafferentation pain (Levy et al., 1987). Neuromodulation has been specifically employed for pain due to phantom-limb, stroke, and anesthesia dolorosa (Bittar et al., 2005). Additionally, after it was appreciated by functional imaging that cluster headache and facial pain syndromes may be related to hypothalamic circuitry, neuromodulation of this region was attempted and has been reported successful in multiple cases (Leone et al., 2006). Follow-up performed for up to 2 years revealed improvement in 50% of patients with cluster headache (Bartsch et al., 2008; Broggi et al., 2007; Schoenen et al., 2005; Starr et al., 2007). A recent study, however, did not show as robust a benefit (Fontaine et al., 2010). Greater occipital nerve stimulation is a less invasive alternative being explored for refractory headaches (Burns et al., 2009).
Epilepsy
Epilepsy affects approximately 50 million people worldwide (Kale, 2002). Despite active antiepileptic drug (AED) development, up to 20% of patients suffer from poor seizure control even with optimal medical therapy (Devinsky, 1999). A subset of these patients may be candidates for anterior temporal lobectomy (ATL), which may result in 80% to 90% seizure freedom (Yoon et al., 2003). For the remaining patients, alternative therapies such as vagal nerve stimulation (VNS) have proven limited in efficacy, although some studies report remarkable improvements (see Table 32D.3). Given the tremendous success of DBS for the treatment of movement and neuropsychiatric disorders, clinicians have begun to explore the potential of electrical stimulation for the treatment of a select group of patients with medication-refractory epilepsy.
The process of empirical trials for epilepsy has to date resulted in the discovery of unlikely DBS targets, including the cerebellum and thalamic nuclei. Additionally, DBS for epilepsy has reinvigorated interest in the possibility of long-term, potentially positive neuronal changes that may occur secondary to chronic stimulation and may have benefits even when stimulation has ceased (e.g., batteries of devices burn out, closed-loop devices are employed). This idea of neuronal-level changes has been based in part on the phenomenon of secondary epileptogenesis in patients with long-term poorly controlled seizures. A challenge that DBS may be utilized to address is in repeated ictal insults that may be initiated from a single site and may eventually induce remote and independent ictal activity (Khalilov et al., 2003; Morrell, 1985; Wilder et al., 1969).
Despite recent FDA hearings for approval of DBS for epilepsy, the treatment remains investigational. The mechanisms by which DBS affects seizures or movement disorders are not completely understood. Some theorize that electrical stimulation results in the release of inhibitory neurotransmitters. Others posit that stimulation inactivates neurons via depolarization blockade, although most movement disorders experts agree this is likely not the mechanism of action. There are likely synaptic-level changes, neurochemical changes, and also neurophysiological changes underpinning the benefits of DBS (Dostrovsky and Lozano, 2002; McIntyre et al., 2004a; Vitek, 2002). Recently it has been discovered that DBS may actually inhibit cells close to an electrical field and excite those farther from it. The timing of delivery of electrical stimulation is also an area of active research. Chronic stimulation with an “open-loop” system that responds to a cue (i.e., seizure) has been recently developed and has been referred to as “closed-loop” treatment (Fisher et al., 2010; Skarpaas and Morrell, 2009). Also, scheduled rather than chronic stimulation has been proposed. Multiple targets have been evaluated for DBS in epilepsy, with variable results. One of the reasons studies have reported variable results with the same DBS target may be that certain seizure types may respond differently to stimulation of a particular target. Multiple targets have been proposed and tried for epilepsy DBS (hippocampus, anterior nucleus, thalamus, CM nucleus, cerebellum, STN, etc.). Despite all the uncertainty, several trials have empirically demonstrated the efficacy of DBS for seizures, even in patients who have failed other therapies. These exciting results have fueled a number of studies designed to firmly establish DBS as an effective treatment for intractable epilepsy. In the largest controlled study of DBS for epilepsy (anterior nucleus stimulation) (Fisher et al., 2010), even though patients with a temporal lobe focus or foci had a significant reduction in seizure frequency, those with diffuse frontal, occipital, or parietal seizure foci failed to benefit. In contrast, STN DBS seems to be more effective in patients with seizures having a frontal focus or spread (Shon et al., 2005).
Closed-Loop Stimulation
Closed-loop devices are programmed to respond to detection of ictal or epileptiform discharges and abort impending seizures. An initial trial of closed-loop stimulation in eight patients with intractable epilepsy involved local closed-loop (n = 4) high-frequency electrical stimulation (HFES) directly to the epileptic focus in response to the abnormal electrocorticographic discharges detected by a seizure-detection algorithm. For patients (n = 4) with multiple remote seizure foci, closed-loop HFES was applied using the anterior nucleus of thalamus. There was significant decrease (>50%) in seizures during the experimental phase in both patient groups (Osorio et al., 2005). Similar experience in four patients (Kossoff et al., 2004) using an external responsive neurostimulator (eRNS; NeuroPace, Inc., Mountain View, California) has encouraged a multicenter trial of an implantable responsive neurostimulator (RNS) that continuously monitors electrographic activity from leads positioned near the seizure foci (up to two). An external programmer is used for detection and stimulation parameters and to retrieve recorded electrographic activity. The RNS delivers electrical stimulation to the seizure focus when it detects the epileptic activity (Skarpaas and Morrell, 2009). An initial feasibility study showed encouraging results, with 50% or greater reduction in 43% of patients with complex partial seizures and 35% reduction in all seizure types (n = 24). Patients with pharmacoresistant partial-onset epilepsy having more than three seizures every month over a period of 4 months were recruited for a randomized, double-blinded, multicenter, sham-controlled clinical trial, which was recently completed to establish safety and efficacy of the RNS system as an adjunctive therapy. Results of this trial should be announced soon (Clinicaltrials.gov NCT00264810).
Vagal Nerve Stimulation
Vagal nerve stimulation (VNS) is the only electrical stimulation–based therapy currently approved by the FDA for nonpharmacological treatment of partial-onset refractory epilepsy in adults and adolescents older than 12 years of age. It is also used in primary generalized epilepsies and in pediatric populations by some epileptologists. It is an open-loop device consisting of a pulse generator placed subcutaneously in the upper left chest. The generator delivers intermittent electrical stimulation via a bipolar lead to the cervical vagus nerve trunk. The electrical stimulation is adjusted based on patient tolerance and clinical response, and it can be modulated using a handheld magnet (Lulic et al., 2009). Although not clearly understood, the electrical stimulation to the vagus nerve involves the locus coeruleus–noradrenergic system and seems to result in modification of forebrain activity. Furthermore, norepinephrine and serotonin concentrations seem to change by modulation of the locus coeruleus and modulation of the raphe nuclei, respectively (George et al., 2010). Changes in concentrations of these neurotransmitters and enhanced activity in the brain regions associated with depression also may result in improved mood and cognition, as was reported in patients with VNS. The FDA approved the adjunctive application of VNS for treatment-resistant depression on the basis of studies (Rush et al., 2005) that showed better antidepressant effects in the VNS group (George et al., 2005; Rush et al., 2005); however, many experts dispute this antidepressant effect.
Common side effects from VNS include hoarseness, cough, paresthesias (that generally occur with delivery of stimulus), and headaches. Transient complications due to implantation of the VNS device include vocal cord injury, facial paralysis, and local infection. VNS is usually well tolerated, and results from retrospective studies suggest significant reduction in seizure frequency (Morris and Mueller, 1999; Murphy, 1999; Sakas et al., 2007b; Spanaki et al., 2004; Uthman et al., 2004).
Spinal Cord Stimulation
The rationale for spinal cord stimulation (SCS) for pain syndromes can be linked to the gate control theory of pain proposed by Melzack and Wall. In this theory, the wide dynamic range (WDR) neurons of the dorsal horn are thought to be abnormally excitable in neuropathic pain, and this may then be neuromodulated and inhibited by SCS. SCS may result in pre- and postsynaptic inhibition of the abnormal peripheral neurons responsible for maintaining excitability of the WDR cells. GABA-ergic function may be similarly augmented. SCS has been shown to result in the release of vasodilatory neurotransmitters and to have resultant reductions in sympathetic tone, especially when utilized for ischemic pain syndromes such as those resulting from angina and peripheral vascular disease (Kunnumpurath et al., 2009).
SCS has been the most commonly applied neuromodulatory approach in the treatment of spinal pain syndromes and especially in the failed back surgery syndrome (FBSS). FBSS can be diagnosed after recurrence of lower back/leg pain and follows a previously successful surgery. Although randomized control trials have revealed that SCS is more effective than conventional medical treatment for FBSS, with sustained pain relief over prolonged periods, the level of evidence remains moderate, and the experts remain somewhat skeptical, since the efficacy is in comparison to a failed treatment (Kumar K et al., 2007; Kumar K et al., 2008; Taylor, 2006). Treatment effects are shown to diminish in other patient populations (i.e., those with chronic complex regional pain syndrome) when followed over a 5-year period (Kemler et al., 2008). An interesting trial of thoracic SCS showed improvement in coughing and better breathing control in cervical spinal cord injury survivors, which would be helpful in improving their quality of life (DiMarco et al., 2009).
SCS can be a useful option when conventional medical therapies have failed. SCS requires a highly experienced team in both the operation and the follow-up programming and medical management. Patients and clinicians should carefully consider the risk/benefit ratio when considering SCS. There is a potential for adverse events including infection, bleeding, cerebrospinal fluid leak from dural puncture, and hardware-related failures (Kunnumpurath et al., 2009).
Transcutaneous Electrical Nerve Stimulation
Transcutaneous electrical nerve stimulation (TENS) has long been used as a means of analgesia in chronic pain, including neuropathic conditions. It has also been used in acute low back pain, labor pain, and postoperative pain. Electrodes can be applied to the skin to deliver modulated current output that can be varied according to different stimulus parameters (frequency, duration, amplitude) and by connecting to a battery-operated device. TENS inhibits the nociceptive C-fiber evoked responses within the dorsal horn by activating the large-diameter afferents (Fields and Levine, 1984). The effects of TENS are mediated by endorphins, and they also cause decreased levels of glutamate and increased release of GABA and serotonin (DeSantana et al., 2008).
While TENS has been popularly used in pain clinics, its application has not been substantiated by careful studies. A recent analysis by the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology did not recommend TENS for treatment of chronic low back pain. There is, however, level B evidence supporting TENS for painful diabetic neuropathy (Dubinsky and Miyasaki, 2010).
Sacral Neuromodulation and Incontinence of Bowel and Bladder
Sacral neuromodulation targets the somatic afferent axons in the spinal cord that modulate the voiding and continence reflex pathways (Leng and Chancellor, 2005). Direct inhibition of bladder preganglionic neurons, as well as inhibition of interneuronal transmission in the bladder reflex pathway, may suppress detrusor overactivity while inhibiting the guarding reflex. This may help with urinary retention and dysfunctional voiding (Leng and Chancellor, 2005).
Selection of patients should involve a careful history, physical examination, and review of a voiding diary (with evidence of having failed traditional measures) (Koldewijn et al., 1994; Scheepens et al., 2002). The surgical procedure usually involves a test stimulation staged percutaneous nerve evaluation prior to placement of a quadripolar electrode in a sacral foramen. When a DBS lead is placed, it is most commonly attached to S3.
Sacral neuromodulation is an FDA-approved treatment to improve urinary control. Long-term (≥2 years) follow-up of patients has revealed a reasonable success rate, with more than 50% improvement reported in 40% to 80% of patients (Oerlemans and van Kerrebroeck, 2008). With improvements in technology and expertise and low rates of complications, this approach appears to be an attractive option for patients, even for those with complete spinal cord injury (Sievert et al., 2010).
Additionally, outcome studies in patients with another disorder (fecal incontinence) followed for 2 years or more have been encouraging, with better than 80% reduction in incontinence episodes in 80% of patients (Kenefick, 2006; Melenhorst et al., 2007). Recent results showing improved bowel frequency in constipated patients have also raised this approach as an option in the most severe cases (Holzer et al., 2008; Kamm et al., 2010).
Albert G.C., Cook C.M., Prato F.S., et al. Deep brain stimulation, vagal nerve stimulation and transcranial stimulation: An overview of stimulation parameters and neurotransmitter release. Neurosci Biobehav Rev. 2009;33:1042-1060. doi:10.1016/j.neubiorev.2009.04.006
Alberts J.L., Okun M.S., Vitek J.L. The persistent effects of unilateral pallidal and subthalamic deep brain stimulation on force control in advanced Parkinson’s patients. Parkinsonism Relat Disord. 2008;14:481-488. doi:10.1016/j.parkreldis.2007.11.014
Albin R.L., Mink J.W. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175-182. doi:DOI: 10.1016/j.tins.2006.01.001
Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-381. doi:10.1146/annurev.ne.09.030186.002041
Alterman R.L., Miravite J., Weisz D., et al. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology. 2007;69:681-688. doi:10.1212/01.wnl.0000267430.95106.ff
Anderson V.C., Burchiel K.J., Hogarth P., et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554-560. doi:10.1001/archneur.62.4.554
Anderson W.S., Kossoff E.H., Bergey G.K., et al. Implantation of a responsive neurostimulator device in patients with refractory epilepsy. Neurosurg Focus. 2008;25:E12. doi:10.3171/FOC/2008/25/9/E12
Andrade P., Carrillo-Ruiz J.D., Jimenez F. A systematic review of the efficacy of globus pallidus stimulation in the treatment of Parkinson’s disease. J Clin Neurosci. 2009;16:877-881. doi:10.1016/j.jocn.2008.11.006
Andre-Obadia N., Peyron R., Mertens P., et al. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol. 2006;117:1536-1544. doi:10.1016/j.clinph.2006.03.025
Andrews R.J. Neuroprotection trek–the next generation: Neuromodulation I. techniques–deep brain stimulation, vagus nerve stimulation, and transcranial magnetic stimulation. Ann N Y Acad Sci. 2003;993:1-13. discussion 48-53
Arle J.E., Apetauerova D., Zani J., et al. Motor cortex stimulation in patients with Parkinson disease: 12-month follow-up in 4 patients. J Neurosurg. 2008;109:133-139. doi:10.3171/JNS/2008/109/7/0133
Avery D.H., Holtzheimer P.E.3rd, Fawaz W., et al. Transcranial magnetic stimulation reduces pain in patients with major depression: a sham-controlled study. J Nerv Ment Dis. 2007;195:378-381. doi:10.1097/NMD.0b013e31802f58d1
Avery D.H., Isenberg K.E., Sampson S.M., et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry. 2008;69:441-451.
Awan N.R., Lozano A., Hamani C. Deep brain stimulation: current and future perspectives. Neurosurg Focus. 2009;27:E2. doi:10.3171/2009.4.FOCUS0982
Bajbouj M., Merkl A., Schlaepfer T.E., et al. Two-year outcome of vagus nerve stimulation in treatment-resistant depression. J Clin Psychopharmacol. 2010;3:273-281. doi:10.1097/JCP.0b013e3181db8831
Bartsch T., Pinsker M.O., Rasche D., et al. Hypothalamic deep brain stimulation for cluster headache: experience from a new multicase series. Cephalalgia. 2008;28:285-295. doi:10.1111/j.1468-2982.2007.01531.x
Benabid A.L., Pollak P., Louveau A., et al. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344-346.
Benabid A.L., Pollak P., Gao D., et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203-214.
Beric A. Transcranial electrical and magnetic stimulation. Adv Neurol. 1993;63:29-42.
Bewernick B.H., Hurlemann R., Matusch A., et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110-116. doi:10.1016/j.biopsych.2009.09.013
Birdno M.J., Cooper S.E., Rezai A.R., et al. Pulse-to-pulse changes in the frequency of deep brain stimulation affect tremor and modeled neuronal activity. J Neurophysiol. 2007;98:1675-1684. doi:10.1152/jn.00547.2007
Birdno M.J., Grill W.M. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics. 2008;5:14-25. doi:10.1016/j.nurt.2007.10.067
Bittar R.G., Kar-Purkayastha I., Owen S.L., et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515-519. doi:10.1016/j.jocn.2004.10.005
Blomstedt P., Hariz M.I. Hardware-related complications of deep brain stimulation: a ten year experience. Acta Neurochir. 2005;147:1061-1064. discussion 1064. doi:10.1007/s00701-005-0576-5
Blomstedt P., Hariz M.I. Are complications less common in deep brain stimulation than in ablative procedures for movement disorders? Stereotact Funct Neurosurg. 2006;84:72-81. doi:10.1159/000094035
Blomstedt P., Hariz M.I. Deep brain stimulation for movement disorders before DBS for movement disorders. Parkinsonism Relat Disord. 2010;16:429-433. doi:10.1016/j.parkreldis.2010.04.005
Blomstedt P., Hariz G.M., Hariz M.I., et al. Thalamic deep brain stimulation in the treatment of essential tremor: a long-term follow-up. Br J Neurosurg. 2007;21:504-509. doi:10.1080/02688690701552278
Boon P., Vonck K., De Herdt V., et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551-1560.
Bressman S.B. Dystonia: phenotypes and genotypes. Rev Neurol. 2003;159:849-856.
Broggi G., Franzini A., Leone M. Update on neurosurgical treatment of chronic trigeminal autonomic cephalalgias and atypical facial pain with deep brain stimulation of posterior hypothalamus: results and comments. Neurol Sci. 2007;28(Suppl 2):S138-S145. doi:1
Brouwer R., Duthie G. Sacral nerve neuromodulation is effective treatment for fecal incontinence in the presence of a sphincter defect, pudendal neuropathy, or previous sphincter repair. Dis Colon Rectum. 2010;53:273-278. doi:10.1007/DCR.0b013e3181ceeb22
Brown J.A., Pilitsis J.G. Motor cortex stimulation for central and neuropathic facial pain: a prospective study of 10 patients and observations of enhanced sensory and motor function during stimulation. Neurosurgery. 2005;56:290-297. discussion 290-297
Burdick A., Foote K.D., Goodman W., et al. Lack of benefit of accumbens/capsular deep brain stimulation in a patient with both tics and obsessive-compulsive disorder. Neurocase. 2010;16:321-330. doi:10.1080/13554790903560422
Burns B., Watkins L., Goadsby P.J. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology. 2009;72:341-345. doi:10.1212/01.wnl.0000341279.17344.c9
Canavero S., Paolotti R., Bonicalzi V., et al. Extradural motor cortex stimulation for advanced Parkinson disease. Report of two cases. J Neurosurg. 2002;97:1208-1211. doi:10.3171/jns.2002.97.5.1208
Chang S.Y., Shon Y.M., Agnesi F., et al. Microthalamotomy effect during deep brain stimulation: potential involvement of adenosine and glutamate efflux. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3294-3297. doi:10.1109/IEMBS.2009.5333735
Chen R., Classen J., Gerloff C., et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398-1403.
Cheung M.Y., Shahed J., Jankovic J. Malignant Tourette syndrome. Mov Disord. 2007;22:1743-1750. doi:10.1002/mds.21599
Cioni B. Motor cortex stimulation for Parkinson’s disease. Acta Neurochir Suppl. 2007;97:233-238.
Colicchio G., Policicchio D., Barbati G., et al. Vagal nerve stimulation for drug-resistant epilepsies in different age, aetiology and duration. Childs Nerv Syst. 2010;26:811-819. doi:10.1007/s00381-009-1069-2
Coubes P., Cif L., El Fertit H., et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg. 2004;101:189-194. doi:10.3171/jns.2004.101.2.0189
Coubes P., Echenne B., Roubertie A., et al. Treatment of early-onset generalized dystonia by chronic bilateral stimulation of the internal globus pallidus. apropos of a case. [Traitement de la dystonie generalisee a debut precoce par stimulation chronique bilaterale des globus pallidus internes. A propos d’un cas]. Neuro-Chirurgie. 1999;45:139-144.
Delavallee M., Abu-Serieh B., de Tourchaninoff M., et al. Subdural motor cortex stimulation for central and peripheral neuropathic pain: a long-term follow-up study in a series of eight patients. Neurosurgery. 2008;63:101-105. discussion 105-108. doi:10.1227/01.NEU.0000335076.24481.B6
DeSantana J.M., Walsh D.M., Vance C., et al. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492-499.
Destexhe A., Marder E. Plasticity in single neuron and circuit computations. Nature. 2004;431:789-795. doi:10.1038/nature03011
Deuschl G., Raethjen J., Lindemann M., et al. The pathophysiology of tremor. Muscle Nerve. 2001;24:716-735.
Devinsky O. Patients with refractory seizures. N Eng J Med. 1999;340:1565-1570.
Dieckmann G., Schneider H. Influence of stereotactic hypothalamotomy on alcohol and drug addiction. Appl Neurophysiol. 1978;41:93-98.
DiMarco A.F., Kowalski K.E., Geertman R.T., et al. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a national institutes of health-sponsored clinical trial. Part II: clinical outcomes. Arch Phys Med Rehabil. 2009;90:726-732. doi:10.1016/j.apmr.2008.11.014
Dostrovsky J.O., Lozano A.M. Mechanisms of deep brain stimulation. Mov Disord. 2002;17(Suppl 3):S63-S68.
Dubinsky R.M., Miyasaki J. Assessment: efficacy of transcutaneous electric nerve stimulation in the treatment of pain in neurologic disorders (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2010;74:173-176. doi:10.1212/WNL.0b013e3181c918fc
Elahi B., Elahi B., Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function–systematic review of controlled clinical trials. Mov Disord. 2009;24:357-363. doi:10.1002/mds.22364
Feldman R.P., Goodrich J.T. Psychosurgery: a historical overview. Neurosurgery. 2001;48:647-657. discussion 657-659
Fields H.L., Levine J.D., et al. Pain–mechanics and management. West J Med. 1984;143:347-357.
Fisher R., Salanova V., Witt T., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899-908. doi:10.1111/j.1528-1167.2010.02536.x
Flaherty A.W., Williams Z.M., Amirnovin R., et al. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery. 2005;57:E403. discussion E403
Follett K.A., Weaver F.M., Stern M., et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Eng J Med. 2010;362:2077-2091. doi:10.1056/NEJMoa0907083
Fontaine D., Hamani C., Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110:251-256.
Fontaine D., Lazorthes Y., Mertens P., et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11:23-31. doi:10.1007/s10194-009-0169-4
Foote K.D., Okun M.S. Ventralis intermedius plus ventralis oralis anterior and posterior deep brain stimulation for posttraumatic Holmes tremor: two leads may be better than one: Technical note. Neurosurgery. 2005;56:E445. discussion E445
Foote K.D., Seignourel P., Fernandez H.H., et al. Dual electrode thalamic deep brain stimulation for the treatment of posttraumatic and multiple sclerosis tremor. Neurosurgery. 58, 2006. ONS-280-5; discussion ONS-285-6. doi:10.1227/01.NEU.0000192692.95455.FD
Forget B., Pushparaj A., Le Foll B., et al. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265-271. doi:10.1016/j.biopsych.2010.01.029
Fountas K.N., Smith J.R., Murro A.M., et al. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotac Funct Neurosurg. 2005;83:153-158. doi:10.1159/000088656
Fukaya C., Katayama Y., Kano T., et al. Thalamic deep brain stimulation for writer’s cramp. J Neurosurg. 2007;107:977-982. doi:10.3171/JNS-07/11/0977
Gao G., Wang X., He S., et al. Clinical study for alleviating opiate drug psychological dependence by a method of ablating the nucleus accumbens with stereotactic surgery. Stereotact Funct Neurosurg. 2003;81:96-104. doi:10.1159/000075111
Garcia-Larrea L., Peyron R., Mertens P., et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83:259-273.
George M.S., Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology. 2010;35:301-316. doi:10.1038/npp.2009.87
George M.S., Lisanby S.H., Avery D., et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507-516. doi:10.1001/archgenpsychiatry.2010.46
George M.S., Rush A.J., Marangell L.B., et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364-373. doi:10.1016/j.biopsych.2005.07.028
Ghaemi K., Elsharkawy A.E., Schulz R., et al. Vagus nerve stimulation: outcome and predictors of seizure freedom in long-term follow-up. Seizure. 2010;19:264-268. doi:10.1016/j.seizure.2010.03.002
Goodman W.K., Foote K.D., Greenberg B.D., et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67:535-542. doi:10.1016/j.biopsych.2009.11.028
Grant B.F., Dawson D.A., Stinson F.S., et al. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223-234. doi:10.1016/j.drugalcdep.2004.02.004
Greenberg B.D., Gabriels L.A., Malone D.A.Jr, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Mol Psychiatry. 2010;15:64-79. doi:10.1038/mp.2008.55
Greenberg B.D., Malone D.A., Friehs G.M., et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384-2393. doi:10.1038/sj.npp.1301165
Greenberg B.D., Price L.H., Rauch S.L., et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin N Am. 2003;14:199-212.
Guridi J.L., Andres M. A brief history of pallidotomy. Neurosurgery. 1997;41:1169-1183.
Gutierrez J.C., Seijo F.J., Alvarez Vega M.A., et al. Therapeutic extradural cortical stimulation for Parkinson’s disease: report of six cases and review of the literature. Clin Neurol Neurosurg. 2009:111-703.
Halpern C.H., Samadani U., Litt B., et al. Deep brain stimulation for epilepsy. Neurotherapeutics. 2008;5:59-67. doi:10.1016/j.nurt.2007.10.065
Hamani C., Schwalb J.M., Rezai A.R., et al. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain. 2006;125:188-196. doi:10.1016/j.pain.2006.05.019
Hariz M.I., Krack P., Alesch F., et al. Multicentre European study of thalamic stimulation for parkinsonian tremor: a 6 year follow-up. J Neurol Neurosurg Psychiatry. 2008;79:694-699. doi:10.1136/jnnp.2007.118653
Hariz M.I., Rehncrona S., Quinn N.P., et al. Multicenter study on deep brain stimulation in Parkinson’s disease: an independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23:416-421. doi:10.1002/mds.21888
Hatzis A., Stranjalis G., Megapanos C., et al. The current range of neuromodulatory devices and related technologies. Acta Neurochir Suppl. 2007;97:21-29.
Hemond C.C., Fregni F. Transcranial magnetic stimulation in neurology: What we have learned from randomized controlled studies. Neuromodulation. 2007;10:333-344. doi:10.1111/j.1525-1403.2007.00120.x
Holsheimer J. Letters to the Editor. Neuromodulation. 2003;6:270-273.
Holzer B., Rosen H.R., Novi G., et al. Sacral nerve stimulation in patients with severe constipation. Dis Colon Rectum. 2008;51:524-529. discussion 529-30. doi:10.1007/s10350-007-9160-9
Hosomi K., Saitoh Y., Kishima H., et al. Electrical stimulation of primary motor cortex within the central sulcus for intractable neuropathic pain. Clin Neurophysiol. 2008;119:993-1001. doi:10.1016/j.clinph.2007.12.022
Houeto J.L., Karachi C., Mallet L., et al. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76:992-995. doi:10.1136/jnnp.2004.043273
Hua Z., Guodong G., Qinchuan L., et al. Analysis of complications of radiofrequency pallidotomy. Neurosurgery. 2003;52:89-99. discussion 99-101
Huff W., Lenartz D., Schormann M., et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: outcomes after one year. Clin Neurol Neurosurg. 2010;112:137-143. doi:10.1016/j.clineuro.2009.11.006
Hung S.W., Hamani C., Lozano A.M., et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457-459. doi:10.1212/01.wnl.0000252932.71306.89
Isaias I.U., Alterman R.L., Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol. 2009;66:465-470. doi:10.1001/archneurol.2009.20
Jankovic J. Tourette’s syndrome. N Eng J Med. 2001;345:1184-1192. doi:10.1056/NEJMra010032
Jeong S.G., Lee M.K., Kang J.Y., et al. Pallidal deep brain stimulation in primary cervical dystonia with phasic type: clinical outcome and postoperative course. J Korean Neurosurg Soc. 2009;46:346-350. doi:10.3340/jkns.2009.46.4.346
Jimenez F., Velasco F., Salin-Pascual R., et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97:393-398.
Kale R. Global campaign against epilepsy: the treatment gap. Epilepsia. 2002;43(Suppl 6)):31-33.
Kalivas P.W., Volkow N.D. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403-1413. doi:10.1176/appi.ajp.162.8.1403
Kamm M.A., Dudding T.C., Melenhorst J., et al. Sacral nerve stimulation for intractable constipation. Gut. 2010;59:333-340. doi:10.1136/gut.2009.187989
Kanaka T.S., Balasubramaniam V. Stereotactic cingulumotomy for drug addiction. Appl Neurophysiol. 1978;41:86-92.
Kang B.S., Shin H.I., Bang M.S., et al. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Arch Phys Med Rehabil. 2009;90:1766-1771. doi:10.1016/j.apmr.2009.04.008
Kapural L., Nagem H., Tlucek H., et al. Spinal cord stimulation for chronic visceral abdominal pain. Pain Med. 2010;11:347-355. doi:10.1111/j.1526-4637.2009.00785.x
Katayama Y., Fukaya C., Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. J Neurosurg. 1998;89:585-591.
Katsakiori P.F., Kefalopoulou Z., Markaki E., et al. Deep brain stimulation for secondary dystonia: Results in 8 patients. Acta Neurochir. 2009;151:473-478. discussion 478. doi:10.1007/s00701-009-0281-x
Katz P.S., Frost W.N. Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci. 1996;19:54-61.
Kemler M.A., de Vet H.C., Barendse G.A., et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292-298. doi:10.3171/JNS/2008/108/2/0292
Kenefick N.J. Sacral nerve neuromodulation for the treatment of lower bowel motility disorders. Ann R Coll Surg Engl. 2006;88:617-623. doi:10.1308/003588406X149174
Khalilov I., Holmes G.L., Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079-1085. doi:10.1038/nn1125
Kim H.J., Paek S.H., Kim J.Y., et al. Chronic subthalamic deep brain stimulation improves pain in Parkinson disease. J Neurol. 2008;255:1889-1894. doi:10.1007/s00415-009-0908-0
Kim H.J., Paek S.H., Kim J.Y., et al. Two-year follow-up on the effect of unilateral subthalamic deep brain stimulation in highly asymmetric Parkinson’s disease. Mov Disord. 2009;24:329-335. doi:10.1002/mds.22211
Kiss Z.H., Doig-Beyaert K., Eliasziw M., et al. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879-2886. doi:10.1093/brain/awm229
Kleiner-Fisman G., Fisman D.N., Kahn F.I., et al. Motor cortical stimulation for parkinsonism in multiple system atrophy. Arch Neurol. 2003;60:1554-1558. doi:10.1001/archneur.60.11.1554
Kluger B.M., Klepitskaya O., Okun M.S. Surgical treatment of movement disorders. Neurol Clin. 2009;27:633-677. v. doi:10.1016/j.ncl.2009.04.006
Knight G. Chronic depression and drug addiction treated by stereotactic surgery. Nurs Times. 1969;65:583-586.
Koldewijn E.L., Rosier P.F., Meuleman E.J., et al. Predictors of success with neuromodulation in lower urinary tract dysfunction: results of trial stimulation in 100 patients. J Urol. 1994;152:2071-2075.
Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217-238. doi:10.1038/npp.2009.110
Kossoff E.H., Ritzl E.K., Politsky J.M., et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45:1560-1567. doi:10.1111/j.0013-9580.2004.26104.x
Kovacs N., Pal E., Balas I., et al. Neurosurgical treatment of tremor in mitochondrial encephalopathy. Mov Disord. 2006;21:2227-2230. doi:10.1002/mds.21128
Krack P., Batir A., Van Blercom N., et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925-1934. doi:10.1056/NEJMoa035275
Krames E.S., Pekham P.H., Rezai R., editors. Neuromodulation, vol. 1. London: Academic Press, 2009.
Kreek M.J. Neurobiology of opiates and opioids. In: Galanter M., Kleber H.D. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. Inc.: American Psychiatric Publishing, 2008. doi:10.1176/appi.books.9781585623440.350941
Kuhn J., Lenartz D., Huff W., et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78:1152-1153. doi:10.1136/jnnp.2006.113092
Kumar R., Dagher A., Hutchison W., et al. Globus pallidus deep brain stimulation for generalized dystonia: Clinical and PET investigation. Neurology. 1999;53:871.
Kumar K., Taylor R.S., Jacques L., et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179-188. doi:10.1016/j.pain.2007.07.028
Kumar K., Taylor R.S., Jacques L., et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762-770. discussion 770. doi:10.1227/01.NEU.0000325731.46702.D9
Kunnumpurath S., Srinivasagopalan R., Vadivelu N. Spinal cord stimulation: Principles of past, present and future practice: a review. J Clin Monit Comput. 2009;23:333-339. doi:10.1007/s10877-009-9201-0
Kupsch A., Benecke R., Muller J., et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978-1990. doi:10.1056/NEJMoa063618
Kupsch A., Kuehn A., Klaffke S., et al. Deep brain stimulation in dystonia. J Neurol. 2003;250(Suppl:I):47-52. doi:10.1007/s00415-003-1110-2
Lam R.W., Chan P., Wilkins-Ho M., et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53:621-631.
Leckman J.F., Zhang H., Vitale A., et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14-19. doi:10.1542/peds.102.1.14
Lee H.O., Koh E.J., Oh Y.M., et al. Effect of vagus nerve stimulation in post-traumatic epilepsy and failed epilepsy surgery: preliminary report. J Korean Neurosurg Soc. 2008;44:196-198. doi:10.3340/jkns.2008.44.4.196
Lee K.H., Chang S.Y., Roberts D.W., et al. Neurotransmitter release from high-frequency stimulation of the subthalamic nucleus. J Neurosurg. 2004;101:511-517. doi:10.3171/jns.2004.101.3.0511
Leng W.W., Chancellor M.B. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32:11-18. doi:10.1016/j.ucl.2004.09.004
Leone M., Franzini A., Broggi G., et al. Hypothalamic stimulation for intractable cluster headache: long-term experience. Neurology. 2006;67:150-152. doi:10.1212/01.wnl.0000223319.56699.8a
Levy R.M., Lamb S., Adams J.E. Treatment of chronic pain by deep brain stimulation: Long term follow-up and review of the literature. Neurosurgery. 1987;21:885-893.
Liang G.S., Chou K.L., Baltuch G.H., et al. Long-term outcomes of bilateral subthalamic nucleus stimulation in patients with advanced Parkinson’s disease. Stereotact Funct Neurosurg. 2006;84:221-227. doi:10.1159/000096495
Lim S.N., Lee S.T., Tsai Y.T., et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia. 2007;48:342-347. doi:10.1111/j.1528-1167.2006.00898.x
Lima M.C., Fregni F., et al. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329-2337. doi:10.1212/01.wnl.0000314649.38527.93
Lisanby S.H., Husain M.M., Rosenquist P.B., et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522-534. doi:10.1038/npp.2008.118
Loher T.J., Capelle H.H., Kaelin-Lang A., et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255:881-884. doi:10.1007/s00415-008-0798-6
Lozano A.M., Lang A.E., Galvez-Jimenez N., et al. Effect of GPi pallidotomy on motor function in Parkinson’s disease. Lancet. 1995;346:1383-1387.
Lulic D., Ahmadian A., Baaj A.A., et al. Vagus nerve stimulation. Neurosurg Focus. 2009;27:E5.
Lyons K.E., Wilkinson S.B., Overman J., et al. Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology. 2004;63:612-616.
Maciunas R.J., Maddux B.N., Riley D.E., et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107:1004-1014. doi:10.3171/JNS-07/11/1004
Magis D., Schoenen J. Neurostimulation in chronic cluster headache. Curr Pain Headache Rep. 2008;12:145-153.
Mallet L., Polosan M., Jaafari N., et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121-2134. doi:10.1056/NEJMoa0708514
Mann J.M., Foote K.D., Garvan C.W., et al. Brain penetration effects of microelectrodes and DBS leads in STN or GPi. J Neurol Neurosurg Psychiatry. 2009;80:794-797. doi:10.1136/jnnp.2008.159558
Manola L., Holsheimer J., Veltink P., et al. Anodal vs cathodal stimulation of motor cortex: a modeling study. Clin Neurophysiol. 2007;118:464-474. doi:10.1016/j.clinph.2006.09.012
Mayberg H.S., Lozano A.M., Voon V., et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651-660. doi:10.1016/j.neuron.2005.02.014
McIntyre C.C., Grill W.M., Sherman D.L., et al. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457-1469. doi:10.1152/jn.00989.2003
McIntyre C.C., Hahn P.J. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38:329-337. doi:10.1016/j.nbd.2009.09.022
McIntyre C.C., Savasta M., Walter B.L., et al. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40-50.
Melenhorst J., Koch S.M., Uludag O., et al. Sacral neuromodulation in patients with faecal incontinence: results of the first 100 permanent implantations. Colorectal Dis. 2007;9:725-730. doi:10.1111/j.1463-1318.2007.01241.x
Melzack R., Wall P.D., et al. Pain mechanisms: a new theory. Science. 1965;150:971-979.
Mikos A., Zahodne L., Okun M.S., et al. Cognitive declines after unilateral deep brain stimulation surgery in Parkinson’s disease: a controlled study using reliable change, part II. Clin Neuropsychol. 2010;24:235-245. doi:10.1080/13854040903277297
Mink J.W., Walkup J., Frey K.A., et al. Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord. 2006;21:1831-1838. doi:10.1002/mds.21039
Montavont A., Demarquay G., Ryvlin P., et al. Long-term efficiency of vagus nerve stimulation (VNS) in non-surgical refractory epilepsies in adolescents and adults. [Efficacite de la stimulation intermittente du nerf vague dans les epilepsies pharmaco-resistantes non chirurgicales de l’adolescent et de l’adulte] Rev Neurol (Paris). 2007;163:1169-1177. doi:10.1016/S0035-3787(07)78401-1
Morishita T., Foote K.D., Burdick A.P., et al. Identification and management of deep brain stimulation intra- and postoperative urgencies and emergencies. Parkinsonism Relat Disord. 2010;16:153-162. doi:10.1016/j.parkreldis.2009.10.003
Moro E., Lozano A.M., Pollak P., et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25:578-586. doi:10.1002/mds.22735
Morrell F. Secondary epileptogenesis in man. Arch Neurol. 1985;42:318-335. doi:10.1001/archneur.1985.04060040028009
Morris G.L.3rd, Mueller W.M., et al. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. the vagus nerve stimulation study group E01-E05. Neurology. 1999;53:1731-1735.
Murata J., Kitagawa M., Uesugi H., et al. Deep brain stimulation of the posterior subthalamic area (Zi/Raprl) for intractable tremor. No Shinkei Geka. 2007;35(4):355-362.
Murphy J.V. Left vagal nerve stimulation in children with medically refractory epilepsy. the pediatric VNS study group. J Pediatr. 1999;134:563-566.
Nguyen J.P., Lefaucheur J.P., Decq P., et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245-251.
Nuttin B.J., Gabriels L.A., Cosyns P.R., et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2008;62:966-977. doi:10.1227/01.neu.0000333764.20575.d6
Oerlemans D.J., van Kerrebroeck P.E. Sacral nerve stimulation for neuromodulation of the lower urinary tract. Neurourol Urodyn. 2008;27:28-33. doi:10.1002/nau.20459
Oh M.Y., Abosch A., Kim S.H., et al. Long-term hardware-related complications of deep brain stimulation. Neurosurgery. 2002;50:1268-1276.
Okun M.S., Rodriguez R.L., Foote K.D., et al. A case-based review of troubleshooting deep brain stimulator issues in movement and neuropsychiatric disorders. Parkinsonism Relat Disord. 2008;14:532-538. doi:10.1016/j.parkreldis.2008.01.001
Okun M.S., Fernandez H.H., Foote K.D., et al. Avoiding deep brain stimulation failures in tourette syndrome. J Neurol Neurosurg Psychiatry. 2008;79:111-112. doi:10.1136/jnnp.2007.135715
Okun M.S., Fernandez H.H., Pedraza O., et al. Development and initial validation of a screening tool for Parkinson disease surgical candidates. Neurology. 2004;63:161-163.
Okun M.S., Fernandez H.H., Rodriguez R.L., et al. Identifying candidates for deep brain stimulation in Parkinson’s disease: the role of the primary care physician. Geriatrics. 2007;62:18-24.
Okun M.S., Fernandez H.H., Wu S.S., et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65:586-595. doi:10.1002/ana.21596
Okun M.S., Foote K.D. A mnemonic for Parkinson disease patients considering DBS: a tool to improve perceived outcome of surgery. Neurologist. 2004;10:290.
Okun M.S., Foote K.D. Enough is enough: moving on to deep brain stimulation in patients with fluctuating Parkinson disease. Arch Neurol. 2009;66:778-780. doi:10.1001/archneurol.2009.82
Okun M.S., Tagliati M., Pourfar M., et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62:1250-1255. doi:10.1001/archneur.62.8.noc40425
Ondo W., Almaguer M., Jankovic J., et al. Thalamic deep brain stimulation: comparison between unilateral and bilateral placement. Arch Neurol. 2001;58:218-222. doi:10.1001/archneur.58.2.218
Ondo W.G., Desaloms J.M., Jankovic J., et al. Pallidotomy for generalized dystonia. Mov Disord. 1998;13:693-698.
Ondo W.G., Bronte-Stewart H. DBS Study Group: the North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact Funct Neurosurg. 2005;83:142-147. doi:10.1159/000088654
O’Reardon J.P., Solvason H.B., Janicak P.G., et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208-1216. doi:10.1016/j.biopsych.2007.01.018
Osorio I., Frei M.G., Sunderam S., et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57:258-268. doi:10.1002/ana.20377
Owen S.L., Green A.L., Nandi D.D., et al. Deep brain stimulation for neuropathic pain. Acta Neurochir Suppl. 2007;97:111-116.
Pahwa R., Lyons K.E., Wilkinson S.B., et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg. 2006;104:506-512.
Pahwa R., Wilkinson S.B., Overman J., et al. Preoperative clinical predictors of response to bilateral subthalamic stimulation in patients with Parkinson’s disease. Stereotact Funct Neurosurg. 2005;83:80-83. doi:10.1159/000086866
Panescu D. Emerging technologies. IEEE Eng Med Biol Mag. 2008;27:100-113.
Papavassiliou E., Rau G., Heath S., et al. Thalamic deep brain stimulation for essential tremor: Relation of lead location to outcome. Neurosurgery. 2008;62(Suppl 2):884-894. doi:10.1227/01.neu.0000316290.83360.7e
Pascual-Leone A., Valls-Sole J., Wassermann E.M., et al. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847-858.
Plaha P., Khan S., Gill S.S. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79:504-513. doi:10.1136/jnnp.2006.112334
Porta M., Brambilla A., Cavanna A.E., et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375-1380. doi:10.1212/WNL.0b013e3181bd809b
Powell C.R., Kreder K.J. Long-term outcomes of urgency-frequency syndrome due to painful bladder syndrome treated with sacral neuromodulation and analysis of failures. J Urol. 2010;183:173-176. doi:10.1016/j.juro.2009.08.142
Rasche D., Rinaldi P.C., Young R.F., et al. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21:E8.
Reed K.L., Black S.B., Banta C.J.2nd, et al. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: Initial experience. Cephalalgia. 2009. doi:10.1111/j.1468-2982.2009.01996.x
Robertson M.M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123:425-462. doi:10.1093/brain/123.3.425
Rodriguez R.L., Fernandez H.H., Haq I., et al. Pearls in patient selection for deep brain stimulation. Neurologist. 2007;13:253-260. doi:10.1097/NRL.0b013e318095a4d5
Rossini P.M., Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484-488. doi:10.1212/01.wnl.0000250268.13789.b2
Rouaud T., Lardeux S., Panayotis N., et al. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A. 2010;107:1196-1200. doi:10.1073/pnas.0908189107
Rush A.J., Sackeim H.A., Marangell L.B., et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58:355-363. doi:10.1016/j.biopsych.2005.05.024
Saint-Cyr J.A., Albanese A. STN DBS in PD: selection criteria for surgery should include cognitive and psychiatric factors. Neurology. 2006;66:1799-1800. doi:10.1212/01.wnl.0000227468.17113.07
Sakas D.E., Panourias I.G., Simpson B.A., et al. An introduction to operative neuromodulation and functional neuroprosthetics, the new frontiers of clinical neuroscience and biotechnology. Acta Neurochir Suppl. 2007;97:3-10.
Sakas D.E., Korfias S., Nicholson C.L., et al. Vagus nerve stimulation for intractable epilepsy: outcome in two series combining 90 patients. Acta Neurochir Suppl. 2007;97:287-291.
Sako W., Goto S., Shimazu H., et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov Disord. 2008;23:1929-1931. doi:10.1002/mds.22100
Scheepens W.A., Jongen M.M., Nieman F.H., et al. Predictive factors for sacral neuromodulation in chronic lower urinary tract dysfunction. Urology. 2002;60:598-602.
Schoenen J., Di Clemente L., Vandenheede M., et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128:940-947. doi:10.1093/brain/awh411
Schulder M., Sernas T.J., Karimi R. Thalamic stimulation in patients with multiple sclerosis: long-term follow-up. Stereotact Funct Neurosurg. 2003;80:48-55. doi:10.1159/000075160
Schüpbach W.M., Chastan N., Welter M.L., et al. Stimulation of the subthalamic nucleus in Parkinson’s disease: a 5 year follow up. J Neurol Neurosurg Psychiatry. 2005;76:1640-1644. doi:10.1136/jnnp.2005.063206
Schuurman P.R., Bosch D.A., Bossuyt P.M.M., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461-468. doi:10.1056/NEJM200002173420703
Sensi M., Cavallo M.A., Quatrale R., et al. Pallidal stimulation for segmental dystonia: long term follow up of 11 consecutive patients. Mov Disord. 2009;24:1829-1835. doi:10.1002/mds.22686
Servello D., Porta M., Sassi M., et al. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79:136-142. doi:10.1136/jnnp.2006.104067
Shields D.C., Cheng M.L., Flaherty A.W., et al. Microelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg. 2008;86:87-91. doi:10.1159/000112429
Shon Y.M., Chang S.Y., Tye S.J., et al. Comonitoring of adenosine and dopamine using the wireless instantaneous neurotransmitter concentration system: proof of principle. J Neurosurg. 2010;112(3):539-548. doi:10.3171/2009.7.JNS09787
Shon Y.M., Lee K.J., Kim H.J., et al. Effect of chronic deep brain stimulation of the subthalamic nucleus for frontal lobe epilepsy: subtraction SPECT analysis. Stereotact Funct Neurosurg. 2005;83:84-90. doi:10.1159/000086867
Sievert K.D., Amend B., Gakis G., et al. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol. 2010;67:74-84. doi:10.1002/ana.21814
Skarpaas T.L., Morrell M.J. Intracranial stimulation therapy for epilepsy. Neurotherapeutics. 2009;6:238-243. doi:10.1016/j.nurt.2009.01.022
Skidmore F.M., Rodriguez R.L., Fernandez H.H., et al. Lessons learned in deep brain stimulation for movement and neuropsychiatric disorders. CNS Spectr. 2006;11:521-536.
Slotema C.W., Blom J.D., Hoek H.W., et al. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010. doi:10.4088/JCP.08m04872gre
Slowinski J.L., Putzke J.D., Uitti R.J., et al. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2007;106(4):626-632. doi:10.3171/jns.2007.106.4.626
Spanaki M.V., Allen L.S., Mueller W.M., et al. Vagus nerve stimulation therapy: 5-year or greater outcome at a university-based epilepsy center. Seizure. 2004;13:587-590. doi:10.1016/j.seizure.2004.01.009
Spiegel E.A., Wycis H.T., Marks M., et al. Stereotaxic apparatus for operations on the human brain. Science. 1947;106:349-350. doi:10.1126/science.106.2754.349
Starr P.A., Barbaro N.M., Raskin N.H., et al. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106:999-1005. doi:10.3171/jns.2007.106.6.999
Stefani A., Fedele E., Galati S., et al. Subthalamic stimulation activates internal pallidus: evidence from cGMP microdialysis in PD patients. Ann Neurol. 2005;57:448-452. doi:10.1002/ana.20402
Stelten B.M., Noblesse L.H., Ackermans L., et al. The neurosurgical treatment of addiction. Neurosurg Focus. 2008;25:E5. doi:10.3171/FOC/2008/25/7/E5
Stieglitz T. Neural prostheses in clinical practice: biomedical microsystems in neurological rehabilitation. Acta Neurochir Suppl. 2007;97:411-418.
Stillings D. The Piscean origin of medical electricity. Med Instrum. 1973;7(2):163-164.
Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2008 national survey on drug use and health: National findings. Retrieved 05/05, 2010, from http://www.oas.samhsa.gov/NSDUH/2K8NSDUH/tabs/toc.htm, 2008.
Sydow O., Thobois S., Alesch F., et al. Multicentre European study of thalamic stimulation in essential tremor: a six year follow up. J Neurol Neurosurg Psychiatry. 2003;74:1387-1391. doi:10.1136/jnnp.74.10.1387
Taylor R.S. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(4 Suppl):S13-S19. doi:10.1016/j.jpainsymman.2005.12.010
Tronnier V.M., Fogel W. Pallidal stimulation for generalized dystonia. Report of three cases. J Neurosurg. 2000;92:453-456. doi:10.3171/jns.2000.92.3.0453
Tsubokawa T., Katayama Y., Yamamoto T., et al. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl. 1991;52:137-139.
Tye S.J., Frye M.A., Lee K.H. Disrupting disordered neurocircuitry: treating refractory psychiatric illness with neuromodulation. Mayo Clin Proc. 2009;84:522-532. doi:10.4065/84.6.522
Uthman B.M., Reichl A.M., Dean J.C., et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124-1126.
Vassoler F.M., Schmidt H.D., Gerard M.E., et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Clin Neurosci. 2008;28:8735-8739. doi:10.1523/JNEUROSCI.5277-07.2008
Velasco F., Carrillo-Ruiz J.D., Castro G., et al. Motor cortex electrical stimulation applied to patients with complex regional pain syndrome. Pain. 2009;147:91-98. doi:10.1016/j.pain.2009.08.024
Vercueil L., Pollak P., Fraix V., et al. Deep brain stimulation in the treatment of severe dystonia. J Neurol. 2001;248:695-700.
Vesper J., Haak S., Ostertag C., et al. Subthalamic nucleus deep brain stimulation in elderly patients–analysis of outcome and complications. BMC Neurol. 2007;7:7. doi:10.1186/1471-2377-7-7
Vidailhet M., Vercueil L., Houeto J.L., et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459-467. doi:10.1056/NEJMoa042187
Vidailhet M., Vercueil L., Houeto J.L., et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223-229. doi:10.1016/S1474-4422(07)70035-2
Vilensky J.A., Gilman S. Horsley was the first to use electrical stimulation of the human cerebral cortex intraoperatively. Surg Neurol. 2002;58:425-426.
Visser-Vandewalle V., Temel Y., Boon P., et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. report of three cases. J Neurosurg. 2003;99:1094-1100. doi:10.3171/jns.2003.99.6.1094
Vitek J.L. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69-S72.
Ward H.E., Hwynn N., Okun M.S. Update on deep brain stimulation for neuropsychiatric disorders. Neurobiol Dis. 2010. doi:10.1016/j.nbd.2010.01.011
Weaver F.M., Follett K., Stern M., et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63-73. doi:10.1001/jama.2008.929
Wichmann T., Delong M.R. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52(1):197-204. doi:10.1016/j.neuron.2006.09.022
Wider C., Pollo C., Bloch J., et al. Long-term outcome of 50 consecutive Parkinson’s disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. 2008;14(2):114-119. doi:DOI: 10.1016/j.parkreldis.2007.06.012
Wilder B.J., King R.L., Schmidt R.P. Cortical and subcortical secondary epileptogenesis. Neurology. 1969;19:643.
Windels F., Bruet N., Poupard A., et al. Influence of the frequency parameter on extracellular glutamate and gamma-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J Neurosci Res. 2003;72:259-267. doi:10.1002/jnr.10577
Wishart H.A., Roberts D.W., Roth R.M., et al. Chronic deep brain stimulation for the treatment of tremor in multiple sclerosis: review and case reports. J Neurol Neurosurg Psychiatry. 2003;74:1392-1397.
Witjas T., Baunez C., Henry J.M., et al. Addiction in Parkinson’s disease: Impact of subthalamic nucleus deep brain stimulation. Mov Disord. 2005;20:1052-1055. doi:10.1002/mds.20501
Woehrle J.C., Blahak C., Kekelia K., et al. Chronic deep brain stimulation for segmental dystonia. Stereotact Funct Neurosurg. 2009;87:379-384. doi:10.1159/000249819
Yoon H.H., Kwon H.L., Mattson R.H., et al. Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology. 2003;61:445-450.
Yoshor D., Hamilton W.J., Ondo W., et al. Comparison of thalamotomy and pallidotomy for the treatment of dystonia. Neurosurgery. 2001;48:818-826.
Zabek M., Sobstyl M., Koziara H., et al. Bilateral subthalamic nucleus stimulation in the treatment of advanced Parkinson’s disease. Five years’ personal experience. Neurol Neurochir Pol. 2010;44:3-12.
Zahodne L.B., Okun M.S., Foote K.D., et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol. 2009;256:1321-1329. doi:10.1007/s00415-009-5121-7
Zhang J.G., Zhang K., Wang Z.C., et al. Deep brain stimulation in the treatment of secondary dystonia. Chin Med J. 2006;119:2069-2074.
Zhang K., Bhatia S., Oh M.Y., et al. Long-term results of thalamic deep brain stimulation for essential tremor. J Neurosurg. 2010;112:1271-1276. doi:10.3171/2009.10.JNS09371