Clinical Laboratory Studies
After reading this chapter, you will be able to:
1. Explain the three phases of laboratory testing.
2. Describe the composition of blood.
3. Explain the importance of specimen integrity and effects on laboratory test results.
4. Define laboratory test sensitivity, specificity, and positive and negative predictive value.
5. Discuss the meaning of the term reference range.
6. Describe the clinical applications and general clinical significance of increases and decreases for each component of the complete blood count and for the reticulocyte count and erythrocyte sedimentation rate.
7. Define leukocytosis, leukopenia, relative and absolute count, neutrophilia, neutropenia, lymphocytosis, lymphopenia, monocytosis, eosinophilia, basophilia, leukemia, anemia, and hemostasis.
8. Describe the body’s response to anemia and the potential effects on the body of uncompensated anemia.
9. Differentiate primary, secondary, and relative polycythemia and describe the adverse effects of polycythemia on the body.
10. Describe the clinical applications of the activated partial thromboplastin time, prothrombin time, and platelet count in assessing hemostasis.
11. Explain the clinical application and significance of the quantitative D-dimer assay.
12. Describe the clinical applications and general clinical significance of increases and decreases in electrolyte concentrations, glucose levels, blood urea nitrogen and creatinine.
13. Discuss the importance of renal and hepatic panel tests as related to the management of patients with respiratory disorders.
14. Relate lipid panel measures to the risk for atherosclerosis and heart disease.
15. Identify the current cardiac biomarkers used to help identify acute coronary syndrome and congestive heat failure.
16. Describe the pre-analytical phase of testing in clinical microbiology.
17. Discuss the common methods of examination of microbiology specimens (e.g., Gram stain, acid-fast stains).
18. Explain the purpose of a microbiology culture and antimicrobial sensitivity test.
19. Describe the collection and transport protocols for pulmonary secretions.
20. Discuss the importance of macroscopic and microscopic examination of sputum.
21. List the microscopic criteria used to assess the quality of a sputum sample.
22. Explain the significance of sputum eosinophilia.
23. Describe the indications and method of performing a bronchoalveolar lavage.
24. Describe the macroscopic, microscopic, and chemical significance of pleural fluid examination.
25. Explain the purpose of histologic and cytologic examinations.
26. List the malignant tumors responsible for producing most primary lung cancers.
27. List the types of pulmonary samples that can be examined cytologically.
28. Discuss the general concept of skin testing and two methods to screen for tuberculosis.
29. Given a variety of patient presentations, identify the common laboratory tests helpful in assessing the problem.
Clinical Laboratory Overview
Specimen Integrity and Effect on Test Results
Specimen integrity is critical to quality, accurate laboratory testing. Test results can be affected by hemolysis, storage times and temperature, method of transport, interfering conditions of the blood (icterus, lipemia, turbidity), inadequate blood volume when using tubes with additives, and using the wrong collection tube. For example, a specimen that is hemolyzed can yield falsely elevated results for several analytes, including potassium, magnesium, calcium, and iron. Maintenance of specimen integrity for microbiologic samples commonly gathered by RTs is discussed later in this chapter. Specimen integrity for arterial blood gas samples is covered in Chapter 8.
Laboratory Test Parameters
• Sensitivity: frequency of positive test results of patients with disease. Example: A sensitivity of 98% means that 98% of the patients with the disease will be detected by the test (TP), and 2% of the patients with the disease will be negative with the test (FN). Another way to express sensitivity is: “if the patient has the disease, the test will be positive.” If, on the other hand, the results of a test with high sensitivity are negative, one can confidently rule out the disorder in question.
• Specificity: frequency of negative test results of patients without disease. Example: A specificity of 98% means that 98% of the patients without the disease will be negative for the test (TN), and 2% of the patients without the disease will be positive for the test (FP). Another way to express specificity is: “if the patient does not have the disease, the test will be negative.” Conversely, if the results of a test with high specificity are positive, one can confidently rule-in the disorder in question.
Hematology
Complete Blood Count
The CBC is an overall assessment of the quantity and morphology (appearance) of the white blood cells (WBCs), red blood cells (RBCs), and platelets. The CBC includes a total WBC count, a count of the different types of WBCs (called a differential count), the RBC count, hemoglobin (Hb) level, hematocrit (Hct), the RBC indices, a platelet count, and sometimes a reticulocyte count. Table 7-1 contains sample adult reference ranges for the CBC components in both common units and standard international (SI) units.
TABLE 7-1
Sample Reference Ranges for the Complete Blood Count (CBC) in Adults
| CBC Component Conventional Units (SI units) | Reference Ranges |
| WBC count × 103/μL (× 109/L) | 4.5-11.5 |
| RBC count × 106/μL (× 1012/L) | M: 4.60-6.00 F: 4.00-5.40 |
| Hemoglobin g/dL (g/L) | M: 14.0-18.0 (140-180) F: 12.0-15.0 (120-150) |
| Hct % (L/L) | M: 40-54 (0.40-0.54) F: 35-49 (0.35-0.49) |
| Erythrocyte Indices | |
| Mean cell volume (MCV) (fL) | 80-100 |
| Mean cell hemoglobin (pg) | 26-32 |
| Mean cell Hb concentration (MCHC) (g/dL) | 32-36 |
| Platelet count × 103/μL (× 109/L) | 150-450 |
| Reticulocytes % | 0.5-1.5 |
| Reticulocytes × 103/μL (× 109/L) | 25-75 |
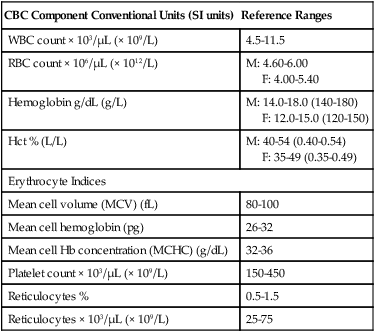
M, male; F, female; RBC, Red blood cell; SI, standard international; WBC, white blood cell.
White Blood Cells
White Blood Cell Differential Count
The relative count is the percentage of a particular cell among all the WBCs counted. The absolute count for that cell type is determined by multiplying its percentage or relative count by the total WBC count. CBC analyzers automatically calculate the absolute counts for each type of WBC. Table 7-2 provides sample reference ranges for the relative and absolute counts of each type of WBC.
TABLE 7-2
Sample Reference Ranges for the Differential White Blood Cell Count in Adults
| Cell Type | Relative | Absolute∗ |
| Segmented neutrophils | 50-70 | 2.3-8.1 |
| Bands | 0-5 | 0-0.6 |
| Eosinophils | 1-3 | 0-0.4 |
| Basophils | 0-1 | 0-0.1 |
| Lymphocytes | 20-45 | 0.8-4.8 |
| Monocytes | 2-11 | 0.45-1.3 |
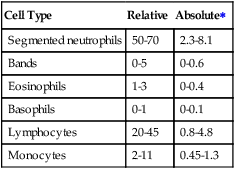
Because the relative count is influenced by increases or decreases of the other WBC types, the absolute count provides a more useful quantitative assessment. In a simplified example, if an adult patient had a total WBC count of 20 ×109/L with a relative count of 85% neutrophils and 15% lymphocytes, the absolute counts would be 17 × 109/L for neutrophils and 3 × 109/L for lymphocytes. Comparing these results to the reference ranges in Table 7-2, there is both a relative and an absolute increase in neutrophils. On the other hand, although the relative count for the lymphocytes is decreased, the absolute count is well within the reference range.
Nonmalignant White Blood Cell Abnormalities
Neutrophilia is a common response to acute bacterial infections, such as bacterial pneumonia (Box 7-1). Neutrophilia also may occur in response to fungal, parasitic, or early viral infections. In addition, neutrophilia also occurs in inflammation due to autoimmune disorders or tissue damage that occurs after surgery, burns, myocardial infarction, or traumatic injury. Acute hemorrhage or hemolysis, metabolic disorders (such as acidosis or uremia), and certain drugs, toxins, and chemicals also cause neutrophilia. When the bone marrow is stimulated to release neutrophils at a rate faster than it can produce them, it releases them at increasingly more immature stages. This is called a left shift. The degree of the left shift and the severity of the neutrophilia usually correlate with the severity of the infection.
Neutropenia may occur as a result of decreased bone marrow production or increased destruction of circulating neutrophils (Box 7-2). Many drugs, some chemicals, and radiation therapy can cause neutropenia by destroying the hematopoietic cells in the bone marrow, thus decreasing bone marrow production. One of the most common causes of neutropenia is cancer chemotherapy. Because of their short half-life, the neutrophils are the first blood cell type to decrease with chemotherapy. The absolute neutrophil count is closely monitored during chemotherapy, and adjustments in therapy are made if it drops too low. Other causes of decreased production include acquired and hereditary defects in hematopoietic stem cells, infiltration of cancer cells into the bone marrow, and deficiencies of vitamin B12 or folate. Neutrophils can also be quickly depleted by overwhelming infections or be destroyed as a result of the production of antibodies against them. Pseudoneutropenia is a transient shift of the neutrophils in the circulating pool to the marginated pool. It may be caused by bacterial endotoxins or hypersensitivity reactions.
Red Blood Cells
Normal RBCs assume the shape of a biconcave disk to facilitate their primary function of carrying oxygen and to provide maximal deformability to bend as they pass through small capillaries. The major component of mature RBCs (erythrocytes) is hemoglobin, which imparts to blood its normal red color when carrying oxygen. The RBC count is reported in number of cells per microliter or liter of blood (see Table 7-1 for reference ranges). An adequate number of RBCs with an adequate concentration of hemoglobin and a functionally normal hemoglobin molecule are needed for transport of sufficient oxygen from the lungs to the tissues.
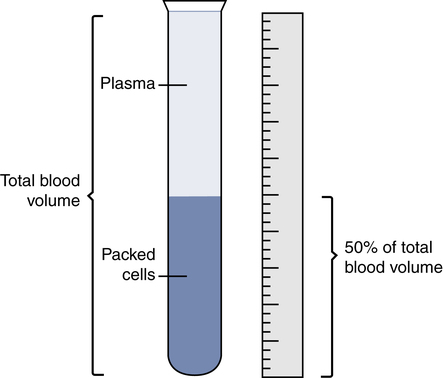
Hematocrit (Packed Cell Volume)
The Hct is the ratio of the packed RBC volume to the volume of whole blood. A manual Hct is performed by centrifuging whole blood to pack the blood cells to the bottom of a small capillary tube and then measuring the percentage or fraction of the total blood volume occupied by the RBCs (Fig. 7-1). CBC analyzers determine the Hct using various automated technologies. Because it measures the volume of the hemoglobin-containing RBCs, one can estimate the Hct by multiplying the hemoglobin content times a factor of three.
Hemoglobin
Hemoglobin is reported in grams per deciliter or liter of blood. The reference ranges for the RBC count, Hct, and hemoglobin vary by gender, with men having higher levels than women because of the positive influence of testosterone on RBC production (see Table 7-1). Age also affects hemoglobin levels, with higher values in newborns and slightly lower values in elderly people.
Red Blood Cell Abnormalities
Hemoglobin may be converted to an inactive form through oxidation, denaturation, or other chemical reaction. The most common forms of inactive hemoglobins are carboxyhemoglobin (COHb) and methemoglobin (metHb). The measurement of these abnormal Hb combinations and their impact on oxygen transport are discussed in detail in Chapter 8.
Reticulocyte Count
The reticulocyte is the final erythrocyte development stage before the RBC is fully mature. These slightly immature RBCs lack a nucleus and differ from mature RBCs in that they are slightly larger and have not yet assumed a biconcave shape. A reticulocyte count is performed by obtaining the percentage of reticulocytes among the RBCs. Normally about 1% of the circulating RBCs are reticulocytes, with slightly higher values in newborns. The reticulocyte count is also reported as an absolute count by multiplying the reticulocyte percentage by the RBC count. Reference ranges are provided in Table 7-1.
Platelet Count
Platelets are the smallest cells in the peripheral blood. After injury to a blood vessel, they participate in clot formation at the site of the injury to stop the bleeding. The platelet count routinely is provided as part of the CBC. Table 7-1 provides the reference range for the platelet count. Like other blood cells, platelets may be decreased as a result of increased destruction in the peripheral blood or decreased production in the bone marrow. A reduction in the platelet count below the reference range is called thrombocytopenia.
An increase in platelets (thrombocytosis