Chapter 81 Clinical Application of New Antiarrhythmic Drugs for Atrial Fibrillation
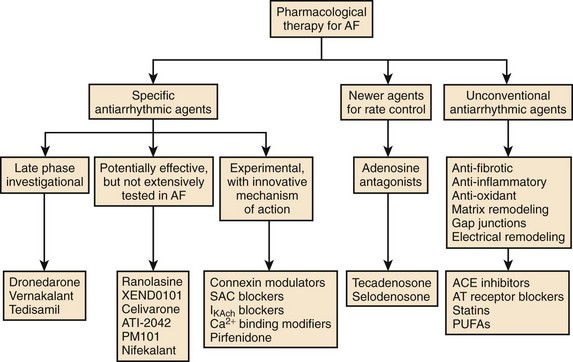
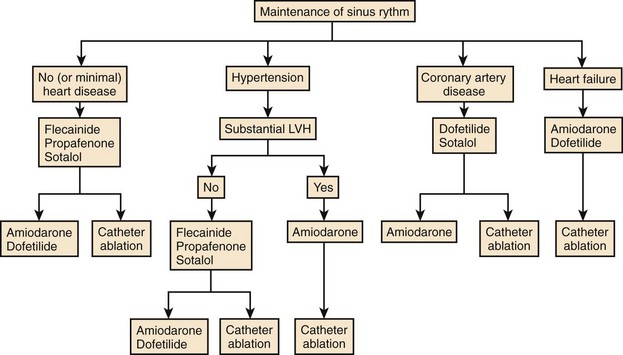
Atrial fibrillation (AF) is the most common sustained arrhythmia requiring medical care. Oral antiarrhythmic drugs are effective in controlling AF in 50% to 65% of cases but have limitations, including subjective adverse effects, ventricular proarrhythmia, and end-organ toxicity.1 In addition, therapeutic options available to the majority of patients with AF associated with significant structural heart disease are limited. Therefore, more effective and safer antiarrhythmic drugs for the termination and prevention of AF are needed (Figure 81-1).2 Although many new antiarrhythmic drugs are at various degrees of development, this chapter will limit discussion to a recently released antiarrhythmic, dronedarone, a commercially released anti-anginal medication with antiarrhythmic properties; vernakalant; several drugs furthest along in development, such as celivarone, budiodarone, and ranolazine.
Dronedarone (Multaq)
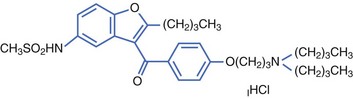
Dronedarone is a non-iodinated benzofurane derivative, structurally related to amiodarone, developed by Sanofi-Aventis. The addition of a methyl-sulfonamide grouping made the drug less lipophilic with a shorter half-life than that of amiodarone (Figure 81-2).
Electrophysiology
In vitro, dronedarone blocks IKr, IKs, Ito, INa, and ICa-L.3 Dronedarone prolongs the action potential duration in the atria and ventricles with no significant reverse-use dependence. In vivo, dronedarone has been shown to actively block the following channels: INa, IKr, IKs, IKACh, and ICa-L and inhibit α-adrenoceptors and β-adrenoceptors. Although its effect is similar to that of amiodarone, the magnitude of the effect is different. For example, dronedarone is 10 times more effective in blocking INa, and 100 times more effective in blocking IKACh, compared with amiodarone. In addition, blockade of isoprenaline β2-adrenoceptor–mediated decrease in blood pressure is more pronounced with dronedarone than with amiodarone. Other amiodarone-like electrophysiological effects include alpha, beta, and muscarinic blocking effects.3 Dronedarone slows sinus rate, prolongs atrioventricular (AV) nodal refractory periods, and slows AV nodal conduction. Dronedarone increases the Q-T interval but has a low propensity to cause torsades de pointes, since dronedarone reduces the transmural dispersion of ventricular refractoriness and protects from class III antiarrhythmic-induced early after depolarizations.3
Dronedarone exhibits significant antiarrhythmic properties in the ventricle and is effective against arrhythmias arising as a result of myocardial ischemia; in murine studies, it has also been shown to be effective against arrhythmias arising as a result of sudden reperfusion of the ischemic myocardium. In ischemic porcine hearts, dronedarone appears to be more potent than amiodarone in suppressing ventricular arrhythmias.3
Pharmacokinetics and Metabolism
Similar to amiodarone, over a two-fold increase is seen in the serum concentration of dronedarone when taken with food. With twice-daily dosing, the drug reaches steady-state levels in 4 to 7 days. The elimination half-life of dronedarone ranges from 13 to 30 hours.3 Dronedarone is a substrate for and an inhibitor of CYP3A4. Therefore, dronedarone should not be used concomitantly with potent CYP3A4 inhibitors such as ketokonazole or macrolide antibiotics. Dronedarone has similar amiodarone-like drug interactions with simvastatin and digoxin, but, importantly, no significant dronedarone interaction with warfarin occurs. Dronedarone increases the tubular secretion of creatinine by about 10%, with no effect on the glomerular filtration rate.
Clinical Efficacy
Several clinical trials have assessed the efficacy of dronedarone in suppressing AF in humans. The Dronedarone Atrial Fibrillation study after Electrical Cardioversion (DAFNE) was the first prospective randomized trial evaluating the efficacy and safety of dronedarone.4 In this dose-ranging study, placebo or dronedarone 400 mg, 600 mg, or 800 mg twice daily was randomly given to 199 patients with AF longer than 3 days but less than 365 days. Patients were then followed up for 6 months to measure the primary endpoint of time to AF recurrence. In comparison with placebo, dronedarone 400 mg twice daily significantly prolonged the time to recurrence of AF (median time 60 days in the dronedarone group versus 5.3 days in the placebo group, P < .001; relative risk reduction, 55%; confidence interval [CI], 28% to 72%). At doses higher than 400 mg twice daily, no efficacy in preventing the recurrence of AF was noted; an adverse effect—a dose-response curve of gastrointestinal side effects (diarrhea, nausea) requiring drug discontinuation—was, however, noted. The DAFNE demonstrated that dronedarone, at a dose of 400 mg twice daily, was safe and effective in preventing AF recurrences following conversion of persistent AF but was not very effective in the medical conversion of persistent AF.
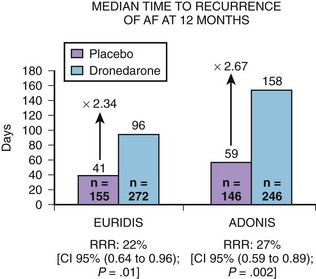
Two pivotal phase III trials assessed the efficacy of dronedarone in the maintenance of sinus rhythm in patients with AF or atrial flutter: (1) The Australian-American-African Trial with Dronedarone in Atrial Fibrillation or Flutter for the Maintenance of Sinus Rhythm (ADONIS) and (2) the European Trial in Atrial Fibrillation or Flutter in Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm (EURIDIS).5 These blinded, placebo-controlled trials randomized patients in a 2 : 1 ratio of either placebo or dronedarone 400 mg twice daily (Figure 81-3). Patients had a history of AF or atrial flutter in the previous 3 months and had to be in sinus rhythm for at least 1 hour before randomization. In the two trials, 1237 patients were randomized to either dronedarone or placebo (612 in the EURIDIS trial and 625 in the ADONIS trial). In the EURIDIS trial, dronedarone prolonged the median time to recurrence of AF or atrial flutter from 41 days in the placebo group to 96 days (relative risk [RR], 0.784; P = .0138). In ADONIS, the median time to recurrence increased from 59 days in the placebo group to 158 days in the dronedarone group (RR, 0.725; P < .0017). Dronedarone also significantly reduced symptomatic AF recurrences and significantly slowed the ventricular response rate by about 12 to 15 beats/min compared with placebo (P < .001) in those who had AF recurrence.
In the Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation (ERATO) trial, in addition to other AV node–blocking agents, dronedarone had therapeutic use in further slowing ventricular response during permanent AF or atrial flutter as assessed by Holter monitoring and stress testing.6
As part of the European regulatory filing, the active control Randomized, Double-Blind Trial to Evaluate the Efficacy and Safety of Dronedarone (400 mg bid) Versus Amiodarone (600 mg qd for 28 Days, then 200 mg qd Thereafter) for at Least 6 Months for the Maintenance of Sinus Rhythm in Patients with AF (DIONYSOS), which compared dronedarone with amiodarone, was performed in 504 patients with persistent AF.7 The primary endpoint of this trial was a composite of electrocardiographically documented AF recurrence or premature discontinuation of drug for lack of efficacy or adverse events. The trial had a short follow-up period (mean, 7 months) but was able to show that amiodarone was more effective in suppressing the recurrence of AF but tended to having more adverse events, including thyroid abnormalities and bleeding secondary to amiodarone-warfarin interaction problems.
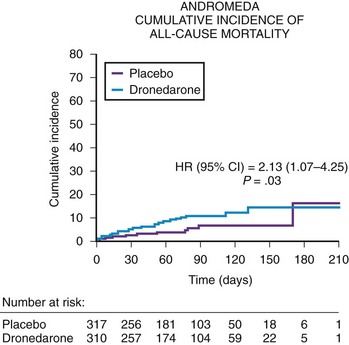
The safety of dronedarone in moderate to severe congestive heart failure (CHF) was assessed in the Antiarrhythmic Trial with Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease (ANDROMEDA) trial.8 The ANDROMEDA trial was a double-blind, placebo-controlled study evaluating dronedarone (400 mg twice daily) in high-risk patients with CHF and ventricular dysfunction (Figure 81-4). The primary endpoint of the ANDROMEDA trial was the composite endpoint of all-cause mortality or hospitalization for heart failure. The ANDROMEDA trial focused on patients at the highest possible risk of major cardiovascular events and enrolled recently hospitalized patients having current symptomatic New York Heart Association (NHYA) class II to IV heart failure with at least one decompensation of heart failure (class III to IV) in the last month and a wall motion index (WMI) by echocardiography of 1.2 or less, which correlates to a left ventricular ejection fraction of 35% or lower. Except for the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) trials with dofetilide, patients hospitalized for decompensated heart failure generally had not been enrolled in survival trials to evaluate the safety of antiarrhythmic drugs.9 After enrolling 627 of the 1000 planned patients, the trial was stopped by the Data and Safety Monitoring Board because of the excess risk of death (risk ratio, 2.13) in patients treated with dronedarone. In a review of the cause of deaths in this trial, the majority of deaths were secondary to nonsudden death or worsening of heart failure in the dronedarone arm. The primary composite endpoint of mortality and cardiovascular hospitalization trended adversely for dronedarone but was not statistically different (hazard ratio [HR], 1.38; CI, 0.92 to 2.09). The most likely explanation is that the ANDROMEDA trial finding is a true finding that can be explained by the deleterious effect of dronedarone in NYHA class III to IV patients with left ventricular ejection fractions of less than 35% who also had a recent hospitalization for decompensated heart failure. Similar concern has been raised by the termination of the recent Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS) trial.
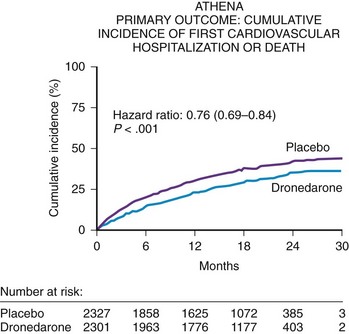
The A Placebo-Controlled, Double-Blind Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter (ATHENA) trial was conducted to focus on patients at risk of AF recurrence who may or may not have had heart failure but who would not have been randomized in the ANDROMEDA trial.10 Thus, key exclusion criteria for the ATHENA trial were pulmonary edema within 12 hours, cardiogenic shock requiring intravenous pressors, mechanical ventilation or class IV heart failure within 4 weeks, or all. The ATHENA trial was also performed for several other regulatory reasons: (1) to verify the post hoc result from the ADONIS and EURIDIS trials that dronedarone prospectively could reduce the composite endpoint of cardiovascular hospitalizations or death; (2) to create a large database to show that dronedarone would be safe in a large number of patients with structural heart disease; and, (3) to define a point estimate to show that this drug could be used safely in high-risk patients with AF or atrial flutter excluding the ANDROMEDA trial population.
The ATHENA trial demonstrated a statistical reduction in the composite endpoint of all-cause mortality or cardiovascular hospitalization in the dronedarone group (HR, 0.76; CI, 0.69 to 0.84; P < .001)] (Figure 81-5). Although dronedarone reduced cardiovascular hospitalization rates (HR, 0.75; CI, 0.67 to 0.82), no effect was seen on reducing hospitalizations for noncardiovascular reasons (HR, 0.98; CI, 0.87 to 1.11). The decrease in re-admission for cardiovascular hospitalization was mainly determined by the suppression of AF and other supraventricular disorders (HR, 0.62; CI, 0.53 to 0.71). All-cause mortality trended favorably in the dronedarone group with an HR of 0.84 (CI, 0.66 to 1.08); cardiovascular death relative risk was 0.70 (CI, 0.51 to 0.96). A retrospective analysis of the ATHENA trial reported that dronedarone reduced the incidence of stroke by 34% (HR, 0.66; CI, 0.46 to 0.96).11
Regulatory Affairs
In the United States, dronedarone’s approved indication is for the reduction of cardiovascular hospitalization in patients similar to those in the ATHENA trial. The warning in the package insert excludes patients with class IV heart failure and those recently hospitalized for heart failure within the past month from an acute decompensation. In Europe, dronedarone is approved for the suppression of AF recurrences and for rate control. The 2001 update of the ACC/AHA guidelines now includes dronedarone in the first tier of antiarrhythmic drug therapy for rhythm control (Figure 81-6).
Vernakalant (Kynapid, Brinavess)
Vernakalant is a new amino-cyclohexyl ether antiarrhythmic agent discovered by Cardiome Pharma. The drug has some selective atrial-channel–blocking properties that minimize QT prolongation and torsades de pointes.12,13 Cardiome and Astellas Pharma are co-developing intravenous vernakalant for the acute termination of AF in the United States. Merck and Cardiome are developing the intravenous compound outside North America and developing the oral compound worldwide.
Electrophysiological Effects of Vernakalant
Vernakalant (RSD-1235) is an atrial selective (IKACh, IKur) potassium channel blocker with little effect on ventricular repolarization and frequency-dependent and voltage-dependent INa–blocking activity (Table 81-1).12,13 The combined effects of blockade of these channels include prolongation of atrial refractory periods and rate-dependent slowing of atrial conduction. Vernakalant also blocks the atrial expressed transient outward potassium current (Ito) carried by the Kv4.3 channels, which may prolong atrial refractoriness but has minimal effects on hERG. Vernakalant has been shown to be a rate-dependent blocker (fast onset, fast offset) of late INa which should attenuate any QT-prolonging effect from IKr inhibition.
Table 81-1 New Antiarrhythmic Agents for Managing Atrial Fibrillation
| AGENT | MECHANISM OF ACTION | COMMENTS |
|---|---|---|
| Dronedarone | Vaughn Williams class I, II, III, and IV effects |
AF, Atrial fibrillation.
In in vivo studies, vernakalant showed an increase in the left atrial effective refractory period and the QRS complex; however, it did not change the Q-T interval of the right ventricular effective refractory period and terminated AF.13 In basic animal studies in which the clofilium or methhoxamine model was used, vernakalant has been shown to decrease dofetilide-induced action potential duration and early after-depolarizations (EADs) and to prevent torsades de pointes. In humans, vernakalant appears to prolong the atrial effective refractory periods, with no significant effect on ventricular repolarization.
Pharmacokinetics and Dosing
Intravenous vernakalant demonstrates linear kinetics and follows a two-compartment model with rapid first-order elimination from the central compartment.12,13 Mean plasma concentrations peak at the end of a 10-minute infusion. In the Atrial Arrhythmia Conversion Trial 1 (ACT 1) and ACT 3, vernakalant 3 mg/kg was intravenously infused over 10 minutes, followed by 2 mg/kg if AF persisted 15 minutes after the first infusion was completed.12,14,15 Vernakalant is primarily metabolized by the cytochrome P450 system (CYP2D6). In poor metabolizers, the half-life is approximately 2.7 hours in normal volunteers and 8.5 hours in patients with AF. Two demethylated metabolites have some bioactivity, but these are rapidly conjugated, inactivated, and eliminated by renal excretion.
Clinical Efficacy: Intravenous Vernakalant for the Conversion of Atrial Fibrillation
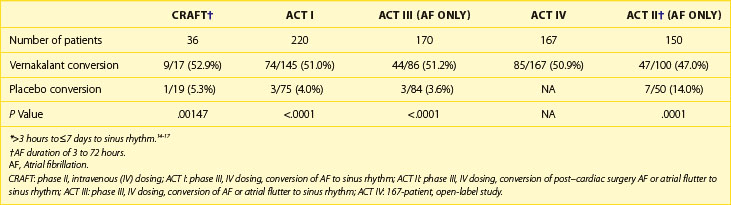
Intravenous vernakalant was developed to be an effective therapy for the acute termination of recent-onset AF. In the Controlled Randomized Atrial Fibrillation Trial (CRAFT), a phase IIa dosing study, patients were randomized to placebo versus infusions of 2 + 3 mg/kg intravenous vernakalant.16 This study demonstrated that vernakalant had a dose-related ability to terminate AF of 3 to 72 hours’ duration. The subgroup given 2 mg/kg followed by 3 mg/kg of intravenous vernakalant showed superior results compared with the placebo group in termination of AF (61% vs. 5%, P < .0005), patients in sinus rhythm at 30 minutes (56% vs. 5%, P < .001) and 1 hour (53% vs. 5%, P = .0014), and median time to conversion (14 vs. 162 minutes, P = .016).
The efficacy of intravenous vernakalant for converting AF was studied in several prospective, placebo-controlled trials (Table 83-2). The ACT 1 trial compared intravenous vernakalant to placebo in 416 patients with AF onset of 3 hours’ to 7 days’ duration.14 The primary endpoint was conversion to sinus rhythm within 90 minutes of injection, and 52% of the patients with recent-onset AF (duration of 3 hours to 7 days) converted to sinus rhythm compared with 4% of those in the placebo group (P < .001). However, in the subset of patients with AF duration of 3 hours to 45 days, only 38% of patients receiving intravenous vernakalant had their AF terminated compared with 3% of patients in the placebo group (P < .001). Of note, intravenous vernakalant was ineffective in converting atrial flutter.
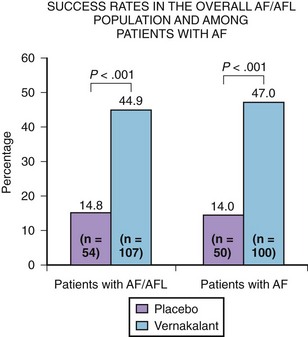
The ACT 2 trial evaluated the efficacy and safety and intravenous vernakalant in 190 patients who developed AF or atrial flutter between 24 hours and 7 days following coronary artery bypass graft or valve replacement surgery (Figure 81-7).17 In the AF group, 47% of patients dosed with intravenous vernakalant converted to sinus rhythm within 90 minutes compared with only 14% of the placebo group (P = .0001) (see Table 81-2). The median time to conversion was 12.3 minutes for the vernakalant responders. Similar to the other two ACT trials, no torsades de pointes was reported, and the drug was ineffective in the medical conversion of atrial flutter in 0 of 10 patients.
The ACT 4 trial, a phase III open-label study, evaluated the safety of intravenous vernakalant in the conversion of 236 patients with AF (<3 hours but ≤45 days).12 Of these patients, 167 had AF of less than 3 hours but 7 days or longer. This subgroup had a 50.9% conversion rate, similar to the other ACT trials. In patients who converted within 90 minutes, the median time to conversion was 14 minutes.
Clinical Efficacy of Oral Vernakalant
A phase IIa trial demonstrated that oral vernakalant at 300 mg and 600 mg twice daily was superior to placebo in maintaining sinus rhythm after cardioversion of persistent AF over a 28-day treatment.12 In the 300-mg twice-daily group, 61% (33 of 54) of patients maintained sinus rhythm 28 days after cardioversion compared with 43% (24 of 56) of patients in the placebo group (P = .048) and 61% (30 of 49) in the 600-mg twice-daily group. A phase IIb, placebo-controlled trial of more than 670 patients measured the safety and efficacy of 150-mg, 300-mg, and 500-mg twice-daily oral vernakalant over a 90-day period after conversion of persistent AF. In this study, the 500-mg dose was most effective in suppressing recurrences of AF. Further phase II trials with the oral compound are planned.
Other Amiodarone Analog Compounds (Celivarone, Budiodarone)
Celivarone (SSR149744C), developed by Sanofi-Aventis, has electrophysiological effects similar to those of dronedarone, with a longer half-life of 28 to 35 hours, so the drug can be given once a day.2 No iodine moieties are present in the celivarone molecule, and thus thyroid adverse effects should not be an issue. An early phase II human trial with oral celivarone reported no dose effect (50 mg, 100 mg, 200 mg, 300 mg once a day) in preventing the recurrence of persistent AF following cardioversion. The 50-mg once-daily dose had a recurrence rate of 52.1% at 3 months compared with 67.1% for the placebo group (P = .055). Celivarone holds some promise for suppressing implantable cardioverter-defibrillator discharges in patients.2
Budiodarone (ATI-2042), developed by ARYX, is an amiodarone-like compound (addition of a sec-butyl acetate side chain at position 2 of the benzofuran moiety) with a short half-life of 7 hours (Figure 81-8).2 Budiodarone has multi-channel–blocking electrophysiological effects with increased effects on atrial refractory periods compared with amiodarone. Preliminary patient trials have shown that budiodarone twice daily is effective in suppressing the AF burden in a dose-dependent fashion, as assessed by pacemaker monitoring logs. Larger scale AF trials are needed to determine budiodarone’s safety and efficacy.
Ranolazine (Ranexa)
Ranolazine is a piperazine derivative that is approved for the treatment of angina at a dose of 500 mg to 100 mg twice daily.17 Ranolazine reduces calcium overload in the ischemic myocyte via late sodium channel blockade. The drug is extensively metabolized in the gut and liver (CYP3A4), and its elimination half-life is 7 hours. Steady state is reached after 3 days of twice-daily dosing. CYP3A4 inhibitors such as diltiazem and verapamil will increase ranolazine levels. Because ranolazine is a substrate of P-glycoprotein, inhibitors of P-glycoprotein such as verapamil may increase the absorption of ranolazine and increase plasma levels. In addition, ranolazine increases digoxin levels up to twofold probably through competition in the P-glycoprotein pathway in the intestine and kidneys. The most common adverse effects with ranolazine include dizziness, nausea, dyspepsia and headache.
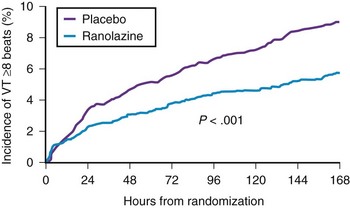
In relation to its potential in suppressing AF, ranolazine inhibits IKr and late INa. Although ranolazine increases the Q-T interval in a dose-related manner, drug-induced torsades de pointes is uncommon via a protective effect similar to that of dronedarone and amiodarone, with no increase in the dispersion of repolarization. Basic studies have demonstrated that the inhibition of late INa mitigates the inhibition of IKr. In the Metabolic Efficiency with Ranolazine for Less Ischemia in Non−ST-Elevation Acute Coronary Syndromes (MERLIN) trial, the drug was shown to be safe for use in patients with acute coronary syndromes, and some antiarrhythmic effect was seen in the atria and ventricles (Figure 81-9).19 Basic electrophysiological data suggest that ranolazine may be useful in suppressing AF and have shown that ranolazine can abolish ectopic activity from pulmonary vein sleeves as a result of re-entry, delayed after-depolarization, and late phase 3 EAD triggered activity.20 The clinical efficacy of ranolazine in suppressing AF is documented in small patient trials and in the MERLIN study.21 Further clinical trials will be required to establish ranolazine’s effectiveness in suppressing AF.
Antzelevitch C. Ranolazine: A new antiarrhythmic agent for patients with non-ST segment elevation acute coronary syndromes. Nat Clin Pract Cardiovasc Med. 2008;5(5):248-249.
Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiac conditions. Circulation. 2006;113:2462-2472.
Connolly SJ, Crijns HJGM, Torp-Pedersen C, et al. for the ATHENA Investigators: Analysis of stroke in ATHENA: A placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation. 2009;120:1174-1180.
Fedida D. Vernakalant (RSD1235): A novel, atrial selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16(4):519-532.
Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668-678.
Køber L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678-2687.
Kowey PR, Dorian P, Mitchell LB, et al. Vernakalant hydrochloride for the rapid conversion of atrial fibrillation following cardiac surgery. A randomized, double-blind, placebo-controlled trial. Circulation Arrhythmia Electrophysiol. 2009;2:652-659.
Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: The DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21:597-605.
Murdock DK, Oveton N, Kersten M, Kaliebe J, DeVecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pace Electrophysiol J. 2008;8(3):175-181.
Naccarelli GV, Wolbrette DL, Samii S, et al. New antiarrhythmic treatment of atrial fibrillation. Expert Rev Cardiovasc Ther. 2007;5(4):707-714.
Naccarelli GV, Wolbrette DL, Samii S, et al. Vernakalant—a promising therapy for conversion of recent-onset atrial fibrillation. Expert Opin Investig Drugs. 2008;17(5):805-810.
Patel C, Kowey PR. Dronedarone. Circulation. 2009;120:636-644.
Roy D, Pratt C, Torp-Pedersen C, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation. A phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1516-1525.
Scirica B, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiologic properties, on the incidence of arrhythmias in patients with non-ST segment elevation acute coronary syndrome: Results from the metabolic efficiency with ranolazine for less ischemia in non-ST segment elevation acute coronary syndrome thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647-1652.
Singh BN, Connolly SJ, Crijns HJGM, et al. for the EURIDIS and ADONIS Investigators: Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987-999.
1 Naccarelli GV, Wolbrette DL, Khan M, et al. Old and new antiarrhythmic drugs for converting and maintaining sinus rhythm in atrial fibrillation: Comparative efficacy and results of trials. Am J Cardiol. 2003;91:15D-26D.
2 Naccarelli GV, Wolbrette DL, Samii S, et al. New antiarrhythmic treatment of atrial fibrillation. Expert Rev Cardiovasc Ther. 2007;5(4):707-714.
3 Patel C, Kowey PR. Dronedarone. Circulation. 2009;120:636-644.
4 Touboul P, Brugada J, Capucci A, et al. Dronedarone for prevention of atrial fibrillation: A dose-ranging study. Eur Heart J. 2003;24:1481-1487.
5 Singh BN, Connolly SJ, Crijns HJGM, et al. for the EURIDIS and ADONIS Investigators: Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987-999.
6 Davy JM, Herold M, Hoglund C, et al. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: The Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J. 2008;156:527.e1-527.e9.
7 Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: The DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21:597-605.
8 Køber L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678-2687.
9 Torp-Pedersen C, Moller M, Bloch-Thomsom PE, et al. for the Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group: Dofetilide in patients with congestive heart failure and left ventricular dysfunction. N Engl J Med. 1999;341:857-865.
10 Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668-678.
11 Connolly SJ, Crijns HJGM, Torp-Pedersen C, et al. for the ATHENA Investigators: Analysis of stroke in ATHENA: A placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation. 2009;120:1174-1180.
12 Naccarelli GV, Wolbrette DL, Samii S, et al. Vernakalant—a promising therapy for conversion of recent-onset atrial fibrillation. Expert Opin Investig Drugs. 2008;17(5):805-810.
13 Fedida D. Vernakalant (RSD1235): A novel, atrial selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16(4):519-532.
14 Roy D, Pratt C, Torp-Pedersen C, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation. A phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1516-1525.
15 Pratt C, Roy D, Juul-Moller S, et al. Efficacy and tolerance of RSD1235 in the treatment of atrial fibrillation or atrial flutter: Results of a phase III, randomized, placebo-controlled, multicenter trial. J Am Coll Cardiol. 2006;47:10A. Abst.
16 Roy D, Rowe BH, Stiell IG, et al. A randomized, controlled trial of RSD 1235, a novel anti-arrhythmic agent, in the treatment of recent onset atrial fibrillation, for the CRAFT Investigators. J Am Coll Cardiol. 2004;44:2355-2361.
17 Kowey PR, Dorian P, Mitchell LB, et al. Vernakalant hydrochloride for the rapid conversion of atrial fibrillation following cardiac surgery. A randomized, double-blind, placebo-controlled trial. Circulation Arrhythmia Electrophysiol. 2009;2:652-659.
18 Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiac conditions. Circulation. 2006;113:2462-2472.
19 Scirica B, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiologic properties, on the incidence of arrhythmias in patients with non-ST segment elevation acute coronary syndrome: Results from the metabolic efficiency with ranolazine for less ischemia in non-ST segment elevation acute coronary syndrome thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647-1652.
20 Antzelevitch C. Ranolazine: A new antiarrhythmic agent for patients with non-ST segment elevation acute coronary syndromes. Nat Clin Pract Cardiovasc Med. 2008;5(5):248-249.
21 Murdock DK, Oveton N, Kersten M, Kaliebe J, DeVecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pace Electrophysiol J. 2008;8(3):175-181.