Chapter 19 Circulatory Assist Devices
4 How is IABP inflation-deflation timing coordinated?
Three possible triggers are routinely used to coordinate IABP inflation-deflation:
5 What are the indications for IABP placement?
Clinical conditions that may warrant initiation of IABP therapy can be broken down into two categories: those conditions that would benefit primarily from increased diastolic coronary perfusion and those that would benefit mainly from afterload reduction. Balloon pumps may also be placed prophylactically in high-risk patients undergoing cardiac surgery (Box 19-1). The most common condition for which IABP therapy is started is cardiogenic shock, accounting for approximately 20% of placements.
6 What are the contraindications for IABP placement?
The contraindications for IABP placement include the following:
7 What are possible complications of IABP placement?
 Vascular complications include hematoma, perforation, dissection, pseudoaneurysm, and aneurysm. These complications are more common in patients with diabetes or peripheral vascular disease, smaller patients, and females.
Vascular complications include hematoma, perforation, dissection, pseudoaneurysm, and aneurysm. These complications are more common in patients with diabetes or peripheral vascular disease, smaller patients, and females.
 Complications resulting from poor positioning of the balloon occur because of the unintended obstruction of arterial branches of the aorta. This results in compromised perfusion and/or ischemia to the upper extremities and brain (if the balloon is too proximal) or to the mesentery (if it is too distal). Poor positioning may also affect the ability of the IABP to maximally augment.
Complications resulting from poor positioning of the balloon occur because of the unintended obstruction of arterial branches of the aorta. This results in compromised perfusion and/or ischemia to the upper extremities and brain (if the balloon is too proximal) or to the mesentery (if it is too distal). Poor positioning may also affect the ability of the IABP to maximally augment.
 Balloon-related complications include balloon rupture, gas embolization, and traumatic thrombocytopenia. Of note, the reduction in platelet count caused by the balloon pump is predictable and generally stabilizes after 4 days. Platelet counts return to baseline quickly after removal of IABP.
Balloon-related complications include balloon rupture, gas embolization, and traumatic thrombocytopenia. Of note, the reduction in platelet count caused by the balloon pump is predictable and generally stabilizes after 4 days. Platelet counts return to baseline quickly after removal of IABP.
10 What are the clinical criteria for IABP removal?
11 What does an arterial pressure tracing look like in a patient being assisted by an IABP?
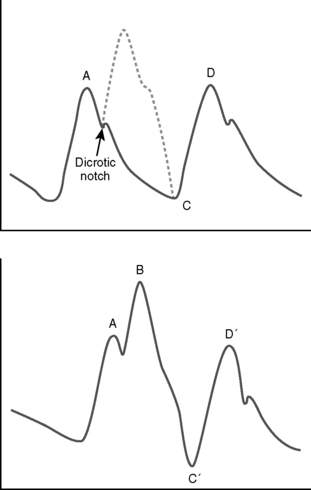
The function of an IABP during diastole results in an upswing in the arterial pressure waveform during diastole (Fig. 19-1). The peak created by the IABP is known as the peak augmented diastolic pressure. The upstroke created by the IABP, if timing is correct, correlates with the dicrotic notch. When maximal augmentation by the IABP is achieved, the peak augmented diastolic pressure will be greater than the unassisted systolic pressure. In addition, both the assisted aortic end-diastolic pressure and the assisted systolic pressure should be less than their unassisted counterparts, given appropriate IABP function.
12 What are some common problems that may result in failure of the IABP to augment?
Failure to augment may be associated with inadequate inflated balloon size, as a result of either a balloon that is too small or an appropriately sized balloon that is being underfilled. A balloon that is misplaced too proximal or too distal in the aorta might not augment appropriately, as will a balloon inadvertently placed in a false passage (possibly created with insertion of the device). Inappropriate timing of inflation or deflation of the balloon, which may be either too early or too late, can impair augmentation (Table 19-1). Improper timing can often be corrected manually on the IABP console. Both tachycardia and bradycardia can result in failure to augment, as can certain arrhythmias such as atrial fibrillation. These rhythm abnormalities should be treated to allow for maximal effectiveness of the IABP.
| Timing issue | Resulting problems |
|---|---|
| Early IABP inflation | Early closure of aortic valve |
| Reduction in cardiac output | |
| Increased oxygen consumption | |
| Early IABP deflation | Reduction in duration of diastolic augmentation |
| Late IABP inflation | Reduction in duration of diastolic augmentation |
| Late IABP deflation | Increased left ventricular afterload |
| Increased oxygen consumption |
13 What is a ventricular assist device (VAD)?
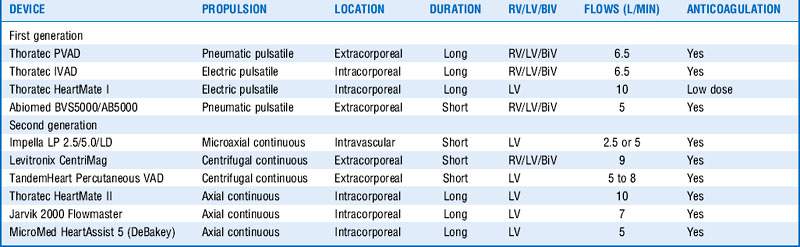
In general, VADs are mechanical devices inserted to assist cardiac function by offloading part or all of the pumping responsibilities from the ventricle. Placement of a VAD can be done on the left side of the heart to assist in left ventricular function (LVAD) or on the right to help with the right ventricle (RVAD). The presence of both an LVAD and an RVAD is referred to as biventricular support (BiVAD). A number of different VAD constructs exist, with major differentiators being pulsatile versus nonpulsatile (continuous), external versus internal, degree of assistance provided, ability to help the left or right sides, and the length of time it can be used (Table 19-2). Some VADs are intended to be used for only 7 to 10 days, whereas others have supported patients over a 7-year period.
18 What are the indications for VAD placement?
22 List the major considerations for intensive care unit management of a patient directly after an LVAD placement
25 What is the REMATCH study?
Key Points Circulatory Assist Devices
1. IABPs are effective circulatory assist devices because they both increase diastolic blood pressure and decrease systolic afterload.
2. Improper timing and/or positioning of an IABP can result in ineffective blood pressure augmentation, as well as ischemia to the end organs of blocked arteries.
3. VADs are placed for three reasons:
4. A VAD can be used to augment the left ventricle (LVAD), the right ventricle (RVAD), or both ventricles (BiVAD) but does not help with blood oxygenation or ventilation.
5. With the incidence in the United States of new patients with cardiac failure each year being around 40,000 and the number of available donor hearts at only 2300, the role of VADs continues to increase, as does the pressure for technologic and industrial advances that will increase device effectiveness and improve the safety profile.
1 Campbell L.J. Circulatory assist devices. Parsons P.E., Wiener-Kronish J.P. Critical Care Secrets, 4th ed, Philadelphia: Mosby, 2007.
2 Cohn L. Perioperative/intraoperative care. In Cardiac Surgery in the Adult, 3rd ed, New York: McGraw-Hill; 2008:507–533.
3 . Counterpulsation Applied: An Introduction to Intra-Aortic Balloon Pumping. N.J. Mount Holly. Arrow International, Inc. 2005:1–158.
4 Donelli A., Jansen J.R., Hoeksel B., et al. Performance of a real-time dicrotic notch detection and prediction algorithm in arrhythmic human aortic pressure signals. J Clin Monit Comput. 2002;17:181–185.
5 Fitzsimons M.G., Ennis S., MacGillivray T. Devices for cardiac support. In: Sandberg W.S., Urman R., Ehrenfeld J. The MGH Textbook of Anesthetic Equipment. 1st ed. Philadelphia: Saunders; 2010:247–262.
6 Laish-Farkash A., Hod H., Matetzky S., et al. Safety of intra-aortic balloon pump using glycoprotein IIb/IIIa antagonists. Clin Cardiol. 2009;32:99–103.
7 Mitter N., Sheinberg R. Update on ventricular assist devices. Curr Opin Anaesthesiol. 2010;23:57–66.
8 Nicolosi A.C., Pagel P.S. Perioperative considerations in the patient with a left ventricular assist device. Anesthesiology. 2003;98:565–570.
9 Rose E.A., Gelijns A.C., Moskowitz A.J., et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443.
10 Roy S.K., Howard E.W., Panza J.A., et al. Clinical implications of thrombocytopenia among patients undergoing intra-aortic balloon pump counterpulsation in the coronary care unit. Clin Cardiol. 2010;33:30–35.
11 Slaughter M.S., Rogers J.G., Milano C.A., et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251.
12 Song X., Throckmorton A.L., Untaroiu A., et al. Axial flow blood pumps. ASAIO J. 2003;49:355–364.
13 Thunberg C.A., Gaitan B.D., Arabia F.A., et al. Ventricular assist devices today and tomorrow. J Cardiothorac Vasc Anesth. 2010;24:656–680.
14 Trost J.C., Hillis L.D. Intra-aortic balloon counterpulsation. Am J Cardiol. 2006;97:1391–1398.
15 Wilson S.R., Mudge G.H.Jr. Stewart GC, et al. Evaluation for ventricular assist device: selecting the appropriate candidate. Circulation. 2009;119:2225–2232.