CHAPTER 346 Circulatory Arrest with Deep Hypothermia
Most aneurysms are amenable to endovascular or traditional microsurgical treatment with general neurovascular anesthesia. However, there is a small subset of aneurysms that present the surgeon with an unusually complex technical challenge, and pose a higher risk of surgical morbidity and mortality when traditional techniques are employed.1 Large size, calcified, or atherosclerotic walls, intimacy with critical perforator branches, and the incorporation of afferent or efferent arteries in the dome, are characteristics that often preclude safe clipping while the aneurysm remains tense with high-pressure flow. Circumferential dissection or freeing of adhesions may be impossible with a full, tense dome. Further, calcified or thrombosed aneurysms may be impossible to occlude with a clip without first removing thrombus or calcified components from inside of the aneurysm.
Deep hypothermic circulatory arrest for aneurysm clipping was first employed more than 50 years ago. Initially, the technique carried an unacceptably high incidence of systemic complications, limiting its application to neurosurgery.2 Over time, however, the refinement of bypass techniques via evolving cardiac procedures has allowed for safer employment of circulatory arrest for aneurysm surgery. Coupled with a better understanding of cerebral physiology under hypothermic conditions, these techniques have enabled neurosurgeons to approach otherwise untreatable complex cerebral aneurysms with acceptable morbidity and mortality. Deep hypothermic circulatory arrest is still a relatively high-risk procedure, however, and must performed for appropriate indications at centers able to provide sophisticated neurosurgical and cardiothoracic care. The morbidity and mortality must be weighed against the surgical risks of treating complicated intracerebral aneurysms with traditional techniques.
Temporary Clipping: Limitations of Focal Circulatory Arrest
The utility and complications of temporary clipping for focal circulatory arrest have been explored extensively since its inception in 1961.3–13 Though all cerebral arteries can withstand temporary occlusion for a brief duration, vascular territories vary in the length of occlusion time they may tolerate before ischemia and/or permanent infarction ensue.6 In some cases, the proximal internal carotid artery can be occluded for an hour or more, whereas the perforator-bearing segments of the proximal middle cerebral artery or the basilar apex may tolerate occlusion for only 5 to 15 minutes.8,12 In one recent series, increasing duration of temporary occlusion was shown to correlate significantly with the development of radiographic or clinical infarction.12 Permanent vessel damage and consequent infarction can result from injury to individual perforators captured in the temporary clip because these vessels are not strong enough to withstand the closing force of the clip itself. Additionally, temporarily trapped arterial segments may be subject to intravascular thromboembolism. Finally, a steal phenomenon may occur if a hole is created in the aneurysm while the vessel is temporarily proximally occluded, further exacerbating ischemic intolerance.
Cerebral Ischemia: Pathophysiology and Protection
Cerebral arterial occlusion, either by design or a pathologic event, is known to produce two distinct zones of tissue immediately after occlusion: (1) a central core of densely ischemic tissue, and (2) a penumbra that receives collateral circulation and thus possesses prolonged viability compared to the core. The ischemic core undergoes anaerobic glycolysis, acidosis, adenosine triphosphate (ATP) depletion, and ultimately dysregulated ion homeostasis. The potassium-sodium pump loses function in the absence of ATP, intracellular sodium accumulates, and eventually chloride and toxic calcium accumulate intracellularly causing acidosis, edema, and cell death. If the penumbral zone continues without perfusion, it too is progressively recruited into the ischemic core. Over time the penumbra is exposed to increasing concentrations of toxic neurotransmitters, such as glutamate and nitric oxide released from the actively infarcting ischemic core, which lead to cellular depolarization and spreading neuronal death.14,15
Broadly, the concept of protection from ischemic injury has evolved to include traditional, well-described measures employed during the ischemic insult, and more recently in experimental models, measures implemented before the ischemic insult that provide endogenously mediated protection, known as “ischemic preconditioning.” Preconditioning is a method by which a noxious stimulus near to but below the threshold of damage is applied to cerebral tissue, and shortly thereafter the tissue develops tolerance or resistance. So when the same noxious stimulus is applied at or above the threshold of damage, the tissue is resistant to or protected from injury. Preconditioning has been studied in ischemic stroke, carotid endarterectomy, and myocardial infarction, but has yet to find evidence-supported therapeutic application in these diseases or the ischemia induced in aneurysm surgery.16
Barbiturates are the best studied agent for decreasing brain electrical activity and have been shown to reduce CMRO2 by as much as 50%.17 However, other CMRO2-reducing agents have been used because barbiturates are associated with adverse effects pertinent to hypothermic cardiac arrest. Propofol and isoflurane, for example, are shorter acting and produce less myocardial depression than barbiturates.18 Whichever combination of agents the anesthesiologist uses, one must be mindful that CMRO2 is reduced by only about 50% once electrical activity is eliminated, therefore adding other agents does not effectively lower CMRO2 any further.
Several groups have examined drugs that act by interfering with the toxic events that follow ischemia and deranged ion flux, including free radical scavengers, calcium channel blockers, and glutamate receptor blockers.19–21 Others have examined the beneficial effects of adhesion molecule blockers (anti-ICAM-1 antibody), nonspecific anti-inflammatories (steroids), and rheologic enhancers (mannitol) in preventing progressive microvascular failure and in stabilizing plasma membranes.8 Though these agents tend to be useful for protecting the penumbral zone, they provide little benefit for the already damaged ischemic core.
Hypothermia is one of the few interventions that has proven capable of sufficiently reducing CMRO2 and protecting ionic gradients and structural homeostasis in the setting of prolonged ischemia. Even mild hypothermia down to 33°C has been shown to have demonstrable protective effects, but deep hypothermia to 18°C allows the brain to be totally deprived of blood flow for up to 1 hour without noticeable damage.17
Hypothermia: Cerebroprotective Effects
Hypothermia as a cerebroprotective agent has been used extensively in neurotrauma, cardiac arrest, neurovascular surgery, and cardiac surgery. Deep hypothermia with circulatory arrest has been routinely used in the correction of pediatric cardiac malformations and aortic arch reconstruction.22,23 Ischemic protection provided by hypothermia is attributed primarily to a decrease in metabolic demand (CMRO2), and more recently hypothermia has been shown to reduce proteolysis and excitotoxic damage caused by glutamate toxicity and neuronal calcium influx.17,24,25 Hypothermia has also been suggested to reduce free radical production and eliminate spreading depression in ischemic tissue.26,27 Hypothermia tends to affect the at-risk penumbral tissue but has a minimal effect on the core of infarcted tissue.
Hypothermic tolerance is directly related to the degree of temperature reduction and occurs in the presence of proportional reductions in cerebral blood flow (CBF).28 In cell culture, oxygen consumption has been shown to decrease 50% for every 10°C drop in temperature. However, in vivo reductions in metabolism are even greater (approximately 7% for each 1°C drop in temperature), likely related to hypothermia’s combined effect on basal metabolic rate, electrical activity, and excitatory neurotransmitter and inflammatory cytokine release.29–35 Temporal limitations still exist, however, as humans cannot tolerate deep hypothermia for much longer than 90 minutes. Extended hypothermia results in IQ reduction and neuropsychological deficits as membrane stability becomes threatened.35,36 Therefore, surgeons recommend temporary reperfusion for periods of arrest longer than this.
Hypothermia: Physiologic Considerations
The entire multidisciplinary team, including the neurosurgeon, must understand the widespread systemic effects of profound hypothermia to optimally manage the patient’s intraoperative and postoperative course. Of concern to the anesthesia team is hypothermia’s ability to increase both oxygen and CO2 solubility, which has created controversy among anesthesiologists with regards to temperature-correction of blood gases by adding CO2. Recent evidence suggests it may unnecessarily increase cerebral blood volume, swelling, and intracranial pressure that may be successfully avoided by titrating ventilation to uncorrected gases.37,38 Another major physiologic effect of hypothermia is its role in increasing blood viscosity. Plasma viscosity increases approximately 3% for every 1°C reduction in temperature. The anesthesiologist must therefore counteract the changing viscosity by aggressive isovolemic hemodilution to preserve the integrity of the microcirculatory bed, with the final hematocrit titrated approximately to temperature (i.e., 18°C = HCT of 18).39 Hypothermia may also induce a metabolic acidosis as tissues become relatively underperfused. Metabolic acidosis can then lead to dilation of cerebral vasculature and increased cerebral blood flow, which may be important in the setting of cerebral edema.40,41
Careful glucose monitoring and avoidance of glucose-containing solutions should be maintained during profound hypothermia because hypoinsulinemia and resultant cerebrotoxic hyperglycemia can occur. Endogenous corticosteroid production may be diminished after prolonged hypothermia, necessitating perioperative replacement and continued hypothalamic-adrenal axis surveillance for up to a year. Profound hypothermia can occasionally cause a complement-mediated pneumonitis, which may necessitate prolonged postoperative intubation. Though frank hepatic dysfunction is extraordinarily rare, hypothermia can lead to mild hepatic dysfunction with decreased dilantin metabolism and resultant supratherapeutic levels. Rarely, postoperative renal failure occurs because of transient decreases in glomerular filtration rate, hemolysis, and blood product reactions.41–44 Hemoglobinuria may be managed with osmotic alkalinization of the urine.45,46 Hypothermia may also have profound effects on myocardial contractility and conduction, which persist into the postoperative period. Widening of the PR interval, QRS complex changes, systolic and diastolic dysfunction, sinus bradycardia, and sensitization to pharmacologic challenge have all been reported.47–49
Of greater concern than these rare complications is the more common coagulopathy caused by circulatory arrest techniques and deep hypothermia. The surgeon encounters difficulty with hemostasis because of both platelet dysfunction and slowing of the enzymatic coagulation cascade.50 Fortunately, platelet dysfunction is mostly due to sequestration that resolves within an hour of rewarming, and major clotting factors are mostly unaffected. Heparinization and extracorporeal circuitry account for the remainder of the coagulopathy, and while hypothermia may prolong the anticoagulant effects of the former, its half-life generally is less than 2 hours. As for the latter, removal of the circuits results in an immediate reduction in turbulence and shear-induced red blood cell and platelet destruction. Progressive hemoconcentration during warming also helps to correct the coagulopathy, and warming causes a decrease in plasmin-dependent fibrinolysis.51–53
Although hypothermia is intended to be neuroprotective, neurologic dysfunction can still occur after prolonged, profound hypothermia.54–57 Global hypoperfusion and macroembolization and microembolization may all contribute to the development of neurological injury. Age and preexisting cerebrovascular disease are major risk factors for neurological complications.58 Some recent improvement in neurological complication rates can be found with the use of barbiturate alternatives, which more effectively reduce cerebral metabolism and have fewer cardiovascular effects.38,52,59–61
Deep Hypothermic Circulatory Arrest: Indications
Aneurysm Characteristics
Anatomic Location: Parent Artery
Aneurysms of the posterior circulation have predominated in previously published series of aneurysms treated with hypothermic circulatory arrest.62–64 Some argue that advances in surgical approaches, particularly the orbitozygomatic craniotomy and drilling of the anterior clinoid, provide improved access to the internal carotid artery and give the aneurysm surgeon total proximal control,62 negating the need for circulatory arrest. In fact, before our recently published series of 66 patients outlined below, in which 50% of aneurysms treated with circulatory arrest resided in the anterior circulation, the largest series previously published reported only 4 of 58 aneurysms treated were in the anterior circulation.62 We maintain, however, that particularly risky aneurysms of the anterior circulation may be best treated with hypothermic circulatory arrest.
Clinical Techniques
Neuroanesthetic Management
Once these special monitoring devices have been arranged, the case may begin, and the neurosurgeon may concentrate on the superficial dissection as per routine. Nevertheless it is critical that the neurosurgeon understands the routine in order to help solve problems. All patients are given perioperative antibiotics, steroids, and anticonvulsants. Steroids purportedly stabilize membranes, prevent vasogenic edema, and minimize complement generation.65 Antibiotics are especially important as hypothermia increases the risk of infection (both independently and in association with prolonged operative exposure). Anticonvulsants are employed only in the immediate perioperative period, and while their use has little scientific merit, Dilantin (phenytoin) especially may be mildly cerebroprotective. Anesthesia is induced with intravenous midazolam, fentanyl, and thiopental. Vecuronium, lidocaine, and esmolol are used immediately before intubation and maintenance anesthesia consists of a balanced technique of narcotics, oxygen, and isoflurane. Ventilation is controlled to maintain a normal PaCO2, and the concentration of isoflurane is minimized (less than 0.75%) to facilitate electrophysiologic monitoring. In general, aneurysm patients should have their fluids conservatively managed; however, hypothermic circulatory arrest often makes this difficult. The vasodilatory effects of warming, together with third space losses because of membrane dysfunction, often renders the patient relatively hypovolemic. Euvolemic correction is guided by both central pressures and urine output, and maintained primarily with glucose-free isotonic crystalloids. However, colloid, both as packed cells and albumin, is occasionally necessary.
Low-flow cardiopulmonary bypass has been described as an alternative to complete circulatory arrest, providing a means to maintain continuous cerebral oxygenation while still reducing intravascular perfusion pressure and cerebral blood flow. Studies comparing low-flow bypass with complete circulatory arrest have suggested that low-flow bypass actually results in a lower likelihood of clinical and electroencephalogram (EEG) seizures, a shorter time to recovery of normal EEG activity, and a lesser release of BB isoenzyme in the immediate postoperative period.66 For selected cases in which complete cessation of flow is not required for the entirety of the aneurysm dissection, the surgeon may choose to employ low-flow cardiopulmonary bypass for a period of time.
Complication Avoidance and Management
Clinical Results: 15-Year Experience
Treatment Complications
CASE 1
Giant Carotid Bifurcation Aneurysm
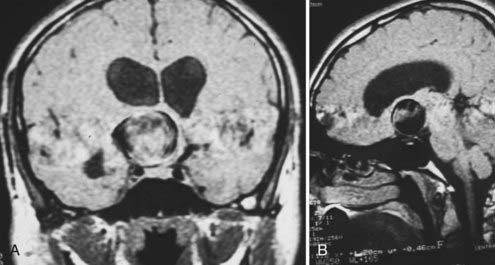
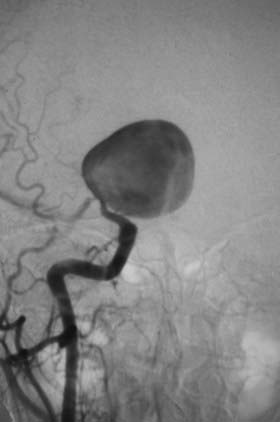
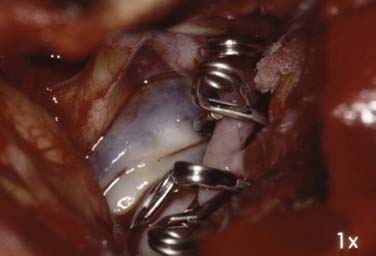
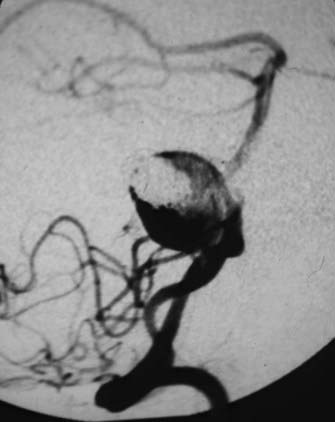
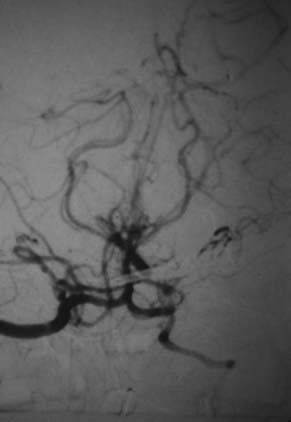
This is the case of a 34-year-old truck driver who had left focal motor seizures. MRI revealed a giant right carotid bifurcation aneurysm abutting the right basal ganglia, hypothalamus, and foramen of Monro with mild hydrocephalus (Fig. 346-1, A and B). After confirming a 3.5 cm giant aneurysm with DSA (Fig. 346-2), the decision was made to perform surgical clipping under deep hypothermic circulatory arrest because of the aneurysm size and involvement with eloquent structures. At surgery, the aneurysm was collapsed after complete circulatory arrest, and multiple clips were successfully placed (Fig. 346-3) with no residual filling on postoperative angiogram. Postoperatively he had transient, mild left-sided weakness that resolved within days, and thereafter remained deficit free.
CASE 2
Giant Midbasilar Aneurysm
This is the case of a 22-year-old woman who presented complaining of intermittent headaches, vomiting, and double vision. MRI and angiogram revealed a large, thrombosed 2.7 cm midbasilar aneurysm without evidence of hemorrhage (Fig. 346-4, A). The aneurysm was coiled at an outside institution with good result (Fig. 346-4, B). However, an 8-month follow-up angiogram demonstrated migration of the coils with refilling of the entire aneurysm dome and resultant brainstem compression (Fig. 346-5). Because of the aneurysm size and involvement with the brainstem, the decision was made to perform surgical clipping under complete circulatory arrest. At surgery, exposure was achieved via left temporal craniotomy and suboccipital craniectomy with a presigmoid approach. After inducing hypothermia and complete circulatory arrest, the aneurysm dome was collapsed, partially dissected away from the brainstem, and clipped using fenestrated and straight clips (Fig. 346-6). Postoperatively her symptoms resolved and she remained deficit free.
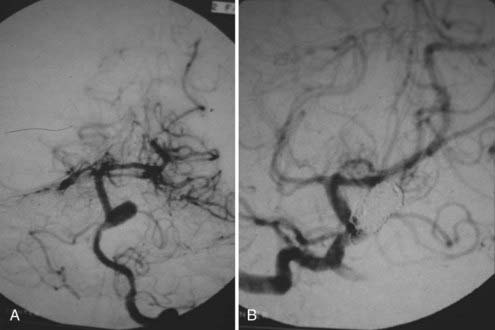
FIGURE 346-4 A, Pretreatment angiogram showing the partially thrombosed midbasilar aneurysm.
B, Postcoiling angiogram revealing near complete cessation of filling in the aneurysm dome.
1 Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-95.
2 Patterson RHJr, Ray BS. Profound hypothermia for intracranial surgery: laboratory and clinical experiences with extracorporeal circulation by peripheral cannulation. Ann Surg. 1962;156:377-393.
3 Boecher-Schwarz HG, et al. Pre- and intraoperative methods of controlling cerebral circulation in giant aneurysm surgery. Neurosurg Rev. 1995;18(2):85-93.
4 Fujita S, Kawaguchi T. Monitoring of direct cortical responses during temporary arterial occlusion at aneurysm surgery. Acta Neurochir (Wien). 1989;101(1-2):23-28.
5 Momma F, Wang AD, Symon L. Effects of temporary arterial occlusion on somatosensory evoked responses in aneurysm surgery. Surg Neurol. 1987;27(4):343-352.
6 Mizoi K, Yoshimoto T. Permissible temporary occlusion time in aneurysm surgery as evaluated by evoked potential monitoring. Neurosurgery. 1993;33(3):434-440.
7 Mooij JJ, Buchthal A, Belopavlovic M. Somatosensory evoked potential monitoring of temporary middle cerebral artery occlusion during aneurysm operation. Neurosurgery. 1987;21(4):492-496.
8 Ogilvy CS, et al. Temporary vessel occlusion for aneurysm surgery: risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996;84(5):785-791.
9 Ohmoto T, et al. Monitoring of cortical blood flow during temporary arterial occlusion in aneurysm surgery by the thermal diffusion method. Neurosurgery. 1991;28(1):49-54.
10 Pool JL. Aneurysms of the anterior communicating artery. Bifrontal craniotomy and routine use of temporary clips. J Neurosurg. 1961;18:98-112.
11 Sako K, et al. Aneurysm surgery using temporary occlusion under SEP monitoring. No Shinkei Geka. 1995;23(1):35-41.
12 Samson D, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34(1):22-28.
13 Steinberg GK, et al. Cerebral damage caused by interrupted, repeated arterial occlusion versus uninterrupted occlusion in a focal ischemic model. J Neurosurg. 1994;81(4):554-559.
14 Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: pathophysiology. J Neurosurg. 1992;77(2):169-184.
15 Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part II: mechanisms of damage and treatment. J Neurosurg. 1992;77(3):337-354.
16 Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8(4):398-412.
17 Smith AL, Wollman H. Cerebral blood flow and metabolism: effects of anesthetic drugs and techniques. Anesthesiology. 1972;36(4):378-400.
18 Ridenour TR, et al. Comparative effects of propofol and halothane on outcome from temporary middle cerebral artery occlusion in the rat. Anesthesiology. 1992;76(5):807-812.
19 Gill R, Foster AC, Woodruff GN. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J Neurosci. 1987;7(10):3343-3349.
20 Sheardown MJ, et al. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990;247(4942):571-574.
21 Steen PA, et al. Nimodipine improves outcome when given after complete cerebral ischemia in primates. Anesthesiology. 1985;62(4):406-414.
22 Crawford ES, Saleh SA. Transverse aortic arch aneurysm: improved results of treatment employing new modifications of aortic reconstruction and hypothermic cerebral circulatory arrest. Ann Surg. 1981;194(2):180-188.
23 Tharion J, et al. Profound hypothermia with circulatory arrest: nine year’ clinical experience. J Thorac Cardiovasc Surg. 1982;84(1):66-72.
24 Feigin VL, et al. The emerging role of induced hypothermia in the management of acute stroke. J Clin Neurosci. 2002;9(5):502-507.
25 Fay T. Early experiences with local and generalized refrigeration of the human brain. J Neurosurg. 1959;16(3):239-259.
26 Ji X, et al. Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: a mechanism for neuroprotection. Front Biosci. 2007;12:1737-1747.
27 Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke. 1996;27(5):913-918.
28 Schettini A, Stahurski B, Young HF. Osmotic and osmotic-loop diuresis in brain surgery. Effects on plasma and CSF electrolytes and ion excretion. J Neurosurg. 1982;56(5):679-684.
29 Busto R, et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7(6):729-738.
30 Dempsey RJ, et al. Moderate hypothermia reduces postischemic edema development and leukotriene production. Neurosurgery. 1987;21(2):177-181.
31 Farrar JK, et al. Effects of profound hypotension on cerebral blood flow during surgery for intracranial aneurysms. J Neurosurg. 1981;55(6):857-864.
32 Fox LS, et al. Relationship of whole body oxygen consumption to perfusion flow rate during hypothermic cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1982;83(2):239-248.
33 Michenfelder JD, Milde JH. The effect of profound levels of hypothermia (below 14 degrees C) on canine cerebral metabolism. J Cereb Blood Flow Metab. 1992;12(5):877-880.
34 Steen PA, et al. Hypothermia and barbiturates: individual and combined effects on canine cerebral oxygen consumption. Anesthesiology. 1983;58(6):527-532.
35 Swan H. The importance of acid-base management for cardiac and cerebral preservation during open heart operations. Surg Gynecol Obstet. 1984;158(4):391-414.
36 Rittenhouse EA, et al. Deep hypothermia in cardiovascular surgery. Ann Thorac Surg. 1974;17(1):63-98.
37 Henriksen L. Brain luxury perfusion during cardiopulmonary bypass in humans. A study of the cerebral blood flow response to changes in CO2, O2, and blood pressure. J Cereb Blood Flow Metab. 1986;6(3):366-378.
38 Murkin JM, et al. Cerebral autoregulation and flow/metabolism coupling during cardiopulmonary bypass: the influence of Paco2. Anesth Analg. 1987;66(9):825-832.
39 Tommasino C, Moore S, Todd MM. Cerebral effects of isovolemic hemodilution with crystalloid or colloid solutions. Crit Care Med. 1988;16(9):862-868.
40 Delin NA, et al. Redistribution of regional blood flow in hypothermia. J Thorac Cardiovasc Surg. 1965;49:511-516.
41 Zarins CK, Skinner DB. Circulation in profound hypothermia. J Surg Res. 1973;14(2):97-104.
42 Litwin MS, Walter CW, Jackson N. Experimental production of acute renal tubular necrosis. I. The role of gram-negative bacteria. II. The toxicity of acid hematin. III. Acid hematin—the etiologic pigment. Ann Surg. 1960;152:1010-1025.
43 Boylan JW, Hong SK. Regulation of renal function in hypothermia. Am J Physiol. 1966;211(6):1371-1378.
44 Sears DA. Disposal of plasma heme in normal man and patients with intravascular hemolysis. J Clin Invest. 1970;49(1):5-14.
45 Soffer O, et al. Intraoperative hemodialysis during cardiopulmonary bypass in chronic renal failure. J Thorac Cardiovasc Surg. 1979;77(5):789-791.
46 Flores J, et al. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972;51(1):118-126.
47 Okada M. The cardiac rhythm in accidental hypothermia. J Electrocardiol. 1984;17(2):123-128.
48 Chenoweth DE, et al. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304(9):497-503.
49 Kirklin JK, et al. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86(6):845-857.
50 Patt A, McCroskey BL, Moore EE. Hypothermia-induced coagulopathies in trauma. Surg Clin North Am. 1988;68(4):775-785.
51 Patten BM, et al. Double-blind study of the effects of dexamethasone on acute stroke. Neurology. 1972;22(4):377-383.
52 Cold GE, et al. CBF and CMRO2 during continuous etomidate infusion supplemented with N2O and fentanyl in patients with supratentorial cerebral tumour. A dose-response study. Acta Anaesthesiol Scand. 1985;29(5):490-494.
53 Spetzler RF, et al. Microsurgical endarterectomy under barbiturate protection: a prospective study. J Neurosurg. 1986;65(1):63-73.
54 Aberg T, et al. Adverse effects on the brain in cardiac operations as assessed by biochemical, psychometric, and radiologic methods. J Thorac Cardiovasc Surg. 1984;87(1):99-105.
55 Messmer BJ, et al. Psychomotor and intellectual development after deep hypothermia and circulatory arrest in early infancy. J Thorac Cardiovasc Surg. 1976;72(4):495-502.
56 Settergren G, et al. Cerebral blood flow and cerebral metabolism in children following cardiac surgery with deep hypothermia and circulatory arrest. Clinical course and follow-up of psychomotor development. Scand J Thorac Cardiovasc Surg. 1982;16(3):209-215.
57 Treasure T, et al. The effect of hypothermic circulatory arrest time on cerebral function, morphology, and biochemistry. An experimental study. J Thorac Cardiovasc Surg. 1983;86(5):761-770.
58 Slogoff S, Girgis KZ, Keats AS. Etiologic factors in neuropsychiatric complications associated with cardiopulmonary bypass. Anesth Analg. 1982;61(11):903-911.
59 Churchill-Davidson HC, et al. Hypothermia: an experimental study of surface cooling. Lancet. 1953;265(6794):1011-1013.
60 Newberg LA, Michenfelder JD. Cerebral protection by isoflurane during hypoxemia or ischemia. Anesthesiology. 1983;59(1):29-35.
61 Nugent M, Artru AA, Michenfelder JD. Cerebral metabolic, vascular and protective effects of midazolam maleate: comparison to diazepam. Anesthesiology. 1982;56(3):172-176.
62 Lawton MT, et al. Hypothermic circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. Neurosurgery. 1998;43(1):10-20.
63 Spetzler RF, et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68(6):868-879.
64 Sullivan BJ, et al. Profound hypothermia and circulatory arrest with skull base approaches for treatment of complex posterior circulation aneurysms. Acta Neurochir (Wien). 1999;141(1):1-11.
65 Anderson DC, Cranford RE. Corticosteroids in ischemic stroke. Stroke. 1979;10(1):68-71.
66 Rebeyka IM, et al. The effect of low-flow cardiopulmonary bypass on cerebral function: an experimental and clinical study. Ann Thorac Surg. 1987;43(4):391-396.
67 Baumgartner WA, et al. Reappraisal of cardiopulmonary bypass with deep hypothermia and circulatory arrest for complex neurosurgical operations. Surgery. 1983;94(2):242-249.
68 Silverberg GD, Reitz BA, Ream AK. Hypothermia and cardiac arrest in the treatment of giant aneurysms of the cerebral circulation and hemangioblastoma of the medulla. J Neurosurg. 1981;55(3):337-346.
69 Stone JG, et al. Consequences of electroencephalographic-suppressive doses of propofol in conjunction with deep hypothermic circulatory arrest. Anesthesiology. 1996;85(3):497-501.
70 Casey WF, et al. Intravenous meperidine for control of shivering during caesarean section under epidural anaesthesia. Can J Anaesth. 1988;35(2):128-133.
71 Sladen RN, et al. Comparison of vecuronium and meperidine on the clinical and metabolic effects of shivering after hypothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1995;9(2):147-153.
72 Guffin A, Girard D, Kaplan JA. Shivering following cardiac surgery: hemodynamic changes and reversal. J Cardiothorac Anesth. 1987;1(1):24-28.
73 Baker KZ, et al. Deliberate mild intraoperative hypothermia for craniotomy. Anesthesiology. 1994;81(2):361-367.
74 Gravlee GP, et al. Heparin management protocol for cardiopulmonary bypass influences postoperative heparin rebound but not bleeding. Anesthesiology. 1992;76(3):393-401.
75 Pifarre R, et al. Management of postoperative heparin rebound following cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1981;81(3):378-381.
76 Smith EE, et al. Microvascular permeability after cardiopulmonary bypass. An experimental study. J Thorac Cardiovasc Surg. 1987;94(2):225-233.