CHAPTER 98 Chronic Pulmonary Embolism
Pulmonary hypertension is defined as a mean arterial pressure of greater than 25 mm Hg at rest and 30 mm Hg with exercise.1 Symptoms are often nonspecific and include dyspnea on exertion and easy fatigability. When it is severe, pulmonary hypertension can be severely debilitating and even fatal. During the past decade, advances in diagnosis and therapeutics have markedly affected the mortality of pulmonary hypertension.2 What was once a lethal condition without transplantation has become a condition that may be managed with various medications.
In 2003, an international multidisciplinary conference held in Venice, Italy, revisited the classification of pulmonary hypertension with a goal of classifying pulmonary hypertension into categories based on treatment algorithms that may be beneficial.3 The result was five main categories: pulmonary arterial hypertension, pulmonary hypertension due to left-sided heart disease, pulmonary hypertension from chronic hypoxia, pulmonary hypertension due to chronic embolic disease, and pulmonary hypertension from miscellaneous causes. Of these categories, pulmonary hypertension due to chronic embolic disease is the only one that may benefit from pulmonary thromboendarterectomy and one of the only forms of pulmonary hypertension that may be cured.4 The goal of this chapter is to highlight the imaging findings of chronic pulmonary embolism and to help delineate those patients who may benefit from surgical intervention.
CHRONIC PULMONARY EMBOLISM
Definition
Chronic pulmonary embolism is more accurately referred to as chronic thromboembolic pulmonary hypertension (CTPH) to distinguish it from chronic emboli from foreign materials, such as talc, or parasitic ova, such as schistosomiasis.3 CTPH represents cytokine-mediated scarring of the pulmonary circulation from even one episode of acute pulmonary embolism.5,6 The net effect of the scarring is decreased luminal volume for the pulmonary circulation. This decrease in arterial volume results in increased pulmonary pressures.
Prevalence and Epidemiology
In the age of cross-sectional imaging, CTPH may be more common than previously thought. Prior reports suggested that less than 0.1% of patients with an episode of acute pulmonary embolism will go on to develop CTPH and pulmonary hypertension.7 More recent studies suggest that this number is an underestimation of the true incidence and prevalence. In one prospective follow-up study of 78 patients with acute pulmonary embolism, 4 patients (5%) went on to develop definite CTPH, and in another of 223 patients with acute pulmonary embolism, 3.8% went on to develop symptomatic CTPH at 2 years.8,9 Estimations of true incidence are hindered by the large number of patients with CTPH who may not have had any history or memory of symptomatic pulmonary embolism in the past. As many as two of three patients with CTPH may not have had a documented history of acute pulmonary embolism.6
Etiology and Pathophysiology
CTPH is a cytokine-mediated condition that may follow even one episode of acute pulmonary embolism. This is important to consider so that it is not confused with recurrent acute pulmonary embolism, which represents either failure of anticoagulation or a manifestation of a hypercoagulable state.6 CTPH also does not represent failure of an acute pulmonary embolism from resolution. Instead, CTPH represents the end of a cycle of in situ thrombus, scarring, and vascular remodeling that occurs after a vascular insult, usually acute pulmonary embolism.5
Manifestations of Disease
Clinical Presentation
Like other causes of pulmonary hypertension, CTPH usually is manifested with vague symptoms and nonspecific findings.10,11 Patients tend to notice decrease in exercise tolerance, easy fatigability, and dyspnea on exertion. As the condition progresses, they may find that they develop dyspnea at rest. The mean time from onset of symptoms to diagnosis is about 3 years, highlighting the insidious, nonspecific nature of this process.6,10
Imaging Indications and Algorithm
Imaging of a patient with suspected pulmonary hypertension from CTPH is aimed at answering four main questions: (1) What is the degree of pulmonary hypertension? (2) What is the etiology of the pulmonary hypertension—is chronic pulmonary embolism the cause or is another entity more likely? (3) Are changes of chronic pulmonary embolism surgically accessible? (4) Are any new pulmonary emboli present? Imaging usually begins with a chest radiograph, which might suggest pulmonary hypertension, and quickly moves to an echocardiogram, which is used to estimate pulmonary pressures and to assess cardiac function.12 Traditionally, ventilation-perfusion scintigraphy (V/Q scan) has been used to assess for CTPH in combination with CT to exclude interstitial lung disease.6,12,13 At many institutions, however, the pulmonary embolism protocol CT has replaced both studies.14 This single study, especially when it is performed on a multidetector row CT (MDCT) scanner, allows the simultaneous assessment for CTPH and interstitial lung disease.
MR may allow a noninvasive assessment of pulmonary pressures, but its routine use for exclusion of CTPH remains somewhat experimental.15 If pulmonary hypertension is confirmed on any of the modalities, patients tend to be referred for right-sided heart catheterization so that accurate measurements of pulmonary pressures and right-sided heart hemodynamics may be performed. If CTPH is uncovered, patients may go on to pulmonary angiography to assess whether pulmonary thromboendarterectomy may be beneficial. Multiplanar postprocessing of MDCT data and thin maximum intensity projections (usually 4 to 7 mm) may provide equivalent information and avoid angiography altogether.
Imaging Techniques and Findings
Radiography
Conventional radiography may show enlargement of the right side of the heart, with increased convexity of the right border of the heart on the frontal projection and filling in of the retrosternal clear space on the lateral projection (Figs. 98-1 and 98-2). The pulmonary arteries may be enlarged. Features unique to CTPH are rarely observed.
Ultrasonography
Echocardiography will show increase in the size of the right atrium and the right ventricle as well as the main pulmonary artery. Echocardiography may be used to estimate pulmonary pressures.16 This estimate is based on calculating the peak velocity of the tricuspid regurgitation jet and calculating a pressure based on a modified Bernoulli equation [pressure = 4 × velocity2 (velocity in meters/sec)]. This value is added to an estimate of right atrial pressure, which is based on the size of the inferior vena cava and its variation during respiration.
Computed Tomography
CT findings can be divided into three main categories: cardiac findings, pulmonary arterial findings, and parenchymal or other findings.17–19
Cardiac findings, as with echocardiography, center on the effect of increased pulmonary pressures on the right ventricle and right atrium. Both chambers tend to be enlarged, and the right ventricle tends to be hypertrophied (see Figs. 98-1 and 98-2). The observation of muscle hypertrophy is important because right ventricular enlargement alone may be seen in the setting of acute pulmonary embolism. In this situation, right ventricular enlargement may be a finding of right-sided heart strain and may require more aggressive intervention or closer observation. With right-sided heart enlargement and right ventricle hypertrophy, the interventricular septum tends to straighten at first and then to bow toward the left. The interatrial septum also may eventually straighten and then bow toward the left. Rarely, intravenous contrast material may be seen entering the left atrium through a septal defect, or two thin membranes may be seen in the region of the atrial septum. These CT features of a patent foramen ovale can be subtle and, as with echocardiography, may be secondary to pulmonary hypertension from CTPH. One should not expect to see a defect on a single transaxial image in a patent foramen ovale. If one is observed, a true atrial septal defect may be present.
Pulmonary artery findings on CT are similar to the vascular findings on angiography.18,19 These include thrombus along the vessel wall, abrupt vessel caliber change, abrupt vessel cutoff or thrombosed vessel, webs within the arteries, beaded vessels, and enlarged central vessels. The eccentric nature of the thrombus is key in distinguishing CTPH from acute pulmonary embolism (see Figs. 98-1 and 98-2). CTPH tends to be present along the wall of the vessel, with a flat or concave interface with the lumen.17 Conversely, acute pulmonary embolism tends to be central with vessel expansion. When it is eccentric, along the vessel wall, acute pulmonary embolism is convex toward the lumen. CTPH rarely calcifies. In fact, if calcification is seen, one must strongly consider in situ thrombus formation as can be seen in pulmonary artery aneurysms, familial pulmonary arterial hypertension, intracardiac shunts, or even mitral disease (Fig. 98-3). Many of these features are better appreciated on coronal reconstructions.
Parenchymal or other findings are useful in confirming that the patient has CTPH as an explanation for pulmonary hypertension. These include enlarged bronchial arteries, mosaic perfusion, and bronchiectasis.20–22 The enlarged bronchial arteries tend to be variable, with only some of the arteries being enlarged. In general, these may be seen at the level of the carina as serpentine, enhancing structures near the descending aorta. They are much better delineated on coronal reconstructions. Mosaic perfusion in CTPH is a reflection of the small-vessel disease, with the darker areas representing abnormal lung with decreased vascularity. The darker lung should not demonstrate air trapping. Bronchiectasis in CTPH is a poorly understood phenomenon that has been clearly observed in the setting of CTPH. In general, the bronchiectasis is cylindrical.
Magnetic Resonance
The role of MR in the evaluation of CTPH is very much under current investigation.15 Ideally, MR would provide a one-test answer to the severity of hypertension and the location of the emboli, if present. As with echocardiography, estimation of pulmonary pressures depends on visualization of a tricuspid regurgitation jet. The excursion of the tricuspid annulus (up to 4 cm) during the cardiac cycle can make imaging of a clear dephasing jet difficult. Using a velocity-encoded phase contrast sequence, one can then obtain images orthogonal to the jet to calculate a peak velocity, which can then be used to calculate a pressure gradient. This number can be added to an estimation of right atrial pressure based on the size of the inferior vena cava and its respiratory variation.15,16
Nuclear Medicine/Positron Emission Tomography
Nuclear medicine for CTPH primarily relies on ventilation and perfusion (V/Q) scans. There is no role for positron emission tomography in the evaluation of CTPH. As with acute pulmonary embolism, the V/Q scan in CTPH tends to show multiple perfusion defects without ventilation abnormalities.13,23 These “mismatches” can be used to diagnose CTPH in the presence of pulmonary hypertension. A potential pitfall is that subsegmental defects may be seen in veno-occlusive disease, fibrosis, and idiopathic pulmonary hypertension (Fig. 98-4).24 On occasion, these defects can be difficult to separate from those of CTPH. Another shortcoming of V/Q scintigraphy is its inability to delineate location of the scars of CTPH.23 No information about the surgical resectability of the lesions is provided by this examination. Despite these potential pitfalls, V/Q scintigraphy offers a safe, lower radiation alternative to MDCT and is still frequently used in the evaluation of pulmonary hypertension that may be secondary to CTPH.25
Angiography
Like MDCT and contrast-enhanced MR, angiography relies on the vascular findings of CTPH: intravascular webs, eccentric thrombus, beaded vessels, vessel cutoff, and enlarged main pulmonary artery.26 Because imaging is performed at various points in time, another well-described sign, known as pouching, may be seen. In pouching, a convex, enlarged vessel is seen without initial filling of peripheral vessels. Over time, the thready peripheral vessels opacify.
An advantage of angiography is that it allows simultaneous measurements of right-sided heart and pulmonary pressures. These are still considered the gold standard measurements and are used for determination of prognosis and for follow-up of the patients.6
During the parenchymal phase, mosaic perfusion may be observed.
Differential Diagnosis
From Clinical Presentation
Any cause of pulmonary hypertension may mimic CTPH clinically.6,23 These include idiopathic pulmonary hypertension, Eisenmenger syndrome, pulmonary vasculitis, left-sided heart failure, chronic hypoxia, sarcoidosis, interstitial lung disease, fibrosing mediastinitis, and central bronchogenic cancer.
From Imaging Findings
Other conditions can mimic the angiographic findings. These include fibrosing mediastinitis and pulmonary vasculitis, such as Takayasu arteritis.23 Both of these conditions may cause thready vessels with beading and vascular cutoffs. The key in excluding these other conditions is to look for soft tissue attenuation or lymphadenopathy on CT or MR (Fig. 98-5). Enhancement of the vessel wall may also be helpful. This finding is not present with CTPH.
Synopsis of Treatment Options
Medical
Treatment of CTPH very much depends on the location of the scars and webs. If it is central enough, patients may be referred for surgery, which can be curative.2,4,18 If it is peripheral, CTPH will be treated similarly to pulmonary arterial hypertension. Vasodilator therapy may be all that can be offered to the patient. Because isolated peripheral disease will be treated like other causes of pulmonary arterial hypertension, one should not fear that tiny webs in small arterioles that may be missed on CT will alter treatment.
Surgical/Interventional
Surgical treatment consists of removal of the scar tissue so that the pulmonary vascular volume can be returned.2,4,18 The surgical procedure is a pulmonary thromboendarterectomy. The boundary between what is accessible and what is not depends on the experience of the surgeon. Usually, subsegmental artery thromboendarterectomy marks the limit to where a patient may be cured. This procedure is quite different from an embolectomy, which may be used in the setting of a large acute or subacute saddle pulmonary embolism. This procedure does not require stripping of the pulmonary artery intima and is quicker.
Reporting: Information for the Referring Physician
KEY POINTS
 CT, MR, and angiography can show the vascular findings of CTPH, including vessel cutoff, webs, beaded vessels, and eccentric thrombus.
CT, MR, and angiography can show the vascular findings of CTPH, including vessel cutoff, webs, beaded vessels, and eccentric thrombus. CT also allows corroborative evidence of CTPH, including mosaic perfusion, bronchiectasis, and enlarged bronchial arteries.
CT also allows corroborative evidence of CTPH, including mosaic perfusion, bronchiectasis, and enlarged bronchial arteries.Frazier AA, Galvin JR, Franks TJ, Rosado-de-Christenson ML. From the archives of the AFIP: pulmonary vasculature—hypertension and infarction. Radiographics. 2000;20:491-524.
Han D, Lee KS, Franquet T, et al. Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. Radiographics. 2003;23:1521-1539.
Hoeper MH, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011-2020.
Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(Suppl S):5S-12S.
Wittram C, Kalra MK, Maher MM, et al. Acute and chronic pulmonary emboli: angiography-CT correlation. AJR Am J Roentgenol. 2006;186(Suppl 2):S421-S429.
1 Frazier AA, Galvin JR, Franks TJ, Rosado-de-Christenson ML. From the archives of the AFIP: pulmonary vasculature—hypertension and infarction. Radiographics. 2000;20:491-524.
2 Sahara M, Takahashi T, Imai Y, et al. New insights in the treatment strategy for pulmonary arterial hypertension. Cardiovasc Drugs Ther. 2006;20:377-386.
3 Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(Suppl S):5S-12S.
4 Wray CJ, Auger WR. Evaluation of patients for pulmonary endarterectomy. Semin Thorac Cardiovasc Surg. 2006;18:223-229.
5 Moser KM, Braunwald NS. Successful surgical intervention in severe chronic thromboembolic pulmonary hypertension. Chest. 1973;64:29-35.
6 Hoeper MH, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011-2020.
7 Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345:1465-1472.
8 Ribeiro A, Lindmarker P, Johnsson H, et al. Pulmonary embolism: one-year follow-up with echocardiography Doppler and five-year analysis. Circulation. 1999;99:1325-1330.
9 Pengo V, Lensing AW, Prins MH, et al. Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
10 Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med. 2005;143:282-292.
11 Nauser TD, Stites SW. Diagnosis and treatment of pulmonary hypertension. Am Fam Physician. 2001;63:1789-1798.
12 Galiè N, Manes A, Branzi A. Evaluation of pulmonary arterial hypertension. Curr Opin Cardiol. 2004;19:575-581.
13 Worsley DF, Palevsky HI, Alavi A. Ventilation-perfusion lung scanning in the evaluation of pulmonary hypertension. J Nucl Med. 1994;35:793-796.
14 Coulden R. State-of-the-art imaging techniques in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:577-583.
15 Kreitner KF, Kunz RP, Ley S, et al. Chronic thromboembolic pulmonary hypertension—assessment by magnetic resonance imaging. Eur Radiol. 2007;17:11-21. Epub 2006
16 Sallach SM, Peshock RM, Reimold S. Noninvasive cardiac imaging in pulmonary hypertension. Cardiol Rev. 2007;15:97-101.
17 Wittram C, Kalra MK, Maher MM, et al. Acute and chronic pulmonary emboli: angiography-CT correlation. AJR Am J Roentgenol. 2006;186(Suppl 2):S421-S429.
18 Heinrich M, Uder M, Tscholl D, et al. CT scan findings in chronic thromboembolic pulmonary hypertension: predictors of hemodynamic improvement after pulmonary thromboendarterectomy. Chest. 2005;127:1606-1613.
19 Han D, Lee KS, Franquet T, et al. Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. Radiographics. 2003;23:1521-1539.
20 Hasegawa I, Boiselle PM, Hatabu H. Bronchial artery dilatation on MDCT scans of patients with acute pulmonary embolism: comparison with chronic or recurrent pulmonary embolism. AJR Am J Roentgenol. 2004;182:67-72.
21 King MA, Bergin CJ, Yeung DW, et al. Chronic pulmonary thromboembolism: detection of regional hypoperfusion with CT. Radiology. 1994;191:359-363.
22 Remy-Jardin M, Remy J, Louvegny S, et al. Airway changes in chronic pulmonary embolism: CT findings in 33 patients. Radiology. 1997;203:355-360.
23 Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637-648.
24 Thadani U, Burrow C, Whitaker W, Heath D. Pulmonary veno-occlusive disease. Q J Med. 1975;44:133-159.
25 Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48:680-684.
26 Auger WR, Fedullo PF, Moser KM, et al. Chronic major-vessel thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology. 1992;182:393-398.

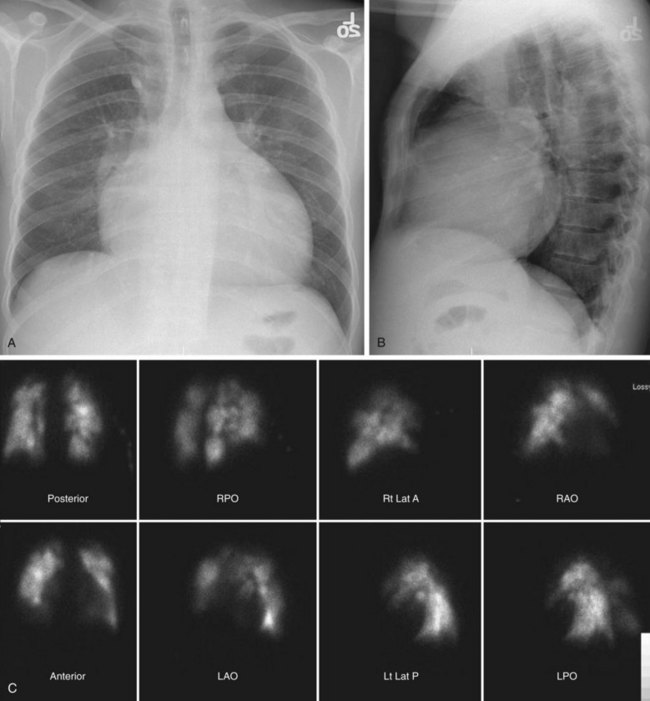
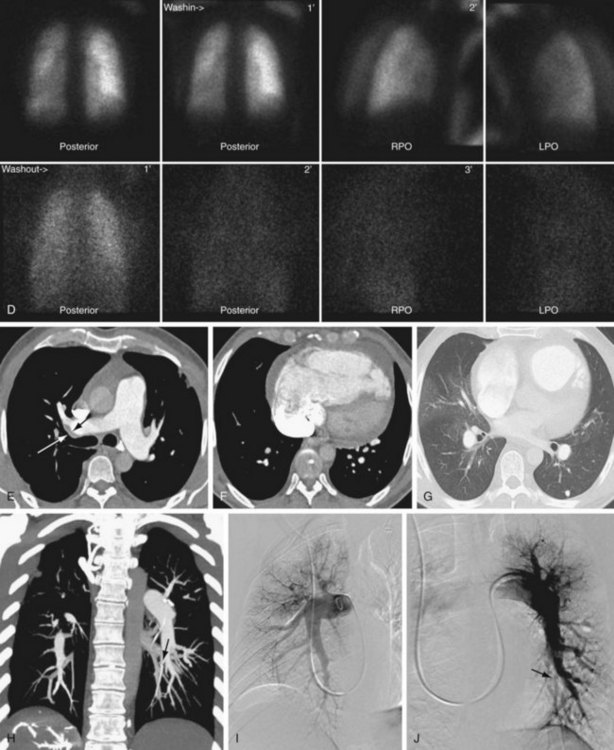
 FIGURE 98-1
FIGURE 98-1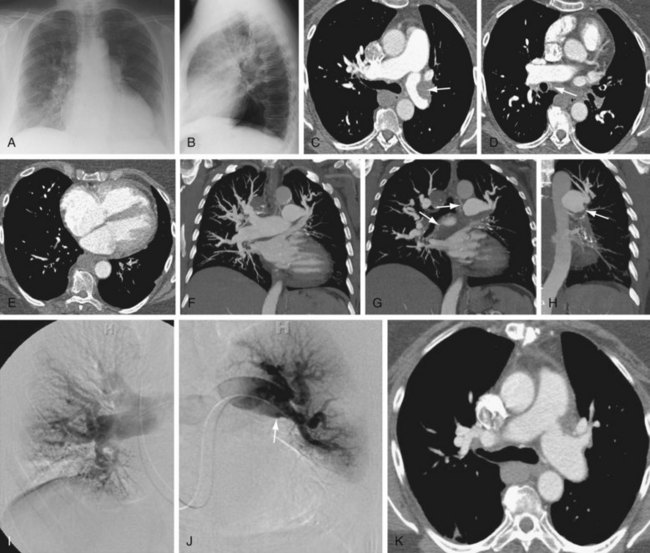
 FIGURE 98-2
FIGURE 98-2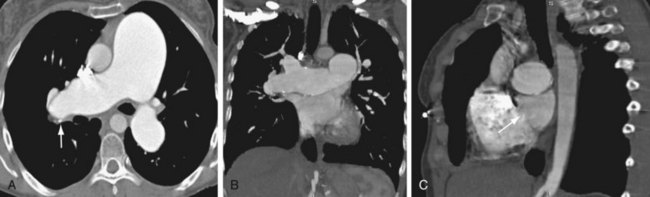
 FIGURE 98-3
FIGURE 98-3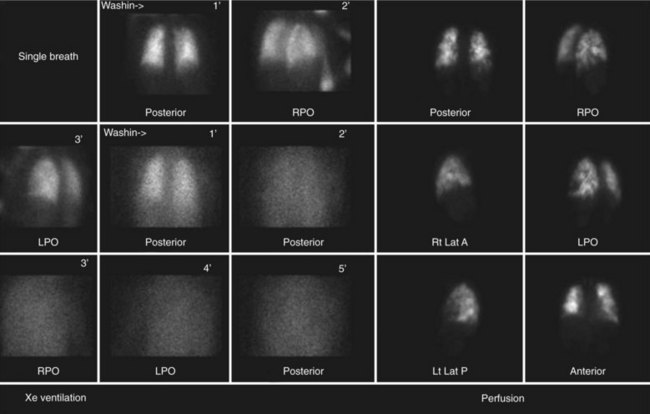
 FIGURE 98-4
FIGURE 98-4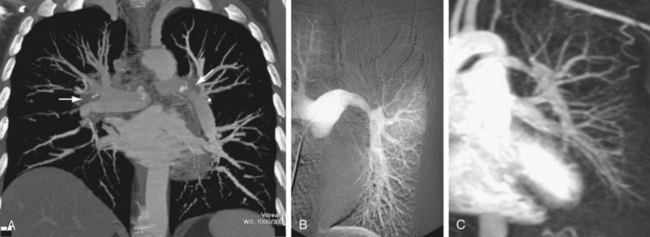
 FIGURE 98-5
FIGURE 98-5



