Chronic Obstructive Pulmonary Disease, Chronic Bronchitis, and Emphysema
After reading this chapter, you will be able to:
• Describe the American Thoracic Society (ATS) guidelines for chronic obstructive pulmonary disease (COPD), chronic bronchitis, and emphysema.
• Discuss the Global Initiative for Chronic Obstructive Lung Disease (GOLD) definition of COPD.
• List the etiology and epidemiology and risk factors associated with COPD.
• Describe the GOLD global strategy for diagnosing COPD.
• Describe the key indicators for considering a COPD diagnosis.
• History of exposure to risk factors
• Describe the three main pulmonary function study measurements used to confirm the clinical suspicion of COPD:
• Forced expiratory volume in 1 second (FEV1)
• Forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC ratio)
• Differentiate the following four stages of COPD as outlined by GOLD:
• Identify additional diagnostic studies for patients identified as having Stage II, Stage III, or Stage IV COPD:
• Bronchodilator reversibility testing
• Arterial blood gas measurement
• Alpha1-antitrypsin deficiency screening
• List the anatomic alterations of the lungs caused by both chronic bronchitis and emphysema.
• List the cardiopulmonary clinical manifestations caused by the anatomic alterations and pathophysiologic mechanisms associated with chronic bronchitis and emphysema.
• Identify the key distinctive differences between chronic bronchitis and emphysema—the “pink puffer” and the “blue bloater.”
• Describe the GOLD global strategy for the management and prevention of COPD.
• Describe additional treatment considerations for emphysema, including the following:
• Alpha1-antitrypsin replacement therapy
• Lung volume reduction surgery
• Describe the clinical strategies and rationales of the SOAPs presented in the case studies.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs Associated with Chronic Bronchitis
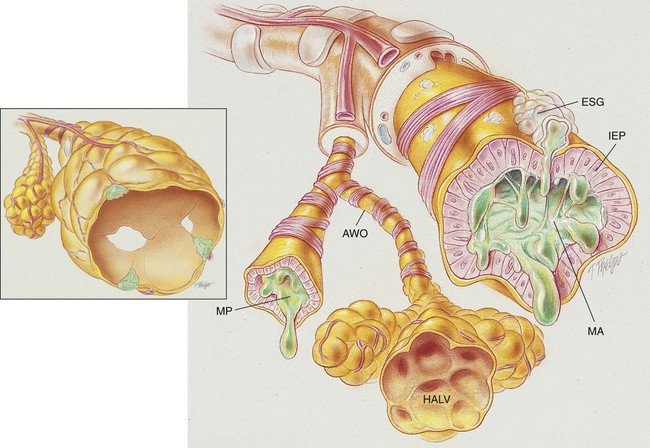
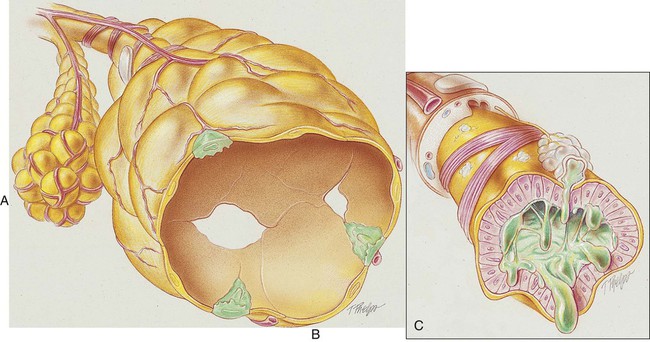
The conducting airways (particularly the bronchi) are the primary structures that undergo change in chronic bronchitis. As a result of chronic inflammation the bronchial walls are narrowed by vasodilation, congestion, and mucosal edema. This condition is often accompanied by bronchial smooth muscle constriction. In addition, continued bronchial irritation causes the submucosal bronchial glands to enlarge and the number of goblet cells to increase, resulting in excessive mucous production. The number and function of cilia lining the tracheobronchial tree are diminished, and the peripheral bronchi are often partially or totally occluded by inflammation and mucous plugs, which in turn leads to hyperinflated alveoli (see Figure 11-1).
The following major pathologic or structural changes are associated with chronic bronchitis:
• Chronic inflammation and swelling of the wall of the peripheral airways
• Excessive mucous production and accumulation
• Partial or total mucous plugging of the airways
• Smooth muscle constriction of bronchial airways (bronchospasm)
• Air trapping and hyperinflation of alveoli—occasionally in late stages
Anatomic Alterations of the Lungs Associated with Emphysema
In panacinar emphysema, or panlobular emphysema, there is an abnormal weakening and enlargement of all alveoli distal to the terminal bronchioles, including the respiratory bronchioles, alveolar ducts, alveolar sacs, and alveoli—the entire acinus is affected by dilatation and destruction. The alveolar-capillary surface area is significantly decreased (see Figure 11-2). Panlobular emphysema commonly is found in the lower parts of the lungs and often is associated with a deficiency of alpha1-antitrypsin. Panlobular emphysema is the most severe type of emphysema and therefore the most likely to produce significant clinical manifestations.
In centriacinar emphysema, or centrilobular emphysema, the pathology involves the respiratory bronchioles in the proximal portion of the acinus. The respiratory bronchiolar walls enlarge, become confluent, and are then destroyed. A rim of parenchyma remains relatively unaffected (see Figure 11-3). Centriacinar emphysema is the most common form of emphysema and is strongly associated with cigarette smoking and with chronic bronchitis.
The following are the major pathologic or structural changes associated with emphysema:
Etiology and Epidemiology
Risk Factors
• Tobacco smoke—including smoke from cigarette, pipe, cigar, and other types of tobacco smoking popular in many countries, as well as environmental tobacco smoke. According to GOLD, cigarette smoking is the most commonly encountered risk factor for COPD worldwide.
• Occupational dusts and chemicals—vapors, irritants, and fumes, when the exposures are sufficiently intense or prolonged.
• Indoor air pollution—from biomass fuel used for cooking and heating in poorly vented dwellings, a risk factor that particularly affects women in developing countries.
• Outdoor air pollution—also contributes to the lungs’ total burden of inhaled particles and gases (e.g., silicates, sulfur dioxide, the nitrogen oxides, and ozone), although it appears to have a relatively small effect in causing COPD.
• Conditions that affect normal lung growth—any condition that affects lung growth during gestation and childhood (e.g., low birth weight, respiratory infections) has the potential for increasing an individual’s risk for developing COPD.
• Genetic predisposition (alpha1-antitrypsin deficiency)—in about 1 out of every 50 cases of emphysema, there is a specific hereditary basis for panlobular emphysema called alpha1 (or α1)-antitrypsin deficiency. Alpha1-antitrypsin is a major protein in the blood. It is produced by the liver. Alpha1-antitrypsin protects the lungs by blocking the effects of a powerful enzyme called elastase. Elastase is carried by the body’s white cells to help kill invading bacteria and to neutralize small particles inhaled into the lung. When old white cells are destroyed in the lungs, elastase is released. Under normal circumstances, alpha1-antitrypsin works to inactivate the released elastase. However, when the alpha1-antitrypsin level is low, the elastase is free to attack and destroy the elastic tissue of the lungs.
• The normal level of alpha1-antitrypsin is 200 to 400 mg/dL. Patients with normal levels of alpha1-antitrypsin are referred to genetically as having an MM phenotype or simply an M phenotype (homozygote). The phenotype associated with severely low serum concentrations is the ZZ phenotype, or simply Z. The heterozygous offspring of parents with the M and Z phenotypes have an MZ phenotype. The MZ phenotype results in an intermediate deficiency of alpha1-antitrypsin. The precise effect of the intermediate level of alpha1-antitrypsin is unclear. It is strongly recommended, however, that individuals with this phenotype not smoke or work in areas having significant environmental air pollution.
Diagnosis of Chronic Obstructive Pulmonary Disease
Pulmonary Function Study in the Diagnosis of Chronic Obstructive Pulmonary Disease
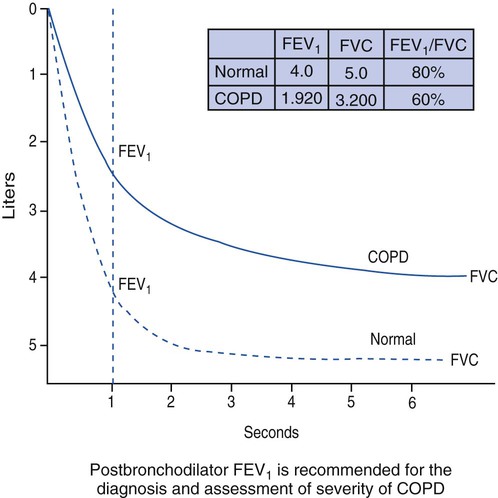
The three main spirometry tests used to identify COPD are the forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC ratio). Clinically, the FEV1/FVC ratio is also commonly called the forced expiratory volume 1 second percentage (FEV1%). Figure 11-4 illustrates a normal spirogram and a spirogram typical of patients with mild to moderate COPD.
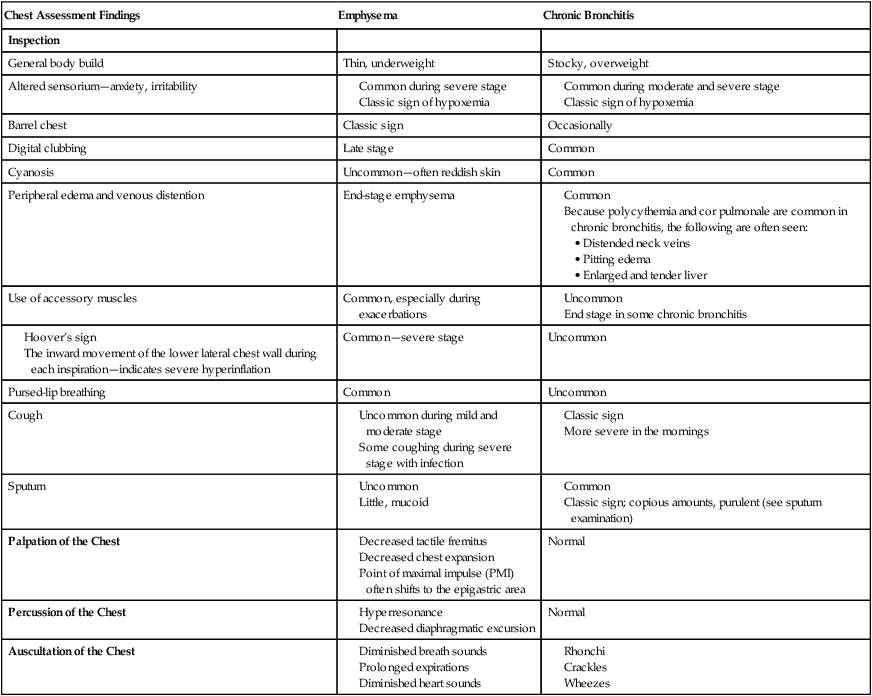
| Chest Assessment Findings | Emphysema | Chronic Bronchitis |
| Inspection | ||
| General body build | Thin, underweight | Stocky, overweight |
| Altered sensorium—anxiety, irritability |
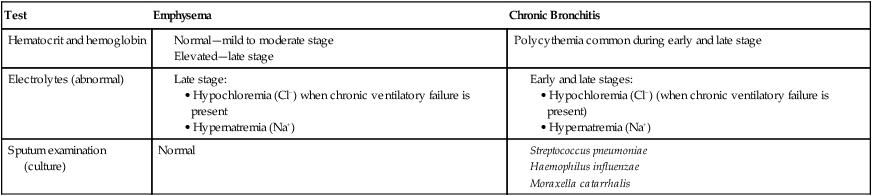
LABORATORY TESTS AND PROCEDURES
| Test | Emphysema | Chronic Bronchitis |
| Hematocrit and hemoglobin |
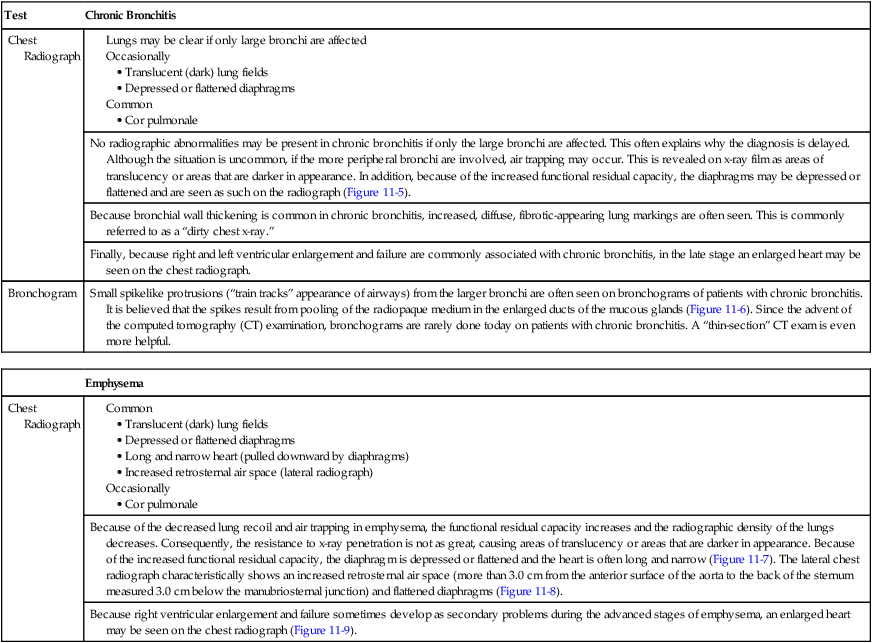
| Test | Chronic Bronchitis |
| Chest Radiograph |
| Emphysema | |
| Chest Radiograph | |
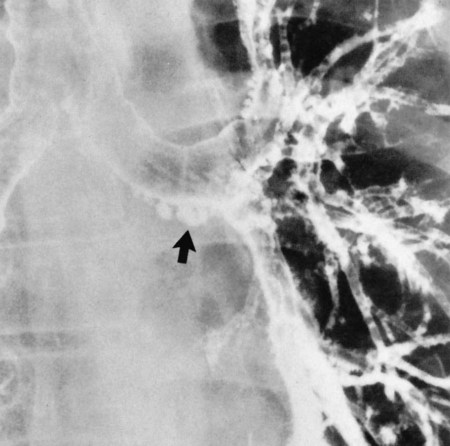
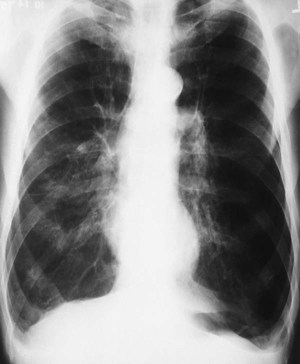
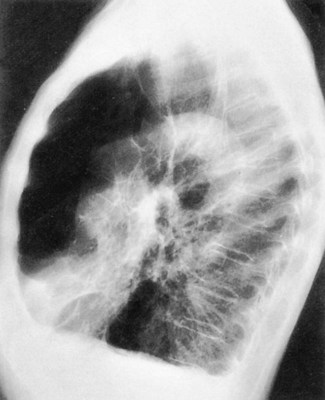
| Because of the decreased lung recoil and air trapping in emphysema, the functional residual capacity increases and the radiographic density of the lungs decreases. Consequently, the resistance to x-ray penetration is not as great, causing areas of translucency or areas that are darker in appearance. Because of the increased functional residual capacity, the diaphragm is depressed or flattened and the heart is often long and narrow (Figure 11-7). The lateral chest radiograph characteristically shows an increased retrosternal air space (more than 3.0 cm from the anterior surface of the aorta to the back of the sternum measured 3.0 cm below the manubriosternal junction) and flattened diaphragms (Figure 11-8). | |
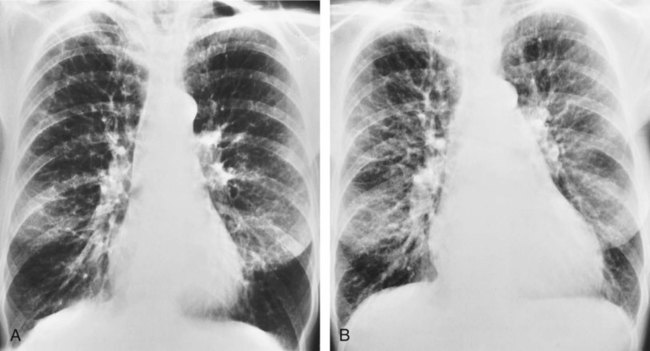
| Because right ventricular enlargement and failure sometimes develop as secondary problems during the advanced stages of emphysema, an enlarged heart may be seen on the chest radiograph (Figure 11-9). |
aChronic bronchitis and emphysema frequently occur together as a disease complex referred to as chronic obstructive pulmonary disease (COPD). Patients with COPD typically demonstrate clinical manifestations of both chronic bronchitis and emphysema.
Stages of chronic obstructive pulmonary disease.
GOLD now recognizes the following four stages of COPD:
Stage I, mild COPD, consists of mild airflow limitation (FEV1/FVC < 70%; FEV1 ≥ 80% predicted). At this stage the symptoms may be so mild that an individual may not recognize abnormal lung function.
Stage II, or moderate COPD consists of worsening airflow limitation (FEV1/FVC < 70%; FEV1 50% to <80% of predicted). The patient often complains of shortness of breath upon exertion. The patients usually will seek medical attention at this stage because of their symptoms.)
Stage III, severe COPD, includes further worsening of the airflow limitation (FEV1/FVC < 70%; FEV1 30% to <50% of predicted). At this stage the symptoms have an impact on the patient’s quality of life.
Stage IV, very severe COPD, includes severe airflow limitation (FEV1/FVC < 70%; FEV1 < 30% predicted, or FEV1 < 50% predicted, plus chronic ventilatory failure). Quality of life is very impaired and exacerbations may be life threatening.
Additional Diagnostic Studies for Chronic Obstructive Pulmonary Disease
• Bronchodilator reversibility testing: To rule out a diagnosis of asthma, particularly in patients with an atypical history (e.g., asthma in childhood and regular nocturnal night waking with cough and wheeze).
• Chest x-ray examination: Seldom diagnostic in COPD but valuable to exclude alternative and/or additional diagnoses, such as pulmonary tuberculosis, and pneumonia, and to identify comorbidities such as cardiac failure.
• Arterial blood gas measurement: Perform in patients with FEV1 <50% predicted or with clinical signs suggestive of ventilatory failure or right-sided heart failure. The major clinical sign of ventilatory failure is cyanosis. Clinical signs of right-sided heart failure include ankle edema and an increase in the jugular venous pressure. Ventilatory failure is indicated by a Pao2 <60 mm Hg, with or without a Paco2 >50 mm Hg while breathing room air.
• Alpha1-antitrypsin deficiency screening: Perform when COPD develops in patients of Caucasian descent under 45 years of age or with a strong family history of COPD.
Key Distinguishing Features between Emphysema and Chronic Bronchitis
Pink Puffer (Type A Chronic Obstructive Pulmonary Disease)
• Emphysema is caused by the progressive destruction of the distal airways and pulmonary capillaries.
• The progressive elimination of the pulmonary capillaries leads to a reduced pulmonary blood flow throughout the lungs—that is, an overall increased ventilation-perfusion ratio.
• To compensate for the increased ventilation-perfusion ratio in the patient who has emphysema hyperventilates.
• The increased respiratory rate, in turn, works to maintain a relatively normal arterial oxygenation level and causes a ruddy or flushed skin complexion. During the end stage of emphysema, however, the patient’s oxygenation status decreases and the carbon dioxide level increases.
• Thus, the patient with emphysema, who has both a red complexion and a rapid respiratory rate, is called a pink puffer.
Blue Bloater (Type B Chronic Obstructive Pulmonary Disease)
• Unlike emphysema, the pulmonary capillaries in the patient with chronic bronchitis are not damaged. The patient with chronic bronchitis responds to the increased airway obstruction by decreasing ventilation and increasing cardiac output—that is, a decreased ventilation-perfusion ratio.
• The chronic hypoventilation and increased cardiac output (decreased ventilation-perfusion ratio) leads to a decreased arterial oxygen level, an increased arterial carbon dioxide level, and a compensated (normal) pH—or chronic ventilatory failure arterial blood gases (also called compensated respiratory acidosis). The respiratory drive is depressed in patients with chronic ventilatory failure.
• The persistent low ventilation-perfusion ratio and depressed respiratory drive both contribute to a chronically reduced arterial oxygenation level and polycythemia—which, in turn, causes cyanosis.
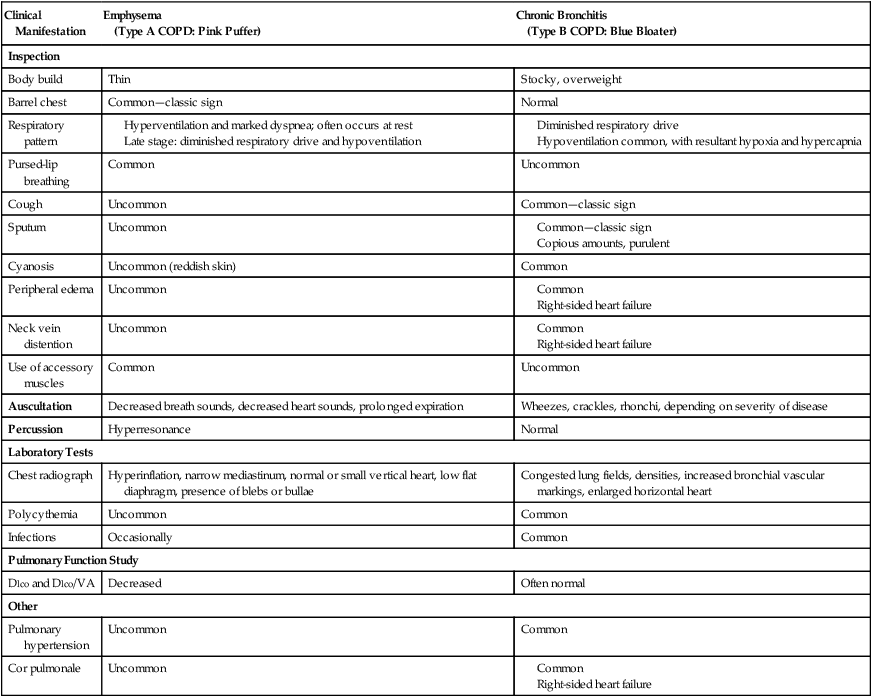
Table 11-1 provide an overview of the more common distinguishing features between emphysema and chronic bronchitis.
TABLE 11-1
Key Features Distinguishing Emphysema from Chronic Bronchitis*
| Clinical Manifestation | Emphysema (Type A COPD: Pink Puffer) |
Chronic Bronchitis (Type B COPD: Blue Bloater) |
| Inspection | ||
| Body build | Thin | Stocky, overweight |
| Barrel chest | Common—classic sign | Normal |
| Respiratory pattern | ||
General Management of Chronic Obstructive Pulmonary Disease
Global Initiative for Chronic Obstructive Lung Disease
• past medical history (including asthma and allergies)
• pattern of symptom development
• appropriateness of current medical treatments
• impact of the disease on patient’s life
The third component is to manage the stable COPD patient. In order to achieve this you must determine the disease severity on an individual basis, implement a treatment plan that reflects the severity, and choose treatments according to national and cultural preferences, the patient’s abilities and preferences and the availability of medications. Patient education is important to improve skills and the patient’s ability to deal with the disease. Pharmacologic treatments used to control and prevent symptoms, reduce exacerbation, and improve exercise tolerance and quality of life can be found in Table 11-2. Bronchodilators are medications that are frequently used in symptom management of COPD. The inhaled method is preferred and these medications are given as needed to relieve and prevent worsening of symptoms. Regular treatment with long-acting bronchodilators is more effective and convenient than treatment with short-acting bronchodilators.
TABLE 11-2
Medications Commonly Used in the Treatment of Chronic Obstructive Pulmonary Disease
| Drug | Inhaler (µg) | Solution for Nebulizer (mg/mL) | Oral | Vials for Injection (mg) | Duration of Action (hours) |
| Beta2-Agonists | |||||
| Short-Acting Beta2-Agonists | |||||
| Fenoterol | 100-200 (MDI) | 1 | 0.05% (syrup) | 4-6 | |
| Levalbuterol | 0.63, 1.25 | 4-6 | |||
| Salbutamol (albuterol) | 100, 200 (MDI and DPI) | 5 | 5 mg (pill) syrup 0.024% | 0.1, 0.5 | 4-6 |
| Terbutaline | 400, 500 (DPI) | 2.5, 5 (pill) | 0.2, 0.25 | 4-6 | |
| Long-Acting Beta2-Agonists | |||||
| Formoterol | 4.5-12 (MDI and DPI) | 12+ | |||
| Salmeterol | 25-50 (MDI and DPI) | 12+ | |||
| Anticholinergics | |||||
| Short-Acting Anticholinergics | |||||
| Ipratropium bromide | 20, 40 (MDI) | 0.25-0.5 | 6-8 | ||
| Oxitropium bromide | 100 (MDI) | 1.5 | 7-9 | ||
| Long-Acting Anticholinergics | |||||
| Tiotropium | 18 (DPI) | 24+ | |||
| Combination Short-Acting Beta2-Agonists Plus Anticholinergic in One Inhaler | |||||
| Fenoterol and ipratropium | 200/80 (MDI) | 1.25/0.5 | 6-8 | ||
| Oxitropium bromide | 75/15 (MDI) | 0.75/4.5 | 6-8 | ||
| Methylxanthines | |||||
| Aminophylline | 200-600 mg (pill) | 240 mg | Variable, up to 24 | ||
| Theophylline (SR) | 200-600 mg (pill) | Variable, up to 24 | |||
| Inhaled Glucocorticosteroids | |||||
| Beclomethasone | 50-400 (MDI and DPI) | 0.2-0.4 | |||
| Budesonide | 100, 200, 400 (DPI) | 0.20, 0.25, 0.5 | |||
| Fluticasone | 50-500 (MDI and DPI) | ||||
| Triamcinolone | 100 (MDI) | 40 | 40 | ||
| Combination Long-Acting Beta2-Agonists Plus Glucocorticosteroids in One Inhaler | |||||
| Formoterol and budesonide | 4.5/160, 9/320 (DPI) | ||||
| Salmeterol and fluticasone | 50/100, 250, 500 (DPI) 25/50, 125, 250 (MDI) |
||||
| Systemic Glucocorticosteroids | |||||
| Prednisone | 5-60 mg (pill) | ||||
| Methyl-prednisolone | 4, 8, 16 mg (pill) | ||||
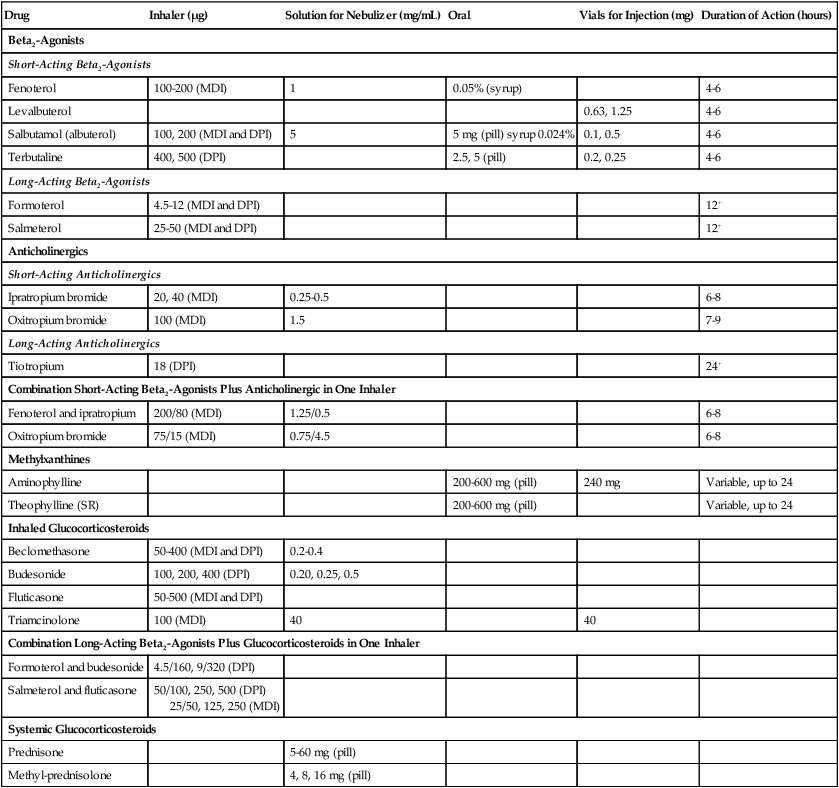
Data from Global Initiative for Chronic Obstructive Lung Disease (GOLD): Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professionals, 2007, GOLD, available at: www.goldcopd.org; and Gardenshire DS: Rau’s respiratory care pharmacology, ed 7, St. Louis, 2008, Elsevier.
• marked increase in intensity of signs or symptoms, such as resting dyspnea
• onset of new physical signs (cyanosis, peripheral edema)
• failure of exacerbations to respond to initial medical management
Figure 11-10 provides an overview of a chronic obstructive pulmonary disease (COPD) management algorithm recommended by GOLD.
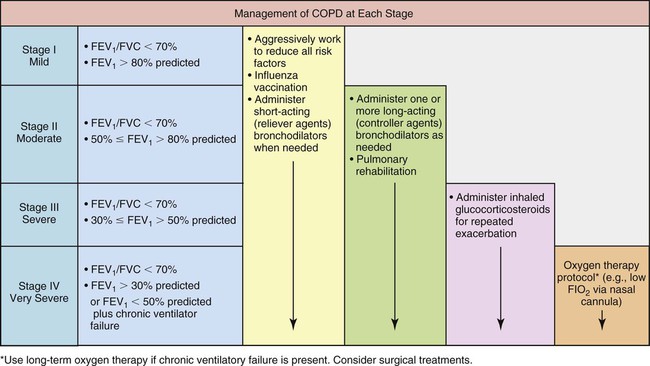
• Global Strategy for the Diagnosis, Management, and Prevention of COPD. Scientific information and recommendations for COPD programs. (November 2008).
• Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professionals. (2008).
These publications are freely available on the Internet at www.goldcopd.org.
Respiratory Care Treatment Protocols
Oxygen Therapy Protocol
Oxygen therapy is used to treat hypoxemia, decrease the work of breathing, and decrease myocardial work. The hypoxemia that develops in cystic fibrosis is caused by the pulmonary shunting associated with the disorder. When the patient demonstrates chronic ventilatory failure during the advanced stages of COPD, caution must be taken not to overoxygenate the patient (see Oxygen Therapy Protocol, Protocol 9-1).
Mechanical Ventilation Protocol
Mechanical ventilation may be necessary to provide and support alveolar gas exchange and eventually return the patient to spontaneous breathing. Because acute ventilatory failure superimposed on chronic ventilatory failure is often seen in patients with severe COPD, continuous mechanical ventilation is justified when the acute ventilatory failure is thought to be reversible; e.g., when acute pneumonia exists as a complicating factor (see Mechanical Ventilation Protocols 9-5, 9-6, and 9-7).
CASE STUDY
Chronic Bronchitis
Admitting History and Physical Examination
Respiratory Assessment and Plan
S “Smoker’s cough,” sputum production, dyspnea
O Strong productive cough. Sputum: Yellow-gray. Breath sounds: Rhonchi and crackles at lung bases. ABG: pH—7.36, Paco2—87, —48, Pao2—64. X-ray: increased markings at bases. PFTs: decreased FEV1/FVC ratio (55% of predicted), decreased FEV1 (35% of predicted).
Severe, Stage III, chronic bronchitis (history, physical exam, and PFT results)
Moderate airway secretions (rhonchi and crackles)
Good ability to mobilize secretions (strong cough and sputum production)
P Bronchopulmonary Hygiene Therapy Protocol (cough and deep breathing, prn). Patient education on smoking. Refer to Smoking Cessation Clinic. Aerosolized Medication Protocol (med. neb. treatment with Levalbuterol q6h).
Respiratory Assessment and Plan
S Complains of productive cough and exertional dyspnea (history)
O Bibasilar crackles, wheezing, rhonchi, cyanosis, obesity. Vital signs: HR 116, BP 165/90, RR 26/min. Cough: productive with copious yellow green sputum. PFT: FEV1/FVC 55% and FEV1 of 35%. CXR: diffuse fibrotic lung markings and cor pulmonale. ABG: pH 7.51, Paco2 51, 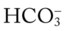 39, Pao2 41. Spo2 89% on 2 L/min O2. Hemoglobin 17.8 g%.
39, Pao2 41. Spo2 89% on 2 L/min O2. Hemoglobin 17.8 g%.
Acute exacerbation of chronic bronchitis (history, physical examination)
Exacerbation of chronic bronchitis (history, PFT)
Excessive mucous accumulation (sputum, rhonchi, and crackles)
Infection (yellow and green sputum)
Acute alveolar hyperventilation superimposed on chronic ventilatory failure with moderate to severe hypoxemia (ABG, Spo2)
P Aerosolized Medication Therapy Protocol (MDI with combined albuterol and ipratropium, 2 puffs qid). Inhaled glucocorticosteroid (beclomethasone) qid. Bronchopulmonary Hygiene Therapy Protocol (cough and deep breathing under supervision qid, cautious trial of CPT with postural drainage to lower lobes, 3 times a day). Call physician about impending ventilatory failure and chest x-ray report of cor pulmonale. Also check to see whether the doctor wants to schedule complete pulmonary function test. Recheck Spo2 on room air. Again, advise and facilitate smoking cessation program.
Discussion
In the first portion of this case study, some of the clinical manifestations of chronic Excessive Airway Secretions (see Figure 9-11) were present. These findings were clearly documented in the first SOAP note when the therapist charted the presence of productive cough, rhonchi, and crackles sounds and pulmonary function findings that indicated airway obstruction.
In the second part of this case study, there were more of the classic clinical manifestations associated with chronic bronchitis. For example, the patient’s Excessive Bronchial Secretions (see Figure 9-11) not only resulted in hypoxia and cyanosis secondary to a decreased 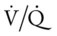 ratio and pulmonary shunting but also produced increased airway resistance that resulted in rhonchi and crackles and a decreased FEV1/FVC ratio and FEV1. In addition, the second part of this case study started with the patient’s failure to stop smoking and with increased symptoms and worsening of his obstructive pulmonary disease (dyspnea and productive cough). The findings on the chest x-ray film also suggested cor pulmonale, which often occurs in bronchitis. His pulmonary function was worsening, and he had acute alveolar hyperventilation superimposed on chronic ventilatory failure with moderate to severe hypoxemia. Impending ventilatory failure was a serious concern.
ratio and pulmonary shunting but also produced increased airway resistance that resulted in rhonchi and crackles and a decreased FEV1/FVC ratio and FEV1. In addition, the second part of this case study started with the patient’s failure to stop smoking and with increased symptoms and worsening of his obstructive pulmonary disease (dyspnea and productive cough). The findings on the chest x-ray film also suggested cor pulmonale, which often occurs in bronchitis. His pulmonary function was worsening, and he had acute alveolar hyperventilation superimposed on chronic ventilatory failure with moderate to severe hypoxemia. Impending ventilatory failure was a serious concern.
In addition to treating the acute symptoms with a simple MDI bronchodilator regimen (see Protocol 9-4) and Bronchopulmonary Hygiene Therapy Protocol (see Protocol 9-2) in the form of cough and deep breathing, a cautious trial of chest physiotherapy, and postural drainage, the respiratory therapist does not give up on the longer-term but extremely important goal of modifying behavior (smoking cessation) in the patient. A complete pulmonary function test in the near future would further define the patient’s disease process, both in its nature and severity. Such data are often helpful to the patient’s understanding of just how ill he is and may constitute a “teachable moment” for the physician and therapist. Although the patient was discharged from the hospital 5 days later, he unfortunately died from another acute exacerbation of chronic bronchitis, ventilatory failure, and cardiac arrest 7 months later.
CASE STUDY
Emphysema
Admitting History and Physical Examination
Respiratory Assessment and Plan
S “I’m short of breath with any exercise at all.” Cough for past 6 weeks.
O HR 120, BP 140/70, RR 32, and temp 100° F. Use of accessory muscles of inspiration, increased AP diameter, cyanosis. Hyperresonant percussion note and diminished breath sounds. I:E ratio 1 : 4. Lower lung infiltrates, hyperinflation of lungs on CXR. PFT: FEV1/FVC ratio of 57% and FEV1 of 40%. ABGs: pH 7.26, Paco2 82, 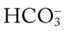 36, and Pao2 of 48. Sao2: 75%. Elevated WBC and gram-positive organisms in the sputum, alpha1-antitrypsin = 30 mg/dL.
36, and Pao2 of 48. Sao2: 75%. Elevated WBC and gram-positive organisms in the sputum, alpha1-antitrypsin = 30 mg/dL.
Panacinar emphysema (history, alpha1-antitrypsin deficiency)
Pulmonary hyperinflation (x-ray, diminished breath sounds, barrel chest)
Acute ventilatory failure on chronic ventilatory failure with moderate/severe hypoxemia (ABGs)
P Notify doctor about acute ventilatory failure. Oxygen Therapy Protocol (HAFOE mask at Fio2 = 0.35). Place intubation equipment and ventilator on standby. Monitor and evaluate per ICU standing orders (Spo2, vital signs). Check ABG in 30 min.
Discussion
The patient’s emphysema or Distal Airway and Alveolar Weakening (see Figure 9-12) was complicated by additional anatomic alterations of the lungs (i.e., Alveolar Consolidation [see Figure 9-8]). The alveolar consolidation was reflected in the patient’s immune-inflammatory response (fever and increased WBC), alveolar infiltrates (x-ray), low Pao2 (caused by a decreased 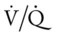 ratio and intrapulmonary shunting), and abnormal vital signs (see Figure 9-8).
ratio and intrapulmonary shunting), and abnormal vital signs (see Figure 9-8).
The effects of distal airway and alveolar weakening were reflected in the patient’s increased AP diameter, use of accessory muscles of inspiration, hyperresonant percussion note, diminished breath sounds, PFT results, and the chest x-ray film, which showed alveolar hyperinflation (see Figure 9-12).
CASE STUDY
Chronic Obstructive Pulmonary Disease (COPD)
Admitting History and Physical Examination
Respiratory Assessment and Plan
S:“I can’t take a deep breath.”
O:Moderate to severe respiratory distress. Vital signs: RR 35 bpm, HR: 145 bpm, BP: 145/90, T: 37° C. Barrel chest, cyanotic, frequent weak cough, moderate amount of purulent, gray-yellow sputum, using accessory muscles of inspiration, prolonged exhalation, pursed-lip breathing. 3+ pitting edema on legs, ankles, and feet. Distended neck veins, digital clubbing. Decreased chest expansion. Aus: diminished heart and breath sounds. Bilateral wheezes, rhonchi, and crackles could be heard over all lung fields. CXR: hyperinflation, depressed diaphragms, increased bronchial vascular markings, and an enlarged right heart. Arterial blood gas values on a 2 L/min O2 cannula were pH 7.24, Paco2 110, 55, Pao2 47, Spo2 77%. Hemoglobin level of 19 g%.
Excessive airway secretions (COPD history, rhonchi, crackles, purulent, gray-yellow secretions)
Pulmonary infection (yellow sputum)
Poor ability to mobilize secretions (weak cough effort )
Air trapping (hyperresonant percussion notes, hyperinflation on x-ray film, barrel chest)
Acute ventilatory failure on top of chronic ventilatory failure with moderate to severe hypoxemia (ABGs)
Cor pulmonale (swollen feet, ankles, and legs; enlarged right heart on x-ray film)
P:Notify physician stat regarding acute ventilatory failure superimposed on chronic ventilatory failure. Also inform the physician about the clinical manifestations associated with cor pulmonale. Recommend Mechanical Ventilation Protocol and Oxygen Therapy Protocol. Start Bronchopulmonary Hygiene Therapy Protocol (chest physical therapy qid; suctioning prn). Start Aerosolized Medication Protocol (combination short-acting beta2-agonist plus long-acting anticholinergic—e.g., salbutamol/ipratropium). Supplement these agents with an inhaled glucocorticosteroid (beclomethasone tid).
*GOLD (www.goldcopd.org) is recognized as a worldwide leading authority for the diagnosis, management, and prevention of COPD.
*The clinical features of emphysema and chronic bronchitis are not always clear-cut because many patients have a combined disease process (COPD—this is especially the case during the late stages of emphysema and chronic bronchitis).




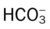

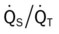
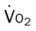
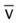 )02
)02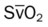

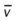 )O2, arterial-venous oxygen difference; O2ER, oxygen extraction ratio;
)O2, arterial-venous oxygen difference; O2ER, oxygen extraction ratio; 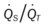 , pulmonary shunt fraction;
, pulmonary shunt fraction; 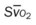 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 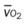 , oxygen consumption.
, oxygen consumption.
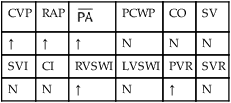
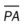 , mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.
, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.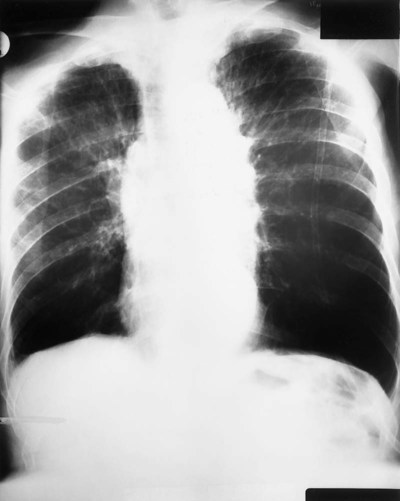
 39, Pa
39, Pa 36, and Pa
36, and Pa 34, and Pa
34, and Pa