Chronic Medical Illness during Pregnancy
Specific Disorders
Asthma is the most common pulmonary problem in pregnancy; it affects up to 8% of gestations, and the prevalence appears to be increasing.1 The overall effect of pregnancy on asthma seems to be that one third of patients will experience a worsening of the disease, one third will experience improvement, and the other third will remain unchanged.2 The exact effect of asthma on the fetus is likely to depend on the severity of disease and how well it is managed during pregnancy. Studies show that pregnant asthmatics have good perinatal outcomes in the setting of well-controlled symptoms.3–6 On the other hand, women with asthma exacerbations during pregnancy are at increased risk for complications, including congenital malformations and preterm delivery7–9 (see Table 179-1).
Table 179-1
Gestational Effects and Treatment of Medical Illnesses during Pregnancy
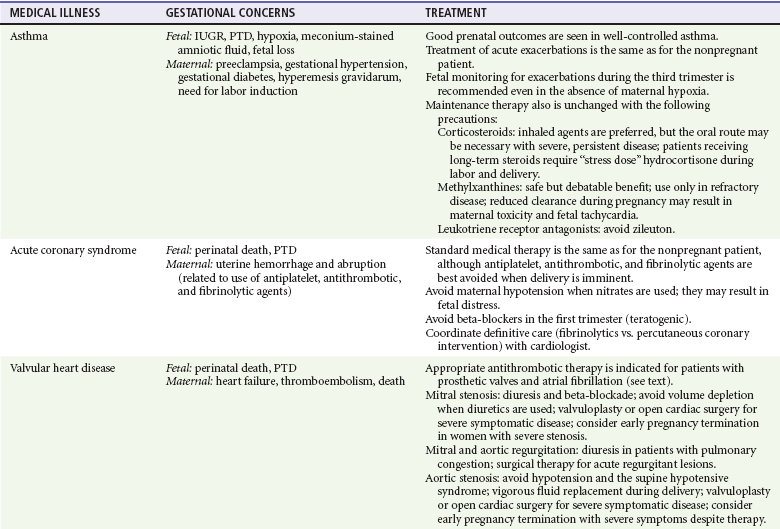


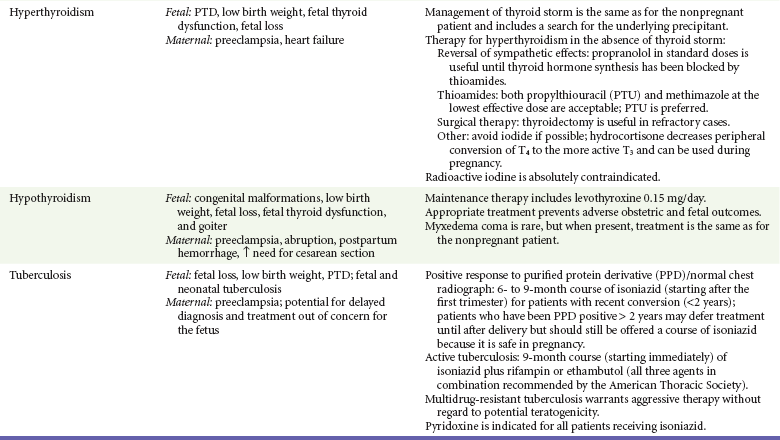
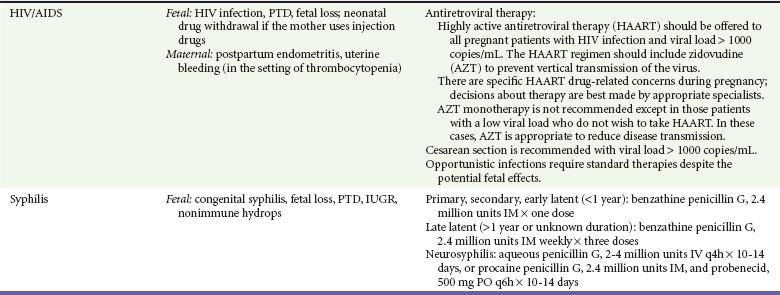
AEM, antiepileptic medicine; IUGR, intrauterine growth restriction; PTD, preterm delivery.
Several changes in respiratory physiology during normal pregnancy affect the management of asthma in this context; both the tidal volume and the minute ventilation rise, increasing up to 50% by term. This increase in minute ventilation, or “hyperventilation of pregnancy,” lowers the resting PCO2 by an average of 8 to 10 mm Hg to a PCO2 of 32 mm Hg. In response to chronic hyperventilation, there is a compensatory increase in the renal excretion of bicarbonate, with serum levels averaging 19 mEq/mL (19 mmol/L). The net result is a slight respiratory alkalosis, with a serum pH ranging from 7.40 to 7.45. It is important to keep these “normal” values in mind in gauging the severity of an asthma exacerbation in the pregnant patient. Acute asthma exacerbations pose a significant maternal and fetal risk, and the pregnant patient should be managed as aggressively as the nonpregnant patient.10
The inhaled beta-agonist agents are generally considered safe in pregnancy (Tables 179-1 and 179-2). As in the nonpregnant patient, inhaled short-acting beta-agonists are first-line agents for acute asthma exacerbations.3,10 Long-acting agonists (in conjunction with inhaled corticosteroids) are also recommended for maintenance use in patients with moderate or severe disease.3,10 Selective agonists are preferred because nonselective agents such as epinephrine theoretically decrease uteroplacental blood flow through beta-mediated effects. In addition, although they are safe from a teratogenic perspective, beta-agonists are tocolytics and will usually halt active labor.
Table 179-2
Drugs Used in the Treatment of Acute Asthma Exacerbations during Pregnancy
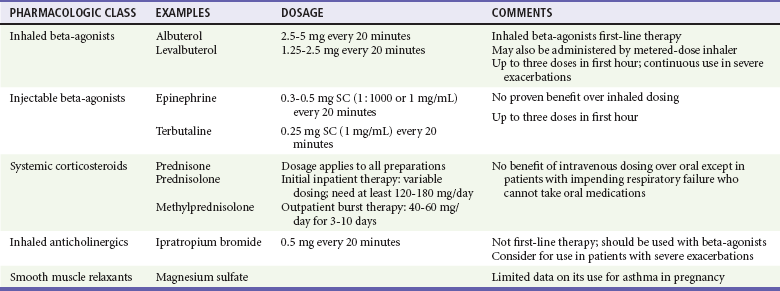
Modified from National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Asthma and Pregnancy Working Group: NAEPP Expert Panel Report. Managing asthma during pregnancy: Recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol 115:34, 2005.
Corticosteroids remain the core component for maintenance treatment of asthma in the pregnant patient. In fact, current data support the safety of inhaled corticosteroids,11 and their chronic use is recommended in all asthmatic patients except those with mild intermittent disease.3,10 Use of systemic corticosteroids is sometimes necessary on a chronic basis in patients with severe persistent asthma and as a short burst in patients with exacerbations that exhibit inadequate or poor response to initial beta-agonist therapy.
The methylxanthine theophylline has been shown to be safe in pregnancy at therapeutic doses and is an acceptable alternative medication for maintenance therapy for patients with moderate to severe disease.3,10 There are limited data for cromolyn sodium, but this agent is considered class B (presumed safety) during pregnancy, and its use as an alternative agent is acceptable.10 Likewise, the leukotriene receptor antagonists (montelukast and zafirlukast) have not been widely investigated. However, an initial small study in pregnant women did not find a significant increase in adverse outcomes, and these medications are also considered class B in pregnancy.12 Magnesium sulfate has received a good deal of attention in the acute treatment of refractory asthma exacerbations because it has a wide safety profile and potent bronchodilator properties. Although large doses of magnesium have been used for the treatment of eclampsia, at this time there are insufficient data to support routine use of magnesium in this population of patients.
Perhaps the most important tenet of treatment is that if the mother is hypoxic, the fetus is hypoxic as well. In addition, the fetus is more sensitive to hypoxia; thus normal maternal oxygen saturation does not preclude fetal distress. Supplemental oxygen should be administered to all pregnant patients with an acute asthma exacerbation, and assessment of fetal well-being is indicated for viable pregnancies.3
Hypertension
Chronic Hypertension and Hypertensive Emergencies
Chronic hypertension is defined as elevated blood pressure that is present before the onset of pregnancy or that begins before the 20th week of gestation. It affects up to 7% of pregnancies and represents a significant source of maternal and fetal mortality and morbidity (see Table 179-1).13,14 The emergency physician may be required to provide therapy for hypertensive emergencies in patients with chronic hypertension and to differentiate between the various hypertensive disorders of pregnancy (Table 179-3).13–15
Table 179-3
Hypertensive Disorders of Pregnancy
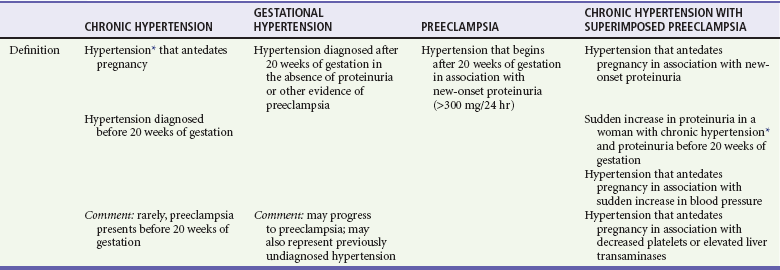
*Defined as blood pressure > 140 mm Hg systolic or > 90 mm Hg diastolic.
Modified from Roberts JM, et al: Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 41:437, 2003; and the National Institutes of Health: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md, U.S. Department of Health and Human Services.
In the treatment of chronic hypertension in pregnancy, the physician should try to balance the goal of reducing maternal blood pressure with the requirements to maintain cardiac output and to minimize adverse effects for both mother and fetus.13,14,16,17 Precipitous and marked decreases in blood pressure may significantly diminish uteroplacental blood flow. Treatment of patients with mild to moderate disease does not have an appreciable effect on perinatal outcomes, nor does it reduce the incidence of superimposed preeclampsia. In addition, these patients are likely to have a decrease in blood pressure without pharmacologic agents because of normal physiologic changes that occur in pregnancy, and they are often successfully managed without medications.13,14,17 On the contrary, patients with more severe hypertension, patients with evidence of end-organ damage, and patients taking more than one pregestational antihypertensive medication probably need maintenance medical therapy (see Table 179-1).13,17 Lifestyle modification is a potential mode of therapy, but it is unclear whether strict dietary and weight restrictions are appropriate in the gravid patient.18
Decisions about maintenance therapy are most appropriately made by the patient’s primary care practitioner in conjunction with her obstetrician. However, the emergency physician may be called on to make treatment decisions for patients with severely elevated blood pressure and for patients with superimposed eclampsia. Methyldopa remains the preferred maintenance agent, but nearly all of the major classes of antihypertensive agents are acceptable in the pregnant patient, with the exception of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. Diuretics are considered second-line agents because of concerns about plasma volume constriction (see Table 179-1).13,14,15,17 Hydralazine and labetalol are the agents most commonly used for hypertensive emergencies associated with eclampsia and are also appropriate for such emergencies in the patient with chronic hypertension. Sodium nitroprusside can cause fetal cyanide toxicity after several hours of infusion and is considered a second-line agent (Table 179-4).13,17
Table 179-4
Antihypertensive Agents for Hypertensive Emergencies
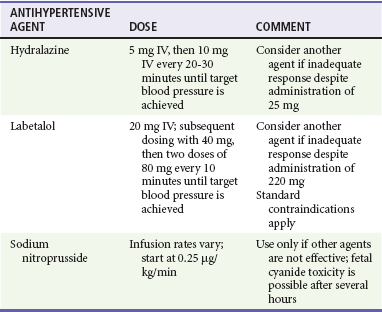
From the National Institutes of Health: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md, U.S. Department of Health and Human Services; August 2004. NIH Publication No. 04-5230.
Patients with chronic hypertension are more likely to have preeclampsia, a situation that results in increased morbidity and mortality compared with either process in isolation. Coexistence of the two disorders should be suspected in the following situations: (1) new onset of proteinuria after 20 weeks of gestation in a patient with known hypertension and (2) pregnant patients with hypertension and proteinuria before 20 weeks of gestation who have thrombocytopenia, increased transaminase levels, or an acute increase in proteinuria or blood pressure (see Table 179-3).18
Pulmonary Hypertension
The cardiovascular changes associated with pregnancy and delivery are poorly tolerated in patients with pulmonary hypertension. Data for pregnancy in women with pulmonary hypertension are generally limited to case series, but maternal mortality rates have classically been reported to range from 30 to 56%, depending on the underlying cause (primary pulmonary hypertension, secondary vascular pulmonary hypertension, or Eisenmenger’s syndrome). Recent case series suggest that a lower mortality rate is possible in the setting of aggressive monitoring and care, including use of prostacyclin analogues and phosphodiesterase inhibitors.19,20 Unfortunately, however, a recent literature review shows that mortality remains unacceptably high (17-33%).21
Cardiac Disorders
Coronary artery disease is rare in pregnant women; a population-based study noted a diagnosis of acute myocardial infarction (AMI) in up to 6.2 per 100,000 deliveries.22 Prior mortality rates were reported to be approximately 20%, but more recent studies reveal rates ranging from 5 to 11%.22–24 Normal physiologic changes of pregnancy, such as increased cardiac output and reduced oxygen-carrying capacity secondary to physiologic anemia, have the potential to exceed the threshold for angina if a fixed coronary artery stenosis is present. Certain conditions are also associated with an increased risk of pregnancy-related AMI, including advanced maternal age, thrombophilia, hypertensive disorders, anemia, diabetes mellitus, and tobacco use.22,23 A review found that comorbid conditions such as tobacco use and diabetes are relatively common in gravid patients with AMI.24 AMI can occur anytime during the gestational period but peaks during the last trimester and peripartum period.22,23 Because increases in cardiac output peak during labor and delivery, maternal mortality from AMI is higher in the intrapartum period than during the antepartum or postpartum period.23,24
There are a number of potential causes of AMI in pregnant women. Despite their relatively young age, a review found that only 13% of gravidas with AMI have normal coronary arteries.24 Other pathophysiologic mechanisms besides atherosclerosis that cause AMI in the pregnant patient include coronary artery dissection, vasospasm, and coronary artery thrombosis in otherwise normal vessels. The relative significance of these lesions varies according to the gestational period. Atherosclerotic disease causes the majority of AMIs in the antepartum period, whereas coronary artery dissection is the most common causative lesion during the intrapartum and postpartum stages.24 Pulmonary embolus, reflux esophagitis, biliary colic, and aortic dissection are all more common than myocardial ischemia during pregnancy and need to be considered in the differential diagnosis. Unfortunately, some women continue to use illicit drugs during their pregnancy, and it is important to consider cocaine use in the pregnant patient presenting with chest pain.
The diagnosis of acute coronary syndrome is similar to that in nonpregnant patients with certain exceptions. Electrocardiographic changes sometimes occur in normal pregnancies and delivery. These include T wave flattening and T wave inversion (mainly in lead III) and nonspecific ST changes during pregnancy as well as ST depression during labor induction for cesarean section.24 As a result, additional evaluation may be necessary. Echocardiography is useful in correlation of suspicious electrocardiographic findings with wall motion abnormalities. The enzymatic diagnosis of myocardial infarction is unchanged except during and immediately after delivery, when troponin is preferred to creatine kinase, which rises above baseline during this time. Angiography is appropriate in high-risk patients with acute coronary syndrome but is generally avoided in low-risk and stable gravidas.24 There have been reported cases of dissection resulting from percutaneous intervention during pregnancy.24 Radionuclide studies carry a lower radiation risk than does angiography but are also generally avoided in stable and low-risk patients.
Treatment of AMI during pregnancy is similar in most respects to treatment of the nonpregnant patient. Survival of the mother is the primary concern, and therapy to improve maternal outcome should not be withheld. Standard treatment includes antiplatelet agents, nitroglycerin, beta-blockers, and antithrombotic agents. However, there are potential adverse effects of such therapy on the mother and fetus (see Table 179-1), and emergent consultation with a cardiologist is recommended. Regarding antiplatelet agents, aspirin remains the first-line agent. There is limited experience with clopidogrel and eptifibatide, but both have been used successfully and are considered safe from a teratogenic standpoint. Heparin has long been considered the antithrombotic agent of choice in pregnant patients, but newer low-molecular-weight agents, specifically dalteparin and enoxaparin, appear to be efficacious and safe and are deemed appropriate for use.25 Heparin is preferable for patients in the late third trimester because there is a more predictable response to protamine sulfate should labor begin.
Experience with thrombolytic therapy in pregnancy is extremely limited; most experience has occurred in the setting of stroke, prosthetic valve thrombosis, and massive pulmonary embolism.26,27 The majority of pregnant patients receiving tissue plasminogen activator have had favorable maternal and fetal outcomes, but most were being treated for indications other than AMI.26 Reported adverse effects have included maternal hemorrhage, maternal death, placental abruption, preterm delivery, fetal death, and fetal intracranial hemorrhage, although the causal relationship in many of these cases is unclear because neither tissue plasminogen activator nor streptokinase crosses the placenta. Because thrombolytic therapy precludes major surgery and epidural anesthesia in the hours to days immediately after administration, the emergency physician must carefully consider whether to use these agents in pregnant women who are close to term, especially if the need for cesarean delivery is anticipated. In addition, the use of thrombolytics in coronary artery dissection is not advised. Because of the lack of data and potential risks of fibrinolytics in gravidas, emergent percutaneous intervention is the treatment of choice for definitive management of AMI.24,28
In the setting of peripartum AMI, labor should be conducted with continuous monitoring of both the mother’s hemodynamic status and fetal well-being. There are benefits and risks of both vaginal and operative delivery. Cesarean section avoids prolonged exertion by the mother but subjects the patient to general anesthesia if use of antithrombotic agents precludes epidural catheter placement. In addition, surgical delivery places the patient at risk for typical postoperative complications, such as infection, hemorrhage, and thromboembolism. Therefore, assisted vaginal delivery is preferred unless there is an obstetric reason for cesarean section.24 Resolution of angina may occur during the postpartum period as physiologic demands lessen, but its occurrence during pregnancy merits a complete cardiac evaluation for acute coronary syndrome.
Valvular Heart Disease
Maternal valvular heart disease can be congenital or acquired and is one of the leading causes of nonobstetric death. Acquired valvular disease is mainly the result of rheumatic fever and endocarditis. In the United States, most cases of significant congenital heart disease are identified and corrected surgically before puberty. The ability of patients to tolerate pregnancy without significant adverse effects depends on the type and severity of the lesion. Mild to moderate lesions (New York Heart Association classes I and II) are often associated with good outcomes. On the other hand, mitral stenosis (beyond class I), advanced aortic stenosis, aortic and mitral lesions associated with moderate to severe ventricular dysfunction or pulmonary hypertension, and mechanical prosthetic valves requiring anticoagulation can result in significant maternal mortality and require directed therapy (see Table 179-1).29
Mitral Stenosis: Mitral stenosis is the most commonly encountered valvular lesion in pregnancy and is also the most important lesion to detect in early pregnancy because maternal mortality is not insignificant.30 The increased resting heart rate and stroke volume in normal pregnancy increase the pressure gradient across the mitral valve. The result is high left atrial pressures, pulmonary vascular congestion, and the symptoms and physical findings typical of left ventricular failure. Patients may also develop atrial dysrhythmias that have the potential to decrease cardiac output and worsen symptoms.30 The likelihood of maternal symptoms and worsening of cardiovascular status is directly related to the severity of disease. Pregnancy in women with mild mitral stenosis is generally well tolerated.30
In patients with symptomatic mitral stenosis, treatment is aimed at reduction of the plasma volume and slowing of the heart rate (see Table 179-1). Surgical intervention is indicated in patients with refractory symptoms despite optimal medical management and in patients with pulmonary hypertension. The preferred procedure is percutaneous balloon valvotomy, which is associated with good maternal and fetal outcomes when it is performed in experienced centers.28–30
Aortic and Mitral Regurgitation: In most cases, chronic regurgitant lesions are well tolerated during pregnancy and may even improve as the reduced systemic vascular resistance of pregnancy allows more forward and less regurgitant flow. In addition, this effect is aided by the increase in heart rate and shortened diastole that occur in pregnancy.28–30 When necessary, medical therapy consists of diuresis, digoxin, and vasodilators. Patients with acute mitral regurgitation due to ruptured chordae do not fare as well and may require surgical therapy.28–30
Aortic Stenosis: Symptomatic aortic stenosis during pregnancy usually occurs in the setting of a congenital bicuspid valve.28,29 Patients with mild to moderate aortic stenosis tend to have uncomplicated pregnancies,30,31 and conservative management is often possible. Severely symptomatic patients may need percutaneous valvotomy in an experienced institution, although this procedure is risky for both the mother and the fetus.28,29
Prosthetic Heart Valves: Anticoagulation for the pregnant patient with a prosthetic heart valve is complicated. Warfarin is considered to be contraindicated during weeks 6 through 12 of gestation, although the risk of embryopathy is likely to be low when the dose is less than 5 mg/day.28,29 In addition, because warfarin crosses the placenta, there is a risk of fetal bleeding. If warfarin is used after the first trimester, it should be replaced by a heparin compound several weeks before anticipated delivery.29,32 Neither unfractionated heparin (UFH) nor low-molecular-weight heparin (LMWH) crosses the placenta or is teratogenic, but there are problems with their use in pregnancy. The response to UFH is more unpredictable in the pregnant patient, and aggressive dosing with frequent monitoring of the activated partial thromboplastin time is indicated.32 LMWH is an acceptable alternative to UFH in pregnant patients with thromboembolic disease, but there are still only limited data on its use in the gravida with a mechanical valve.29 In addition, there is an increased incidence of valve thrombosis in the setting of inadequate dosing and monitoring of anticoagulation.32 If the practitioner elects to use LWMHs, frequent monitoring of anti-Xa levels is recommended with the goal of achieving a level of approximately 1.0 unit/mL 4 hours after injection.29,32 In addition, intravenous UFH is substituted for LMWH 24 hours before delivery in anticipation of epidural or spinal anesthesia.32
Hematologic Disorders
Iron Deficiency Anemia: Iron deficiency anemia is common, occurring in approximately 20 to 25% of pregnancies in industrialized countries.33 Apart from chronically low or marginal iron stores in many women, diversion of maternal iron to the fetus for development of its own RBCs and iron stores and increased maternal demand for iron exacerbate the deficiency during pregnancy. Pregnant patients are also subject to other causes of iron deficiency anemia, such as malnutrition, chronic underlying disease, and blood loss from the gastrointestinal or genitourinary tracts.
Anemia in pregnancy is defined by the World Health Organization as a hemoglobin concentration of less than 11 g/dL (110 g/L). Because of physiologic dilution, this level may be slightly lower after the 25th week of gestation. The risks of adverse pregnancy outcomes (see Table 179-1) have been noted to be related to the severity of the anemia. This generally is manifested as a proportionate increase in the risk of preterm delivery and low-birth-weight babies for women with mild to moderate anemia.33–35 Severe anemia (<6 to 7 g/dL or 60 to 70 g/L), especially occurring early in pregnancy, is associated with even higher rates of preterm delivery and low birth weights as well as increased fetal mortality, abnormal fetal oxygenation, premature rupture of membranes, gestational hypertension, and reduced volume of amniotic fluid.36,37
It is currently recommended that all pregnant patients be screened for iron deficiency anemia.36 The diagnosis is most accurately made in the very early stages of pregnancy because serum ferritin, the most sensitive and specific (and preferred) test, is affected by the dilutional effect of increased plasma volume occurring later in pregnancy. After the first trimester, other blood tests performed in combination may be necessary. The use of RBC indices is not a reliable screening tool because microcytosis and hypochromia are not reliably present in pregnant patients. Other supporting laboratory evidence includes low plasma iron levels, increased free erythrocyte protoporphyrin, and elevated total iron-binding capacity.
Iron supplementation with non–enteric-coated preparations is recommended to increase maternal iron stores (see Table 179-1) in women with iron deficiency,36 even though it remains unclear whether such therapy improves perinatal outcomes.38 Patients with an uncomplicated physiologic anemia who are not iron deficient can be expected to have good obstetric outcomes without therapy and do not require treatment. The use of prophylactic supplementation in women with normal hemoglobin levels (>11 g/dL or 110 g/L) and normal iron stores (ferritin > 20 mg/dL or 20 µg/L) to prevent anemia in late pregnancy remains controversial. One randomized controlled trial found that iron supplements for such women before 28 weeks of gestation did not reduce the prevalence of preterm delivery or development of anemia later in pregnancy but did result in a decreased incidence of low-birth-weight infants.39 Another trial noted no effect on iron status but found that the incidence of both preterm delivery and low birth weight was reduced in women receiving prophylactic iron.40 On the other hand, a more recent randomized controlled trial found that nonanemic women receiving oral iron supplementation were more likely to have infants small for gestational age as well as hypertension during gestation.41 Other investigators have noted that pregnant patients with hemoglobin values typically considered “normal” in the nongravid patient have an increase in adverse outcomes, and the practitioner should consider that gravid patients with hemoglobin values of 13 to 15 g/dL (130 to 150 g/L) have inadequate expansion of their plasma volume.42
Folate Deficiency Anemia: Folate deficiency is one of a number of causes of megaloblastic anemia, a condition characterized by abnormal DNA synthesis and ineffective RBC production. The incidence of folate deficiency in pregnancy is low in developed countries but remains higher in other populations. The risk for development of folate deficiency is increased in patients with multiple gestations, short interpregnancy intervals, preexisting malnutrition, hyperemesis gravidarum, malabsorption syndromes, alcoholism, and diets lacking green leafy vegetables and animal protein.36,43 Use of certain antiepileptic medications also places women at increased risk for deficiency.
As is the case for iron deficiency, effects on the fetus depend on the degree of anemia; the most significant complications are neural tube defects (NTDs). Folate supplementation reduces the risk of NTDs,44,45 and oral folate supplementation with 0.4 mg daily is routinely recommended for all women during pregnancy and before conception as the requirement for this micronutrient increases during gestation.36 The American College of Obstetricians and Gynecologists recommends 1.0 mg for those women who have a known pregnancy-related deficiency of folate.36 Women at higher risk for NTDs (e.g., NTDs in prior pregnancy) are advised to take much higher doses of folate at 4 mg daily under the close supervision of their obstetrician.46
Sickle Cell Anemia: Sickle cell disease (SCD) is one of the major sources of maternal and fetal complications in the United States. The details of the pathophysiologic mechanism and genetics of SCD are discussed in Chapter 121, but it is useful to review the most common phenotypes that affect pregnancy. The sickle gene can be homozygous (hemoglobin SS or SCD), and this form of the disease is responsible for most pregnancy complications. The sickle gene can also be heterozygous with normal hemoglobin A (sickle cell trait or hemoglobin SA), in which case symptoms are rare except under extreme environmental conditions. The hemoglobin S can also be heterozygous with a large number of abnormal hemoglobins, such as hemoglobin C, several variants of thalassemia, and other rare hemoglobin variants, and each variant has its own complication profile. Of these, the most relevant in terms of pregnancy complications is hemoglobin SC.
Patients with SCD are subject to many chronic medical problems secondary to a variety of pathophysiologic mechanisms, including sickling of RBCs, anemia, immunosuppression caused by autosplenectomy, and repeated transfusion. Median life expectancy is in the fifth decade for both sexes affected by SCD, and female fertility is generally unaffected, so it is likely that the emergency physician will encounter pregnant patients with the disease. Maternal complications are common in patients with SCD and include preterm labor, eclampsia, premature rupture of membranes, maternal infections, more frequent pain crises, thrombosis, and increased need for cesarean delivery.47–52 Despite these complications, the maternal mortality rate is less than 1% with current treatment.48,49,51,52
SCD also results in adverse effects on the fetus (see Table 179-1).47 Placental infarction and insufficiency are common, and the incidence of small-for-gestational-age and low-birth-weight infants is significantly increased in SCD pregnancies compared with normal controls.48–51,53 The reported perinatal mortality rate varies but is low in the setting of appropriate maternal and neonatal care.49,50,52
Management of SCD during pregnancy is similar to that of the nonpregnant patient (see Table 179-1). Folate supplementation is standard even in the nonpregnant state because of the increased turnover of RBCs, and the recommended daily dose of folate increases to 4 mg daily during pregnancy.47 The use of supplemental iron and transfusion is controversial because of the potential for iron overload. The need for therapy is best determined by the appropriate specialists after a complete analysis of relevant hematologic parameters. Transfusion or exchange transfusion is generally reserved for patients with symptomatic anemia, cardiopulmonary instability, acute chest syndrome, intrapartum hemorrhage, and preeclampsia and possibly for patients with increasingly frequent pain crises. It is also used preoperatively for anticipated blood loss in patients undergoing cesarean section.49 In general, the goal with transfusion or exchange transfusion is to lower the percentage of hemoglobin S to 40% and to achieve hemoglobin values of approximately 10 g/dL (110 g/L).47 Hydroxyurea is not recommended for use in pregnancy because of potential teratogenicity.47
Neurologic Disorders
The effect of pregnancy on epilepsy is variable. Most (50-76%) epileptic patients experience no change in their seizure frequency, whereas approximately 15% experience more frequent seizures.54–56 A decrease in plasma drug concentrations is expected with certain AEMs, such as phenytoin, lamotrigine, oxcarbazepine, and levetiracetam.54 This results from a number of potential pregnancy-related physiologic changes, including increased plasma volume, decreased protein binding, and increased renal clearance. In addition, some patients engage in voluntary noncompliance with medications to avoid teratogenic effects on the fetus.
Although gravid patients with epilepsy may be at increased risk for cesarean section, postpartum hemorrhage, and other adverse outcomes (see Table 179-1),57 the primary complication in these pregnancies is congenital malformations.56 Of primary concern is the risk for NTDs, facial clefts, and cardiac anomalies with the older generation agents: valproate, carbamazepine, and phenytoin. There is a twofold or threefold increase in the incidence of serious congenital malformations in offspring of epileptic mothers taking these agents. The risk is greatest with valproate and is also increased with AEM polypharmacy.55,56,58,59 Of all the older agents, carbamazepine appears to be the safest for use as monotherapy.55,56,58 Controversy exists as to whether infants of epileptic patients not taking AEMs have an increased incidence of congenital malformations compared with the general population. Studies comparing these infants with infants born to mothers without epilepsy noted a similar incidence of malformations.58 Data are limited, but preliminary results suggest that the rate of congenital malformations with the newer agents lamotrigine and levetiracetam is similar to that in the general population, and these agents are being used with increased frequency in gravid patients.59–62
New-Onset Seizure: Pregnant patients may seek treatment for idiopathic new-onset seizures; however, drug toxicity or withdrawal, head injury, meningitis, stroke, and eclampsia should be considered possible causes. The most important of these is eclampsia. Patients in the immediate postictal phase often have hypertension resulting from increased sympathetic neural activity, and even those with normal pregnancies may have mild edema of the lower extremities. Consequently, urinalysis is indicated to search for proteinuria, which may be the only differentiating factor in the initial assessment of these patients. After a period of observation, elevated blood pressure in the noneclamptic patient will likely revert to normal. If the patient remains hypertensive or has other signs of eclampsia, magnesium sulfate and other agents are indicated to prevent further seizures and to control blood pressure. In patients who do not have signs of eclampsia, investigation of the cause of the seizure should proceed as with the nonpregnant patient.
Status Epilepticus: Any potential cause of seizure, including eclampsia, may result in status epilepticus. Despite this, status epilepticus in pregnancy is relatively rare, and limited data are available about its occurrence and therapy. Observations from the European Epilepsy Pregnancy Registry note that status epilepticus may occur at any time during gestation and even at delivery. It may also occur in patients who have been seizure free throughout their pregnancy, and no specific risk factors for its occurrence have been identified.54 Older reports noted a high fetal and maternal mortality, but recent data support a much lower complication rate.54
Pregnant Epileptic Patient: Patients with epilepsy coming to the emergency department for unrelated reasons may be found to be pregnant. Although no immediate change in their therapeutic regimen needs to be made, these patients should be advised of the potential risk of AEMs in pregnancy and be referred to appropriate specialists. Unintentional pregnancy is seen even in patients taking oral contraceptives because AEMs can cause increased clearance of these medications, thereby reducing their efficacy.
There are significant obstetric complications related to prolonged seizure activity, and long-term treatment with an AEM for most patients with seizures is warranted (see Table 179-1). Patients who have nonconvulsive seizure disorders or who are seizure free for a sufficient period before conception are candidates for nonpharmacologic observation, but this decision should be deferred to the patient’s primary physician or neurologist. Because phenytoin, carbamazepine, valproate, and possibly other AEMs interfere with folate metabolism, oral supplementation with at least 0.4 mg/day is recommended for all women of childbearing age taking these drugs as it may have some efficacy in preventing malformations associated with these agents.63 Enzyme-inducing AEMs such as carbamazepine, phenytoin, and phenobarbital have been reported to cause neonatal vitamin K deficiency and hemorrhagic complications. However, results from a prospective study of 667 neonates in 662 women taking enzyme-inducing AEMs (none of whom received antenatal vitamin K) indicate that the actual risk of hemorrhage is low and without significant relationship to AEM use.64 The American Academy of Neurology and the American Epilepsy Society note that there is inadequate evidence to determine a definitive relationship between AEM use and neonatal hemorrhage.63
Multiple Sclerosis
The impact of pregnancy on the course of MS has been closely studied in various cohorts of women, and a pattern has emerged. As in other autoimmune diseases, the frequency and severity of exacerbations of MS improve because of the immunosuppressant effects of pregnancy. This effect is most pronounced in the third trimester. During the 3 months after delivery, the rate of relapse increases and then returns to the pre-pregnancy baseline.65 The risk of postpartum relapse is increased in those women with relapses during the pre-pregnancy year and during pregnancy. Relapses are also more likely in MS patients with higher disability at the time of pregnancy onset. On the other hand, it does not seem that postpartum relapses are related to the duration of disease or the total number of relapses before conception.65 In addition, pregnancy does not appear to have any significant long-term adverse effects on disease progression.65 In fact, pregnancies occurring after onset of MS may confer a protective effect in regard to disease course.66
MS patients with disease exacerbation are often treated with immunomodulators such as intravenous immune globulin (IVIG), corticosteroids, and interferon beta. Although concerns exist about the use of interferon during pregnancy, small studies in gravid patients have shown that use of IVIG during pregnancy and the postpartum period is safe and may decrease the relapse rate.67–69 Likewise, the use of intermittent steroids in the postpartum period may decrease the likelihood of disease relapse.70
Maternal relapse rate is unaffected by epidural anesthesia,65 and decisions about anesthesia should be based solely on obstetric considerations. Labor may be complicated by fatigue and uncoordinated voluntary motor activity in pushing, and MS patients may be more likely to require assisted vaginal delivery.71 These pregnancies may also be at increased risk for preterm delivery and small-for-gestational-age infants.72 In general, however, pregnancies in the setting of MS have outcomes similar to those in unaffected patients.71
Spinal Cord Injuries
The increased coagulability of pregnancy combined with chronic immobilization results in an increased incidence of thromboembolic disease.73 The incidence of urinary tract infection is also markedly increased as a result of neurogenic changes and the need for catheterization.73 Infections are even more likely during pregnancy74 and may progress to pyelonephritis, with the subsequent increased risk of fetal loss, prematurity, and maternal sepsis.
A unique problem in the patient with SCI is the detection of the onset of labor, which may be painless and precipitous. Patients with spinal cord lesions below T10 to T12 have an intact uterine nerve supply and will experience labor pains; however, with lesions above T10, labor may be imperceptible or experienced as only mild abdominal discomfort.73 In addition, up to 85% of patients with high lesions (above T5 to T6) experience potentially life-threatening autonomic dysreflexia.75 This is manifested as severe paroxysmal hypertension, headache, tachycardia, diaphoresis, piloerection, mydriasis, and nasal congestion. Because the response is not specific to labor and may be precipitated by distention of bowel or bladder, other causes may need to be pursued as well. Pregnant patients with SCI who have these symptoms should be assessed for cervical dilation and have uterine contractions monitored. Emergency department treatment is directed at restoration of normal blood pressure with standard agents. Definitive therapy is with regional anesthesia. Both spinal anesthesia and epidural anesthesia obliterate and prevent this response and should be used as soon as possible during labor for all women with SCI.75 Because of the difficulty in detecting labor, pregnant patients with SCI are sometimes admitted for observation near term.
Myasthenia Gravis
The effect of pregnancy and the postpartum state on myasthenia gravis is unpredictable in the individual patient, but overall approximately 25 to 40% of patients experience exacerbation of disease, with the remainder having improvement or no change in disease severity.76–78 Several important pregnancy complications are associated with myasthenia gravis. Because of weight gain, anemia, and other physiologic adjustments of pregnancy that may result in fatigue, the distinction between normal pregnancy symptoms and myasthenia may be difficult. Most deliveries are accomplished vaginally without complication in adequately treated patients; assisted and surgical delivery in these women is indicated mainly for obstetric reasons rather than for specific myasthenia-related care.76,77,79
Up to 30% of neonates born to mothers with myasthenia gravis have a transient neonatal myasthenic syndrome through placental transport of acetylcholine receptor antibodies.77 The risk of neonatal myasthenia gravis is decreased in those infants born to mothers who have undergone thymectomy.77,80 Onset of neonatal myasthenia is typically within the first hours of life but may be delayed by a period of days. Manifestations include poor feeding and suck, diminished reflexes, hypotonia, and respiratory failure. As in adults, the symptoms respond to cholinesterase inhibitors, but treatment should be done in an intensive care unit setting. The duration of the syndrome depends on its severity; severely affected infants sometimes require therapy for several months.77
Fortunately, myasthenic crises during pregnancy are rare.77,78 When exacerbations do occur, treatment is no different from treatment of nonpregnant patients (see Table 179-1). Assessment of pulse oximetry, forced vital capacity, and arterial blood gas parameters will guide respiratory therapy. For patients presenting with weakness, an edrophonium (Tensilon) challenge test to distinguish myasthenic from cholinergic crisis is appropriate after initiation of appropriate ventilatory support. Standard medical treatment is continued during labor and delivery to maximize motor strength. Epidural anesthesia is also recommended to reduce pain and fatigue. Sedatives and other agents that increase fatigue are best avoided during this time. The physician should be aware that 30% of patients experience an exacerbation during the postpartum period as the protective immunosuppressant effect of pregnancy dissipates.77 In addition, puerperal infections place the patient at risk for disease exacerbation and warrant aggressive therapy.77
Renal Disorders
The primary factors that determine how renal disease affects pregnancy outcome are the degree of underlying dysfunction and the presence of associated hypertensive disorders. In general, patients with mild insufficiency (creatinine < 1.4 mg/dL or 106.76 µmol/L) and no hypertension can expect good pregnancy outcomes and preserved renal function. On the other hand, patients with moderate to severe renal dysfunction have a much higher risk of further decline in renal function as well as adverse obstetric outcomes, including preeclampsia, placental abruption, fetal loss, preterm delivery, low birth weight, polyhydramnios, and increased need for cesarean section and neonatal intensive care.81–87 Worsening of underlying renal function is more likely in patients with decreased glomerular filtration rate who also have associated proteinuria or hypertension.84 Because worsening renal function is manifested by hypertension and proteinuria, differentiation from preeclampsia can be difficult. In this setting, it is best to treat the patient for presumed preeclampsia with the caveat that magnesium administration be performed judiciously on the basis of serum magnesium levels.
Pregnant women with chronic renal failure require aggressive and timely management to optimize their chances for a successful gestation without causing further deterioration in renal function. Baseline renal function studies are done early in pregnancy and then reassessed every 4 to 6 weeks. Evidence of renal function deterioration or the development or exacerbation of hypertension warrants admission for specialized inpatient care. Hemodialysis is indicated for creatinine levels above 3.5 to 5 mg/dL (266.91 to 381.3 µmol/L). Pregnancy in women who are already dialysis dependent has been associated with poor outcomes, and pregnancy has previously been discouraged in this population of patients. However, studies including small numbers of women have noted live birth rates approaching 60 to 85% when mothers receive specialized prenatal care.82,85,86 Such care includes correction of anemia, appropriate antihypertensive therapy, and more aggressive dialysis with more frequent or prolonged sessions.81,86,88
Metabolic and Endocrine Disorders
Three types of diabetes are involved in pregnancy: type 1, or insulin-dependent diabetes mellitus (IDDM); type 2, or non–insulin-dependent diabetes mellitus (NIDDM); and gestational diabetes mellitus (GDM). Although NIDDM is sometimes considered a more benign form of disease, the risk of malformations is the same for both NIDDM and IDDM.89 Maternal and fetal complications relate to inadequate glycemic control as well as to the presence of vascular complications or severe renal insufficiency more than to the type of diabetes. All pregnant patients with diabetes are considered “brittle” and require regular follow-up by appropriate specialists. In addition, all patients, including those with GDM, should perform routine self-monitoring of glucose concentration.
Maternal Complications: The physiology of glucose regulation during pregnancy is complex. During the first half of gestation, some patients have an increased sensitivity to insulin,90 although this is not a universal finding and the change in insulin requirements in early pregnancy may be variable. In addition, hormonal changes of pregnancy combined with emesis, increased use of glucose by the placenta and fetus, and decreased hepatic glucose production predispose the gravid diabetic to more rapid onset of hypoglycemia. During later gestation, there is progressive insulin resistance that peaks during the third trimester and then falls again during labor and the immediate postpartum period. Obesity and underlying insulin resistance in NIDDM may exacerbate hyperglycemia during pregnancy.91 Pregnancy also predisposes to ketosis, and this effect is exacerbated in the setting of emesis. Specific adverse pregnancy-related outcomes are more common and include stillbirth, preeclampsia, preterm delivery, and the requirement for cesarean delivery.92–94
The effects of pregnancy on underlying diabetes vary by the organ system. The data are limited, but pregnancy is not advised for diabetic patients with significant coronary artery disease because of the cardiovascular demands of pregnancy and the high mortality of AMI during pregnancy.92 Patients with diabetic nephropathy are at increased risk for preeclampsia and the subsequent requirement for preterm delivery.91,92 The renal effects of pregnancy depend on the severity of underlying disease. Patients with mild to moderate renal dysfunction do not seem to experience permanent disease progression. On the other hand, women with more severe renal dysfunction are at increased risk for progression of their nephropathy.91,92 Retinopathy may worsen acutely during pregnancy, especially in patients with high hemoglobin A1c (HbA1c) levels, hypertension, nephropathy, and active nonproliferative or proliferative retinopathy.91,95 Laser therapy of preexisting retinopathy is recommended before conception as well as in pregnant patients with severe disease.91,92,95 Autonomic neuropathy does not accelerate during pregnancy, with the exception of a possible increase in symptomatic severity of gastroparesis,91 and the combination of hyperemesis gravidarum and gastroparesis often causes problems. Frequent vomiting results in dehydration and inadequate intake of nutrients that can cause hypoglycemic episodes if the insulin dosage is not adjusted accordingly.
Diabetic ketoacidosis (DKA) occurs in up to 9% of diabetic patients during pregnancy and may be the initial presentation of diabetes. DKA is most commonly seen in patients with IDDM but also complicates pregnancies in women with NIDDM and GDM.91,92,96 Common precipitating events include the typical factors seen in nonpregnant patients, such as insulin noncompliance and infection. Other pregnancy-specific factors are increased insulin resistance, hyperemesis, use of beta-mimetic medications for tocolysis, and use of corticosteroids to hasten fetal lung maturity. The serum pH may be deceptively normal in a pregnant patient with DKA because the initial pH tends to be higher in pregnancy as a result of physiologic hyperventilation. In addition, serum glucose concentration may be normal or only moderately elevated, and screening for DKA is indicated in gravid diabetics with vomiting or with persistent hyperglycemia.91,96 Maternal mortality is rare in appropriately treated DKA. Fetal mortality rates are relatively high, ranging from 10 to 35%.92
Fetal Complications: Diabetes has many deleterious effects on the fetus (see Table 179-1). The rate of congenital malformations in patients with pre-pregnancy diabetes is increased threefold or fourfold compared with the nondiabetic population, with anomalies being more likely in pregnancies with poor glycemic control.91,92,97–99 Glucose crosses the placenta, and prolonged fetal exposure to maternal hyperglycemia induces fetal pancreatic hyperplasia and high insulin production. Elevated insulin levels in turn promote fetal growth, resulting in macrosomia and the need for cesarean section. Conversely, preeclampsia and placental infarction secondary to vascular disease may result in impaired fetal development and stillbirth.94 After delivery, continued high insulin secretion in the absence of a maternal glucose supply results in a significant rate of neonatal hypoglycemia. Because these infants are more likely to be preterm, hyperbilirubinemia and respiratory problems are also more frequent.92,93
Management: Treatment of NIDDM and IDDM requires individualized and carefully adjusted insulin administration with the goal of maintaining strict glycemic control while avoiding hypoglycemia. Several studies suggest that sulfonylureas and metformin are safe and effective in pregnancies complicated by GDM,100,101 but experts recommend that NIDDM patients taking oral agents be transitioned to insulin during pregnancy.91,92,102 Ideally, HbA1c values should not be higher than 6%91,92 (see Table 179-1). Frequent self-monitoring is recommended, and these patients should also have frequent office visits with their practitioner throughout the duration of pregnancy. It is preferable to achieve glycemic control as manifested by a normal or nearly normal HbA1c level before conception to minimize the risks of congenital malformations and other complications.91,93,97,98 Treatment of DKA does not differ from treatment given in the nonpregnant state except that fetal viability and well-being should be assessed.
The timing and mode of delivery depend on whether obstetric or maternal complications exist. In the absence of suspected problems, vaginal delivery at term is recommended. Elective delivery is indicated in the setting of poor metabolic control, significant diabetic complications, and fetal macrosomia with suspected birth weight greater than 4500 g.92 Delivery is timed to avoid fetal growth into the macrosomic range while allowing sufficient fetal lung maturity. If corticosteroids are administered, close glucose monitoring is required because insulin requirements are likely to increase. Pregnancy progression beyond term is not recommended.
Gestational Diabetes
GDM is defined as glucose intolerance that begins (or is first detected) during pregnancy. As the incidences of both NIDDM and IDDM have increased, so has that of gestational disease.103 It is important to detect and to address glycemic disorders because maternal glucose intolerance increases the likelihood of adverse perinatal outcomes, such as fetal macrosomia and maternal hypertensive disorders, even at glycemic levels that are considered below the threshold for diagnosis of diabetes.104–107 GDM is also a risk factor for the subsequent development of nongestational diabetes; an increased risk is seen with obesity, higher HbA1c values in pregnancy, advanced age at time of GDM diagnosis, lower beta cell function, and need for insulin dosing in late pregnancy.108–111 It is recommended that all postpartum patients with GDM be educated about diet and exercise and undergo routine postnatal screening at repeated intervals long term.108,109,112
The exact protocol to follow for the diagnosis of GDM and preexisting, undiagnosed diabetes mellitus is controversial. It is recommended that pregnant patients undergo screening at the beginning of prenatal care in an effort to identify those patients who are at risk for GDM and pregestational diabetes mellitus to minimize adverse pregnancy outcome and progression of diabetic complications.105 However, it remains unclear whether universal laboratory testing at the first prenatal visit (as opposed to clinical screening) is cost-effective or if this type of screening should be applied only to those populations with a high prevalence of diabetes.105 The International Association of the Diabetes and Pregnancy Study Groups recommends laboratory screening of all women (if prevalence of disease is high in the population being tested) or only high-risk women at the first prenatal visit with subsequent testing at 24 to 28 weeks’ gestation for all women unless they have already been diagnosed with a glycemic disorder.105 The American Diabetes Association advocates selective laboratory assessment as soon as possible in gestation for patients at high risk for GDM. This group includes patients older than 25 years; certain high-risk ethnic groups; and patients with obesity, known first-degree relatives with diabetes, personal history of diabetes or glucose intolerance, and personal history of poor pregnancy outcome.109 Average-risk women and those high-risk women not diagnosed with GDM early in pregnancy then undergo laboratory screening at 24 to 28 weeks’ gestation.111 On the other hand, the American College of Obstetricians and Gynecologists recommends universal laboratory screening.108
The majority of patients with GDM achieve metabolic control with dietary therapy. Traditionally, insulin administration is indicated if dietary control is unsuccessful108,109 (see Table 179-1). Standard practice has been to avoid oral medications because of concerns for placental transfer. However, glyburide has minimal transfer, and studies comparing insulin with glyburide in GDM found that glyburide provided similar glycemic control without an increase in adverse pregnancy outcomes.110,113,114 A summary from the Fifth International Workshop-Conference on GDM noted that glyburide may be considered in patients with GDM as long as care is taken to prevent maternal hypoglycemia.115 Similarly, initial investigations of metformin in the setting of GDM have not noted any increase in perinatal complications, although some patients taking these agents have required supplemental insulin.101,116 At this time, however, further study is recommended before its widespread use.115 There are limited data for other oral medications.
Thyroid Disorders
Hyperthyroidism
There are several obstetric concerns for both the mother and the fetus in the setting of untreated clinical hyperthyroidism (see Table 179-1).117 Thyroid storm is the most serious manifestation of the disease. It may be precipitated by stressors such as infection and delivery, and it is manifested with fever, dysrhythmias, myocardial dysfunction, mental status changes, and circulatory collapse. In addition to the more general complications stemming from thyroid hormone excess, Graves’ disease places the fetus at risk for autoimmune-mediated thyroid dysfunction through placental transfer of maternal thyroid-stimulating immunoglobulins. Up to 17% of neonates of mothers with Graves’ disease and positive thyroid-stimulating immunoglobulin values have transient hyperthyroidism lasting 3 to 12 weeks. The condition gradually clears as maternal antibodies are metabolized.118 Manifestations are potentially severe and include irritability, tachycardia, goiter, cardiomegaly, congestive heart failure, premature craniosynostosis, low birth weight, and failure to thrive.119 These infants also have an increased mortality rate, so routine thyroid assessment is indicated in all infants born to mothers with autoimmune thyroid disease.120
The mainstay of treatment of hyperthyroidism (see Table 179-1) consists of antithyroid drugs. Propylthiouracil is preferred to methimazole because of an increased potential for adverse congenital drug effects from methimazole.120,121 However, both medications cross the placenta and can stimulate the fetal thyroid, so maternal thyroid function should be assessed periodically during pregnancy with the goal of keeping free thyroxine in the high-normal range.120 Most patients respond to pharmacologic manipulation, although thyroidectomy may be considered in severe cases in which patients cannot tolerate antithyroid medication or in the setting of medication failure.120 Use of iodine-131 radionuclide to ablate the maternal thyroid is contraindicated because it will also destroy the fetal thyroid gland.
Additional therapy with beta-blockade to mitigate the hemodynamic effects of sympathetic stimulation may be required in certain cases pending adequate disease control with antithyroid medications. In the nonpregnant patient, iodides may be used to transiently block the release of stored T4 from the thyroid gland and to inhibit organification of iodide. However, the fetal thyroid is extremely sensitive to iodide, which may result in neonatal goiter and hypothyroidism. Therefore, iodide is considered class D in pregnancy, and its use should be reserved for severe cases with duration of therapy limited to a period of days. As with other autoimmune conditions, transient improvement of Graves’ disease during pregnancy is common, with rebound and clinical deterioration occurring in a majority of patients after delivery.122
Postpartum Thyroiditis: Postpartum thyroiditis is a relatively common condition that develops in approximately 4 to 8% of parturients within 9 months of delivery.117,123,124 The risk for development of postpartum thyroiditis is increased in patients with diabetes as well as in patients who have thyroid peroxidase antibodies.120 Patients experience transient hyperthyroidism, transient hypothyroidism, or transient hyperactivity followed by transient hypoactivity. Symptoms are typical for the type of impairment. The need for medication seems to be confined to those patients with postpartum hypothyroidism123; thyroid replacement is recommended for patients who are symptomatic or who have a markedly elevated TSH. Hypothyroid patients trying to conceive another pregnancy should be treated as well.120 On occasion, patients with transient hyperthyroidism require a short course of beta-blockers, but antithyroid drugs are not helpful because the pathophysiologic process relates to release of preformed thyroid rather than to excess thyroid hormone production. Approximately 30% of patients with postpartum thyroiditis develop permanent thyroid failure, and this percentage is increased to 50% or more in patients with postpartum hypoactivity.124,125
Hypothyroidism
As with hyperthyroidism, there is an increased incidence of adverse maternal and fetal effects in women with clinical hypothyroidism (see Table 179-1).117,126 The majority of patients who are already undergoing treatment for hypothyroidism will require an increased dosage of levothyroxine during pregnancy, and close monitoring of thyroid function is recommended as soon as possible after conception.120,127,128 Treatment of newly diagnosed patients consists of prompt initiation of thyroid hormone replacement with the goal being to achieve a normal TSH level.117,120
Approximately 3 to 5% of women of childbearing age have subclinical hypothyroidism,129 as defined by an elevated TSH level and a normal T4 level.130 It has been noted that clinical and subclinical hypothyroidism result in adverse perinatal outcomes and adverse neurologic outcomes for affected infants.120,130 One study also found an increased risk of placental abruption and preterm delivery in subclinical hypothyroidism, and the authors suggested that premature birth may have a direct impact on cognitive outcome in affected infants.131 Conversely, another more recent analysis found no increase in adverse outcomes in subclinical hypothyroidism.132
The issue of whether to provide routine prenatal screening for hypothyroidism remains controversial. At present, both the American College of Obstetricians and Gynecologists and the Endocrine Society note that there is insufficient evidence to confirm that diagnosis and treatment of subclinical disease significantly improve perinatal neurologic outcomes.120,130 These organizations state that screening should include symptomatic patients and those at risk for disease (prior thyroid diagnoses or other endocrine or autoimmune problems).120,130 On the other hand, other sources recommend assessment of TSH levels in all women before conception or as soon as possible after becoming pregnant because targeted screening may miss up to one third of affected women.133,134
Congenital hypothyroidism, associated with severe mental retardation, occurs in approximately 4 in 10,000 births in the United States.135 It is most often the result of sporadic fetal thyroid dysgenesis or ectopy rather than maternal thyroid dysfunction.136 Diagnosis is often difficult because clinical signs and symptoms may be masked by maternal thyroid hormone. Congenital hypothyroidism is successfully treated with hormone replacement begun in the first days of life, and screening for congenital hypothyroidism is routinely performed in most developed countries.
Systemic Infections
Acquisition and presentation of tuberculosis are unchanged during pregnancy. However, the effect of tuberculosis on pregnancy is unclear. Some studies reveal an increase in gestational complications137–139 (see Table 179-1), but these outcomes are likely to be significantly influenced by the site of disease and specifics of treatment. In addition, conclusions are limited by the fact that these studies are often small or performed retrospectively. Other small studies found no significant increase in gestational complications in 32 and 111 pregnant patients with properly treated tuberculosis.140,141 Complications are more likely in patients with inadequate or delayed treatment, delayed diagnosis, and extrapulmonary (extranodal) tuberculosis.137,138 Neonatal tuberculosis acquired by exposure to undiagnosed and untreated active disease places infants at significant risk for acquiring tuberculosis during the first year of life, with significant mortality. In addition, congenital tuberculosis is possible after the fetus becomes infected through the placenta or aspiration of infected amniotic fluid. The latter is rare if the mother has received appropriate therapy.
Current recommendations are to administer a tuberculin skin test early in pregnancy to all patients at high risk for disease and to obtain a chest radiograph if the purified protein derivative (PPD) skin test response is positive or if the patient’s signs and symptoms suggest tuberculosis. Universal screening should be considered in urban areas with a high prevalence of disease. Definitive treatment is indicated in all pregnant patients with confirmed tuberculosis as well as in those high-risk women with suspected disease (see Table 179-1).142 Isoniazid, ethambutol, and rifampin in their usual doses have not been shown to be teratogenic to human fetuses and are acceptable during pregnancy and breast-feeding.143 On the other hand, streptomycin causes fetal ototoxicity, and little is known about the safety of other second-line agents during pregnancy. These less commonly used agents should be avoided except in the case of multidrug-resistant disease (MDR-TB). One retrospective study of 38 gravidas with MDR-TB found no significant increase in perinatal complications, and even patients with drug-resistant disease can be considered for continuation of pregnancy.144
HIV Infection and AIDS
Human immunodeficiency virus (HIV) infection is one of the leading health problems in pregnancy. In 2008, 25% of reported cases of HIV infection and acquired immunodeficiency syndrome (AIDS) in the United States were in women, with a significant percentage being in women of childbearing age.145 Estimates of the seroprevalence of HIV infection in pregnant women vary on a regional basis. In the United States, the overall prevalence and number of perinatally infected infants are low but also vary according to the population, with increased rates seen in the southern states and in the black and Hispanic populations.145,146
The mechanism of vertical transmission is multifactorial. The majority of cases are thought to occur during delivery through exposure to maternal blood and secretions; other infants are likely to be infected in utero or through breast-feeding. Various factors influence the rate of transmission. The most important is maternal viral load, although infection can occur even with low maternal viral load.147–149 Other contributing factors for transmission include vaginal infections, injection drug use, premature delivery, low birth weight, and prolonged rupture of membranes.147,149–151 Vertical transmission of HIV in the United States and other developed countries has declined significantly since its peak in the early 1990s because of the implementation of a number of interventions, including routine voluntary testing, antiretroviral therapy (ART), use of elective cesarean section, and avoidance of breast-feeding.152 However, vertical transmission remains a concern in women without adequate prenatal care as well as a global concern. It is estimated that perinatal infection occurs in approximately 20% of deliveries if the mother is untreated, whereas the previously mentioned interventions reduce this rate to less than 1 or 2%.149,150,152–154 Because of this beneficial effect, it is recommended that all pregnant women undergo screening during their routine prenatal evaluation, with repeated screening in the third trimester for women engaging in high-risk behavior or those who have concurrent sexually transmitted diseases (STDs). Rapid HIV screening is recommended for women in labor whose HIV status is unknown.147,155,156
Treatment of the pregnant patient with HIV infection includes appropriate ART as well as standard therapy for opportunistic infections (see Table 179-1).147,157 There are limited data on specific therapy for opportunistic infections during pregnancy, but the practitioner should take into account the fact that medication clearance may be affected by various pregnancy-related changes, including increased renal clearance, dilutional anemia, and fetal metabolism of medications.157 Periodic ultrasonographic assessments of fetal growth are indicated if required medications have an unknown safety profile in pregnancy.
Optimal therapy to prevent vertical transmission includes three stages: antepartum administration of highly active antiretroviral therapy (HAART), intrapartum intravenous zidovudine dosing, and treatment of the infant with 4 to 6 weeks of zidovudine.147,158 The specific ART regimen to follow depends on a number of variables. In patients who are already taking ART with good disease control (viral load < 1000 copies/mL) at the time or pregnancy diagnosis, the current medication regimen should be continued with the exception of efavirenz, which should be discontinued.147 Women who have never received ART are started on a standard multidrug HARRT regimen that avoids use of efavirenz in the first trimester. Delayed initiation of HAART until the second trimester may be considered in women if the only goal of therapy is to prevent mother-to-child transmission (maternal viral load < 1000 copies/mL).147,158 In HIV-infected mothers without prenatal care, intrapartum ART followed by postexposure zidovudine treatment of the infant reduces the likelihood of infection but is less effective than the recommended three-stage regimen.147 Elective cesarean section is recommended in mothers with a viral load greater than 1000 copies/mL.147 It is reasonable to present cesarean delivery as an option for patients with lower viral loads, although the benefit for these lower risk mothers in uncertain,147,159 and the risk of mother-to-child transmission by vaginal delivery is very low when mothers have received effective HAART.148,160
Whereas breast-feeding is associated with mother-to-child transmission of HIV infection and is not recommended in urban areas with access to formula preparations, it remains the preferred mode of feeding in resource-poor areas where formula use is not possible. Studies have shown that continued infant prophylaxis with ART is effective in reducing mother-to-child transmission through breast-feeding, with the absolute risk of transmission being very low.161,162 Exclusive breast-feeding has also been shown to confer a mortality benefit and to reduce the risk of acquired infections,163,164 and the World Health Organization now encourages exclusive breast-feeding in women when adequate and safe formula-feeding is not possible.165 Ideally, breast-feeding mothers should be maintained with ART. If ART is not available, breast-feeding is preferable when replacement nutrition is not an option.165
Even in the setting of ART, commonly used serologic tests to detect antibodies for HIV yield positive results in virtually all neonates born to HIV-positive mothers because of placental transfer of maternal antibodies. These antibodies may be present for up to 18 months and are not necessarily indicative of infection. The preferred means of diagnosis of perinatal HIV infection is by use of assays to detect viral RNA or DNA. Infants are considered noninfected if there have been two negative viral assays after 1 month of age, with one of the assays being done after 4 months of age, or with negative results of two antibody tests done on separate occasions after 6 months of age. In addition, there should be no clinical or other laboratory findings to suggest infection.166 Clinical assessment for possible HIV infection is also warranted. Infants with AIDS typically present with recurrent bacterial infections, Pneumocystis pneumonia, encephalopathy, extrapulmonary tuberculosis, and generalized wasting. Recent analysis reveals that HAART therapy in infants and children is extremely effective in reducing HIV-related morbidity and mortality.167
The effects of pregnancy in women with symptomatic HIV infection include an increased incidence of infant mortality, prematurity, stillbirth, and low birth weight and also gestational diabetes.164,168,169 Seropositive mothers who undergo cesarean delivery generally have an uncomplicated postoperative course, although some studies note a slightly increased risk of postpartum endometritis and other maternal infections, with the highest rate of infection seen in women who have low CD4+ cell counts.170–172 It is also important to consider the effect of ART on pregnancy outcomes. It is recommended that efavirenz be avoided in the first trimester of pregnancy because of potential teratogenicity and fetal anomalies.147,173–175 Overall, however, most antiretroviral medications are considered safe in this regard. Several large studies have noted no increase in adverse outcomes in women taking ART, with the possible exception of preterm delivery, although this remains a matter of controversy.176–178
The effect of pregnancy is unclear. An observational cohort study in which a majority of women were using HAART found that pregnancy had no impact on the rate of disease progression.179 Additional studies are necessary for definitive conclusions to be drawn.
Syphilis
The incidence of primary and secondary syphilis among reproductive-age women in the United States increased during 2004 through 2008, with a current rate of 1.5 cases per 100,000 women.180 Unfortunately, the incidence of congenital syphilis has shown a similar increase from 2005 to 2008, with a current rate of 8.2 per 100,000 live births.181 Of all the congenital syphilis cases for 2008, half were born to black mothers and an additional 30% to Hispanic mothers, highlighting the need for better screening in these high-risk populations.181
Syphilis causes numerous gestational complications (see Table 179-1), but its most significant sequela is congenital syphilis. This syndrome is characterized by clinical abnormalities such as hepatosplenomegaly, osteochondritis, jaundice, rash, lymphadenopathy, rhinitis, Hutchinson’s teeth, and anemia. Infant mortality for cases occurring in 2008 was 6.5%.181 Screening for all pregnant patients is indicated at the first prenatal visit and again in the early third trimester and at delivery for high-risk patients156 because vertical transmission rates can be reduced significantly with appropriate penicillin therapy.156,182 Either the Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test can be used to detect nontreponemal antibody. Pregnancy may cause false-positive results for nontreponemal studies, so confirmation with specific treponemal tests is indicated for a positive VDRL or RPR study. Patients with latent syphilis and those whose titers fail to respond to therapy should undergo cerebrospinal fluid analysis to screen for tertiary syphilis. In addition, fetal ultrasonography before the 20th week of gestation is indicated to assess for abnormalities consistent with congenital syphilis.156
Treatment is identical to that given to nonpregnant patients with use of benzathine penicillin G appropriate for the disease stage156 (see Table 179-1). Penicillin-allergic patients are sometimes treated with macrolides.182 However, it is preferable that these patients undergo skin testing and desensitization if the skin test result is positive because alternative therapy is not reliably effective in preventing congenital syphilis.156,183,184 Treatment failures are rare with appropriately administered penicillin but do occur. Failures leading to congenital syphilis are more likely in mothers with secondary syphilis, high VDRL or RPR levels, and an interval from treatment to delivery of less than 30 days.156,182,184,185
Hepatitis
Hepatitis B: The prevalence of hepatitis B virus (HBV) infection among pregnant women varies by the population studied, with the highest rates seen in Asians and women of African descent.186,187 The rate of vertical transmission depends on the acuity of maternal infection and when it occurs during the gestation. Perinatal transmission is approximately 10 to 20% in women seropositive for HBsAg alone but approaches 90% in mothers who are seropositive for HBsAg and HBeAg, and it is also more likely if the mother has acute infection during the third trimester.188 Of infants who have HBV infection, up to 90% become chronic carriers as adults and are at risk for complications such as cirrhosis and hepatocellular carcinoma.188
Routine screening for HBV during early pregnancy is recommended because treatment with hepatitis B immune globulin and hepatitis B vaccine is effective in reducing the rate of vertical transmission.156,188–190 Repeated testing is indicated at the time of delivery for women at high risk for infection.156 Although these therapies are effective in reducing neonatal infection, treatment failures do occur in the setting of maternal seropositivity for HBeAg and high viral loads of HBV DNA.191,192 Studies suggest that lamivudine given in late pregnancy to these high-risk gravidas reduces viral transmission when it is given in conjunction with HBV vaccine and immune globulin.193,194 Vaccination against HBV is currently recommended for all infants in the United States. The treatment schedule and the need for additional therapy with immune globulin vary according to maternal seropositivity. Infants of HBsAg-positive mothers should receive hepatitis B immune globulin and the first dose of vaccine within 12 hours of birth. Two additional doses of vaccine are administered at a later date.188,195 Pregnancy is not a contraindication to either therapy. All HBsAg-negative gravidas at risk for HBV infection or other STDs or who are seeking STD treatment should receive the vaccine.156 Pregnant patients who are exposed to HBV should receive both hepatitis B immune globulin and vaccine.
Hepatitis C: As with HBV, the prevalence of hepatitis C virus (HCV) infection among pregnant women varies by the population, ranging from less than 1% to approximately 4%. Vertical transmission is rare in mothers with anti-HCV antibodies and no circulating HCV RNA. However, perinatal transmission is significantly increased by the presence of HCV viremia, occurring in approximately 4 to 6% of cases.196 The transmission rate is even higher in the setting of coinfection with HIV.197,198 Unfortunately, cesarean delivery has not been shown to prevent HCV transmission.156,188,197 No available vaccine or immune globulin exists to prevent hepatitis C, and routine prenatal screening is not indicated.156
Inflammatory Disorders
Systemic Lupus Erythematosus
SLE primarily affects women of reproductive age, and fertility is usually unaffected. The disease course during pregnancy varies, but studies indicate that acute flares occur in less than one third of patients in clinical remission at the time of conception.199–201 Disease activity tends to involve the skin and musculoskeletal system, although renal involvement is not uncommon, especially in those patients with active lupus nephritis.202–204 The gestational effects of SLE also depend on the underlying severity of disease. Patients with active disease, especially lupus nephritis, have a higher incidence of disease flares and pregnancy complications,199,201,203–205 and it is best for women to achieve good disease control before pregnancy. Many patients have acceptable outcomes, but lupus pregnancies are associated with an increased maternal mortality as well as an increased rate of other complications, including preeclampsia, preterm delivery, thrombocytopenia, anemia, intrauterine growth retardation, fetal loss, and need for cesarean section.199,205–207 Other comorbid conditions that adversely affect pregnancy, such as diabetes and thrombophilia, are also seen more commonly in SLE patients.207 The risk of preeclampsia is especially increased in patients with active lupus nephritis.201,203 As with other renal disease, increasing proteinuria warrants a careful evaluation to distinguish between lupus glomerulonephritis and preeclampsia. The presence of abnormal urine sediment, increasing titers of anti-DNA antibody, and decreasing levels of C3 and C4 suggest lupus nephritis.
Other Rheumatologic Diseases
RA is characterized by chronic, destructive, symmetrical joint inflammation. Less common manifestations include the development of subcutaneous nodules, neuropathy, pleuropericarditis, and vasculitis. Systemic symptoms, including weight loss, lymphadenopathy, and fatigue, are common. Because the median age at onset is later with RA, this disorder is seen less frequently than SLE in the pregnant population.208 Disease improvement has classically been estimated to occur in approximately two thirds of patients with RA. However, a prospective study noted disease improvement in about half of pregnant patients, with almost 40% of patients experiencing a subsequent exacerbation after delivery.209
Patients with RA tend to have good pregnancy outcomes in the setting of well-controlled disease. Those women with active disease are more likely to have small-for-gestational-age infants,210 possibly as a result of underlying vasculopathy and associated effects on the placenta.210
Treatment
The rheumatologic diseases are similar with respect to the therapeutic approach. Corticosteroids form the basis of treatment for most disease complications and exacerbations. These drugs may result in a minimally increased risk of cleft deformities (data are inconclusive) but have not been found to have any other significant teratogenic effects and are considered relatively safe to use for disease control in pregnant patients.211,212 Steroid use during pregnancy does have a dose-related effect on intrauterine growth retardation and predisposes the patient to GDM and hypertension, so close monitoring is advised.
Aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) are also a mainstay of therapy in many patients with rheumatic diseases and may be used in pregnancy. There are potential adverse effects of aspirin and NSAIDs on gestation, including miscarriage when they are taken early in pregnancy, premature closure of the fetal ductus arteriosus, increased maternal hemorrhage, and prolongation of gestation and labor when they are taken later in pregnancy. There are also conflicting data about an increased risk for gastroschisis, cleft defects, and cardiac defects with NSAID use.213–217 If a true association exists, the risk for these anomalies remains extremely small in absolute terms. Use of these agents after 32 weeks of gestation should be avoided to minimize the risks of maternal and fetal hemorrhage and premature ductus closure.
Acute flares often require institution of cytotoxic drugs. Cyclophosphamide and methotrexate are contraindicated during the first trimester and should be used only in extreme circumstances because of their teratogenicity and abortifacient properties. Azathioprine has a much more favorable safety profile, and it is the cytotoxic agent of choice during pregnancy. Although the drug and its metabolites cross the placenta, it does not appear to have any major teratogenic effects.218,219 There is an association between azathioprine use and preterm delivery and low birth weight, but it is likely that these adverse outcomes are also related to the underlying disease.219,220 Cyclosporine also seems to lack teratogenicity and is an acceptable alternative to azathioprine.221
The antimalarial agents chloroquine and hydroxychloroquine are also useful in the treatment of SLE and RA and have been found to be safe during pregnancy. Gravis patients using antimalarials have a decrease in lupus activity, and as a result, these agents may allow a decrease in corticosteroid dosage.222,223 In addition, patients who have been maintained with hydroxychloroquine before conception experience an increased rate of disease flares when the agent is discontinued.222
There are limited numbers of pregnant patients using the newer anti–tumor necrosis factor medications, such as infliximab and etanercept. A review of the Food and Drug Association database noted an increase in congenital malformations with anti–tumor necrosis factor agents.224 However, other data suggest that these agents have an acceptable risk profile and that they may be continued in pregnancy.225,226 Because the safety of these medications remains uncertain, their use should be reserved for patients with severe disease who are not adequately treated by other more established medications.225
References
1. Kwon, HL, et al. Asthma prevalence among pregnant and childbearing-aged women in the United States: Estimates from national health surveys. Ann Epidemiol. 2003;13:317.
2. Schatz, M, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112:283.
3. Dombrowski, MP, Schatz, M. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists number 90, February 2008: Asthma in pregnancy. Obstet Gynecol. 2008;111:457.
4. Bracken, MB, et al. Asthma symptoms, severity, and drug therapy: A prospective study of effects on 2205 pregnancies. Obstet Gynecol. 2003;102:739.
5. Dombrowski, MP, et al. Asthma during pregnancy. Obstet Gynecol. 2004;103:5.
6. Tata, LJ, et al. A comprehensive analysis of adverse obstetric and pediatric complications in women with asthma. Am J Respir Crit Care Med. 2007;175:991.
7. Bakhireva, LN, et al. Asthma control during pregnancy and the risk of preterm delivery or impaired fetal growth. Ann Allergy Asthma Immunol. 2008;101:137.
8. Blais, L, Forget, A. Asthma exacerbations during the first trimester of pregnancy and the risk of congenital malformations among asthmatic women. J Allergy Clin Immunol. 2008;121:1379.
9. Enriquez, R, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120:625.
10. National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP Expert Panel Report. Managing asthma during pregnancy: Recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol. 2005;115:34.
11. Blais, L, et al. High doses of inhaled corticosteroids during the first trimester of pregnancy and congenital malformations. J Allergy Clin Immunol. 2009;124:1229.
12. Bakhireva, LN, et al. Safety of leukotriene receptor antagonists in pregnancy. J Allergy Clin Immunol. 2007;119:618.
13. Lindheimer, M, et al. ASH Position Paper: Hypertension in pregnancy. J Clin Hypertens. 2009;11:214.
14. Podymow, T, August, P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960.
15. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1.
16. Abalos, E, et al, Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007;(24):CD002252.
17. National Institutes of Health. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md: U.S. Department of Health and Human Services; August 2004.
18. Roberts, JM, et al. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437.
19. Goland, S, et al. Favorable outcome of pregnancy with an elective use of epoprostenol and sildenafil in women with severe pulmonary hypertension. Cardiology. 2010;115:205.
20. Kiely, DG, et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG. 2010;117:565.
21. Bédard, E, et al. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. 2009;30:256.
22. James, AH, et al. Acute myocardial infarction in pregnancy: A United States population-based study. Circulation. 2006;113:1564.
23. Ladner, HE, et al. Acute myocardial infarction in pregnancy and the puerperium: A population-based study. Obstet Gynecol. 2005;105:480.
24. Roth, A, Elkayam, U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52:171.
25. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 276: Safety of Lovenox in pregnancy. Obstet Gynecol. 2002;100:845.
26. Leonhardt, G, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis. 2006;21:271.
27. Te Raa, GD, et al. Treatment options in massive pulmonary embolism during pregnancy: A case-report and review of literature. Thromb Res. 2009;124:1.
28. Task Force on the Management of Cardiovascular Diseases During Pregnancy of the European Society of Cardiology. Expert consensus document on management of cardiovascular diseases during pregnancy. Eur Heart J. 2003;24:761.
29. Bonow, RO, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;118:e523.
30. Elkayam, U, Bitar, F. Valvular heart disease and pregnancy: Part I: Native valves. J Am Coll Cardiol. 2005;46:223.
31. Yap, SC, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol. 2008;126:240.
32. Elkayam, U, Bitar, F. Valvular heart disease and pregnancy: Part II: Prosthetic valves. J Am Coll Cardiol. 2005;46:403.
33. Ren, A, et al. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynaecol Obstet. 2007;98:124.
34. Kidanto, HL, et al. Risks for preterm delivery and low birth weight are independently increased by severity of maternal anaemia. S Afr Med J. 2009;99:98.
35. Ronnenberg, AG, et al. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. J Nutr. 2004;134:2586.
36. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: Anemia in pregnancy. Obstet Gynecol. 2008;112:201.
37. Rohilla, M, et al. Severe anemia in pregnancy: A tertiary hospital experience from northern India. J Obstet Gynaecol. 2010;30:694.
38. Peña-Rosas, JP, Viteri, FE. Effects and safety of preventive oral iron or iron + folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev. (7):2009.
39. Cogswell, ME, et al. Iron supplementation during pregnancy, anemia, and birth weight: A randomized controlled trial. Am J Clin Nutr. 2003;78:773.
40. Siega-Riz, AM, et al. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: A randomized controlled trial. Am J Obstet Gynecol. 2006;194:512.
41. Ziaei, S, et al. A randomized placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG. 2007;114:684.
42. Von Tempelhoff, GF, et al. Mean maternal second-trimester hemoglobin concentration and outcome of pregnancy: A population-based study. Clin Appl Thromb Hemost. 2008;14:19.
43. Van Eijsden, M, et al. Association between short interpregnancy intervals and term birth weight: The role of folate depletion. Am J Clin Nutr. 2008;88:147.
44. Berry, RJ, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485.
45. De-Regil, LM, et al, Effects and safety of preconceptual folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2010;(6):CD007950.
46. American College of Obstetricians and Gynecologists. Nutrition in Pregnancy. www.acog.org/publications/patient_education/bp001.cfm.
47. ACOG Committee on Obstetrics. ACOG Practice Bulletin No. 78: Hemoglobinopathies in pregnancy. Obstet Gynecol. 2007;109:229.
48. Al Jama, FE, et al. Pregnancy outcome in patients with homozygous sickle cell disease in a university hospital, Eastern Saudi Arabia. Arch Gynecol Obstet. 2009;280:793.
49. Ngô, C, et al. Pregnancy in sickle cell disease: Maternal and fetal outcomes in a population receiving prophylactic partial exchange transfusions. Eur J Obstet Gynecol Reprod Biol. 2010;152:138.
50. Sun, PM, et al. Sickle cell disease in pregnancy: Twenty years of experience at Grady Memorial Hospital, Atlanta, Georgia. Am J Obstet Gynecol. 2001;184:1127.
51. Villers, MS, et al. Morbidity associated with sickle cell disease in pregnancy. Am J Obstet Gynecol. 2008;199:125.
52. Yu, CK, et al. Outcome of pregnancy in sickle cell disease patients attending a combined obstetric and haematology clinic. J Obstet Gynaecol. 2009;29:512.
53. Serjeant, GR, et al. Outcome of pregnancy in homozygous sickle cell disease. Obstet Gynecol. 2004;103:1278.
54. The EURAP Study Group. Seizure control and treatment in pregnancy: Observations from the EURAP Epilepsy Pregnancy Registry. Neurology. 2006;66:354.
55. Eroglu, E, et al. Pregnancy and teratogenicity of antiepileptic drugs. Acta Neurol Belg. 2008;108:53.
56. Mawer, G, et al. Pregnancy with epilepsy: Obstetric and neonatal outcome of a controlled study. Seizure. 2010;19:112.
57. Borthen, I, et al. Delivery outcome of women with epilepsy: A population-based cohort study. BJOG. 2010;117:1537.
58. Morrow, J, et al. Malformation risks of antiepileptic drugs in pregnancy: A prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193.
59. Vajda, FJ, et al. Foetal malformations and seizure control: 52 months data of the Australian Pregnancy Registry. Eur J Neurol. 2006;13:645.
60. Cunnington, M, et al. Effect of dose on the frequency of major birth defects following fetal exposure to lamotrigine monotherapy in an international observational study. Epilepsia. 2007;48:1207.
61. Hunt, S, et al. Levetiracetam in pregnancy: Preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology. 2006;67:1876.
62. Longo, B, et al. Levetiracetam use in pregnancy. Ann Pharmacother. 2009;43:1692.
63. Harden, CL, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50:1247.
64. Kaaja, E, et al. Enzyme-inducing antiepileptic drugs in pregnancy and the risk of bleeding in the neonate. Neurology. 2002;58:549.
65. Vukusic, S, et al. Pregnancy and multiple sclerosis (the PRIMS study): Clinical predictors of post-partum relapse. Brain. 2004;127:1353.
66. D’hooghe, MB, et al. Long-term effects of childbirth in MS. J Neurol Neurosurg Psychiatry. 2010;81:38.
67. Achiron, A, et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004;251:1133.
68. Haas, J, Hommes, OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007;13:900.
69. Hellwig, K, et al. Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disord. 2009;2:7.
70. De Seze, J, et al. Intravenous corticosteroids in the postpartum period for reduction of acute exacerbations in multiple sclerosis. Mult Scler. 2004;10:596.
71. Jalkanen, A, et al. Pregnancy outcome in women with multiple sclerosis: Results from a prospective nationwide study in Finland. Mult Scler. 2010;16:950.
72. Chen, YH, et al. Does multiple sclerosis increase risk of adverse pregnancy outcomes? A population-based study. Mult Scler. 2009;15:606.
73. Ghidini, A, et al. Pregnancy and women with spinal cord injuries. Acta Obstet Gynecol Scand. 2008;87:1006.
74. Skowronski, E, Hartman, K. Obstetric management following traumatic tetraplegia: Case series and literature review. Aust N Z J Obstet Gynaecol. 2008;48:485.
75. American College of Obstetricians and Gynecologists. ACOG Committee Opinion: Number 275, September 2002. Obstetric management of patients with spinal cord injuries. Obstet Gynecol. 2002;100:625.
76. Almeida, C, et al. Myasthenia gravis and pregnancy: Anaesthetic management—a series of cases. Eur J Anaesthesiol. 2010;27:985.
77. Djelmis, J, et al. Myasthenia gravis in pregnancy: Report on 69 cases. Eur J Obstet Gynecol. 2002;104:21.
78. Téllez-Zenteno, JF, et al. Myasthenia gravis and pregnancy: Clinical implications and neonatal outcome. BMC Musculoskelet Disord. 2004;5:42.
79. Wen, JC, et al. No increased risk of adverse pregnancy outcomes for women with myasthenia gravis: A nationwide population-based study. Eur J Neurol. 2009;16:889.
80. Hoff, JM, et al. Myasthenia gravis in pregnancy and birth: Identifying risk factors, optimising care. Eur J Neurol. 2007;14:38.
81. Bahadi, A, et al. Pregnancy during hemodialysis: A single center experience. Saudi J Kidney Dis Transpl. 2010;21:646.
82. Chou, CY, et al. Pregnancy in patients on chronic dialysis: A single center experience and combined analysis of reported results. Eur J Obstet Gynceol Reprod Biol. 2008;136:165.
83. Fischer, MJ, et al. Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis. 2004;43:415.
84. Imbasciati, E, et al. Pregnancy in CKD stages 3 to 5: Fetal and maternal outcomes. Am J Kidney Dis. 2007;49:753.
85. Luders, C, et al. Obstetric outcome in pregnant women on long-term dialysis: Case series. Am J Kidney Dis. 2010;56:77.
86. Piccoli, GB, et al. Pregnancy in dialysis patients: Is the evidence strong enough to lead us to change our counseling policy? Clin J Am Soc Nephrol. 2010;5:62.
87. Piccoli, GB, et al. Pregnancy and chronic kidney disease: A challenge in all CKD stages. Clin J Am Soc Nephrol. 2010;5:844.
88. Tan, LK, et al. Obstetric outcomes in women with end-stage renal failure requiring dialysis. Int J Gynaecol Obstet. 2006;94:17.
89. Balsells, M, et al. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: A systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94:4284.
90. Garcia-Patterson, A, et al. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: Three changes of direction. Diabetologia. 2010;53:446.
91. Kitzmiller, JL, et al. Managing preexisting diabetes for pregnancy: Summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31:1060.
92. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Pregestational diabetes mellitus. Number 60, March 2005. Obstet Gynecol. 2005;105:675.
93. Gonzalez-Quintero, VH, et al. The impact of glycemic control on neonatal outcome in singleton pregnancies complicated by gestational diabetes. Diabetes Care. 2007;30:467.
94. Persson, M, et al. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care. 2009;32:2005.
95. Rahman, W, et al. Progression of retinopathy during pregnancy in type I diabetes mellitus. Clin Experiment Ophthalmol. 2007;35:231.
96. Guo, RX, et al. Diabetic ketoacidosis in pregnancy tends to occur at lower blood glucose levels: Case-control study and a case report of euglycemic diabetic ketoacidosis in pregnancy. J Obstet Gynaecol Res. 2008;34:324.
97. American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(Suppl 1):S4.
98. Jensen, DM, et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care. 2009;32:1046.
99. Lapolla, A, et al. A multicenter Italian study on pregnancy outcome in women with diabetes. Nutr Metab Cardiovasc Dis. 2008;18:291.
100. Dhulkotia, JS, et al. Oral hypoglycemic agents vs insulin in management of gestational diabetes: A systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:457.
101. Rowan, JA, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003.
102. Ekpebegh, CO, et al. A 10-year retrospective analysis of pregnancy outcome in pregestational type 2 diabetes: Comparison of insulin and oral glucose-lowering agents. Diabet Med. 2007;24:253.
103. Getahun, D, et al. Gestational diabetes in the United States: Temporal trends 1989 through 2004. Am J Obstet Gynecol. 2008;198:525.
104. Metzger, BE, et al. HAPO Study Cooperative Research Group: Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991.
105. International Association of Diabetes and Pregnancy Study Groups Consensus PanelMetzger, BE, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676.
106. Ferrara, A, et al. Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycemia, and hyperbilirubinaemia. Diabetologia. 2007;50:298.
107. Landon, MB, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;364:1339.
108. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Gestational diabetes. Number 30, September 2001. Obstet Gynecol. 2001;98:525.
109. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2003;26:S103.
110. Ekelund, M, et al. Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia. 2010;53:452.
111. Kim, SH, et al. Nutritional risk factors of early development of postpartum prediabetes and diabetes in women with gestational diabetes mellitus. Nutrition. 2011;27:782.
112. Committee on Obstetric Practice. ACOG Committee Opinion No. 435: Postpartum screening for abnormal glucose tolerance in women who had gestational diabetes. Obstet Gynecol. 2009;113:1419.
113. Moretti, ME, et al. Safety of glyburide for gestational diabetes: A meta-analysis of pregnancy outcomes. Ann Pharmacother. 2008;42:483.
114. Nicholson, W, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes. Obstet Gynecol. 2009;113:193.
115. Metzger, BE, et al. Summary and recommendations of the Fifth International Workshop-Conference on gestational diabetes mellitus. Diabetes Care. 2007;30:S251.
116. Moore, LE, et al. Metformin compared with glyburide in gestational diabetes: A randomized controlled trial. Obstet Gynecol. 2010;115:55.
117. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists. Number 37, August 2002. Thyroid disease in pregnancy. Obstet Gynecol. 2002;100:387.
118. Rosenfeld, H, et al. Pregnancy outcome, thyroid dysfunction and fetal goiter after in utero exposure to propylthiouracil: A controlled study. Br J Clin Pharmacol. 2009;68:609.
119. Peleg, D, et al. The relationship between maternal serum thyroid-stimulating immunoglobulin and fetal and neonatal thyrotoxicosis. Obstet Gynecol. 2002;99:1040.
120. Abalovich, M, et al. Clinical practice guideline: Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1.
121. Clementi, M, et al. Treatment of hyperthyroidism in pregnancy and birth defects. J Clin Endocrinol Metab. 2010;95:E337.
122. Rotondi, M, et al. The effect of pregnancy on subsequent relapse from Graves’ disease after a successful course of antithyroid drug therapy. J Clin Endocrinol Metab. 2008;93:3985.
123. Lucas, A, et al. Postpartum thyroiditis: Long term follow-up. Thyroid. 2005;15:1177.
124. Stagnaro-Green, A, et al. High rate of persistent hypothyroidism in a large-scale prospective study of postpartum thyroiditis in southern Italy. J Clin Endocrinol Metab. 2011;96:652.
125. Azizi, F. The occurrence of permanent thyroid failure in patients with subclinical postpartum thyroiditis. Eur J Endocrinol. 2005;153:367.
126. Sahu, MT, et al. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet. 2010;281:215.
127. Alexander, EK, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241.
128. Hallengren, B, et al. Pregnant women on thyroxine substitution are often dysregulated in early pregnancy. Thyroid. 2009;19:391.
129. Aoki, Y, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211.
130. Committee on Patient Safety and Quality Improvement; Committee on Professional Liability. ACOG Committee Opinion No. 381: Subclinical hypothyroidism in pregnancy. Obstet Gynecol. 2007;110:959.
131. Casey, BM, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239.
132. Cleary-Goldman, J, et al. Maternal thyroid dysfunction and pregnancy outcome. Obstet Gynecol. 2008;112:85.
133. Gharib, H, et al. Consensus statement: Subclinical thyroid dysfunction: A joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90:581.
134. Vaidya, B, et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203.
135. Hinton, CF, et al. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: Data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125:S37.
136. Dias, VM, et al. Congenital hypothyroidism etiology. J Pediatr Endocrinol Metab. 2010;23:815.
137. Jana, N, et al. Perinatal outcome in pregnancies complicated by pulmonary tuberculosis. Int J Gynaecol Obstet. 1994;44:119.
138. Jana, N, et al. Obstetrical outcomes among women with extrapulmonary tuberculosis. N Engl J Med. 1999;341:645.
139. Lin, HC, et al. Increased risk of low birthweight and small for gestational age infants among women with tuberculosis. BJOG. 2010;117:585.
140. Kothari, A, et al. Tuberculosis and pregnancy—results of a study in a high prevalence area in London. Eur J Obstet Gynecol Repod Biol. 2006;126:48.
141. Tripathy, SN, Tripathy, SN. Tuberculosis and pregnancy. Int J Obstet Gynecol. 2003;80:247.
142. American Thoracic Society; CDC; Infectious Diseases Society of America, Treatment of tuberculosis. MMWR Recomm Rep. 2003;(RR-11):52.
143. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603.
144. Palacios, E, et al. Drug-resistant tuberculosis and pregnancy: Treatment outcomes of 38 cases in Lima, Peru. Clin Infect Dis. 2009;48:1413.
145. Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. www.cdc.gov/hiv/surveillance/resources/reports/2008report/pdf/2008SurveillanceReport.pdf, 2008.
146. Centers for Disease Control and Prevention. Racial/ethnic disparities among children with diagnoses of perinatal HIV infection—34 states, 2007-2007. MMWR Morb Mortal Wkly Rep. 2010;59:97.
147. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1–Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf, May 24, 2010.
148. Townsend, CL, et al. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000-2006. AIDS. 2008;22:973.
149. Warszawski, J, et al. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS French Perinatal Cohort. AIDS. 2008;22:289.
150. Garcia-Tejedor, A, et al. Influence of highly active antiretroviral treatment (HAART) on risk factors for vertical HIV transmission. Acta Obstet Gynecol Scand. 2009;88:882.
151. Peters, VB, et al. Trends in perinatal HIV prevention in New York City, 1994-2003. Am J Public Health. 2008;98:1857.
152. Centers for Disease Control and Prevention. Achievements in public health: Reduction in perinatal transmission of HIV infection, 1985-2005. MMWR Morb Mortal Wkly Rep. 2006;55:592.
153. Shapiro, RL, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282.
154. Townsend, CL, et al. Trends in management and outcome of pregnancies in HIV-infected women in the UK and Ireland, 1990-2006. BJOG. 2008;115:1078.
155. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 418: Prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Obstet Gynecol. 2008;112:739.
156. Workowski, KA, Berman, S. Centers for Disease Control and Prevention: Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1.
157. Kaplan, JE, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1.
158. World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants, 2010 version. http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf.
159. Boer, K, et al. Mode of delivery in HIV-infected pregnant women and prevention of mother-to-child transmission: Changing practices in Western Europe. HIV Med. 2010;11:368.
160. Boer, K, et al. The AmRo study: Pregnancy outcome in HIV-1–infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG. 2007;114:148.
161. Kesho Bora Study Group. Eighteen-month follow-up of HIV-1–infected mothers and their children enrolled in the Kesho Bora Study Group. J Acquir Immune Defic Syndr. 2010;54:533.
162. Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV (Kesho Bora study): A randomized controlled trial. Lancet Infect Dis. 2011;11:171.
163. Coovadia, HM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: An intervention cohort study. Lancet. 2007;369:1107.
164. Kurewa, EN, et al. Effect of maternal HIV status on infant mortality: Evidence from 9-month follow-up of mothers and their infants in Zimbabwe. J Perinatol. 2010;30:88.
165. World Health Organization. Guidelines on HIV and Infant Feeding. http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf, 2010.
166. Schneider, E, et al. Centers for Disease Control and Prevention: Revised surveillance: Infection among adults, adolescents, and children aged < 18 months and for HIV infection and AIDS among children aged 18 month to < 13 years—United States, 2008. MMWR Recomm Rep. 2008;57:1.
167. Mahdavi, S, et al. Treatment and disease progression in a birth cohort of vertically-infected children in Ukraine. BMC Pediatr. 2010;10:85.
168. Aebi-Popp, K, et al. Pregnancy and delivery outcomes of HIV infected women in Switzerland. J Perinat Med. 2010;38:353.
169. Martí, C, et al. Obstetric and perinatal complications in HIV-infected women. Analysis of a cohort of 167 pregnancies between 1997 and 2003. Acta Obstet Gynecol Scand. 2007;86:409.
170. Cavasin, H, et al. Postoperative infectious morbidities of cesarean delivery in human immunodeficiency virus–infected women. Infect Dis Obstet Gynecol. 2009;2009:827405.
171. Louis, J, et al. Perioperative morbidity and mortality among human immunodeficiency virus infected women undergoing cesarean delivery. Obstet Gynecol. 2007;110:385.
172. Maiques, V, et al. Perioperative cesarean delivery morbidity among HIV-infected women under highly active antiretroviral treatment: A case-control study. Eur J Obstet Gynecol Reprod Biol. 2010;153:27.
173. Brogly, SB, et al. Birth defects among children born to human immunodeficiency virus–infected women: Pediatric AIDS clinical trials protocols 219 and 219C. Pediatr Infect Dis J. 2010;29:721.
174. Joao, EC, et al. Maternal antiretroviral use during pregnancy and infant congenital anomalies: The NISDI perinatal study. J Acquir Immune Defic Syndr. 2010;53:176.
175. Watts, DH, et al. Birth defects among a cohort of infants born to HIV-infected women on antiretroviral medication. J Perinat Med. 2011;39:163.
176. Briand, N, et al. No relation between in-utero exposure to HAART and intrauterine growth retardation. AIDS. 2009;23:1235.
177. Townsend, C, et al. Antiretroviral therapy and preterm delivery—a pooled analysis of data from the United States and Europe. BJOG. 2010;117:1399.
178. Patel, K, et al. Prenatal protease inhibitor use and risk of preterm birth among HIV-infected women initiating antiretroviral drugs during pregnancy. J Infect Dis. 2010;201:1035.
179. Tai, JH, et al. Pregnancy and HIV disease progression during the era of highly active antiretroviral therapy. J Infect Dis. 2007;196:1044.
180. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance. www.cdc.gov/std/stats08/main.htm, 2008.
181. Centers for Disease Control and Prevention. Congenital syphilis—United States, 2003-2008. MMWR Morb Mortal Wkly Rep. 2010;59:413.
182. Cheng, JQ, et al. Syphilis screening and intervention in 500,000 pregnant women in Shenzhen, the People’s Republic of China. Sex Transm Infect. 2007;83:347.
183. Zhou, P, et al. Occurrence of congenital syphilis after maternal treatment with azithromycin during pregnancy. Sex Transm Dis. 2007;34:472.
184. Sheffield, JS, et al. Congenital syphilis after maternal treatment for syphilis during pregnancy. Am J Obstet Gynecol. 2002;186:569.
185. Zhu, L, et al. Maternal and congenital syphilis in Shanghai, China, 2002 to 2006. Int J Infect Dis. 2010;14(Suppl 3):e45.
186. Lobstein, S, et al. Prevalence of hepatitis B among pregnant women and its impact on pregnancy and newborn complications at a tertiary hospital in the eastern part of Germany. Digestion. 2011;83:76.
187. Salleras, L, et al. Seroepidemiology of hepatitis B virus infection in pregnant women in Catalonia (Spain). J Clin Virol. 2009;44:329.
188. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 86: Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941.
189. Lee, C, et al. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: Systematic review and meta-analysis. BMJ. 2006;332:328.
190. Shi, Z, et al. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother-to-child transmission—a meta-analysis. Int J Infect Dis. 2010;14:e622.
191. Song, YM, et al. Factors associated with immunoprophylaxis failure against vertical transmission of hepatitis B virus. Eur J Pediatr. 2007;166:813.
192. Wiseman, E, et al. Perinatal transmission of hepatitis B virus: An Australian experience. Med J Aust. 2009;190:489.
193. Shi, Z, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: A systematic review and meta-analysis. Obstet Gynecol. 2010;116:147.
194. Xu, WM, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: A multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94.
195. National Center for Immunization and Respiratory Diseases. General Recommendations on Immunization—Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1.
196. McMenamin, MB, et al. Obstetric management of hepatitis C–positive mothers: Analysis of vertical transmission in 559 mother-infant pairs. Am J Obstet Gynecol. 2008;199:315.
197. Marine-Barjoan, E, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: Risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811.
198. Polis, CB, et al. Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: A meta-analysis. Clin Infect Dis. 2007;44:1123.
199. Al Arfaj, AS, et al. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus. 2010;19:1665.
200. Carvalheiras, G, et al. Pregnancy and systemic lupus erythematosus: Review of clinical features and outcome of 51 pregnancies at a single institution. Clin Rev Allergy Immunol. 2010;38:302.
201. Smyth, A, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5:2060.
202. Ambrósio, P, et al. Lupus and pregnancy—15 years of experience in a tertiary center. Clin Rev Allergy Immunol. 2010;38:77.
203. Gladman, DD, et al. The effect of lupus nephritis on pregnancy outcome and fetal and maternal complications. J Rheumatol. 2010;37:754.
204. Imbasciati, E, et al. Pregnancy in women with pre-existing lupus nephritis: Predictors of fetal and maternal outcome. Nephrol Dial Transplant. 2009;24:519.
205. Wagner, SJ, et al. Maternal and fetal outcomes in pregnant patients with active lupus nephritis. Lupus. 2009;18:342.
206. Chen, CY, et al. Increased risk of adverse pregnancy outcomes for hospitalisation of women with lupus during pregnancy: A nationwide population-based study. Clin Exp Rheumatol. 2010;28:49.
207. Clowse, MEB, et al. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008;199:127.
208. Chakravarty, EF, et al. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:899.
209. De Man, YA, et al. Disease activity of rheumatoid arthritis during pregnancy: Results from a nationwide prospective study. Arthritis Rheum. 2008;59:1241.
210. De Man, YA, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: Results of a national prospective study. Arthritis Rheum. 2009;60:3196.
211. Park-Wyllie, L, et al. Birth defects after maternal exposure to corticosteroids: Prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385.
212. Pradat, P, et al. First trimester exposure to corticosteroids and oral clefts. Birth Defects Res A Clin Mol Teratol. 2003;67:968.
213. Cassina, M, et al. First trimester diclofenac exposure and pregnancy outcome. Reprod Toxicol. 2010;30:401.
214. da Silva, DP, et al. Dipyrone use during pregnancy and adverse perinatal events. Arch Gynecol Obstet. 2009;279:293.
215. Ericson, A, Källén, BA. Non-steroidal anti-inflammatory drugs in early pregnancy. Reprod Toxicol. 2001;15:371.
216. Kallen, BA, Otterblad, OP. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol. 2003;17:255.
217. Nielsen, GL, et al. Risk of adverse birth outcome and miscarriage in pregnant users of nonsteroidal anti-inflammatory drugs: Population based observational study and case-control study. BMJ. 2001;322:266.
218. Goldstein, LH, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696.
219. Langagergaard, V, et al. Birth outcome in women treated with azathioprine or mercaptopurine during pregnancy: A Danish nationwide cohort study. Aliment Pharmacol Ther. 2007;25:73.
220. Cleary, BJ, et al. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defects Res A Clin Mol Teratol. 2009;85:647.
221. Bar Oz, B, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: A meta-analysis. Transplantation. 2001;27:1051.
222. Clowse, ME, et al. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54:3640.
223. Ruiz-Irastorza, G, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Ann Rheum Dis. 2010;69:20.
224. Carter, JD, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: A review of the Food and Drug Administration database. J Rheumatol. 2009;36:635.
225. Clowse, MEB. The use of anti-TNFα medications for rheumatologic disease in pregnancy. Int J Womens Health. 2010;2:199.
226. Schnitzler, F, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm Bowel Dis. 2011;17:1846.








