7 Chronic lower abdominal pain or discomfort
Case
A 35-year-old florist presents to her doctor because of a 6-year history of recurrent episodes of lower abdominal pain associated with an erratic bowel habit. Her symptoms have been present intermittently for this period but over the past 12 months have become more frequent and severe, with pain occurring every few weeks and lasting for 4-5 days. The pain is situated in the left iliac fossa and left hypochondrium, and has a cramping nature. The pain can cause her to cease her current activities. Her bowel habit is characterised by an alternating pattern of loose frequent motions for several days followed by a hard dry motion every few days with associated straining and a sensation of incomplete evacuation. She complains of abdominal bloating, which has become more of a problem recently, and this is often accompanied by visible abdominal distension. She has no ‘alarm’ symptoms, with her appetite and weight well-maintained and no evidence of blood or mucus in her stools. She has no other significant medical illnesses, is a non-smoker and eats a balanced diet. There is no family history of gastrointestinal disease. Physical examination is normal with no evidence of abdominal tenderness, mass or other abnormality. Rectal examination is normal.
History
Compared to acute abdominal pain, the origin of chronic abdominal pain is often more difficult to diagnose, because the characteristics of the pain tend to be less specific and can be difficult for patients to describe. For example, even the terms used by patients to describe chronic abdominal pain vary greatly. In particular, some refer to pain, while others to discomfort, fullness or even indigestion. Nevertheless a skilled physician can make an accurate diagnosis, or at least narrow the range of possible diagnoses, even before the physical examination. At the outset, it is important to remember that the experience of pain includes several components: (1) nociception, whereby a noxious stimulus conveys an impulsive centrally; (2) conscious perception of this sensation; (3) an affective response such as distress; and (4) a behavioural response. The essential features that need to be elicited in the history are described below.
Modifying factors
The associations of the pain are relevant; for example, colonic pain can be temporally associated with a change in stool form or frequency, a key feature of IBS. Episodes of pain related to menstruation raise the possibility of pelvic inflammatory disease or endometriosis; it should be remembered, however, that abdominal discomfort from a wide variety of causes can be aggravated premenstrually, and alterations in stool pattern are also common at this time.
Differential Diagnosis
The differential diagnosis of chronic or recurrent lower abdominal pain is wide. Traditionally, the two main categories are various ‘organic’ disorders and various ‘functional’ bowel disorders (Table 7.1). In the case of organic disorders, the cause can be identified and, if improved or eliminated, symptoms improve. In the case of functional disorders, there is no structural or biochemical explanation for the symptoms, although in some there may be an identifiable pathophysiological dysfunction present. The distinction between ‘organic’ and ‘functional’ disorders has become increasingly blurred, however, because of the finding of low-grade intestinal inflammation in some cases of IBS.
Table 7.1 Differential diagnosis of chronic or recurrent lower abdominal pain
| ‘Organic’ disorders | ‘Functional’ disorders |
|---|---|
‘Organic’ disorders
Diverticular disease
Uncomplicated diverticular disease of the colon is very common, and is not normally associated with symptoms. A proportion of individuals with diverticulosis, however, experience recurrent lower abdominal pain, predominantly in the left iliac fossa, and occasionally a change in bowel habit. These symptoms are similar to, and indeed can be indistinguishable from, IBS, and it is probable that the symptoms in these instances are due to the presence of concomitant irritable bowel. In severe diverticular disease, in contrast, the colonic lumen can become distorted and narrowed in the sigmoid colon; symptoms of partial bowel obstruction may then develop and produce recurrent lower abdominal, often left iliac fossa, pain. Diverticulitis is discussed in Chapter 4.
Gynaecological disorders
Endometriosis
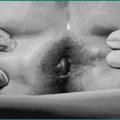
Endometriosis may cause recurrent abdominal pain and bowel symptoms in women. Usually patients are under the age of 45 years and two-thirds are nulliparous. Symptoms may sometimes occur with the period (because the endometrial implants are influenced by hormonal changes; at termination of the menstrual cycle, endometrial engorgement and sloughing occurs). However, in many cases symptoms do not coincide with periods. Common symptoms include bloating, abdominal or pelvic pain, constipation or diarrhoea, proctalgia and lower back pain; indeed, irrespective of any bowel involvement, gastrointestinal symptoms have been shown to be nearly as frequent as gynaecological symptoms. Thus the gynaecological symptoms usually co-exist with the gastrointestinal symptoms (a clinical clue) and can include menstrual irregularity, dysmenorrhoea and dyspareunia. There may also be a history of infertility. Rectal examination may sometimes detect tender nodules or irregular induration. At sigmoidoscopy or colonoscopy, there may occasionally be findings of a submucosal mass, usually with overlying intact mucosa; biopsy may not be diagnostic because endometriosis is usually in the deeper layers. Barium studies may provide useful indirect evidence of the disease. Laparoscopy, the main diagnostic tool, allows direct visualisation and biopsy of serosal lesions, and also ablative therapy. Recurrence after surgical treatment is common. Hormonal therapy (usually oral contraceptives) may be useful in mild disease. Drugs such as danazol, a synthetic androgen, and antigonadotropins, such as gestrinone, are effective, but have a greater incidence of side effects than hormonal therapy. In cases with complicated bowel disease, surgical resection may be required. In incapacitating cases, a total abdominal hysterectomy and oophorectomy may be considered.
Adhesive enteropathy
Crohn’s disease
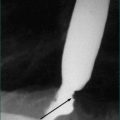
Crohn’s disease of the small intestine may produce recurrent mid to lower abdominal pain from inflammation, or symptoms of intermittent partial bowel obstruction from acute inflammation or stricturing disease (see Ch 15). Localised and minimal disease can be associated with delay before eventual diagnosis. Terminal ileal biopsy at colonoscopy enables confirmation of Crohn’s ileitis. Barium follow-through or CT or MRI enteroclysis can reveal evidence of likely small bowel Crohn’s disease. Capsule endoscopy of the small bowel avoids irradiation but is usually reserved for those where the clinical suspicion of Crohn’s disease remains despite negative other investigations.
Chronic intestinal pseudo-obstruction
This refers to a heterogeneous group of rare disorders affecting the neuromuscular apparatus of the bowel (Box 7.1). Recurrent symptoms of small or large bowel obstruction occur in the absence of luminal or extrinsic causes. Some cases can be associated with the ingestion of certain medications or secondary to rare metabolic or systemic disorders; others are idiopathic. Laparoscopic full-thickness jejunal biopsy is becoming increasingly utilised in order to obtain appropriate tissue for histological examination. Histology can provide clues as to the presence of myopathy, neuropathy or both, but in some cases no specific abnormalities can be demonstrated despite impressive clinical features. Therapies for the disorder are limited, and include symptomatic treatment, especially analgesia, nutritional support and treatment of complications. Drug therapy with prokinetic agents is not usually effective, especially in visceral myopathy. Antibiotics can be given for small bowel bacterial overgrowth. Surgical therapies include jejunostomy to facilitate enteral nutrition, venting gastrostomy or enterostomy to relieve abdominal distension, and resection of localised disease.
Box 7.1 Chronic intestinal pseudo-obstruction
Diagnosis
Abdominal angina (chronic mesenteric ischaemia)
This is an important condition to recognise. Usually this is due to atherosclerosis but the condition can also occur with vasculitis or other lesions of the splanchnic vessels. Abdominal pain occurs 10–30 minutes after eating, gradually increasing in severity and then slowly resolving over 1–3 hours. The pain can occur in the periumbilical region or in the epigastrium. The patient becomes afraid to eat and reduces the meal size to avoid pain, leading to substantial weight loss. The pain may be due to an increase in gastric blood flow after food enters the stomach, leading to ‘stealing’ of blood from the small intestine. On examination, there may be a systolic bruit in the abdomen, but this is non-diagnostic. Doppler flow studies of the celiac axis and superior mesenteric arteries are useful screening tests. Angiography is helpful to confirm the diagnosis if there is involvement of at least two of the three major arteries. However, as such abnormalities can occur in the absence of symptoms, this entity remains a largely clinical diagnosis.
‘Functional’ bowel disorders
Based on characteristic symptom clusters, a number of functional bowel disorders can be recognised.
Irritable bowel syndrome
The presence of the following symptoms increases confidence in the diagnosis: abnormal stool frequency; abnormal stool form (lumpy/hard or loose/watery stool); abnormal stool passage (straining, urgency of feeling of incomplete evacuation); passage of mucus; and bloating or a feeling of abdominal distension. These symptoms often occur in discrete episodes, varying in frequency and severity, and are present without abnormalities on radiological, endoscopic and laboratory investigations. IBS accounts for 5% or more of attendances to general practitioners and 20–50% of referrals to gastroenterologists. It is twice as common in females as males, and half of the patients are younger than 35 years of age.
Clinical Evaluation
The extent of the evaluation will depend on the age of the patient and the duration and severity of symptoms. Based on a detailed history and general abdominal examination, the organ and disease process most likely to be involved should be defined. In the history, ‘alarm features’ such as the presence of fever, weight loss, rectal bleeding or steatorrhoea are all strong pointers towards the presence of organic disease (Box 7.2). As well as the aspects of the pain discussed earlier, further questions are often required to elicit specific information, such as new exacerbating factors to the pain (e.g. dietary change or change in medication), worry about serious disease (especially cancer), new life stresses, the presence of psychological or psychiatric disorders, or impairment in the patient’s daily functioning. The family history can be very relevant. Because the functional bowel disorders cannot be established by any investigation, it is important to take a structured history, noting the features that support these diagnoses (see the definitions earlier), as well as those that suggest another cause for the symptoms. In some of these disorders (e.g. IBS), upper gastrointestinal symptoms, such as heartburn, dyspepsia, nausea and excessive belching, are frequently present, and non-gastrointestinal symptoms such as fatigue, dysmenorrhoea, migraine and symptoms of bladder irritability are also common. Moreover, in IBS for example, the history is often prolonged with symptoms dating from a relatively early age, and the course is characterised by exacerbations and remissions.
Irritable Bowel Syndrome (IBS)
Normal small bowel and colonic motility
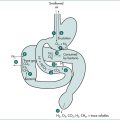
In the small intestine, intermittent segmenting and propulsive contractions occur after ingestion of food, mixing it with digestive secretions and transporting the chyme aborally. Each propagated contraction is preceded by a propagated relaxation, a phenomenon termed the ‘peristaltic reflex’. The overall duration and intensity of postprandial motor activity depends upon the caloric content and the proportion of fat, carbohydrate and protein in the meal. The jejunum acts primarily as a mixing and conduit segment, while the ileum, which has specialised absorptive properties, retains chyme until digestion and absorption are largely complete. The terminal ileum and ileocolonic junction control the rate of emptying of ileal contents into the colon. In between meals, and particularly during sleep, motility in the stomach and small intestine undergoes regular cycles of activity every few hours, termed ‘migrating motor complexes’. These complexes migrate slowly along the small bowel, clearing away residual food and secretions.
Gastrointestinal sensorimotor dysfunction
Dietary factors
Eating often provokes symptoms in patients with IBS. In general, meals high in fat move slowly through the gut, and in IBS this may exacerbate symptoms such as bloating and constipation. Foods such as baked beans, cabbage and brussels sprouts, and sugars such as fructose and sorbitol, may exacerbate symptoms in patients with complaints of excessive bloating and rectal flatus.
Treatment
Management is outlined in Box 7.3. Therapy begins at the initial consultation, where it is essential to establish rapport with patients and secure their confidence. This is achieved by a process of reassurance regarding the genuine nature of the symptoms. Many patients have been told that they are not suffering from a significant illness; the patient should be reassured that IBS is a well recognised and common, though benign and chronic, clinical entity.
Box 7.3 Management of IBS
Diet and the role of fibre
Although it is controversial whether dietary fibre supplementation is of any greater overall benefit, a stool bulking by a regular increased intake of fibre-rich foods (e.g. cereals, wholemeal bread and unprocessed wheat bran) and/or proprietary bulking agents should be trialled in most patients. Fibre supplementation should be introduced gradually and continued for at least 1 month before its effect is judged. Patients should be made aware that too much fibre can produce excessive intestinal gas and cause bloating and flatus. Proprietary bulking agents (e.g. those containing psyllium or ispaghula) may be less likely to cause this problem than bran. A trial of stool bulking is less likely to be effective in patients with diarrhoea-predominant IBS. In selected cases, where there is a high suspicion of food intolerance, a symptom and food diary may enable the patient to recognise specific items more readily.
If there is no improvement
There is increasing evidence that non-absorbable antibiotics improve diarrhoea-predominant IBS.
Key Points
Brandt L.J., Chey W.D., Foxx-Orenstein A.E., et al. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35.
Brenner D.M., Moeller M.J., Chey W.D., et al. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033-1049.
Clouse R.E., Mayer E.A., Aziz Q., et al. Functional abdominal pain syndrome. In Drossman D.A., editor: Rome III: The Functional Gastrointestinal Disorders, 3rd edn, McLean: Degnon Associates, 2006.
Ford A.C., Chey W.D., Talley N.J., et al. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651-658.
Kellow J.E., Azpiroz F., Delvaux M., et al. Principles of applied neurogastroenterology: physiology/motility sensation. In: Drossman D.A., editor. Rome III: The Functional Gastrointestinal Disorders. 3rd edn. McLean: Degnon Associates; 2006:40-63.
Longstreth G.F., Thompson W.G., Chey W.D., et al. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491.
Shepherd S.J., Parker F.C., Muir J.G., et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765-771.
Spiegel B.M., Chey W.D., Chang L. Bacterial overgrowth and irritable bowel syndrome: unifying hypothesis or a spurious consequence of proton pump inhibitors? Am J Gastroenterol. 2008;103:2972-2976.
Spiller R.C. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662-1671.
Talley N.J., Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet. 2002;360:555-564.