21 Chronic heart failure
Treatment is aimed at improving left ventricular function, controlling the secondary effects that lead to the occurrence of symptoms, and delaying disease progression. Drug therapy is indicated in all patients with heart failure to control symptoms (where present), improve quality of life and prolong survival. Patients with heart failure usually have their functional status assessed and categorised using the New York Heart Association (NYHA) classification system shown in Table 21.1.
Table 21.1 New York Heart Association (NYHA) classification of functional status of the patient with heart failure
| I | No symptoms with ordinary physical activity (such as walking or climbing stairs) |
| II | Slight limitation with dyspnoea on moderate to severe exertion (climbing stairs or walking uphill) |
| III | Marked limitation of activity, less than ordinary activity causes dyspnoea (restricting walking distance and limiting climbing to one flight of stairs) |
| IV | Severe disability, dyspnoea at rest (unable to carry on physical activity without discomfort) |
Aetiology
Atrial fibrillation often accompanies hyperthyroidism and mitral valve disease, where a rapid and irregular ventricular response can compromise cardiac efficiency. Improved management of the underlying causes, where appropriate, may alleviate the symptoms of heart failure, whereas the presence of mechanical defects may require the surgical insertion of prosthetic valve(s). While around 50% of patients with heart failure have significant left ventricular systolic dysfunction, the other half is comprised of patients who have either a normal or insignificantly reduced left ventricular ejection fraction (EF), although there is no consensus on the threshold for compromised EF and assessment of each patient relies mainly on clinical symptoms. These patients are referred to as having heart failure with preserved left ventricular ejection fraction (HFPEF). However, most of the available evidence from clinical trials regarding the pharmacological treatment of heart failure to date relates to those patients with heart failure due to left ventricular systolic dysfunction. Clinical symptomatic description of chronic heart failure is mild, moderate, or severe heart failure. ‘Mild’ is used for patients who are mobile with no important limitations of dyspnoea or fatigue, ‘severe’ for patients who are markedly symptomatic in terms of exercise intolerance and ‘moderate’ for those with restrictions in between. Trials tend to formalise these categories into NYHA Categories I–IV (Table 21.1).
Clinical manifestations
Patients with heart failure may appear pale and their hands cold and sweaty. Reduced blood supply to the brain and kidney can cause confusion and contribute to renal failure, respectively. Hepatomegaly occurs from congestion of the gastro-intestinal tract, which is accompanied by abdominal distension, anorexia, nausea and abdominal pain. Oedema affects the lungs, ankles and abdomen. Signs of oedema in the lungs include crepitations heard at the lung bases. In acute heart failure, symptoms of pulmonary oedema are prominent and may be life-threatening. The sputum may be frothy and tinged red from the leakage of fluid and blood from the capillaries. Severe dyspnoea may be complicated by cyanosis and shock. Table 21.2 presents the clinical manifestations of heart failure.
| Venous (congestion) | Cardiac (cardiomegaly) | Arterial (peripheral hypoperfusion) |
|---|---|---|
| Dyspnoea | Dilation | Fatigue |
| Oedema | Tachycardia | Pallor |
| Hypoxia | Regurgitation | Renal impairment |
| Hepatomegaly | Cardiomyopathy | Confusion |
| Raised venous pressure | Ischaemia, arrhythmia | Circulatory failure |
Investigations
Echocardiography is important when investigating patients with a suspected diagnosis of heart failure. An echocardiogram allows visualisation of the heart in real time and will identify whether heart failure is due to systolic dysfunction, diastolic dysfunction or heart valve defects. With the provision of direct access echocardiography services to doctors in primary care, an increasing number of patients can now be quickly referred to confirm or exclude heart failure due to left ventricular systolic dysfunction or other structural abnormalities. Some reports suggest that between 50% and 75% of patients referred to direct access clinics may have normal left ventricular function, which has important implications for the selection of appropriate drug treatment. Table 21.3 shows a number of investigations that are routinely performed in the assessment of heart failure symptoms. The use of serum natriuretic peptide measurement in the diagnosis of patients with heart failure is currently limited by the lack of defined cut-off values and, therefore, measurements are only considered in combination with ECG/chest X-ray data prior to echocardiography.
Table 21.3 Investigations performed to confirm a diagnosis of heart failure
| Investigation | Comment |
|---|---|
| Blood test | The following assessments are usually performed: |
Treatment of heart failure
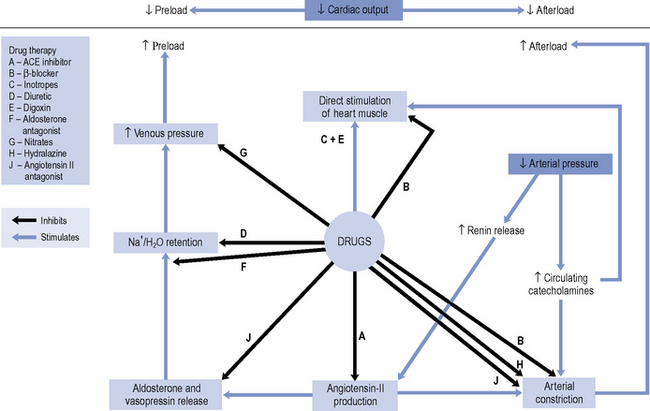
Until the 1980s, pharmacotherapy was driven by the aim to control symptoms, when diuretics and digoxin were the mainstay of treatment. While relieving the symptoms of heart failure remains decisive in improving a patient’s quality of life, a better understanding of the underlying pathophysiology has led to major advances in the pharmacological treatment of heart failure. With the introduction of angiotensin converting enzyme (ACE) inhibitors, β-blockers, angiotensin II receptor blockers (ARBs) and aldosterone antagonists, delaying disease progression and ultimately improving survival have become realistic goals of therapy. An outline of the site of action of the various drugs is schematically presented in Fig. 21.1.
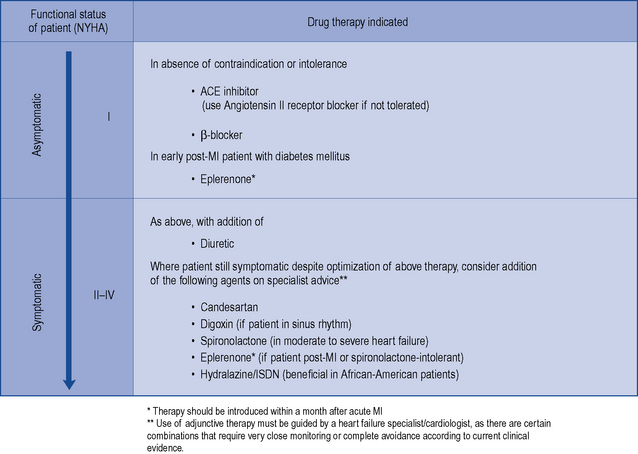
There is consensus that all patients with left ventricular systolic dysfunction should be treated with both an ACE inhibitor and a β-blocker in the absence of intolerance or contraindications. The evidence base for treatment clearly shows that use of an ACE inhibitor (or angiotensin receptor blocker) and β-blocker therapy in patients with heart failure due to left ventricular systolic dysfunction leads to an improvement in symptoms and reduction in mortality. There is some evidence to suggest that either agent can be initiated first, as both appear to be just as effective and well tolerated (CARMEN 2004, CIBIS III, 2005). Beneficial effects on morbidity and mortality have also been shown for the use of ARBs, aldosterone antagonists and hydralazine/nitrate combinations when used in the treatment of chronic heart failure. Digoxin has been shown to improve morbidity and reduce the number of hospital admissions in patients with heart failure, although its effect on mortality has not been demonstrated. Table 21.4 describes the treatment of acute heart failure in the hospital setting, while Fig. 21.2 highlights the possible treatment options for patients with chronic heart failure due to left ventricular systolic dysfunction.
Table 21.4 Treatment of acute heart failure due to left ventricular systolic dysfunction in patients requiring hospitalisation
| Problem | Drug therapy indicated |
|---|---|
| Anxiety | Use of intravenous opiates to reduce anxiety and reduce preload through venodilation |
| Breathlessness | High-flow oxygen (60–100%) may be required in conjunction with i.v. furosemide as either direct injection or 24-h infusion (5–10 mg/h). |
| Venodilation with i.v. GTN is also effective at doses titrated every 10–20 min against systolic BP ≤ 110 mmHg | |
| Arrhythmia | Digoxin useful in control of atrial fibrillation. Amiodarone is the drug of choice in ventricular arrhythmias |
| Expansion of blood volume following blood transfusion | An elevation in preload, such as can occur acutely by expansion of blood volume after a transfusion, can exacerbate the degree of systolic dysfunction. Therefore, it is necessary to continue or increase diuretic dosage during this time |
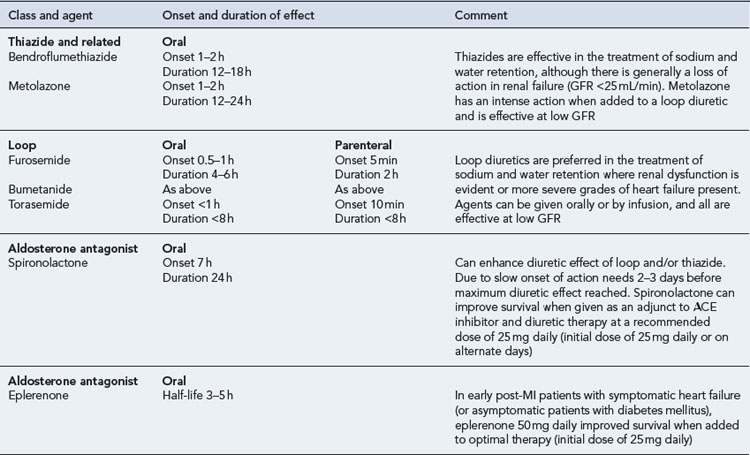
Diuretics
Details of diuretic therapy used in left ventricular systolic dysfunction are summarised in Table 21.5.
ACE inhibitors
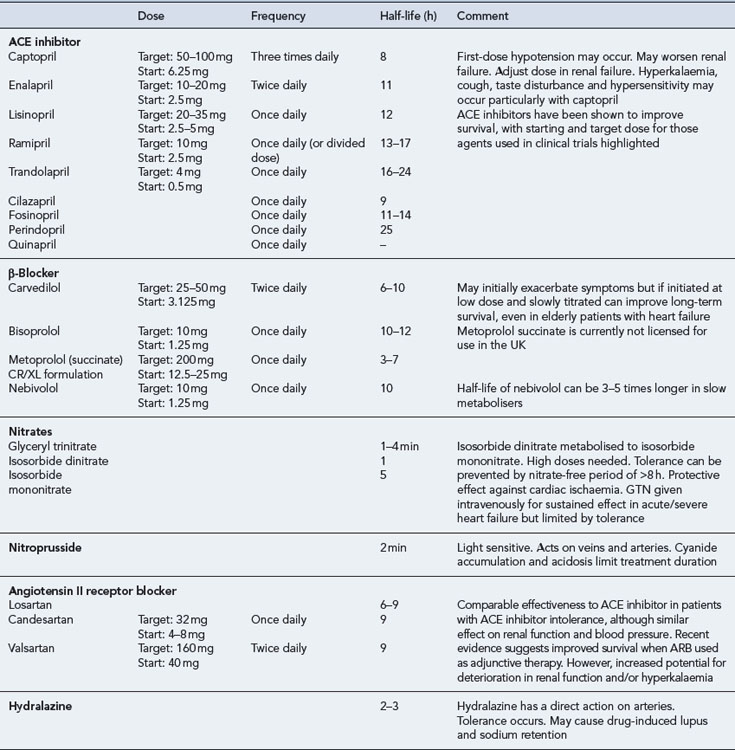
ACE inhibitors are generally well tolerated by most patients and have been shown to improve the quality of life and survival in patients with mild-to-severe systolic dysfunction (CONSENSUS, 1987; CONSENSUS II, 1992; SOLVD-P, 1992; SOLVD-T, 1991; V-HeFT II, 1991), including those patients who have experienced a myocardial infarction (AIRE), 1993; SAVE, 1992; TRACE, 1995). When an ACE inhibitor is prescribed, it is important to ensure that the dose is started low and increased gradually, paying close attention to renal function and electrolyte balance. The dose should be titrated to achieve the target dose that has been associated with long-term benefits shown in clinical trials or (if not possible) the maximum tolerable dose. There is some evidence to suggest that high doses of ACE inhibitor are more effective than low doses in relation to reduction in mortality, although it is uncertain whether this is a general class effect (ATLAS, 1999). In clinical practice, it is possible that some patients may be treated with ACE inhibitors at doses below those used in clinical trials. As a consequence, actual outcomes in heart failure treatment may not be as good as expected from the trial findings.
The introduction of an ACE inhibitor may produce hypotension, which is most pronounced after the first dose and is sometimes severe. Patients at risk include those already on high doses of loop diuretics, where the diuretics cannot be stopped or reduced beforehand, and patients who may have a low-circulating fluid volume (due to dehydration) and an activated renin–angiotensin system. Hypotension can also occur where the ACE inhibitor has been initiated at too high a dose or where the dose has been increased too quickly after initiation. In the primary care setting, treatment must be started with a low dose which is usually administered at bedtime. In patients at particular risk of hypotension, a test dose of the shorter-acting agent captopril can be given to assess suitability for treatment before commencing long-term treatment with a preferred ACE inhibitor. Once it has been established that the ACE inhibitor can be initiated safely, the preferred option would be to switch to a longer acting agent with once- or twice-daily dosing, starting with a low dose which would be gradually titrated upwards to the recommended target (Table 21.6). Monitoring of fluid balance, blood biochemistry and blood pressure are essential safety checks during initiation and titration of ACE inhibitor therapy.
ACE inhibitors are potentially hazardous in patients with pre-existing renal disease, as blockade of the renin-angiotensin system may lead to reversible deterioration of renal function. In particular, ACE inhibitors are contraindicated in patients with bilateral renal artery stenosis, in whom the renin-angiotensin system is highly activated to maintain renal perfusion. Since most ACE inhibitors or their active metabolites rely on elimination via the kidney, the risk of other forms of dose-related toxicity is also increased in the presence of renal failure. Fosinopril, which is partially excreted by metabolism, may be the preferred agent in patients with renal failure. ACE inhibitors are also contraindicated in patients with severe aortic stenosis because their use can result in a markedly reduced cardiac output due to decreased filling pressure within the left ventricle. Table 21.6 summarises the activity and use of ACE inhibitors.
Angiotensin II receptor blockers
The use of ARBs as an adjunct to ACE inhibitor and β-blocker therapy has been associated with significant reductions in cardiovascular events and hospitalisation rate (CHARM Added, 2003). Although this finding is encouraging, the impact on mortality alone remains inconsistent and there is no clear consensus on when to use an ARB as adjunctive therapy. In studies involving patients unable to tolerate an ACE inhibitor, ARBs have been shown to be comparable to ACE inhibitors in reducing the risk of cardiovascular death and rate of hospitalisation, and in the control of symptoms in heart failure patients (CHARM Alternative, 2003; Val-HeFT, 2002). Therefore, ARBs are recommended for use as an alternative to ACE inhibitor therapy where intolerance has been confirmed. It is important to note that in patients who have renal failure secondary to ACE inhibitors, switching to an ARB is of no theoretical or practical benefit, as similar adverse effects are likely.
A recent meta-analysis has raised concerns about a possible increase of cancer in people taking ARBs (Sipahi et al., 2010). Although the implications of this are unclear it adds weight to the recommendation that ACE inhibitors, not ARBs, should be the first-line agent when selecting a drug to act on the renin-angiotensin system.
β-Blockers
Formerly, β-blockers have been contraindicated in patients with heart failure. However, the sympathetic neurohormonal overactivity that occurs in response to the failing heart has been identified as a decisive factor in the progression of ventricular dysfunction. Consequently, β-blockers have been tested in a number of clinical trials. There is now substantial evidence that β-blockers reduce mortality among patients with mild-to-moderate symptomatic heart failure (ANZ Carvedilol, 1997; CAPRICORN, 2001; CIBIS II, 1999; MERIT-HF, 1999; US Carvedilol, 1996) and those with severe heart failure (COPERNICUS, 2001). This beneficial effect also extends to the elderly heart failure population (SENIORS, 2005).
The use of β-blockers is, therefore, recommended for all patients with heart failure due to left ventricular systolic dysfunction, irrespective of age and the degree of dysfunction. However, due to their negative inotropic effects, β-blockers should only be initiated when the patient’s condition is stable. There is insufficient evidence for a class effect to be assumed illustrated by the fact that in one trial, metoprolol tartrate was found to be inferior to carvedilol (COMET, 2003). Currently, nebivolol, bisoprolol and carvedilol are the only licensed β-blockers for the treatment of heart failure in the UK.
It is likely that patients will experience a worsening of symptoms during initiation of therapy and, therefore, patients are started on very low doses of β-blocker (e.g. carvedilol 3.125 mg daily) with careful titration occurring over a number of weeks or months with careful monitoring. The goal is to titrate the dose towards those used in clinical trials that have been associated with morbidity and mortality benefits (carvedilol 25–50 mg daily). Table 21.6 summarises the activity and use of β-blockers in heart failure.
Despite the demonstrated benefits, there is ongoing concern that certain subgroups of patients with heart failure continue to be undertreated with β-blockers. These groups include patients with chronic obstructive pulmonary disease (COPD), peripheral vascular disease, diabetes mellitus, erectile dysfunction and older adults. With the exception of patients with reversible pulmonary disease, who have typically been excluded from β-blocker trials (CIBIS II, 1999; MERIT-HF, 1999), there is now sufficient evidence to justify the use of β-blockers licensed for heart failure in these patients. In addition, a systematic review of trials on cardio-selective β-blockers found no clinically significant adverse respiratory effects in patients with reversible COPD, although it would be prudent to use these agents in such patients with caution and with appropriate monitoring in place (Salpeter S. et al., 2005).
Aldosterone antagonists
The use of aldosterone antagonists as an adjunct to standard treatment has been shown to have an effect on morbidity and mortality in patients with heart failure. Spironolactone has been shown to reduce mortality and hospitalisation rates in patients with moderate-to-severe heart failure (RALES, 1999). The use of eplerenone has also been shown to be associated with similar benefits in early post-MI patients with symptomatic heart failure or early post-MI diabetic patients with asymptomatic heart failure (EPHESUS, 2003, EMPHASIS-HF, 2010).
Aldosterone can cause sodium and water retention, sympathetic activation and parasympathetic inhibition, all of which are associated with harmful effects in the patient with heart failure. Aldosterone antagonists counteract these effects by directly antagonising the activity of aldosterone, providing a more complete blockade of the renin–angiotensin–aldosterone system when used in conjunction with an ACE inhibitor. Although the combination of spironolactone (at a dose of 50 mg daily or more) and an ACE inhibitor is associated with an increased risk of developing hyperkalaemia, the use of a 25-mg daily dose has been shown to have little effect on serum potassium and provides a significant reduction in mortality. The use of spironolactone is, however, contraindicated in those patients with a serum potassium >5.5 mmol/L or serum creatinine >200 µmol/L. With eplerenone, similar contraindications exist and, therefore, close monitoring of blood biochemistry and renal function must be undertaken for use of either agent. The activity and use of spironolactone and eplerenone are summarised in Table 21.5.
Digoxin
Although digoxin has an established role in the control of atrial fibrillation, its place in the treatment of heart failure is still the subject of debate. There is evidence to show that when digoxin has been used to treat heart failure in patients in sinus rhythm, as an adjunct to ACE inhibitor and diuretic therapy, then worsening of symptoms occurs on withdrawal of digoxin (PROVED, 1993; RADIANCE, 1993). While the use of digoxin in heart failure in patients in sinus rhythm has no measurable impact on mortality, it reduces the number of hospital admissions (DIG, 1997). Consequently, digoxin is currently recommended for use as add-on therapy at low doses in patients with moderate-to-severe heart failure who remain symptomatic despite adequate doses of ACE inhibitor, β-blocker and diuretic treatment. Due to the lack of effect on mortality, it is unlikely that digoxin would be considered before the other adjunctive therapies available.
Digoxin treatment is potentially hazardous due to its low therapeutic index and so all patients receiving this drug should be regularly reviewed to exclude clinical signs or symptoms of adverse effects. Digoxin may cause bradycardia and lead to potentially fatal cardiac arrhythmias. Other symptoms associated with digoxin toxicity include nausea, vomiting, confusion and visual disturbances. Digoxin toxicity is more pronounced in the presence of metabolic or electrolyte disturbances and in patients with cardiac ischaemia. Those patients who develop hypokalaemia, hypomagnesaemia, hypercalcaemia, alkalosis, hypothyroidism or hypoxia are at particular risk of toxicity. Treatment may be required to restore serum potassium, and in emergency situations intravenous digoxin-specific antibody fragments can be used to treat life-threatening digoxin toxicity. Table 21.7 summarises the activity and use of digoxin.
Table 21.7 Inotropic agents used in the treatment of heart failure
| Class and agent | Pharmacological half-life | Comment |
|---|---|---|
| Cardiac glycosides | ||
| Digoxin | 39 h | In renal failure, half-life of digoxin is prolonged. Dosage individualisation required. Serum drug concentration monitoring used to confirm or exclude toxicity or effectiveness. Dose of digitoxin unaffected by renal failure. CNS, visual and GI symptoms linked to digoxin toxicity. No benefit in terms of mortality, but use associated with improved symptoms and reduced hospitalisation for heart failure. Beneficial in AF, although risk of arrhythmias with high doses. If given i.v. must be administered slowly (20 min) to avoid cardiac ischaemia |
| Digitoxin | 5–8 days | |
| Phosphodiesterase inhibitors | ||
| Enoximone | 4.2 h | Used only in severe heart failure as adjunctive therapy. Associated with arrhythmias and increased mortality with chronic use |
| Milrinone | 2.4 h | |
| Sympathomimetics | ||
| Dobutamine | 2 min | Continuous intravenous use only. Require close monitoring in critical care setting |
| Dopamine | 2 min | |
| Dopexamine | 6–7 min | |
| Isoprenaline | >1 min | |
Nitrates/hydralazine
Nitrates exert their effects in heart failure predominantly on the venous system where they cause venodilation, thereby reducing the symptoms of pulmonary congestion. The preferred use of nitrates is in combination with an arterial vasodilator such as hydralazine, which reduces the afterload, to achieve a balanced effect on the venous and arterial circulation. The combined effects of these two drugs lead to an increase in cardiac output, and there is evidence to show the combination is effective and associated with a reduction in mortality in patients with heart failure (V-HeFT I, 1986). Although the combination can improve survival, the reduction in mortality is much smaller than that seen with ACE inhibitors (V-HeFT II, 1991), especially in the white population. The combination has been shown to reduce mortality, heart failure hospitalisation rates and quality of life in patients of African descent, when added as an adjunct to optimum medical therapy (A-HeFT, 2004) and this benefit is sustained (A-HeFT, 2007).
ISDN can be given orally and is completely absorbed; however, only 25% of a given dose appears as ISDN in serum with 60% of an oral dose being rapidly converted to ISMN. ISMN is longer acting and, therefore, most of the accumulated effects of a dose of ISDN are attributable to the 5-isosorbide mononitrate metabolite. Consequently, a 20-mg dose of ISDN is approximately equivalent to a 10-mg dose of ISMN. In practice, nitrate preparations are usually given orally in the form of ISMN, and intravenously in the form of GTN (see Table 21.6).
Inotropic agents
The use of inotropic agents (except digoxin) is almost exclusively limited to hospital practice, where acute heart failure may require the use of one or more inotropic agents, particularly the sympathomimetic agents dobutamine and dopamine, in an intravenous continuous infusion. These agents have inotrope-vasodilator effects which differ according to their action on α, β1, β2 and dopamine receptors (β1-agonists increase cardiac contractility, β2-agonists produce arterial vasodilation, dopamine agonists enhance renal perfusion). With dopamine, low doses (0–2 µcg/kg/min) have a predominant effect on dopamine receptors within the kidneys to improve urine output, intermediate doses (2–5 µcg/kg/min) affect β1-receptors, producing an inotropic effect, and high doses (10 µcg/kg/min) have a predominant action on α-adrenoreceptors. Dobutamine has a predominantly inotropic and vasodilator action due to the action of the (+) isomer selectively on β-adrenoreceptors (see Table 21.7). Tolerance to sympathomimetic inotropic agents may develop on prolonged administration, particularly in patients with underlying ischaemia, and is also associated with a risk of precipitating arrhythmias.
Phosphodiesterase inhibitors are rarely used in clinical practice as a consequence of trials showing an increased risk of mortality (PROMISE, 1991).
Guidelines
Several groups have produced evidence-based consensus clinical guidelines for the management of chronic heart failure. The focus of the various guidelines tends to be on chronic medication use (National Institute for Health and Clinical Excellence, 2010; American College of Cardiology/American Heart Association Task Force on Practice Guidelines, 2009; European Society of Cardiology, 2008; Scottish Intercollegiate Guidelines Network, 2007). All guidelines confirm that ACE inhibitors and β-blockers should be given to all patients with all grades of heart failure, whether symptomatic or asymptomatic, in the absence of contraindication or intolerance.
Patient care
Patient education and self-monitoring
Timing of doses is also important. If a nitrate regimen is being used, then patients must be made aware that the last dose of the nitrate should be taken mid to late afternoon to ensure that a nitrate-free period occurs overnight, thus, reducing the risk of nitrate tolerance. However, patients with prominent nocturnal symptoms require separate consideration. Where β-blockers are introduced, it is important that the patient is aware of the need for gradual dose titration due to the risk of the medication aggravating heart failure symptoms. Certain medicines for the treatment of minor ailments that are available for purchase over the counter without a prescription can aggravate heart failure, such as ibuprofen, antihistamines and effervescent formulations. It is important that patients know what action to take if their symptoms become progressively worse, and whom to contact when necessary for advice. Table 21.8 provides a general patient education and self-monitoring checklist, highlighting the typical areas where advice should be given.
Table 21.8 Patient education and self-monitoring in the treatment of heart failure
| Topic | Advice | Comment |
|---|---|---|
| Diuretics |
Monitoring effectiveness of drug treatment
In an effort to systematically identify whether a patient’s therapeutic plan adheres to the current evidence base for treatment, and whether any changes might be required to optimise therapy, the audit tool shown in Box 21.1 could be used in routine practice. The tool has been derived from published consensus-based clinical guidelines, and could underpin a more comprehensive medication review.
Box 21.1 Criteria for the assessment of drug treatment in a patient with chronic heart failure (Scottish Intercollegiate Guidelines Network, 2007)
Need for drug therapy (as appropriate)
Monitoring safety of drug treatment
A number of issues around the safe use of medication must be considered, especially in those patients with co-morbidity and/or a high number of prescribed medicines. In these patients there is an increased risk of drug–drug and drug–disease interactions (Tables 21.9–21.11). It is important to be aware of clinically important interactions and to investigate potentially problematic combinations, as well as to regularly assess the patient for any signs or symptoms of drug therapy problems. Monitoring for problems such as negative inotropic effects, excessive blood pressure reduction, and salt and fluid retention should be undertaken and, where appropriate, laboratory measurement of serum drug concentration (digoxin) or physiological markers (potassium, creatinine) should be performed to confirm or exclude adverse effects. Patients started on an ACE inhibitor should have renal function and serum electrolytes checked at 1 and 3 months after starting therapy, and 6 monthly once a maintenance dose is reached.
Table 21.9 Monitoring the effectiveness of drug treatment in patients with heart failure
| Consider | Monitor for | Comment |
|---|---|---|
| Clinical markers |
Table 21.10 Common drug–drug interactions with prescribed heart failure medication
| Drug | Interacts with | Result of interaction |
|---|---|---|
| Diuretic | NSAIDs | Decreased effect of diuretic and increased risk of renal impairment |
| Carbamazepine | Increased risk of hyponatraemia | |
| Lithium | Excretion of lithium impaired (thiazides worse than loop diuretics) | |
| ACE inhibitor or ARB | NSAIDs | Antagonism of hypotensive effect. Increased risk of renal impairment |
| Ciclosporin | Increased risk of hyperkalaemia | |
| Lithium | Excretion of lithium impaired | |
| Diuretics | Enhanced hypotensive effect. Increased risk of hyperkalaemia with potassium-sparing drugs | |
| Digoxin | Amiodarone | Increased digoxin level (need to halve maintenance dose of digoxin) |
| Propafenone | Increased digoxin level (need to halve maintenance dose of digoxin) | |
| Quinidine | Increased digoxin level (need to halve maintenance dose of digoxin) | |
| Verapamil | Increased risk of AV block | |
| Diuretics | Increased risk of hypokalaemia and therefore toxicity | |
| Amphotericin | Increased cardiac toxicity if hypokalaemia present | |
| Nitrates | Sildenafil | Increased hypotensive effect |
| Heparin | Increased excretion of heparin | |
| Spironolactone | Digoxin | Spironolactone may interfere with measurement of digoxin serum levels, resulting in inaccurate interpretation |
| β-Blocker | Amiodarone | Increased risk of bradycardia |
| Diltiazem | Increased risk of AV block and bradycardia | |
| Verapamil | Increased risk of hypotension, heart failure and asystole |
Table 21.11 Common drug–disease interactions with prescribed heart failure medication
| Drug | Concurrent disease | Potential outcome |
|---|---|---|
| Diuretic | Prostatism | Urinary retention/incontinence |
| Hyperuricaemia | Exacerbation of gout | |
| Liver cirrhosis | Encephalopathy | |
| ACE inhibitor | Renal artery stenosis | Renal failure |
| Severe aortic stenosis | Exacerbation of heart failure | |
| Renal impairment | Renal failure | |
| Hypotension | Hypotension and cardiogenic shock | |
| β-Blocker | Asthma | Bronchoconstriction/respiratory arrest |
| Bradyarrhythmias | Exacerbation of heart failure | |
| Hypotension | Further hypotension and cardiogenic shock | |
| Digoxin | Bradyarrhythmias | Exacerbation of heart failure |
| Renal impairment | Exacerbation of heart failure and digoxin toxicity leading to cardiac arrhythmias |
Potential problems with ACE inhibitor and ARB therapy
The use of an aldosterone antagonist as adjunctive therapy with an ACE inhibitor (or ARB if the patient is ACE inhibitor intolerant) can be safely undertaken with minimal effects on the serum potassium concentration, provided that recommended target doses for the aldosterone antagonist are not exceeded (see Table 21.5). Although this is usually the case, laboratory monitoring of potassium is mandatory to ensure patient safety. Heparin therapy has also been shown to increase the risk of hyperkalaemia when used alongside ACE inhibitor or ARB therapy, and therefore a similar approach to monitoring should be taken when co-prescribed.
Potential problems with other cardiovascular drugs
A summary of monitoring activity required to ensure the safety of drug use is outlined in Table 21.12.
Table 21.12 Monitoring the safety of drug treatment in patients with heart failure
| Consider | Monitor | Comment |
|---|---|---|
| Clinical markers | Side effects | There is a need to monitor for signs/symptoms of overtreatment with prescribed medication, such as diuretics (dehydration) and digoxin (nausea and vomiting). Look for signs of patient intolerance, allergy, serious adverse effects or troublesome side effects. Document unexpected adverse drug reactions if reported |
| Toxicity | ||
| Adverse drug reactions | ||
| Laboratory markers | Changes in organ function | Renal function assessment and implications for drug choice and dosage individualisation required, especially in the elderly and for initiation or titration of ACE inhibitor therapy (creatinine, potassium, urea). Hypokalaemia can lead to digoxin toxicity, and serum drug concentration measurement may be performed to confirm or exclude toxicity. Haematological side effects with some drugs have been reported, for example, ACE inhibitors, therefore, laboratory checks may be required in response to clinical signs/symptoms presented |
| Biochemical changes | ||
| Haematological changes | ||
| Suspected digoxin toxicity | ||
| Interactions | Drug–drug interactions | Some interactions may result in harm to the patient |
| Drug–disease interactions | ||
| Co-morbidity | Drug selection for concomitant conditions | The presence of heart failure may influence treatment choice for co-existing diseases or conditions, for example, coronary artery disease, thyroid disease, respiratory disease. Where possible, ensure drugs known to worsen heart failure are avoided or used with caution, for example, non-steroidal anti-inflammatory agents or corticosteroids in rheumatoid arthritis |
Potential problems with non-cardiovascular agents
A number of agents should be avoided or used with caution in patients with heart failure because of their known negative inotropic or pro-arrhythmic effects that may aggravate symptoms of heart failure (see Box 21.1). In particular, the use of non-steroidal anti-inflammatory drugs (NSAIDs) should be actively discouraged where possible. Not only do NSAIDs cause fluid retention and put patients at increased risk of bleeding, especially if they are already taking antiplatelets or anticoagulants, there is also an increased risk of acute renal failure, particularly in those on long-term use and in the elderly. Recent articles have described the synergistic/cumulative adverse renal effects of combinations of ACE inhibitors or ARBs with diuretics and NSAIDs, which are particularly common in patients with heart failure.
Answer
Answer
Questions
Answers
Answers
Answers
Neither the ACE inhibitor nor β-blocker is prescribed at the recommended target dose (see Table 21.6); therefore, there is scope to titrate the dose of either agent to the target dose which should result in improvement of symptoms and a reduced need for diuretic therapy. However, there may be reluctance to increase the dose of ACE inhibitor possibly due to the fact that Mr CH has a relatively low blood pressure and compromised renal function (estimated creatinine clearance 20 mL/min). However, it is unclear whether Mr CH is receiving maximally tolerated doses and whether his apparent hypotension is indeed symptomatic. Similarly, there may be reluctance to increase the dose of β-blocker due to a low blood pressure and heart rate (54 bpm). As both options may adversely affect the patient, the doctor has decided to treat the symptoms with additional diuretic when required as a short-term solution prior to Mr CH’s appointment with the cardiology consultant. Advice should be sought from a heart failure specialist where a patient may be poorly tolerant of ACE inhibitor or β-blocker, or where there is a risk of hypotension or renal failure in susceptible individuals. In Mr CH’s case, specialist supervision is required for optimisation of therapy.
A-HeFT, Taylor A.L., Ziesche S., Yancy C., et alfor the African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl. J. Med.. 2004;351:2049-2057.
A-HeFT, Taylor A.L., Ziesche S., Yancy C., et alfor the African-American Heart Failure Trial Investigators. Early and sustained benefit on event-free survival and heart failure hospitalization from fixed-dose combination of isosorbide dinitrate/hydralazine: consistency across subgroups in the African-American Heart Failure Trial. Circulation. 2007;115:1747-1753.
Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet. 1993;342:821-828.
American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Guideline update for the diagnosis and management of chronic heart failure in the adult – summary article. Circulation. 2009;112:e154-235.
ANZ Carvedilol. Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease, Australia/New Zealand Heart Failure Research Collaborative Group. Lancet, 349. 1997: 375-380.
ATLAS, Packer M., Poole-Wilson P.A., Armstrong P.W., et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure, ATLAS Study Group. Circ., 100. 1999: 2312-2318.
CAPRICORN Investigators. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385-1390.
CARMEN, Komajda M., Lutiger B., Madeira H., et al. Tolerability of carvedilol and ACE-inhibition in mild heart failure, Results of CARMEN (Carvedilol ACE-Inhibitor Remodelling Mild CHF Evaluation). Eur. J. Heart Fail., 6. 2004: 467-475.
CHARM Added, McMurray J.J., Ostergren J., Swedberg K., et al. Effects of candesartan in patients with CHF and reduced left-ventricular systolic dysfunction taking angiotensin-converting-enzyme inhibitors: the CHARM Added trial. Lancet. 2003;362:767-771.
CHARM Alternative, Granger C.B., McMurray J.J., Yusuf S., et al. Effects of candesartan in patients with CHF and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM Alternative trial. Lancet. 2003;362:772-776.
CIBIS II: Investigators Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS II): a randomised trial. Lancet. 1999;353:9-13.
CIBIS III, Willenheimer R., van Veldhuisen D.J., Silke B., et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426-2435.
COMET, Poole-Wilson P.A., Swedberg K., Cleland J.G.F., et alfor the COMET Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): a randomised controlled trial. Lancet. 2003;362:7-13.
CONSENSUS I: CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N. Engl. J. Med.. 1987;316:1429-1435.
CONSENSUS II, Swedberg K., Held P., Kjekshus J., et al. Effects of the early administration of enalapril on the mortality in patients with acute myocardial infarction. Results from the co-operative new Scandinavian enalapril survival study II. N. Engl. J. Med.. 1992;327:678-684.
COPERNICUS, Packer M., Coats A.J., Fowler M.B., et alfor the Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med.. 2001;334:1651-1658.
DIG: Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med.. 1997;336:525-533.
EPHESUS, Pitt B., Remme W., Zannad F., et al. Eplerenone, a selective aldosterone blocker, in 38 patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med.. 2003;348:1309-1321.
European Society of Cardiology. Guidelines for the diagnosis and treatment of acute and chronic heart failure. The task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. J. Heart Fail.. 2008;10:933-989.
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353:2001-2007.
National Institute of Health and Clinical Excellence. Chronic heart failure CG108. NICE, London. Available at:. 2010. http://www.nice.org.uk/nicemedia/live/13099/50514/50514.pdf.
PROMISE, Packer M., Carver J.R., Rodeheffer R.J., et alfor the PROMISE Study Research Group. Effect of oral milrinone on mortality in severe chronic heart failure. N. Engl. J. Med.. 1991;325:1468-1475.
PROVED, Uretsky B.F., Young J.B., Shahidi F.E., et alfor the PROVED Investigative Group. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. J. Am. Coll. Cardiol.. 1993;22:955-962.
RADIANCE, Packer M., Gheorghiade M., Young J.B., et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors, RADIANCE Study. N. Engl. J. Med., 329. 1993: 1-7.
RALES, Pitt B., Zannad F., Remme W.J., et alfor the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med.. 1999;341:709-717.
SAVE, Pfeffer M.A., Braunwald E., Moye L.A., et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction, Results of the Survival and Ventricular Enlargement trial (SAVE). N. Engl. J. Med., 327. 1992: 669-677.
Scottish Intercollegiate Guidelines Network. Management of Chronic Heart Failure (Guideline 95). Edinburgh: SIGN; 2007. Available at www.sign.ac.uk
SENIORS, Flather M.D., Shibata M.C., Coats A.J., et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J.. 2005;26:215-225.
Sipahi I., Debanne S.M., Rowland D.Y., et al. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol.. 2010;11:627-636.
SOLVD-P: SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N. Engl. J. Med.. 1992;327:685-691.
SOLVD-T: SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med.. 1991;325:293-302.
TRACE: Trandolapril Cardiac Evaluation Study Group. A clinical trial of the angiotensin-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med.. 1995;333:1670-1676.
US Carvedilol, Packer M., Bristow M.R., Cohn J.N., et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure, US Carvedilol Heart Failure Study Group. N. Engl. J. Med., 334. 1996: 1349-1355.
V-HeFT I, Cohn J., Archibald D., Ziesche S., et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure, Results of a Veterans Administration Cooperative Study. N. Engl. J. Med., 314. 1986: 1547-1552.
V-HeFT II, Cohn J.N., Johnson G., Ziesche S., et alfor the V-HeFT II Study. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N. Engl. J. Med.. 1991;325:303-310.
Val-HeFT, Maggioni A.P., Anand I., Gottlieb S.O., et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J. Am. Coll. Cardiol.. 2002;40:1422-1424.
Zannad F., McMurray J.J.V., Krum H., van Veldhuisen D.J., et alfor the EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med.. 2011;364:11-21.
Koshman S.L., Charrois T.L., Simpson S.H., et al. Pharmacist care of patients with heart failure. A systematic review of randomized trials. Arch. Intern. Med.. 2008;168:687-694.
McMurray J., Cohen-Solal A., Dietz R., et al. Practical recommendations for the use of ACE inhibitors, β-blockers, aldosterone antagonists and angiotensin receptor blockers in heart failure: putting guidelines into practice. Eur. J. Heart Fail.. 2005;7:710-721.