Chapter 138 Choroidal Nevi
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Introduction
Choroidal nevi are common tumors composed of benign-appearing atypical uveal melanocytes called nevus cells.1,2 When encountered clinically, they are widely regarded as trivial lesions; however, they have the potential to cause visual loss and, most importantly, they can resemble or transform into a choroidal melanoma. In fact, these lesions are the source of many unanswered questions, unresolved controversies, and much confusion as to their definition, incidence, histopathologic classification, and their relationship to uveal melanomas.
Definitions
Nevus
The term “nevus” in its historical context, is synonymous with hamartoma and denotes any congenital tumor-like tissue malformation.3 However, except in a few cases (e.g., nevus sebaceous or nevus flammeus), this word is now used restrictively to designate benign acquired or congenital tumors of neural crest-derived cells, including atypical melanocytes.2,3 A melanocyte is a mature melanin-producing and melanin-containing cell.2,3 Melanocytes derive embryologically from melanoblasts.2,4 These cells migrate during closure of the neural tube, and melanization starts between the 24th and 27th weeks of gestation, proceeding anteriorly until birth.4 Nevus cells are believed to be modified, or atypical, melanocytes.3 Zimmerman2 has emphasized that the morphologic appearance of cutaneous nevus cells may vary considerably, making it difficult to characterize a typical nevus cell.
Halo nevus
Halo nevus is a rarely encountered subgroup of choroidal nevus, and it is named after the depigmented annulus that surrounds the central pigmented portion of the nevus. It is believed that the halo is composed of large, polygonal cells with foamy cytoplasm, termed balloon nevus cells, similar to those found in halo nevi in other anatomic locations.5
Giant choroidal nevus
There are no definitive criteria to designate which nevus falls under the category of “giant” choroidal nevus; however, Shields et al.6 included those with a basal diameter greater than or equal to 10 mm. Based on their size, this rare subgroup of choroidal nevi may be particularly difficult to distinguish from malignant melanoma.6
Melanocytoma
Among nevi, melanocytomas are those that are composed mostly of uniform, densely pigmented, and plump polyhedral cells (magnocellular nevi).2,7
Ocular melanocytosis
Melanocytosis designates a congenital hyperpigmentation of the uveal tract caused by an increased number of uveal melanocytes.2,8 When accompanied by dermal melanocytosis in a distribution similar to that of the first or second division of the trigeminal nerve, this condition is termed oculodermal melanocytosis or nevus of Ota (nevus fuscoceruleus ophthalmomaxillaris (Fig. 138.1A).1,8
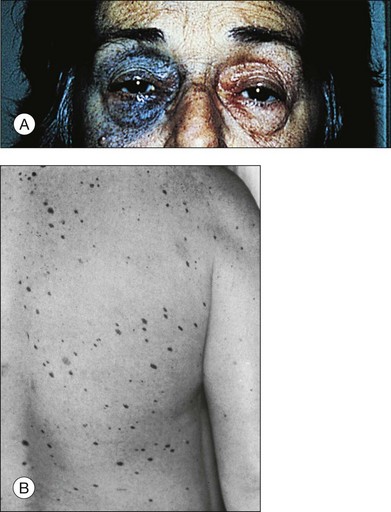
Fig. 138.1 (A) Nevus of Ota. (B) Cutaneous dysplastic nevi.
(Part A reproduced with permission from Gonder JR, Shields JA, Albert DM, et al. Uveal malignant melanoma associated with ocular and oculodermal melanocytosis. Ophthalmology 1982; 89:953–60. Part B reproduced with permission from Albert DM, Chang MA, Lamping K, et al. The dysplastic nevus syndrome. A pedigree with primary malignant melanomas of the choroid and skin. Ophthalmology 1985;92:1728–34.)
Prevalence
Nevus
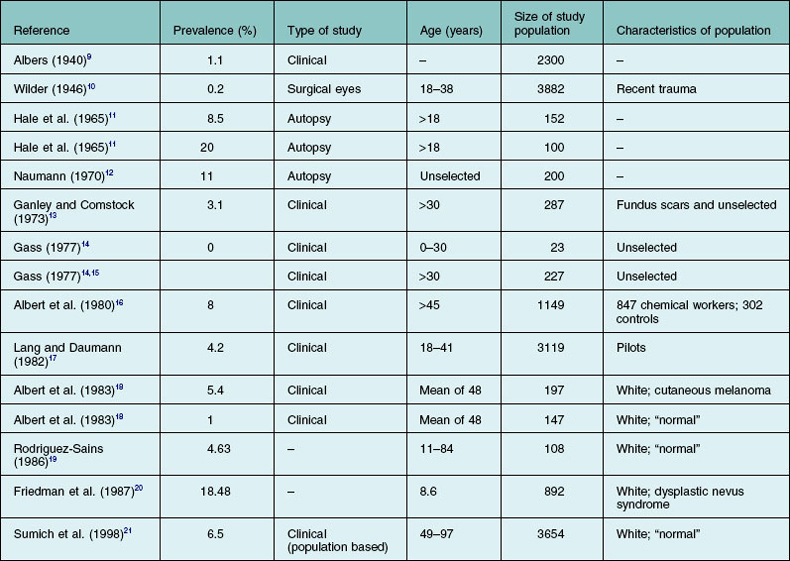
The exact prevalence of choroidal nevi has been variably estimated in clinical and histopathologic studies (Table 138.1).9–21 The Blue Mountain Eye Study, a population-based study, demonstrated a 6.5% prevalence in an older, largely white population.21 Generally, higher rates are found in histopathologic studies. Naumann et al.22 attributed the clinical underevaluation to the obscuring of lesions by retinal pigment epithelium (RPE) and choriocapillaris, lack of adequate contrast between the nevus and the surrounding normal choroid, and the hypopigmentation of many nevi. In their series, only 10 out of 29 nevi were clinically detected in eyes with clear media. Other factors contributing to the discrepancy are probably related to the examining technique, variations in the criteria for identifying a nevus, and characteristics of the populations under study (Table 138.1). Like cutaneous nevi, uveal nevi are rare among blacks. Except for melanocytosis and, presumably, melanocytomas, choroidal nevi are not seen at birth and, like acquired cutaneous nevi, develop or become pigmented in the first three decades of life.14 No significant epidemiologic data establish a relation to sex or color of skin, hair, or iris.11–14,16 The influence of solar exposure, chemicals, and endogenous or therapeutic hormones on the formation of nevi is still to be established.16,23
Halo nevus
In a review of 3422 eyes with choroidal nevi by Shields et al., 4.7% were halo nevi.24 As with most other choroidal nevi, they were found almost exclusively in Caucasians. A predilection for women (71%) was also found.24
Giant choroidal nevus
In the Blue Mountain Eye Study, only 1.5% of choroidal nevi were larger than 4 mm, although measurements above this cutoff were not specified.21 In a review of 4100 patients with choroidal nevi by Shields et al., 8% met the criteria for giant choroidal nevus; however, this probably represents a referral bias as large nevi are likely to be referred to ocular oncology centers for melanoma evaluation.6
Melanocytoma
Melanocytomas represented only five of 907 pigmented intraocular tumors examined histopathologically by Howard and Forrest.25 Melanocytomas differ from choroidal nevi or melanomas in that they occur relatively more frequently in blacks and tend to grow more rapidly than typical nevi.25–28 Although probably congenital, they usually are not seen before the third decade of life.
Ocular melanocytosis
The prevalence of ocular melanocytosis in clinical series was determined to be 0.038% (two cases per 5251 persons) in whites, 0.014% in blacks (one case per 6915 persons), and 0.4% to 0.84% in Japanese.29–32 However, the diagnosis of melanocytosis in blacks is difficult to ascertain, and the prevalence of ocular melanocytosis in blacks is believed to be higher than the figure stated above.
Choroidal nevi and systemic disease
Uveal nevi and neurofibromatosis
Uveal nevi are considered to be quite common in association with neurofibromatosis. Melanocytes, composing the nevi, share a neural crest origin with the other tumors found in von Recklinghausen disease (neurocristopathies).33–35
Dysplastic nevus syndrome
Reese36 reported an association of uveal nevi with cutaneous nevi. The observation that cutaneous dysplastic nevi are related to increased risk of cutaneous melanoma gave a new impetus to the investigation of the association of ocular and cutaneous nevi. The importance of early diagnosis and prompt intervention in cutaneous melanoma made necessary an intensive evaluation of melanoma-prone patients.23,37 This led to the identification of families genetically predisposed to melanoma and to the characterization of clinicopathologically distinctive nevi – the dysplastic nevi. Clinically, a dysplastic nevus must be suspected if a melanocytic nevus harbors at least two of the following four features1,38,39: (1) ill-defined or irregular borders; (2) irregular pigmentation; (3) accentuated skin markings, and (4) large size (>5 mm).1 They tend to occur on sun-shielded skin (e.g., the scalp or bathing trunk area) (Fig. 138.1B).
Histopathologically, dysplastic nevi are divided into two types: (1) a mild type with aberrant differentiation (e.g., lentiginous melanocytic hyperplasia), and (2) a severe type with melanocytic nuclear atypia, in addition to aberrant differentiation.1 These dysplastic nevi are markers of both familial and nonfamilial melanoma risk.40
That the uvea might be involved in familial melanoma or dysplastic nevus patients is suggested by the following: (1) a report of a type of dysplastic nevus syndrome with primary malignant melanomas of the choroid and the uvea41; (2) an increased (although not statistically significant) frequency of iris nevi and choroidal nevi in patients with cutaneous melanoma,18 and (3) a statistically significant increase in the percentage of dysplastic nevus syndrome in patients harboring iris and choroidal nevi.19 Although inconclusive and controversial,42 these data should lead to an attentive ophthalmologic evaluation in patients with dysplastic nevi.
Paraneoplastic bilateral diffuse uveal melanocytic proliferations
Bilateral diffuse uveal melanocytic proliferation (BDUMP) is a rare paraneoplastic syndrome described in elderly patients with advanced systemic carcinomas.43–46 Gass47 does not consider these tumors to be malignant on the basis of the relatively benign appearance of spindle cells and the rare mitotic figures. However, Zimmerman32 mentions foci of plump spindle B and epithelioid cells in these tumors. No metastases from these uveal tumors have been found at autopsy. In these cases, patchy areas of degeneration of the RPE and photoreceptors account for the rapid irreversible visual loss, although a bilateral cataract is generally present. (Bilateral diffuse melanocytic proliferation is discussed in Chapter 154, Miscellaneous uveal tumors.)
Halo nevi
The development of the depigmented halo has been postulated to be secondary to an immune response, such as that incited by prior antigenic exposure to cutaneous melanoma, as there is a higher association of a prior diagnosis of cutaneous melanoma in patients with choroidal halo nevi. No increased association with systemic autoimmune diseases has been found.1,5,48
Histopathology
Cytology
Adult melanocytes are normally found in the suprachoroidal lamellae, around blood vessels and in the outer layer of the choroidal stroma.4 These stellate cells with long processes contain oval membrane-bound melanin granules.49 Immunohistochemical staining against S-100 antigen is the most sensitive way to label melanocytic cells of neural crest origin, such as nevus and melanoma cells. Nevus cells are larger and show other morphologic differences from normal melanocytes.4 Naumann et al.50 classified nevus cells into four main cell types.
Plump polyhedral nevus cells
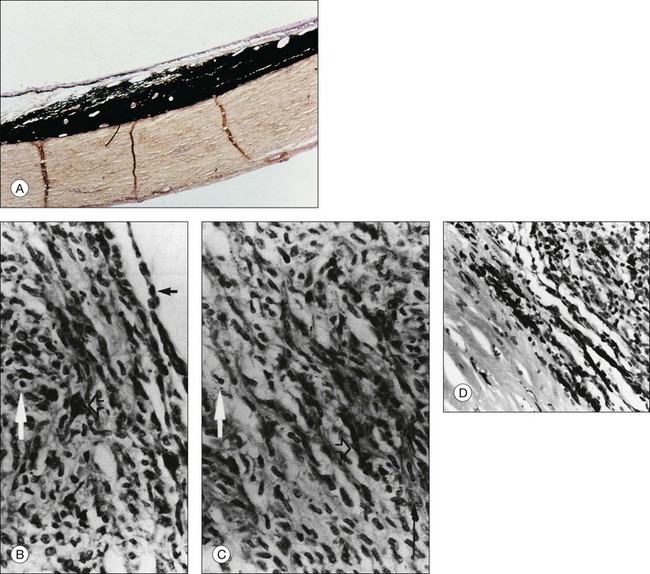
Plump polyhedral nevus cells are the most common nevus cells and usually represent about two-thirds of the mass. Their voluminous cytoplasm is filled with melanin. Bleaching with potassium permanganate is necessary to visualize the small, round, and uniform basophilic nuclei that lack prominent nucleoli (Fig. 138.2A).22
Electron microscopic studies have demonstrated ultrastructural differences in the cells composing melanocytomas. Juarez and Tso,51 studying enlarging melanocytomas of the optic disc or uvea, disclosed the following two cell types:
1. Type 1 cells are large, polyhedral, and heavily pigmented. They possess round nuclei without nucleoli, few cytoplasmic organelles, and prominent giant melanosomes. These characteristics suggest an inactive state.
2. Type 2 cells are small, spindle-shaped, and sparsely pigmented. They possess marked infoldings of the nuclear membrane, conspicuous nucleoli, abundant cytoplasmic organelles, and small melanosomes. These characteristics suggest high metabolic activity.
Slender spindle nevus cells
Slender spindle nevus cells are the second most common cell type. These small, spindle-shaped, lightly pigmented or unpigmented cells have a slender, intensely basophilic nucleus, and are often distributed in the outer portions of nevi (Fig. 138.2B).1,22 When they make up the bulk of a nevus, the nevus is often amelanotic.1,22,52
Intermediate nevus cells
Between plump polyhedral and slender spindle-shaped nevus cells, there exists a spectrum of cells with larger nuclei showing a less marked chromatin pattern and occasionally small nucleoli. The cytoplasmic volume and pigmentation are at intermediate amounts between those of the principal nevus cell types. According to the shape of their cytoplasmic border, they are called either plump fusiform or plump dendritic nevus cells (Fig. 138.2B,C).1,22
Balloon cells
Balloon cells are large, nonpigmented cells with abundant foamy cytoplasm (Fig. 138.2B,C). They are found in the peripheral halo surrounding skin nevi, cutaneous and choroidal melanomas, and, presumably, halo nevi in the choroid.1,5,53 The significance of the cytoplasmic lipoid content is not certain.1 Some authors,48 reviewed by Green,1 have interpreted balloon cell transformation and halo nevi as the consequence of an autoimmune response to the antigens of abnormal melanocytic cells. The finding of a halo nevus associated with cutaneous melanoma and vitiligo-like leukoderma is consistent with these hypotheses.53,54
Secondary histologic changes in the neighboring tissues
Choriocapillaris
Thick nevi induce slight narrowing and, in some cases, complete obliteration of the choriocapillaris.22,55
Drusen
Drusen formation is frequently seen in the overlying Bruch’s membrane; it was seen in 27 of the 101 nevi reported by Naumann et al.22 Drusen most often are an indication of chronicity and an inactive tumor.14
Retina and retinal pigment epithelium
The RPE overlying nevi can either degenerate or proliferate slightly. In choroidal nevi and melanomas, clumps of lipofuscin have been shown in the RPE and in macrophages overlying the tumor.22,56–58 This is more likely to occur in melanomas,22,56–58 the orange pigment being most commonly associated with risk for tumor growth. Serous detachment of the RPE15,59 and the neurosensory retina can be observed. In rare instances, choroidal neovascularization may cause these detachments.1,22,57,60–62 Disturbances of the retina are sometimes seen, which include thinning, loss of photoreceptors, and intraretinal edema.22,63
Controversial aspects
Do all choroidal melanomas arise from nevi?
In 1966, Yanoff and Zimmerman,64 reviewing the previous literature and 100 consecutive malignant melanomas of the choroid or ciliary body, found a significant component of small, benign-appearing, and spindle-shaped nevus cells at the periphery and along the scleral edge in 73 cases. They inferred that “most malignant melanomas, perhaps all such neoplasms, have their origin in pre-existing nevi”.64 Subsequent additional cases of uveal melanomas associated with nevi have been reported.32 This has led many clinicians to undertake follow-up of quiescent nevi.
In 1972, Albert et al.65 examined the eyes of a 36-year-old white man with multiple bilateral choroidal metastases of a skin melanoma and found a nevus-like configuration at the base of the metastatic lesion. On previous fundus examination, no nevus was seen. Albert et al.66 subsequently induced nevus-like structures at the base of experimental choroidal tumors. In a case report,67 a large choroid melanoma arose over a 16-month period. Previous fundus photographs and angiograms documented the absence of any pigmented tumor in this fundus. A pigmented nevus-like structure again was found at the base of the tumor (Fig. 138.2D).67 The possibility that pre-existent nevi had escaped clinical examination, as claimed in a similar case by Yanoff and Zimmerman,64 was unlikely.67
A nevus-like configuration associated with a melanoma may have several interpretations:
1. In many instances, including skin melanomas, melanomas may arise from nevi.
2. In other instances, choroidal or skin melanomas arise de novo. At their base and periphery, these can induce a nevus-like structure by flattening normal uveal melanocytes or by the modification of the tumor cells while they infiltrate the sclera (Fig. 138.2D).
3. Nevus-like structures may result as a secondary proliferative effect of the malignancy or because of a common oncogenic stimulus.32 These mechanisms have been postulated in a few cases of bilateral diffuse melanocytic tumors of the uvea composed of relatively benign-looking, spindle-shaped cells in patients with systemic carcinoma.68,69
Ocular and oculodermal melanocytosis are conditions that predispose to choroidal melanoma. Malignant transformation was described in 4.6% of the reported cases of nevus of Ota8,29,35,70–72 and, with rare exception,70 the melanoma occurred in the pigmented eye. Orbital, meningeal, and cerebral melanomas have also been reported in patients with oculodermal melanocytosis.35,49,73–76 Zimmerman2 states that ocular melanocytosis, unlike racial uveal pigmentation, is associated with an increased risk of melanomas, even in blacks.
The occurrence of uveal melanomas in patients with neurofibromatosis has been reported in rare cases34,35; one of these was associated with nevus of Ota.33
Malignant changes in melanocytomas are unusual; these tumors are described as being at the more benign end of the nevus spectrum.1,32 Zimmerman called attention to Roth’s report, of eight uveal melanomas arising from melanocytomas and questioned the reported presence of plump polyhedral cells in these tumors. He suggested that the melanomas probably arose from populations of spindle-shaped nevus cells. Yet, a few reports of malignant transformation of melanocytomas do exist.32,34,77–79 In these cases, the type 1 cell population described in the section on plump polyhedral nevus cells is more likely to account for the malignant potential. (Additional material concerning the malignant transformation of melanocytomas is included in Chapter 135, Melanocytoma of the optic disc.)
Reappraisal of the Callender classification
The second controversial point is related to the modification of the Callender classification in which the spindle A and spindle B subgroups were combined as the spindle cell type.14,80 The usefulness of Callender’s classification of choroidal melanomas into the categories of spindle A, spindle B, epithelioid, or mixed tumors has been called into question by Jensen,81 Gass,14 and McLean et al.45 for many reasons. One of the criticisms was that, although none of the initial spindle A melanomas proved fatal, Callender did not classify them as benign or describe a spindle cell nevus type.82 Gass14 was reluctant to accept the survival curve reported by Paul et al.83 from the mortality data of the spindle A portion of 2652 patients (i.e., 81.2% were survivors at 15 years). He argued that: (1) only a small sample of the tumor is generally studied, leaving many areas that could contain epithelioid cells unexamined; (2) many differences exist in the interpretation of the cell types by pathologists; (3) no more than 50% of the deaths are tumor-related because most patients are elderly and are prone to multiple neoplasms.14 McLean et al.80 re-evaluated the sections of 105 tumors, previously called spindle A melanomas, which had been followed up for at least 5 years after their original diagnosis. They found that 15 tumors contained epithelioid cells and should be classified as mixed tumors. The other 90 tumors were divided into the following two groups:
1. The first group, consisting of 15 tumors, had the following benign cytologic features: small size, slender nucleus, fine structure of nuclear chromatin, inconspicuous nucleoli, and no mitotic activity. These tumors measured no more than 10 mm in diameter and 3 mm in elevation. They were consequently reclassified as spindle cell nevi. No tumor-related death occurred in this group of patients.
2. The second group of tumors had atypical spindle cells, increased nuclear-to-cytoplasmic ratio, clumped chromatin, and distinct nucleoli. These were called spindle cell melanomas. In this group, 11 tumor-related deaths occurred, and metastases composed of spindle A cells were documented.80
According to the interpretation of McLean et al.,80 not all spindle A melanomas are benign, as was also suggested by Gass.14
Updated classification
As with iris tumors, histopathologic descriptions of choroidal melanocytic proliferations should be re-evaluated, and the spectrum of lesions should be reclassified.84 The difficulty lies in the fact that iris tumors can be detected early and accurately followed-up, and, when indicated, a biopsy can be performed. In contrast, most choroidal nevi are examined histopathologically after enucleation, when a prognostic evaluation is less crucial. A suggested histopathologic classification could be as follows:
• Melanocytosis composed of normal melanocytes and plump polyhedral cells (low risk of transformation).
• Melanocytomas composed exclusively of plump polyhedral cells. Without type 2 cells they are quiescent and have no risk of malignant transformation. With type 2 cells they are active and growing and have an exceptional risk of malignant change.
• Mixed nevus with both plump polyhedral, spindle-shaped, and intermediate cells. Subclassification should be considered regarding the presence of balloon cells, which may indicate an immunologic response.
• Spindle cell nevi composed of cells with the above-described benign features.22,32,80
In a few instances, the histopathologic diagnosis of a lesion will be between a spindle cell nevus and a spindle cell melanoma. In such cases, the clinical data, with particular attention to the growth of the tumor, would be critical for determining malignant potential.32,43 In 1994, Rummelt and coworkers85 showed that the vascular pattern of choroidal tumors seen on light microscopy might help to differentiate malignant and benign features. They found that the presence of “normal” vessels, a “silent” pattern (i.e., zones of avascularity), straight and parallel vessels, and the lack of closed vascular loops and networks were correlated with the diagnosis of a nevus. With the major advances in recognizing significant genetic mutations in choroidal melanomas, it seems likely this will be applied to more definitively define nevi in the future.
Clinical findings
Functional repercussions
Most nevi are incidental findings made during systematic ocular examination. However, some nevi can lead to decreased visual acuity and induce a visual field defect. In patients with choroidal nevi, the percentage of patients with defects in the visual field attributed to the nevi has varied considerably. Visual field defects were found in 16 (38%) of the 42 patients of Tamler and Maumenee86,87; in 19 (86%) of 22 patients of Flindall and Drance88; in 20 (24%) of 84 patients of Naumann et al.50; in 11% of 206 patients of Gonder et al.,63 and in 148 (4.3%) of 3422 eyes in a review by Shields et al.24 Decreased visual acuity related to choroidal nevi was found in 215 (6.5%) of 3422 eyes.24 Visual acuity loss occurred in 2% of patients with extrafoveal nevi and 26% of patients with subfoveal nevi in 2334 eyes in reviews by Shields et al.89 Mechanisms of visual loss are related to functional or anatomic disturbances in the overlying RPE and retina.22,45,63
Clinical presentation
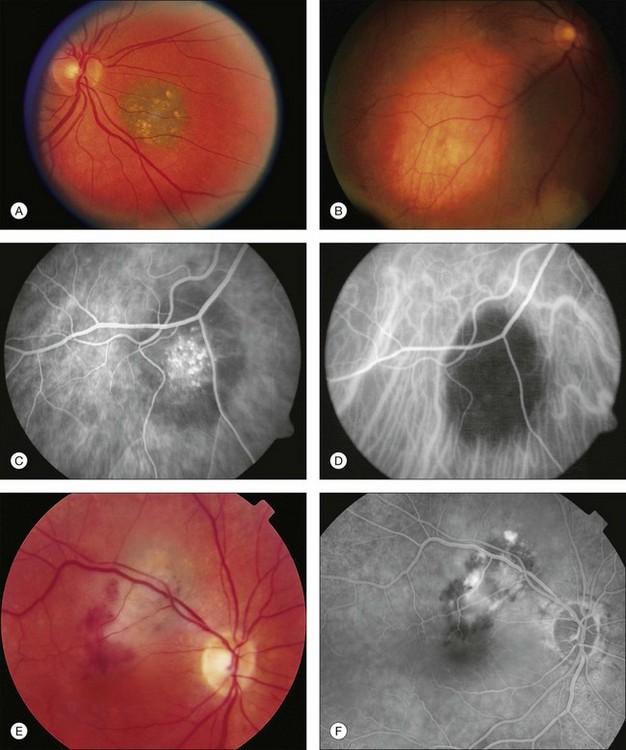
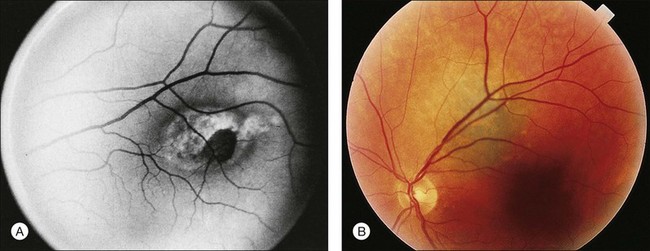
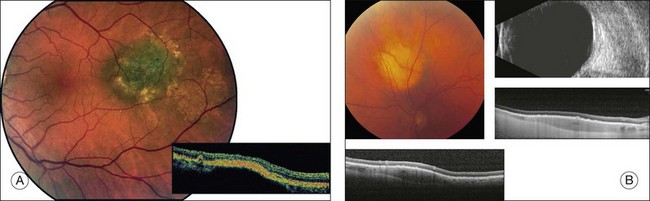
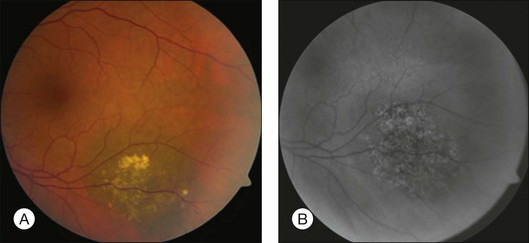
Choroidal nevi usually appear on ophthalmoscopy as flat or slightly elevated slate-gray tumors, with defined but not sharply demarcated margins (Fig. 138.3A). They are generally located at the posterior pole and vary in size between one-third disc diameter and seven disc diameters. They are usually oval and not more than 2 mm thick.21,22 Exceptional cases of giant choroidal nevi, diffuse uveal nevi, or melanocytomas have been reported.6,57 The pigmentation of nevi may vary considerably; melanocytomas are usually heavily pigmented, while in white patients, amelanotic nevi (Fig. 138.3B) are not unusual: 10% in 3422 eyes of patients evaluated by Shields et al.24 and 5.1% in the series of 373 patients of Brown et al.52 In halo nevi, the lesion is surrounded by a depigmented yellow ring (Fig. 138.4A). Other associated findings include the presence of drusen (Fig. 138.3A), RPE disturbances, orange pigment (Fig. 138.4B), subretinal fluid, and choroidal neovascularization (Fig. 138.3E,F), which will be discussed further in the following sections. These findings were present in similar percentages among all age groups except for drusen, which is more prevalent in older patients.24
Secondary changes in the overlying tissues
Secondary changes in the overlying tissues can result in the appearance of irregular pigmentation of the nevus. Green light photographs have proved helpful in demonstrating some of these changes.90 Fluorescein angiography or indocyanine green angiography of nevi may demonstrate hypofluorescence induced either by the pigmentation of the nevus or by a local circulatory disturbance in the choroid. A mottled appearance of the nevus or hyperfluorescence from drusen and retinal pigment epithelial defects is sometimes seen (Fig. 138.3C,D).55,58
Retinal pigment epithelial and Bruch’s membrane changes
Drusen overlying pigmented nevi are common and were seen in 50–60% of the patients in the series of Naumann et al.,22 Shields et al.,24 and Mashayekhi et al. (Fig. 138.3A).91 Discrete areas of pigment epithelial atrophy or bone-spicule pattern of pigment migration at the margin of a nevus may occur due to prolonged associated serous detachment. Gass suggests that this damage may be greatest during the period of maximum tumor growth early in a patient’s life.14
Atrophy, hyperplasia, and fibrous metaplasia of the RPE can be seen above and adjacent to choroidal nevi, and each was observed in 6–10% of cases in a review by Shields et al.24 and Mashayekhi.91 These findings may indicate chronicity of the lesion and are more likely to be associated with choroidal nevi than melanomas.
Orange pigment, corresponding to lipofuscin, can be seen overlying melanocytic lesions (Fig. 138.4B), and was observed in 6% of 3422 eyes in a review by Shields et al.24 and in 4% of 240 small choroidal melanocytic lesions in a study by Singh et al.92 Large geographic areas of orange pigment are more commonly found above uveal melanomas56 and, according to Smith and Irvine,58 Gass,57 and Shields and Shields93 may indicate malignant transformation or the malignant nature of a suspicious lesion.
Serous detachment
In rare instances, a serous detachment of the overlying RPE or even of the neurosensory retina can be found; 2.14% of 933 nevi in the series of Pro et al.59 and 10.3% of 3422 eyes in a review by Shields et al.24 were found to have subretinal fluid. Fluorescein angiograms demonstrate the classic features of these detachments with localized or diffuse leaks. These leaks may be related to the chronic, degenerative changes in the RPE.15,59
Clinical differential diagnosis
The diagnosis of choroidal nevi is usually easily made with ophthalmoscopic examination. In rare instances, various other types of lesions can mimic nevi. In addition to the conditions discussed in the following paragraphs, material on the differential diagnoses of choroidal nevi can be found in the following chapters: Chapter 136, Congenital hypertrophy of the retinal pigment epithelium; Chapter 137: Combined hamartoma of the retinal pigment epithelium and retina; Chapter 151, Choroidal metastases; Chapter 153, Circumscribed choroidal hemangioma, and in the sections examining choroidal melanomas, see Chapter 144, Enucleation for choroidal melanomas.
Freckles
Freckles are flat foci of increased choroidal pigmentation with irregular borders. Histologically, there is no hyperplasia of uveal melanocytes, but there is an increase in volume and pigmentation of the melanocytes. The main relevancy of this distinction is the lack of malignant potential of these lesions.93
Subretinal hemorrhages
While red-colored hemorrhages are rarely mistaken for nevi, large areas of dark-colored hemorrhagic detachment of the RPE may be mistaken for a large nevus or melanoma.57 Diagnostic ultrasonography is usually helpful in demonstrating high reflectivity of hemorrhage versus the medium to low reflectivity of a melanocytic tumor. Optical coherence tomography (OCT) can be helpful as well.
Congenital hypertrophy of the retinal pigment epithelium
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) and CHRPE-like lesions may be confused with nevi, but are usually able to be distinguished on ophthalmoscopic examination. CHRPE are typically unilateral, darkly pigmented, round, well-circumscribed, flat lesions at the level of the RPE. They often have a surrounding pigmented or nonpigmented halo, or a double halo consisting of pigmented and nonpigmented rings. Intralesional lacunae are often present and may enlarge. CHRPE-like lesions, associated with familial adenomatous polyposis (FAP), are similar in appearance, but are bilateral, multiple, and pisciform in shape.95
Small melanomas
The clinical distinction between a small melanoma and a large nevus, which may also be histopathologically difficult, must be considered both at the time of initial examination and during the follow-up examinations. While small melanocytic lesions classified as choroidal nevi may show slow, limited growth, especially in a younger patient,14 the growth of an indeterminate lesion (large nevus versus small melanoma) is generally the determinate factor in re-classifying the lesion as a melanoma and considering treatment.86,87,96,97 Clinical observations that may help differentiate a small melanoma from a choroidal nevus are indications of dormancy (nevus) or growth (melanoma). A melanoma should be suspected if: (1) the thickness of the tumor is >2 mm86,87,98–100; (2) there is orange pigment overlying the tumor (Fig. 138.4B)14,57,58; (3) a neurosensory detachment is present without evidence of choroidal neovascularization, or (4) visual symptoms are present.21,99 Evidence of biologic dormancy favoring a diagnosis of nevus includes: (1) drusen overlying the tumor14; (2) choroidal neovascularization,101 and (3) smaller size.
Ancillary studies
Careful follow-up observation with photography is most important for accurate determination of growth and the malignant nature of melanocytic tumors. However, slight misalignment off axis of the fundus camera can filter the pigmented edges of melanocytic tumors, while the details of the retina are unaffected. If the camera is misaligned in subsequent photographs, the edges would not be filtered and the lesion would appear larger. This may lead to an erroneous diagnosis of growth or malignant transformation, with subsequent unnecessary treatment.102 Other diagnostic tests may be helpful in the initial evaluation and follow-up of a presumed nevus. Ultrasound is most valuable in determining tumor thickness, ruling-out extraocular extension, and documenting subsequent growth. As mentioned above, the demonstration of growth is important for the determination of malignancy. Additionally, accurate determination of lesion height may help the initial classification of the lesion; a lesion >2.0 mm in height is more likely to be a small melanoma.14,86,87,98–100 However, it is not generally helpful for distinguishing a nevus that is <2.0 mm in height from a melanoma. Acoustic hollowness on ultrasound is also more suggestive of melanoma than a choroidal nevus.103 Magnetic resonance imaging is rarely helpful in the evaluation of a nevus.104
Redlight photography has been described as helpful in the analysis of choroidal vascularization.55 The vascularization of choroidal tumors has been studied with indocyanine green (ICG) angiography. Choroidal melanomas may demonstrate abnormal vascular patterns such as dilation, tortuosity, vascular loops, and branching105; delayed maximal fluorescence106; and marginal late dye leakage.107 Confocal ICG scanning enables serial sectioning through the tumor. This technique may be capable of further detecting microvascular patterns predictive of growth.108 A small flat choroidal nevus may appear hypofluorescent throughout the ICG angiogram (Fig. 138.3D).109
Fluorescein angiography of choroidal nevi without overlying RPE changes, occupying the inner or full thickness of the choroid, will demonstrate hypofluorescence throughout the angiogram. Overlying drusen usually fluoresce early in the angiogram and stain in the late stages (Fig. 138.3C). Fluorescein angiography is helpful in demonstrating choroidal neovascularization (Fig. 138.3F), RPE alterations or permeability of the RPE overlying a nevus.57,110 Multiple points of leakage (hot spots) from the surface of a tumor may indicate more acute pigment epithelial damage and a higher probability of growth.14,99
Standard OCT is not helpful for detecting the internal characteristics of melanocytic tumors.111 OCT readily demonstrates retinal and RPE/choriocapillaris abnormalities associated with choroidal nevi. Retinal findings include intraretinal cysts, retinal edema, retinal thinning, photoreceptor attenuation, and subretinal fluid (Fig. 138.5A).112 Abnormalities of the RPE/choriocapillaris include drusen, RPE fragmentation and detachments, and thickening of the RPE/choriocapillaris (Fig. 138.5A).112 In a review by Shields et al.112 regarding the OCT qualities in 120 patients with choroidal nevi, two-thirds of the nevi were hyporeflective, one-third were isoreflective, about 10% were hyperreflective, and about half revealed hyperreflectivity at the level of the RPE/choriocapillaris. OCT is more sensitive than ophthalmoscopy in detecting almost all retinal/RPE abnormalities except for the presence of drusen.112 Retinal edema, photoreceptor attenuation, and drusen suggest chronicity, whereas subretinal fluid without retinal atrophy may suggest a more active lesion.111,112 EDI SD-OCT (enhanced depth imaging spectral-domain optical coherence tomography), a method that allows for the evaluation of deeper portions of the choroid, may be useful in measuring the diameter and height of lesions that are too small to be detected by ultrasonography (Fig. 138.5B).113 The usefulness of EDI SD-OCT to differentiate choroidal nevi from melanomas remains to be seen.113
Fundus autofluorescence (FAF) imaging is a relatively new technique that allows visualization of light emitted from certain substances, particularly lipofuscin, that possess autofluorescent properities.114 No characteristic autofluorescent patterns were found for choroidal nevi or melanoma in one small study.115 However, factors that favor for or against malignancy may be seen. Drusen may be slightly hyper-, iso- or hypofluorescent, while orange pigment is brightly autofluorescent and much brighter than the fluorescence seen with drusen (Fig. 138.6).116,117 In amelanotic lesions, lipofuscin may appear clinically as hyperpigmented areas and go unrecognized without the use of FAF imaging.118 Subretinal fluid is hyperfluorescent with the peripheral rim of fluid being slightly more hyperfluorescent. Hypofluorescence was found in more chronic RPE degenerative changes, such as RPE hyperplasia, fibrous metaplasia, and RPE atrophy.116,117
Other imaging techniques, such as duplex ultrasound and color Doppler, may help in the differential diagnosis of choroidal tumors by quantifying tumor blood flow. Wolff-Korman and colleagues119 detected a pulsatile blood flow at the tumor base of 62 choroidal melanomas where no Doppler signals were elicited in a series of 18 choroidal nevi.
As advocated by most authors,14,32,57 the best way to determine the malignant nature of a melanocytic tumor is a careful follow-up, showing either significant growth or a virtually unchanged appearance. However, benign nevi may show limited, slow growth,14,91,103 and a definite distinction between a nevus and a small melanoma may be problematic.
Natural history
In a review by Shields et al., the presence of subretinal fluid, orange pigment, or RPE changes was similar throughout all age groups; however, nevus thickness and the presence of drusen were greater with increasing age.24 In reviews by Masheyehki et al.91 and Shields et al.,103 slow growth of choroidal nevi over many years or decades, without the presence of other risk factors, may not be indicative of malignant transformation. Gass noted that growth occurred more often in younger patients.16 The finding that, like some cutaneous melanomas, choroidal melanomas could arise from pre-existent benign nevi has led to follow-up studies of choroidal nevi. However, according to the calculations of Ganley and Comstock,13 only one of 4800 patients with choroidal nevi will develop a malignant change in a 10-year period. Singh et al. estimated that one in 8845 will undergo malignant transformation.120 A population-based study in Australia utilizing stereoscopic photos of the posterior pole demonstrated growth in only one of 160 nonsuspicious choroidal nevi (0.6%) over a 5-year follow-up period.121
An attempt has been made to establish the criteria for follow-up of nevi by more accurately defining suspicious nevi.96 The presence of any two of the following features is considered evidence of a suspicious nevus: (1) largest diameter between two and five disc diameters96; (2) thickness >2 mm,93 and (3) significant effects on the overlying structures,14,57,93,96 with, most importantly, the presence of orange pigment on the tumor surface (Fig. 138.4B).57
Augsburger and coworkers98 concluded that several clinical parameters can be used to predict lesion enlargement: (1) lesion thickness (exceeding 1.5 mm); (2) presence of exudative retinal detachment; (3) presence of symptoms; (4) proximity to the optic nerve (within two disc diameters), and (5) the presence of orange pigment clumps on the surface of the nevus.98 Augsburger et al.98 showed that the incidence of enlargement was 90.6% in patients with unfavorable prognostic parameters, although patients with none of these features had only a 5.8% 5-year incidence of lesion enlargement.
Shields et al. have similarly identified risk factors predictive of growth of small choroidal melanocytic tumors.122 If three or more factors are present, they estimate a greater than 50% risk of growth.123 Additional factors predictive of growth were later found by Shields et al., and include ultrasonographic hollowness and the absence of drusen or surrounding halo. The mnemonic TFSOM UHHD for the phrase: “To Find Small Ocular Melanomas Using Helpful Hints Daily” correlates to Thickness >2 mm; subretinal Fluid; visual Symptoms; Orange pigment; tumor Margin within 3 mm of the optic disc; Ultrasonographic Hollowness; absence of a surrounding Halo, and absence of Drusen.103 Factors predictive of metastases include posterior tumor margin touching the optic disc, documented growth, and greater tumor thickness.123
Management of nevi
Suspicious nevi
Evaluate suspicious nevi by performing fundus photographs (Fig. 138.4B) and ultrasound. Fluorescein angiography (Fig. 138.3C), ICG angiography (Fig. 138.3D), fundus autofluorescence (Fig. 138.6A,B) and OCT (Fig. 138.5A) may be of value. Perform ophthalmoscopy and fundus photography every 6 months to evaluate for suspicious changes, such as growth.
Serous detachment and choroidal neovascular membrane
Due to the rarity of choroidal neovascularization (Fig. 138.3E,F) and serous retinal detachments associated with choroidal nevi, treatment options of these conditions has not been well studied. Intravitreal injection of anti-VEGF agents has widely been used for treatment of CNVM associated with a variety of conditions, particularly exudative macular degeneration and degenerative myopia, and also appears to be effective for treatment of CNVM associated with choroidal nevi.124 Photodynamic therapy with verteporfin may also be effective for the treatment of choroidal neovascularization secondary to choroidal nevus.124,125 In cases of angiographically demonstrated leaks, argon laser photocoagulation can be directed to those areas, according to criteria similar to those used in the treatment of central serious choroidopathy.15,59 Successful treatment of CNVM using transpupillary thermotherapy has also been reported.126
![]() For online acknowledgments visit http://www.expertconsult.com
For online acknowledgments visit http://www.expertconsult.com
1 Green W. Uveal tract. In: Spencer W, ed. Ophthalmic pathology: an atlas and text. Philadelphia: WB Saunders, 1997.
2 Zimmerman L. Melanocytes, melanocytic nevi, and melanocytomas. Invest Ophthalmol. 1965;4:11–44.
3 Robbins S, Cotran R, Kumar V. Pathologic basis of disease. Philadelphia: WB Saunders; 1984.
4 Torczynski E. Choroid and suprachoroid. In: Jakobiec F, ed. Ocular anatomy, embryology, and teratology. Philadelphia: Harper & Row, 1982.
5 Shields CL, Maktabi AM, Jahnle E, et al. Halo nevus of the choroid in 150 patients. Arch Ophthalmol. 2010;7:859–864.
6 Li HK, Shields CL, Mashayekhi A, et al. Giant choroidal nevi: clinical features and natural course in 322 cases. Ophthalmology. 2010;117:324–333.
7 Cogan D. Discussion of pigmented ocular tumors. In: Boniuk M, ed. Ocular and adnexal tumors: new and controversial aspects. St Louis: Mosby, 1964.
8 Dutton J, Anderson R, Schleper R, et al. Orbital malignant melanoma and oculodermal melanocytosis: report of two cases and review of the literature. Ophthalmology. 1984;90:497–507.
9 Albers E. Benign melanomas of the choroid and their malignant transformation. Am J Ophthalmol. 1940;23:779–783.
10 Wilder H. Intraocular tumors in soldiers: World War II. Milit Surg. 1946;99:459–490.
11 Hale P, Allen R, Straatsma B. Benign melanoma (nevi) of the choroid and ciliary body. Arch Ophthalmol. 1965;74:532–538.
12 Naumann G. Pigmentierte naevi der aderhaut und des ciliarkorpers. Adv Ophthalmol. 1970;23:187–272.
13 Ganley J, Comstock G. Benign nevi and malignant melanomas of the choroid. Am J Ophthalmol. 1973;76:19–25.
14 Gass J. Problems in the differential diagnosis of choroidal nevi and malignant melanomas. Am J Ophthalmol. 1977;83:299–323.
15 Folk J, Weingeist T, Coonan P, et al. The treatment of serous macular detachment secondary to choroidal melanoma and nevi. Ophthalmology. 1989;96:547–551.
16 Albert D, Robinson N, Fulton A, et al. Epidemiologic investigation of increased incidence of choroidal melanoma in a single population of chemical workers. Int Ophthalmol Clin. 1980;20:71–92.
17 Lang G, Daumann F. The peripheral retina and choroid in “healthy eyes” (airline pilots). Klin Monatsbl Augenheilkd. 1982;181:493–495.
18 Albert D, Searl S, Forget B, et al. Uveal findings in patients with cutaneous melanoma. Am J Ophthalmol. 1983;95:474–479.
19 Rodriguez-Sains R. Ocular findings in patients with dysplastic nevus syndrome. Ophthalmology. 1986;93:661–665.
20 Friedman R, Rodriguez-Sains R, Jakobiec F. Ophthalmologic oncology: conjunctival malignant melanoma in association with sporadic dysplastic nevus syndrome. J Dermatol Surg Oncol. 1987;13:31–34.
21 Sumich P, Mitchell P, Wang J. Choroidal nevi in a white population: The Blue Mountain Eye Study. Arch Ophthalmol. 1998;116:645–650.
22 Naumann G, Yanoff M, Zimmerman L. Histogenesis of malignant melanomas of the uvea. I. Histopathologic characteristics of nevi of the choroid and ciliary body. Arch Ophthalmol. 1966;6:784–796.
23 Consensus Conference. Precursors to malignant melanoma. JAMA. 1984;251:1864–1866.
24 Shields CL, Furuta M, Mashayekhi A, et al. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology. 2008;115:546–552.
25 Howard G, Forrest A. Incidence and location of melanocytomas. Arch Ophthalmol. 1967;77:61–66.
26 Shields J, Shields C, Eagle R, et al. Malignant melanoma associated with melanocytoma of the optic disc. Ophthalmology. 1990;97:225–230.
27 Yoshida K. Nevus fuscocaerulus ophthalmomaxillaris Ota. Tohoku J Exp Med. 1952;55:34–43.
28 Joffe L, Shields JA, Osher JH, et al. Clinical and follow up studies of melanocytomas of the optic disc. Ophthalmology. 1979;86:1067–1078.
29 Gonder J, Ezell P, Shields J, et al. Ocular melanocytosis: a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89:950–952.
30 Mishima Y, Mevorah B. Nevus of Ota and nevus of Ito in American Negroes. J Invest Dermatol. 1961;36:133–154.
31 Ota M, Tanino H. Nevus fuscocaerulus ophthalmomaxillaris. Tokyo Med J. 1939;63:1243–1245.
32 Zimmerman L. Malignant melanoma of the uveal tract. In: Spencer W, ed. Ophthalmic pathology: an atlas and text. Philadelphia: WB Saunders, 1986.
33 Croxatto J, Charles D, Malbran E. Neurofibromatosis associated with nevus of Ota and choroidal melanoma. Am J Ophthalmol. 1981;92:578–589.
34 Nordmann J, Brini A. Von Recklinghausen’s disease and melanoma of the uvea. Br J Ophthalmol. 1970;54:641–648.
35 Yanoff M, Zimmerman L. Histogenesis of malignant melanomas of the uvea. III. Relationship of congenital ocular melanocytosis and neurofibromatosis to uveal melanomas. Arch Ophthalmol. 1967;77:331–336.
36 Reese A. Association of uveal nevi with skin nevi. Arch Ophthalmol. 1952;48:271–275.
37 Greene M, Clark WJ, Tucker M, et al. Acquired precursors of cutaneous malignant melanoma: the familial dysplastic nevus syndrome. N Engl J Med. 1985;312:91–97.
38 Liu K, Peyman G, Mafee M, et al. False positive magnetic resonance imaging of choroidal nevus simulating a choroidal melanoma. Int Ophthalmol. 1989;13:265–268.
39 Kraemer KH, Tucker M, Tarone R, et al. Risk of cutaneous melanoma in dysplastic nevus syndrome types A and V. N Engl J Med. 1986;315:1615–1616.
40 Caporaso N, Greene M, Tsai S, et al. Cytogenetics in hereditary malignant melanoma and dysplastic nevus syndrome: is dysplastic nevus syndrome a chromosome instability disorder? Cancer Genet Cyogenet. 1987;24:299–314.
41 Albert D, Chang M, Lamping K, et al. The dysplastic nevus syndrome: a pedigree with primary malignant melanoma of the choroid and skin. Ophthalmology. 1985;92:1728–1734.
42 Taylor M, Guerry DI, Bondi E, et al. Lack of association between intraocular melanoma and cutaneous dysplastic nevi. Am J Ophthalmol. 1984;98:478–482.
43 Gass J, Geiser R, Wilkinson C, et al. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol. 1990;108:527–533.
44 Margo C, Pavan P, Gendelman D, et al. Bilateral melanocytic uveal tumors associated with systemic nodular malignancy: malignant melanomas or benign paraneoplastic syndrome. Retina. 1987;7:137–141.
45 McLean I, Zimmerman L, Evans R. Reappraisal of Callender’s spindle A type of malignant melanoma of the choroid and ciliary body. Am J Ophthalmol. 1978;86:557–564.
46 Rohrbach J, Roggendorf W, Thanos S, et al. Simultaneous bilateral diffuse melanocytic uveal hyperplasia. Am J Ophthalmol. 1990;110:49–56.
47 Gass J. Observations of suspected choroidal and ciliary body melanomas for evidence of growth prior to enucleation. Ophthalmology. 1987;80:1523.
48 Schrader W, Helwig E. Balloon cell nevi. Cancer. 1967;20:1502–1514.
49 Kojima K, Natsume C, Honda A, et al. A melanoma of the optic chiasm in a case of Ota’s nevus and melanosis oculi. Jpn J Clin Ophthalmol. 1959;13:502–504.
50 Naumann G, Zimmerman L, Yanoff M. Visual field defect associated with choroidal nevus. Am J Ophthalmol. 1966;62:914–917.
51 Juarez C, Tso M. An ultrastructural study of melanocytomas (magnocellular nevi) of the optic disc and uvea. Am J Ophthalmol. 1980;90:48–62.
52 Brown G, Shields J, Augsburger J. Amelanotic choroidal nevi. Ophthalmology. 1981;88:1116–1121.
53 Fournier G, Albert D, Wagoner M. Choroidal halo nevus occurring in a patient with vitiligo. Surv Ophthalmol. 1984;28:671–672.
54 Chang M, Fournier G, Koh H, et al. Ocular abnormalities associated with cutaneous melanoma and vitiligo-like leukoderma. Graefes Arch Clin Exp Ophthalmol. 1986;224:529–535.
55 Oosterhuis J, von Winning C. Naevus of the choroid. Ophthalmologica. 1979;178:156–165.
56 Font R, Zimmerman L, Armaly M. The nature of the orange pigment over a choroidal melanoma: histochemical and electron microscopical observations. Arch Ophthalmol. 1974;91:359–362.
57 Gass J. Stereoscopic atlas of macular disease. St Louis: Mosby; 1997.
58 Smith L, Irvine A. Diagnostic significance of orange pigment accumulation over choroidal tumors. Mod Probl Ophthalmol. 1974;12:536–543.
59 Pro M, Shields J, Tomer T. Serous detachment of the macula associated with presumed choroidal nevi. Arch Ophthalmol. 1978;96:1374–1377.
60 Waltman D, Gitter K, Yannuzzi L, et al. Choroidal neovascularization associated with choroidal nevi. Am J Ophthalmol. 1978;85:704–710.
61 Rubin M. Disciform lesions overlying melanocytoma simulating progression of choroidal melanoma. Trans Am Ophthalmol Soc. 1976;74:282–294.
62 Snip R, Green W, Jaegers K. Choroidal nevus with subretinal pigment epithelial neovascular membrane and a positive P-32 test. Ophthalmic Surg. 1978;9:35–42.
63 Gonder J, Augsberger J, McCarthy E, et al. Visual loss associated with choroidal nevi. Ophthalmology. 1982;89:961–965.
64 Yanoff M, Zimmerman L. Histogenesis of malignant melanomas of the uvea. II. Relationship of uveal nevi to malignant melanomas. Cancer. 1967;20:493–507.
65 Albert D, Gaasterland D, Caldwell J, et al. Bilateral metastatic choroidal melanoma, nevi and cavernous degeneration of the optic nerve head. Arch Ophthalmol. 1972;87:39–47.
66 Albert D, Lahav M, Packer S, et al. Histogenesis of malignant melanomas of the uvea: occurrence of nevus-like structures in experimental choroidal tumors. Arch Ophthalmol. 1974;92:318–323.
67 Sahel J, Pesavento R, Frederick A, et al. Melanoma arising de novo over a 16 month period. Arch Ophthalmol. 1988;106:381–386.
68 Barr C, Zimmerman L, Curtin V, et al. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms: a recently recognized syndrome. Arch Ophthalmol. 1982;100:249–255.
69 Mullaney J, Mooney D, O’Connor M, et al. Bilateral ovarian carcinoma with bilateral uveal melanomas. Br J Ophthalmol. 1984;68:261–267.
70 Blodi F. Ocular melanocytosis and melanoma. Am J Ophthalmol. 1975;80:389–395.
71 Albert D, Scheie H. Nevus of Ota with malignant melanoma of the choroid: report of a case. Arch Ophthalmol. 1963;69:774–777.
72 Bordon A, Wray M, Belfort R, et al. Choroidal malignant melanoma in association with oculodermal melanocytosis in a black patient. Br J Ophthalmol. 1995;79:191–192.
73 Enriquez R, Egbert B, Bullock J. Primary malignant melanoma of the central nervous syndrome: pineal involvement in a patient with nevus of Ota and multiple pigmented skin nevi. Arch Pathol. 1973;98:392–395.
74 Gomez-Gonzalez J, Roselli A, Rodriguez A. Melanosis oculodermica melanoblastosis leptomeninges y melanoma intracerebral primario. Universitats Media. 1959;7:47–52.
75 Haim T, Meyer K, Kerner Het, et al. Oculodermal melanocytosis (nevus of Ota) and orbital malignant melanoma. Ann Ophthalmol. 1982;14:1132–1136.
76 Sang DN, Albert DM, Sober AJ, et al. Nevus of Ota with contralateral cerebral melanoma. Arch Ophthalmol. 1977;95:1820–1827.
77 Barker-Griffith A, McDonald P, Green W. Malignant melanoma arising in a choroidal magnocellular nevus (melanocytoma). Can J Ophthalmol. 1976;11:140–146.
78 Cialdini A, Sahel J, Jalkh A, et al. Malignant transformation of an iris melanocytoma. Graefes Arch Clin Exp Ophthalmol. 1989;227:348–354.
79 Loeffler K, Tecklenborg H. Melanocytoma-like growth of a juxtapapillary malignant melanoma. Retina. 1992;12:29–34.
80 McLean I, Foster W, Zimmerman L, et al. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983;96:502–509.
81 Jensen O. Malignant melanoma of the human uvea: recent follow-up of cases in Denmark, 1943–1952. Acta Ophthalmol. 1970;48:1113–1128.
82 Callender G. Malignant melanocytic tumors of the eye: a study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolarnyngol. 1936;36:131–142.
83 Paul EL, Parnell B, Fraker M. Prognosis of malignant melanomas of the choroid and ciliary body. Int Ophthal Clin. 1962;2:387–410.
84 Jakobiec F, Silbert G. Are most iris “melanomas” really nevi? A clinicopathologic study of 189 lesions. Arch Ophthalmol. 1981;99:2117–2132.
85 Rummelt V, Folberg R, Rummelt C, et al. Microcirculation architecture of melanocytic nevi and malignant melanoma of the ciliary body and choroid: a comparative histopathologic and ultrastructural study. Ophthalmology. 1994;101:718–727.
86 Tamler E. A clinical study of choroidal nevi: a follow-up report. Arch Ophthalmol. 1970;84:29–32.
87 Tamler E, Maumenee A. A clinical study of choroidal nevi. Arch Ophthalmol. 1959;62:196–202.
88 Flindall R, Drance S. Visual field studies of benign choroidal melanoma. Arch Ophthalmol. 1969;81:41–44.
89 Shields CL, Furuta M, Mashayekhi A, et al. Visual acuity in 3422 consecutive eyes with choroidal nevus. Arch Ophthalmol. 2007;11:1501–1507.
90 Parver L. The clinical characteristics of presumed choroidal nevi observed in green light. Ophthalmology. 1979;86:1924–1930.
91 Mashayekhi A, Siu S, Shields CL, et al. Slow enlargement of choroidal nevi: a long-term follow-up study. Ophthalmology. 2011;118:382–388.
92 Singh AD, Mokashi AA, Bena JF, et al. Small choroidal melanocytic lesions: features predictive of growth. Ophthalmology. 2006;113:1032–1039.
93 Shields J, Shields C. Intraocular tumors: a text and atlas. Philadelphia: WB Saunders; 1992.
94 Mines J, Freilich D, Friedman A, et al. Choroidal (sub-retinal) neovascularization secondary to choroidal nevus and successful treatment with argon laser photocoagulation: case report and review of the literature. Ophthalmologica. 1985;190:210–218.
95 Shields CL, Mashayekhi A, Ho T, et al. Solitary congenital hypertrophy of the retinal pigment epithelium: clinical features and frequency of enlargement in 330 patients. Retinal Cases & Brief Reports 2007;1:95–7.
96 Mims J, Shields J. Follow-up studies of suspicious choroidal nevi. Ophthalmology. 1978;85:929–943.
97 Shields JA, Augsburger JJ, Brown GC, et al. The differential diagnosis of posterior uveal melanoma. Ophthalmology. 1980;87:518–522.
98 Augsburger J, Schroeder R, Territo C, et al. Clinical parameters predictive of enlargement of melanocytic choroidal lesions. Br J Ophthalmol. 1989;73:911–917.
99 Butler P, Char D, Zarbin M, et al. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology. 1994;101:710–716.
100 The Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma. Arch Ophthalmol. 1997;115:1537–1544.
101 Callanan DG, Lewis ML, Byrne SF, et al. Choroidal neovascularization associated with choroidal nevi. Arch Ophthalmol. 1993;111:789–794.
102 Johnson RN, McDonald HR, Ai E, et al. Camera artifacts producing the false impression of growth of choroidal melanocytic lesions. Am J Ophthalmol. 2003;135:711–713.
103 Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127:981–987.
104 Loeffler K, Tecklenborg H. Melanocytoma-like growth of a juxtapapillary malignant melanoma. Retina. 1992;12:29–34.
105 Schneider U, Gelisken F, Inhoffen W, et al. Indocyanine green videoangiography of malignant melanomas of the choroid using the scanning laser ophthalmoscope. German J Ophthalmol. 1996;5:6–11.
106 Shields C, Shields J, DePotter P. Patterns of indocyanine green videoangiography of choroidal tumors. Br J Ophthalmol. 1995;79:237–245.
107 Sallet G, Amoaku W, Laufaut B, et al. Indocyanine green angiography of choroidal tumors. Graefes Arch Clin Exp Ophthalmol. 1995;233:677–689.
108 Mueller AJ, Bartsch D-U, Folberg R, et al. Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol. 1998;116:31–39.
109 Andersen M, Scherfig E, Prause J. Differential diagnosis of choroidal melanomas and nevi using scanning laser ophthalmoscopical indocyanine green angiography. Acta Ophthalmol Scand. 1995;73:453–456.
110 Gass JDM. Fluorescein angiography: an aid in the differential diagnosis of intraocular tumors. Int Ophthalmol Clin. 1972;12:85–120.
111 Espinoza G, Rosenblatt B, Harbour JW. Optical coherence tomography in the evaluation of retinal changes associated with suspicious choroidal melanocytic tumors. Am J Ophthalmol. 2004;137:90–95.
112 Shields CL, Mashayekhi A, Materin MA, et al. Optical coherence tomography of choroidal nevus in 120 patients. Retina. 2005;25:243–252.
113 Torres VL, Brugnoni N, Kaiser PK, et al. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol. 2011;151:586–593.
114 Yanuzzi LA, Ober MD, Slakter JS, et al. Ophthalmic fundus imaging: today and beyond. Am J Ophthalmol. 2004;137:511–524.
115 Lavinsky D, Belfort RN, Navajas E, et al. Fundus autofluorescence of choroidal nevus and melanoma. Br J Ophthalmol. 2007;91:1299–1302.
116 Shields CL, Pirondini C, Bianciotto C, et al. Autofluorescence of choroidal nevus in 64 cases. Retina. 2008;28:1035–1043.
117 Gunduz K, Pulido JS, Bakri SJ, et al. Fundus autofluorescence in choroidal melanocytic lesions. Retina. 2007;27:681–687.
118 Gunduz K, Pulido JS, Ezzat K, et al. Review of fundus autofluorescence in choroidal melanocytic lesions. Eye. 2009;23:497–503.
119 Wolff-Korman P, Korman B, Hazeneratz G. Duplex and color Doppler ultrasound in the differential diagnosis of choroidal tumors. Acta Ophthalmol. 1992;204:66–70.
120 Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005;112:1784–1789.
121 Thiagalingam S, Wang JJ, Mitchell P. Absence of change in choroidal nevi across 5 years in an older population. Arch Ophthalmol. 2004;122:89–93.
122 Shields CL, Cater J, Shields JA, et al. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol. 2000;118:360–364.
123 Shields CL, Shields JA, Kiratli H, et al. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102:1351–1361.
124 Pilotto E, Parrozzani R, Midena E. Treatment of choroidal neovascularization associated with choroidal nevus. Acta Ophthalmolgica. 2010:88.
125 Parodi MB, Boscia F, Ferrari TM, et al. Variable outcome of photodynamic therapy for choroidal neovascularization associated with a choroidal nevus. Retina. 2005;25:438–442.
126 Parodi MB. Transpupillary thermotherapy for subfoveal choroidal neovascularization associated with choroidal nevus. Am J Ophthalmol. 2004;138:1074–1075.