Chapter 151 Choroidal Metastases
Introduction
Choroidal metastases are the most common of adult intraocular tumors.1,2 Autopsy studies suggest that approximately 10% of cancer patients have ocular metastases, most frequently to the choroid.1,3 Pathologies such as breast cancer are associated with higher rates approaching 40% late in the course of the disease.4 Of the cases, 20–40% are bilateral, and multifocal involvement of one eye occurs in approximately 20% of affected cases.5 Frequency of metastases does not differ for the right or the left eye.5
Symptoms and clinical findings
Intraocular metastases may be asymptomatic. When symptomatic, choroidal metastases cause painless visual loss by involvement of the macular area or peripapillary retina or because of an associated, generally exudative, retinal detachment.6,7 Retinal detachment may cause visual deficits, floaters and flashes. Larger retinal detachments may be associated with peripheral field deficits. Tumors located anteriorly may tilt the lens, thereby causing visual loss. Rarely, these patients may have painful visual loss as a result of neovascular glaucoma or metastatic iritis.
In a review of 70 patients with choroidal metastases, symptoms at presentation included: blurred vision in 80%; pain in 14%; photopsias in 13%; red eye and floaters in 7%; field defects in 3%, and photophobia in 1%. A total of 6% of patients were asymptomatic.8
The most common location for choroidal metastases is the posterior pole of the globe. Up to 40% of lesions have been reported to be in the macular region.9 The reason for this may be due to differential blood flow to that area, although macular metastases are also more likely to be symptomatic, therefore increasing the likelihood of diagnosis.
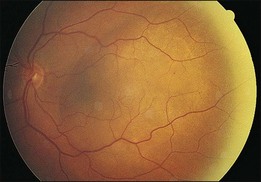
In contrast to choroidal melanomas which are generally darkly pigmented, choroidal metastases more commonly present as yellow or white lesions (Fig. 151.1). For lesions of a comparable basal size, choroidal metastases are often flatter than choroidal melanomas. Serous retinal detachment is commonly associated and frequently not in proportion with the tumor size.8,10
Frequency of primary cancer site
The relative frequency of primary cancer site for patients with choroidal metastases varies by gender. A series of more than 500 patients from the Wills Eye Hospital11 revealed primary sites for women as follows: breast, 68%; lung, 12%; unknown, 12%; gastrointestinal, 2%; skin, 1%; renal, <1%; and other, 4%. For men, primary sites were: lung, 40%; unknown, 29%; gastrointestinal, 9%; prostate, 6%; renal, 6%; skin, 4%; breast, 1%; and other, 4%.
Diagnostic evaluation
Differential diagnosis
Metastatic tumors usually have a creamy yellow appearance; purely amelanotic lesions are more likely to represent amelanotic choroidal melanomas. Metastatic lesions are usually flatter and rarely show the mushroom-shaped configuration that is often seen with melanomas. Choroidal metastases are also generally located in the posterior pole. Metastatic lesions anterior to the equator and metastases that involve the ciliary body are uncommon, and therefore amelanotic choroidal melanoma is more likely to be diagnosed in those locations. Bilateral or multifocal lesions are more likely to be metastatic, infectious, or uveitic. Combined A- and B-scan ultrasonography is of value in differentiating metastases from primary choroidal melanomas. Choroidal melanomas usually have low to moderate internal reflectivity, whereas metastatic lesions usually have higher internal reflectivity.12,13 More recently, optical coherence tomography has become useful to assist with the differential diagnosis of choroidal tumors.14
Choroidal osteomas may be bilateral and may have a color similar to that of metastatic tumors. Osteomas are rarely significantly elevated, and associated choroidal neovascularization is more common in patients with choroidal osteomas than in those with metastases. Ultrasound examinations15 and computed tomography (CT) scans are extremely valuable diagnostic tools in these patients because the bony change in the choroid shows very high reflectivity on ultrasound examination, and bone density can be confirmed with the CT scan.16 Optical coherence tomography may show overlying photoreceptor changes.17
Circumscribed choroidal hemangiomas are almost always unilateral and unifocal. Their characteristic reddish orange coloration is an important diagnostic clue. Fluorescein angiography shows more prominent early choroidal filling than is usually seen with metastatic lesions.16 Optical coherence tomography features of hemangioma have also been reported.18
Although some inflammation may be associated with a choroidal metastasis, inflammation is generally not prominent, but it can be seen, sometimes associated with choroidal detachment.19 A number of inflammatory conditions should be distinguishable, including Harada disease, the uveal effusion syndrome, posterior scleritis, and similar entities.16 In these patients, associated vitreous inflammation is often seen, and diffuse thickening of the choroid usually can be demonstrated on ultrasound examination.
Rarely do patients with large disciform scars or subretinal hemorrhages present a confusing picture. There is usually a history of previous visual loss related to choroidal neovascularization, and a history of systemic cancer is much less common. Drusen are usually apparent in the fellow eye. The echograms may be difficult to interpret, and the eyes are occasionally enucleated owing to a mistaken diagnosis of choroidal melanoma.20 The whitish yellow color of choroidal metastases is usually quite different from the darker appearance of these lesions, in which admixed blood is a prominent feature.
Ophthalmic evaluation and ancillary tests
Anticipating that radiation therapy may be recommended and may be cataractogenic, the status of the lens should be carefully evaluated.21 If the patient is diabetic or has retinal microangiopathy, this should be noted because radiation therapy exacerbates pre-existing retinal vascular disease (see Chapter 58, Radiation retinopathy). Documentary fundus photographs should be obtained to compare with later examinations to assess growth or treatment response.
Fluorescein angiography
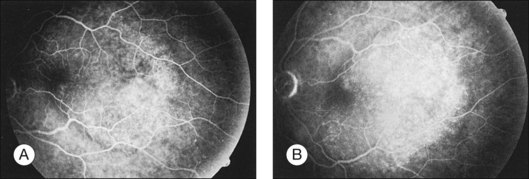
Metastatic choroidal tumors are usually hypofluorescent in the early phases of the study and become progressively hyperfluorescent in late phases (Figs 151.2, 151.3). The so-called double circulation pattern, thought to be characteristic of choroidal melanomas that have broken through Bruch’s membrane, is a rare finding in metastatic tumors.22 Prominent early choroidal filling is a feature more typical of circumscribed choroidal hemangiomas.16
A- and B-scan ultrasonography
B-scan ultrasonography is of value in evaluating patients with media opacities or bullous retinal detachments when a choroidal lesion is suspected but cannot be clearly seen. The B-scan ultrasonogram shows an echogenic subretinal mass with diffuse, ill-defined borders. Overlying retinal detachment is common, and sound attenuation in the lesion is usually moderate.13 In unusual cases, choroidal detachment may be seen.19
The A-scan ultrasonogram usually demonstrates moderate to high internal reflectivity. Vascularity is not prominent, and the consistency is solid.12,13 It is useful to determine the height of the metastatic tumor by ultrasonography, so that shrinkage of the lesion can be documented after treatment. A more complete discussion of the ultrasonographic features of choroidal metastases is provided in Chapter 9 (Diagnostic ophthalmic ultrasound), as well as in standard texts.15 Magnetic resonance imaging and spectroscopy may also prove to be valuable differential diagnostic aids.23,24
Optical coherence tomography
Optical coherence tomography (OCT) imaging, particularly with enhanced depth imaging (EDI) of the choroid as popularized by Spaide and others may help with the differential diagnosis. Torres and colleagues have recently summarized the EDI features of various choroidal tumors.25
Fine-needle aspiration biopsy
Diagnostic fine-needle aspiration biopsy (FNAB) of intraocular tumors should be considered only under special circumstances.26 The technique may be appropriate in a patient who has what appears to be a characteristic metastatic tumor but in whom a primary lesion cannot be found despite an extensive systemic evaluation. Augsburger and Shields26 include, as an additional indication, the use of the technique in some patients who refuse treatment without histopathologic verification of the lesion (rare) or in patients who have lesions that present major diagnostic uncertainty.
In general, oncologists require a histologic diagnosis before initiation of treatment of nonocular sites. Because of the potential threat to vision, it is not a routine procedure to biopsy intraocular lesions before treatment. Useful reports provide updates on FNAB and choroidal tumors.27–29
Systemic evaluation
Two-thirds of choroidal metastases are diagnosed in patients with a prior history of malignancy.11 Of the remaining patients, the primary site of malignancy was subsequently detected in about half, with approximately 70% originating from lung cancer. This high prevalence of lung primaries is likely due to the increased propensity of lung cancer to present with brain/eye metastases relative to other malignancies. In general, the work-up for a patient with a known malignancy will vary depending on the location and status of the primary tumor as well as a history of prior metastatic disease. The staging evaluation should include an MRI of the brain since studies have suggested central nervous system metastases may develop in close to 30% of patients.5 In addition, it often consists of laboratory data including serum chemistries, liver function tests, alkaline phosphatase, chest computerized tomography, and bone scan.
Management
Treatment of patients with choroidal metastases benefits from multidisciplinary collaboration and careful evaluation of multiple factors including the intraocular site, presence of visual symptoms, primary tumor histology and site, presence and status of other metastases, and anticipated efficacy of systemic treatment options. Close observation may be considered for small metastases in the periphery of the retina that may not enlarge rapidly or lead to visual symptoms.5 Similarly, rapid or complete response to systemic therapy especially in patients with breast or lung cancer may allow postponement or avoidance of local therapy to the choroidal metastases. By contrast, lesions causing visual symptoms or encroaching upon the optic disc or macula generally require local therapy. Some reports suggest improved outcomes with a combination of local and systemic therapies.32 The following section addresses radiotherapeutic management options for choroidal metastases.
Conventional external beam radiation therapy
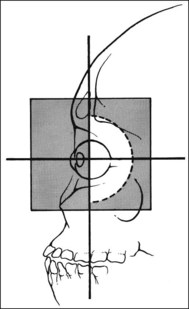
Historically, the conventional local treatment for choroidal metastases has been external beam radiation therapy using megavoltage photons. Treatment planning for modern external beam treatment begins with computerized tomographic (CT) simulation to delineate the target as well as adjacent normal tissues. The patient is positioned in a supine position on a flat table with a mask fitting over the face and shoulders for immobilization. The treatment field depends on the location and geometry of the tumor. Since approximately 80% of tumors are located in the posterior segment of the uvea,33,34 it is generally possible to use a D-shaped field designed to cover the posterior globe, bony roof, floor of orbit, and a portion of the optic nerve but shield the cornea and lens (Fig. 151.4). It may not be possible to block the lens if the metastasis is anterior to the equator. For unilateral metastases, it is possible to achieve adequate coverage with only a single ipsilateral beam. The minimal exit dose to the contralateral globe does not preclude subsequent treatment for development of contralateral metastases. As long as the dose to the contralateral orbit is limited in the initial field, it is relatively simple to treat the contralateral orbit as needed at a later date. A total of 20–40% of patients have bilateral choroidal metastases requiring treatment of both globes.8,10 The dose typically used for palliation is 3000 cGy in 300 cGy per fraction delivered as a single fraction 5 days per week for 10 treatments. If prolonged survival is anticipated, treatment to a higher dose of radiation is administered to improve long-term local control. Doses of 4500–5000 cGy in fractions of 200–250 cGy have been reported.35–40 Local control with these regimens is excellent.
Brachytherapy plaques
An alternative local therapy for choroidal metastases involves plaque brachytherapy; radioisotopes such as iodine-125, ruthenium-106, or palladium-103 are temporarily surgically placed adjacent to the lesion designed to cover the tumor plus 2–3 mm margin. These treatments typically last about 3 days, after which the plaque is removed. Data to support plaque brachytherapy may be extrapolated from choroidal melanoma in which the technique is believed to have comparable survival to enucleation.41 A large series of 650 melanoma cases was recently reported by the Wills eye hospital.42 Local recurrence was 14% at 5 years, and 24% at 10 years.
Plaque brachytherapy may only be used for patients with solitary choroidal metastases. In addition, they must have lesions of appropriate size, depth, and location relative to the macula and fovea. A major disadvantage of this approach is that it involves hospitalization and two surgical procedures for placement and removal of the brachytherapy plaque. (For additional information concerning melanoma-related brachytherapy, the reader is referred to Chapter 145, Brachytherapy for choroidal melanoma.)
Stereotactic radiosurgery
Stereotactic radiosurgery (SRS) utilizes multiple precisely focused small radiation beams and high doses for a limited number of fractions. This technique administers ablative doses of radiation to a small volume thereby maximizing local control while sparing critical adjacent normal tissues. There are multiple technologies that may be used to deliver SRS, including linear accelerator-based approaches or Gamma Knife. When using conformal radiation techniques, precise and reproducible immobilization of the eye is critical. Several techniques exist. For example, eye gaze may be directed by asking the patient to visualize a flashing light at a set position. A light field projected onto the sclera may be used to ensure that the gaze during treatment planning matches the treatment setup. The positioning is then verified by an ophthalmologist.43
A series from China reviewed patients treated with Gamma Knife radiosurgery for orbital tumors.44 Of the 202 cases included, 18 had malignant choroidal tumors. Vision was preserved in 129 patients, with 72 experiencing improvement in vision and 18 experiencing deterioration.
Gamma Knife radiosurgery utilizes 201 individual cobalt-60 sources in a collimator to which the patient is adhered using an invasive frame for immobilization. The radiation beam is focused by different-sized collimators which are controlled by a program which allows them to be quickly changed. Gamma Knife is extremely accurate and may be used to treat small lesions (Fig. 151.5).
Protons
A final treatment option for choroidal metastases is proton beam irradiation in which charged particles are used to deliver radiation with a very sharp dose fall-off, allowing a potential improvement in vision preservation over conventional radiation therapy. Like radiosurgery, it typically allows for fewer treatments than conventional radiation therapy. A retrospective series from Harvard44 included 63 patients treated with protons for choroidal metastases. Tumor regression was documented in 84% of patients and stable disease was reported in 14%. Resolution of retinal detachment was reported in 82%. Some 58% of patients had visual acuity at last follow-up of 20/200 or better. (More detail on proton beam treatment is included in Chapter 146, Charged-particle irradiation of uveal melanoma.)
Toxicity
Ocular toxicity
Ocular radiation therapy is well tolerated with minimal acute toxicities40,45 including dry eye, epiphora, or irritative symptoms. These symptoms may be managed with artificial tears or lubricating ointments. Topical antibiotics may be indicated for epithelial damage. Long-term ocular toxicity includes cataract formation which was reported in 3% of patients in one series.46 Cataract formation is expected if the lens is not shielded and there is a reasonable period of survival following treatment. Damage to the retina and optic nerve are the primary toxicities resulting in reduced visual acuity. Because the dose to critical structures (optic nerve and macula) is below the known radiation tolerance and due to the relatively poor prognosis of patients with choroidal metastases, many patients do not survive to develop radiation retinopathy which has a median onset of 18 months to 2 years.2,8,47 A large series using conventional radiation therapy reported retinopathy and optic neuropathy in 2% of patients.46
Prognosis
Development of choroidal metastases usually represents advanced systemic disease48 with limited potential for cure. Studies suggest a median survival of approximately 16 months39,44,49,50 with survival varying by primary tumor type as well as the availability of effective systemic chemotherapeutic options.
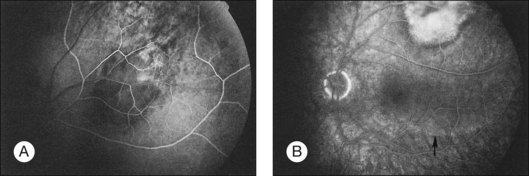
Most choroidal metastases respond to local therapy with response rates of approximately 80% in some series.45,51 Local response is often associated with tumor flattening (Fig. 151.6) and pigmentary proliferation. Improved response rates were associated with: (1) symptoms for <49 days prior to initiation of radiation therapy, and (2) small tumor extent less than half of a quadrant with minimal or no retinal detachment.45
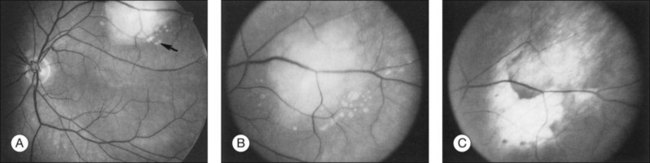
Fig. 151.6 (A) Fundus appearance of metastatic tumor before treatment. The angiogram from this patient is illustrated in Figure 151.3. Small tumor deposits (arrow) are present in subretinal fluid surrounding the inferior aspect of the tumor. (B) Higher-magnification view. (C) The tumor has flattened completely 1 month after external beam radiation.
Globe preservation is possible in the majority of patients and complete resolution of visual symptoms has been reported in 25–50% of patients.5,8,10 Time to improvement of visual symptoms ranges from a few days to 10 weeks following initiation of radiation therapy and typically persists for the remainder of the patient’s life.35
1 Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Opthalmol. 1971;85:673–675.
2 Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. A clinicopathologic study of 227 cases. Arch Opthalmol. 1974;92:276–286.
3 Nelson CC, Hertzberg BS, Klintworth GK. A histopathologic study of 716 unselected eyes in patients with cancer at the time of death. Am J Ophthalmol. 1983;95:788–793.
4 Mewis L, Young SE. Breast carcinoma metastatic to the choroid. Analysis of 67 patients. Ophthalmology. 1982;89:147–151.
5 Shields JA. Diagnosis and management of intraocular tumors. St Louis: Mosby; 1983.
6 Gonvers M, Zografos L. Choroidal metastases and rhegmatogenous retinal detachment. Retina. 1991;11:426–429.
7 Haimovici R, Mukai S, Schachat AP, et al. Rhegmatogenous retinal detachment in eyes with uveal melanoma. Retina. 1996;16:488–496.
8 Stephens RF, Shields JA. Diagnosis and management of cancer metastatic to the uvea: a study of 70 cases. Ophthalmology. 1979;86:1336–1349.
9 Freedman MI, Folk JC. Metastatic tumors to the eye and orbit: patient survival and clinical characteristics. Arch Ophthalmol. 1987;105:1215–1219.
10 Mewis L, Young SE. Breast carcinoma metastatic to the choroid: analysis of 67 patients. Ophthalmology. 1982;89:147–151.
11 Shields CL, Shields JA, Gross NE, et al. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–1276.
12 Fuller DG, Snyder WB, Hutton WI, et al. Ultrasonographic features of choroidal malignant melanomas. Arch Ophthalmol. 1979;97:1465–1472.
13 Shammas JH. Atlas of ophthalmic ultrasonography and biometry. St Louis: Mosby; 1984.
14 Sayanagi K, Pelayes DE, Kaiser PK, et al. 3D Spectral domain optical coherence tomography findings in choroidal tumors. Eur J Ophthalmol. 2011;21:271–275.
15 Dibernardo C, Schachat AP, Fekrat S. Ophthalmic ultrasound: a diagnostic atlas. New York: Thieme; 1998.
16 Gass JDM. Stereoscopic atlas of macular diseases: diagnosis and treatment, 3rd edn. St Louis: Mosby; 1987.
17 Shields CL, Perez B, Materin MA, et al. Optical coherence tomography of choroidal osteoma in 22 cases: evidence for photoreceptor atrophy over the decalcified portion of the tumor. Ophthalmology. 2007;114:e53–e58.
18 Liu W, Zhang Y, Xu G, et al. Optical coherence tomography for evaluation of photodynamic therapy in symptomatic circumscribed choroidal hemangioma. Retina. 2011;31:336–343.
19 Sneed SR, Byrne SF, Mieler WF, et al. Choroidal detachment associated with malignant choroidal tumors. Ophthalmology. 1991;98:963–970.
20 El Baba F, Jarrett WH, II., Harbin TS, Jr., et al. Massive hemorrhage complicating age-related macular degeneration: clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986;93:1581–1592.
21 Macfaul PA, Bedford MA. Ocular complications after therapeutic irradiation. Br J Ophthalmol. 1970;54:237–247.
22 Augsburger JJ, Golden MI, Shields JA. Fluorescein angiography of choroidal malignant melanomas with retinal invasion. Retina. 1984;4:232–241.
23 Haik BG, Saint Louis L, Smith ME, et al. Magnetic resonance imaging in choroidal tumors. Ann Ophthalmol. 1987;19:218–238.
24 Mafee MF, Peyman GA, Peace JH, et al. Magnetic resonance imaging in the evaluation and differentiation of uveal melanoma. Ophthalmology. 1987;94:341–348.
25 Torres VL, Brugnoni N, Kaiser PK, et al. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol. 2011;151:586–593.
26 Augsburger JJ, Shields JA. Fine-needle biopsy of solid intraocular tumors; indications, instrumentation, and techniques. Ophthalmic Surg. 1984;15:34–40.
27 Eide N, Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review. Acta Ophthalmol. 2009;87:588–601.
28 Pelayes DE, Zárate JO. Fine needle aspiration biopsy with liquid-based cytology and adjunct immunohistochemistry in intraocular melanocytic tumors. Eur J Ophthalmol. 2010;20:1059–1065.
29 Shields CL, Ganguly A, Bianciotto CG, et al. Prognosis of uveal melanoma in 500 cases using genetic testing of fine-needle aspiration biopsy specimens. Ophthalmology. 2011;118:396–401.
30 Eide N, Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review. Acta Ophthalmol. 2009;87:588–601.
31 Shields JA, Shields CL, Ehya H, et al. Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993;100:1677–1684.
32 Amer R, Pe’er J, Chowers I, et al. Treatment options in the management of choroidal metastases. Ophthalmologica. 2004;218:372–377.
33 Castro PA, Albert DM, Wang WJ, et al. Tumors metastatic to the eye and adnexa. Int Ophthalmol Clin. 1982;22:189–223.
34 Hutchison DS, Smith TR. Ocular and orbital metastatic carcinoma. Ann Ophthalmol. 1979;11:869–873.
35 Thatcher N, Thomas PRM. Choroidal metastases from breast carcinoma: a survey of 42 patients and the use of radiation therapy. Clin Radiol. 1975;26:549–553.
36 Hoogenhout J, Brink HM, Verbeek AM, et al. Radiotherapy of choroidal metastases. Strahlenther Onkol. 1989;165:375–379.
37 Phillips TL. More on managing metastatic choroidal tumors. Oncology. 1989;3:14.
38 Brady LW, Shields JA, Augsburger JJ, et al. Malignant intraocular tumors. Cancer. 1982;49:578–585.
39 Maor M, Chan RC, Young SE. Radiotherapy of choroidal metastases breast cancer as primary site. Cancer. 1977;40:2081–2086.
40 Burmeister BH, Benjammin CS, Childs WJ. The management of metastases to eye and orbit from carcinoma of the breast. Aust NZ J Ophthalmol. 1990;18:187–190.
41 De Potter P, Shields CL, Shields JA, et al. Impact of enucleation versus plaque radiotherapy in the management of juxtapapillary choroidal melanoma on patient survival. Br J Ophthalmol. 1994;78:109–114.
42 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: tumor control in 650 consecutive cases. Ophthalmology. 2011;118:402–407.
43 Tsina EK, Lane AM, Zacks DN, et al. Treatment of metastatic tumors of the choroid with proton beam irradiation. Ophthalmology. 2005;112:337–343.
44 Xu D, Liu D, Zhang Y, et al. Gamma knife surgery in the management of orbital tumors. J Neurosurg. 2010;113:34–38.
45 Panizzoni GA, Gasparini G, Dal Fior S, et al. Radiotherapeutic treatment for breast cancer choroidal metastases. Tumori. 1990;76:563–565.
46 Rudoler SB, Corn BW, Shields CL, et al. External beam irradiation for choroid metastases: identification of factors predisposing to long term sequelae. Int J Radiat Oncol Biol Phys. 1997;38:251–256.
47 Brown GC, Shields JA, Sanborn G, et al. Radiation retinopathy. Ophthalmology. 1982;89:1494–1501.
48 Char DH. Posterior uveal tumors. In: Tracy TM, Otway M. Clinical ocular oncology. New York: Churchill Livingstone; 1989:102.
49 Wiegel T, Bottke D, Kreusel KM, et al. External beam radiotherapy of choroidal metastases – final results of a prospective study of the German Cancer Society (ARO 95–08). Radiother Oncol. 2002;64:13–18.
50 Amichetti M, Caffo O, Minatel E, et al. Ocular metastases from breast carcinoma: a multicentric retrospective study. Oncol Rep. 2000;7:761–765.
51 Hoogenhout J, Brink HM, Verbeek AM, et al. Radiotherapy of choroidal metastases. Strahlenther Onkol. 1989;165:375–379.