CHAPTER 197 Choroid Plexus Tumors
Clinical Background
Choroid plexus tumors are rather infrequent neoplasms, representing less than 1% of all tumors.1–3 However, they are much more frequent in the pediatric population and can represent a sizeable percentage of the tumors in children younger than 1 year of age.4,5 Choroid plexus and its tumors are derived from the specialized neuroepithelial cells in the ventricles. The tumors can recapitulate the characteristics of normal choroid plexus in their structure and appearance and in their function, with the overproduction of cerebrospinal fluid resulting in hydrocephalus. There are also more aggressive forms, with clearly anaplastic features that more closely resemble carcinomas. Significant glial characteristics can also be expressed.
Choroid plexus tumors must be included in the differential diagnosis of the young child or adult presenting with an intraventricular mass. Surgical resection has become an essential part of the treatment of these lesions, with the specific approach based on the tumor location and characteristics. Although the outcome is a function of the tumor type and required treatments, cures and long-term survival are quite possible. See Case 197-1![]() for an illustrative example of evaluation of an intraventricular mass in a 1-year-old child.
for an illustrative example of evaluation of an intraventricular mass in a 1-year-old child.
Case 197-1
Case provided by Dr. Frederick A. Boop, LeBonheur Children’s Hospital and St. Jude Children’s Cancer Center Research Hospital.
One-Year-Old with an Intraventricular Mass
Imaging
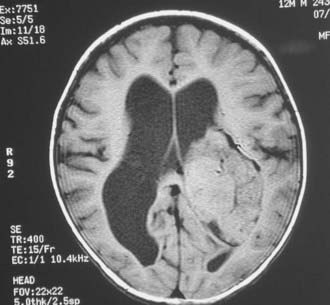
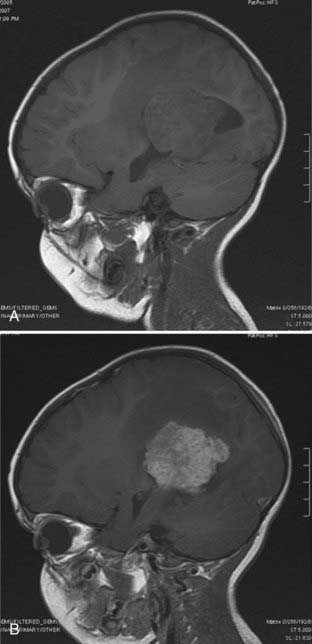
Magnetic resonance imaging clearly showed generalized hydrocephalus and a left intraventricular mass at the atrium consistent with a choroid plexus tumor (Fig. 197-E1). The mass appears to be completely within the left lateral ventricle, with no obvious brain edema, nor any invasion of the parenchyma. Several flow voids are seen within the tumor suggesting large vessels.
The tumor enhances brightly with gadolinium, showing a structure along the superior and lateral margins of the mass that resembles normal choroid plexus. However, more medially, there are definite structural differences (Fig. 197-E2). These differences are seen much better on the T2-weighted images (Fig. 197-E3). In addition, there is a small area of increased signal in the lateral wall of the ventricle that is consistent with brain invasion.
History
Guerard in 1832 provided the first description of a choroid plexus tumor found at autopsy of a 3-year-old girl.6 The first surgical resection was reported in 1906, with the first long-term survival in an adult in 1919.7,8 Until the 1930s, a number of reports focused on the rarity of these lesions and their association with hydrocephalus.8–17 Van Wagenen17 reported excision and good survival of a lateral ventricle tumor in a 3-month-old child in 1930. Dandy18 reported his transcallosal approach to the third ventricle to remove a choroid plexus tumor in a 14-year-old girl. Masson19 reported a transfrontal approach to the third ventricle to successfully remove a third ventricular papilloma in 1934.
Presentation
Incidence
Choroid plexus tumors may occur during any stage of life and thus represent only 0.5% to 0.6% of all intracranial tumors.2,3,20 They are clearly more frequent in the pediatric age range, representing up to 2.9% of all pediatric brain tumors, but account for between 10% and 20% of tumors in infants younger than 1 year.4,20,21 Considering only choroid plexus tumors, 70% are found in children and 50% in children younger than 2 years.5,21–26 Congenital examples have been reported, including tumors detected on prenatal ultrasound.20,27–31 There does not appear to be clear evidence of a sex predilection, with a reported male-to-female ratio of 1.2 : 1.32 Some reports have noted a slight male predominance, whereas others have seen an equal distribution.1,21,25,33–35
The malignant forms (choroid plexus carcinoma) account for 15% to 20% of choroid plexus tumors, but 80% of these malignant tumors are found in children.24,36–39 Although these tumors tend to have a shorter duration of symptoms, there does not appear to be a difference from the benign tumors with respect to age at presentation, sex, type of symptoms, or location of the tumor. The carcinomas have a marked propensity to metastasize.40 Metastasis is the exception for the benign forms, but the satellite lesion retains the benign histology.
Symptoms
The most common symptoms are associated with increased intracranial pressure (ICP).20,22,26,35 The increased ICP may result directly from the large tumor size or from the tumor location obstructing the outflow of cerebrospinal fluid (CSF) and the development of obstructive hydrocephalus. Interestingly, not all choroid plexus papillomas produce hydrocephalus. In younger children, the signs of increased ICP include the sometimes subtle observations of increased head circumference or split sutures before the more obvious signs of vomiting and lethargy. In adult patients, the symptoms can be ataxia, nausea, and vomiting.
In many cases, there is generalized hydrocephalus with ventricular enlargement, not only from obstruction but also possibly from an overproduction of CSF, with up to 800 mL per hour reported in one case.22,23,26,41–47 The overproduction of CSF is also supported by electron microscopic morphologic findings consistent with CSF production, with the frequent resolution of hydrocephalus after tumor removal, and with a report of ascites after shunting but before tumor resection.48 However, increased rate of CSF production has been actually shown in only a few patients.42,43,46,47
Alternative causes for hydrocephalus have been suggested, including the effects of subtle hemorrhage or ventriculitis occurring before any surgery. There is a failure to resolve hydrocephalus after tumor removal in up to 50% of patients, and this could be due to operation-induced hemorrhage or inflammation.21,26,35,46,49,50 It is likely that there are multiple factors of CSF production and absorption that affect the occurrence and resolution of hydrocephalus.
Some patients will manifest focal neurological signs as a result of hemorrhage or focal invasion. For example, tumors located in the fourth ventricle can present with brainstem and lower cranial nerve symptoms related to direct brainstem or cerebellar compression. Patients with tumors in the third ventricle have been reported with various endocrine disturbances, the bobble-head doll phenomenon, and diencephalic dysfunction.16,51–53
Location
Choroid plexus tumors are generally found where the choroid plexus is located—the lateral ventricle in 40% to 50%, third ventricle in 5% to 10%, fourth ventricle in about 40%, and more than one ventricle in about 5%.17,21,25,26,37,40,48,54–57 Primary extraventricular locations have been rarely described, including the cerebellopontine angle, cerebellomedullary cistern, suprasellar cistern, foramen magnum, and spinal subarachnoid space.58–61
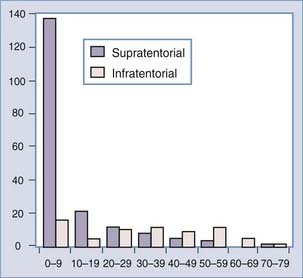
There is a correlation of ventricular location with age, however, that is without an obvious pathophysiologic cause. The lateral ventricular location is most common and the fourth ventricular location least common in children4,20,23,32,56,58,62,63 (Fig. 197-1). In a meta-analysis, the median ages at diagnosis were 1.5 years, 1.5 years, 22.5 years, and 35.5 years for patients with lateral, third, and fourth ventricle and cerebellopontine angle tumors, respectively.32 Bilateral tumors have been reported, but the sheer size of some tumors precludes accurately defining the multifocal origin versus direct extension.23,48,55
The lateral ventricle tumors are typically located at the atrium, although they can be found more anteriorly near the foramen of Monro and in the temporal horn. These tumors are usually very large at presentation, often greater than 4 to 6 cm.21 There may be a predilection for lateral ventricle tumors to occur on the left side, but this is an inconsistent finding. The fourth ventricle tumors are usually in the midline. There can be extension into and through the lateral recesses of the ventricle.32
There are rare reports of metastatic masses derived from the benign forms of choroid plexus tumors. The metastases retain the same pathologic structure and are assumed to be “drop” lesions, disseminating through the CSF.64 It is possible that this group of choroid plexus papillomas showing metastases is a unique and more aggressive variant called an atypical choroid plexus papilloma. There has been one case of pulmonary metastasis.65 However, metastases are a notable feature in the malignant forms of choroid tumors.
The blood supply to the intraventricular tumors is the same as for normal choroid plexus in that ventricle. The principal arterial supply to the choroid of the lateral and third ventricles comes from the anterior and posterior choroidal arteries.66,67 The anterior choroidal artery comes off the internal carotid artery, courses through the choroidal fissure, and supplies the choroid and tumors primarily in the atrium and temporal horn. The posterior choroidal arteries arise from the posterior cerebral artery. The lateral posterior choroidal artery enters the ventricle near the crus of the fornix and supplies the choroidal tissues in the temporal horn, atrium, and body of the lateral ventricle. The medial posterior choroidal artery has a variable supply to the lateral ventricle through the choroidal fissure and foramen of Monro, but it does supply the choroid in the roof of the third ventricle. Thus, tumors of the third ventricle and some in the lateral ventricle can be supplied by branches of this vessel. Because of the significant anastomosis and overlap in arterial supply, both the anterior and posterior choroidal arteries can contribute feeders to these intraventricular tumors.66,67 The choroid of the fourth ventricle is supplied by branches from both the posterior inferior cerebellar and the superior cerebellar arteries.
Diagnosis
Imaging
The evolution of imaging for brain lesions has also affected the diagnosis of choroid plexus lesions. Plain radiography, while done infrequently in the modern era, can show nonspecific calcification within the tumor and nonspecific signs of increased intracranial pressure such as split sutures.1,55,68,69 In the past, pneumoencephalography and ventriculograms were used to indirectly demonstrate these tumors but with significant morbidity and mortality.50 These studies have been replaced with direct imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) usually obtained for the initial evaluation of symptoms in these patients.
Angiography used to be routinely performed to demonstrate the vascular supply of the tumor. The lateral ventricular tumors would consistently show enlarged anterior or lateral posterior choroidal arteries. The third ventricular tumors would be shown supplied by the medial posterior choroidal arteries. The tumors would have an irregular blush. Raimondi and Gutierrez50 provide excellent examples of these angiographic findings. However, currently, angiography is rarely indicated for diagnostic purposes; the vascular supply to these tumors both is consistent and can be effectively demonstrated on MRI.
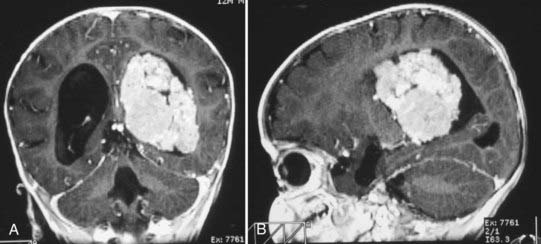
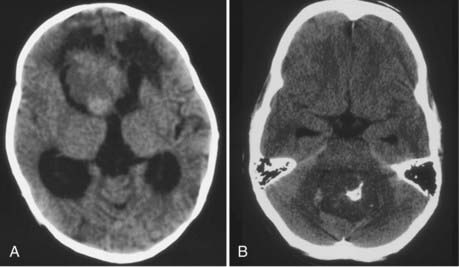
CT will show a variable density to these tumors, ranging from isodense to hyperdense relative to the brain.22,26 Calcification occurs in about 10%26 (Fig. 197-2). The tumor is usually well demarcated from the brain tissue and has rather dramatic enhancement.26 Areas of cystic degeneration can suggest the more malignant choroid plexus carcinoma.22
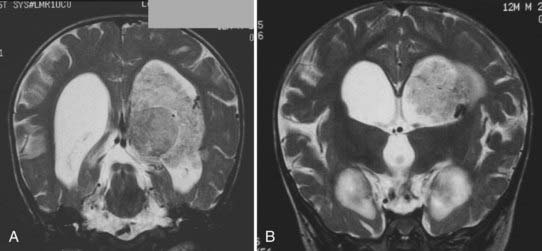
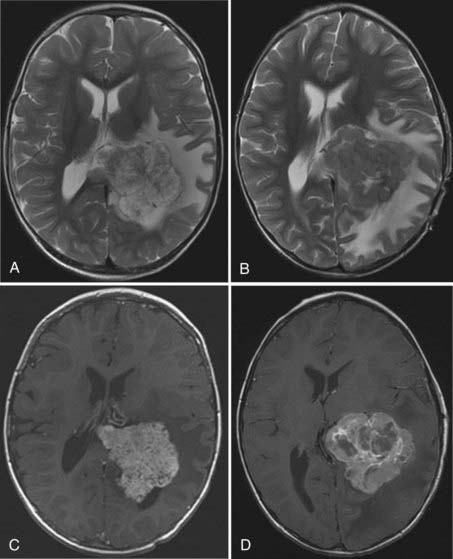
MRI will provide excellent anatomic delineation of the tumor and surrounding brain. The choroid plexus papilloma appears lobulated and separate from the surrounding brain tissue (Fig. 197-3). They are usually isointense to brain on T1-weighted imaging and enhance uniformly. The T2-weighted images show an intermediate to high signal intensity, and the serpentine vascular supply and drainage can be easily seen as flow voids.40,70
The distinction between papillomas and carcinomas, however, is not always clear-cut. Some papillomas demonstrate adjacent cerebral edema and invasion, whereas some carcinomas do not (Fig. 197-4). Choroid plexus carcinomas often have lost the lobulated appearance and have invasion of the parenchyma with associated vasogenic edema.40,70 There is frequently significant hemosiderin signal change and extensive enhancement with administration of gadolinium-DTPA, although with more variability than seen in papillomas.
Magnetic resonance spectroscopy has shown consistently a prominent choline peak with an absent N-acetyl aspartate peak. Choroid plexus papillomas appear to have a significantly higher myoinositol signal than both choroid plexus carcinomas and all other brain tumors.71 The carcinomas had markedly elevated choline peaks.71,72
All the tumors in the differential diagnosis, including ependymoma, primitive neuroectodermal tumor, astrocytoma, germinoma, teratoma, and meningioma, can have similar imaging characteristics. It can even be difficult to define the difference between the benign choroid plexus papilloma and the malignant choroid plexus carcinoma. Modern imaging cannot yet accurately define the pathologic diagnosis. However, the magnetic resonance spectroscopy findings of papillomas compared with carcinomas suggest that preoperative differentiation is possible. This could have a tremendous effect on planning, with a greater emphasis on embolization or chemotherapy for the identified carcinoma.71
Pathology
Choroid Plexus Papilloma
Choroid plexus papillomas correlate with World Health Organization (WHO) grade I classification.73 On gross inspection, the choroid plexus papillomas are well circumscribed and globular, with a pink to reddish-gray color. They are frequently described as cauliflower like, and tend to expand and fill the ventricular cavity. There is limited if any direct brain invasion, but the tumors can cause extensive compression of the brain.
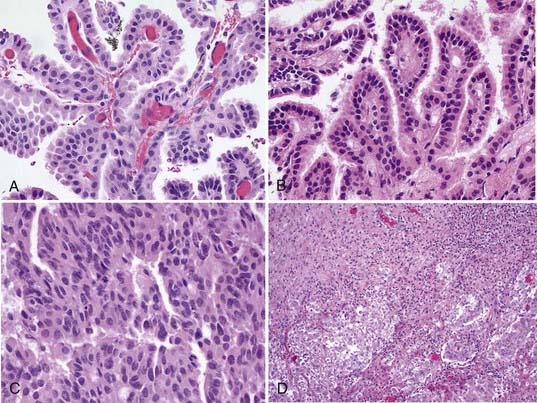
Microscopically, the papillomas are easy to identify as they recapitulate the structure of normal choroid plexus tissue74,75 (Fig. 197-5). There is a single layer of cuboidal to columnar cells, usually without cilia or blepharoplasts as in normal choroidal epithelium, covering a stroma of delicate fibrovascular connective tissue. The nuclei are monomorphic and toward the base of the cells, and mitoses are rarely seen in papillomas but if present can signify a more aggressive form. It is sometimes difficult to identify papilloma from normal choroid plexus, but the papillomas tend to have higher cellularity and more cellular and nuclear pleomorphism.58 Papillary ependymomas of the ventricle must be distinguished from choroid plexus papillomas. However, the ependymoma has a fibrillary neuroglial stroma that is absent in the papillomas.
If there is also brain invasion, a malignant change to a carcinoma can be considered. There have been reports of papillomas undergoing malignant transformation, but the pathology was also reported to show mitoses and may actually represent a previously unrecognized variant of the tumor (atypical choroid plexus papilloma).76–80
Choroid Plexus Carcinoma
Choroid plexus carcinomas correlate with WHO grade III.73 The gross structure of the choroid plexus carcinoma is not similar to normal choroid, and the tumor at surgery is usually friable. There is usually clear-cut invasion of adjacent tissue. The changes on microscopic examination are equally obvious, with loss of the papillary structures into ill-defined patterns, and a loss of the fibrous supporting stroma seen in papillomas (see Fig. 197-5). The cells are variable in size and shape, densely packed or heaped up, with frequent mitoses and nuclear variability.74,81
The malignant changes in the carcinomas are usually obvious, although in the older literature, there has been debate about which feature is most important in making the diagnosis. Retrospective reviews that applied more recent criteria for diagnosis of a carcinoma identified that only half of the reported cases fit the definition.82,83 The most consistent histologic criteria for diagnosis of a carcinoma will have a tumor with cellular anaplasia, loss of the papillary fibrous stroma, frequent mitosis and necrosis, and giant cell formation.21,40
The presence of brain invasion has been suggested as a required component for the diagnosis of choroid plexus carcinoma (see Fig. 197-5).84 However, there can be tumors with otherwise benign histologic features showing some brain invasion, and others with anaplastic features appearing to have circumscribed borders.21,85 The technique of surgical resection could obliterate the tumor-brain interface and render that distinction moot.21 MRI may give the best indication of brain invasion, but it is does not seem realistic to require the demonstration of brain invasion as the sole diagnostic criterion for choroid plexus carcinoma.
The choroid plexus carcinoma can be confused with metastatic carcinoma in adults or with neuroepithelial embryonal tumors in children.58 A combination of cytologic, ultrastructural, and immunohistochemical evaluations can be used to differentiate choroid plexus carcinomas from atypical teratoid/rhabdoid tumors (AT/RT).86
Atypical Choroid Plexus Papillomas
Recent attention has been focused on the few patients with tumors that for the most part appear pathologically just like papillomas but behave in a more aggressive fashion with a higher rate of recurrence and demonstrating dissemination.80 These have been recognized as atypical choroid plexus papillomas and may represent up to 15% of all choroid plexus papillomas.37 They can have more invasion than expected with papillomas, more cellular pleomorphism, and increased cellularity. The only histologic feature that consistently defines this group of tumors is having 2 or more mitoses per 10 high-power fields.37 These tumors correlate with WHO grade II.73
Choroid Villous Hypertrophy
It is not clear whether there is a distinct pathologic entity of choroid villous hypertrophy. Davis10 described this in 1924 as a bilateral choroid plexus lesion. It is hard to determine, however, whether this entity is not really the same as a bilateral papilloma, given the relatively small pathologic difference between normal choroid plexus and papillomas. The overproduction of CSF could occur with either entity. Treatment of these bilateral lesions has resulted in the resolution of CSF overproduction, whether through resection or endoscopic coagulation of the plexus.46,87,88
Immunohistochemistry and Molecular Biology
The immunohistochemistry of choroid plexus tumors has not shown a clear-cut distinction among the various forms.74 The tumors can show both epithelial and glial characteristics. Normal choroidal tissue can demonstrate expression of S-100, low-molecular-weight keratin (CKER), and keratin. S-100 staining is seen in 55% to 90% of papillomas and is found variably in carcinomas.74,78 Significant S-100 staining (>50% of cells) has been associated with a more benign histologic appearance and a better outcome. Glial fibrillary acidic protein (GFAP) and cytokeratin are expressed in both papillomas and carcinomas but can also be seen in normal choroid plexus and some carcinomas.74,89 GFAP-positive cells are usually absent in normal choroid plexus but can be seen in infant choroidal tissue.75 GFAP positivity can be seen in 25% to 55% of papillomas and in up to 20% of carcinomas. Carcinoembryonic antigen positivity has been seen in up to 100% of choroid plexus carcinomas but also in 29% of papillomas. Its presence is associated with a more aggressive behavior.74 As might be expected, the cellular proliferation indices (Ki-67, MIB-1) are typically low in papillomas and higher in carcinomas.90,91
Chromosomal analysis of papillomas and carcinomas by comparative genomic hybridization showed a multitude of chromosomal gains and losses.92 However, there was a significant difference in the aberrations in the carcinomas compared with the papillomas. There was also a difference between papillomas in adult and pediatric patients. This supports the theory that these tumors develop from different genetic abnormalities and suggests why malignant degeneration from papilloma to carcinoma seems so rare.
Surgical Treatment and Complications
Surgical treatment of these lesions through the 1980s consistently resulted in high operative mortality and morbidity rates (25% to 65%).1,22,63,69,93 This was due to the less sophisticated imaging, with difficulty in accurately defining the tumors preoperatively, and to the often very significant blood loss in infants. Modern surgical treatment has improved through the use of enhanced preoperative delineation of the tumors and use of steroids, anesthetic techniques, and microsurgical techniques that provide better direct vision and lighting to the tight confines of intraventricular surgery. The more recent results have been much better, but perioperative death still occurs.21,35,39,50
The surgical approach is planned to maximize the exposure of the tumor, with the aim of gaining early access to the vascular supply to the lesion. The goals are to achieve a gross total resection of the lesion and to alleviate any hydrocephalus (temporarily or permanently). The tumor locations within the ventricular system virtually ensure an approach through normal neurological tissue, some inferior fourth ventricular lesions excepted.66,67
The standard technique of internal debulking is fraught with danger in these tumors with significant vascularity. An attempt at extensive debulking often results in unacceptable blood loss, morbidity, and the risk for death from rapid exsanguination.39 Early “control of the vascular pedicle” has been stressed but may not be allowed by the required approaches, which often place the vascular pedicle on the opposite side of the tumor from the surgeon.26,69 This has led to recommendations for preoperative embolization, which is more feasible in this age of superselective interventional angiography.26 However, the option for embolization may be limited in very small children and infants because of complications of vascular access.26
As previously discussed, hydrocephalus may persist even after tumor removal, ranging in frequency from 24% to 50%.21,26,39,49,50 It is treated with shunting in a standard fashion. Each surgical approach will entail risks specific to that approach, but in general these include postoperative hematoma formation, developmental and neuropsychological deficits, visual field defects, and cranial nerve deficits. Seizure problems can persist in 25% of papilloma patients after resection. Hemiparesis can remain in another 25%, but 25% will have no morbidity.
The development of large, symptomatic subdural fluid collections occurs regularly with these tumors.5,25,26 They occur not as a result of a specific surgical approach but as a function of the suddenly decompressed brain (from hydrocephalus or tumor evacuation).25,57 Shunting does not always resolve the collections but will treat any pressure effects.
Subdural collections may be avoided by use of a modified “brain patch” technique.25,26,94 A small corticotomy is used, kept at a superior position, allowing the decompressed ventricular cavity to be filled and the brain “floated” with saline. The corticotomy is sealed, either with Gelfoam surrounded by fibrin glue or with pial sutures and sealant. After the tissue sealant has set, a ventricular catheter inserted into the cavity can be used to slowly infuse more saline and “inflate” the brain. The catheter is withdrawn from the brain, the hole is sealed, and the catheter is then used as an externalized subdural drain. Although subdural collections may still form, the tissue sealant closure allows subsequent direct treatment and resolution of only the subdural collection.26
Outcomes
The extent of surgical resection remains the most important factor in determining long-term survival in patients with tumors of the choroid plexus.21,22,26,32,39,56,95–98 Adjuvant therapy is used for malignant tumors and those that have shown leptomeningeal spread. Radiation therapy is reasonable in adults and older children, especially in the context of dissemination or residual malignant tumor. It has no significant role in younger children, in whom severe developmental delay would result from such treatment.
Choroid plexus papillomas have an excellent long-term survival after only gross-total resection, ranging from 90% to 100%.21,26,56 This group of patients may have some major morbidity, with up to 25% having a seizure disorder and 25% having hemiparesis. About 25% will have no significant morbidity.
Patients with choroid plexus carcinomas treated only with surgery have had a very poor outcome: disease progresses rapidly, and patients often die within 1 year.21,82,84,99,100 The early use of radiation therapy did extend survival but at a significant developmental cost because most of these patients are younger than 2 years.32,38 This result led to the use of combination chemotherapy after surgery, with a notable improvement in survival rate to 26% to 50% at 5 years.21,39,79,95,96,101,102
As the combination of improved surgery and aggressive chemotherapy was used consistently on choroid plexus carcinomas, the long-term survival rate began to approach 75%.26,84,103 In turn, this prompted use of chemotherapy as preoperative adjunctive treatment.21,24,84,103,104 A biopsy or subtotal resection of the tumor would confirm the diagnosis, followed by multidrug chemotherapy. After tumor regression on chemotherapy, a second surgery to attempt complete excision would be performed. The tumor vascularity was decreased, which aided resection and correlated with the significant fibrosis seen on pathology.
It appears that the key to long-term survival with choroid plexus carcinoma is the achievement of gross total resection, even if multiple surgeries are required.21,26,39,95,103 Recurrences tend to manifest early.37 It has been controversial whether chemotherapy is required after gross total resection, but generally it has been undertaken owing to the aggressive nature of these tumors.79 Chemotherapy without complete resection does not provide the same long-term survival.
Burger PC, Scheithauer BW. Tumors of neuroglia and choroid plexus epithelium. In: Burger PC, Scheithauer BW, editors. Tumors of the Central Nervous System. Washington DC: Armed Forces Institute of Pathology; 1994:136-161.
Ellenbogen RG, Winston KR, Kupsky WJ. Tumors of the choroid plexus in children. Neurosurgery. 1989;25:327-335.
Hirsch JF, Sante-Rose C. A new surgical approach to subcortical lesions; balloon inflation and cortical gluing. J Neurosurg. 1991;74:1014-1017.
Jeibmann A, Hasselblatt M, Gerss J, et al. Prognostic implications of atypical histologic features in choroid plexus papilloma. J Neuropathol Exp Neurol. 2006;65:1069-1073.
Krieger MD, Panigraphy A, McComb JG, et al. Differentiation of choroid plexus tumors by advanced magnetic resonance spectroscopy. Neurosurg Focus. 2005;18:E4.
Laurence KM. The biology of choroid plexus papilloma in infancy and childhood. Acta Neurochir. 1979;50:79-90.
Meyers SP, Khademian ZP, Chuang SH, et al. Choroid plexus carcinomas in children: MRI features and patient outcomes. Neuroradiology. 2004;46:770-780.
Newbould MJ, Kelsey AM, Arango JC, et al. The choroid plexus carcinomas of childhood: histopathology, immunocytochemistry and clinicopathological correlations. Histopathology. 1995;26:137-143.
Paulus W, Brandner S. Choroid plexus tumors. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumors of the Central Nervous System. Lyon, France: IARC Press; 2007:82-85.
Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, et al. Papillomas and carcinomas of the choroid plexus in children. J Neurosurg. 1998;88:521-528.
Raimondi AJ, Gutierrez FA. Diagnosis and surgical treatment of choroid plexus papillomas. Child Brain. 1975;1:81-115.
Rhoton ALJr. Microsurgical anatomy of the lateral ventricles. In: Wilkins RH, Rengachary SS, editors. Neurosurgery Update I: Diagnosis, Operative Technique, and Neuro-Oncology. New York: McGraw-Hill; 1990:354-368.
Rickert CH, Wiestler OD, Paulus W. Chromosomal imbalances in choroid plexus tumors. Am J Pathol. 2002;160:1105-1113.
Russell DS, Rubinstein LJ. Pathology of Tumors of the Nervous System, 5th ed. Baltimore: Williams & Wilkins; 1989.
Souweidane M, Johnson JJ, Lis E. Volumetric reduction of a choroid plexus carcinoma using preoperative chemotherapy. J Neurooncol. 1999;43:167-171.
Timurkaynak E, Rhoton A, Barry M. Microsurgical anatomy and approaches to the lateral ventricles. Neurosurgery. 1986;19:685-723.
Tomita T, McLone DG, Flannery AM. Choroid plexus papillomas of neonates, infants and children. Pediatr Neurosci. 1988;14:23-30.
Wolff JEA, Sajedi M, Brant R, et al. Choroid plexus tumours. Br J Cancer. 2002;87:1086-1091.
Wrede B, Liu P, Wolff JE. Chemotherapy improves the survival of patients with choroid plexus carcinoma: a meta-analysis of individual cases with choroid plexus tumors. J Neurooncol. 2007;85:345-351.
1 Matson D. Tumors of the choroid plexus. In: Matson DD, editor. Neurosurgery of Infancy and Childhood. 2nd ed. Springfield IL: Charles C Thomas; 1969:581-595.
2 Cushing H. Intracranial Tumors. Springfield IL: Charles C Thomas; 1932.
3 Zulch K. Biologie und pathologie der hirngeschwulste. In: Olvecrona H, Tonnis W, editors. Handbuch der Neruochirurgie, Vol 3. Berlin: Springer-Verlag; 1956.
4 Laurence KM. The biology of choroid plexus papilloma in infancy and childhood. Acta Neurochir. 1979;50:79-90.
5 Matson D, Crofton F. Papilloma of the choroid plexus in childhood. J Neurosurg. 1960;17:1002-1027.
6 Guerard M. Tumeur Fongeuse dans le ventricle droit du cerveau chez une petite fille de trois ans. Anat Paris. 1832;8:211-214.
7 Bielschowsky M, Unger E. Kenntnis der primaren Epithelgeschwulste der Adergeflechte des Gehirns. Arch Klin Chir. 1906;81:61-82.
8 Perthes G. Entfernung eines Tumors des Plexus Choriodeus an dem Seitenventrikel des Cerebrums. Munch Med Wschr. 1919;66:677-678.
9 Boudet G, Clunet J. Contribution a l’etude des tumeurs epitheliales primitives de l’encephale. Arch Med Exp Anat Pathol. 1910;22:379-411.
10 Davis L. A physiopathological study of the choroid plexus with the report of a case of villous hypertrophy. Med Res. 1924;44:521-534.
11 Davis L, Cushing H. Papillomas of the choroid plexus: a report of six cases. Arch Neurol Psych. 1925;13:681-710.
12 Garrod: Papillomatous tumor in the fourth ventricle of the brain. Lancet. 1873;1:303.
13 Rand C, Reeves D. Choroid plexus tumors in infancy and childhood: report of four cases. Bull L A Neurol Soc. 1940;5:405-410.
14 Sachs E. Papillomas of the fourth ventricle. Arch Neurol Psych. 1922;8:379-382.
15 Slaymaker S, Elias F. Papilloma of the choroid plexus with hydrocephalus: report of a case. Arch Intern Med. 1909;3:289-294.
16 Turner O, Simon M. Malignant papillomas of the choroid plexus: report of two cases with review of the literature. Am J Cancer. 1937;30:289-297.
17 Van Wagenen WP. Papillomas of the choroid plexus: report of two cases, one with removal of tumor at operation and one with “seeding” of the tumor in the ventricular system. Arch Surg. 1930;20:199-231.
18 Dandy W. Benign Encapsulated Tumors of the Lateral Ventricle. Baltimore: Williams & Wilkins; 1934.
19 Masson C. Complete removal of two tumors of the third ventricle with recovery. Arch Surg. 1934;28:527-537.
20 Strojan P, Popovic M, Surlan K, et al. Choroid plexus tumors: a review of 28-year experience. Neoplasma. 2004;51:306-312.
21 Ellenbogen RG, Winston KR, Kupsky WJ. Tumors of the choroid plexus in children. Neurosurgery. 1989;25:327-335.
22 Johnson DL. Management of choroid plexus tumors in children. Pediatr Neurosci. 1989;15:195-206.
23 Pascual-Castroviejo I, Villarejo F, Perez-Higueras A. Childhood choroid plexus neoplasms: a study of 14 cases less than 2 years old. Eur Pediatr. 1983;140:51-56.
24 Allen J, Wisoff J, Helson L, et al. Choroid plexus carcinoma: responses to chemotherapy alone in newly diagnosed young children. J Neurooncol. 1992;12:69-74.
25 Boyd M, Steinbok M. Choroid plexus tumors: problems in diagnosis and management. J Neurosurg. 1987;66:800-805.
26 Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, et al. Papillomas and carcinomas of the choroid plexus in children. J Neurosurg. 1998;88:521-528.
27 Drucker GA. Papillary tumor of the choroid plexus in a newborn infant. Arch Pathol. 1939;28:390-395.
28 Braunstein H, Martin FJr. Congenital papilloma of choroid plexus: report of a case, with observations on pathogenesis of associated hydrocephalus. AMA Arch Neurol Psychiatry. 1952;68:475-480.
29 Matson DD. Hydrocephalus in a premature infant caused by papilloma of the choroid plexus with report of surgical treatment. J Neurosurg. 1953;10:416-420.
30 Body G, Darnis E, Pourcelot D. Choroid plexus tumors: antenatal diagnosis and follow-up. J Clin Ultrasound. 1990;18:575-578.
31 Tomita T, Naidich TP. Successful resection of choroid plexus papilloma diagnosed at birth: report of two cases. Neurosurgery. 1987;20:774-779.
32 Wolff JEA, Sajedi M, Brant R, et al. Choroid plexus tumours. Br J Cancer. 2002;87:1086-1091.
33 Kahn E, Luros J. Hydrocephalus from overproduction of cerebrospinal fluid (and experiences with other papilloma of the choroid plexus). J Neurosurg. 1952;9:59-67.
34 Knierim DS. Choroid plexus tumors in infants. Pediatr Neurosurg. 1990;16:276-280.
35 Tomita T, McLone DG, Flannery AM. Choroid plexus papillomas of neonates, infants and children. Pediatr Neurosci. 1988;14:23-30.
36 Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumors. Brain Pathol. 1993;3:255-268.
37 Jeibmann A, Hasselblatt M, Gerss J, et al. Prognostic implications of atypical histologic features in choroid plexus papilloma. J Neuropathol Exp Neurol. 2006;65:1069-1073.
38 Shinoda J, Kawaguchi M, Matsuhisa T, et al. Choroid plexus carcinoma in infants: report of two cases and review of the literature. Acta Neurochir (Wein). 1998;140:557-563.
39 Due-Tonnessen B, Helseth E, Skullerud K, et al. Choroid plexus tumors in children and young adults: report of 16 consecutive cases. Childs Nerv Syst. 2001;17:252-256.
40 Meyers SP, Khademian ZP, Chuang SH, et al. Choroid plexus carcinomas in children: MRI features and patient outcomes. Neuroradiology. 2004;46:770-780.
41 Ray BS, Peck FCJr. Papilloma of the choroid plexus of the lateral ventricles causing hydrocephalus in an infant. J Neurosurg. 1956;13:405-410.
42 Milhorat T, Hammock M, Davis D, et al. Choroid plexus papilloma: proof of cerebrospinal overproduction. Childs Brain. 1976;2:273-289.
43 Eisenberg H, McComb G, Lorenzo A. Cerebrospinal fluid overproduction and hydrocephalus associated with choroid plexus papilloma. J Neurosurg. 1974;40:380-385.
44 Portnoy H, Croissant P. A practical method of measuring hydrodynamics of cerebrospinal fluid. Surg Neurol. 1976;5:173-177.
45 Sahar A, Feinsod M, Beller A. Choroid plexus papilloma: hydrocephalus and cerebrospinal fluid dynamics. Surg Neurol. 1980;13:476-478.
46 Welch K, Strand R, Bresnan M, et al. Congenital hydrocephalus due to villous hypertrophy of the telencephalic choroid plexuses: case report. J Neurosurg. 1983;59:172-175.
47 Gudeman S, Sullivan H, Rosner M, et al. Surgical removal of bilateral papillomas of the choroid plexus of the lateral ventricles with resolution of hydrocephalus. J Neurosurg. 1979;50:677-681.
48 Schijman E, Monges J, Raimondi A, et al. Choroid plexus papillomas of the III ventricle in childhood. Childs Nerv Syst. 1990;6:331-334.
49 McDonald J. Persistent hydrocephalus following the removal of papillomas of the choroid plexus of the lateral ventricles. J Neurosurg. 1969;30:736-740.
50 Raimondi AJ, Gutierrez FA. Diagnosis and surgical treatment of choroid plexus papillomas. Childs Brain. 1975;1:81-115.
51 Pollack IF, Schor NF, Martinez AJ, et al. Bobble-head doll syndrome and drop attacks in a child with a cystic choroid plexus papilloma of the third ventricle. Case report. J Neurosurg. 1995;83:729-732.
52 Pecker J, Ferrand B, Javalet A. Tumeurs du troisieme ventricule. Neurochirurgia. 1966;12:1-36.
53 Cassinari V. Tumori della parte anteriore del terzo ventricolo. Acta Neurochir (Wein). 1963;11:236-271.
54 Seifert V, Stolke D, Mehdorn HM, et al. Clinical and radiological evaluation of long-term results of stereotactic proton beam radiosurgery in patients with cerebral arteriovenous malformations. J Neurosurg. 1994;81:683-689.
55 Rovit RL, Schechter MM, Chodroff P. Choroid plexus papillomas: observations on radiographic diagnosis. AJR Am J Roentgenol. 1970;110:608-617.
56 McGirr SJ, Ebersold MJ, Scheithauer BW, et al. Choroid plexus papillomas: long-term follow-up results in a surgically treated series. J Neurosurg. 1988;69:843-849.
57 Jooma R, Grant D. Third ventricle choroid plexus papillomas. Childs Brain. 1983;10:242-250.
58 Burger PC, Scheithauer BW. Tumors of neuroglia and choroid plexus epithelium. In: Burger PC, Scheithauer BW, editors. Tumors of the Central Nervous System. Washington DC: Armed Forces Institute of Pathology; 1994:136-161.
59 Talacchi A, DeMicheli E, Lombardo C, et al. Choroid plexus papilloma of the cerebellopontine angle: a twelve patient series. Surg Neurol. 1999;51:621-629.
60 Kimura M, Takayasu M, Suzuki Y, et al. Primary choroid plexus papilloma located in the suprasellar region: case report. Neurosurgery. 1992;31:563-566.
61 Kieserman S, Linstrom C, McCormick S, et al. Choroid plexus papilloma of the cerebellopontine angle. Am J Otolaryngol. 1996;17:119-122.
62 Bohm J, Strange R. Choroid plexus papillomas. J Neurosurg. 1961;18:493-500.
63 Guidetti B, Spallone A. The surgical treatment of choroid plexus papillomas: the results of 27 years’ experience. Neurosurgery. 1981;6:380-384.
64 Leblanc R, Bekhor S, Melanson D, et al. Diffuse craniospinal seeding from a benign fourth ventricle choroid plexus papilloma: case report. J Neurosurg. 1998;88:757-760.
65 Vraa-Jensen G. Papilloma of the choroid plexus with pulmonary metastases. Acta Psychiatr (Koln). 1950;25:299-306.
66 Rhoton ALJr. Microsurgical anatomy of the lateral ventricles. In: Wilkins RH, Rengachary SS, editors. Neurosurgery Update I. Diagnosis, Operative Technique, and Neuro-Oncology. New York: McGraw-Hill; 1990:354-368.
67 Timurkaynak E, Rhoton A, Barry M. Microsurgical anatomy and approaches to the lateral ventricles. Neurosurgery. 1986;19:685-723.
68 Crofton F, Matson D. Roentgenologic study of choroid plexus papillomas in children. AJR Am J Roentgenol. 1950;84:273-311.
69 Hawkins JCI. Treatment of choroid plexus papillomas in children: a brief analysis of twenty years’ experience. Neurosurgery. 1980;6:380-384.
70 Coates TL, Hinshaw DBJr, Peckman N, et al. Pediatric choroid plexus neoplasms: MR, CT, and pathologic correlation. Radiology. 1989;173:81-88.
71 Krieger MD, Panigraphy A, McComb JG, et al. Differentiation of choroid plexus tumors by advanced magnetic resonance spectroscopy. Neurosurg Focus. 2005;18:E4.
72 Horska A, Ulug AM, Melhem ER, et al. Proton magnetic resonance spectroscopy of choroid plexus tumors in children. J Magn Reson Imag. 2001;14:78-82.
73 Paulus W, Brandner S. Choroid plexus tumors. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumors of the Central Nervous System. Lyon, France: IARC Press; 2007:82-85.
74 Coffin CC, Wick MR, Braun JT, et al. Choroid plexus neoplasms: clinicopathologic and immunohistochemical studies. Am J Surg Pathol. 1986;10:394-404.
75 Russell DS, Rubinstein LJ. Pathology of Tumors of the Nervous System, 5th ed. Baltimore: Williams & Wilkins; 1989.
76 Chow E, Jenkins JJ, Burger PC, et al. Malignant evolution of choroid plexus papilloma. Pediatr Neurosurg. 1999;31:127-130.
77 Gullotta F, de Melo A. Plexus choriodeus: Klinishe, lightmikroscopische und elektroneneoptische untersuchungen. Neurochirurgia. 1979;22:1-9.
78 Paulus W, Janisch W. Clinicopathologic correlations in epithelial choroid plexus neoplasms: a study of 52 cases. Acta Neuropathol. 1990;80:635-641.
79 Chow E, Reardon DA, Shah AB, et al. Pediatric choroid plexus neoplasms. Int J Radiat Oncol Biol Phys. 1999;44:249-254.
80 Jeibmann A, Wrede B, Peters O, et al. Malignant progression in choroid plexus papillomas. J Neurosurg. 2007;107(3 suppl):199-202.
81 Newbould MJ, Kelsey AM, Arango JC, et al. The choroid plexus carcinomas of childhood: histopathology, immunocytochemistry and clinicopathological correlations. Histopathology. 1995;26:137-143.
82 Dohrmann GJ, Collias JC. Choroid plexus carcinoma: case report. J Neurosurg. 1975;43:225-232.
83 Lewis P. Carcinoma of the choroid plexus. Brain. 1967;90:177-186.
84 St. Clair SK, Humphreys RP, Pillay PK, et al. Current management of choroid plexus carcinoma. Pediatr Neurosurg. 1991;17:225-233.
85 Ausman J, Shrontz C, Chason J, et al. Aggressive choroid plexus papilloma. Surg Neurol. 1984;22:472-476.
86 Tena-Suck ML, Gomez-Amador JL, Ortiz-Plata A, et al. Rhabdoid choroid plexus carcinoma. Arq Neuropsiquiatr. 2007;65:705-709.
87 Philips M, Shanno G, Duahime A. Treatment of villous hypertrophy of the choroid plexus by endoscopic contact coagulation. Pediatr Neurosurg. 1998;28:252-256.
88 Hirano H, Hirahara K, Tetsuhiko A, et al. Hydrocephalus due to villous hypertrophy of the choroid plexus in the lateral ventricles. J Neurosurg. 1994;80:321-323.
89 Cruz-Sanchez F, Rossi M, Hughes J, et al. Choroid plexus papillomas: an immunohistological study of 16 cases. Histopathology. 1989;15:61-69.
90 Vajtai I, Varga Z, Aguzzi A. MIB-1 immunoreactivity reveals different labelling in low grade and malignant epithelial neoplasms of the choroid plexus. Histopathology. 1996;29:147-151.
91 Wyatt-Ashmead J, Kleinschmidt-DeMasters B, Mierau GW, et al. Choroid plexus carcinomas and rhabdoid tumors: phenotypic and genotypic overlap. Pediatr Dev Pathol. 2001;4:545-549.
92 Rickert CH, Wiestler OD, Paulus W. Chromosomal imbalances in choroid plexus tumors. Am J Pathol. 2002;160:1105-1113.
93 Fortuna A, Celli P, Ferrante L, et al. A review of papillomas of the third ventricle. J Neurosurg. 1979;23:61-72.
94 Hirsch JF, Sante-Rose C. A new surgical approach to subcortical lesions: balloon inflation and cortical gluing. J Neurosurg. 1991;74:1014-1017.
95 Berger C, Thiesse P, Lellouch-Tubiana A. Choroid plexus carcinomas in childhood: clinical features and prognostic factors. Neurosurgery. 1998;42:470-475.
96 Packer R, Perilongo G, Johnson D, et al. Choroid plexus carcinoma of childhood. Cancer. 1992;69:580-585.
97 Pierga J, Kalifa C, Terrier-Lacombe MJ, et al. Carcinoma of the choroid plexus: a pediatric experience. Med Pediatr Oncol. 1993;21:480-487.
98 Sharma R, Rout D, Gupta A, et al. Choroid plexus papillomas. Br J Neurosurg. 1994;8:169-177.
99 Humphreys R, Nemoto S, Hendrick EB, et al. Childhood choroid plexus tumors. Concepts Pediatr Neurosurg. 1987;7:1-18.
100 Carpenter D, Michelsen W, Hays A. Carcinoma of the choroid plexus. J Neurosurg. 1982;56:722-777.
101 Lena G, Genitori L, Molina J, et al. Choroid plexus tumors in children: review of 24 cases. Acta Neurochir (Wein). 1990;106:68-72.
102 Wrede B, Liu P, Wolff JE. Chemotherapy improves the survival of patients with choroid plexus carcinoma: a meta-analysis of individual cases with choroid plexus tumors. J Neurooncol. 2007;85:345-351.
103 Duffner PK, Kun LE, Burger PC, et al. Postoperative chemotherapy and delayed radiation in infants and very young children with choroid plexus carcinomas. Pediatr Neurosurg. 1995;22:189-196.
104 Souweidane M, Johnson JJ, Lis E. Volumetric reduction of a choroid plexus carcinoma using preoperative chemotherapy. J Neurooncol. 1999;43:167-171.