Chapter 138 Childhood Asthma
Etiology
Although the cause of childhood asthma has not been determined, contemporary research implicates a combination of environmental exposures and inherent biologic and genetic vulnerabilities (Fig. 138-1). Respiratory exposures in this causal environment include inhaled allergens, respiratory viral infections, and chemical and biologic air pollutants such as environmental tobacco smoke. In the predisposed host, immune responses to these common exposures can be a stimulus for prolonged, pathogenic inflammation and aberrant repair of injured airways tissues. Lung dysfunction (i.e., AHR and reduced airflow) develops. These pathogenic processes in the growing lung during early life adversely affect airways growth and differentiation, leading to altered airways at mature ages. Once asthma has developed, ongoing exposures appear to worsen it, driving disease persistence and increasing the risk of severe exacerbations.
Epidemiology
Approximately 80% of all asthmatic patients report disease onset prior to 6 yr of age. However, of all young children who experience recurrent wheezing, only a minority go on to have persistent asthma in later childhood. Early childhood risk factors for persistent asthma have been identified (Table 138-1). Prediction of asthma includes major (parent asthma, eczema, inhalant allergen sensitization) and minor (allergic rhinitis, wheezing apart from colds, ≥4% eosinophils, food allergen sensitization) risk factors. Allergy in young children has emerged as a major risk factor for the persistence of childhood asthma.
Types of Childhood Asthma
Asthma is considered to be a common clinical presentation of intermittent, recurrent wheezing and/or coughing, resulting from different airways pathologic processes underlying different types of asthma. There are 2 main types of childhood asthma: (1) recurrent wheezing in early childhood, primarily triggered by common viral infections of the respiratory tract, and (2) chronic asthma associated with allergy that persists into later childhood and often adulthood. A 3rd type of childhood asthma typically emerges in females who experience obesity and early-onset puberty (by 11 yr of age). Some children may be hypersensitive to common air pollutants (environmental tobacco smoke, ozone, endotoxin) such that exposures to these pollutants might not only make existing asthma worse but may also have a causal role in the susceptible. The most common persistent form of childhood asthma is associated with allergy and susceptibility to common respiratory virus–induced exacerbations (Table 138-2).
Table 138-2 RECURRENT COUGHING/WHEEZING PATTERNS IN CHILDHOOD, BASED ON NATURAL HISTORY
TRANSIENT EARLY WHEEZING
PERSISTENT ATOPY-ASSOCIATED ASTHMA
NONATOPIC WHEEZING
ASTHMA WITH DECLINING LUNG FUNCTION
LATE-ONSET ASTHMA IN FEMALES, ASSOCIATED WITH OBESITY AND EARLY-ONSET PUBERTY
OCCUPATIONAL-TYPE ASTHMA IN CHILDREN
Children with asthma associated with occupational-type exposures known to trigger asthma in adults in occupational settings (e.g., endotoxin exposure in children raised on farms)
From Taussig LM, Landau LI, et al, editors: Pediatric respiratory medicine, ed 2, Philadelphia, 2008, Mosby/Elsevier, p 822.
Pathogenesis
Airflow obstruction in asthma is the result of numerous pathologic processes. In the small airways, airflow is regulated by smooth muscle encircling the airways lumens; bronchoconstriction of these bronchiolar muscular bands restricts or blocks airflow. A cellular inflammatory infiltrate and exudates distinguished by eosinophils, but also including other inflammatory cell types (neutrophils, monocytes, lymphocytes, mast cells, basophils), can fill and obstruct the airways and induce epithelial damage and desquamation into the airways lumen. Helper T lymphocytes and other immune cells that produce proallergic, proinflammatory cytokines (IL-4, IL-5, IL-13), and chemokines (eotaxin) mediate this inflammatory process. Pathogenic immune responses and inflammation may also result from a breach in normal immune regulatory processes (such as regulatory T lymphocytes that produce IL-10 and transforming growth factor [TGF]-β) that dampen effector immunity and inflammation when they are no longer needed. Hypersensitivity or susceptibility to a variety of provocative exposures or triggers (Table 138-3) can lead to airways inflammation, AHR, edema, basement membrane thickening, subepithelial collagen deposition, smooth muscle and mucous gland hypertrophy, and mucus hypersecretion—all processes that contribute to airflow obstruction (Chapter 134).
Clinical Manifestations and Diagnosis
Asthma symptoms can be triggered by numerous common events or exposures: physical exertion and hyperventilation (laughing), cold or dry air, and airways irritants (see Table 138-3). Exposures that induce airways inflammation, such as infections (rhinovirus, respiratory syncytial virus, metapneumovirus, torque teno virus, parainfluenza virus, influenza virus, adenovirus, Mycoplasma pneumonia, Chlamydia pneumoniae), and inhaled allergens, also increase AHR to irritant exposures. An environmental history is essential for optimal asthma management (Chapter 135).
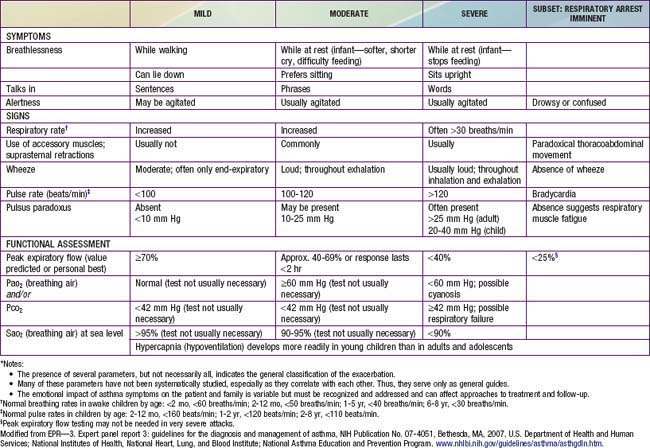
During asthma exacerbations, expiratory wheezing and a prolonged expiratory phase can usually be appreciated by auscultation. Decreased breath sounds in some of the lung fields, commonly the right lower posterior lobe, are consistent with regional hypoventilation owing to airways obstruction. Crackles (or rales) and rhonchi can sometimes be heard, resulting from excess mucus production and inflammatory exudate in the airways. The combination of segmental crackles and poor breath sounds can indicate lung segmental atelectasis that is difficult to distinguish from bronchial pneumonia and can complicate acute asthma management. In severe exacerbations, the greater extent of airways obstruction causes labored breathing and respiratory distress, which manifests as inspiratory and expiratory wheezing, increased prolongation of exhalation, poor air entry, suprasternal and intercostal retractions, nasal flaring, and accessory respiratory muscle use. In extremis, airflow may be so limited that wheezing cannot be heard (Table 138-4).
Differential Diagnosis
Many childhood respiratory conditions can present with symptoms and signs similar to those of asthma (Table 138-5). Besides asthma, other common causes of chronic, intermittent coughing include gastroesophageal reflux (GER) and rhinosinusitis. Both GER and chronic sinusitis can be challenging to diagnose in children. Often, GER is clinically silent in children, and children with chronic sinusitis do not report sinusitis-specific symptoms, such as localized sinus pressure and tenderness. In addition, both GER and rhinosinusitis are often co-morbid with childhood asthma and, if not specifically treated, may make asthma difficult to manage.
Table 138-5 DIFFERENTIAL DIAGNOSIS OF CHILDHOOD ASTHMA
UPPER RESPIRATORY TRACT CONDITIONS
MIDDLE RESPIRATORY TRACT CONDITIONS
LOWER RESPIRATORY TRACT CONDITIONS
Laboratory Findings
Lung function tests can help to confirm the diagnosis of asthma and to determine disease severity.
Pulmonary Function Testing
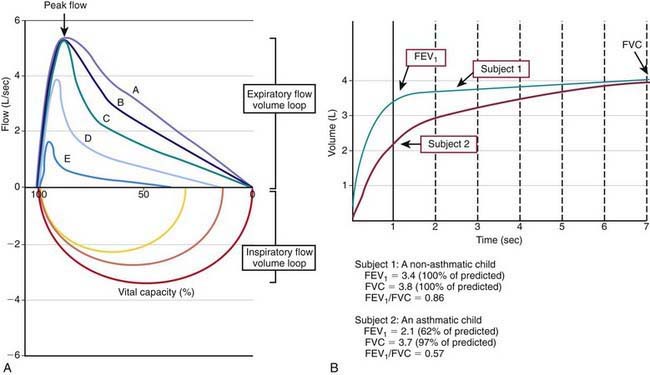
Many asthma guidelines promote spirometric measures of airflow and lung volumes during forced expiratory maneuvers as standard for asthma assessment. Spirometry is helpful as an objective measure of airflow limitation (Fig. 138-2). Knowledgeable personnel are needed to perform and interpret findings of spirometry tests. Valid spirometric measures depend on a patient’s ability to properly perform a full, forceful, and prolonged expiratory maneuver, usually feasible in children > 6 yr of age (with some younger exceptions). Reproducible spirometric efforts are an indicator of test validity; if the FEV1 (forced expiratory volume in 1 sec) is within 5% on 3 attempts, then the highest FEV1 effort of the 3 is used. This standard utilization of the highest of 3 reproducible efforts is indicative of the effort dependence of reliable spirometric testing.
In asthma, airways blockage results in reduced airflow with forced exhalation and smaller partial-expiratory lung volumes (see Fig. 138-2). Because asthmatic patients typically have hyperinflated lungs, FEV1 can be simply adjusted for full expiratory lung volume—the forced vital capacity (FVC)—with an FEV1/FVC ratio. Generally, an FEV1/FVC ratio <0.80 indicates significant airflow obstruction (Table 138-6). Normative values for FEV1 have been determined for children on the basis of height, gender, and ethnicity. Abnormally low FEV1 as a percentage of predicted norms is 1 of 6 criteria used to determine asthma severity in the National Institutes of Health (NIH)–sponsored asthma guidelines.
Table 138-6 LUNG FUNCTION ABNORMALITIES IN ASTHMA
FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity.
Such measures of airflow alone are not diagnostic of asthma, because numerous other conditions can cause airflow reduction. Bronchodilator response to an inhaled β-agonist (e.g., albuterol) is greater in asthmatic patients than nonasthmatic persons; an improvement in FEV1 ≥12% or >200 mL is consistent with asthma. Bronchoprovocation challenges can be helpful in diagnosing asthma and optimizing asthma management. Asthmatic airways are hyperresponsive and therefore more sensitive to inhaled methacholine, histamine, and cold or dry air. The degree of AHR to these exposures correlates to some extent with asthma severity and airways inflammation. Although bronchoprovocation challenges are carefully dosed and monitored in an investigational setting, their use is rarely practical in a general practice setting. Exercise challenges (aerobic exertion or “running” for 6-8 min) can help to identify children with exercise-induced bronchospasm. Although the airflow response of non-asthmatic persons to exercise is to increase functional lung volumes and improve FEV1 slightly (5-10%), exercise often provokes airflow obstruction in persons with inadequately treated asthma. Accordingly, in asthmatic patients, FEV1 typically decreases during or after exercise by >15% (see Table 138-6). The onset of exercise-induced bronchospasm is usually within 15 min after a vigorous exercise challenge and can spontaneously resolve within 30-60 min. Studies of exercise challenges in school-aged children typically identify an additional 5-10% with exercise-induced bronchospasm and previously unrecognized asthma. There are two caveats regarding exercise challenges: first, treadmill challenges in the clinic are not completely reliable and can miss exertional asthma that can be demonstrated on the playing field; and second, treadmill challenges can induce severe exacerbations in at-risk patients. Careful patient selection for exercise challenges and preparedness for severe asthma exacerbations are required.
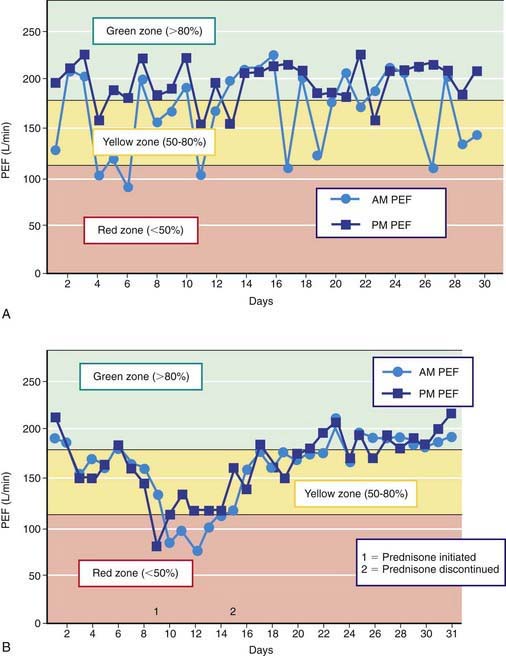
Peak expiratory flow (PEF) monitoring devices provide simple and inexpensive home-use tools to measure airflow and can be helpful in a number of circumstances (Fig. 138-3). “Poor perceivers” of airflow obstruction due to asthma can benefit by monitoring PEFs daily to assess objectively airflow as an indicator of asthma control or problems that would be more sensitive than their symptom perception. PEF devices vary in the ability to detect airflow obstruction: they are generally less sensitive than spirometry to airflow obstruction such that, in some patients, PEF values decline only when airflow obstruction is severe. Therefore, PEF monitoring should be started by measuring morning and evening PEFs (best of 3 attempts) for several weeks for patients to practice the technique, to determine a “personal best,” and to correlate PEF values with symptoms (and ideally spirometry). PEF variation >20% is consistent with asthma (see Fig. 138-3 and Table 138-6).
Radiology
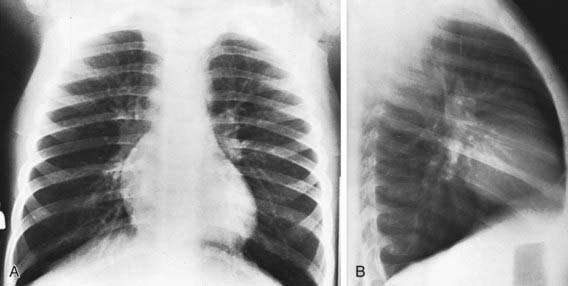
The findings of chest radiographs (posteroanterior and lateral views) in children with asthma often appear to be normal, aside from subtle and nonspecific findings of hyperinflation (flattening of the diaphragms) and peribronchial thickening (Fig. 138-4). Chest radiographs can be helpful in identifying abnormalities that are hallmarks of asthma masqueraders (aspiration pneumonitis, hyperlucent lung fields in bronchiolitis obliterans), and complications during asthma exacerbations (atelectasis, pneumomediastinum, pneumothorax). Some lung abnormalities can be better appreciated with high-resolution, thin-section chest CT scans. Bronchiectasis, which is sometimes difficult to appreciate on chest radiograph but is clearly seen on CT scan, implicates an asthma masquerader, such as cystic fibrosis, allergic bronchopulmonary mycoses (aspergillosis), ciliary dyskinesias, or immune deficiencies.
Treatment
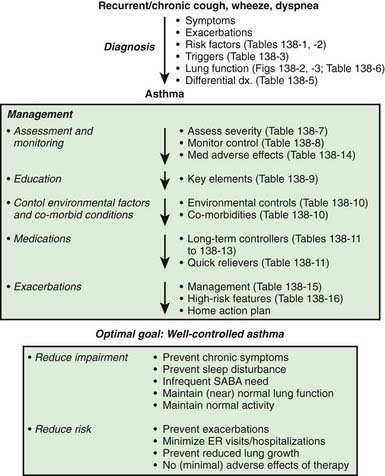
The National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma 2007 is available online (www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm), and the highlights of significant changes from the previous version of the guidelines have been published. The key components to optimal asthma management are specified (Fig. 138-5). Management of asthma should have the following components: (1) assessment and monitoring of disease activity; (2) provision of education to enhance the patient’s and family’s knowledge and skills for self-management; (3) identification and management of precipitating factors and co-morbid conditions that may worsen asthma; and (4) appropriate selection of medications to address the patient’s needs. The long-term goal of asthma management is attainment of optimal asthma control.
Component 1: Regular Assessment and Monitoring
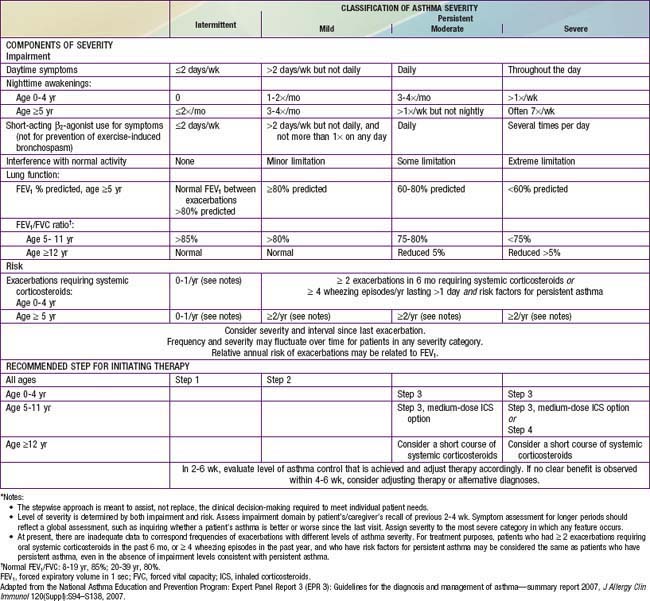
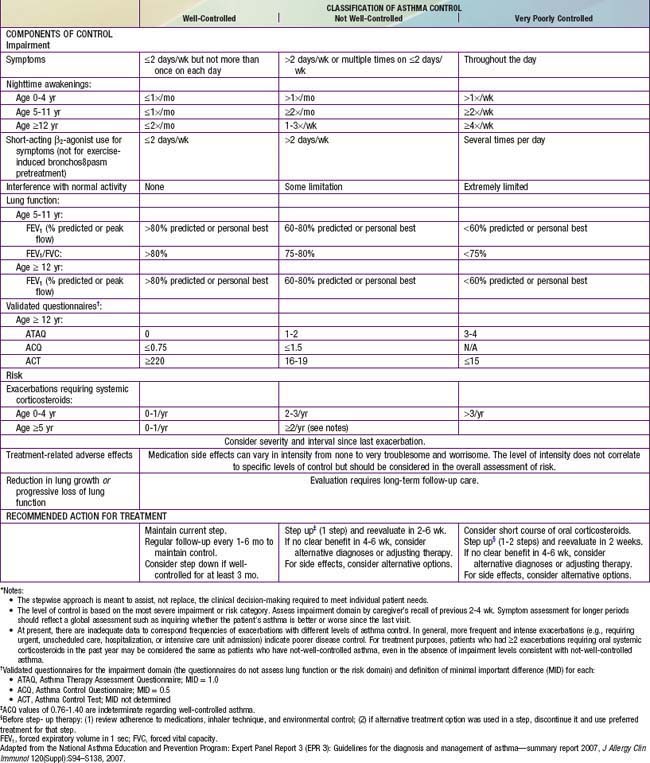
Classification of asthma severity and control is based on the domains of Impairment and Risk. These domains may not correlate with each other and may respond differently to treatment. The NIH guidelines have criteria for 3 age groups—0-4 yr, 5-11 yr, and ≥12 yr—for the evaluation of both severity (Table 138-7) and control (Table 138-8). The level of asthma severity or control is based on the most severe impairment or risk category. In assessing asthma severity, impairment consists of an assessment of the patient’s recent symptom frequency (daytime and nighttime with subtle differences in numeric cutoffs between the 3 age groups), need for short-acting β2-agonists for quick relief, ability to engage in normal or desired activities, and airflow compromise, which is evaluated with spirometry in children 5 yr and older. Risk refers to an evaluation of the likelihood of developing asthma exacerbations for the individual patient. Of note, in the absence of frequent symptoms, persistent asthma should be considered, and therefore long-term controller therapy should be initiated for infants or children who have risk factors for asthma (see earlier) and 4 or more episodes of wheezing over the past year that lasted longer than 1 day and affected sleep, or 2 or more exacerbations in 6 months requiring systemic corticosteroids.
Table 138-7 ASSESSING ASTHMA SEVERITY AND INITIATING TREATMENT FOR PATIENTS WHO ARE NOT CURRENTLY TAKING LONG-TERM CONTROL MEDICATIONS*
Asthma management can be optimized through regular clinic visits every 2-6 wk until good asthma control is achieved. For children already on controller medication therapy, management is tailored to the child’s level of control. The NIH guidelines provide tables for evaluating asthma control for the 3 age groups (see Table 138-8). In evaluation of asthma control, as in severity assessment, impairment includes an assessment of the patient’s symptom frequency (daytime and nighttime), need for short-acting β2-agonists for quick relief, ability to engage in normal or desired activities, and, for older children, airflow measurements. In addition, determination of quality of life measures for older children is included. Furthermore, with respect to risk assessment, besides considering severity and frequency of exacerbations requiring systemic corticosteroids, tracking of lung growth in older children and monitoring of untoward effects of medications are also warranted. As already mentioned, the degree of impairment and risk are used to determine the patient’s level of asthma control as well-controlled, not well-controlled, or very poorly controlled. Children with well-controlled asthma have: daytime symptoms ≤2 days/week and need a rescue bronchodilator ≤2 days/week; an FEV1 of >80% of predicted (and FEV1/FVC ratio >80% for children 5-11 yr of age); no interference with normal activity; and <2 exacerbations in the past year. The impairment criteria vary slightly depending on age group: there are different thresholds in the frequency of nighttime awakenings; addition of FEV1/FVC ratio criteria for children 5-11 yr old, and addition of validated questionnaires in evaluating quality of life for older children. Children whose status does not meet all of the criteria defining well-controlled asthma are determined to have either not well-controlled or very poorly controlled asthma, which is determined by the single criterion with the lowest rating.
Two to four asthma checkups per year are recommended for reassessing and maintaining good asthma control. During these visits, asthma control can be assessed by determining the: (1) frequency of asthma symptoms during the day, at night, and with physical exercise; (2) frequency of “rescue” SABA medication use and refills; (3) quality of life for youths with an assessment tool; (4) lung function measurements for older children and youths; (5) number and severity of asthma exacerbations; and (6) presence of medication adverse effects since the last visit (see Fig. 138-5). Lung function testing (spirometry) is recommended at least annually and more often if asthma is inadequately controlled or lung function is abnormally low. PEF monitoring at home can be helpful in the assessment of asthmatic children with poor symptom perception, other causes of chronic coughing in addition to asthma, moderate to severe asthma, or a history of severe asthma exacerbations. PEF monitoring is feasible in children as young as 4 yr who are able to master this skill. Use of a stoplight zone system tailored to each child’s “personal best” PEF values can optimize effectiveness and interest (see Fig 138-3): The green zone (80-100% of personal best) indicates good control; the yellow zone (50-80%) indicates less than optimal control and necessitates increased awareness and treatment; the red zone (<50%) indicates poor control and greater likelihood of an exacerbation, requiring immediate intervention. In actuality, these ranges are approximate and may need to be adjusted for many asthmatic children by raising the ranges that indicate inadequate control (in the yellow zone, 70-90%). The NIH guidelines recommend at least once-daily PEF monitoring, preferably in the morning when peak flows are typically lower.
Component 2: Patient Education
Specific educational elements in the clinical care of children with asthma are believed to make an important difference in home management and in adherence of families to an optimal plan of care and eventually impacting patient outcomes (Table 138-9). Every visit presents an important opportunity to educate the child and family, allowing them to become knowledgeable partners in asthma management, because optimal management depends on their daily assessments and implementation of any management plan. Effective communications take into account sociocultural and ethnic factors of children and their families, address concerns about asthma and its treatment, and include patients and families as active participants in the development of treatment goals and selection of medications. Self-monitoring and self-management skills should be reinforced regularly.
Table 138-9 KEY ELEMENTS OF PRODUCTIVE CLINIC VISITS FOR ASTHMA
During initial patient visits, a basic understanding of the pathogenesis of asthma (chronic inflammation and AHR underlying a clinically intermittent presentation) can help children with asthma and their parents understand the importance of recommendations aimed at reducing airways inflammation. It is helpful to specify the expectations of good asthma control resulting from optimal asthma management (see Fig. 138-5). Explaining the importance of steps to reduce airways inflammation in order to achieve good asthma control and addressing concerns about potential adverse effects of asthma pharmacotherapeutic agents, especially their risks relative to their benefits, are essential in achieving long-term adherence with asthma pharmacotherapy and environmental control measures.
Component 3: Control of Factors Contributing to Asthma Severity
Controllable factors that can significantly worsen asthma can be generally grouped as (1) environmental exposures and (2) co-morbid conditions (Table 138-10).
Table 138-10 CONTROL OF FACTORS CONTRIBUTING TO ASTHMA SEVERITY
Treat Co-Morbid Conditions
Rhinitis is usually co-morbid with asthma, detected in ≈90% of children with asthma. Rhinitis can be seasonal and/or perennial, with allergic and nonallergic components. Rhinitis complicates and worsens asthma via numerous direct and indirect mechanisms. Nasal breathing may improve asthma and reduce exercise-induced bronchospasm by humidifying and warming inspired air and filtering out allergens and irritants that can trigger asthma and increase AHR. Reduction of nasal congestion and obstruction can help the nose to perform these humidifying, warming, and filtering functions. In asthmatic patients, improvement in rhinitis is also associated with improvement in AHR, lower airways inflammation, asthma symptoms, and asthma medication use. Optimal rhinitis management in children is similar to asthma management in regard to the importance of interventions to reduce nasal inflammation (Chapter 137).
Component 4: Principles of Asthma Pharmacotherapy
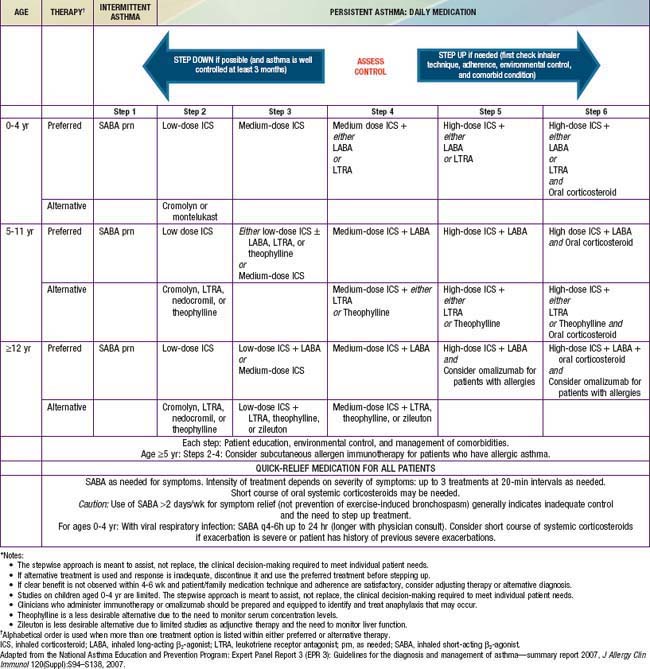
The current version of NIH asthma guidelines (2007) proposes an expanded stepwise treatment approach to assist, not replace, the clinical decision-making required to meet individual patient needs. The recommendations vary by age groups and are based on current evidence (Table 138-11). The goals of therapy are to reduce the components of both impairment (e.g., preventing chronic and troublesome symptoms, allowing infrequent need of quick-reliever medications, maintaining “normal” lung function, maintaining normal activity levels including physical activity and school attendance, meeting families’ expectations and satisfaction with asthma care) and risk (e.g., preventing recurrent exacerbations, reduced lung growth, and medications’ adverse effects). The choice of initial therapy is based on assessment of asthma severity, and for patients who are already using controller therapy, modification of treatment is based on assessment of asthma control and responsiveness to therapy. A major objective of this approach is to identify and treat all “persistent” and uncontrolled asthma with anti-inflammatory controller medication. Daily controller therapy is not recommended for children with “intermittent asthma.” Management of intermittent asthma is simply the use of a short-acting inhaled β-agonist as needed for symptoms and for pre-treatment in those with exercise-induced bronchospasm (Step 1 therapy; see Table 138-11).
Long-Term Controller Medications
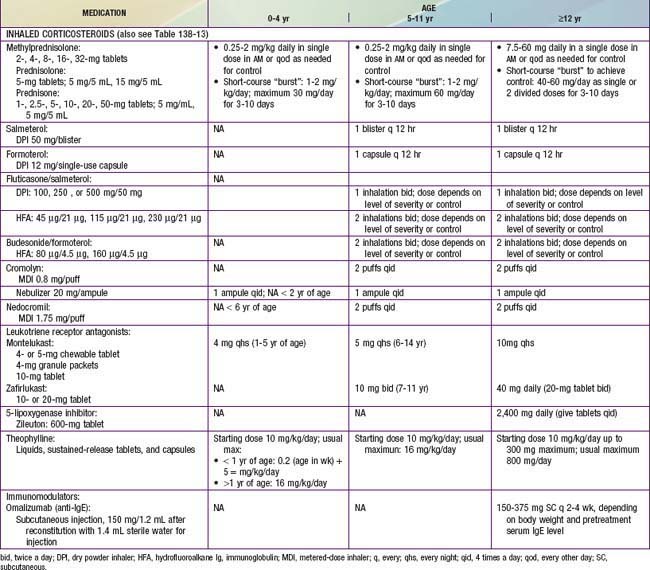
All levels of persistent asthma should be treated with daily medications to improve long-term control (see Table 138-11). Such medications include ICSs, LABAs, leukotriene modifiers, nonsteroidal anti-inflammatory agents, and sustained-release theophylline. An anti-IgE preparation, omalizumab (Xolair), has been approved by the U.S. Food and Drug Administration (FDA) for use as an add-on therapy in children ≥12 yr who have moderate to severe allergic asthma that is difficult to control. Corticosteroids are the most potent and most effective medications used to treat both the acute (administered systemically) and chronic (administered by inhalation) manifestations of asthma. They are available in inhaled, oral, and parenteral forms (Tables 138-12 and 138-13).
Inhaled Corticosteroids
The NIH guidelines recommend daily ICS therapy as the treatment of choice for all patients with persistent asthma (see Table 138-11). ICS therapy has been shown to improve lung function as well as to reduce asthma symptoms, AHR, and use of “rescue” medications; most important, it has been found to reduce urgent care visits, hospitalizations, and prednisone use for asthma exacerbations by about 50%. ICS therapy may lower the risk of death due to asthma. It can achieve all of the goals of asthma management and, as a result, is viewed as first-line treatment for persistent asthma.
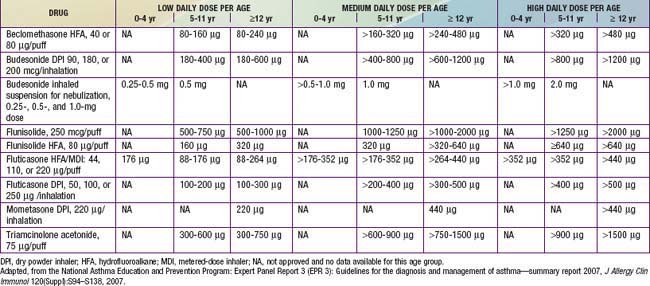
Currently, 6 ICSs are approved for use in children by the FDA, and the NIH guidelines provide an equivalence classification (see Table 138-13), although direct comparisons of efficacy and safety outcomes in children are lacking. ICSs are available in metered-dose inhalers (MDIs), in dry powder inhalers (DPIs), or in suspension for nebulization. Fluticasone propionate, mometasone furoate, ciclesonide, and, to a lesser extent, budesonide are considered “2nd-generation” ICSs, in that they have greater anti-inflammatory potency and diminished systemic bioavailability for potential adverse effects, owing to extensive first-pass hepatic metabolism. The selection of the initial ICS dose is based on the determination of disease severity. A fraction of the initial ICS dose is often sufficient to maintain good control after this goal has been achieved.
Although ICS therapy has been widely used in adults with persistent asthma, its application in children has lagged because of concerns about the potential for adverse effects with long-term use. Generally, clinically significant adverse effects that occur with long-term systemic corticosteroid therapy have not been seen or have only very rarely been reported in children receiving ICSs in recommended doses. The risk of adverse effects from ICS therapy is related to the dose and frequency with which ICSs are given (Table 138-14). High doses (≥1,000 µg/day in children) and frequent administration (4 times/day) are more likely to have local and systemic adverse effects. Children who receive maintenance therapy with higher ICS doses are also likely to require systemic corticosteroid courses for asthma exacerbations, further increasing the risk of corticosteroid adverse effects.
Table 138-14 RISK ASSESSMENT FOR CORTICOSTEROID ADVERSE EFFECTS
| CONDITIONS | RECOMMENDATIONS | |
|---|---|---|
| Low risk |
• Measure height annually (stadiometry); monitor periodically for declining growth rate and pubertal developmental delay
|
DEXA, dual-energy x-ray absorptiometry; ICS, inhaled corticosteroid; TSH, thyroid-stimulating hormone.
* Risk factors for osteoporosis: Presence of other chronic illness(es), medications (corticosteroids, anticonvulsants, heparin, diuretics), low body weight, family history of osteoporosis, significant fracture history disproportionate to trauma, recurrent falls, impaired vision, low dietary calcium and vitamin D intake, and lifestyle factors (decreased physical activity, smoking, and alcohol intake).
The potential for growth suppression and osteoporosis with long-term ICS use has been a concern. In the long term, prospective NIH-sponsored CAMP study of children with mild to moderate asthma, after ≈4.3 yr of ICS therapy and 5 yr after the trial, there was a significant 1.7-cm decrease in height in girls, but not in boys. There was also a slight dose-dependent effect of ICS therapy on bone mineral accretion in boys, but not girls. A greater effect on bone mineral accretion was observed with increasing numbers of courses of oral corticosteroid burst therapy for asthma, as well as an increase in risk for osteopenia, again limited to boys. Although this study cannot predict a significant effect of ICS therapy in childhood on osteoporosis in later adulthood, improved asthma control with ICS therapy may result in a need for fewer courses of oral corticosteroid burst therapy over time. These findings were with use of budesonide at doses of about 400 µg/day; higher ICS doses, especially of agents with increased potency, have a greater potential for adverse effects. Hence, corticosteroid adverse effects screening and osteoporosis prevention measures are recommended for patients receiving higher ICS doses, as these patients are also likely to require systemic courses for exacerbations (see Table 138-14).
Systemic Corticosteroids
ICS therapy has allowed the large majority of children with asthma to maintain good disease control without maintenance oral corticosteroid therapy. Oral corticosteroids are used primarily to treat asthma exacerbations and, rarely, in patients with severe disease who remain symptomatic despite optimal use of other asthma medications. In these severely asthmatic patients, every attempt should be made to exclude any co-morbid conditions and to keep the oral corticosteroid dose at ≤20 mg qod. Doses exceeding this amount are associated with numerous adverse effects (Chapter 571). To determine the need for continued oral corticosteroid therapy, tapering of the oral corticosteroid dose (over several weeks to months) should be considered, with close monitoring of the patient’s symptoms and lung function.
Children who require long-term oral corticosteroid therapy are at risk for development of associated adverse effects over time. Essentially all major organ systems can be adversely affected by long-term oral corticosteroid therapy (Chapter 571). Some of these effects occur immediately (metabolic effects). Others can develop insidiously over several months to years (growth suppression, osteoporosis, cataracts). Most adverse effects occur in a cumulative dose- and duration-dependent manner. Children who require routine or frequent short courses of oral corticosteroids, especially with concurrent high-dose ICSs, should receive corticosteroid adverse effects screening (see Table 138-14) and osteoporosis preventive measures (Chapter 698).
Quick-Reliever Medications
Quick-reliever or “rescue” medications (SABAs, inhaled anticholinergics, and short-course systemic corticosteroids) are used in the management of acute asthma symptoms (Table 138-15).
TABLE 138-15 MANAGEMENT OF ASTHMA EXACERBATION (STATUS ASTHMATICUS)
| RISK ASSESSMENT ON ADMISSION | ||
| Focused history | ||
IM, intramuscular; MDI, metered-dose inhaler; PEF, peak expiratory flow; SABA, short-acting β-agonist; SC, subcutaneous; 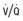 , ventilation-perfusion.
, ventilation-perfusion.
Asthma Exacerbations and Their Management
A severe exacerbation of asthma that does not improve with standard therapy is termed status asthmaticus. Immediate management of an asthma exacerbation involves a rapid evaluation of the severity of obstruction and assessment of risk for further clinical deterioration (see Tables 138-14 and 138-15). For most patients, exacerbations improve with frequent bronchodilator treatments and a course of systemic (oral or intravenous) corticosteroid. However, the optimal management of a child with an asthma exacerbation should include a more comprehensive assessment of the events leading up to the exacerbation and the underlying disease severity. Indeed, the frequency and severity of asthma exacerbations help define the severity of a patient’s asthma. Whereas most children who experience life-threatening asthma episodes have moderate to severe asthma by other criteria, some children with asthma appear to have mild disease except when they suffer severe, even near-fatal exacerbations. The biologic, environmental, economic, and psychosocial risk factors associated with asthma morbidity and death can further guide this assessment (Table 138-16).
Table 138-16 RISK FACTORS FOR ASTHMA MORBIDITY AND MORTALITY
BIOLOGIC
ENVIRONMENTAL
ECONOMIC AND PSYCHOSOCIAL
Emergency Department Management of Asthma Exacerbations
In the emergency department, the primary goals of asthma management include correction of hypoxemia, rapid improvement of airflow obstruction, and prevention of progression or recurrence of symptoms. Interventions are based on clinical severity on arrival, response to initial therapy, and presence of risk factors that are associated with asthma morbidity and mortality (see Table 138-16). Indications of a severe exacerbation include breathlessness, dyspnea, retractions, accessory muscle use, tachypnea or labored breathing, cyanosis, mental status changes, a silent chest with poor air exchange, and severe airflow limitation (PEF or FEV1 value <50% of personal best or predicted values). Initial treatment includes supplemental oxygen, inhaled β-agonist therapy every 20 min for 1 hr, and, if necessary, systemic corticosteroids given either orally or intravenously (see Table 138-15). Inhaled ipratropium may be added to the β-agonist treatment if no significant response is seen with the 1st inhaled β-agonist treatment. An intramuscular injection of epinephrine or other β-agonist may be administered in severe cases. Oxygen should be administered and continued for at least 20 min after SABA administration to compensate for possible ventilation-perfusion abnormalities caused by SABAs.
Hospital Management of Asthma Exacerbations
For patients with moderate to severe exacerbations that do not adequately improve within 1-2 hr of intensive treatment, observation and/or admission to the hospital, at least overnight, is likely to be needed. Other indications for hospital admission include high-risk features for asthma morbidity or death (see Table 138-16). Admission to an intensive care unit is indicated for patients with severe respiratory distress, poor response to therapy, and concern for potential respiratory failure and arrest.
Supplemental oxygen, frequent or continuous administration of an inhaled bronchodilator, and systemic corticosteroid therapy are the conventional interventions for children admitted to the hospital for status asthmaticus (see Table 138-15). Supplemental oxygen is administered because many children hospitalized with acute asthma have or eventually have hypoxemia, especially at night and with increasing SABA administration. SABAs can be delivered frequently (every 20 min to 1 hr) or continuously (at 5-15 mg/hr). When administered continuously, significant systemic absorption of β-agonist occurs and, as a result, continuous nebulization can obviate the need for intravenous β-agonist therapy. Adverse effects of frequently administered β-agonist therapy include tremor, irritability, tachycardia, and hypokalemia. Patients requiring frequent or continuous nebulized β-agonist therapy should have ongoing cardiac monitoring. Because frequent β-agonist therapy can cause ventilation-perfusion mismatch and hypoxemia, oximetry is also indicated. Inhaled ipratropium bromide is often added to albuterol every 6 hr if patients do not show a remarkable improvement, although there is little evidence to support its use in hospitalized children receiving aggressive inhaled β-agonist therapy and systemic corticosteroids. In addition to its potential to provide a synergistic effect with a β-agonist agent in relieving severe bronchospasm, ipratropium bromide may be beneficial in patients who have mucous hypersecretion or are receiving β-blockers.
Rarely, a severe asthma exacerbation in a child results in respiratory failure, and intubation and mechanical ventilation become necessary. Mechanical ventilation in severe asthma exacerbations requires the careful balance of enough pressure to overcome airways obstruction while reducing hyperinflation, air trapping, and the likelihood of barotrauma (pneumothorax, pneumomediastinum) (Chapter 65.1). To minimize the likelihood of such complications, mechanical ventilation should be anticipated, and asthmatic children at risk for the development of respiratory failure should be managed in a pediatric intensive care unit (ICU). Elective tracheal intubation with rapid-induction sedatives and paralytic agents is safer than emergency intubation. Mechanical ventilation aims to achieve adequate oxygenation while tolerating mild to moderate hypercapnia (PCO2 50-70 mm Hg) to minimize barotrauma. Volume-cycled ventilators, using short inspiratory and long expiratory times, 10-15 mL/kg tidal volume, 8-15 breaths/min, peak pressures < 60 cm H2O, and without positive end-expiratory pressure are starting mechanical ventilation parameters that can achieve these goals. As measures to relieve mucous plugs, chest percussion and airways lavage are not recommended because they can induce further bronchospasm. One must consider the nature of asthma exacerbations leading to respiratory failure; those of rapid or abrupt onset tend to resolve quickly (hours to 2 days), whereas those that progress gradually to respiratory failure can require days to weeks of mechanical ventilation. Such prolonged cases are further complicated by muscle atrophy and, when combined with corticosteroid-induced myopathy, can lead to severe muscle weakness requiring prolonged rehabilitation. This myopathy should not be confused with the rare occurrence of an asthma-associated flaccid paralysis (Hopkins syndrome), which is of unknown etiology but prolongs the intensive care stay.
Special Management Circumstances
Management of Infants and Young Children
Recurrent wheezing episodes in preschool-aged children are very common, occurring in as much as one third of this population. Of them, most improve and even become asymptomatic during the prepubescent school-age years, whereas others have lifelong persistent asthma. All require management of their recurrent wheezing problems (see Tables 138-5, 138-6, and 138-11). The updated NIH guidelines recommend risk assessment to identify preschool-aged children who are likely to have persistent asthma. One implication of this recommendation is that these at-risk children may be candidates for conventional asthma management, including daily controller therapy and early intervention with exacerbations (see Tables 138-7, 138-8, and 138-11). Nebulized budesonide and montelukast appear to be more effective than cromolyn. For young children with a history of moderate to severe exacerbations, nebulized budesonide is FDA-approved, and its use as a controller medication could prevent subsequent exacerbations.
Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123:S131-S145.
The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1498.
Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107-1119.
Anderson M, Thomas DA. Drug therapy for chronic asthma in children. Arch Dis Child Educ Prac Ed. 2010;95:145-150.
Andrews T, McGintee E, Mittal MK, et al. High-dose continuous nebulized levalbuterol for pediatric status asthmaticus: a randomized trial. J Pediatr. 2009;155:205-210.
Arita J, Nakae Y, Matsushima H, et al. Hopkins syndrome: T2−weighted high intensity of anterior horn on spinal MR imaging. Pediatr Neurol. 1995;13:263-265.
Armann JP, von Mutius E. Bacteria in the respiratory tract and wheeze in children. BMJ. 2010;341:741-742.
Arnold DH, Hartert TV. What we need to know about long-acting β-2-agonists. Am J Respir Crit Care Med. 2010;182:1219-1220.
Barr RG. Does paracetamol cause asthma in children? Time to remove the guesswork. Lancet. 2008;372:1011-1012.
Beasley R, Clayton T, Crane J, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from phase three of the ISAAC programme. Lancet. 2008;372:1039-1048.
Bernard A, Nickmilder M, Voisin C, et al. Impact of chlorinated swimming pool attendance on the respiratory health of adolescents. Pediatrics. 2009;124:1110-1118.
Bleecker ER, Postma DS, Lawrence RM, et al. Effect of ADRB2 polymorphisms on response to longacting β2-agonist therapy: a pharmacogenetic analysis of two randomized studies. Lancet. 2007;370:2118-2125.
Brodlie M, McKean MC. Exhaled nitric oxide in the diagnosis of childhood asthma. BMJ. 2010;340:113-114.
Bush A, Hedlin G, Carlsen KH, et al. Severe childhood asthma: a common international approach? Lancet. 2008;372:1019-1020.
Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814-822.
Busse WW, Lemanske RFJr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826-834.
Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2010;364(11):1005-1014.
Checkley W. New insights into the treatment of persistent asthma. Lancet. 2011;377:614-616.
Chowdhury BA, Pan GD. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med. 2010;362:1169-1171.
2008 Ciclesonide (Alvesco)—a new inhaled corticosteroid for asthma. Med Lett. 2008;50:75-76.
Coffman JM, Cabana MD, Yelin EH. Do school-based asthma education programs improve self-management and health outcomes? Pediatrics. 2009;124:729-742.
Covar RA, Strunk R, Zeiger RS, et al. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010;125:359-366.
Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701-709.
. EPR—3. Expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication No. 07-4051. Bethesda, MA, 2007, U.S. Department of Health and Human Services; National Institutes of Health, National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm
Etminan M, Sadatsafavi M, Jafaro S, et al. Acetaminophen use and the risk of asthma in children and adults. Chest. 2009;136:1316-1323.
Fairs A, Agbetile J, Hargadon B, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung infection in asthma. Am J Respir Crit Care Med. 2010;182:1362-1368.
Fanta C. Asthma. N Engl J Med. 2009;360:1002-1012.
Fisher EB, Strunk R, Highstein GR, et al. A randomized controlled evaluation of the effect of community health workers on hospitalizations for asthma. Arch Pediatr Adolesc Med. 2009;163:225-232.
Flores G, Bridon C, Torres S, et al. Improving asthma outcomes in minority children: a randomized, controlled trial of parent mentors. Pediatrics. 2009;124:1522-1532.
Gimenez LM, Zafra H. Vocal cord dysfunction: an update. Ann Allergy Asthma Immunol. 2010. (in press)
Global Initiative for Asthma. Global strategy for asthma management and prevention. www.ginasthma.com, 2008. update
Harley KG, Macher JM, Lipsett M, et al. Fungi and pollen exposure in the first months of life and risk of early childhood wheezing. Thorax. 2009;64:353-358.
Henderson J, Granell R, Sterne J. The search for new asthma phenotypes. Arch Dis Child. 2009;94:333-336.
Hernández-Cadena L, Holguin F, Barraza-Villarreal A, et al. Increased levels of outdoor air pollutants are associated with reduced bronchodilation in children with asthma. Chest. 2009;136:1529-1536.
Kelly HW, Van Natta ML, Covar RA, et al. CAMP Research Group: Effect of long-term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the Childhood Asthma Management Program (CAMP) study. Pediatrics. 2008;122:e53-e61.
Kercsmar CM, McDowell KM. Love it or lev it: Levalbuterol for severe acute asthma—for now, leave it. J Pediatr. 2009;155:162-164.
Lai CK, Beasley R, Crane J, et al. International Study of Asthma and Allergies in Childhood Phase Three Study Group: Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009;64:476-483.
Lau S. Transition from childhood to adult asthma. Lancet. 2008;372:1014-1015.
Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
Lemanski RFJr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975-985.
Liberty KA, Pattermore P, Reid J, et al. Beginning school with asthma independently predicts low achievement in a prospective cohort of children. Chest. 2010;138(6):1349-1355.
Martinez FD, Chinchilli V, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomized, double-blind, placebo-controlled trial. Lancet. 2001;377:650-657.
McBride JT. Genes, environment, child care, and asthma. J Pediatr. 2009;155:771-772.
The Medical Letter. Bronchial thermoplasty for asthma. Med Lett. 2010;52(1345):65-66.
The Medical Letter. Mometasone/formoterol (dulera) for asthma. Med Lett. 2010;52(1348):83-84.
The Medical Letter. Stopping long-acting beta-2 agonists. Med Lett. 2010;52(1334):21.
Mehta R, Fisher LEJr, Segeleon JE, et al. Acute rhabdomyolysis complicating status asthmaticus in children. Pediatr Emerg Med. 2006;22:587-591.
Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211-1221.
Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253-1258.
Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985-993.
Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95-100.
Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on-therapy for corticosteroids for children and adults with asthma. Chest. 2011;139(1):28-35.
Schuh S, Willan AR, Stephens D, et al. Can montelukast shorten prednisolone therapy in children with mild to moderate acute asthma? A randomized controlled trial. J Pediatr. 2009;155:795-800.
Simpson AB, Yousef E, Hossain J. Association between peanut allergy and asthma morbidity. J Pediatr. 2010;156:777-781.
Sivan Y, Gadish T, Fireman E, et al. The use of exhaled nitric oxide in the diagnosis of asthma in school children. J Pediatr. 2009;155:211-216.
Sleiman PMA, Flory J, Imielinski M, et al. Variants of DENND1B associated with asthma in children. N Engl J Med. 2010;362:36-44.
Sly PD, Boner AL, Björksten B, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100-1106.
Stern DA, Morgan WJ, Halonen M, et al. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058-1064.
2010 Stopping long-acting beta-2 agonists. Med Lett. 2010;52:21-22.
Strunk RC, Sternberg AL, Szefler SJ, et al. Childhood Asthma Management Program (CAMP) Research Group: Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mold to moderate asthma in children and adolescents. J Pediatr. 2009;154:682-687.
Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomized controlled trial. Lancet. 2008;372:1065-1072.
Takaro TK, Krieger J, Song L, et al. The breathe-easy home: the impact of asthma-friendly home construction on clinical outcomes and trigger exposure. Am J Pub Health. 2011;101(1):55-62.
Taylor DR, Hall IP. ADRB2 polymorphisms and β2 agonists. Lancet. 2007;379:2075-2076.
Thakkar K, Boatright RD, Gilger MA, et al. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125(4):e925-e930.
Thomsen SF, van der Sluis S, Stensballe LG, et al. Exploring the association between severe respiratory syncytial virus infection and asthma. Am J Respir Crit Care Med. 2009;179:1091-1097.
Vaessen-Verberne AAPH, van den Berg NJ, van Nierop JC, et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. Am J Respir Crit Care Med. 2010;182:1221-1227.
Wang C, Abou El-Nour MM, Bennett GW. Survey of pest infestations, asthma, and allergy in low-income housing. J Community Health. 2008;33:31-39.
Wechsler ME, Kunselman SJ, Chinchilli VM, et al. Effect of β2-adrenergic receptor polymorphism on response to long acting β2 agonist in asthma (LARGE trial): a genotype-stratified, randomized, placebo-controlled, crossover trial. Lancet. 2009;374:1754-1764.
Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120:855-864.

 mismatch, and hypoxemia
mismatch, and hypoxemia